Summary
Invasive freshwater mussels, such as the zebra (Dreissena polymorpha), quagga (Dreissena rostriformis bugensis), and golden (Limnoperna fortunei) mussel have spread outside their native ranges throughout many regions of the North American, South American, and European continents in recent decades, damaging infrastructure and the environment. This review describes ongoing efforts by multiple groups to develop genetic biocontrol methods for invasive mussels. First, we provide an overview of genetic biocontrol strategies that have been applied in other invasive or pest species. Next, we summarize physical and chemical methods that are currently in use for invasive mussel control. We then describe the multidisciplinary approaches our groups are employing to develop genetic biocontrol tools for invasive mussels. Finally, we discuss the challenges and limitations of applying genetic biocontrol tools to invasive mussels. Collectively, we aim to openly share information and combine expertise to develop practical tools to enable the management of invasive freshwater mussels.
Subject areas: Ecology, Biological sciences, Evolutionary biology
Graphical abstract

Ecology; Biological sciences; Evolutionary biology
Overview of genetic biocontrol strategies for invasive species
As a result of intentional and accidental human activities (e.g., travel, tourism, trade, and so forth), invasive species have steadily gained ground by spreading in non-native ranges. These opportunistic organisms can damage ecosystems and infrastructure, and impair agriculture, water treatment, fisheries, forestry, recreation, and many other activities.1,2 Although the full toll of damage caused by invasive species is challenging to calculate, their impacts are understood to be widespread. A wide variety of control measures have been developed, with varying efficacy depending on the technology and the target species. For invasive freshwater mussels, available treatments are generally limited to application in small-scale settings, such as controlling fouling of infrastructure. Technologies for the control or eradication of large populations in open water are not currently available. In recent years, genetic biocontrol techniques have become a focus of research and strategies for combating invasive species, pest species, and human disease vectors have been developed. Such genetic biocontrol approaches have the potential to allow for large-scale control of invasive mussel populations. In this review, we summarize the efforts to develop genetic biocontrol tools and how different groups are working to apply these tools with the ultimate goal of controlling the spread of invasive freshwater mussels.
Sterile male technique: the case of the New World screwworm
While many genetic biocontrol tools rely on relatively new technologies such as RNA interference or CRISPR/Cas9, practical genetic biocontrol methods have been successfully employed in the field for many decades now. During the last sixty years, several strategies have been developed to impair the reproductive performance of invasive insects.3,4 One of the most successful such applications is the sterile male technique, which was used to eradicate the New World screwworm (Cochliomyia hominivorax) from the United States and continues to be applied to prevent the spread of screwworms north from the isthmus of Panama.5,6,7 The sterile male technique involves the use of ionizing irradiation at sub-lethal doses to sterilize male insects prior to their environmental release.8 In this pest control method, the γ-radiation induces sterility but does not significantly affect the ability of males to fly, mate, and transfer sperm to females.9 However, the adaptation of this technique to other organisms has been challenging, principally due to the high costs of growing and distributing irradiated insects, the difficulty of isolating treated males, and difficulties in establishing doses of ionizing radiation that induce sterility but do not interfere with the ability to successfully mate in the wild.10
More recently, focused genetic biocontrol strategies have been developed using sequence-guided technologies to selectively sterilize pest insects. These next-generation approaches have several advantages over radiation-induced mutagenesis. Genetic biocontrol techniques for sterile insects are more scalable than radiation-mediated mutagenesis, more consistent in their results, and likely to produce cost savings in the long-term. Transgenic male-only strains have been obtained for screwworm control.11 For this approach, a tetracycline-regulated female conditional-lethal system has been developed.3 It is estimated that this transgenic strain could save as much as a million U.S. dollars per year in maintenance costs for the screwworm barrier zone.11
Among crustaceans, crayfish from the family Astacidea are recognized as one of the principal threats to freshwater ecosystems.12 For many decades, efforts to control crayfish were based only on trapping this species at infested sites. Other approaches that have been explored for the management of these crustaceans include the sterile male release technique,13 autocidal controls and sexual attractants,14,15 monosex populations,16,17 and RNA interference.16,18
RNA interference (RNAi) control
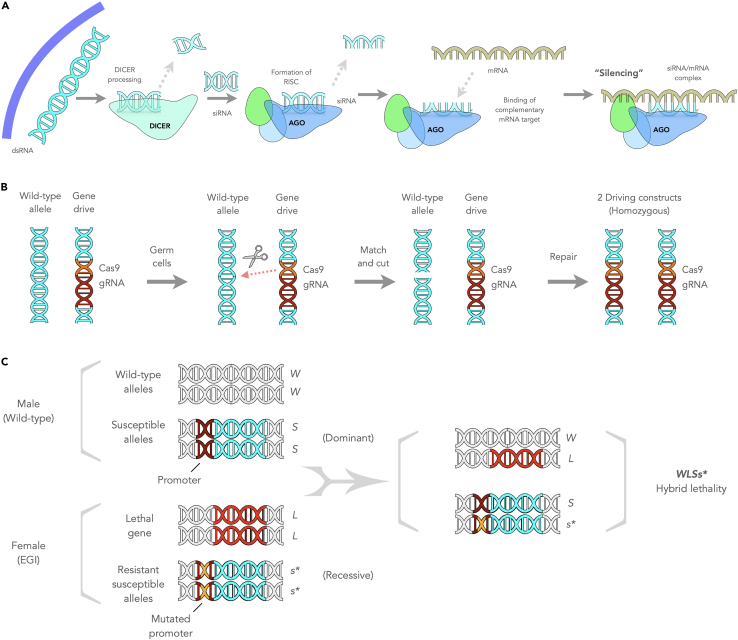
The RNA interference (RNAi) mechanism is hypothesized to have evolved as an immune pathway by which eukaryotic cells degrade viral transcripts to protect against infections,19 but this system also contributes to many aspects of endogenous gene regulation. In the canonical RNAi pathway (Figure 1A), messenger RNA (mRNA) is inactivated by small RNA molecules produced from endogenous long double-stranded RNA (dsRNA) cleaved by dicer enzymes.20 However, RNAi can be triggered by exogenous dsRNA, long single-stranded hairpin RNA, short hairpin RNA (shRNA), short interfering RNA (siRNA), and microRNA (miRNA).
Figure 1.
Approaches developed for the genetic biocontrol of invasive species
(A) RNA interference (RNAi). Silencing RNA precursors are trimmed into short double-stranded RNA (dsRNA) by a Dicer enzyme. An Argonaute protein binds and unwinds the short dsRNA and selectively retains one of the two RNA strands (the guide strand). A messenger RNA (mRNA)-silencing ribonucleoprotein complex, known as RNA-induced silencing complex (RISC), is formed between Argonaute proteins, the small single-strand guide RNA, and the mRNA target to induce mRNA silencing. RISC drives the silencing of a target mRNA via mRNA degradation or translational repression.
(B) Gene drive. CRISPR-Cas9-based gene drives utilize an expression cassette containing the Cas9 and guide RNA (gRNA) genes inserted into a target gene. The insertion disrupts the wild-type target gene and creates a self-propagating gene drive construct, where the gRNA directs Cas9 to cleave the wild-type target gene copy in the germ cells of heterozygous organisms. During the repair, the cleaved copy uses the driving construct as a template. This process converts the wild-type allele to the gene driving construct, resulting in the germ cells becoming homozygous for this allele and favoring its inheritance.
(C) Engineered genetic incompatibility (EGI). Outcrossing of wild-type male with Engineered Genetic Incompatibility (EGI) female strain produces hybrid lethality.
RNAi has been adapted for use as a tool for post-transcriptional gene silencing in many eukaryotes.21 But not every class of small RNA are found in every organism. For example, Capitella teleta lacks endogenous siRNA, so long dsRNA would presumably be a poor RNAi trigger.22 This annelid has a dicer with a mutant helicase domain. Similar dicer variants are specific for miRNA and show reduced processivity and altered RNA binding specificities. Exogenous dsRNA is critical for inducing RNAi and there are differences in the processivity of dsRNA and the responses induced by different RNAi triggers. For example, studies conducted on the two-spotted spider mite, Tetranychus urticae, have revealed that the length of the exogenous dsRNA determines the ability to induce phenotypic changes in vivo.23 Susceptibility to extra-organismal RNA found in an environmental medium, also known as environmental RNAi (eRNAi), can vary between species. For example, in herbivorous insects, eRNAi has been demonstrated to be effective in Coleopterans but not in Lepidopterans.24
A major area for the application of RNAi is the management of insect pest species, which adversely impact human health, agriculture, and the environment.25 In North America, the emerald ash borer (EAB), Agrilus planipennis, is an invasive insect that has decimated forests and urban ash trees.26 Methods for controlling EAB using double-stranded RNAi have been reported. EAB larvae process long dsRNA to produce siRNA. Oral delivery of dsRNA can silence the inhibitor of apoptosis (IAP) or COPI coatomer (COP) genes in EAB, resulting in larval mortality.27 RNAi treatments have shown a high mortality rate in both EAB larvae and adults. The oral administration of dsRNA constructs targeting different genes is lethal to EAB after ingestion and no phenotypic effects have been observed for non-targeted organisms.28,29 In other cases, RNAi approaches can silence gene expression but not elicit mortality, as was observed for paper wasp (Polistes dominula) control.30
In mosquitoes, the knockdown of target genes related to reproduction and survival has been widely tested with RNAi.31 RNAi treatments reduced the expression of sex differentiation genes such as doublesex (dsx) in Anopheles gambiae, and the loss of dsx expression prevented female larvae from reaching adulthood.32 Moreover, mosquito RNAi-based bioinsecticides have targeted important processes involved in larval survival, such as chitin synthesis.33 Through expression in E. coli, dsRNA targeting chitin synthases (CHs) A and B can cause significantly lower viability in larvae associated with decreased expression of CHs. Other potential mosquito insecticides have been tested using microalgae-based expression systems. Essential genes such as hydroxykynurenine transaminase (3-HKT) have been suppressed in mosquito larvae using both Chlamydomonas and Chlorella algal strains as vectors for dsRNA delivery.34,35,36
RNAi has also been successfully applied in tephritid fruit flies such as Bactrocera tryoni. Strong RNAi phenotypes have been observed after RNAi-mediated knockdown of the dsRNase and yellow genes involved in dsRNA degradation and melanin synthesis, respectively.37 Sterile insects can also be created using RNAi, without the need for irradiation. For instance, spermless male flies have been obtained by knocking down azoospermia and germ cell differentiation genes such as boul, zpg, dsxM, fzo, and gas8.38 The downregulated expression induced by these dsRNA constructs has resulted in up to 85% male sterility in B. dorsalis.38 In addition, using RNAi against transformer genes (tra and tra-2) in B. dorsalis, nearly 80% of the RNAi males mated, and the mated females laid eggs, but only about 50% of the RNAi males produced progeny.39
RNAi-based gene knockdown has been tested in a variety of aquatic organisms, such as sea lamprey (Petromyzon marinus40) and oysters (Crassostrea gigas41,42,43,44,45 and Crassostrea virginica46). One representative study on RNAi knockdown in bivalves was conducted on C. gigas; these oysters were injected with HIF-α-targeted long dsRNAs, and after the injection, the knock-down of HIF-α resulted in the production of reactive oxygen species (ROS).43 Another study utilized Platymonas subcordiformis and Nitzschia closterium f. minutissima as vectors to feed oysters with engineered E. coli to express dsRNAs targeting pigmentation genes. Feeding C. gigas dsRNA targeting tyrosinase resulted in the knockdown of this gene and hindered shell growth. Feeding oysters with dsRNA of the peroxidase gene showed a knockdown of the expression of the corresponding gene and caused diminished black pigmentation in the newly developed shell.42 Aside from oysters, beetles (Leptinotarsa decemlineata47 and Tribolium castaneum48), aphids (Sitobion miscanthi,49 Sitobion avenae,50 and Acyrthosiphon pisum51), brown planthopper (Nilaparvata lugens52), western corn rootworm (Diabrotica virgifera virgifera53), among many other organisms have been subjected to RNAi studies.
Gene drive: the case of the malaria-transmitting mosquito Anopheles gambiae
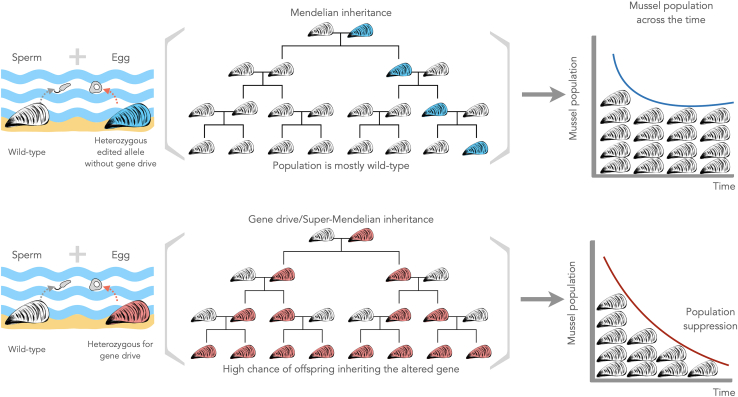
Gene drives can occur naturally. For instance, in several fly species, a naturally occurring gene drive acts during sperm development to abolish competition from non-driver carrying sperm.54 A gene drive biases its inheritance, resulting in its transmission to offspring at frequencies greater than 50%, a pattern known as super-Mendelian inheritance. As a result, the prevalence of the genetic element increases over generations, even in the absence of selective pressure.55 Synthetic gene drives have been proposed to spread traits for controlling crop pests, disease vectors, and invasive species.56 CRISPR/Cas9-based gene drives for population suppression require inserting an expression cassette containing the Cas9 gene and single guide RNA (sgRNA) into a region of interest to impair reproduction, for example (Figure 1B). The insertion disrupts the wild-type target gene and creates a self-propagating gene drive construct, where the sgRNA directs Cas9 to cleave the wild-type target gene copy in heterozygous organisms. The homology-directed repair pathway repairs the cleaved copy using the driving construct as a template. This process, called homing, changes the wild-type allele to the gene drive construct, resulting in homozygosity for this allele and favoring its inheritance.1
Creating a gene drive requires a deep understanding of the species' life cycle, the ability to breed the organism in captivity, and the application of advanced genetic modification techniques to promote the site-specific insertion of a large construct, which will form the gene drive construct. This biocontrol technology also has additional specific prerequisites. To suppress invasive populations by disseminating sterility, it is crucial to maintain the fertility of heterozygous organisms, ensuring the transmission of the driving construct to offspring. To achieve this, the Cas9 gene in the gene drive construct must be placed under the control of a germline-specific promoter that restricts homing to gamete precursor cells. Additionally, the target gene must be expressed solely in somatic cells, where homing will not occur, and be haplosufficient, meaning that only one functional copy is required to maintain a fertile phenotype.57 Consequently, heterozygotes remain fertile while only producing gametes with the driving construct, leading to the more frequent formation of sterile homozygous organisms and resulting in population decline over generations.
Currently, the most well-developed synthetic gene drives aim to suppress populations of Anopheles gambiae, malaria-transmitting mosquitoes, by disrupting a gene essential for female reproduction and restricting the homing process to the germ cells. The first generation of such a gene drive for A. gambiae demonstrated transmission rates of up to 99.6%.58 However, the emergence and positive selection of cleavage-resistant variants prevented it from achieving population suppression in longer laboratory experiments.59 To address this limitation, a second generation of gene drive targeted a highly conserved splice junction in the doublesex gene, which does not tolerate sequence variations due to functional constraints.60 Large cage experiments resulted in the complete elimination of mosquito populations within a year (8–12 generations) without the emergence of gene-drive-resistant variants.61
The third generation of gene drive technology combines this doublesex gene strategy with the super-Mendelian transmission of the X-chromosome-shredding I-PpoI nuclease gene, thus shifting the sex ratio toward males. Experimental and modeling data suggest that this strategy outperforms the second generation in terms of speed to achieve population suppression.62 To advance in the phased testing pathway proposed by the World Health Organization (WHO) for genetically modified mosquitoes, WHO recommends field tests that assess physical, geographical, or ecological containment of the GMO.63 The main challenges in taking this next step are risk assessment, regulation, and addressing ethical and social concerns. The remarkable outcomes obtained thus far offer hope in the fight against malaria, a disease that causes over half a million deaths annually.64 Success in laboratory and field trials with this method may help to pave the way for further development and application of gene drive technology to control other species.
Engineered genetic incompatibility methods
Engineered genetic incompatibility (EGI) methods represent another new approach for genetic population control. In this approach, genetically modified species express heritable genes that are incompatible with wild-type organisms, thus limiting the production or survival of their progeny upon mating (Figure 1C).
As an initial proof-of-concept for this approach, genetic barriers to sexual reproduction have been designed using CRISPR/Cas9 in Saccharomyces cerevisiae. Programmable transcription factors employing dCas9-based activators can induce the lethal overexpression of the actin gene (ACT1) in strains containing the wild-type actin promoter after mating with engineered strains.65 In genetically engineered insects, such as Drosophila melanogaster, it has been possible to design proof-of-concept transgenes with dominant lethality and recessive resistance.66 In D. melanogaster, EGI can be combined with conditional female lethality to create a sex-sorting incompatible male system. A tetracycline-controlled genetic construct controlled female viability,67 while an independently functioning EGI construct targeted the pyramus gene to produce lethal ectopic expression in the hybrid offspring of the control agent and wild-type. The sex-sorted incompatible males can compete with wild-type males for mating and reproduction and were effective in population suppression in cage trials.68 Strategies such as the field-amplified male sterility system (FAMSS), utilizing combined female lethality with EGI, could provide additional robustness. In simulations, FAMSS showed efficacy as a spatially and temporally self-limiting strategy for mosquito control.69 Overall, modifying male genes by EGI methods in invasive species could lead to sterile offspring and the eventual control of these species.
Impacts of invasive mussels
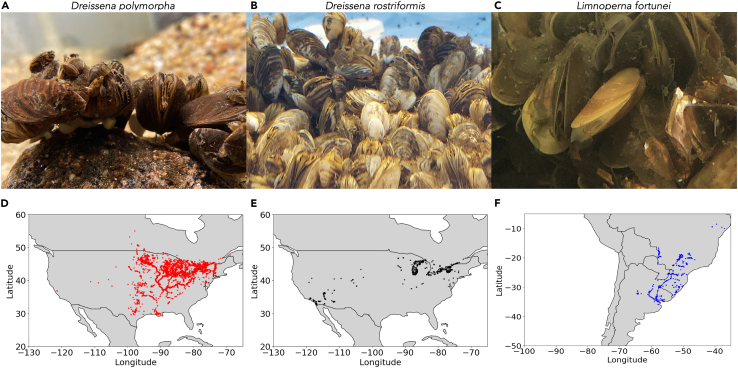
Invasive freshwater D. polymorpha, D. rostriformis bugensis, and L. fortunei species possess high fecundity.70 They can disperse rapidly thanks to their free-floating larval stage.71 The prolific zebra (D. polymorpha) (Figure 2A) and quagga (D. rostriformis bugensis) (Figure 2B) mussels native to Eurasia were likely introduced to the Laurentian Great Lakes in the 1980s, and they have expanded throughout the United States.72 In South America, the Asiatic River golden mussel (Figure 2C) was first reported in 1991.73 These problematic species have provoked widespread problems related to water quality, ecosystem imbalance, and macrofouling issues.
Figure 2.
Zebra, quagga, and golden invasive mussel distribution in North and South America
(A) Zebra mussel (Dreissena polymorpha). Photo credit: Victor H. Hernandez Elizarraga, 2023.
(B) Quagga mussel (Dreissena rostriformis bugensis). Photo credit: Steve Suhr, 2023.
(C) Golden mussel (Limnoperna fortunei). Photo credit: Fábio Sendim, 2023.
(D) Zebra mussel invasion in North America. Red dots indicate where the occurrence of this species has been confirmed according to the USGS database: https://nas.er.usgs.gov/viewer/omap.aspx?SpeciesID=95 (Accessed May 2023).
(E) Quagga mussel invasion in North America. Black dots indicate where the occurrence of this species has been confirmed according to the USGS database: https://nas.er.usgs.gov/viewer/omap.aspx?SpeciesID=5 (Accessed May 2023).
(F) Golden mussel invasion in South America. Blue dots indicate where the occurrence of this species has been confirmed according to the GISD and the CBEIH databases: http://www.iucngisd.org/gisd/species.php?sc=416 (Accessed May 2023), https://base.cbeih.org/index.php?lan=en (Accessed May 2023).
Invasive mussels have significant adverse economic and ecological impacts in ecosystems where they establish.74 Users of freshwater, wastewater treatment, agriculture, industry, and hydroelectric generation can be directly impacted by macrofouling invasive mussels. Biofouling reduces water flow through intake pipelines and cooling systems and causes the deterioration of surfaces. Costs for treatment plants in the United States ranged from ¢ 0.0001 to ¢ 0.0013 per gallon, depending on the location and extent of the mussel infestation.75 In addition, invasive freshwater mussels have negative impacts related to the prevention and control of these organisms that infest hydropower facilities, where annual costs for increased maintenance can reach approximately $464,000 per affected facility.76
Modeling work has shown that the zebra mussel invasion in the United States presents a high risk of continuing spreading across the Midwest, and it is likely throughout the Southeast and along the eastern seaboard.77 Comparable with what has been observed for zebra mussels, the quagga mussel has expanded rapidly in non-indigenous regions, including the United States, Western Europe, and the Netherlands.78
Dreissenids are efficient filter feeders that can outcompete many native species for food resources while shifting energy pathways from the pelagic to benthic zones.79,80,81 These shifts can reduce phytoplankton production82 while promoting cyanobacteria, potentially harmful algal blooms,83 and filamentous nuisance algae, such as Cladophora.84,85,86,87 Dreissenid Invasive mussels have been shown to induce high mortality in native freshwater mussel (Unionidae) populations. Particularly, they have also significantly reduced and even extirpated native unionid mussels.88,89 Invasive Dreissenids can encrust unionid surfaces using their byssal threads, preventing the opening and closing of valves, and limiting feeding in native mussels, resulting in decreased energy reserves and causing the arrest of essential processes for survival such as reproduction and shell formation.90 In lakes and rivers from North America, zebra mussels can reach high densities (>3000 organisms per square meter), which leads to the extirpation of Unionoida native mussels within 4–8 years after the invasion.91 Besides, D. polymorpha has contributed to native mussels' population decline in Europe. Some affected native species are Anosonta anatina, Anodonta cygnea, Pseudanodonta complanata, Unio tumidus, and Unio pictorum.92 As per the IUCN red list (https://www.iucnredlist.org/), many native mussels, such as Pseudanodonta complanata, are currently recognized as vulnerable species that can be seriously threatened by invasive mussels. However, the impacts of invasive mussels on Unionoida populations are not fully understood.
The golden mussel (Limnoperna fortunei) (Figure 2C) is the most aggressive invasive freshwater species in Latin America (Figure 2D). It alters both biotic and abiotic factors, leading to significant impacts on freshwater ecosystems and biota.79,93 Since its initial detection in Argentina in 1991, it has spread to Uruguay, Paraguay, Bolivia, and Brazil, where its northernmost range is in the northeast.94 Predictive models suggest that by 2050, there is a risk that the golden mussel may extend its range to the Amazon basin.95 The species reproduces year-round96 and can reach high densities of up to 200 individuals/m2, particularly on man-made structures such as those in hydroelectric power plants and fish farms.97 Golden mussel biofouling has caused significant economic impacts, with estimated losses of up to $128 million annually in the Brazilian electric sector alone.98 Controlling the golden mussel remains an unsolved challenge. While existing methods can mitigate biofouling in man-made structures,97 they are unable to eliminate the species from the environment or prevent further geographic spread. This is evidenced by the lack of reports of eradication in areas where the golden mussel has been established and the continued emergence of new occurrences.
Overview of existing control methods for invasive mussels
Invasive bivalves are spreading quickly across freshwater ecosystems (Figures 2D–2F).99 To mitigate the impacts elicited by non-native mussels, a wide assortment of treatments and molluscicides have been tested for controlling these problematic species in closed systems. Efforts to eradicate or reduce invasive mussels have used chemical, physical, and biological tools.
Regarding chemical methods, chlorine compounds, such as hypochlorite and chlorine dioxide, are substances capable of killing mussels. These agents disrupt cell integrity through a general denaturation of proteins and the arrest of cellular enzymatic activity.100 Chlorides have shown a dose-dependent effect in mussels, but their effectiveness depends on the right exposure concentration and duration.101 Studies have shown that larval and juvenile freshwater mussels can have low susceptibility to chlorination.102 Additionally, chlorine compounds can generate harmful byproducts such as trihalomethanes, pose a human safety risk, and involve neutralization steps before discharge into the environment.
In addition to chlorine compounds, non-chloride oxidizing compounds such as ammonia, activated bromine, and potassium permanganate, can be used for population suppression of invasive mussels. These chemicals are mainly used for the prevention of biological fouling on the surfaces of boats, docks, or other infrastructure. There are many oxidizing agent treatments for the control of mussel macrofouling within piping systems. Several control tools for internal piping systems have been developed for golden,103 quagga,104 and zebra mussels.105
The non-oxidizing molluscicides are a group of chemicals that mainly consist of commercial and non-generic formulations (e.g., BULAB, MEXEL, EVAC, Clam-Trol CT-2, VeliGON, among many others105). Non-oxidizing treatments are more selective than oxidizing molluscicides, do not have a general mechanism of action, do not readily react with organics, and target specific cellular components. Mulloscicides are applied at regular intervals. Non-oxidizing molluscicides are generally compatible with materials and metallurgies found in pipe and water systems. These molluscicides are also non-corrosive, non-volatile, non-flammable, easy to transport, and safe to handle compared with oxidizing agents. High variability in the efficacy of these formulations has been observed against mussels, and their application is limited due to several factors such as formulation type, mode of action, feasibility of application in waterways, cost of treatment, effective concentrations, regulatory restrictions, toxicological data, and precautions on product handling.106
The copper-based molluscicides EarthTec QZ and Natrix are also EPA-registered for invasive mussel control in open water. Although effective for killing invasive mussels, copper can be toxic to aquatic plants, algae, fish, and other invertebrates and persists in the environment. Potassium chloride (KCl), or potash, is not a registered molluscicide, but has also been used in open water treatments with regulatory exemption.107 Similar to copper, potassium can harm non-target species and does not degrade in the environment. Carbon dioxide, currently registered as a piscicide, can prevent the settlement and attachment of dreissenids108 and readily off-gasses, but application methods in open water are in the early stages of investigation.
Physical control strategies include hand removal, desiccation by water drawdown, and anoxia with benthic mats. Mussels can be removed manually from water bodies by scraping and suction removal. Manual removal is highly resource- and time-consuming but effectively withdraws live mussels and dead shells. Desiccation exposes the shorelines and decreases the ability of the water to retain heat. Depending on the drawdown duration, the water can reach freezing temperatures, causing the mussels to die. Benthic mats kill mussels by withholding oxygen and food and restricting the water flow. In addition, these systems block light to prevent photosynthesis. Benthic mats may also prevent larval mussels from spreading.
The naturally derived pesticide, Zequanox, is an approved biopesticide by the U.S. Environmental Protection Agency (EPA) for zebra and quagga mussel control.109 The active ingredient in Zequanox is killed Pseudomonas fluorescens strain CL145A cells110,111 which cause the degradation of the digestive system epithelium.112 This biopesticide can reduce invasive mussel biomass, degrades rapidly, and is relatively selective, but it can affect water quality, and when it is applied in large doses can be harmful to some fish species and other organisms.113
In summary, invasive mussel control technologies that are effective, specific, safe for the environment, and scalable for large water bodies are currently lacking. Furthermore, control tools for invasive mussels in open water are more limited than those used in closed systems because of the large treatment areas, associated environmental concerns, and costs for materials and application. These gaps could be addressed with novel biocontrol tools, as reviewed here.
Current approaches in development for the genetic biocontrol of invasive mussels
RNA interference for the control of zebra mussels
Zebra mussels (Figure 2A) have caused significant changes in food webs, including the loss of diversity of zooplankton and phytoplankton in invaded waters, impacting aquatic ecosystems across North America (Figure 2D).114 Chemical control methods have thus far been insufficient to eradicate mussels from infested water and to prevent additional water bodies from becoming infested, and such control methods often have non-specific toxic effects on a wide range of other organisms.115,116,117 RNAi represents a strategy for controlling invasive mussels that has the potential to be highly species-specific, deployable in both closed and open systems, and that does not require genetic modification of the target invasive mussel species.
A critical constraint in developing genetic biocontrol tools for zebra mussels at present is the lack of robust laboratory culture methods. The inability to culture zebra mussels in the lab throughout their full life cycle and over multiple generations precludes the use of tools such as gene drives or EGI that require the construction and maintenance of strains with heritable genetic modifications. One strategy that has been employed for RNAi-based pest control, which does not require breeding or transgenesis, is feeding with genetically engineered microbes (e.g., RNAi feeding). The RNAi feeding method was first demonstrated in the nematode C. elegans,118 but has been applied in a wide variety of organisms. Feeding with modified microbes that express dsRNA can be effective only if the target species internalizes the genetic material through the digestive system.119
As filter feeders, mussels are known to consume bacteria in addition to algae and other phytoplankton.120 It may be possible to knock down molecular targets essential for survival, growth, reproduction, attachment, or other key processes in invasive mussels using RNAi triggers delivered by genetically modified microorganisms (Figure 3). Bacteria, such as E. coli, have been widely used to produce and deliver silencing RNA by feeding.118 E. coli is relatively easy to genetically modify, methods for maximizing the expression are well established, and it grows quickly to high cell densities.121 At the same time, E. coli bacteria is an acceptable food source for many animals and has been found in the zebra mussel gut.122 Several alternative microbial vectors have also been tested as vectors for feeding RNAi in other systems, including bacterial symbionts,123 yeast,124 and microalgae.35,36 Due to the variation in eRNAi susceptibility between species and the fact that zebra mussels can selectively filter different particles when feeding,87,125 it will be important to determine whether the feeding of RNAi trigger-expressing microorganisms is a viable delivery strategy for zebra mussels or if the amount of consumed microbe in the wild is enough to induce RNAi phenotypes.
Figure 3.
RNA interference (RNAi) for control of zebra mussels
RNAi can potentially be employed to create species-specific biocides that selectively inhibit essential gene functions in zebra mussels. Engineered microbes, such as bacteria and algae, could be utilized to treat infested waterways to produce and deliver dsRNA to zebra mussels through feeding.
Recently, the sequence of the D. polymorpha genome was published.126 This resource, together with tissue-specific transcriptomic data, provided a rich starting point to identify genes involved in key processes that could be potential targets for RNAi-based genetic biocontrol. To begin, we identified nearly two hundred zebra mussel candidate biocontrol genes that were predicted to impact survival (housekeeping genes involved in basic cell biological functions), as well as other processes such as shell formation, feeding, byssal thread formation/surface attachment, reproduction, and stress response. We have begun to clone these target genes into E. coli expression vectors and test their effects on zebra mussel gene expression and other phenotypes. Our ongoing project aims to test whether RNAi can be used to manipulate the expression of genes in zebra mussels through the delivery of RNAi triggers such as dsRNAs or shRNAs, delivered either by direct injection or by engineered bacteria or algal expression strains. By identifying RNAi reagents that disrupt key processes, we aim to develop RNAi tools that could be deployed to safely and specifically eradicate zebra mussels from non-native waterways.
One set of molecular targets that are particularly intriguing from the standpoint of RNAi-based zebra mussel biocontrol are genes controlling reproductive capacity. For example, silencing sex determination genes, such as doublesex (dsx)32 or transformer (tra)39,48 have been used to generate sterile males in insect species. However, this strategy may be challenging due to the lack of information on the mechanisms of sex determination in zebra mussels. Another option to impair reproduction is to silence spawning-related genes. It is known that serotonin triggers spawning in zebra mussels.127,128,129,130,131 However, while the silencing of zebra mussel genes involved in serotonin signaling could limit their spawning, impairing this critical pathway may also elicit more widespread effects.
Other delivery mechanisms, such as synthetic RNA, can also be employed for gene silencing.132 Synthetic RNA can directly induce RNAi responses in organisms without using a live genetic vector, which makes it easy to analyze standard toxicology, dosing, and effectiveness. Thanks to advances in RNA synthesis and stabilization, driven partly by agricultural applications for RNAi, synthetic RNA production costs have significantly decreased and yields have significantly increased in recent years.133 However, it likely remains cost-prohibitive to deliver synthetic RNA in open water systems. First, it would be challenging and extremely expensive to reach effective concentrations of synthetic RNA in treated waterways. Second, “naked” dsRNA is unstable and susceptible to environmental nucleases. Thus, more sophisticated mechanical delivery routes are required to ensure the effectiveness of synthetic RNA or other toxicants against mussels. Techniques such as biocide microencapsulation may present a solution that makes synthetic RNA delivery more feasible, and microencapsulation approaches have shown promising results for invasive mussel control.116,117
The molecular mechanisms of the RNAi pathways employed in zebra mussels are not yet well characterized. For instance, the susceptibility or effectiveness of RNAi at different life stages has not been assessed. In addition, we are still assessing the effectiveness of different RNAi triggers and delivery methods. Insights into the function of the RNAi system in zebra mussels will shed new light on a relatively underexplored facet of mussel biology and may ultimately yield practical tools for highly species-specific genetic control of zebra mussels.
Quagga mussel control by disseminated neoplasia
In some marine bivalve species, there is a naturally occurring disease known as disseminated neoplasia (DN) that has been associated with large die-offs of farmed shellfish. DNs are very rare in nature and are a type of cancer where the cancer cells themselves are transmitted from one animal to another of the same species, producing lethality.134 DN in molluscs was first described in the late 1960s and has since been studied by marine biologists concerned with the preservation of farmed marine molluscs of commercial importance.134 DNs are transmitted from bivalves in close proximity through seawater. In the laboratory, transmission can occur by injecting DN cells from an infected bivalve into an uninfected one or by simply co-housing infected and healthy animals together in the same tank (Figure 4).135,136
Figure 4.
Quagga mussel control by disseminated neoplasia (DN)
Disseminated neoplasias (DNs) can be transmitted between bivalves in close proximity through seawater. Given the absence of natural pathogenic DNs in quagga mussels, it may be possible to engineer a DN from healthy quagga mussel cells. After the development of systemic neoplasia, the widespread dissemination of cancer-causing oncogenes could occur. Mussels growing at high density in inland lakes and reservoirs could be highly susceptible to the toxic effects of DNs resulting in the collapse of the target population.
One common perturbation of molluscan DNs is the alteration of the cell cycle and cell death master regulating protein p53.137,138,139 p53 is the subject of hundreds of studies for its role in cancer in many organisms, and mutations in p53 are widely considered to be the most common mutation in human cancers.140 Based on published reports linking changes in p53 to molluscan/mussel DN and the known role of p53 in neoplasia of mammals from mouse to man, it is feasible that the mutation of the tumor suppressor p53 within the mussel genome also has a high probability of producing culturable cancer cells.
The conversion of healthy primary cells to cancerous, “transformed” cells, can be achieved by targeting cancer-causing oncogenes that knock-out cell cycle-regulating genes. This has proved to be a difficult task with dreissenid mussels because methods of live animal culture, live cell culture, and gene transfer are not at an advanced stage compared to other commonly researched species. Nevertheless, using microinjection, it might be possible to introduce DNA encoding known oncogenes, such as SV40 Large-T antigen (Tag), into the quagga mussel genome to induce a cancer cell phenotype. The Tag protein has been shown to work through multiple cellular pathways to induce cellular transformation, most notably through the inhibition of cell cycle control proteins such as p53 and tumor suppressive factor Retinoblastoma-1 (Rb).141 Tag has been used successfully for decades to induce the transformation of mammalian, reptile, and amphibian cells. We predict that Tag could have the same properties of cellular transformation in invasive mussel cells as well.
Engineered DN could possess several advantages over other possible genetic biocontrol solutions. The DN requires a compatible host to survive and replicate, so once the last dreissenid mussel in a target waterway has died, the agent itself is completely removed from the environment. Additionally, DN is progressively toxic and should kill invasive mussels of breeding age at a rate that does not put an undue burden on the environment due to the accumulation of dead mussel biomaterial. Because the anti-dreissenid DN should spread through host populations of any size, a single deployment of the DN may be all that is required, even in the largest water body. Finally, with no capacity for sexual reproduction, prolonged nutrient uptake, or survival in the wild unless engrafted within the body of a host mussel, the DN agent could avoid some of the risks commonly associated with genetically modified organisms.
Gene drive for the control of the golden mussel
Advances in DNA sequencing and genome editing technologies have made it possible to consider using gene drives for non-model species, such as the golden mussel (Figure 5). A genome sequencing program was initiated within our collaborative group to address the knowledge gap for the golden mussel and to identify candidate target genes and promoters required for a gene drive strategy (Figure 1B). We used genome and transcriptome sequencing to identify genes142,143 and RNAseq analysis to find sex-biased genes potentially involved in sex determination and differentiation in golden mussel gonads.144 Although much about these processes in bivalves is still unknown, it is believed that they are oligogenic and influenced by environmental factors.145 Therefore, gene-environment relationships appear to be key.
Figure 5.
Gene drive for the control of the golden mussel
Heterozygous edited alleles without gene drive have a 50% chance of being inherited by offspring when crossed with a wild-type organism. Gene drives increase the probability of transmitting the modified genes to offspring at frequencies greater than 50%, a pattern known as super-mendelian inheritance. Heterozygote offspring remain fertile while only producing gametes with the gene drive construct, leading to the more frequent formation of sterile homozygous organisms, and resulting in population decline over generations.
The transcription factor families Drmt (doublesex and mab3-related-transcription factor) and Fox (forkhead-box) are involved in sex determination and differentiation in model organisms.146 Evidence also suggests their involvement in bivalves. Dmrt1 and Foxl2 have shown sex-biased expression patterns in several bivalve species, including L. fortunei.144,147,148,149 The knockdown of Foxl2 and Dmrt1 genes by RNAi silencing in the oyster C. gigas resulted in a reduction in gonad volume and the failure of gonadal differentiation into females and males, respectively.150 Starvation has also been shown to alter the expression of these genes in C. gigas, leading to a delay in gonadal development and a bias toward a higher proportion of males in the sex ratio.151 This provides further support for the gene-environment hypothesis for sex determination and differentiation in bivalves and for the involvement of these two antagonist genes in these processes. Therefore, in the search for candidate target genes to disseminate sterility in the golden mussel, the focus has been on the Dmrt gene family, which also includes the doublesex gene successfully used as a target for gene drive in A. gambiae.60 The recent assembly of a chromosome-level genome for the golden mussel152 has allowed us to identify and characterize the Dmrt family in L. fortunei, including the main candidate target gene, Dmrt1l (unpublished results). However, it is still necessary to experimentally validate the knockout phenotype and haplosufficiency of this gene in L. fortunei.
To make gene drive viable, it is also crucial to identify and validate functional promoters for obtaining the gene drive construct. In our ongoing project, we are using a GFP-based reporter system to evaluate candidate promoters, including endogenous promoters, which are expected to be specific to germ cells, as well as exogenous promoters such as CMV and U6. We are testing these promoters by transfecting naked plasmid DNA into L. fortunei embryos obtained through induced spawning in captivity. We are also delivering the plasmid DNA into adult mussels through injection in the adductor muscle, which has been shown to work for the delivery of nucleic acid (dsRNA) in other bivalves.43,153 These plasmid DNA delivery methods are also being used to test genetic modification protocols, particularly for genomic insertion via the CRISPR/Cas9 system.
Due to the length of time required for gene drive development, the complexity of population dynamics, and the regulatory challenges involved, mathematical models are commonly used to test scenarios, anticipate risks, and assess requirements associated with gene drive technology.154,155 While most current models tend to focus on gene drive efficiency, the inclusion of other important ecological aspects would enhance assessments of gene drive risk. Informative aspects include organisms life histories and those required by the European Food Safety Authority (not regionally applicable) and WHO guidelines for genetically modified organisms.156 We are currently building a model specific for the golden mussel using the SLiM framework,157 which allows for the customization of reproductive strategy and is considered one of the most powerful models available for gene drives.
Despite all the challenges, gene drive technology offers a promising avenue for the control of the golden mussels and the reduction of associated environmental and economic impacts. Further research would improve our understanding of this mussel’s biology and inform effective gene drive strategies to control its populations. These efforts will be enhanced by engaging multidisciplinary groups in golden mussel research and collaborating within the community of researchers currently addressing invasive freshwater mussel challenges worldwide.
Limitations and barriers to applying genetic biocontrol tools in invasive mussels
Genomic resource tools
Knowledge of the genome is essential for the design of new targeted genetic biocontrol techniques to eradicate invasive mussel species.158 Recently, the D. polymorpha,126 D. rostriformis bugensis,159 and L. fortunei152 genomes were reported. Some features of these genomes, such as their large size and a high proportion of repetitive DNA regions, large-scale structural variants, and high levels of heterozygosity reflect a very complex genomic architecture of bivalves.
These genomes, along with a large number of other recently published bivalve genomes, provide a resource for comparative genomic studies that could shed light on common mechanisms of response against stressors, pesticides, or biocontrol treatments. Nevertheless, current methods for sequence annotation are imperfect and may produce errors in the accurate identification of exons and untranslated mRNA regions. We encountered limitations in automated annotation when characterizing the D. polymorpha byssus genes.126 The primary amino acid sequences of these byssal proteins were known, but in many cases the genome annotation algorithms failed to predict the corresponding transcripts. For this reason, manual annotation was required to identify many of the exons that encode the known byssal proteins.160
Transcriptomic data from adult tissues and developing embryos have also been used to annotate these mussel genomes, but these transcriptomic datasets and the resulting annotations are far from complete. Regardless, a diverse transcriptome repertoire that comprehensively represents the expressed RNA during different life stages and in different tissues is desirable for modern genetic biocontrol strategies that rely upon genome editing. Small RNA (∼20–30 nt) is found in all eukaryotes and is processed through RNAi pathways. Small RNAs and their regulatory regions are not currently well annotated in invasive freshwater mussel genomes. A better understanding of small RNA pathways could contribute to developing efficient inhibitory mechanisms such as RNAi.
Furthermore, because it is imperative that any developed RNAi control tool is highly specific to the target species, it is also important to have genomic data for cohabitating non-target species that could be exposed to the treatment. Often, these species can be underrepresented in available genomic repositories, which can make interfering RNA design challenging and may necessitate more extensive specificity testing after candidates are produced.
Lack of robust lab culture methods
Although a vast literature exists describing the reproduction and developmental processes of diverse mussels,161 few laboratories have successfully cultured larvae of D. polymorpha, D. rostriformis bugensis, and L. fortunei through metamorphosis and settlement due to the need for space, equipment, and expert staff to (1) properly care for systems, (2) rear algae as food and ensure adequate larvae nutrition, (3) prevent the contamination of culture water by predators or bacteria, and (4) keep water quality conditions optimal.130,162,163,164,165,166
To begin rearing, adult mussels can be encouraged to release gametes in the lab with a temperature increase, the introduction of a gonad slurry, or the addition of serotonin hydrochloride for D. polymorpha and rostriformis bugensis128,166 and Spectrus CT1300 for L. fortunei,167 which results in the highest spawning rate in reproductively ripe individuals. Females typically take twice as long to spawn compared to males and fresh sperm results in the best rates of fertilization. Thus, successful fertilization in the lab requires proper timing and planning.128,166,167 The settlement of planktonic larvae is highly variable and depends on temperature and nutrition. For instance, if larvae are held at 22°C, settlement occurs at 21 days for D. polymorpha and 32 days for D. rostriformis bugensis163,168, while L. fortunei settles in 11 days at 28°C.167 Larvae develop best in the absence of turbulence and other hydrodynamic forces that cause mechanical damage, particularly during the trochophore, D-shaped veliger, veliconcha, and pediveliger stages, prior to metamorphosis.169,170 Preventing interactions with other veligers is also important and a density of <1 larva/ml is recommended.163,166
Moreover, significant mortality occurs during the transitions between the D-shaped, umbonal,171,172 and the veliconcha stages166 likely due to shifts in metabolic demand toward organ development as seen in marine bivalves.173,174 To meet nutritional needs, larvae require a diet of algae that are the appropriate size (<6 μm) for selection by gill cilia and ingestion at each developmental stage and are comprised of a diverse fatty acid composition, particularly polyunsaturated fatty acids.168,175,176,177,178 To reduce the presence of predators or competitive protozoa and rotifers, broodstock can be treated with sodium hypochlorite solution.163,167 The impacts of fouling bacteria can also be reduced by the addition of antibiotics.163,166
Larval mussel development in static or recirculating systems is further supported by high-quality water, defined as as low total ammonia nitrogen (NH4+), hardness (Ca(HCO3)2) above 31 ppm,179 pH 7.4–9.4,180 Ca2+ concentrations above 12 mg/L,172,179,180 and Na+, Cl, K+, and Mg2+ above a minimal threshold181 to maintain conditions viable for respiration, waste abatement, and growth of shell material.
These exacting requirements, along with the need for a large space and the staff time to culture food and maintain larval tanks makes successful culture of invasive mussel larvae challenging.
Lack of existing molecular genetic tools and transgenesis methods
In general, non-model organisms lack advanced molecular techniques. The sequences that control fundamental processes such as DNA replication, transcription, RNA processing, and translation have not been well defined for these organisms. Regulatory sequences that could be used to control reporter gene expression have not been characterized. There are developed techniques for monitoring endogenous mRNA, but there are no established reporter genes or cell lines that allow for more rapid gene expression studies.
There are currently no established methods for transgenesis or targeted mutagenesis in invasive mussels. There has been some progress in developing techniques for monitoring and manipulating genes in commercially important marine bivalves (i.e., introducing nucleic acid via injection182 or soaking183, as well as CRISPR184 gene editing). However, at present, most techniques for the genetic manipulation of invasive mussels are being developed de novo. Current culture limitations may require alternative transgenesis approaches.185
Phenotypic assays for assessing effects of manipulations
Phenotypic screening is an essential aspect of assessing the effects of genetic manipulations. A wide variety of methods have been developed to assess toxicology in dreissenid mussels and efforts are currently underway to harmonize these methods so that comparisons can be made more easily between laboratories.186 Our groups are also currently working on establishing quantitative phenotypic assays for attachment, filtration of food particles, growth, reproduction, and survival. In addition, the assessment of molecular phenotypes by RT-qPCR or RNA-sequencing will also provide valuable insights into the efficacy of genetic manipulations. Moreover, methods for the robust testing of potential off-target effects on native mussels or non-target organisms will help to establish that the observed changes in the phenotype are specific to the disruption of the target gene and not due to off-target effects, and that the genetic biocontrol tools under development will not have undesired impacts on other organisms.
Concluding remarks
Here we summarize existing methods for genetic biocontrol and our efforts to apply these tools to control freshwater invasive mussels. We aim to overcome the many challenges to developing effective, specific, and safe genetic biocontrol tools through collaborative interactions amongst our groups and by employing the diverse, multidisciplinary approaches described above. In addition to surmounting the many technical hurdles to demonstrate laboratory-based proof-of-concept, defined regulatory pathways and stakeholder engagement and education will be needed to enable the safe and responsible field deployment of these potentially powerful tools.
Acknowledgments
D.M.G. and S.B. gratefully acknowledge funding from the Minnesota Environment and Natural Resources Trust Fund, the Minnesota Department of Natural Resources (MN DNR) and the Minnesota Aquatic Invasive Species Research Center (MAISRC). B.M. was supported by funding from the Minnesota Zoo, MAISRC, and MN DNR. Y.J.P. was supported by a Science & Technology Research Project from the Bureau of Reclamation Research and Development Office. S.T.S and M.C.S. were supported in part by a grant from the U.S Bureau of Reclamation, Department of the Interior, R19AC00021. M.F.R. and J.A.A. were funded by CTG Brasil, Tijoá Energia, and SPIC Brasil through the Brazilian National Electric Energy Agency ANEEL R&D program (grant PD-10381-0419/2019). C.A.R., T.M.E., K.K., C.M.M., and D.L.W. were funded by the USGS Ecosystems Mission Area, Invasive Species Program. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author contributions
Conceptualization, V.H.H.E and D.M.G; writing – original draft, V.H.H.E., S.B., L.G., J.A.A., S.T.S., M.C.S., B.M., C.M.M., T.M.E., K.K., C.A.R., and D.L.W.; writing –review & editing, V.H.H.E, Y.J.P., M.F.R., and D.M.G.; supervision, D.M.G.
Declaration of interests
S.T.S and M.C.S. are co-founders of Biomilab LLC and are members of its scientific advisory board and have management/advisory roles and a financial interest. Biomilab LLC has patents related to this work. Bio Bureau Biotecnologia is a private, for-profit company with commercial interests in invasive mussel biocontrol. J.A. A. is a shareholder of Bio Bureau Biotecnologia and its Scientific Director. M.F.R. is a shareholder, founder, and member of the scientific advisory board of Bio Bureau Biotecnologia. The remaining authors have no competing interests to declare.
Contributor Information
Yale J. Passamaneck, Email: ypassamaneck@usbr.gov.
Mauro F. Rebelo, Email: mrebelo@biof.ufrj.br.
Daryl M. Gohl, Email: dmgohl@umn.edu.
References
- 1.Teem J.L., Alphey L., Descamps S., Edgington M.P., Edwards O., Gemmell N., Harvey-Samuel T., Melnick R.L., Oh K.P., Piaggio A.J., et al. Genetic Biocontrol for Invasive Species. Front. Bioeng. Biotechnol. 2020;8:452. doi: 10.3389/fbioe.2020.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuthbert R.N., Diagne C., Haubrock P.J., Turbelin A.J., Courchamp F. Are the “100 of the world’s worst” invasive species also the costliest? Biol. Invasions. 2022;24:1895–1904. doi: 10.1007/s10530-021-02568-7. [DOI] [Google Scholar]
- 3.Scott M.J., Concha C., Welch J.B., Phillips P.L., Skoda S.R. Review of research advances in the screwworm eradication program over the past 25 years. Entomol. Exp. Appl. 2017;164:226–236. doi: 10.1111/eea.12607. [DOI] [Google Scholar]
- 4.Knipling E.F. Sterile-Male Method of Population Control. Science. 1959;130:902–904. doi: 10.1126/science.130.3380.902. [DOI] [PubMed] [Google Scholar]
- 5.Dyck V.A., Hendrichs J., Robinson A.S., editors. Impact of screwworm eradication programmes using the sterile insect technique. Sterile insect technique. CRC Press; 2021. pp. 949–978. [Google Scholar]
- 6.Wyss J.H. Screwworm eradication in the Americas. Ann. N. Y. Acad. Sci. 2000;916:186–193. doi: 10.1111/j.1749-6632.2000.tb05289.x. [DOI] [PubMed] [Google Scholar]
- 7.Gillman H. Food & Agriculture Org.; 1992. The New World Screwworm Eradication Programme: North Africa, 1988-1992. [Google Scholar]
- 8.Dyck V.A., Hendrichs J., Robinson A.S., editors. Sterile Insect Technique: Principles And Practice In Area-Wide Integrated Pest Management. Taylor & Francis; 2021. [DOI] [Google Scholar]
- 9.Hendrichs J., Robinson A. In: Encyclopedia of Insects. Second Edition. Resh V.H., Cardé R.T., editors. Academic Press; 2009. Chapter 243 - Sterile Insect Technique; pp. 953–957. [DOI] [Google Scholar]
- 10.Marec F., Vreysen M.J.B. Advances and Challenges of Using the Sterile Insect Technique for the Management of Pest Lepidoptera. Insects. 2019;10:371. doi: 10.3390/insects10110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concha C., Palavesam A., Guerrero F.D., Sagel A., Li F., Osborne J.A., Hernandez Y., Pardo T., Quintero G., Vasquez M., et al. A transgenic male-only strain of the New World screwworm for an improved control program using the sterile insect technique. BMC Biol. 2016;14:72. doi: 10.1186/s12915-016-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfrin C., Souty-Grosset C., Anastácio P.M., Reynolds J., Giulianini P.G. Detection and Control of Invasive Freshwater Crayfish: From Traditional to Innovative Methods. Diversity. 2019;11:5. doi: 10.3390/d11010005. [DOI] [Google Scholar]
- 13.Aquiloni L., Becciolini A., Berti R., Porciani S., Trunfio C., Gherardi F. Managing invasive crayfish: use of X-ray sterilisation of males. Freshw. Biol. 2009;54:1510–1519. [Google Scholar]
- 14.Holdich D.M., Gydemo R., Rogers W.D. A review of possible methods for controlling nuisance populations of alien crayfish. Crayfish Eur. Alien Species. 2017:245–270. [Google Scholar]
- 15.Gherardi F., Aquiloni L., Diéguez-Uribeondo J., Tricarico E. Managing invasive crayfish: is there a hope? Aquat. Sci. 2011;73:185–200. [Google Scholar]
- 16.Savaya A., De Leo G., Aalto E., Levy T., Rosen O., Manor R., Aflalo E., Tricarico E., Sagi A. The IAG gene in the invasive crayfish Procambarus clarkii-towards sex manipulations for biocontrol and aquaculture. Manag. Biol. Invasion. 2020;11:237–258. [Google Scholar]
- 17.Levy T., Sagi A. The “IAG-switch”—A key controlling element in decapod crustacean sex differentiation. Front. Endocrinol. 2020;11:651. doi: 10.3389/fendo.2020.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savaya-Alkalay A., Sagi A. Biotechnology, biocontrol and conservation: potential approaches—harnessing RNAi-based sex-differentiation manipulations in decapods. Glob. Overv. Conserv. Freshw. Decapod Crustac. 2016:323–338. [Google Scholar]
- 19.Bagasra O., Prilliman K.R. RNA interference: the molecular immune system. J. Mol. Histol. 2004;35:545–553. doi: 10.1007/s10735-004-2192-8. [DOI] [PubMed] [Google Scholar]
- 20.Nejepinska J., Flemr M., Svoboda P. In: Regulatory RNAs: Basics, Methods and Applications. Mallick B., Ghosh Z., editors. Springer; 2012. The Canonical RNA Interference Pathway in Animals; pp. 111–149. [DOI] [Google Scholar]
- 21.Flynt A.S. Insecticidal RNA interference, thinking beyond long dsRNA. Pest Manag. Sci. 2021;77:2179–2187. doi: 10.1002/ps.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanal S., Zancanela B.S., Peter J.O., Flynt A.S. The small RNA universe of Capitella teleta. Front. Mol. Biosci. 2022;9:802814. doi: 10.3389/fmolb.2022.802814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bensoussan N., Dixit S., Tabara M., Letwin D., Milojevic M., Antonacci M., Jin P., Arai Y., Bruinsma K., Suzuki T., et al. Environmental RNA interference in two-spotted spider mite, Tetranychus urticae, reveals dsRNA processing requirements for efficient RNAi response. Sci. Rep. 2020;10:19126. doi: 10.1038/s41598-020-75682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivashuta S., Zhang Y., Wiggins B.E., Ramaseshadri P., Segers G.C., Johnson S., Meyer S.E., Kerstetter R.A., McNulty B.C., Bolognesi R., Heck G.R. Environmental RNAi in herbivorous insects. RNA. 2015;21:840–850. doi: 10.1261/rna.048116.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradshaw C.J.A., Leroy B., Bellard C., Roiz D., Albert C., Fournier A., Barbet-Massin M., Salles J.-M., Simard F., Courchamp F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016;7:12986. doi: 10.1038/ncomms12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herms D.A., McCullough D.G. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts, and Management. Annu. Rev. Entomol. 2014;59:13–30. doi: 10.1146/annurev-ento-011613-162051. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues T.B., Rieske L.K., J Duan J., Mogilicherla K., Palli S.R. Development of RNAi method for screening candidate genes to control emerald ash borer, Agrilus planipennis. Sci. Rep. 2017;7:7379. doi: 10.1038/s41598-017-07605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues T.B., Duan J.J., Palli S.R., Rieske L.K. Identification of highly effective target genes for RNAi-mediated control of emerald ash borer, Agrilus planipennis. Sci. Rep. 2018;8:5020. doi: 10.1038/s41598-018-23216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pampolini F., Rieske L.K. Emerald Ash Borer Specific Gene Silencing Has No Effect on Non-target Organisms. Front. Agron. 2020;2 [Google Scholar]
- 30.Bulgarella M., Baty J.W., McGruddy R., Lester P.J. Gene silencing for invasive paper wasp management: Synthesized dsRNA can modify gene expression but did not affect mortality. PLoS One. 2023;18:e0279983. doi: 10.1371/journal.pone.0279983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munawar K., Alahmed A.M., Khalil S.M.S. Delivery Methods for RNAi in Mosquito Larvae. J. Insect Sci. 2020;20:12. doi: 10.1093/jisesa/ieaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taracena M.L., Hunt C.M., Benedict M.Q., Pennington P.M., Dotson E.M. Downregulation of female doublesex expression by oral-mediated RNA interference reduces number and fitness of Anopheles gambiae adult females. Parasit. Vectors. 2019;12:170. doi: 10.1186/s13071-019-3437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez S.B.G., Guimarães-Ribeiro V., Rodriguez J.V.G., Dorand F.A.P.S., Salles T.S., Sá-Guimarães T.E., Alvarenga E.S.L., Melo A.C.A., Almeida R.V., Moreira M.F. RNAi-based bioinsecticide for Aedes mosquito control. Sci. Rep. 2019;9:4038. doi: 10.1038/s41598-019-39666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fei X., Zhang Y., Ding L., Xiao S., Xie X., Li Y., Deng X. Development of an RNAi-based microalgal larvicide for the control of Aedes aegypti. Parasit. Vectors. Springer. 2021;14:387. doi: 10.1186/s13071-021-04885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A., Wang S., Ou R., Samrakandi M., Beerntsen B.T., Sayre R.T. 2013. Development of an RNAi Based Microalgal Larvicide to Control Mosquitoes; p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fei X., Xiao S., Huang X., Li Z., Li X., He C., Li Y., Zhang X., Deng X. Control of Aedes mosquito populations using recombinant microalgae expressing short hairpin RNAs and their effect on plankton. PLoS Negl. Trop. Dis. 2023;17:e0011109. doi: 10.1371/journal.pntd.0011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tayler A., Heschuk D., Giesbrecht D., Park J.Y., Whyard S. Efficiency of RNA interference is improved by knockdown of dsRNA nucleases in tephritid fruit flies. Open Biol. 2019;9:190198. doi: 10.1098/rsob.190198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali M.W., Zheng W., Sohail S., Li Q., Zheng W., Zhang H. A genetically enhanced sterile insect technique against the fruit fly, Bactrocera dorsalis (Hendel) by feeding adult double-stranded RNAs. Sci. Rep. 2017;7:4063. doi: 10.1038/s41598-017-04431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu G., Wu Q., Li J., Zhang G., Wan F. RNAi-Mediated Knock-Down of transformer and transformer 2 to Generate Male-Only Progeny in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel) PLoS One. 2015;10:e0128892. doi: 10.1371/journal.pone.0128892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath G., Childs D., Docker M.F., McCauley D.W., Whyard S. RNA Interference Technology to Control Pest Sea Lampreys - A Proof-of-Concept. PLoS One. 2014;9:e88387. doi: 10.1371/journal.pone.0088387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu B., Li Q., Yu H. RNA Interference by Ingested Dsrna-Expressing Bacteria to Study Porphyrin Pigmentation in Crassostrea gigas. Int. J. Mol. Sci. 2021;22:6120. doi: 10.3390/ijms22116120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng D., Li Q., Yu H. RNA Interference by Ingested dsRNA-Expressing Bacteria to Study Shell Biosynthesis and Pigmentation in Crassostrea gigas. Mar. Biotechnol. 2019;21:526–536. doi: 10.1007/s10126-019-09900-2. [DOI] [PubMed] [Google Scholar]
- 43.Choi S.H., Jee B.Y., Lee S.J., Cho M.Y., Lee S.J., Kim J.W., Jeong H.D., Kim K.H. Effects of RNA interference-mediated knock-down of hypoxia-inducible factor-α on respiratory burst activity of the Pacific oyster Crassostrea gigas hemocytes. Fish Shellfish Immunol. 2013;35:476–479. doi: 10.1016/j.fsi.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y., Li Q., Yu H., Liu S., Kong L. Expression of tyrosinase-like protein genes and their functional analysis in melanin synthesis of Pacific oyster (Crassostrea gigas) Gene. 2022;840:146742. doi: 10.1016/j.gene.2022.146742. [DOI] [PubMed] [Google Scholar]
- 45.Payton L., Perrigault M., Bourdineaud J.-P., Marcel A., Massabuau J.-C., Tran D. Trojan Horse Strategy for Non-invasive Interference of Clock Gene in the Oyster Crassostrea gigas. Mar. Biotechnol. 2017;19:361–371. doi: 10.1007/s10126-017-9761-9. [DOI] [PubMed] [Google Scholar]
- 46.Schwaner C., Pales Espinosa E., Allam B. RNAi Silencing of the Biomineralization Gene Perlucin Impairs Oyster Ability to Cope with Ocean Acidification. Int. J. Mol. Sci. 2023;24:3661. doi: 10.3390/ijms24043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallis S., Alyokhin A., Manley B., Rodrigues T., Barnes E., Narva K. Effects of Low Doses of a Novel dsRNA-based Biopesticide (Calantha) on the Colorado Potato Beetle. J. Econ. Entomol. 2023;116:456–461. doi: 10.1093/jee/toad034. [DOI] [PubMed] [Google Scholar]
- 48.Shukla J.N., Palli S.R. Sex determination in beetles: production of all male progeny by parental RNAi knockdown of transformer. Sci. Rep. 2012;2:602. doi: 10.1038/srep00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Li H., Zhong X., Tian J., Segers A., Xia L., Francis F. Silencing an aphid-specific gene SmDSR33 for aphid control through plant-mediated RNAi in wheat. Front. Plant Sci. 2022;13:1100394. doi: 10.3389/fpls.2022.1100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdellatef E., Will T., Koch A., Imani J., Vilcinskas A., Kogel K.-H. Silencing the expression of the salivary sheath protein causes transgenerational feeding suppression in the aphid Sitobion avenae. Plant Biotechnol. J. 2015;13:849–857. doi: 10.1111/pbi.12322. [DOI] [PubMed] [Google Scholar]
- 51.Ye C., An X., Xie B.-Q., Ding B.-Y., Niu J., Wang J.-J. The involvement of systemic RNA interference deficient-1-like (SIL1) in cellular dsRNA uptake in Acyrthosiphon pisum. Insect Sci. 2022 doi: 10.1111/1744-7917.13167. [DOI] [PubMed] [Google Scholar]
- 52.Wang W., Yang R.-R., Peng L.-Y., Zhang L., Yao Y.-L., Bao Y.-Y. Proteolytic activity of the proteasome is required for female insect reproduction. Open Biol. 2021;11:200251. doi: 10.1098/rsob.200251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyata K., Ramaseshadri P., Zhang Y., Segers G., Bolognesi R., Tomoyasu Y. Establishing an In Vivo Assay System to Identify Components Involved in Environmental RNA Interference in the Western Corn Rootworm. PLoS One. 2014;9:e101661. doi: 10.1371/journal.pone.0101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wedell N., Price T.A.R., Lindholm A.K. Gene drive: progress and prospects. Proc. Biol. Sci. 2019;286:20192709. doi: 10.1098/rspb.2019.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alphey L.S., Crisanti A., Randazzo F.F., Akbari O.S. Opinion: Standardizing the definition of gene drive. Proc. Natl. Acad. Sci. USA. 2020;117:30864–30867. doi: 10.1073/pnas.2020417117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bier E. Gene drives gaining speed. Nat. Rev. Genet. 2022;23:5–22. doi: 10.1038/s41576-021-00386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. Biol. Sci. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammond A., Galizi R., Kyrou K., Simoni A., Siniscalchi C., Katsanos D., Gribble M., Baker D., Marois E., Russell S., et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hammond A.M., Kyrou K., Bruttini M., North A., Galizi R., Karlsson X., Kranjc N., Carpi F.M., D’Aurizio R., Crisanti A., Nolan T. The creation and selection of mutations resistant to a gene drive over multiple generations in the malaria mosquito. PLoS Genet. 2017;13:e1007039. doi: 10.1371/journal.pgen.1007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kyrou K., Hammond A.M., Galizi R., Kranjc N., Burt A., Beaghton A.K., Nolan T., Crisanti A. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018;36:1062–1066. doi: 10.1038/nbt.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hammond A., Pollegioni P., Persampieri T., North A., Minuz R., Trusso A., Bucci A., Kyrou K., Morianou I., Simoni A., et al. Gene-drive suppression of mosquito populations in large cages as a bridge between lab and field. Nat. Commun. 2021;12:4589. doi: 10.1038/s41467-021-24790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simoni A., Hammond A.M., Beaghton A.K., Galizi R., Taxiarchi C., Kyrou K., Meacci D., Gribble M., Morselli G., Burt A., et al. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nat. Biotechnol. 2020;38:1054–1060. doi: 10.1038/s41587-020-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guidance Framework for Testing of Genetically Modified Mosquitoes, second edition. https://www.who.int/publications-detail-redirect/9789240025233
- 64.World malaria report 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022
- 65.Maselko M., Heinsch S.C., Chacón J.M., Harcombe W.R., Smanski M.J. Engineering species-like barriers to sexual reproduction. Nat. Commun. 2017;8:883. doi: 10.1038/s41467-017-01007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maselko M., Feltman N., Upadhyay A., Hayward A., Das S., Myslicki N., Peterson A.J., O’Connor M.B., Smanski M.J. Engineering multiple species-like genetic incompatibilities in insects. Nat. Commun. 2020;11:4468. doi: 10.1038/s41467-020-18348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu G., Condon K.C., Epton M.J., Gong P., Jin L., Condon G.C., Morrison N.I., Dafa’alla T.H., Alphey L. Female-specific insect lethality engineered using alternative splicing. Nat. Biotechnol. 2007;25:353–357. doi: 10.1038/nbt1283. [DOI] [PubMed] [Google Scholar]
- 68.Upadhyay A., Feltman N.R., Sychla A., Janzen A., Das S.R., Maselko M., Smanski M. Genetically engineered insects with sex-selection and genetic incompatibility enable population suppression. Elife. 2022;11:e71230. doi: 10.7554/eLife.71230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maselko M., Heinsch S., Das S., Smanski M.J. Genetic incompatibility combined with female-lethality is effective and robust in simulations of Aedes aegypti population control. bioRxiv. 2018 doi: 10.1101/316406. Preprint at. [DOI] [Google Scholar]
- 70.Keller R.P., Drake J.M., Lodge D.M. Fecundity as a basis for risk assessment of nonindigenous freshwater molluscs. Conserv. Biol. 2007;21:191–200. doi: 10.1111/j.1523-1739.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 71.Bossenbroek J.M., Kraft C.E., Nekola J.C. Prediction of Long-Distance Dispersal Using Gravity Models: Zebra Mussel Invasion of Inland Lakes. Ecol. Appl. 2001;11:1778–1788. doi: 10.1890/1051-0761(2001)011[1778:POLDDU]2.0.CO;2. [DOI] [Google Scholar]
- 72.Barnes M.A., Patiño R. U.S. Geological Survey; 2020. Predicting Suitable Habitat for Dreissenid Mussel Invasion in Texas Based on Climatic and Lake Physical Characteristics.https://www.usgs.gov/publications/predicting-suitable-habitat-dreissenid-mussel-invasion-texas-based-climatic-and-lake [Google Scholar]
- 73.Brugnoli E., Clemente J., Boccardi L., Borthagaray A., Scarabino F. Golden mussel Limnoperna fortunei (Bivalvia: Mytilidae) distribution in the main hydrographical basins of Uruguay: update and predictions. An. Acad. Bras. Ciênc. 2005;77:235–244. doi: 10.1590/S0001-37652005000200004. [DOI] [PubMed] [Google Scholar]
- 74.Haubrock P.J., Cuthbert R.N., Ricciardi A., Diagne C., Courchamp F. Economic costs of invasive bivalves in freshwater ecosystems. Divers. Distrib. 2022;28:1010–1021. [Google Scholar]
- 75.Chakraborti R.K., Madon S., Kaur J. Costs for controlling dreissenid mussels affecting drinking water infrastructure: Case studies. J.-Am. Water Works Assoc. 2016;108:E442–E453. [Google Scholar]
- 76.Rumzie N., Christopherson R., O’Meara S., Passamaneck Y., Murphy A., Pucherelli S., Trujillo J. Costs Associated with Invasive Mussels Impacts and Management. Wiley; 2021. [Google Scholar]
- 77.Drake J.M., Bossenbroek J.M. The potential distribution of zebra mussels in the United States. Bioscience. 2004;54:931–941. [Google Scholar]
- 78.Matthews J., Van der Velde G., Bij de Vaate A., Collas F.P.L., Koopman K.R., Leuven R.S.E.W. Rapid range expansion of the invasive quagga mussel in relation to zebra mussel presence in The Netherlands and Western Europe. Biol. Invasions. 2014;16:23–42. [Google Scholar]
- 79.Boltovskoy D., Correa N. Ecosystem impacts of the invasive bivalve Limnoperna fortunei (golden mussel) in South America. Hydrobiologia. 2015;746:81–95. [Google Scholar]
- 80.Karatayev A.Y., Boltovskoy D., Padilla D.K., Burlakova L.E. The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. J. Shellfish Res. 2007;26:205–213. [Google Scholar]
- 81.Karatayev A.Y., Burlakova L.E., Karatayev V.A., Boltovskoy D. Limnoperna fortunei versus Dreissena polymorpha: population densities and benthic community impacts of two invasive freshwater bivalves. J. Shellfish Res. 2010;29:975–984. [Google Scholar]
- 82.Vanderploeg H.A., Liebig J.R., Nalepa T.F., Fahnenstiel G.L., Pothoven S.A. Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. J. Great Lakes Res. 2010;36:50–59. [Google Scholar]
- 83.Cataldo D., Vinocur A., O′Farrell I., Paolucci E., Leites V., Boltovskoy D. The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia. 2012;680:25–38. [Google Scholar]
- 84.Francoeur S.N., Peters Winslow K.A., Miller D., Peacor S.D. Mussel-derived stimulation of benthic filamentous algae: The importance of nutrients and spatial scale. J. Great Lakes Res. 2017;43:69–79. [Google Scholar]
- 85.Howell E.T. Cladophora (green algae) and dreissenid mussels over a nutrient loading gradient on the north shore of Lake Ontario. J. Great Lakes Res. 2018;44:86–104. [Google Scholar]
- 86.Raikow D.F., Sarnelle O., Wilson A.E., Hamilton S.K. Dominance of the noxious cyanobacterium Microcystis aeruginosa in low-nutrient lakes is associated with exotic zebra mussels. Limnol. Oceanogr. 2004;49:482–487. [Google Scholar]
- 87.Vanderploeg H.A., Liebig J.R., Carmichael W.W., Agy M.A., Johengen T.H., Fahnenstiel G.L., Nalepa T.F. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can. J. Fish. Aquat. Sci. 2001;58:1208–1221. [Google Scholar]
- 88.Martel A.L., Pathy D.A., Madill J.B., Renaud C.B., Dean S.L., Kerr S.J. Decline and regional extirpation of freshwater mussels (Unionidae) in a small river system invaded by Dreissena polymorpha: the Rideau River, 1993 2000. Can. J. Zool. 2001;79:2181–2191. [Google Scholar]
- 89.Schloesser D.W., Nalepa T.F., Mackie G.L. Zebra mussel infestation of unionid bivalves (Unionidae) in North America. Am. Zool. 1996;36:300–310. [Google Scholar]
- 90.Schloesser D.W., Metcalfe-Smith J.L., Kovalak W.P., Longton G.D., Smithee R.D. Extirpation of freshwater mussels (Bivalvia: Unionidae) following the invasion of dreissenid mussels in an interconnecting river of the Laurentian Great Lakes. Am. Midl. Nat. 2006;155:307–320. [Google Scholar]
- 91.Ricciardi A., Neves R.J., Rasmussen J.B. Impending extinctions of North American freshwater mussels (Unionoida) following the zebra mussel (Dreissena polymorpha) invasion. J. Anim. Ecol. 1998;67:613–619. [Google Scholar]
- 92.Ożgo M., Urbańska M., Hoos P., Imhof H.K., Kirschenstein M., Mayr J., Michl F., Tobiasz R., von Wesendonk M., Zimmermann S., Geist J. Invasive zebra mussel (Dreissena polymorpha) threatens an exceptionally large population of the depressed river mussel (Pseudanodonta complanata) in a postglacial lake. Ecol. Evol. 2020;10:4918–4927. doi: 10.1002/ece3.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Darrigran G., Damborenea C. Ecosystem engineering impact of Limnoperna fortunei in South America. Zoolog. Sci. 2011;28:1–7. doi: 10.2108/zsj.28.1. [DOI] [PubMed] [Google Scholar]
- 94.Barbosa N., de Carvalho M., Silva F., Neto M., Oliveira M.D., Cardoso A. Limnoperna fortunei (Dunker, 1857) (Mollusca, Bivalvia, Mytilidae): first record in the São Francisco River basin, Brazil. Check List. 2016;12:1–6. doi: 10.15560/12.1.1846. [DOI] [Google Scholar]
- 95.Barbosa N.P., Ferreira J.A., Nascimento C.A., Silva F.A., Carvalho V.A., Xavier E.R., Ramon L., Almeida A.C., Carvalho M.D., Cardoso A.V. Prediction of future risk of invasion by Limnoperna fortunei (Dunker, 1857) (Mollusca, Bivalvia, Mytilidae) in Brazil with cellular automata. Ecol. Indic. 2018;92:30–39. doi: 10.1016/j.ecolind.2018.01.005. [DOI] [Google Scholar]
- 96.Boltovskoy D., Morton B., Correa N., Cataldo D., Damborenea C., Penchaszadeh P.E., Sylvester F. In: Limnoperna Fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Invading Nature - Springer Series in Invasion Ecology. Boltovskoy D., editor. Springer International Publishing; 2015. Reproductive Output and Seasonality of Limnoperna fortunei; pp. 77–103. [DOI] [Google Scholar]
- 97.Boltovskoy D., Xu M., Nakano D. In: Limnoperna Fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Invading Nature - Springer Series in Invasion Ecology. Boltovskoy D., editor. Springer International Publishing; 2015. Impacts of Limnoperna Fortunei on Man-Made Structures and Control Strategies: General Overview; pp. 375–393. [DOI] [Google Scholar]
- 98.Rebelo M.F., Afonso L.F., Americo J.A., da Silva L., Neto J.L.B., Dondero F., Zhang Q. PeerJ Inc.; 2018. A Sustainable Synthetic Biology Approach for the Control of the Invasive Golden Mussel (Limnoperna Fortunei) [DOI] [Google Scholar]
- 99.Amidon S.A., Birch C.J., Brown S.E., Butts K.E., Felix A.L., Fuller S.S., Gao E., Lewis E.M., Michaud A., Proto D.M. Who Invited You? The Complex Story of Aquatic Invasive Species. Bureau of Reclamation; 2021. [Google Scholar]
- 100.Rajagopal S., Van der Velde G., Van der Gaag M., Jenner H.A. How effective is intermittent chlorination to control adult mussel fouling in cooling water systems? Water Res. 2003;37:329–338. doi: 10.1016/s0043-1354(02)00270-1. [DOI] [PubMed] [Google Scholar]
- 101.Matisoff G., Brooks G., Bourland B.I. Toxicity of chlorine dioxide to adult zebra mussels. J. AWWA (Am. Water Works Assoc.) 1996;88:93–106. doi: 10.1002/j.1551-8833.1996.tb06603.x. [DOI] [Google Scholar]
- 102.Salerno J., Gillis P.L., Khan H., Burton E., Deeth L.E., Bennett C.J., Sibley P.K., Prosser R.S. Sensitivity of larval and juvenile freshwater mussels (unionidae) to ammonia, chloride, copper, potassium, and selected binary chemical mixtures. Environ. Pollut. 2020;256:113398. doi: 10.1016/j.envpol.2019.113398. [DOI] [PubMed] [Google Scholar]
- 103.Claudi R., de Oliveira M.D. In: Limnoperna Fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Invading Nature - Springer Series in Invasion Ecology. Boltovskoy D., editor. Springer International Publishing; 2015. Chemical Strategies for the Control of the Golden Mussel (Limnoperna fortunei) in Industrial Facilities; pp. 417–441. [DOI] [Google Scholar]
- 104.Nalepa T.F., Schloesser D.W. Second Edition. CRC Press; 2013. Quagga and Zebra Mussels: Biology, Impacts, and Control. [Google Scholar]
- 105.Glomski L.M. Engineer Research and Development Center Vicksburg MS Environmental Lab; 2015. Zebra Mussel Chemical Control Guide, Version 2.0. [Google Scholar]
- 106.Hlth W. Molluscicide Screening and Evaluation. World Health Organization; 1965. [PMC free article] [PubMed] [Google Scholar]
- 107.Fernald R.T., Watson B.T. Quagga Zebra Mussels Biol. Impacts Control Lewis Publ; 2013. Eradication of Zebra Mussels (Dreissena polymorpha) from Millbrook Quarry, Virginia; pp. 195–214. [Google Scholar]
- 108.Waller D., Bartsch L., Bartsch M., Meulemans M., Severson T., Zolper T. Use of carbon dioxide to prevent zebra mussel (Dreissena polymorpha) settlement and effects on native mussels (Order Unionoida) and benthic communities. Manag. Biol. Invasion. 2021;12:927–951. [Google Scholar]
- 109.Whitledge G., Weber M., DeMartini J., Oldenburg J., Roberts D., Link C., Rackl S., Rude N., Yung A., Bock L., Oliver D. An evaluation Zequanox® efficacy and application strategies for targeted control of zebra mussels in shallow-water habitats in lakes. Manag. Biol. Invasion. 2015;6:71–82. [Google Scholar]
- 110.Meehan S., Gruber B., Lucy F. Zebra mussel control using Zequanox® in an Irish waterway. Manag. Biol. Invasion. 2014;5:279–286. [Google Scholar]
- 111.Meehan S., Shannon A., Gruber B., Rackl S.M., Lucy F.E. Ecotoxicological impact of Zequanox®, a novel biocide, on selected non-target Irish aquatic species. Ecotoxicol. Environ. Saf. 2014;107:148–153. doi: 10.1016/j.ecoenv.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 112.Molloy D.P., Mayer D.A., Gaylo M.J., Morse J.T., Presti K.T., Sawyko P.M., Karatayev A.Y., Burlakova L.E., Laruelle F., Nishikawa K.C., Griffin B.H. Pseudomonas fluorescens strain CL145A–A biopesticide for the control of zebra and quagga mussels (Bivalvia: Dreissenidae) J. Invertebr. Pathol. 2013;113:104–114. doi: 10.1016/j.jip.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 113.Murawski B. Zequanox: A Potential Solution to Zebra Mussels. Aisthesis Honors Stud. J. 2016;7:29–33. [Google Scholar]
- 114.Lovejoy R.T., Kandow A.N., Howeth J.G. Zebra mussels (Dreissena polymorpha) influence reservoir ecosystem attributes along southern invasion front metaecosystems in North America. Hydrobiologia. 2023 doi: 10.1007/s10750-022-05112-3. [DOI] [Google Scholar]
- 115.Calazans S.H.C., Americo J.A., da Costa Fernandes F., Aldridge D.C., de Freitas Rebelo M. Assessment of toxicity of dissolved and microencapsulated biocides for control of the Golden Mussel Limnoperna fortunei. Mar. Environ. Res. 2013;91:104–108. doi: 10.1016/j.marenvres.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Aldridge D.C., Elliott P., Moggridge G.D. Microencapsulated BioBullets for the control of biofouling zebra mussels. Environ. Sci. Technol. 2006;40:975–979. doi: 10.1021/es050614+. [DOI] [PubMed] [Google Scholar]
- 117.Tang F., Aldridge D.C. Microcapsulated biocides for the targeted control of invasive bivalves. Sci. Rep. 2019;9:18787. doi: 10.1038/s41598-019-55392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Timmons L., Court D.L., Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- 119.Huvenne H., Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J. Insect Physiol. 2010;56:227–235. doi: 10.1016/j.jinsphys.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 120.Mistry R., Ackerman J.D. Flow, flux, and feeding in freshwater mussels. Water Resour. Res. 2018;54:7619–7630. [Google Scholar]
- 121.Hannig G., Makrides S.C. Strategies for optimizing heterologous protein expression in Escherichia coli. Trends Biotechnol. 1998;16:54–60. doi: 10.1016/s0167-7799(97)01155-4. [DOI] [PubMed] [Google Scholar]
- 122.Mathai P.P., Bertram J.H., Padhi S.K., Singh V., Tolo I.E., Primus A., Mor S.K., Phelps N.B.D., Sadowsky M.J. Influence of Environmental Stressors on the Microbiota of Zebra Mussels (Dreissena polymorpha) Microb. Ecol. 2021;81:1042–1053. doi: 10.1007/s00248-020-01642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Whitten M.M.A., Facey P.D., Del Sol R., Fernández-Martínez L.T., Evans M.C., Mitchell J.J., Bodger O.G., Dyson P.J. Symbiont-mediated RNA interference in insects. Proc. Biol. Sci. 2016;283:20160042. doi: 10.1098/rspb.2016.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duman-Scheel M. Saccharomyces cerevisiae (Baker’s Yeast) as an Interfering RNA Expression and Delivery System. Curr. Drug Targets. 2019;20:942–952. doi: 10.2174/1389450120666181126123538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Naddafi R., Pettersson K., Eklöv P. The effect of seasonal variation in selective feeding by zebra mussels (Dreissena polymorpha) on phytoplankton community composition. Freshw. Biol. 2007;52:823–842. [Google Scholar]
- 126.McCartney M.A., Auch B., Kono T., Mallez S., Zhang Y., Obille A., Becker A., Abrahante J.E., Garbe J., Badalamenti J.P., et al. The genome of the zebra mussel, Dreissena polymorpha: a resource for comparative genomics, invasion genetics, and biocontrol. G3 (Bethesda) 2022;12:jkab423. doi: 10.1093/g3journal/jkab423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fong P.P. Zebra mussel spawning is induced in low concentrations of putative serotonin reuptake inhibitors. Biol. Bull. 1998;194:143–149. doi: 10.2307/1543044. [DOI] [PubMed] [Google Scholar]
- 128.Ram J.L., Crawford G.W., Walker J.U., Mojares J.J., Patel N., Fong P.P., Kyozuka K. Spawning in the zebra mussel (Dreissena polymorpha): activation by internal or external application of serotonin. J. Exp. Zool. 1993;265:587–598. doi: 10.1002/jez.1402650515. [DOI] [PubMed] [Google Scholar]
- 129.Fong P.P., Wall D.M., Ram J.L. Characterization of serotonin receptors in the regulation of spawning in the zebra mussel Dreissena polymorpha (Pallas) J. Exp. Zool. 1993;267:475–482. [Google Scholar]
- 130.Ram J.L., Nichols S.J. Chemical Regulation of Spawning in the Zebra Mussel (Dreissena polymorpha) Lewis Publishers; 1992. [Google Scholar]
- 131.Ram J.L., Fong P.P., Kyozuka K. Serotonergic mechanisms mediating spawning and oocyte maturation in the zebra mussel, Dreissena polymorpha. Invertebr. Reprod. Dev. 1996;30:29–37. [Google Scholar]
- 132.Liang J.C., Bloom R.J., Smolke C.D. Engineering Biological Systems with Synthetic RNA Molecules. Mol. Cell. 2011;43:915–926. doi: 10.1016/j.molcel.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He L., Huang Y., Tang X. RNAi-based pest control: Production, application and the fate of dsRNA. Front. Bioeng. Biotechnol. 2022;10:1080576. doi: 10.3389/fbioe.2022.1080576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carballal M.J., Barber B.J., Iglesias D., Villalba A. Neoplastic diseases of marine bivalves. J. Invertebr. Pathol. 2015;131:83–106. doi: 10.1016/j.jip.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 135.Elston R.A., Kent M.L., Drum A.S. Transmission of hemic neoplasia in the bay mussel, Mytilusedulis, using whole cells and cell homogenate. Dev. Comp. Immunol. 1988;12:719–727. doi: 10.1016/0145-305x(88)90047-x. [DOI] [PubMed] [Google Scholar]
- 136.Mateo D.R., MacCallum G.S., Davidson J. Field and laboratory transmission studies of haemic neoplasia in the soft-shell clam, Mya arenaria, from Atlantic Canada. J. Fish. Dis. 2016;39:913–927. doi: 10.1111/jfd.12426. [DOI] [PubMed] [Google Scholar]
- 137.Walker C.W., Van Beneden R.J., Muttray A.F., Böttger S.A., Kelley M.L., Tucker A.E., Thomas W.K. p53 superfamily proteins in marine bivalve cancer and stress biology. Adv. Mar. Biol. 2011;59:1–36. doi: 10.1016/B978-0-12-385536-7.00001-7. [DOI] [PubMed] [Google Scholar]
- 138.Díaz S., Cao A., Villalba A., Carballal M.J. Expression of mutant protein p53 and Hsp70 and Hsp90 chaperones in cockles Cerastoderma edule affected by neoplasia. Dis. Aquat. Organ. 2010;90:215–222. doi: 10.3354/dao02231. [DOI] [PubMed] [Google Scholar]
- 139.Vassilenko E.I., Muttray A.F., Schulte P.M., Baldwin S.A. Variations in p53-like cDNA sequence are correlated with mussel haemic neoplasia: A potential molecular-level tool for biomonitoring. Mutat. Res. 2010;701:145–152. doi: 10.1016/j.mrgentox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 140.Duffy M.J., Synnott N.C., Crown J. Mutant p53 as a target for cancer treatment. Eur. J. Cancer. 2017;83:258–265. doi: 10.1016/j.ejca.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 141.Ahuja D., Sáenz-Robles M.T., Pipas J.M. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 142.Uliano-Silva M., Americo J.A., Brindeiro R., Dondero F., Prosdocimi F., Rebelo M.d.F. Gene Discovery through Transcriptome Sequencing for the Invasive Mussel Limnoperna fortunei. PLoS One. 2014;9:e102973. doi: 10.1371/journal.pone.0102973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Uliano-Silva M., Dondero F., Dan Otto T., Costa I., Lima N.C.B., Americo J.A., Mazzoni C.J., Prosdocimi F., Rebelo M.d.F. A hybrid-hierarchical genome assembly strategy to sequence the invasive golden mussel, Limnoperna fortunei. GigaScience. 2018;7:gix128. doi: 10.1093/gigascience/gix128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Afonso L.F., Americo J.A., Soares-Souza G.B., Torres A.L.Q., Wajsenzon I.J.R., Rebelo M.D. Gonad transcriptome of golden mussel Limnoperna fortunei reveals potential sex differentiation genes. Preprint at bioRxiv. 2019 doi: 10.1101/818757. [DOI] [Google Scholar]
- 145.Breton S., Capt C., Guerra D., Stewart D. In: Transitions Between Sexual Systems: Understanding the Mechanisms of, and Pathways Between, Dioecy, Hermaphroditism and Other Sexual Systems. Leonard J.L., editor. Springer International Publishing; 2018. Sex-Determining Mechanisms in Bivalves; pp. 165–192. [DOI] [Google Scholar]
- 146.Huang S., Ye L., Chen H. Sex determination and maintenance: the role of DMRT1 and FOXL2. Asian J. Androl. 2017;19:619–624. doi: 10.4103/1008-682X.194420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li R., Zhang L., Li W., Zhang Y., Li Y., Zhang M., Zhao L., Hu X., Wang S., Bao Z. FOXL2 and DMRT1L Are Yin and Yang Genes for Determining Timing of Sex Differentiation in the Bivalve Mollusk Patinopecten yessoensis. Front. Physiol. 2018;9:1166. doi: 10.3389/fphys.2018.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Evensen K.G., Robinson W.E., Krick K., Murray H.M., Poynton H.C. Comparative phylotranscriptomics reveals putative sex differentiating genes across eight diverse bivalve species. Comp. Biochem. Physiol., Part D: Genomics Proteomics. 2022;41:100952. doi: 10.1016/j.cbd.2021.100952. [DOI] [PubMed] [Google Scholar]
- 149.Sun D., Yu H., Li Q. Early gonadal differentiation is associated with the antagonistic action of Foxl2 and Dmrt1l in the Pacific oyster. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2023;265:110831. doi: 10.1016/j.cbpb.2023.110831. [DOI] [PubMed] [Google Scholar]
- 150.Sun D., Yu H., Li Q. Examination of the roles of Foxl2 and Dmrt1 in sex differentiation and gonadal development of oysters by using RNA interference. Aquaculture. 2022;548:737732. doi: 10.1016/j.aquaculture.2021.737732. [DOI] [Google Scholar]
- 151.Sun D., Yu H., Li Q. Starvation-induced changes in sex ratio involve alterations in sex-related gene expression and methylation in Pacific oyster Crassostrea gigas. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2023;267:110863. doi: 10.1016/j.cbpb.2023.110863. [DOI] [PubMed] [Google Scholar]
- 152.Ferreira J.G.R.N., Americo J.A., do Amaral D.L.A.S., Sendim F., da Cunha Y.R., Uliano-Silva M., Rebelo M.F. The Darwin Tree of Life Project Consortium. A high quality chromosome-level genome assembly for the golden mussel (Limnoperna fortunei) Preprint at bioRxiv. 2022 doi: 10.1101/2022.09.29.509984. [DOI] [Google Scholar]
- 153.Huang Y., Wu L., Jin M., Hui K., Ren Q. A C1qDC Protein (HcC1qDC6) with Three Tandem C1q Domains Is Involved in Immune Response of Triangle-Shell Pearl Mussel (Hyriopsis cumingii) Front. Physiol. 2017;8:521. doi: 10.3389/fphys.2017.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Collins J.P. BMC proceedings (BioMed Central) 2018. Gene drives in our future: challenges of and opportunities for using a self-sustaining technology in pest and vector management; pp. 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Legros M., Marshall J.M., Macfadyen S., Hayes K.R., Sheppard A., Barrett L.G. Gene drive strategies of pest control in agricultural systems: Challenges and opportunities. Evol. Appl. 2021;14:2162–2178. doi: 10.1111/eva.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Frieß J.L., Lalyer C.R., Giese B., Simon S., Otto M. Review of gene drive modelling and implications for risk assessment of gene drive organisms. Ecol. Modell. 2023;478:110285. doi: 10.1016/j.ecolmodel.2023.110285. [DOI] [Google Scholar]
- 157.Haller B.C., Messer P.W. SLiM 3: Forward Genetic Simulations Beyond the Wright–Fisher Model. Mol. Biol. Evol. 2019;36:632–637. doi: 10.1093/molbev/msy228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McCartney M.A., Mallez S., Gohl D.M. Genome projects in invasion biology. Conserv. Genet. 2019;20:1201–1222. doi: 10.1007/s10592-019-01224-x. [DOI] [Google Scholar]
- 159.Calcino A.D., de Oliveira A.L., Simakov O., Schwaha T., Zieger E., Wollesen T., Wanninger A. The quagga mussel genome and the evolution of freshwater tolerance. DNA Res. 2019;26:411–422. doi: 10.1093/dnares/dsz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McCartney M.A. Structure, function and parallel evolution of the bivalve byssus, with insights from proteomes and the zebra mussel genome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021;376:20200155. doi: 10.1098/rstb.2020.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cohen A.N. San Franc. Estuary Inst. Oakl.; 2008. Literature Review of the Exotic Mussels Dreissena polymorpha, Dreissena Bugensis, Limnoperna Fortunei and Mytilopsis Leucophaeata. [Google Scholar]
- 162.Nichols S.J. Variations in the reproductive cycle of Dreissena polymorpha in Europe, Russia, and North America. Am. Zool. 1996;36:311–325. [Google Scholar]
- 163.Wright D.A., Setzler-Hamilton E.M., Magee J.A., Rodger Harvey H. Laboratory culture of zebra (Dreissena polymorpha) and quagga (D. bugensis) mussel larvae using estuarine algae. J. Great Lakes Res. 1996;22:46–54. [Google Scholar]
- 164.Vanderploeg H.A., Liebig J.R., Gluck A.A. Evaluation of different phytoplankton for supporting development of zebra mussel larvae (Dreissena polymorpha): the importance of size and polyunsaturated fatty acid content. J. Great Lakes Res. 1996;22:36–45. [Google Scholar]
- 165.Wacker A., Becher P., von Elert E. Food quality effects of unsaturated fatty acids on larvae of the zebra mussel Dreissena polymorpha. Limnol. Oceanogr. 2002;47:1242–1248. [Google Scholar]
- 166.Stoeckel J.A., Padilla D.K., Schneider D.W., Rehmann C.R. Laboratory culture of Dreissena polymorpha larvae: spawning success, adult fecundity, and larval mortality patterns. Can. J. Zool. 2004;82:1436–1443. [Google Scholar]
- 167.Cataldo D.H. Larval development of Limnoperna fortunei. Limnoperna Fortunei Ecol. Distrib. Control Swiftly Spreading Invasive Fouling Mussel. 2015:43–53. [Google Scholar]
- 168.Ackerman J.D., Sim B., Nichols S.J., Claudi R. A review of the early life history of zebra mussels (Dreissena polymorpha): comparisons with marine bivalves. Can. J. Zool. 1994;72:1169–1179. [Google Scholar]
- 169.Horvath T., Crane L. Hydrodynamic forces affect larval zebra mussel (Dreissena polymorpha) mortality in a laboratory setting. Aquat. Invasions. 2010;5:379–385. [Google Scholar]
- 170.Rehmann C.R., Stoeckel J.A., Schneider D.W. Effect of turbulence on the mortality of zebra mussel veligers. Can. J. Zool. 2003;81:1063–1069. [Google Scholar]
- 171.Schneider D.W., Stoeckel J.A., Rehmann C.R., Douglas Blodgett K., Sparks R.E., Padilla D.K. A developmental bottleneck in dispersing larvae: implications for spatial population dynamics. Ecol. Lett. 2003;6:352–360. [Google Scholar]
- 172.Sprung M. Ecological requirements of developing Dreissena polymorpha eggs. Arch. Für Hydrobiol. Suppl. Monogr. Beitr. 1987;79:69–86. [Google Scholar]
- 173.Fujimura T., Wada K., Iwaki T. Development of digestive system of the pearl oyster larvae, Pinctada fucata. Jpn. J. Malacol. Jpn. 1995 [Google Scholar]
- 174.Sprung M., Widdows J. Rate of heat dissipation by gametes and larval stages of Mytilus edulis. Mar. Biol. 1986;91:41–45. [Google Scholar]
- 175.Wacker A., Von Elert E. Food quality controls reproduction of the zebra mussel (Dreissena polymorpha) Oecologia. 2003;135:332–338. doi: 10.1007/s00442-003-1208-5. [DOI] [PubMed] [Google Scholar]
- 176.Morton B. The anatomy of Dreissena polymorpha and the evolution and success of the heteromyarian form in the Dreissenoidea. Zebra Mussels Biol. Impacts Control. 1993:185–215. [Google Scholar]
- 177.Sprung M. Zebra Mussels Biol. Impacts Control Lewis Publ; 1993. The Other Life: An Account of Present Knowledge of the Larval Phase of Dreissena polymorpha; pp. 39–53. 2 Tab 73 Ref. [Google Scholar]
- 178.Darrigran G. Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biol. Invasions. 2002;4:145–156. [Google Scholar]
- 179.Hincks S.S., Mackie G.L. Effects of pH, calcium, alkalinity, hardness, and chlorophyll on the survival, growth, and reproductive success of zebra mussel (Dreissena polymorpha) in Ontario lakes. Can. J. Fish. Aquat. Sci. 1997;54:2049–2057. [Google Scholar]
- 180.Baker P., Baker S., Mann R. Criteria for Predicting Zebra Mussel Invasions in the Mid-Atlantic Region. Elsevier Digital Commons; 1993. [Google Scholar]
- 181.Dietz T.H., Lessard D., Silverman H., Lynn J.W. Osmoregulation in Dreissena polymorpha: the importance of Na, Cl, K, and particularly Mg. Biol. Bull. 1994;187:76–83. doi: 10.2307/1542167. [DOI] [PubMed] [Google Scholar]
- 182.Huvet A., Béguel J.P., Cavaleiro N.P., Thomas Y., Quillien V., Boudry P., Alunno-Bruscia M., Fabioux C. Disruption of amylase genes by RNA interference affects reproduction in the Pacific oyster Crassostrea gigas. J. Exp. Biol. 2015;218:1740–1747. doi: 10.1242/jeb.116699. [DOI] [PubMed] [Google Scholar]
- 183.You Y., Huan P., Liu B. RNAi assay in primary cells: a new method for gene function analysis in marine bivalve. Mol. Biol. Rep. 2012;39:8209–8216. doi: 10.1007/s11033-012-1668-y. [DOI] [PubMed] [Google Scholar]
- 184.Yu H., Li H., Li Q., Xu R., Yue C., Du S. Targeted Gene Disruption in Pacific Oyster Based on CRISPR/Cas9 Ribonucleoprotein Complexes. Mar. Biotechnol. 2019;21:301–309. doi: 10.1007/s10126-019-09885-y. [DOI] [PubMed] [Google Scholar]
- 185.Terradas G., Macias V.M., Peterson H., McKeand S., Krawczyk G., Rasgon J.L. Receptor-Mediated Ovary Transduction of Cargo–ReMOT Control: a Comprehensive Review and Detailed Protocol for Implementation. Transgenic Insects Tech. Appl. 2022:125–148. [Google Scholar]
- 186.Waller D., Pucherelli S., Barbour M., Tank S., Meulemans M., Wise J., Dahlberg A., Aldridge D.C., Claudi R., Cope W.G., et al. Review and development of best practices for toxicity tests with dreissenid mussels. Environ. Toxicol. Chem. 2023;42:1649–1666. doi: 10.1002/etc.5648. [DOI] [PubMed] [Google Scholar]