Abstract
The in vitro antimalarial activity of the new Chinese synthetic drug, lumefantrine, also known as benflumetol (a fluorene derivative belonging to the aminoalcohol class), was determined by an isotopic microtest against 61 fresh clinical isolates of Plasmodium falciparum and compared with that of other established antimalarial agents. The geometric mean 50% inhibitory concentration of lumefantrine was 11.9 nmol/liter (95% confidence intervals, 10.4 to 13.6 nmol/liter; range, 3.3 to 25.6 nmol/liter). The in vitro activities of lumefantrine against the chloroquine-sensitive and the chloroquine-resistant isolates did not differ (P > 0.05). There was a significant positive correlation of responses between lumefantrine and two other aminoalcohols studied, mefloquine (r = 0.688) and halofantrine (r = 0.677), and between lumefantrine and artesunate (r = 0.420), suggesting a potential for in vitro cross-resistance. Our data suggest high in vitro activity of lumefantrine, comparable to that of mefloquine, and are in agreement with the promising results of preliminary clinical trials.
The spread of resistance to chloroquine and sulfadoxine-pyrimethamine in many regions where malaria is endemic, as well as diminished sensitivity to quinine and mefloquine in some restricted areas, calls for a continuous search for new antimalarial drugs that are effective against drug-resistant Plasmodium falciparum (31). New antimalarial drugs that possess high in vitro activity and show high clinical efficacy for the treatment of multidrug-resistant falciparum malaria include halofantrine, atovaquone, artemisinin derivatives (artesunate, artemether, and arteether), and pyronaridine (23). Halofantrine, a 9-phenanthrenemethanol (aminoalcohol), has several disadvantages, such as rare occurrences of cardiotoxicity and poor absorption (8, 22). Atovaquone, a hydroxynaphthoquinone derivative, is a ubiquinone analog that has a wide antiprotozoal spectrum of activity (17). Despite its high in vivo efficacy, preliminary clinical trials have revealed a few cases of recrudescence, leading to the use of a synergistic combination, atovaquone-proguanil (11, 25). Artemisinin derivatives are sesquiterpene lactones that are useful for both oral treatment of uncomplicated malaria due to multidrug-resistant P. falciparum and emergency treatment of severe and complicated malaria (16). Despite their proven clinical efficacy, there are concerns regarding their potential neurotoxicity (9, 24). Pyronaridine, a benzonnaphthyridine (aminoacridine) derivative, has been used in China for over 2 decades and has been confirmed to be effective, safe, and well tolerated in Thailand and Cameroon (12, 15, 19, 26).
Of these four latest kinds of antimalarial drugs, two of them, artemisinin derivatives and pyronaridine, were drawn from the Chinese pharmacopoeia. A third Chinese type of drug, lumefantrine, has recently been identified as a promising candidate for use in therapy (Fig. 1). Lumefantrine (also called benflumetol, code no. CGP 56695; 1:1 racemate of the dextrogyre and levogyre enantiomers) is a fluorene derivative (2,3-benzindene) that belongs to the aminoalcohol class (32). The compound was synthesized at the Academy of Military Medical Sciences, Beijing, China, and has undergone preliminary clinical studies in China. Lumefantrine, in combination with artemether (called coartemether), was registered for the oral treatment of malaria in China in 1987. In early clinical studies conducted in China, cure rates of 96% (n = 314 patients) and 92.5% (n = 40 patients) were reported with oral lumefantrine alone (a total dose of 2,000 mg in divided doses over 4 days) and lumefantrine-artemether oral combination (1,920 mg of lumefantrine–320 mg of artemether in divided doses over 3 days), respectively (32). The clinical efficacy of the lumefantrine-artemether combination has been confirmed recently in China, Thailand, and The Gambia (28–30, 33).
FIG. 1.
Chemical structures of chloroquine, mefloquine, and lumefantrine.
In view of the highly promising preliminary in vivo data, we have (i) assessed the in vitro activity of lumefantrine against clinical isolates of P. falciparum obtained from symptomatic Cameroonian patients in Yaoundé, Cameroon; (ii) compared its activity with those of chloroquine, monodesethylamodiaquine (a biologically active metabolite of amodiaquine), quinine, mefloquine, halofantrine, artesunate, and pyrimethamine; and (iii) evaluated the potential for in vitro cross-resistance between lumefantrine and other antimalarial compounds.
MATERIALS AND METHODS
Parasites.
Sixty-one fresh clinical isolates of P. falciparum were obtained before treatment from symptomatic African adult and pediatric patients attending the Nlongkak Catholic missionary dispensary in Yaoundé in 1997. The patients were questioned about drug intake and screened for self-medication by the Saker-Solomons urine test (20). Giemsa-stained thin blood smears were examined for Plasmodium species identification, and parasite density was determined by counting the number of infected erythrocytes among 20,000 erythrocytes. Five to ten milliliters of venous blood was collected in a tube coated with EDTA after informed consent of the patients (or that of accompanying parents or guardians of sick children) was obtained. Samples with a monoinfection due to P. falciparum and a parasite count of >0.1% from patients whose urine test result was negative were used in this study. Drug assays were performed within 4 h after blood extraction. The patients were treated with chloroquine, amodiaquine, or sulfadoxine-pyrimethamine. This study was approved by the Cameroonian national ethics committee.
Drugs.
Antimalarial drugs used in this study were obtained from the following sources: chloroquine diphosphate, Sigma Chemical Co. (St. Louis, Mo.); monodesethylamodiaquine hydrochloride, Sapec Fine Chemicals (Lugano, Switzerland); mefloquine hydrochloride, Hoffmann-La Roche (Basel, Switzerland); halofantrine hydrochloride, SmithKline Beecham (Hertfordshire, United Kingdom); lumefantrine, Novartis Pharma (Basel, Switzerland); artesunate, Sanofi Winthrop (Gentilly, France); quinine hydrochloride, Sigma Chemical Co.; and pyrimethamine base, Sigma Chemical Co. Stock solutions of chloroquine and monodesethylamodiaquine were prepared in sterile distilled water. Stock solutions of mefloquine, halofantrine, and quinine were prepared in methanol. Stock solutions of artesunate and pyrimethamine were prepared in ethanol. Due to very low solubility in water, lumefantrine was dissolved in culture-compatible organic tensides (unsaturated fatty acids, Tween 80, and ethanol mixture), according to the protocol kindly provided by Walther Wernsdorfer (University of Vienna, Vienna, Austria) (32). Twofold serial dilutions of the drugs were made in sterile distilled water. The final concentration of methanol did not exceed 0.1%, and the solvent alone did not affect the parasite growth. The final concentrations ranged from 1.25 to 80 nmol/liter for lumefantrine, 25 to 1,600 nmol/liter for chloroquine and quinine, 2.5 to 160 nmol/liter for monodesethylamodiaquine, 2.5 to 400 nmol/liter for mefloquine, 0.25 to 32 nmol/liter for halofantrine, 0.5 to 64 nmol/liter for artesunate, and 0.04 to 51,200 nmol/liter (in fourfold dilutions) for pyrimethamine. Each concentration was distributed in triplicate in 96-well tissue culture plates.
In vitro drug sensitivity assay.
The blood samples were washed three times in RPMI 1640 medium. The erythrocytes were resuspended in the complete RPMI 1640 medium, consisting of 10% human serum (type AB+ obtained from European blood donors without previous history of malaria), 25 mmol of HEPES per liter, and 25 mmol of NaHCO3 per liter, at a hematocrit of 1.5% and an initial parasitemia level of 0.1 to 1.0%. RPMI 1640 medium without folic acid and p-aminobenzoic acid was used to assess sensitivity to pyrimethamine. If the blood sample had a parasitemia level of >1.0%, fresh, uninfected, type A+ erythrocytes were added to adjust the parasitemia level to 0.6%.
The isotopic microtest described by Desjardins et al. (14) was used in this study. Two hundred microliters of the suspension of infected erythrocytes was distributed in each well of the 96-well tissue culture plates. The parasites were incubated at 37°C in 5% CO2 for 18 h. To assess parasite growth, [3H]hypoxanthine (specific activity, 5 Ci/mmol; 1 μCi/well) (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) was added. After an additional 24 h of incubation, the plates were frozen to terminate the in vitro drug sensitivity assays. The plates were thawed, and the contents of each well were collected on glass fiber filter papers, washed, and dried with a cell harvester. The filter disks were transferred into scintillation tubes, and 2 ml of scintillation cocktail (Organic Counting Scintillant; Amersham) was added. The incorporation of [3H]hypoxanthine was quantitated with a liquid scintillation counter (Wallac 1410; Pharmacia, Uppsala, Sweden).
The 50% inhibitory concentrations (IC50), defined as the drug concentration corresponding to 50% of the uptake of [3H]hypoxanthine measured in the drug-free control wells, were determined by linear regression analysis of the logarithm of concentrations plotted against the logit of growth inhibition. The threshold IC50 for in vitro resistance to chloroquine, monodesethylamodiaquine, quinine, mefloquine, halofantrine, and pyrimethamine were estimated to be >100, >60, >800, >30, >6, and >100 nmol/liter, respectively (27). Resistance to pyrimethamine was further classified as moderate resistance (IC50 of 100 to 2,000 nmol/liter) or high resistance (IC50 of >2,000 nmol/liter). These threshold values are the same as those for the semimicrotest that we have been using in past studies (1–5). Our comparative study of the semimicrotest and the microtest with the same clinical isolates has shown that the IC50 determined by the two isotopic in vitro assays are similar and highly concordant (7). The levels of resistance to artesunate and lumefantrine are still undefined. Data were expressed as geometric mean IC50 and 95% confidence intervals. The mean IC50 of lumefantrine against the chloroquine-sensitive and the chloroquine-resistant isolates were compared by the unpaired t test. Correlation of the logarithmic values of IC50 of different drugs was calculated by a linear regression analysis. The significance level was set at 0.05.
RESULTS
A total of 61 fresh clinical isolates of P. falciparum were obtained from patients residing in Yaoundé, Cameroon, for in vitro drug sensitivity assays. The in vitro activities of lumefantrine, halofantrine, and artesunate were determined with all 61 isolates. The in vitro activities of chloroquine, monodesethylamodiaquine, quinine, mefloquine, and pyrimethamine were tested against 38, 40, 54, 32, and 44 isolates, respectively.
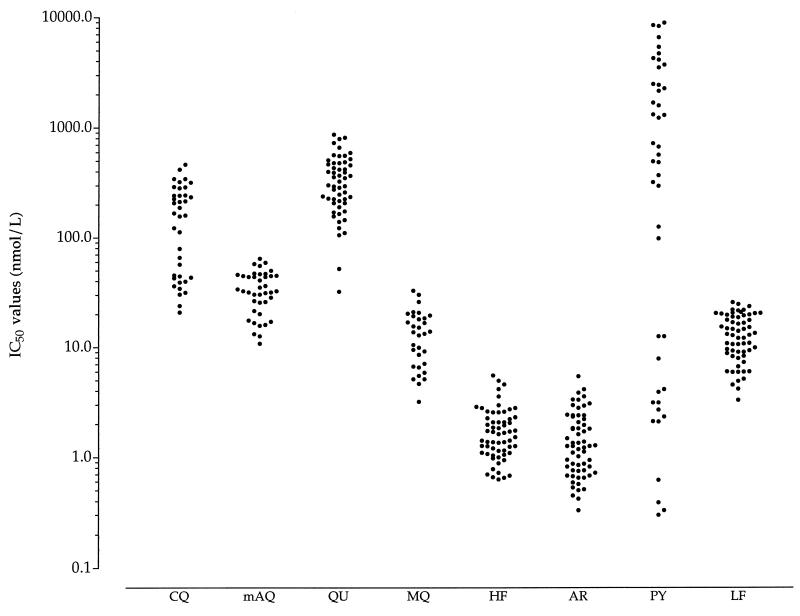
The distribution of the IC50 of the test compounds is presented in Fig. 2. Of the 38 isolates tested against chloroquine, 23 (61%) were resistant in vitro to chloroquine (geometric mean IC50, 237 nmol/liter; 95% confidence intervals, 202 to 277 nmol/liter). The geometric mean IC50 for 15 chloroquine-sensitive isolates was 39.8 nmol/liter (95% confidence intervals, 32.7 to 48.3 nmol/liter). The geometric mean IC50 of monodesethylamodiaquine was 31.1 nmol/liter (95% confidence intervals, 26.8 to 36.1 nmol/liter). Only 1 of 40 isolates was resistant to monodesethylamodiaquine, with an IC50 of 64.3 nmol/liter. The geometric mean IC50 of quinine was 295 nmol/liter (95% confidence intervals, 246 to 352 nmol/liter). Two of 54 isolates were resistant in vitro to quinine, with IC50 of 812 and 866 nmol/liter. Halofantrine was highly active against the Cameroonian isolates, with a geometric mean IC50 of 1.60 nmol/liter (95% confidence intervals, 1.40 to 1.83 nmol/liter; range, 0.63 to 5.52 nmol/liter). None of the isolates was resistant in vitro to halofantrine. The mean IC50 of mefloquine was 11.7 nmol/liter (95% confidence intervals, 9.5 to 14.6 nmol/liter). Two of 32 isolates had IC50 near the threshold (30.4 and 32.6 nmol/liter). Artesunate was highly active against all 61 isolates tested, with a geometric mean IC50 of 1.28 nmol/liter (95% confidence intervals, 1.08 to 1.52 nmol/liter; range, 0.33 to 5.41 nmol/liter). The levels of in vitro activity of halofantrine and artesunate were similar. Of 44 isolates, 16 were pyrimethamine sensitive (mean IC50, 2.8 nmol/liter; 95% confidence intervals, 1.2 to 6.2 nmol/liter). Of 28 pyrimethamine-resistant isolates, 14 were moderately resistant (mean IC50, 630 nmol/liter; 95% confidence intervals, 407 to 975 nmol/liter) and the other 14 isolates were highly resistant (geometric mean IC50, 4,280 nmol/liter; 95% confidence intervals, 3,200 to 5,700 nmol/liter).
FIG. 2.
Distribution of IC50 of chloroquine (CQ), monodesethylamodiaquine (mAQ), quinine (QU), mefloquine (MQ), halofantrine (HF), artesunate (AR), pyrimethamine (PY), and lumefantrine (LF) against Cameroonian P. falciparum isolates.
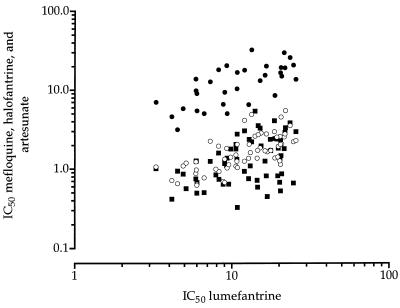
The geometric mean IC50 of lumefantrine was 11.9 nmol/liter (95% confidence intervals, 10.4 to 13.6 nmol/liter; range, 3.3 to 25.6 nmol/liter). Its mean IC50 against the chloroquine-sensitive parasites (n = 15) and the chloroquine-resistant isolates (n = 23) were 12.4 and 10.2 nmol/liter, respectively. The activity of lumefantrine did not differ significantly (P > 0.05) between the chloroquine-sensitive isolates and the chloroquine-resistant isolates. The in vitro responses of lumefantrine and mefloquine (r = 0.688), lumefantrine and halofantrine (r = 0.677), and lumefantrine and artesunate (r = 0.420) were significantly and positively correlated (Fig. 3). There was no correlation between the response to lumefantrine and that to chloroquine, monodesethylamodiaquine, quinine, and pyrimethamine (Table 1).
FIG. 3.
Correlation of in vitro responses, expressed as logarithmic IC50, to lumefantrine and mefloquine (black circles; coefficient of correlation [r] = 0.688; n = 44), lumefantrine and halofantrine (open circles; r = 0.677; n = 61), and lumefantrine and artesunate (black squares; r = 0.420; n = 61).
TABLE 1.
Correlation between the IC50 of lumefantrine and those of other antimalarial drugs
| Drug 1 | Drug 2 | na | rb | Pc |
|---|---|---|---|---|
| Lumefantrine | Chloroquine | 38 | −0.187 | NS |
| Lumefantrine | Monodesethylamodiaquine | 40 | −0.036 | NS |
| Lumefantrine | Quinine | 54 | 0.135 | NS |
| Lumefantrine | Mefloquine | 32 | 0.688 | <0.01 |
| Lumefantrine | Halofantrine | 61 | 0.677 | <0.01 |
| Lumefantrine | Artesunate | 61 | 0.420 | <0.01 |
| Lumefantrine | Pyrimethamine | 44 | −0.013 | NS |
n, number of paired results.
Correlation coefficient (r) was calculated from linear regression analysis of logarithmic IC50.
Probability (P) refers to the significance level of the test (P < 0.05). NS, nonsignificant.
DISCUSSION
Three Chinese types of drugs are currently under various phases of clinical development and may be available for worldwide use for the treatment of chloroquine- or multidrug-resistant falciparum malaria: artemisinin derivatives, pyronaridine, and lumefantrine. Our previous in vitro studies using African isolates of P. falciparum have shown that artemisinin derivatives and pyronaridine are highly active against both the chloroquine-sensitive and the chloroquine-resistant parasites (2, 4). The geometric mean IC50 of artemether were 2.37 and 1.61 nmol/liter against the chloroquine-sensitive and the chloroquine-resistant Cameroonian isolates, respectively (27). Arteether, another artemisinin derivative that is chemically similar to artemether, displayed in vitro activity similar to that of artemether (4, 10). Artesunate, a water-soluble derivative of artemisinin, was shown to be as active as the other artemisinin derivatives against the African isolates in the present work. The geometric mean IC50 of pyronaridine were 5.15 and 4.92 nmol/liter against the chloroquine-sensitive and the chloroquine-resistant Cameroonian isolates, respectively (27).
The level of in vitro activity of lumefantrine was slightly lower than that of two other Chinese types of antimalarial drugs, artemisinin derivatives and pyronaridine. Among other synthetic antimalarial drugs, halofantrine and atovaquone also display higher activity than lumefantrine (1, 6). However, the activity of lumefantrine was similar to that of mefloquine. These drugs had comparable ranges of IC50. Previous in vitro studies using the World Health Organization microtest have shown that the mean IC50 of lumefantrine was 9.84 and 12.44 nmol/liter against 64 and 29 Tanzanian isolates, respectively (30a, 30b). Although the assay systems are different, the IC50 determined against Cameroonian isolates in our study are similar to those against the Tanzanian isolates. The IC50 were largely below the peak concentration of lumefantrine in plasma (0.38 μg/ml after fasting and 5.10 μg/ml after a fatty meal) after administration of single oral doses of coartemether (480 mg of lumefantrine–80 mg of artemether) to healthy volunteers (7a).
An additional feature that favors the development of lumefantrine is that it is equally active in vitro against the chloroquine-sensitive and the chloroquine-resistant clinical isolates of P. falciparum in Yaoundé. The responses to lumefantrine and chloroquine were not correlated. There was a statistically significant positive correlation between the response to lumefantrine and those to mefloquine, halofantrine, and, to a lesser extent, artesunate. Lumefantrine belongs to the aminoalcohol class, as do mefloquine and halofantrine. Despite several differences in the ring structure and side-chain substituents, these three drugs share the basic chemical characteristic—a hydroxyl group near the ring—which has been hypothesized to be the key feature associated with the antimalarial activity of synthetic aminoalcohols (13, 18). It is thus not surprising that the IC50 for lumefantrine, mefloquine, and halofantrine are positively correlated. Our previous in vitro studies have demonstrated the high correlation between mefloquine and halofantrine, suggesting a potential for in vitro cross-resistance between these two drugs (1, 27). Likewise, in vitro responses to artemisinin derivatives were shown to exhibit a statistically significant positive correlation with those to mefloquine and halofantrine (4, 27). The underlying reasons for the potential for in vitro cross-resistance between artemisinin derivatives and aminoalcohols have not been elucidated. The mechanisms of action and intraerythrocytic transport processes of these drugs need to be demonstrated for a clearer understanding of the parallel responses among these drugs.
A positive correlation between the IC50 of two antimalarial drugs suggests in vitro cross-resistance but does not necessarily imply in vivo cross-resistance. The relationship between in vitro and in vivo resistance depends on the level of resistance and the coefficient of correlation. For certain drug pairs in which a high correlation coefficient exists, such as the 4-aminoquinoline chloroquine-monodesethylamodiaquine and the antifolate inhibitor pyrimethamine-cycloguanil (active metabolite of proguanil), in vivo cross-resistance may be observed in zones of endemicity where P. falciparum is highly resistant to one of the drug pair (3, 5, 27, 31). So far, mefloquine resistance has been confined to restricted areas in southeast Asia (21). Thus, it cannot be predicted whether lumefantrine, used alone in a zone of mefloquine resistance, will be clinically effective until clinical trials are conducted.
Initial clinical studies in West Africa have suggested the efficacy of lumefantrine in treatment of uncomplicated falciparum malaria infections (29, 30). Although in vitro data cannot be directly extrapolated to predict in vivo response, our in vitro data showing a high level of lumefantrine activity against clinical isolates of P. falciparum are consistent with its reported in vivo efficacy. Since the high in vitro activity in itself does not necessarily reflect high clinical efficacy, studies correlating in vitro and in vivo responses, dosage studies, pharmacokinetic and pharmacodynamic analysis for humans, and biochemical and genetic studies on the mechanism of action are necessary to evaluate the potential clinical use of the drug. In addition, because lumefantrine (as well as pyronaridine, artesunate, and artemether) was synthesized and registered in China, where regulatory procedures for drug development are considerably different from those of other countries, more preclinical and clinical data are needed before registration in countries where malaria is endemic becomes possible.
ACKNOWLEDGMENTS
We are grateful to Sister Solange and her nursing and laboratory staff at the Nlongkak Catholic missionary dispensary (Yaoundé, Cameroon) for invaluable help in recruiting patients and to Walther H. Wernsdorfer (Institute for Specific Prophylaxis and Tropical Medicine, University of Vienna, Vienna, Austria) for technical advice.
Leonardo K. Basco was supported by a grant from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases.
REFERENCES
- 1.Basco L K, Le Bras J. In vitro activity of halofantrine and its relationship to other standard antimalarial drugs against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1992;47:521–527. doi: 10.4269/ajtmh.1992.47.521. [DOI] [PubMed] [Google Scholar]
- 2.Basco L K, Le Bras J. In vitro activity of pyronaridine against African strains of Plasmodium falciparum. Ann Trop Med Parasitol. 1992;86:447–454. doi: 10.1080/00034983.1992.11812693. [DOI] [PubMed] [Google Scholar]
- 3.Basco L K, Le Bras J. In vitro activities of monodesethylamodiaquine and amopyroquine against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1993;48:120–125. doi: 10.4269/ajtmh.1993.48.120. [DOI] [PubMed] [Google Scholar]
- 4.Basco L K, Le Bras J. In vitro activity of artemisinin derivatives against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1993;49:301–307. doi: 10.4269/ajtmh.1993.49.301. [DOI] [PubMed] [Google Scholar]
- 5.Basco L K, Ramiliarisoa O, Le Bras J. In vitro activity of pyrimethamine, cycloguanil, and other antimalarial drugs against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1994;50:193–199. doi: 10.4269/ajtmh.1994.50.193. [DOI] [PubMed] [Google Scholar]
- 6.Basco L K, Ramiliarisoa O, Le Bras J. In vitro activity of atovaquone (566C80) against the African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1995;53:388–391. doi: 10.4269/ajtmh.1995.53.388. [DOI] [PubMed] [Google Scholar]
- 7.Bickii J, Basco L K, Ringwald P. Assessment of three in vitro tests and an in vivo test for chloroquine resistance in Plasmodium falciparum clinical isolates. J Clin Microbiol. 1998;36:243–247. doi: 10.1128/jcm.36.1.243-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Bindschedler, M., et al. 1996. XIVth International Congress for Tropical Medicine and Malaria, 17 to 22 November 1996, Nagasaki, Japan, abstr. P-01-96.
- 8.Bouchaud O, Basco L K, Gillotin C, Gimenez F, Ramiliarisoa O, Genissel B, Bouvet E, Farinotti R, Le Bras J, Coulaud J P. Clinical efficacy and pharmacokinetics of micronized halofantrine for the treatment of acute uncomplicated falciparum malaria in nonimmune patients. Am J Trop Med Hyg. 1994;51:204–213. doi: 10.4269/ajtmh.1994.51.204. [DOI] [PubMed] [Google Scholar]
- 9.Brewer T G, Grate S J, Peggins J O, Weina P J, Petras J M, Levine B S, Heiffer M H, Schuster B G. Fatal neurotoxicity of arteether and artemether. Am J Trop Med Hyg. 1994;51:251–259. doi: 10.4269/ajtmh.1994.51.251. [DOI] [PubMed] [Google Scholar]
- 10.Brossi A, Venugopalan B, Gerpe L D, Yeh H J C, Flippen-Anderson J L, Buchs P, Luo X D, Milhous W, Peters W. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J Med Chem. 1988;31:645–650. doi: 10.1021/jm00398a026. [DOI] [PubMed] [Google Scholar]
- 11.Canfield C J, Pudney M, Gutteridge W E. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol. 1995;80:373–381. doi: 10.1006/expr.1995.1049. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Tang L H, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Trans R Soc Trop Med Hyg. 1992;86:7–10. doi: 10.1016/0035-9203(92)90414-8. [DOI] [PubMed] [Google Scholar]
- 13.Chien P L, Cheng C C. Difference in antimalarial activity between certain amino alcohol diastereomers. J Med Chem. 1976;19:170–172. doi: 10.1021/jm00223a032. [DOI] [PubMed] [Google Scholar]
- 14.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu S, Xiao S H. Pyronaridine: a new antimalarial drug. Parasitol Today. 1991;7:310–313. doi: 10.1016/0169-4758(91)90267-r. [DOI] [PubMed] [Google Scholar]
- 16.Hien T T, White N J. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 17.Hudson A T. Atovaquone—a novel broad-spectrum anti-infective drug. Parasitol Today. 1993;9:66–68. doi: 10.1016/0169-4758(93)90040-m. [DOI] [PubMed] [Google Scholar]
- 18.Karle J M, Karle I L. Crystal structure and molecular structure of mefloquine methylsulfonate monohydrate: implications for a malaria receptor. Antimicrob Agents Chemother. 1991;35:2238–2245. doi: 10.1128/aac.35.11.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looareesuwan S, Kyle D E, Viravan C, Vanijanonta S, Wilairatana P, Wernsdorfer W H. Clinical study of pyronaridine for the treatment of acute uncomplicated falciparum malaria in Thailand. Am J Trop Med Hyg. 1996;54:205–209. doi: 10.4269/ajtmh.1996.54.205. [DOI] [PubMed] [Google Scholar]
- 20.Mount D L, Nahlen B L, Patchen L C, Churchill F C. Adaptations of the Saker-Solomons test: simple, reliable colorimetric field assays for chloroquine and its metabolites in urine. Bull W H O. 1989;67:295–300. [PMC free article] [PubMed] [Google Scholar]
- 21.Nosten F, Ter Kuile F, Chongsuphajaisiddhi T, Luxemburger C, Webster H K, Edstein M, Phaipun L, Thew K L, White N J. Mefloquine-resistant falciparum malaria on the Thai-Burmese border. Lancet. 1991;337:1140–1143. doi: 10.1016/0140-6736(91)92798-7. [DOI] [PubMed] [Google Scholar]
- 22.Nosten F, Ter Kuile F O, Luxemburger C, Woodrow C, Kyle D E, Chongsuphajaisiddhi T, White N J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993;341:1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- 23.Olliaro P L, Trigg P I. Status of antimalarial drugs under development. Bull W H O. 1995;73:565–571. [PMC free article] [PubMed] [Google Scholar]
- 24.Petras J M, Kyle D E, Gettayacamin M, Young G D, Bauman R A, Webster H K, Corcoran K D, Peggins J O, Vane M A, Brewer T G. Arteether: risks of two-week administration in Macaca mulatta. Am J Trop Med Hyg. 1997;56:390–396. doi: 10.4269/ajtmh.1997.56.390. [DOI] [PubMed] [Google Scholar]
- 25.Radloff P D, Philipps J, Nkeyi M, Hutchinson D, Kremsner P G. Atovaquone and proguanil for Plasmodium falciparum malaria. Lancet. 1996;347:1511–1514. doi: 10.1016/s0140-6736(96)90671-6. [DOI] [PubMed] [Google Scholar]
- 26.Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet. 1996;347:24–28. doi: 10.1016/s0140-6736(96)91558-5. [DOI] [PubMed] [Google Scholar]
- 27.Ringwald P, Bickii J, Basco L K. In vitro activity of antimalarials against clinical isolates of Plasmodium falciparum in Yaoundé, Cameroon. Am J Trop Med Hyg. 1996;55:254–258. doi: 10.4269/ajtmh.1996.55.254. [DOI] [PubMed] [Google Scholar]
- 28.van Vugt M, Brockman A, Gemperli B, Luxemburger C, Gathmann I, Royce C, Slight T, Looareesuwan S, White N J, Nosten F. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42:135–139. doi: 10.1128/aac.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Seidlein L, Bojang K, Jones P, Jaffar S, Pinder M, Obaro S, Doherty T, Haywood M, Snounou G, Gemperli B, Gathmann I, Royce C, McAdam K, Greenwood B. A randomized controlled trial of artemether/benflumetol, a new antimalarial, and pyrimethamine/sulfadoxine in the treatment of uncomplicated falciparum malaria in African children. Am J Trop Med Hyg. 1998;58:638–644. doi: 10.4269/ajtmh.1998.58.638. [DOI] [PubMed] [Google Scholar]
- 30.von Seidlein L, Jaffar S, Pinder M, Haywood M, Snounou G, Gemperli B, Gathmann I, Royce C, Greenwood B. Treatment of African children with uncomplicated falciparum malaria with a new antimalarial drug, CGP 56697. J Infect Dis. 1997;176:1113–1116. doi: 10.1086/516524. [DOI] [PubMed] [Google Scholar]
- 30a.Wernsdorfer, G., et al. 1996. XIVth International Congress for Tropical Medicine and Malaria, 17 to 22 November 1996, Nagasaki, Japan, abstr. O-22-8.
- 30b.Wernsdorfer, W. H., et al. 1996. XIVth International Congress for Tropical Medicine and Malaria, 17 to 22 November 1996, Nagasaki, Japan, abstr. O-26-3.
- 31.Wernsdorfer W H, Payne D. The dynamics of drug resistance in Plasmodium falciparum. Pharmacol Ther. 1991;50:95–121. doi: 10.1016/0163-7258(91)90074-v. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO technical report series. 805. Practical chemotherapy of malaria. Geneva, Switzerland: World Health Organization; 1990. [PubMed] [Google Scholar]
- 33.Xiuquing J, Guang-Yu L, Cheng-Qi S, Xing Z, Xin Wei L, Gathmann I, Royce C. Phase II trial in China of a new, rapidly-acting and effective oral antimalarial, CGP 56697, for the treatment of Plasmodium falciparum malaria. Southeast Asian J Trop Med Public Health. 1997;28:476–481. [PubMed] [Google Scholar]