Abstract
Question
This umbrella review and guidelines aimed to provide evidence to support the rational choice of selected adjunctive therapies for schizophrenia.
Study selection and analysis
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and World Federation of Societies of Biological Psychiatry (WFSBP)-grading recommendations, 63 randomised control trials (RCTs) (of which 4219 unique participants have completed the RCTs) and 29 meta-analyses were analysed.
Findings
Provisional recommendations (WFSBP-grade 1) could be made for two molecules in augmentation to antipsychotics: (1) N-acetyl-cysteine (NAC, 1200–3600 mg/day, for >12 consecutive weeks) in improving negative symptoms, general psychopathology (positive and negative syndrome scale for schizophrenia (PANSS) general psychopathology factor (G)-G subscale), with the RCTs with the longer duration showing the most robust findings; (2) polyunsaturated fatty acids (3000 mg/day of eicosapentaenoic acid, for >12 weeks) in improving general psychopathology. Weaker recommendations (ie, WFSBP-grade 2) could be drawn for sarcosine (2 g/day) and minocycline (200–300 mg/day) for improving negative symptoms in chronic schizophrenia (not early schizophrenia), and NAC for improving positive symptoms and cognition. Weak recommendations are not ready for clinical practice. There is provisional evidence that oestrogens and raloxifene are effective in some patients, but further research is needed to determine their benefit/risk ratio.
Conclusions
The results of this umbrella review should be interpreted with caution as the number of RCTs included in the meta-analyses was generally small and the effect sizes were weak or medium. For NAC, two RCTs with low risk of bias have provided conflicting results and the WFSBP-grade recommendation included also the results of meta-analyses. These drugs could be provisionally prescribed for patients for whom no other treatments have been effective, but they should be discontinued if they prove ineffective.
Keywords: Schizophrenia & psychotic disorders
WHAT IS ALREADY KNOWN ON THIS TOPIC
Antipsychotics are not effective in all patients with schizophrenia and some drugs have shown effectiveness in meta-analyses, but with inconsistent results.
WHAT THIS STUDY ADDS
An international expert committee has analysed the 63 randomised controlled trials for aminoacids, anti-inflammatory drugs and hormonal therapies and has concluded that N-acetyl-cysteine and polyunsatured fatty acids show an excellent benefit/risk ratio and should be recommended in clinical practice. Preliminary evidence for sarcosine and minocycline calls for further research.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
World Federation of Societies of Biological Psychiatry-grade recommendations are needed to guide clinical practice in addition to meta-analyses. These results call for the development of personalised psychiatry to identify which patient may benefit from which treatment.
Introduction
Antipsychotics currently represent the cornerstone treatment for schizophrenia.1 This class of drugs has transformed the course of the disease, essentially by reducing the positive symptoms, the duration of acute episodes and the risk of relapse.2 All antipsychotics to a varying degree block the D2 receptors in the striatum.3 It was hypothesised that this mechanism of action was a universal target in schizophrenia.3 However, the dopamine hypothesis is as simplistic for schizophrenia as the serotonin hypothesis for depression. For example, it has greater therapeutic translational validity for positive than negative or cognitive symptoms.4 Additionally, non-dopaminergic agents such as trace amine-associated receptor 1 (TAAR1) agents show promise.5 Overall, 30% of patients with schizophrenia do not respond to one antipsychotic and only 40% respond to clozapine, the antipsychotic that is indicated in those showing resistance to two antipsychotics.6 In addition, schizophrenia is heterogeneous. Some clinical subpopulations have been identified as potential new targets for precision medicine interventions. Among them, first-episode psychosis/schizophrenia, women, patients with chronic peripheral inflammation and/or oxidative stress, and treatment-resistant schizophrenia were considered in subgroup analyses in order to mitigate the heterogeneity of the response to antipsychotics reported in these subgroups.7–11
During the last two decades, new biological mechanisms acting on psychosis but not directly on dopamine or its receptors are in late-stage development, emerging as effective antipsychotics. These include the M1/M4 muscarinic agonist xanomeline plus the peripherally restricted anticholinergic trospium and several other procholinergic drugs,12 as well as the TAAR1 agent ulotaront.13 14 In addition, multiple meta-analyses have been published reporting the effectiveness of agents added to antipsychotics in schizophrenia targeting clinical subgroups and/or these pathophysiological pathways.15 Adjunctive antidepressants, such as mirtazapine16 17 and lamotrigine,18 have shown effectiveness in improving the symptomatology of schizophrenia and are recommended in some clinical guidelines.19 The role of augmentation strategies with other psychotropic drugs is less clear. Beyond psychopharmacologic medications, some promising non-psychotropic repurposed medicines have also been investigated in meta-analyses.20–49 However, while meta-analyses aim to provide data for effectiveness and tolerance, they are often not designed to readily inform the clinical practice.
Different meta-analyses may yield inconsistent results according to their inclusion criteria or their representativeness as new evidence becomes available for quantitative synthesis over time. Also, some randomised controlled trials (RCTs) with various risks of bias can swing the results in favour or against one treatment. The benefit/risk ratio is often not explored. When there are only small size RCTs for one treatment, a meta-analysis may overestimate or underestimate a treatment effect. Unless controlled via subgroup analyses, studies of high and low quality or studies from heterogeneous samples may be mixed together yielding spurious results. Despite statistical approaches (eg, funnel plot analyses), publication bias can further affect the results of meta-analyses as it can affect the development of recommendations based on clinical trials. The translation of these results into clinical practice prompts a different methodology developed through the 2019 World Federation of Societies of Biological Psychiatry (WFSBP)-grading recommendations. A consensual method to synthesise the evidence in psychiatry was published in 2018 by the WFSBP.50 For example, the WFSBP and the Canadian Network for Mood and Anxiety Treatments (CANMAT) societies have recently published recommendations for using nutrients in severe mental disorders.51 In their recommendations, only N-acetyl-cysteine (NAC) was recommended for treating negative symptoms of schizophrenia, and polyunsaturated fatty acids (PUFAs) were not recommended. However, these guidelines did not use the 2019 WFSBP methodology, and some evidence suggests that these recommendations could be updated or tempered.
Therefore, this work aimed to synthetise the available best-quality evidence on selected adjunctive treatments, including amino acids, hormonal therapies and anti-inflammatory drugs given adjunctively to current antipsychotics in order to guide clinical practice for the management of schizophrenia for clinicians and to provide evidence-based data for stakeholders and public policy makers.
Methods
The detailed methods for literature search, inclusion criteria, data extraction, subgroups, risk of bias assessment and grading process are presented in online supplemental materials 1–7. The present review did not any receive financial support.
bmjment-2023-300771supp001.pdf (2.3MB, pdf)
Results
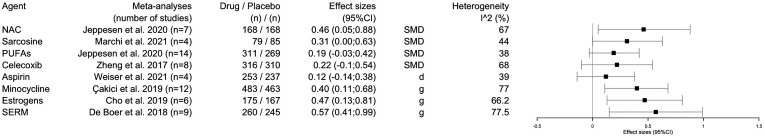
A total of 63 RCTs (4219 patients) and 29 meta-analyses were identified (presented in table 1 and online supplemental material 2). The detailed characteristics of the RCTs, efficacy results, risk of bias and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist are presented in online supplemental materials 3–5. The main effects size of the selected adjunctive agents on total psychopathology is presented in the forest plot of figure 1. The PRISMA flow chart is presented in figure 2. The detailed WFSBP-grade recommendations are presented in online supplemental materials 6 and 7. Importantly, we have changed the wording from ‘strong recommendation’ (corresponding to the GRADE 1 level) to ‘strong provisional recommendation’ to indicate that further RCTs may potentially modify the present recommendations (hence, provisional also applies to moderate and weak recommendations). The influence of sample size, risk of bias, patients’ groups, high-income versus upper middle-income countries are presented in online supplemental materials 8 and 9. We strongly encourage readers to carefully consider the materials on which the present recommendations are based.
Table 1.
Included randomised controlled trials (RCTs) with sample sizes
| Drug | RCTs (N) | RCTs with low/moderate/high risk of bias (N) | Total (N) | Drug (N) | Placebo (N) |
| N-acetyl-cysteine | 8 | 3/4/1 | 523 | 258 | 265 |
| Sarcosine | 6 | 0/4/2 | 211 | 104 | 107 |
| Minocycline | 8 | 4/3/1 | 583 | 298 | 285 |
| PUFA | 14 | 5/5/4 | 809 | 432 | 377 |
| Oestrogens | 9 | 5/3/1 | 677 | 383 | 294 |
| Selective estrogen receptor modulator (raloxifene) | 9 | 6/3/0 | 552 | 275 | 277 |
| Aspirin | 4 | 0/3/1 | 424 | 221 | 203 |
| Celecoxib | 5 | 1/3/1 | 440 | 222 | 218 |
| Total | 63 | 24/28/11 | 4219 | 2193 | 2026 |
PUFA, polyunsaturated fatty acid; SERM, selective estrogen receptor modulator.
Figure 1.
Forest plots of the main effects size of the selected adjunctive agents on total psychopathology. SERM selective estrogen receptor modulator. NAC, N-acetyl-cysteine; PUFAs, polyunsaturated fatty acids; SMD, standardised mean difference.
Figure 2.
Flow diagram.
Discussion
Based on the present umbrella review, we found sufficient evidence to formulate strong provisional recommendations (WFSBP-grade 1) for three adjunctive agents when used in augmentation with antipsychotics in schizophrenia: (1) NAC (when used between 1200 and 3600 mg/day, for at least 12 weeks) with significant improvement in negative symptoms, general psychopathology (here referring to as the symptoms included in the Positive and Negative Syndrome Scale (PANSS) general psychopathology (G) subscale and cognition, with the strongest evidence for the studies going at least 6 months; (2) PUFAs (with doses of 3000 mg/day of eicosapentaenoic acid (EPA), for at least 12 weeks) with significant improvement in general psychopathology; (3) transdermal estradiol (with doses of 0.1–0.2 mg/day) with significant improvement in positive symptoms and general psychopathology in childbearing aged women (especially ≥38 years old). In the latter case, data are, however, limited to 8-week trials. Recommendations regarding NAC and PUFAs are supported by meta-analyses concluding to a significant improvement without publication bias. However, a strong heterogeneity was also reported (67% for NAC and 38% for PUFAs, see figure 1). Weaker recommendations (ie, WFSBP-grade 2) could be made for other adjunctive agents when used in augmentation with antipsychotics for improving (1) negative symptoms: sarcosine (2 g/day), minocycline (200–300 mg/day), oestrogens (either daily 2 mg estradiol valerate or 0.625 mg conjugated oestrogens with 2.5 mg medroxyprogesterone acetate); (2) positive symptoms: NAC 1200 mg/day for at least 12 weeks or (3) general psychopathology: 2 mg estradiol valerate in men but only evaluated for 2 weeks.
The present work adds important findings to the previously published meta-analyses. In the light of these results, patients with respective negative, cognitive or general psychopathological symptoms could be encouraged to take NAC and/or PUFAs, as these agents are available over-the-counter. The findings pertaining to NAC are particularly intriguing, as they demonstrate medium-term effects within the 12-week to 24-week timeframe. This raises important questions about the timing of observation and suggests that long-term assessments may yield additional valuable insights. Of note, in the case of NAC, the level Of Evidence (LoE) is only B but the grade recommendation was 1 due to its excellent acceptability, as recommended in the WFSBP guidelines. However, the off-label prescription of oestrogens and raloxifene in all women with schizophrenia cannot be recommended for sure due to safety issues (even in those aged >38 years in whom oestrogens and raloxifene seem more effective). The present results only confirm the efficacy of these agents that may still be an option in some case of resistance to conventional treatments.
In patients with an acute episode of schizophrenia, no adjunctive treatment in co-initiation to antipsychotics could be recommended with WFSBP-grade 1 evidence due to methodological issues of combining the augmentation of adjunctive agent with varying dosages of antipsychotics during the acute stabilisation phase. Yet, weaker recommendations (WFSBP-grade 2) could be made for improving (1) negative symptoms: sarcosine (2 g/day), minocycline (200–300 mg/day), raloxifene (120 mg/day) in men; (2) positive symptoms: PUFA (at least 2000 mg/day EPA) for patients with low PUFA levels, celecoxib (400 mg/day) or (3) general psychopathology: sarcosine (2 g/day), celecoxib (400 mg/day), raloxifene (120 mg/day) in men.
These recommendations are derived from the careful analysis of RCTs and meta-analyses representing the existing literature. To avoid any misleading interpretation of current practice, we would like to stress that the use of adjunctive treatment should come in second line after all attempts to optimise patients’ current antipsychotic treatment and psychosocial therapies52 have been made (dose optimisation, antipsychotic plasma level monitoring, managing comorbidity such as substance abuse and ruling out of somatic causes for non-response, etc) according to current recommendations.53 It is also important to carefully consider the benefit/risk balance before prescribing any adjunctive treatment. This is crucial to avoid augmentation with ineffective agents that carry a risk of side effects (including more severe negative symptoms). For instance, reducing the dosage of antipsychotics to their minimal effective dose may be safe (some studies suggest that it is not associated with a significantly increased risk of rehospitalisation compared with maintaining the treatment to the same dosage54–56) and it may be an efficient strategy to improve negative symptoms54–56 and cognition.57 This strategy should thus be first discussed with patients who stabilise after a first or multiple episode(s) before envisioning the use of adjunctive treatments for negative symptoms or cognition. There is consistent evidence for oestrogen augmentation in women with schizophrenia especially those over 38 years, but clinical implementation has not yet become common practice. The use of oestrogen needs to be done safely and concordant with existing practice guidelines, for gonadal hormone therapies. Oestrogen can be prescribed clinically as combined oral contraceptives for pre-menopausal women or through hormonal replacement therapy (estradiol patches, with regular progesterone addition) for post-menopausal women. Raloxifene could be an alternative for post-menopausal women. Further clinical research is required to determine the efficacy and safety for the clinical use of oestrogen therapies in the treatment of women with schizophrenia.
A somewhat paradoxical aspect of the present results should be mentioned regarding adjunctive treatment with the selected agents. The adjunctive therapies reviewed here were thought to target (1) particular biological pathways putatively involved in the pathophysiology of schizophrenia or (2) particular patients with schizophrenia presenting alterations of one of those biological pathways. However, almost all studies considered the schizophrenia group as a whole, and only a few examined or stratified for the kind of patients who were most likely to benefit from the tested drug. For example, although lower PUFA blood levels have been shown in schizophrenia as a group,58 only one RCT with a low risk of bias evaluating the effect of PUFA in schizophrenia took the blood level deficiency of PUFAs into account and showed significant improvement of positive symptoms only in this group.59 Similarly, among all RCTs testing the use of anti-inflammatory drugs, only one made the distinction between patients with/without low-grade peripheral inflammation (defined by CRP blood level ≥1 mg/L).60 Also, few studies testing the effect of adjunctive hormonal therapies tested hormone levels in the included patients (eg, Kulkarni et al 61). Similarly, the interpretation of the results for general psychopathology is complex from a precision psychiatry perspective, as the PANSS-G factor encompasses heterogeneous symptoms such as anxiety, depression, lack of insight and attention disorders. All in all, these findings underscore the pressing need to promote the validation of precision psychiatry approaches in future research. However, it is important to acknowledge that this also presents additional challenges in terms of study recruitment and feasibility.
Our results also suggest the benefits of applying the WFSBP-grade recommendations, as our conclusions differ somewhat from those of other meta-analyses. Our findings demonstrate that weak or moderate mean effect sizes in meta-analyses should not be directly translated into recommendations for or against prescribing a specific agent or group of agents. In fact, these effect sizes may correspond to a weak or limited level of evidence. Furthermore, additional complexities arise in trials comparing augmentation and co-initiation approaches. The discrepancies between the results of augmentation and co-initiation trials indicate the relevance of this distinction. In co-initiation trials, the control groups receive an active antipsychotic treatment that reduces psychotic symptoms, while augmentation trials typically involve a stable antipsychotic treatment in the control group, which may result in potentially weaker and clinically significant variations. However, it is important to highlight certain features of the WFSBP-grade system to clarify our recommendations. The designation of ‘limited evidence’ (WFSBP-grade 2) can be assigned when the agents are well tolerated and when one of the following conditions is met: (1) a single RCT with low or moderate risk of bias shows significant improvement without other RCTs of equal quality demonstrating non-significant results, or (2) two RCTs with low risk of bias yield contradictory results, but a meta-analysis demonstrates significant improvement. Therefore, the conclusions may be influenced by both the number of RCTs and the quality of the evidence. This findings should be taken into consideration when interpreting the recommendations for certain adjunctive treatments such as sarcosine, minocycline or oestrogens, as there were generally few RCTs available. It also emphasises the need for new large-scale studies of high quality and low risk of bias to confirm the validity of our recommendations.
It is important to acknowledge that, despite following the SIGN recommendations for grading the risk of bias, there remains a potential for global subjectivity (as mentioned in the SIGN method) regarding the final level of evidence. From this point of view, our grading may appear more stringent compared with the results of some meta-analyses, but it must be acknowledged on the contrary that other guidelines such as the NICE or the GRADE system have a more stringent and may thus come to distinct recommendations than ours. Given that our objective was to support evidence-based practice, we aimed to provide the most rigorous recommendations without dismissing potentially effective agents. Additionally, the risk of bias is also supported by treatment allocation concealment. Blinding between active agents and placebo is not always straightforward, as some active agents may induce noticeable adverse events, which could compromise blinding. This may not be the case for amino acids but may be more common for other active agents included in our work. For example, minocycline could cause diarrhoea, and aspirin may result in easy bruising, which may indicate to participants that they are receiving the active agent. Furthermore, it was not always clear in trials involving PUFAs whether the placebo was comparable with PUFA treatment in terms of flavour. It is worth noting that the same criticism can be raised regarding the side effects of common antipsychotics.
This area of research has several limitations that should be acknowledged. First, we focused this review on augmentation strategies of antipsychotics in schizophrenia, as psychotropic augmentation strategies of antipsychotics have been comprehensively reviewed in a previous umbrella review.62 Second, the number of RCTs included in the meta-analyses assessed in this umbrella review was generally small so that the inclusion of data from new low-risk-of-bias randomised controlled trials carries a high likelihood of potentially influencing the current recommendations in one way or another. Consequently, we have opted to make ‘provisional’ recommendations for some molecules rather than definitive recommendations. To mitigate this limitation as much as possible, we conducted additional literature searches and included any newly published RCTs since the completion of the last meta-analysis. Third, there was heterogeneity in study designs, populations, study durations and intervention doses. To address this relevant heterogeneity, particularly in terms of illness phase and augmentation versus co-initiation strategies, we stratified the results and recommendations based on these important factors. It is important to note that our recommendations were not based on the effect size of each drug, as individual patient responses can vary greatly. Additionally, meta-analyses yielded different effect sizes due to the inclusion of RCTs with a high risk of bias. The focus of our recommendations was solely on the superiority of the add-on strategy with an active agent compared with add-on placebo. While there may have been a potential bias in low sample size studies regarding cognition, we found no evidence of such bias for positive and negative symptoms. The fact that low risk of bias studies had a higher likelihood of showing significant effects on general psychopathology further supports the validity of our results.
Fourth, we also found that the occurrence of positive significant results for negative symptoms was more frequent in studies conducted in upper middle-income countries compared with high-income countries (see online supplemental material 9). This difference was consistently observed when analysing studies with low risk of bias or specifically focusing on NAC, oestrogens and PUFAs. Therefore, one could argue that our recommendations for these drugs may have been biased by those particular studies. This also justifies our use of the term ‘provisional’, as further studies conducted in high-income countries may help confirm our recommendations. Additionally, numerous findings have been provided by Chinese and Iranian research teams, and some authors have shown that accurately assessing the bias risk of these studies can be challenging and can possibly hide high risk of bias.63 The inclusion of Chinese and Iranian researchers in future recommendations could help address this issue.
Fifth, the field of adjunctive treatments is more complex to explore and analyse than antipsychotic monotherapy for multiple reasons exposed in our results. While almost all low risk of bias RCTs used fixed doses, an important number of high risk of bias RCTs did not adequately describe the antipsychotics administered in each arm at baseline and during the trial or did not check if these treatments were comparable between groups at the end of the trial. In these RCTs, the described effect could be due to a significant effect of the adjunctive drug or to a change of antipsychotic dose in one of the arms. WFSBP guidelines ensured that no recommendation was influenced on this kind of bias by downgrading the level of evidence when necessary. In addition, for several adjunctive drugs, the evidence was based on many small studies. However, as the overall meta-regression analyses from the study by Jeppesen et al 45 showed a decreasing effect with the sample size, large low risk of bias studies are needed to confirm our recommendations, thus keeping in mind that sample size had no relevant influence on the probability to find a significant effect on the 63 studies included (see online supplemental material 9). Sixth, while one strength of our systematic umbrella review and recommendations is that we made the distinction between some symptom dimensions of schizophrenia, this may also account for some differences between our recommendations and previous ones, for instance, regarding PUFAs.51 However, we found that no data to evaluate functioning and patient-reported outcomes and other important information on costs or adverse events (including those like irritability or weight gain) were not or not fully assessed due to limited data availability. The present recommendations are therefore only based on clinician-rated symptoms of the PANSS (for most studies) and the effect on quality of life and daily-life functioning is unclear. Given that patient input is crucial, particularly for weak recommendations where the benefit/risk balance is uncertain, future recommendations should include patients.
Seventh, due to lack of data, we were not able to integrate depression or anxiety in our outcomes. Depression is present in at least one-third of patients with schizophrenia64 and has a major impact on prognosis and quality of life.65 Including depression (optimally with the specific Calgary Depression Rating Scale66) is strongly recommended for future research and might be useful to appreciate a putative antidepressant effect of PUFA in schizophrenia, as previously suggested in mood disorders.51 67 Finally, since the search for the study ended in February 2022, it potentially missed more recent evidence that will need to be included in future, up-to-date studies.
Conclusion
Based on the existing literature, the adjunctive use of NAC and PUFA can be tentatively recommended in all phases of schizophrenia, considering their potential benefits for negative symptoms and/or general psychopathology, as well as their high acceptability. NAC also appears to be the only adjunctive treatment that may have potential benefits for cognition. However, it should be emphasised that these recommendations are solely based on a limited number of RCTs, and the current recommendations are derived from significant results and risk of bias rather than effect sizes. These drugs should potentially be prescribed for patients for whom antipsychotics have proven ineffective in addressing negative symptoms and/or general psychopathology and/or cognitive function. Furthermore, if these drugs are found to be ineffective, they should be discontinued. The term ‘provisional’ has been used to underscore this point. Transdermal estradiol, used for short-term, can also be provisionally recommended in women of childbearing age to improve positive symptoms. Other adjunctive agents, such as sarcosine, minocycline, oestrogens or SERMs, may also be effective in certain clinical scenarios, but additional low-risk RCTs are needed. It is important to note that some safety concerns should be considered for these agents, with the exception of sarcosine. These recommendations are provisional and emphasise the need for further research on targeted approaches, such as selecting or stratifying patients based on inflammatory markers or nutrient levels. Large-scale studies with low risk of bias are required to identify the patients who are most likely to benefit from a specific adjunctive agent.
bmjment-2023-300771supp002.pdf (109.4KB, pdf)
Footnotes
Contributors: GF and FB initiated and coordinated the meetings, wrote the initial draft and were responsible for the sarcosine, minocycline and aspirin (GF) and N-acetyl-cysteine and celecoxib (FB) workgroups, respectively. The other leaders of the working groups were: JM (oestrogens), BP (SERM), MU (PUFA). FS, DM and RR participated in the original meetings. JK and IECS reviewed the hormonal therapy section. MB and MS reviewed the N-acetyl-cysteine and minocycline sections. All authors reviewed the final draft and gave their validation for the final content. GF is guarantor.
Funding: Michael Berk is supported by a NHMRC Senior Principal Research Fellowship and Leadership 3 Investigator grant (1156072 and 2017131). No funding for the other authors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: Tweet: NAC and omega-3 fatty acid are provisonnaly recommended in schizophrenia in addition to antipsychotics. Results of an umbrella systematic review and guidelines.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Kane JM, Correll CU. Pharmacologic treatment of schizophrenia. Dialogues Clin Neurosci 2010;12:345–57. 10.31887/DCNS.2010.12.3/jkane [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers 2015;1:15067. 10.1038/nrdp.2015.67 [DOI] [PubMed] [Google Scholar]
- 3. McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci 2019;42:205–20. 10.1016/j.tins.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Revel FG, Moreau J-L, Pouzet B, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 2013;18:543–56. 10.1038/mp.2012.57 [DOI] [PubMed] [Google Scholar]
- 5. Dodd S, F Carvalho A, Puri BK, et al. Trace amine-associated receptor 1 (TAAR1): a new drug target for psychiatry? Neurosci Biobehav Rev 2021;120:537–41. 10.1016/j.neubiorev.2020.09.028 [DOI] [PubMed] [Google Scholar]
- 6. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry 2017;62:772–7. 10.1177/0706743717718167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mustafa FA. Could some patients with treatment-resistant schizophrenia have reversible conditions. Psychother Psychosom 2018;87:369. 10.1159/000493367 [DOI] [PubMed] [Google Scholar]
- 8. Hardingham GE, Do KQ. Linking early-life NMDAR Hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci 2016;17:125–34. 10.1038/nrn.2015.19 [DOI] [PubMed] [Google Scholar]
- 9. Kalinkovich A, Pouyrovsky M, Nasyrova R, et al. Resolution of chronic inflammation as a new adjunctive approach in schizophrenia treatment. Brain Behav Immun 2020;88:867–9. 10.1016/j.bbi.2020.03.024 [DOI] [PubMed] [Google Scholar]
- 10. Brand BA, de Boer JN, Dazzan P, et al. Towards better care for women with schizophrenia-spectrum disorders. Lancet Psychiatry 2022;9:330–6. 10.1016/S2215-0366(21)00383-7 [DOI] [PubMed] [Google Scholar]
- 11. O’Keeffe D, Kinsella A, Waddington JL, et al. 20-year prospective, sequential follow-up study of heterogeneity in associations of duration of untreated psychosis with symptoms, functioning, and quality of life following first-episode psychosis. AJP 2022;179:288–97. 10.1176/appi.ajp.2021.20111658 [DOI] [PubMed] [Google Scholar]
- 12. Yohn SE, Weiden PJ, Felder CC, et al. Muscarinic acetylcholine receptors for psychotic disorders: bench-side to clinic. Trends Pharmacol Sci 2022;43:1098–112. 10.1016/j.tips.2022.09.006 [DOI] [PubMed] [Google Scholar]
- 13. Kane JM. A new treatment paradigm: targeting trace amine-associated receptor 1 (TAAR1) in schizophrenia. J Clin Psychopharmacol 2022;42:S1–13. 10.1097/JCP.0000000000001596 [DOI] [PubMed] [Google Scholar]
- 14. Koblan KS, Kent J, Hopkins SC, et al. A non–D2-receptor-binding drug for the treatment of schizophrenia. N Engl J Med 2020;382:1497–506. 10.1056/NEJMoa1911772 [DOI] [PubMed] [Google Scholar]
- 15. Sinkeviciute I, Begemann M, Prikken M, et al. Efficacy of different types of cognitive enhancers for patients with schizophrenia: a meta-analysis. Npj Schizophr 2018;4:22. 10.1038/s41537-018-0064-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. AJP 2016;173:876–86. 10.1176/appi.ajp.2016.15081035 [DOI] [PubMed] [Google Scholar]
- 17. Galling B, Vernon JA, Pagsberg AK, et al. Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr Scand 2018;137:187–205. 10.1111/acps.12854 [DOI] [PubMed] [Google Scholar]
- 18. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophrenia Research 2009;109:10–4. 10.1016/j.schres.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 19. NICE . Psychosis and schizophrenia in adults: prevention and management. NICE, 2014. [Google Scholar]
- 20. Irving CB, Mumby-Croft R, Joy LA, et al. Polyunsaturated fatty acid supplementation for schizophrenia. Cochrane Database Syst Rev 2006. 10.1002/14651858.CD001257.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai GE, Lin P-Y. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis [DOI] [PubMed]
- 22. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs 2011;25:859–85. 10.2165/11586650-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 23. Begemann MJH, Dekker CF, van Lunenburg M, et al. Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophrenia Research 2012;141:179–84. 10.1016/j.schres.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 24. Fusar-Poli P, Berger G. Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol 2012;32:179–85. 10.1097/JCP.0b013e318248b7bb [DOI] [PubMed] [Google Scholar]
- 25. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry 2012;73:414–9. 10.4088/JCP.10r06823 [DOI] [PubMed] [Google Scholar]
- 26. Sommer IE, van Westrhenen R, Begemann MJH, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 2014;40:181–91. 10.1093/schbul/sbt139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Kemp WJM, Klomp DWJ, Kahn RS, et al. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr Res 2012;141:153–61. 10.1016/j.schres.2012.08.014 [DOI] [PubMed] [Google Scholar]
- 28. Nitta M, Kishimoto T, Müller N, et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull 2013;39:1230–41. 10.1093/schbul/sbt070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stafford MR, Jackson H, Mayo-Wilson E, et al. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ 2013;346:f185. 10.1136/bmj.f185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oya K, Kishi T, Iwata N. Efficacy and tolerability of minocycline augmentation therapy in schizophrenia: a systematic review and meta-analysis of randomized controlled trials. Hum Psychopharmacol 2014;29:483–91. 10.1002/hup.2426 [DOI] [PubMed] [Google Scholar]
- 31. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry Off J Am Acad Clin Psychiatr 2015;27:289–96. [PubMed] [Google Scholar]
- 32. Solmi M, Veronese N, Thapa N, et al. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr 2017;22:415–26. 10.1017/S1092852916000638 [DOI] [PubMed] [Google Scholar]
- 33. Xiang Y-Q, Zheng W, Wang S-B, et al. Adjunctive minocycline for schizophrenia: a meta-analysis of randomized controlled trials. European Neuropsychopharmacology 2017;27:8–18. 10.1016/j.euroneuro.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 34. Zheng W, Cai D-B, Yang X-H, et al. Adjunctive celecoxib for schizophrenia: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Psychiatr Res 2017;92:139–46. 10.1016/j.jpsychires.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 35. Zheng W, Zhang Q-E, Cai D-B, et al. N-Acetylcysteine for major mental disorders: a systematic review and meta-analysis of randomized controlled trials. Acta Psychiatr Scand 2018;137:391–400. 10.1111/acps.12862 [DOI] [PubMed] [Google Scholar]
- 36. Zheng W, Zhu X-M, Zhang Q-E, et al. Adjunctive minocycline for major mental disorders: a systematic review. J Psychopharmacol 2019;33:1215–26. 10.1177/0269881119858286 [DOI] [PubMed] [Google Scholar]
- 37. de Boer J, Prikken M, Lei WU, et al. The effect of raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a systematic review and meta-analysis. NPJ Schizophr 2018;4:1. 10.1038/s41537-017-0043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Q, Dong X, Wang Y, et al. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a meta-analysis of randomized controlled trials. Arch Womens Ment Health 2018;21:31–41. 10.1007/s00737-017-0773-2 [DOI] [PubMed] [Google Scholar]
- 39. Zhu X-M, Zheng W, Li X-H, et al. Adjunctive raloxifene for postmenopausal women with schizophrenia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Schizophrenia Research 2018;197:288–93. 10.1016/j.schres.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 40. Chang C-H, Lane H-Y, Tseng P-T, et al. Effect of N-methyl-D-aspartate-receptor-enhancing agents on cognition in patients with schizophrenia: a systematic review and meta-analysis of double-blind randomised controlled trials. J Psychopharmacol 2019;33:436–48. 10.1177/0269881118822157 [DOI] [PubMed] [Google Scholar]
- 41. Chang C-H, Lin C-H, Liu C-Y, et al. Efficacy and cognitive effect of Sarcosine (N-Methylglycine) in patients with schizophrenia: a systematic review and meta-analysis of double-blind randomised controlled trials. J Psychopharmacol 2020;34:495–505. 10.1177/0269881120908016 [DOI] [PubMed] [Google Scholar]
- 42. Cho M, Lee TY, Kwak YB, et al. Adjunctive use of anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry 2019;53:742–59. 10.1177/0004867419835028 [DOI] [PubMed] [Google Scholar]
- 43. Devoe DJ, Farris MS, Townes P, et al. Attenuated psychotic symptom interventions in youth at risk of psychosis: a systematic review and meta-analysis. Early Interv Psychiatry 2019;13:3–17. 10.1111/eip.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Çakici N, van Beveren NJM, Judge-Hundal G, et al. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med 2019;49:2307–19. 10.1017/S0033291719001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeppesen R, Christensen RHB, Pedersen EMJ, et al. Efficacy and safety of anti-inflammatory agents in treatment of psychotic disorders - A comprehensive systematic review and meta-analysis. Brain, Behavior, and Immunity 2020;90:364–80. 10.1016/j.bbi.2020.08.028 [DOI] [PubMed] [Google Scholar]
- 46. Yolland CO, Hanratty D, Neill E, et al. Meta-analysis of randomised controlled trials with N-Acetylcysteine in the treatment of schizophrenia. Aust N Z J Psychiatry 2020;54:453–66. 10.1177/0004867419893439 [DOI] [PubMed] [Google Scholar]
- 47. Goh KK, Wu T-H, Chen C-H, et al. Efficacy of N-methyl-D-aspartate receptor modulator augmentation in schizophrenia: a meta-analysis of randomised, placebo-controlled trials. J Psychopharmacol 2021;35:236–52. 10.1177/0269881120965937 [DOI] [PubMed] [Google Scholar]
- 48. Goh KK, Chen CY-A, Chen C-H, et al. Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: a meta-analysis of randomized controlled trials. J Psychopharmacol 2021;35:221–35. 10.1177/0269881120981392 [DOI] [PubMed] [Google Scholar]
- 49. Marchi M, Galli G, Magarini FM, et al. Sarcosine as an add-on treatment to antipsychotic medication for people with schizophrenia: a systematic review and meta-analysis of randomized controlled trials. Expert Opin Drug Metab Toxicol 2021;17:483–93. 10.1080/17425255.2021.1885648 [DOI] [PubMed] [Google Scholar]
- 50. Hasan A, Bandelow B, Yatham LN, et al. WFSBP guidelines on how to grade treatment evidence for clinical guideline development. World J Biol Psychiatry 2019;20:2–16. 10.1080/15622975.2018.1557346 [DOI] [PubMed] [Google Scholar]
- 51. Sarris J, Ravindran A, Yatham LN, et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: the world federation of societies of biological psychiatry (WFSBP) and Canadian network for mood and anxiety treatments (CANMAT) taskforce. World J Biol Psychiatry 2022;23:424–55. 10.1080/15622975.2021.2013041 [DOI] [PubMed] [Google Scholar]
- 52. Norman R, Lecomte T, Addington D, et al. Canadian treatment guidelines on psychosocial treatment of schizophrenia in adults. Can J Psychiatry 2017;62:617–23. 10.1177/0706743717719894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shimomura Y, Kikuchi Y, Suzuki T, et al. Antipsychotic treatment in the maintenance phase of schizophrenia: an updated systematic review of the guidelines and algorithms. Schizophrenia Research 2020;215:8–16. 10.1016/j.schres.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 54. Tani H, Takasu S, Uchida H, et al. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacol 2020;45:887–901. 10.1038/s41386-019-0573-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Højlund M, Kemp AF, Haddad PM, et al. Standard versus reduced dose of antipsychotics for relapse prevention in multi-episode schizophrenia: a systematic review and meta-analysis of randomised controlled trials. Lancet Psychiatry 2021;8:471–86. 10.1016/S2215-0366(21)00078-X [DOI] [PubMed] [Google Scholar]
- 56. Leucht S, Bauer S, Siafis S, et al. Examination of dosing of antipsychotic drugs for relapse prevention in patients with stable schizophrenia: a meta-analysis. JAMA Psychiatry 2021;78:1238. 10.1001/jamapsychiatry.2021.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cochrane Schizophrenia Group, Rodolico A, Siafis S, et al. Antipsychotic dose reduction compared to dose continuation for people with schizophrenia. Cochrane Database Syst Rev 2022;2022. 10.1002/14651858.CD014384.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li N, Yang P, Tang M, et al. Reduced erythrocyte membrane polyunsaturated fatty acid levels indicate diminished treatment response in patients with multi- versus first-episode schizophrenia. Schizophr 2022;8:7. 10.1038/s41537-022-00214-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bentsen H, Osnes K, Refsum H, et al. A randomized placebo-controlled trial of an omega-3 fatty acid and vitamins E+C in schizophrenia. Transl Psychiatry 2013;3:e335. 10.1038/tp.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weiser M, Zamora D, Levi L, et al. Adjunctive aspirin vs placebo in patients with schizophrenia: results of two randomized controlled trials. Schizophr Bull 2021;47:1077–87. 10.1093/schbul/sbaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kulkarni J, Riedel A, de Castella AR, et al. Estrogen Ð a potential treatment for schizophrenia. Schizophrenia Research 2001;48:137–44. 10.1016/S0920-9964(00)00088-8 [DOI] [PubMed] [Google Scholar]
- 62. Correll CU, Rubio JM, Inczedy-Farkas G, et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 2017;74:675–84. 10.1001/jamapsychiatry.2017.0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tong Z, Li F, Ogawa Y, et al. Quality of randomized controlled trials of new generation antidepressants and antipsychotics identified in the China National knowledge infrastructure (CNKI): a literature and telephone interview study. BMC Med Res Methodol 2018;18:96. 10.1186/s12874-018-0554-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Etchecopar-Etchart D, Korchia T, Loundou A, et al. Comorbid major depressive disorder in schizophrenia: a systematic review and meta-analysis. Schizophrenia Bulletin 2021;47:298–308. 10.1093/schbul/sbaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andrianarisoa M, Boyer L, Godin O, et al. Childhood trauma, depression and negative symptoms are independently associated with impaired quality of life in schizophrenia. results from the National FACE-SZ cohort. Schizophrenia Research 2017;185:173–81. 10.1016/j.schres.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 66. Addington J, Shah H, Liu L, et al. Reliability and validity of the Calgary depression scale for schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophrenia Research 2014;153:64–7. 10.1016/j.schres.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Firth J, Teasdale SB, Allott K, et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019;18:308–24. 10.1002/wps.20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjment-2023-300771supp001.pdf (2.3MB, pdf)
bmjment-2023-300771supp002.pdf (109.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request.