Summary
The SARS-CoV-2 genome encodes a multitude of accessory proteins. Using comparative genomic approaches, an additional accessory protein, ORF3c, has been predicted to be encoded within the ORF3a sgmRNA. Expression of ORF3c during infection has been confirmed independently by ribosome profiling. Despite ORF3c also being present in the 2002–2003 SARS-CoV, its function has remained unexplored. Here we show that ORF3c localizes to mitochondria, where it inhibits innate immunity by restricting IFN-β production, but not NF-κB activation or JAK-STAT signaling downstream of type I IFN stimulation. We find that ORF3c is inhibitory after stimulation with cytoplasmic RNA helicases RIG-I or MDA5 or adaptor protein MAVS, but not after TRIF, TBK1 or phospho-IRF3 stimulation. ORF3c co-immunoprecipitates with the antiviral proteins MAVS and PGAM5 and induces MAVS cleavage by caspase-3. Together, these data provide insight into an uncharacterized mechanism of innate immune evasion by this important human pathogen.
Subject areas: Molecular biology, Immunology, Microbiology, Proteomics
Graphical abstract

Highlights
-
•
The accessory protein ORF3c is encoded by a “hidden” ORF, which overlaps ORF3a
-
•
ORF3c is expressed by leaky scanning, at approximately the same levels as ORF3a
-
•
ORF3c localizes to mitochondria and interacts with both MAVS and PGAM5
-
•
ORF3c expression leads to a reduction in IFNB transcripts and IFN-β protein levels
Molecular biology; Immunology; Microbiology; Proteomics
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of COVID-19 and the recent pandemic that has had unprecedented effects upon humanity, ranging from numerous casualties to severe economic impact. It is imperative to have a thorough understanding of the pathogen-host interactions that occur during infection. A key first step is to delineate and characterize functionally the full complement of accessory proteins encoded in the SARS-CoV-2 genome. However, most studies have been informed by pre-existing research based upon the closely related SARS-CoV (referred to herein as SARS-CoV-1 to avoid confusion), which caused a relatively minor outbreak in 2002–2003. As a result, proteins that had not been annotated in studies of SARS-CoV-1 were overlooked during the initial scientific response to the COVID-19 pandemic.
SARS-CoV-2 and SARS-CoV-1 belong to the taxon Sarbecovirus, a subgenus of the genus Betacoronavirus in the family Coronaviridae. Members of the Coronaviridae possess large positive-sense, single-stranded RNA genomes (approximately 30 kb in size) which contain 7–16 protein-coding open reading frames (ORFs). The two largest ORFs, ORF1a and ORF1b, are translated from the genomic mRNA directly (with the translation of ORF1b depending on ribosomes making a programmed frameshift near the end of ORF1a) and encode polyproteins pp1a and pp1ab, which are cleaved into individual functional proteins that support viral replication. The remainder of the ORFs are translated from a set of “nested” subgenomic mRNAs (sgmRNAs). The encoded proteins are either structural components of the virion or so-called “accessory” proteins: mostly dispensable for viral replication in cell culture, these latter proteins nonetheless confer significant advantages to replication in hosts, often due to interactions with innate immune pathways. The assemblage of accessory proteins varies substantially across the Coronaviridae, and can vary even between closely related coronaviral species, thus constituting an important field of research. The potential translational repertoire of SARS-CoV-2 has been the subject of many publications, with multiple groups reporting evidence of novel ORFs via a range of approaches.1,2,3,4,5,6,7,8 Collectively, these studies highlight the lack of an established, accepted SARS-CoV-2 viral proteome because the functional relevance of these reports is seldom validated experimentally.9,10
In 2020, making use of the ∼54 then-available sarbecovirus genomes, we and others used comparative genomic approaches to analyze the coding capacity of SARS-CoV-2. Our analysis revealed a previously undetected conserved ORF, overlapping ORF3a in the +1 reading frame, and precisely coinciding with a region of statistically significantly enhanced synonymous site conservation in ORF3a-frame codons, indicative of a functional overlapping gene, ORF3c.11 ORF3c was predicted independently by Cagliani et al. who termed it ORF3h12 and Jungreis et al. who termed it ORF3c.2 A community consensus fixed the name as ORF3c.13 Given ORF3c was previously unknown, it had not been the focus of any pre-existing studies despite being present in SARS-CoV-1. However its continued presence throughout evolution indicates that it is beneficial to successful viral replication, immune evasion or transmission, at least in natural hosts (seemingly mainly Rhinolophus bats).14,15,16,17 In our comparative genomic analysis, we found ORF3c to be the only novel ORF that is conserved across sarbecoviruses and subject to purifying selection.11 Translation of ORF3c is supported by ribosome profiling data,1 which is not the case for the majority of other predicted novel SARS-CoV-2 ORFs. ORF3c thus represents a previously uninvestigated area of sarbecovirus research.
SARS-CoV-2 infection is known to dysregulate host immune responses, specifically the type I interferon (IFN) response, resulting in the severe clinical symptoms characteristic of this pathogen.18,19,20 Type I IFNs are essential innate cytokines that induce a host response that restricts and eliminates SARS-CoV-2 infection.21 Host cells produce type I IFNs in response to the activation of pattern recognition receptors (PRRs): host proteins that recognize molecules from either pathogens or damaged cells. Pathogen-associated molecular patterns (PAMPs) activate PRRs that in turn promote the transcription of type I IFNs and other antiviral genes.22 The two key cytosolic PRRs involved in the antiviral response to RNA viruses are retinoic acid-inducible gene (RIG-I) and melanoma differentiation-associated protein 5 (MDA5). RIG-I senses short dsRNA and 5′-ppp/pp-RNA23 whereas MDA5 senses high molecular weight dsRNA and mRNA that lack 2′-O-methylation at the 5′ cap.24,25 Both RIG-I and MDA5 form filaments with dsRNA to recruit mitochondrial antiviral-signaling protein (MAVS). This interaction promotes the formation of MAVS polymers at the mitochondrial outer membrane that activate transcription factors IFN regulatory factor 3 (IRF3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).26 The activated IRF3 dimer and NF-κB complex translocate into the nucleus and bind to responsive promoters to stimulate the transcription of type I IFNs27 and other pro-inflammatory factors. To suppress the production of these molecules, viruses have evolved different strategies targeting the activation of RIG-I, MDA5 or MAVS, or components of the downstream signaling pathways.
Here we present an initial functional characterization of ORF3c. We find that it localizes to the mitochondrial outer membrane, a platform heavily involved in innate immune signaling. We provide evidence that ORF3c subverts the cascade of cellular antiviral responses via preventing the activation of transcription from the IFN-β gene. This appears to be mediated by interactions of ORF3c with both PGAM5 (mitochondrial protein phosphoglycerate mutant family member 5, previously known as phosphoglycerate mutase family member 5) and MAVS, alongside a subsequent cleavage of MAVS by activated caspases.
Results
ORF3c is conserved across sarbecoviruses
Since we identified ORF3c as a previously undetected and conserved ORF in 2020,11 numerous additional sarbecovirus sequences have been published, including many divergent sequences from bat hosts. We inspected GenBank sequence records for all unique ORF3c sequences (Figures 1A and S1). The ORF3c protein is 39–41 amino acids in length and has a predicted C-terminal transmembrane region and a shorter N-terminal hydrophobic region (Figure 1A).
Figure 1.
ORF3c is a conserved membrane-associated protein expressed by leaky scanning
(A) Representative ORF3c sequences from different sarbecoviruses. See Figure S1 for additional sequences. Sequences are labeled with accession numbers and either name of host (red) or name of virus (blue). Sequence differences from SARS-CoV-2 ORF3c are indicated with white letters. Amino acids are colour-coded according to their physicochemical properties. Asterisks indicate completely conserved columns in the alignment. A transmembrane region predicted by Phobius28 is indicated with a pink bar below the alignment. The helix(H)/coil(C) secondary structure of SARS-CoV-2 3c as predicted with RaptorX29 is shown above the alignment.
(B) Schematic of in vitro transcribed reporter RNAs. RNAs contained the SARS-CoV-2 ORF3a sgmRNA from the leader to the end of the ORF3c coding region. This was followed by FMDV 2A and RLuc sequences, which were in-frame with one of the three AUG codons: two in the ORF3a frame (0 frame), and the last in the ORF3c frame (+1 frame). The AUGs were then mutated systematically to ACG to inhibit ribosomal initiation.
(C) RLuc values were normalized to an internal FLuc control, for each well. Normalized values for each individual experiment were converted to a percentage of ORF3a WT. Individual data points (circles), and means (bars) ± SEM (error bars) based on 3 independent experiments are shown. Statistical analysis (unpaired two sample t-test): ns = not significant, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
(D) Vero cells were transfected with ORF3c-HA or empty plasmid (Mock) and subjected to subcellular fractionation at 24 h post-transfection. Fractions were probed by immunoblot for various markers: alpha-tubulin (cytoplasmic), VDAC (mitochondrial membrane), lamin A/C (nuclear) and the HA tag. A HA-specific band was only observed in the membranous fraction. WCL: whole cell lysate; Cy: cytoplasmic fraction; M: membranous fraction; N: soluble nuclear fraction; L: protein markers (ladder).
ORF3c is expressed via leaky scanning
ORF3c is a small protein (predicted MW = 4.9 kDa) and the ORF entirely overlaps with ORF3a in the +1 frame (with respect to ORF3a). We hypothesized that ORF3c is expressed via ribosomal leaky scanning on the ORF3a sgmRNA, in a similar manner to the ORF7b and ORF9b proteins of SARS-CoV-1.30,31 This would require scanning preinitiation (43S) complexes to proceed past both the AUG start codon of ORF3a and a subsequent in-frame AUG, and then initiate at a third AUG: the start codon of ORF3c (mechanism reviewed in Firth, 201232). Both AUGs in the ORF3a frame have intermediate or weak initiation contexts (thus facilitating leaky scanning) whereas the ORF3c AUG has a strong initiation context.33
To test the leaky scanning hypothesis and measure the ORF3a:ORF3c relative expression levels, an expression cassette was created composed of the 5′ end of the ORF3a sgmRNA transcript (including the 77 nucleotide leader) up to the final coding nucleotide of ORF3c (excluding the stop codon). This was followed by a foot and mouth disease virus (FMDV) 2A sequence (which mediates the co-translational separation of the polypeptide chain) and then the Renilla luciferase (RLuc) sequence. The 2A-RLuc ORF was in-frame with the AUG of either ORF3a or ORF3c. A range of mutants were created based on both of these constructs, which ablated (i) the first AUG of ORF3a; (ii) the second AUG of ORF3a; (iii) both AUGs of ORF3a; or (iv) the AUG of ORF3c (Figure 1B). In all cases, AUGs were mutated to ACG (which, however, may still allow low level initiation32).
Plasmid DNA templates were transcribed in vitro by the T7 RNA polymerase and the transcripts were then purified and transfected into Vero cells in a 96-well plate format. The luciferase values were measured at 20 h post transfection. RLuc was normalized to an internal firefly luciferase (FLuc) control. Values for the ORF3c WT and ORF3a and ORF3c mutant RNAs were then normalized to those for the ORF3a WT RNAs (Figure 1C). Expression of ORF3a or ORF3c was sensitive to, respectively, mutation of the first or both ORF3a-frame AUGs, and mutation of the ORF3c-frame AUG. However, the second AUG was not noticeably utilized for ORF3a expression. As expected, mutation of the ORF3c-frame AUG did not affect ORF3a expression. With the WT sequence, the levels of ORF3c and ORF3a expression appeared to be similar to each other. Consistent with a leaky scanning model, when the first or both ORF3a-frame AUGs were mutated, ORF3c expression approximately doubled. Thus, under the conditions tested, approximately 50% of preinitiation complexes appear to scan past the first two AUGs within the ORF3a sgmRNA and initiate at the next downstream AUG to translate ORF3c. This translational efficiency would result in an approximately equal stoichiometric ratio of ORF3a:ORF3c proteins from the one sgmRNA.
ORF3c associates with cellular membranes
Many mature SARS-CoV-2 proteins possess transmembrane domains or are membrane-associated via a range of topologies and anchors. These include the structural proteins spike (S), membrane (M) and envelope (E), non-structural proteins NSP4 and NSP6 and the accessory proteins ORF3a, ORF6, ORF7a, ORF7b and ORF9b. Other viral proteins possess N-terminal hydrophobic ER-targeting signals that are cleaved following membrane translocation and folding, resulting in a mature secretory-like protein (for example, ORF8). Membrane-tethered viral proteins localize to a range of subcellular locations: NSP3, NSP4 and NSP6 form a complex that rearranges the ER, where viral replication organelles are found34,35; ORF6 localizes to the plasma membrane,36 Golgi and ER37; ORF7a and ORF7b to the Golgi38,39; ORF9b to the mitochondrion and various intracellular vesicles (anchored notably via a hydrophobic cavity,40,41 rather than a prototypic transmembrane helix); and ORF3a to lysosomes, Golgi,42 ER and the plasma membrane.43,44 The three transmembrane structural proteins S, M and E all localize with varying degrees to the ER, Golgi and ERGIC compartments during the viral assembly, budding and trafficking processes.45
ORF3c is predicted to possess a C-terminal ɑ-helix (Figure 1A), which is likely to form a transmembrane domain,11 suggesting that ORF3c is a single-pass integral transmembrane protein. To confirm this predicted membrane association, C-terminally HA-tagged ORF3c (ORF3c-HA) was overexpressed by transfection in Vero cells and the cells were subject to subcellular fractionation. Cytoplasmic, membranous and nuclear fractions were probed for ORF3c-HA via immunoblotting and ORF3c was found to localize primarily within the membranous fraction (Figure 1D). This fraction would contain the plasma membrane and membranes from all membrane-bound organelles.
ORF3c can localize to the ER membrane in vitro
According to the predicted C-terminal helical transmembrane domain,11 ORF3c most likely is a tail-anchored protein. Whilst this group of diverse and functionally important integral membrane proteins are present in all intracellular membranes with a cytosolic surface,46,47 it is generally accepted that most tail-anchored proteins bearing transmembrane domains of substantial hydrophobic character are post-translationally targeted to the endoplasmic reticulum (ER) – the central organelle involved in coronaviral RNA replication48,49,50 and to which multiple SARS-CoV-2 proteins localize. In contrast, tail-anchored proteins containing transmembrane domains of reduced hydrophobicity and increased charge within the extremely short exoplasmic C-terminal region (Cexo), typically target to the mitochondrial outer membrane (MOM).51,52 Thus, we explored sequentially the ability of ORF3c to integrate into the membrane of the ER and mitochondria using in vitro systems specialised for each organelle.
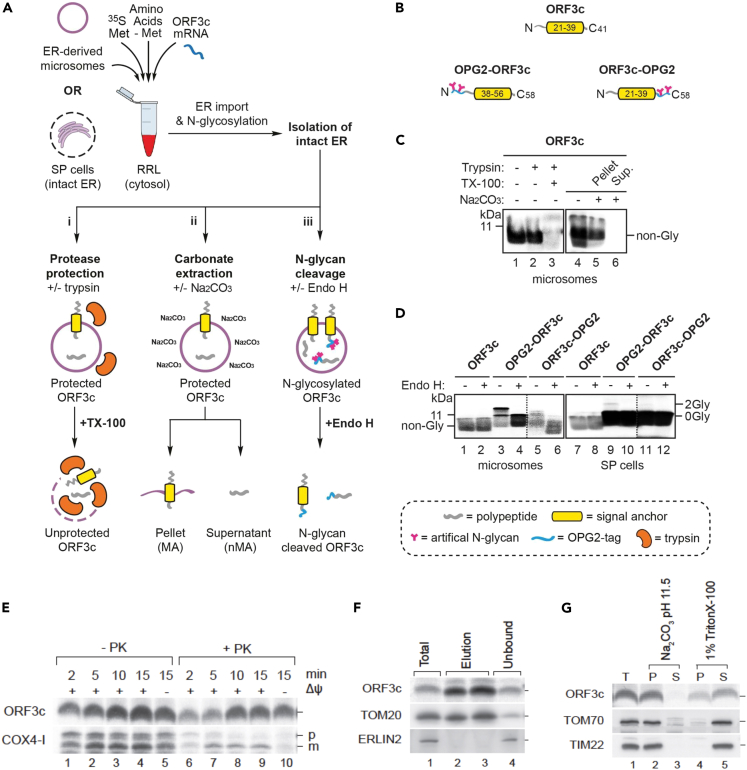
Having exploited canine pancreatic microsomes to study the integration of other SARS-CoV-2 membrane proteins into the ER,53 we studied ORF3c biogenesis using this system (Figure 2A). We found that in vitro synthesized and imported ORF3c (Figure 2B) was protected from added protease (Figure 2C, lanes 1–3) and was resistant to extraction with alkaline sodium carbonate buffer (Figure 2C, lanes 4–6), suggesting that ORF3c can integrate stably into the ER membrane in vitro.
Figure 2.
ORF3c can localize to the ER membrane in vitro but also inserts efficiently into mitochondrial membranes
(A) Outline of the in vitro assay where either canine pancreatic microsomes or semi-permeabilized (SP) HeLa cells are used as sources of ER membrane. Following translation, membrane-associated ORF3c proteins were recovered by centrifugation and subjected to: i) a protease protection assay using trypsin in the presence or absence of Triton X-100 (TX-100), ii) alkaline sodium-carbonate extraction, or iii) endoglycosidase H (Endo H) treatment. Resulting products were analyzed by SDS-PAGE and phosphorimaging.
(B) Schematics of parent ORF3c and its N-terminally (OPG2-ORF3c) and C-terminally (ORF3c-OPG2) OPG2-tagged variants. Comprising residues 1–18 of bovine rhodopsin (Uniprot: P02699), the OPG2 tag is indicated in blue and its N-glycan sites in pink. The putative ORF3c transmembrane domain is indicated in yellow.
(C) Membrane-associated products of ORF3c synthesized in the presence of ER-derived microsomes were treated with trypsin (lanes 2–3) or sodium carbonate (lanes 5–6) as outlined in Ai-Aii.
(D) Using ER-derived microsomes (lanes 1–6) or SP cells (lanes 7–12), parent and OPG2-tagged variants of ORF3c were synthesized as outlined in Aiii. N-glycosylated (2Gly) and non-glycosylated (0Gly) species were confirmed using Endo H. Note that, whilst on the same gel, the signals in lanes 5–6 and 11–12 of panel (D) have been overexposed compared to lanes 1–4 and 7–10 (demarcated by dotted lines) in order to enhance the visibility of the radiolabeled products. RRL, rabbit reticulocyte lysate.
(E) In vitro import and proteinase K (PK) accessibility assay of isolated HEK293T mitochondria upon [35S]ORF3c-5M and COX4-1 import. p, precursor protein; m, mature protein.
(F) Immuno-isolation of mitochondria with anti-Tom22 magnetic beads after [35S]ORF3c-5M import.
(G) Chemical protein extraction following [35S]ORF3c-5M protein import into mitochondria isolated from HEK293T cells. Mitochondria were treated with sodium carbonate (Na2CO3, pH 11.5) or with TBS 1% Triton X-100. T, total; P, pellet; and S, soluble.
To investigate its membrane topology, we modified ORF3c to facilitate the detection of ER import – incorporating an OPG2 tag either at the extreme N or C terminus to generate OPG2-ORF3c and ORF3c-OPG2, respectively (Figure 2B). Since the OPG2 epitope supports efficient ER lumenal N-glycosylation,54 we synthesized radiolabeled ORF3c and its two OPG2-tagged ORF3c variants in the presence of ER membranes and used endoglycosidase H (Endo H) treatment of the resulting membrane-associated products to identify N-glycosylated species (Figure 2D, even numbered lanes). On the basis of these studies, we found that both the N and C termini of OPG2-tagged ORF3c can be N-glycosylated and hence can be translocated into the lumen of ER microsomes (Figure 2D, lanes 1–6). Interestingly, when the same ORF3c proteins were analyzed using semi-permeabilized HeLa cells (SP HeLa cells; Figure 2D, lanes 7–12), the extent of this N-glycosylation was greatly reduced but the total ORF3c signal increased, suggesting it may be targeted to another organelle.Whereas canine pancreatic microsomes are highly enriched in ER-derived membranes, SP cells preserve the integrity of multiple subcellular organelles including both the ER and mitochondria.51,55 Therefore, we considered the possibility that, in contrast to the robust N-glycosylation observed using purified ER membranes, the presence of mitochondria in SP cells may reduce the opportunity for ORF3c to mislocalize to the ER by providing access to the mitochondrial outer membrane (MOM).51,56
ORF3c inserts efficiently into mitochondrial membranes in vitro
Next, we investigated the ability of ORF3c to insert into the membranes of isolated mitochondria by using an in vitro assay comparable to that used to assess ER import. Tail anchored proteins are not found in the inner mitochondrial membrane, whereas a range of endogenous MOM proteins (such as Tom5, Tom6 and Tom7) possess this topology. We radiolabeled ORF3c in reticulocyte lysate and incubated the protein with mitochondria from HEK293T cells. As this fraction was isolated by differential centrifugation, it is highly enriched for mitochondria but could possibly contain low levels of trans-Golgi network (TGN) and ER contaminants. ORF3c increasingly associated in vitro with mitochondria over time (Figure 2E). In contrast to the mitochondrial matrix protein COX4-1, association of ORF3c with mitochondria occurred independently of an inner mitochondrial membrane potential. Following ORF3c import, mitochondria were treated with proteinase K to degrade the non-imported protein. We observed a gradual increase in ORF3c signal intensity with time (Figure 2E, lanes 6–10) suggesting that the protein becomes proteinase K resistant probably due to membrane integration. To rule out that ORF3c targeting occurred due to a distinct contaminating-membrane in the mitochondrial preparation, anti-Tom22 antibodies immobilized on magnetic beads were used to immuno-isolate mitochondria after ORF3c import. The resulting immuno-isolated mitochondria showed clear enrichment for ORF3c and Tom20, while ERLIN2 (an ER marker) was not copurified (Figure 2F). Accordingly, we conclude that ORF3c is targeted selectively to mitochondria.
To address whether imported ORF3c was integrated stably into mitochondrial membranes, we imported ORF3c into purified mitochondria that subsequently were treated with sodium carbonate buffer (pH 11.4). Under these conditions, integral membrane proteins are retained in the membrane pellet, whilst soluble and peripheral membrane proteins are released into the supernatant. Upon carbonate treatment, ORF3c was present exclusively in the membrane pellet fraction, indicating that it was incorporated into the lipid phase (Figure 2G, lanes 2–3). Alternatively, mitochondrial membranes were solubilized in detergent (Triton X-100). Upon detergent treatment, ORF3c was largely released into the supernatant, although a fraction remained unsolubilised (Figure 2G, lanes 4–5). The residual amount of ORF3c recovered in the pellet fraction after Triton X-100 treatment indicated that ORF3c is prone to aggregation in the presence of the ionic detergent. In conclusion, our in vitro data strongly suggest that ORF3c is targeted to and inserted in mitochondrial membranes.
ORF3c localizes to the mitochondria in cells
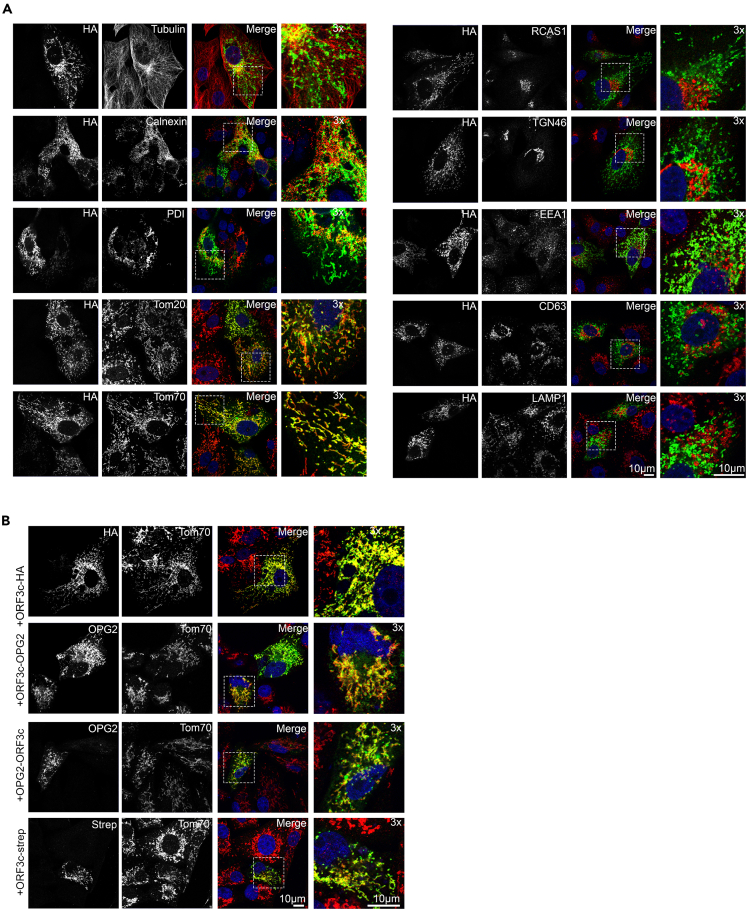
To confirm the mitochondrial localization of ORF3c in intact cells, Vero cells were transfected transiently with ORF3c-HA and immunofluorescence microscopy was performed to identify its subcellular localization. Clear membrane staining was observed and cells were co-stained using antibodies against various sub-cellular markers. No co-localization was observed between ORF3c-HA and tubulin, ER markers (calnexin, PDI), Golgi markers (RCAS1, TGN46), or endolysosomal markers (early: EEA1, late: CD63, lysosome: LAMP1). However, clear co-localization was observed using antibodies against the MOM import receptors Tom20 and Tom70 (Figure 3A).
Figure 3.
ORF3c localizes to the mitochondria in cells
(A) Vero cells were transfected with a plasmid expressing ORF3c-HA and 24 h later the cells were fixed and co-stained using the indicated antibodies raised against a range of endomembrane marker proteins and against HA. Scale bar: 10 μm.
(B) Vero cells were transfected with plasmids expressing alternatively tagged ORF3c proteins, including ORF3c-OPG2, OPG2-ORF3c and ORF3c-Strep. Fixed cells were co-labelled using antibodies against each epitope tag and against Tom70. Scale bar: 10 μm.
To ensure that the C-terminal HA tag was not mis-directing ORF3c-HA localization, alternative epitope tags (OPG2 and Strep) were fused to either the N or C terminus of ORF3c. Ectopic expression of all ORF3c versions displayed clear co-localization with endogenous Tom70 (Figure 3B). Furthermore, the mitochondrial localization of ORF3c did not alter over 48 h post-transfection (Figure S2).
PGAM5 interacts specifically with ORF3c
To identify host interaction partners, we immunoprecipitated ORF3c-HA from transfected Vero cells grown in labeled medium (stable isotope labeling of amino acids in culture, SILAC). ORF3c-HA-binding proteins were compared in triplicate to an HA-only control. Immunoprecipitated samples were subject to trypsin digest and then analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS).
Six proteins were identified as being enriched in ORF3c-HA samples relative to HA-only control samples with a p value, q-value and FDR all <0.05 (Figure S3), and minimum of 0.5 log2 (i.e., 1.41-fold) enrichment. These were: PGAM5, RPL8, EIF6, ARPC5, CAVIN1 and CAPZB. The full set of quantified filtered proteins, and list of significantly enriched proteins are included as supplementary files (Tables S1 and S2). Of these six proteins, PGAM5 was chosen for further investigation due to its recently reported role in antiviral signaling.57,58 The ORF3c:PGAM5 interaction was confirmed by the co-immunoprecipitation of PGAM5-FLAG and ORF3c-HA (Figures S3B and 5B).
Figure 5.
ORF3c interacts with MAVS to restrict IFN-β promoter activation
(A) HEK293T cells were co-transfected with plasmids expressing ORF3c or EV, RLuc, and IFN-β-driven FLuc plasmids, together with increasing doses of plasmids expressing FLAG-tagged CARD domain of RIG-I (RIG-I-CARD), MDA5, MAVS, TRIFΔRIP, IRF3-5D or TBK1. Cell lysates were prepared and analyzed as in Figure 4. Adjacent immunoblots show the expression of the indicated proteins. Data represents the mean ± SEM of three independent biological repeats. Statistical analysis (unpaired two sample t-test): ns = not significant, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
(B) HEK293T cells were seeded in 10-cm dishes and transfected with 2 μg of plasmids encoding FLAG-tagged GFP, MAVS, Tom20 or PGAM5, and 2 μg of ORF3c-HA plasmid. After 48 h, cell lysates were collected, subjected to immunoprecipitation with anti-FLAG affinity beads, and analyzed by SDS-PAGE and immunoblotting.
(C) Schematic illustrating the simplified pathways leading from RNA sensing to the activation of IRF3 and transcription of the IFN-β gene. The position at which ORF3c mediates inhibition is indicated.
The SILAC protocol did not assess whether ORF3c could form homo-oligomeric complexes. Many viruses encode similarly small transmembrane proteins that spontaneously multimerise to form ion channels within host membranes (so-called viroporins, reviewed in Nieva et al.59). Previously, others and we suggested that ORF3c may possess viroporin activity due to its size and hydrophobic nature.11,12 To assess the ability of ORF3c to multimerise, we co-transfected ORF3c-HA and ORF3c-FLAG into HEK293T cells and performed an anti-FLAG immunoprecipitation. FLAG-tagged PGAM5 was included as a positive control. The eluate was then probed for both HA and FLAG tags by immunoblotting (Figure S3B). ORF3c-HA presented as a single band; a fainter, larger band was also seen in the elution fraction due to background/nonspecific staining, and this was only observed in the concentrated elution fractions and not the whole cell lysates (Input). Whereas FLAG-tagged PGAM5 co-immunoprecipitated ORF3c-HA, ORF3c-FLAG did not. Thus, this experiment did not provide evidence for ORF3c homo-oligomerisation in cultured cells, suggesting that ORF3c is unlikely to form ion channels.
PGAM5 is a single pass transmembrane protein with a cytosolic serine/threonine phosphatase domain,60 which localizes to the MOM via its N-terminal transmembrane domain.61 Here it oligomerizes into dodecamers, the catalytically active state.62,63,64 Besides roles in cell death related processes and mitochondrial dynamics, recently PGAM5 was found to play a role in upregulating IFN-β signaling during infection by viruses from multiple families,57,58 acting via a direct interaction with MAVS – a tail-anchored protein that also localizes to the MOM following oligomerisation.65,66 PGAM5 multimerization, induced as a result of viral or poly(I:C) stimulation, causes increased phosphorylation of TBK1 and IRF3 and a subsequent increased transcription of IRF3-responsive genes including IFN-β. Interestingly, this function of PGAM5 appears to be independent of its phosphatase activity, despite the dodecameric form of PGAM5 being catalytically active.57 It has also been reported that SARS-CoV-2 infection results in increased ubiquitylation of PGAM5, accompanied by an overall decrease in PGAM5 protein levels, suggesting it is targeted to the proteasome during infection.67 Therefore, we investigated the ability of ORF3c to dysregulate innate immune signaling in stimulated cells, hypothesizing that the ORF3c:PGAM5 interaction would abrogate the PGAM5-driven antiviral effect.
ORF3c inhibits IFN-β signaling
We co-transfected HEK293T cells with plasmids encoding ORF3c and a range of luciferase reporters, such that FLuc expression was driven by a particular promoter. The cells were then stimulated with a relevant agonist. IFN-ɑ was used to stimulate transcription from the FLuc expression plasmid (pISRE-luc) containing the IFN stimulated responsive element (ISRE); TNF-ɑ was used to stimulate transcription from the NF-κB responsive FLuc plasmid (pNF-κB-luc); PMA was used to stimulate transcription from the activator protein 1 (AP-1) responsive FLuc plasmid (pAP-1-luc); and Sendai virus (SeV) was used to stimulate transcription from FLuc plasmids containing the ISG56 (pISG56.1-luc) or IFN-β (pIFN-β-luc) promoter sequences. Similar approaches have been used extensively to identify SARS-CoV-2 immune antagonists.18,19,68,69,70,71 We observed significantly less FLuc produced by pIFN-β-luc in the presence of ORF3c, whereas FLuc levels produced by pISRE-luc or pNF-κB-luc were unchanged. The results indicated that ORF3c inhibits transcription from the promoter of the IFN-β gene, but not from the NF-κB-responsive promoter or the ISRE (Figure 4A).
Figure 4.
ORF3c inhibits IFN-β signalling
(A) HEK293T cells were co-transfected with a range of FLuc-encoding plasmids (pISRE-luc, pNF-κB-luc, pAP-1-luc, pIFN-β-luc or pISG56.1-luc), together with RLuc and ORF3c-HA plasmids. On the following day, cells were stimulated with a relevant agonist: SeV for pIFN-β and pISG56.1-luc; 1,000 units/mL IFN-α for pISRE-luc; 20 ng/mL TNF-α for pNF-κB-luc; or 20 ng/mL PMA for pAP-1-luc. SeV infection and PMA stimulation were maintained overnight whereas cytokine treatments were maintained for 6 h. The same stimulation conditions were used in all experiments unless otherwise stated. Cell lysates were collected after stimulation to measure luciferase levels and protein expression. FLuc activity was normalized to RLuc and the fold induction is shown relative to unstimulated controls. Data represents the mean ± SEM of three independent biological repeats. Statistical analysis (unpaired two sample t-test): ns = not significant, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001. Later in discussion the figures are representative immunoblots of indicated protein expression.
(B) The same reporter gene assays as described in (A) were performed with ORF3c tagged with HA at either the N or C terminus, or untagged ORF3c. Data represents the mean ± SEM of three independent biological repeats. Statistical analysis (unpaired two sample t-test): ns = not significant, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
(C) After overnight induction with tetracycline, ORF3c-inducible cells were infected with SeV for 6 h, and then collected to extract cellular mRNA. Total mRNA was reverse transcribed into cDNA and the indicated genes were quantified by qPCR. mRNA levels of target genes were normalized to the GAPDH mRNA level, and fold induction was calculated relative to the unstimulated control. Data represents the mean ± SEM of three independent biological repeats. Statistical analysis (unpaired two sample t-test): ns = not significant, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
(D) Tetracycline-inducible A549 cells were induced or mock induced with 100 ng/mL doxycycline to express FLAG-tagged ORF3c. Lysates were probed for the FLAG epitope and actin.
(E) After overnight induction, ORF3c-inducible cells were infected with SeV. The supernatant was collected 16 h post infection. Soluble IFN-β was measured by ELISA (pg/mL). Levels were normalized to the uninfected control cell line. Data represents the mean ± SEM of three independent biological repeats. Statistical analysis (paired t-test): ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.
The pAP-1-luc plasmid also produced lower FLuc levels when co-transfected with ORF3c (Figures 4A and 4B), in a dose-dependent manner (Figure S4A). As the IFN-β promoter is comprised of AP-1, NF-κB and IRF3 responsive elements, we surmise that the IFN-β inhibition is at least partially mediated by the AP-1 element. Other coronaviral proteins reported to antagonize this pathway were also included as controls (Figures S4B and S4C). Importantly, we observed the same trend regardless of the ORF3c tag position, and indeed when using untagged ORF3c (Figure 4B). Intriguingly, we could not detect the N-terminal HA tag, indicating that the N terminus may possess an internal cleavage site (Figure 4B, see also Figure 6A).
Figure 6.
ORF3c induces MAVS cleavage
(A) HEK293T cells were co-transfected with EV or plasmids expressing FLAG-ORF3c, ORF3a-FLAG or ORF3c-FLAG and FLAG-MAVS. At 24 h post-transfection, cell lysates were collected and analyzed by SDS-PAGE and immunoblotting with the antibodies indicated. Positions of full-length and cleaved MAVS are indicated by red lines.
(B) Cells were transfected as described in (A) and treated with 20 μM ABT-737, 40 μM Z-VAD or DMSO overnight. Cell lysates were collected and analyzed as described in (A).
(C) HEK293T cells were co-transfected with plasmids expressing FLAG-tagged MAVS wild type (WT) or mutants Q427A, E463A, C508R or D429A/D490A, with (lanes 7–12) or without (lanes 1–6) ORF3c-FLAG. Cell lysates were collected at 24 h post transfection and processed as described in (A).
(D) A549 +ACE2 +TMPRSS2 cells were infected with SARS-CoV-2 (Wuhan) or delta (B.1.617.2) variant (EPI_ISL_1731019) at MOI 2. At 24 h post infection, infected and mock-infected cells were collected and lysed. The cell lysates were analyzed by immunoblotting using the indicated antibodies. MAVS immunoblots are shown at high and low exposure for clarity. The intensity of full-length MAVS (∼72 kDa) (upper bar graph) and cleaved MAVS (∼57 kDa) (lower bar graph) from 3 independent biological repeats was normalized to GAPDH, and then further normalized relative to mock infection, and shown in percentage. Quantification of band intensity was performed with the LI-COR imaging system. Statistical analysis (paired two-tailed t-test) is based on three biological repeats (∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001).
To decrease the probability of artifacts arising due to the system choice (a concern that has been raised in relation to other potential SARS-CoV-2 immune antagonists72), we performed qRT-PCR analysis of SeV-infected A549 cells expressing ORF3c-FLAG stably (Figure 4D). This showed that the endogenous IFN-β, ISG56 and ISG54 mRNAs were specifically downregulated in ORF3c-expressing SeV-stimulated cells (Figure 4C), indicating this blockade is at the transcriptional level. The downregulation of ISG56 mRNA level in qRT-PCR is inconsistent with the previous pISG56.1-luc reporter gene assay, which might be due to sensitivity differences between the two techniques, and/or differences between HEK293T and A549 cells. Given that A549 cells are derived from human alveolar cells and are broadly used in respiratory virus infection, the qRT-PCR data from these cells that analyses the transcription of endogenous genes is more compelling than the reporter gene assays.
The inhibition of IFN-β production by ORF3c was also confirmed by ELISA (Figure 4E). As expected, uninfected cells produced almost undetectable levels of IFN-β (mean 4.75 and 5.55 pg/mL for +Dox and –Dox, respectively) whereas SeV-infected cells produced excessive amounts of this cytokine (mean 43.6 and 36.1 ng/mL for +Dox and –Dox, respectively). The presence of ORF3c significantly hampered IFN-β production, in accordance with its role as an immune antagonist. The infected uninduced ORF3c-expressing stable cells also released noticeably less IFN-β than the equivalent parental cells. This is presumably due to constitutive expression of ORF3c-FLAG at low levels (Figure 4D).
The levels of NFKBIA mRNA and two downstream NF-κB-induced mRNAs, CXCL8 and CCL2, were unaffected by the presence of ORF3c in the A549 cells (Figure 4C). As NF-κB is a key transcription factor regulating IFN induction during SARS-CoV-2 infection, alongside IRF3 and IRF5, we repeated the qRT-PCR analyses using another cell model, HEK293T, stimulated by TNF-ɑ instead of SeV (Figure S4D). Again, the mRNA levels of NF-κB responsive genes remained unchanged as a result of ORF3c expression. Collectively, these data provide evidence that ORF3c contributes to the suppression of the IFN-β signaling pathway.
ORF3c interacts with mitochondrial antiviral-signaling protein to restrict interferon-β promoter activation
Next, we co-transfected FLAG-tagged proteins from within the IRF3-signaling pathway (RIG-I CARD, MDA5, MAVS, TRIFΔRIP, TBK1 or IRF3-5D73) alongside the IFN-β promoter-driven FLuc plasmid (pIFN-β-luc) and ORF3c. These proteins would activate the IFN-β promoter from various nodes and allow us to elucidate the stage(s) targeted by ORF3c. We found that ORF3c inhibits transcription from the IFN-β promoter induced by MAVS, MDA5 or RIG-I CARD but not by TRIFΔRIP, TBK1 or IRF3-5D (Figure 5A). This indicates that ORF3c acts upstream of the TRAF3/TBK1 nexus. These data, alongside published results that PGAM5 interacts with MAVS,57 led us to hypothesize that ORF3c interacts with MAVS. To investigate this, we performed a co-immunoprecipitation using FLAG-tagged MAVS or PGAM5 and HA-tagged ORF3c. FLAG-tagged Tom20 was included to assess whether we were isolating mitochondrial membrane proteins nonspecifically. Whereas MAVS and PGAM5 co-immunoprecipitated ORF3c, Tom20 and GFP did not (Figure 5B), thus indicating that both MAVS and PGAM5 are specific interaction partners of ORF3c. Immunofluorescence experiments were also strongly supportive of co-localization of ORF3c and MAVS in intact membranes (Figure S5). MAVS overexpression is sufficient to induce expression from the pIFN-β-luc and pAP-1-luc plasmids, indicating it can induce IFN-β transcription.66,74 Thus we hypothesise that the sequestration of MAVS due to interaction with ORF3c may contribute to the observed decrease in transcription from the IFN-β gene (Figure 5C).
ORF3c induces mitochondrial antiviral-signaling protein cleavage
During these experiments, we noticed an additional, lower molecular weight specific band appearing in the MAVS immunoblots upon ORF3c co-transfection. This was reproducible but did not appear upon MAVS/ORF3a co-transfection, indicating this MAVS cleavage was an ORF3c-specific effect (Figure 6A). MAVS is known to be cleaved by the executioner caspases (primarily caspase-3 and to a lesser extent, caspase-6) during some viral infections.75 As we had no evidence that ORF3c itself possesses protease activity, we hypothesized that these caspases may be mediating this cleavage event. Indeed, MAVS cleavage was inhibited by treatment with the pan-caspase inhibitor Z-VAD, and mimicked the effects of apoptosis induction via the Bcl-2 inhibitor ABT-737 (Figure 6B), suggesting that ORF3c-induced MAVS cleavage may be mediated by caspases and accompanied by apoptosis. To further investigate the ORF3c-mediated MAVS cleavage, we examined MAVS mutants that had reported resistance to viral protein or caspase-mediated cleavage.75,76,77 We found that the MAVS point mutations D429A/D490A, but not Q427A, E463A or C508R, blocked the ORF3c-induced cleavage (Figure 6C). As the D429A/D490A mutations were shown to ablate caspase-3-mediated MAVS cleavage,75 we hypothesized that ORF3c-induced cleavage was mediated by this apoptotic caspase. Treatment of the transfected cells with a range of caspase inhibitors indicated that all executioner caspases (i.e., caspase-3, -6 and -7) contributed to the cleavage phenotype (Figure S6A).
The evidence that pro-apoptotic caspases target MAVS when ORF3c and FLAG-MAVS are co-transfected, but not during FLAG-MAVS and EV co-transfection, led us to investigate whether this is accompanied by apoptosis. We utilized two approaches, to assess both early and late stage apoptosis: quantification of cytochrome c release into the cytosol at 24 h post transfection, and measurement of caspase-3/7 cleavage over a 48 h time course post transfection in live cells. We found a statistically significant increase in released cytochrome c levels resulting from the transfection of ORF3c-HA compared to EV (Figure S6B). A similar increase in mean levels was also seen for FLAG-MAVS, or ORF3c-HA and FLAG-MAVS together; however – due to variability in the results – these latter two were not statistically significantly different from the EV. Cytochrome c release is a marker of early apoptosis and is thought to precede and contribute to the initiation of caspase activation. These data suggest ORF3c induces cytochrome c-dependent caspase activation. We measured caspase-3/7 cleavage in live cells using a system whereby cleaved caspase-3/7 binds to a conjugated, initially inert dye, cleaving the dye which is then free to translocate into the nucleus and stain the nuclear DNA of pro-apoptotic cells. When either ORF3c-HA or FLAG-MAVS were expressed alone, there was practically no difference in measured staining compared to EV. When ORF3c-HA and FLAG-MAVS were expressed together, the mean level of staining was ∼1.5-fold higher than for EV (though not statistically significant). Notably, at the relevant timepoint of the MAVS cleavage experiments (i.e., 24 h post transfection), all four transfections had staining levels far below that of the apoptosis-inducing staurosporine positive control (Figure S6C). These data suggest that the overexpression system is not leading to excessive and promiscuous caspase-3/7-mediated cleavage but instead the observed MAVS cleavage may involve more directed ORF3c-mediated recruitment of caspases to MAVS.
Intriguingly, ORF3c is not expressed in the SARS-CoV-2 variant of concern delta (B.1.617.2) lineage due to a CAG to UAG mutation that introduces a premature termination codon at codon 5 (Figure S7B). The delta (B.1.617.2) variant therefore represents an opportunity to examine the effects of ORF3c depletion, albeit with the caveat that many other mutations co-exist in this genome compared to earlier lineages. We utilized this naturally occurring difference to examine MAVS levels in cells infected with either early lineage or delta (B.1.617.2) SARS-CoV-2 (Figures 6D and S6D). The growth characteristics of this particular delta isolate (GISAID accession number: EPI_ISL_1731019) compared to the early lineage (Wuhan) virus have been documented in many studies in multiple cell types and reveal that it replicates more efficiently than early lineage strains and exhibits increased entry rates.78,79
In the experiments utilising infected cell lysates, the relative intensities of the three MAVS-related bands differed from the previous experiment (Figure 6A), possibly as a result of the different cell type (A549 versus HEK293T) or detection of endogenous rather than FLAG-tagged MAVS. We also found the range of MAVS downregulation and cleavage was unusually broad across our biological replicates; whether this represents differences arising in the host cells during passage or variation in the three virus stocks is unknown. All virus stocks were sequenced before use and no novel mutations had arisen. Despite this experimental range, we found that MAVS was consistently downregulated in infected cells compared to mock (Figure 6D, upper graph). There was also less cleaved MAVS in the delta (B.1.617.2) infected lysates, compared to those from the early lineage infection (Figure 6D, lower graph). Finally, the ratio of cleaved MAVS to full-length MAVS was consistently highest in cells infected with the early SARS-CoV-2 lineage virus, possessing an intact ORF3c (Figure S6D).
ORF3c presence does not alter mitochondrial morphology in infected cells
Given the lack of ORF3c expression in the delta (B.1.617.2) variant, we next examined the mitochondrial morphology in A549 +ACE2 +TMPRSS2 cells infected with either delta (B.1.617.2-Por1, B.1.617.2-Por2) or non-delta (VIC1 and B.1.1.7) SARS-CoV-2 variants. Immunofluorescence staining for mitochondrial markers (Tom20 and Tom70) (Figures 7A and S7A) indicated that, although mitochondrial staining was reduced in infected cells compared to uninfected cells, no clear differences were apparent between the variants. Additionally, by transmission electron microscopy, no phenotypic differences in the mitochondria of cells infected with different variants were observed (Figure 7B). This is perhaps unsurprising, due to the multiple redundant mechanisms of IFN dysregulation conferred by different SARS-CoV-2 proteins.
Figure 7.
ORF3c presence does not alter mitochondrial morphology in infected cells
(A) A549 +ACE2 +TMPRSS2 cells were infected with either early lineage (VIC1, B.1.1.7) or delta lineage (B.1.617.2-POR1, B.1.617.2-POR2) isolates and fixed at 24 h post-infection. Cells were stained using antibodies against Spike (green) and Tom70 (red) and imaged by confocal microscopy. Nucleic were stained with DAPI (blue). Scale bar: 20 μm.
(B) As for (A) but cells were processed for electron microscopy imaging. Infected cells were identified by the presence of double membrane vesicles. Mitochondria are identified by white asterisks.
(C) HEK293T cells were transfected with plasmids encoding RLuc, IFN-β driven FLuc, and increasing doses of either WT ORF3c-HA or a Q5Y mutant. The cells were stimulated with SeV, and cell lysates were collected and analyzed as described in Figure 4A. Data represents the mean ± SEM of three independent biological repeats. Statistical analysis (paired two-tailed t-test) is based on three biological repeats (∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001).
(D) SARS-CoV-2 sequences present in the GISAID database that had specific-day collection dates specified were analyzed. Data for all ORF3c variants present in 0.1% or more of the 12,906,225 ORF3c sequences analyzed are shown. Data is on a log scale. PTC (red curve) corresponds to the SARS-CoV-2 delta (B.1.617.2) variant and Q5Y (blue curve) corresponds to the delta ORF3c pseudorevertant.
(E) Pie charts showing the geographic distribution of sequences obtained with the WT ORF3c fifth codon (CAG), PTC variant fifth codon (UAG) and Q5Y “pseudorevertant” fifth codon (UAU).
SARS-CoV-2 ORF3c variants in the human host
As noted above, ORF3c coding capacity is lost in the SARS-CoV-2 delta (B.1.617.2) variant, due to the appearance of a premature termination codon. To further study the appearance and dynamics of different ORF3c variants we queried 12,906,225 SARS-CoV-2 sequences from the GISAID database80 with coverage of the ORF3c region. Seven ORF3c amino acid variants were present at an abundance of ≥0.1% of the total, namely the original variant (WT), besides R36I, L21F/R36I, S22L, Q5Y, K17E and the aforementioned delta premature termination codon variant (PTC) (Figures 7D and S7B). The CAG to UAG substitution at codon 5 of ORF3c that gave rise to the PTC variant pseudoreverted to UAU in the Q5Y variant. This restored expression of full-length ORF3c but with a Q to Y amino acid change at position 5 (the overlapping ORF3a amino acids are SD in WT, LD in PTC, and LY in Q5Y). This Q5Y pseudorevertant increased rapidly in mainland Europe sequencing reports around July 2021 before leveling off and eventually dying away as the delta (B.1.617.2) virus variant was replaced with the omicron virus variant (B.1.1.529) in late 2021 (Figure 7E). Reporter gene assays indicated that the ORF3c Q5Y pseudorevertant is less efficient than the WT at antagonising IFN-β production but still has a marked effect (Figure 7C).
Discussion
Coronaviruses encode a variety of accessory proteins in their genomes, which are non-essential for RNA replication but confer advantageous properties to the virus allowing efficient viral propagation in the host. Many of these accessory proteins are known antagonists of the innate immune response and are useful targets for antiviral treatment strategies. However their variability across the Coronaviridae often means that studies cannot be extrapolated to other members; for example, SARS-CoV-1 ORF3b, which overlaps the 3′ region of ORF3a, is truncated in SARS-CoV-25; and ORF10 in SARS-CoV-2 is entirely lacking in SARS-CoV-1. Previously, using comparative genomics, we identified ORF3c, an accessory protein conserved across the Sarbecovirus subgenus.11 Here we have presented a functional analysis of ORF3c, revealing it to be a tail-anchored transmembrane protein that appears to be inserted into the mitochondrial outer membrane, where it interacts with MAVS and PGAM5, and reduces IFN-β signaling.
To the best of our knowledge, this is the first report of PGAM5 being involved in coronaviral-host protein:protein interactions, although it had been identified as a potential target for viral-induced proteasomal degradation.67 PGAM5 localizes to the MOM,61 although there are also reports of PGAM5 within the inner mitochondrial membrane with the C-terminal catalytic domain facing the intermembrane space.81,82 It has been suggested that its location may depend on cellular stress levels: PGAM5 is known to activate the MAP kinase pathway by dephosphorylating ASK1 (associated with cellular stress)83 and is involved in numerous cell death-related processes. Primarily, it is thought to regulate mitochondrial dynamics (fusion versus fission). Its ability to promote or suppress various cell death pathways is a contentious issue (recently reviewed in Cheng et al.84): whilst reported initially as a pro-necrotic factor,60,85,86 this has been disputed.87 Equally controversial are its role(s) in apoptosis: it has been reported to suppress apoptosis in certain models,88 yet be essential for apoptosis induction in others.89,90,91 What does appear consistent – and dependent on the phosphatase function of PGAM5 – is a role in the induction of mitophagy, an organelle-specific form of autophagy that protects the cell against necroptosis by selectively degrading damaged mitochondria.90,92,93,94,95
Although we do not know whether ORF3c affects the phosphatase function of PGAM5, this function is thought to be independent of the role of PGAM5 in immune signaling. PGAM5 multimerization has been shown to be required for both IFN-β upregulation and induction of many cell death-related events. One possibility is that the ORF3c-induced decrease in IFN-β may be at least partially caused by a reduction in PGAM5 multimerization due to its interaction with ORF3c, and a subsequent redirection of PGAM5 to the proteasome. Equally, it is possible that PGAM5 multimerization continues in the presence of ORF3c, but the PGAM5:MAVS interaction is ablated. A third hypothesis involves the formation of a potential trimeric complex (ORF3c:MAVS:PGAM5), resulting in the functional abrogation of both host proteins. As the self-multimerization of MAVS and PGAM5 are independent events,57 this would be an efficient mechanism of sequestering both potential innate response activators with a single viral protein. However, we currently have no experimental data to support or disprove any of these hypotheses; this will be a focus for future studies.
In addition to the observed ORF3c:MAVS and ORF3c:PGAM5 interactions that may inhibit PGAM5:MAVS stimulation of IFN-β production, we also observed cleavage of MAVS when ORF3c was overexpressed and this cleavage appeared to be driven by caspase-3, suggesting a link to apoptosis. Whether ORF3c-driven apoptosis is an artifact of overexpression outside of the context of virus infection (where other viral proteins might inhibit apoptosis) is currently unknown. While we found a tantalizing suggestion of differences in MAVS cleavage between delta (B.1.617.2) and non-delta infections, the overall strong downregulation of full-length MAVS in either infection compared to mock and the presence of cleaved MAVS even in mock-infected cells in this system, wide variability between biological replicates, and potential effects of other amino acid differences between delta and non-delta virus proteins, all combine to make it difficult to draw robust conclusions at this stage. It is likely that ORF3c does induce some degree of mitochondrial damage during infection, but it is difficult to accurately extrapolate the magnitude of such effects from an overexpression system. Intriguingly, ORF3a of both SARS-CoV-1 and SARS-CoV-2, which localizes to the plasma membrane, has been implicated in apoptosis induction; however in the case of SARS-CoV-1 this was mapped to the cytosolic C-terminal domain of ORF3a and therefore could not have been an incorrectly attributed function of ORF3c.96,97
PGAM5 has been reported to be cleaved (within the N-terminal transmembrane domain) in response to mitochondrial dysfunction and mitophagy, specifically during outer membrane rupture, resulting in its release into the cytosol.81,82,98 Although we did not observe cleavage of PGAM5 in the presence of ORF3c (indicating mitophagy is not occurring), it would be of interest to confirm this with other methods. Equally, it would be of interest to analyze the phosphorylation patterns of PGAM5 when bound to ORF3c given that the phosphorylation status of this protein has numerous effects upon the downstream signaling pathways that it activates. During the preparation of this article, data became publicly available indicating ORF3c overexpression does not affect mitophagy, despite its mitochondrial localization, but rather blocked autophagy by causing autophagosome accumulation.99 This finding supports our preliminary evidence that ORF3c may direct cells toward apoptosis in preference to other cell death pathways, possibly by the sequestration of PGAM5 and prevention of mitophagy activation.
Among SARS-CoV-2 proteins, ORF3c is not alone in having an inhibitory effect on IFN-β expression; however the mode of action differs significantly between the proteins involved. Some proteins directly reduce IFN-β mRNA or protein levels: ORF6, ORF8 and N all have similar effects to ORF3c and reduce IFN-β mRNA (and hence protein) levels although, unlike ORF3c, ORF6 and ORF8 simultaneously reduce expression from ISRE-containing promoters.68,69,100 ORF6 has also been shown independently to reduce IRF3 and STAT1 nuclear translocation18,19,37,101; in comparison, N is thought to inhibit the TRIM25:RIG-I interaction.102,103 NSP136,19 and NSP619 bind TBK1 directly, preventing IRF3 phosphorylation. NSP5 inhibits phosphorylated IRF3 translocation104 and directly cleaves RIG-I.105 Other sarbecoviral proteins inhibit type I IFN activation as an indirect result of their enzymatic function: SARS-CoV-1 NSP16 reduces MDA5 and IFIT activation by capping the viral RNA106; NSP14 reduces IFN levels by shutting down host translation.107 Still others (NSP1 and NSP6) suppress the signaling induced by type I IFN, whilst leaving protein levels unaffected.19,108 The convergent effects of these viral antagonistic proteins, which collectively target multiple layers of the immune signaling cascade, no doubt combine to reduce the host antiviral response and increase virus fitness in the natural host.
Intriguingly, we observed no effect of ORF3c on NF-κB-driven transcripts, despite the known role of MAVS in activating this transcription factor. It is possible that there is sufficient uncleaved MAVS to still activate NF-κB, while the interactions between ORF3c, MAVS and PGAM5 may collectively block the IRF3 pathway. This is especially plausible given the known role of PGAM5 in stimulating IRF3-responsive genes, whilst no equivalent effects upon NF-κB activation have been published. Similarly, although the cleaved fragments of MAVS are not able to induce type I IFN expression, it is not known if they may still induce NF-κB activation.75 Activated caspase-3 is also known to cleave IRF3 and ablate its ability to induce type I IFN transcription, which is thought to be a mechanism of ensuring apoptotic cells remain “immunologically quiet.”75 Although we did not investigate IRF3 cleavage, presumably this is occurring to some level and may be contributing to the IFN-β-specific inhibition. Finally, the stimulants in our assays also activate other pathways, which ORF3c is clearly unable to overcome. For example, SeV (Figure 4C) would activate TRIF by TLR3 (sensor of extracellular dsRNA) and the DDX1/DDX21/DHX36 complex (sensor of cytoplasmic dsRNA), resulting in NF-κB activation via the TRAF6 adaptor. ORF3c did not inhibit the TRIF-activated IRF3 pathway (Figure 5A) indicating this pathway would continue unimpeded during SeV infection despite the induction of ORF3c expression. Similarly, the RIP1/IKK-γ (NEMO) pathway may be activated in the HEK293T luciferase reporter assays, via the TNF-α stimulant (Figure 4A).
Despite the redundancy in IFN antagonists, those that operate directly from a mitochondrial location are uncommon amongst characterized coronaviral proteins. ORF9b and ORF10 are the exceptions. Similar to our observations for ORF3c, these proteins localize to the mitochondria upon overexpression and are able to dampen the immune response in the absence of other coronaviral proteins,41,109,110,111 indicating they may each act in an unassisted fashion and not from within a virally encoded protein complex. ORF9b does, however, interact with host Tom706,109,110,112 which in turn is known to interact with MAVS.113,114 It has been suggested that the ORF9b:Tom70 interaction may lead to either apoptosis or mitophagy, as the levels of functional Tom70 will affect both of these processes; but these hypotheses have not been validated experimentally.110 This provides an interesting parallel to ORF3c, which appears to operate at the same cellular location as Tom70 (specifically, the MOM115), yet we have no evidence that ORF3c and Tom70 interact. It remains possible that an indirect, transient interaction may occur between ORF9b, ORF3c, Tom70 and MAVS. Additionally, unlike ORF3c, ORF9b has been observed to inhibit the IKK-γ (NEMO) cascade,71 suggesting that ORF9b has additional functions downstream of MAVS, specifically inhibiting the NF-κB pathway. Thus it appears that sarbecoviruses have evolved complementary approaches, mediated by ORF9b and ORF3c respectively, to subvert IFN-β signal transduction and reduce mitochondrial innate immune pathway activation from within the mitochondrion itself. The mechanism employed by ORF10 is different yet again; however this protein is not conserved across the subgenus. ORF10 localizes to the mitochondrion where it interacts with the mitophagy receptor NIX, to activate mitophagy and thereby eliminate aggregated MAVS.111 This may in part explain why the downregulation and degradation of MAVS is still visible during infection with a delta (B.1.617.2) variant lacking ORF3c (Figure 6D), although NSP5-mediated degradation may also contribute (see later in discussion). Downregulation of MAVS has also been reported from proteome-wide studies of SARS-CoV-2 infected cells (although the level of reduction appears to depend on the model system).10,116
Prior to the identification of ORF3c, a screen of viral and host protein:protein interactions did not identify either MAVS or PGAM5 as probable interaction partners for any SARS-CoV-2 protein.6 This was reflected in a thorough literature review.117 However NSP5 interacts directly with MAVS during overexpression, leading to ubiquitylation and subsequent degradation which is distinct from the protease function of NSP5.105 The downregulation of MAVS in infected A549 cells, observed herein, mimics that seen in other cell lines and may indeed be partially caused by this mechanism. Additionally, there are some reports of the M protein interacting with MAVS,70,118 although this is not reconciled with the lack of a mitochondrial localization for the M protein, which is found consistently at the ER and Golgi.70,110 As such, the relevance of this potential interaction during an actual viral infection is open to question. In short, ORF3c is the only conserved sarbecoviral protein that has been shown to bind directly to MAVS within the MOM, where the majority of activated MAVS would be located during a viral infection.
A question we have not addressed is whether the specific cytoplasmic dsRNA sensor involved affects ORF3c function. The relative contribution of individual PRRs in controlling SARS-CoV-2 replication and inducing cytokine production has recently been a topic of considerable debate. There is mounting evidence that MDA5 is the central cytoplasmic RLR responsible for the IFN signal cascade during infection,119,120 with RIG-I playing a minor role via a non-canonical RNA recognition mechanism that does not lead to MAVS oligomerisation.121 RIG-I may also be involved in regulating ACE2 levels,122 thereby affecting viral spread in an IFN-independent manner. These results, together with the knowledge that the SARS-CoV-2 main protease NSP5 cleaves RIG-I,105 indicate that MAVS recruitment and subsequent IFN-β induction may primarily be due to MDA5 activation during infection. In contrast to ORF3c, NSP5 inhibits NF-κB signaling, as well as IRF3 translocation.105 This presumably reflects their respective targets within the dsRNA sensing pathway, as NSP5 interacts with and cleaves RIG-I, acting upstream of MAVS, as well as the aforementioned degradation of MAVS following ubiquitylation. However we do not have any evidence that ORF3c function and phenotype would differ depending on the upstream RLR involved.
There are several obstacles currently impeding further progress. For example, analysis of ORF3c-HA transfected cell lysates following digestion with trypsin or chymotrypsin and LC-MS/MS analysis failed to identify ORF3c-derived peptides even when inclusion lists of predicted ORF3c peptides were employed, likely due to high hydrophobicity of the peptides. This explains why multiple published analyses using trypsin digestion have failed to identify the ORF3c protein during infection.9,123 Equally, our own work has been limited by the poor immunogenicity of ORF3c: a peptide-raised rabbit antibody was not reactive against transfected cell lysates, nor was a sheep polyclonal antibody raised against the entire ORF3c protein (data not shown). However, we are confident that these hurdles will be overcome with time. Notably, during the preparation of this article, a newly available preprint reported the identification of an ORF3c-derived peptide within the HLA-II peptidome.124 This discovery is the first experimental evidence of ORF3c protein within infected cells and confirms that some ORF3c peptides are indeed amenable to LC-MS/MS technology, as well as providing evidence that ORF3c elicits T cell responses.
The discovery of ORF3c necessitates a reassessment of previous sarbecoviral ORF3a-targeted studies, which may have also included ORF3c during protein overexpression. DNA-based constructs (although useful and often necessary) create an artificial system, because these vectors generally exclude viral untranslated regions and are designed to optimise expression from the desired AUG codon. Thus the degree of ORF3c expression, alongside the desired ORF3a, remains an unknown factor for most previous studies. It is also probable that ORF3c-mediated effects were overlooked in historical SARS-CoV-1 studies due to the extensive use of Vero cells, which allow efficient replication of many coronaviruses but are deficient in type I IFN production.125 For example, inadvertent deletion of ORF3c via ORF3a mutation results in only minor attenuation in these cells, as measured by viral infectious titer126,127 (although, notably, deletion of the entire ORF3a region reduced cytopathic effect and cell death128).
Sarbecoviruses appear to be primarily bat viruses (Figure S1) and ORF3c appears to be conserved throughout this clade (with the exception of two different sarbecovirus sequences from Rhinolophus hipposideros where ORF3c is truncated; Figure S1B). In the human host, ORF3c is clearly not essential (as demonstrated by the success of the delta (B.1.617.2) variant where ORF3c is disrupted). However, whether or not ORF3c provides a selective advantage in the human host is unclear. It is possible that the observed loss, restoration and variations of ORF3c in the human host may be random events whose effects on virus fitness are outweighed by increases in virus fitness conferred by other mutations (e.g., in the spike protein) with ORF3c variations being “carried along.” The importance of ORF3c in the human host presumably will become clearer as the virus adapts to long-term persistence in the human population. Similarly, other hosts such as the palm civet Paguma larvata and the Malayan pangolin Manis javanica may be intermediate hosts to which these viruses have not fully adapted. The observed Q5Y pseudorevertant of the delta (B.1.617.2) PTC truncation is curious and may reflect a selective advantage of restoring ORF3c protein expression, but it might also have been a random event. Notably Q5 is perfectly conserved across the sarbecovirus ORF3c sequences (Figure S1A) suggesting that a Q at this position is functionally important, at least in bats. Sarbecoviruses have many different ways to antagonize host innate immunity and it may be that ORF3c is redundant in the human host (or its relative importance may also depend on cell type, host genetic background or disease state). Although not essential, ORF3c may still lead to an increase in virus fitness in the human host. Future work will be needed to compare WT and ORF3c knockout viruses in both human and bat cell lines, besides animal models.
Limitations of the study
A major limitation of this study is the focus upon overexpressed ORF3c; that is, expression following the transfection of mammalian cells. This may lead to protein levels that do not correlate with those occurring in infected cells. Whilst this is partially mitigated by our use of a cell line inducibly expressing ORF3c, which recapitulates our observed phenotype, it is nonetheless a concern. Additionally, we did not investigate ORF3c function in bat cells; given the evidence that SARS-CoV-2 is a zoonotic virus, this would be highly informative.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-FLAG | Merck | F7425 |
| mouse anti-HA | BioLegend | 901501 |
| rabbit anti-actin | Sigma Aldrich | A2066 |
| sheep anti-ORF7a | University of Dundee, MRC PPU | N/A |
| sheep anti-ORF3a | University of Dundee, MRC PPU | N/A |
| donkey anti-mouse IgG | LI-COR | 926-68072 |
| goat anti-mouse IgG | LI-COR | 926-32210 |
| donkey anti-rabbit IgG | LI-COR | 926-68073 |
| goat anti-mouse IgG | LI-COR | 926-32211 |
| donkey anti-goat IgG | LI-COR | 926-68074 |
| anti-HA | Abcam | ab20084 |
| anti-alpha-tubulin | Abcam | ab15568 |
| anti-VDAC1 | Abcam | ab14734 |
| anti-lamin A/C | Abcam | ab133256 |
| mouse anti-Tom20 | Abcam | ab283317 |
| rabbit anti-Tom70 | ProteinTech | 14528-1-AP |
| rabbit anti-Calnexin | Cell Signalling Technology | D2B6N |
| rabbit anti-Syntaxin 6 | Cell Signalling Technology | C34B2 |
| mouse anti-alpha-tubulin | ProteinTech | 66031-1 |
| mouse anti-EEA1 | BD Biosciences | 14/EEA1 |
| mouse anti-CD63 | BioLegend | H5C6 |
| mouse anti-LAMP1 | BioLegend | H4A3 |
| rabbit anti-TGN46 | Abcam | ab50595 |
| rabbit anti-HA | Cell Signalling Technology | C29F4 |
| rat anti-HA | Roche | 3F10 |
| mouse anti-OPG2 | McKenna et al., 2016 | N/A |
| mouse anti-Strep | IBA lifesciences | 2-1507-001 |
| mouse anti-Spike | GeneTex | 1A9 |
| rabbit anti-MAVS | Cell Signalling Technology | 3993 |
| rabbit anti-FLAG | Sigma Aldrich | F2555 |
| Alexa Fluor 488 goat anti-rat IgG | Invitrogen | A-11006 |
| Alexa Fluor 488 goat anti-mouse IgG | Invitrogen | A-11001 |
| Alexa Fluor 488 donkey anti-sheep IgG | Invitrogen | A-11015 |
| Alexa Fluor Plus 555 goat anti-rabbit IgG | Invitrogen | A32732 |
| rabbit anti-TIM22 | Proteintech | 14927-1-AP |
| rabbit anti-ERLIN2 | Proteintech | 14781-1-AP |
| Rabbit polyclonal anti-COX4-I | This paper | N/A |
| Bacterial and virus strains | ||
| E. coli DH5a | Thermofisher Scientific | 18258012 |
| SARS-CoV-2 (B lineage) | UK Health Security Agency | BetaCoV/Australia/VIC01/2020 |
| SARS-CoV-2 (B.1.1.7) | UK Health Security Agency | BetaCoV/England/ MIG457/2020 |
| SARS-CoV-2 (B.1.617.2-POR1) | UK Health Security Agency | ASL516 |
| SARS-CoV-2 (B.1.617.2-POR2) | UK Health Security Agency | ASL517 |
| SARS-CoV-2 (B.1.617.2) | Imperial College London | GISAID Accession EPI_ISL_1731019 |
| SARS-CoV-2 (Wuhan) | University of Bristol | Genbank Accession MW041156.1 |
| Sendai Virus | University of London | Cantell strain |
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco’s Modified Eagle’s Medium (DMEM) | Invitrogen | 11965092 |
| Fetal Bovine Serum (FBS) | Gibco | 10500-064 |
| L-glutamine | Gibco | 25030081 |
| Penicillin/Streptomycin | Gibco | 15070063 |
| Trypsin-EDTA | Gibco | R001100 |
| Phosphate-buffered saline (PBS) | Thermoscientific | 10010023 |
| Lipofectamine 2000 | Invitrogen | 11668019 |
| 4% paraformaldehyde | Thermofisher Scientific | 15670799 |
| Saponin | Thermoscientific Chemicals | A18820.14 |
| Triton X-100 | Thermoscientific Chemicals | A16046.AE |
| Bovine serum albumin | Sigma Aldrich | A9418 |
| ProLong Gold Antifade + DAPI | Invitrogen | P36931 |
| NP40 | Thermoscientific | 85124 |
| Halt Protease and Phosphatase Inhibitor Cocktail | Thermofisher Scientific | 78440 |
| SuperScript III | Invitrogen | 18080093 |
| SYBR Green Master Mix | Thermofisher Scientific | 4309155 |
| TNF-α | Peprotech | 300-01A |
| Doxycycline | Sigma Aldrich | D3072 |
| Precast Novex 10–20% tricine protein gels | Thermofisher Scientific | EC66252BOX |
| RIPA buffer | Thermofisher Scientific | 89900 |
| Staurosporine | Abcam | ab120056 |
| Incucyte caspase 3/7 green dye | Sartorius | 4440 |
| ABT-737 | Merck | 197333-10MG |
| Z-VAD | Sigma Aldrich | V116 |
| Z-VEID-FMK | Sigma Aldrich | 218757-250ug |
| Caspase-3/7 Inhibitor I | Merck | 218826-1MG |
| Ac-FLTD-CMK | MedChem Express | HY-111675 |
| Z-LEHD-FMK | Sigma Aldrich | 218728-1MG |
| Z-DEVD-FMK | Santa Cruz | sc-311558 |
| DMSO | Invitrogen | D12345 |
| HEPES | Merck | H0887-100ML |
| nonessential amino acids | Gibco | 11140050 |
| G418 | Gibco | 11811031 |
| Hygromycin B | Roche | 10843555001 |
| OptiMem | Gibco | 31985070 |
| T7 polymerase | Promega | P2075 |
| Puromycin | Gibco | A1113803 |
| IFN-ɑ | Peprotech | 300-02AA |
| PMA | Sigma Aldrich | P8139 |
| cOmplete Mini EDTA-free protease inhibitor | Roche | 11836170001 |
| anti-FLAG agarose | Merck | A2220 |
| MitoTracker Red CM-H2XRos | Invitrogen | M7513 |
| Glycine | Invitrogen | 15527013 |
| glutaraldehyde | Sigma Aldrich | G5882 |
| UA-Zero EM Stain | Agar Scientific | AGR1000 |
| digitonin | Merck Millipore | 300410 |
| Nuclease S7 Micrococcal nuclease | Sigma Aldrich | 10107921001 |
| Trypan blue | Sigma Aldrich | T8154 |
| Flexi rabbit reticulocyte lysate | Promega | L4540 |
| EasyTag EXPRESS 35S Protein Labelling Mix | Perkin Elmer | NEG772002MC |
| endoglycosidase Hf | New England Biolabs | P0703S |
| proteinase K | Carl Roth | 7528.2 |
| PMSF | Carl Roth | 6367.2 |
| [35S] methionine | Hartmann Analytic | SCM-01 |
| Sodium carbonate | Merck | 6398.1 |
| Critical commercial assays | ||
| RNeasy Mini Kit | Qiagen | 74106 |
| HA Tag Magnetic IP/Co-IP kit | Pierce | 88838 |
| Q5 site directed mutagenesis kit | New England Biolabs | E0554S |
| Human IFN-β Quantikine ELISA | Bio-Techne | DIFNB0 |
| Cytochrome c Release Assay | Abcam | ab65311 |
| T7 mMessage kit | Invitrogen | K0441 |
| RNA Clean and Concentrator | Zymo Research | R1014 |
| Dual luciferase kit | Promega | E1910 |
| Miniprep kit | Qiagen | 27104 |
| MycoAlert PLUS Assay | Lonza | LT07-710 |
| Stratagene QuikChange | Agilent Technologies | 200519 |
| Subcellular fractionation | Thermofisher Scientific | 78840 |
| Epoxy Resin (Araldite CY212) Kit | Agar Scientific | AGR1030 |
| SP6 mMESSAGE mMACHINE kit | Invitrogen | AM1340 |
| Mitochondria Isolation Kit, human | Miltenyi Biotec | 130-094-532 |
| DMEM w/High Glucose and w/o Glutamine, Lysine and Arginine | Gibco | A1443101 |
| Deposited data | ||
| Proteomics data | PRIDE | PXD037765 |
| epicov.org | GISAID; Elbe et al., 201780 | http://gisaid.org/EPI_SET_221101zc |
| Experimental models: Cell lines | ||
| HeLA | ATCC | CCL-2 |
| HEK293T | ATCC | CRL-3216 |
| A549 | ATCC | CCL-185 |
| Vero | ATCC | CRL-1586 |
| A549+ACE2+TMPRSS2 | Rihn et al., 2021129 | N/A |
| HEK293T-Flp-In™ T-Rex™ | Thermofisher Scientific | R78007 RRID: CVCL_U421 |
| Vero+ACE2+TMPRSS2 | Rihn et al., 2021129 | N/A |
| ORF3c-FLAG inducible A549 | This paper | N/A |
| Oligonucleotides | ||
| ISG54_FWD ATGAAGACGGTGCTGAATACTAGTGA | This paper | N/A |
| ISG54_REV TGGTGAGGGCTTTCTTTTTCC | This paper | N/A |
| ISG56_FWD ACCATGGGAGAGAATGCTGAT | This paper | N/A |
| ISG56_REV GCCAGGAGGTTGTGC | This paper | N/A |
| IFN-β_FWD CATCAACTATAAGCAGCTCCA | This paper | N/A |
| IFN-β_REV TTCAAGTGGAGAGCAGTTGAG | This paper | N/A |
| ORF3cFLAG_FWD CCGCTCGAGGCCACC ATGCTACTCC | This paper | N/A |
|
ORF3cFLAG_REV TTTGTTCGAACCGCGGTTACTTA TCGTCGTCATCCTTGTAATCGGAGCCTCCAGAGC CTCCTGATTTT |
This paper | N/A |
| GAPDH_FWD ATCAACGACCCCTTCATTGACC | Lu et al., 202220 | N/A |
| GAPDH_REV CCAGTAGACTCCACGACATACTCAGC | Lu et al., 202220 | N/A |
| NFKBIA_FWD CTGCAGGCCACCAACTACAA | Lu et al., 202220 | N/A |
| NFKBIA_REV CAGCACCCAAAGTCACCAAGT | Lu et al., 202220 | N/A |
| CCL2_FWD CTTCTGGGCCTGCTGTTCA | Lu et al., 202220 | N/A |
| CCL2_REV CCAGCCTACTCATTGGGATCA | Lu et al., 202220 | N/A |
| CXCL8_FWD AGAAACCACCGGAAGGAACCATCT | This paper | N/A |
| CXCL8_REV AGAGCTGCAGAAATCAGGAAGGCT | This paper | N/A |
| Recombinant DNA | ||
| pCAG-ORF3cHA | This paper | Addgene ID: 208653 |
| pCAG-ORF3cStrep | This paper | Addgene ID: 208728 |
| pL3cSGRLuc | This paper | Addgene ID: 208729 |
| pcDNA3.1-PGAM5-FLAG | GenScript | CloneID OHu01661D |
| pcDNA3.1-HA-MAVS | Unterholzner et al., 2011130 | N/A |
| pcDNA3.1-FLAG-MAVS | Unterholzner et al., 2011130 | N/A |
| pCAG-ORF3c-FLAG | This paper | N/A |
| pCAG-ORF3c-FLAG-Q5Y | This paper | N/A |
| pISG56.1-luc | Cleveland Clinic, Lerner Research Institute | N/A |
| TBK1 | Unterholzner et al., 2011130 | N/A |
| TRIFΔRIP | Unterholzner et al., 2011130 | N/A |
| IRF3-5D | Unterholzner et al., 2011130 | N/A |
| RIG-I-CARD | University of Surrey | N/A |
| MDA5 | University of St Andrews | N/A |
| pCAG-ORF3a-FLAG | Shandong University | N/A |
| pCAG-NSP6-FLAG | Shandong University | N/A |
| pCAG-NSP9-FLAG | Shandong University | N/A |
| pCAG-NSP12-FLAG | Shandong University | N/A |
| pcDNA3.1-TOMM70A-FLAG | GenScript | Clone ID OHu01661D |
| pLKO.neo.EGFPnls-TetR | MRC University of Glasgow Centre for Virus Research | N/A |
| pLKO.DCMV-TetO.mcs | MRC University of Glasgow Centre for Virus Research | N/A |
| pLKO.DCMV.TetO.ORF3c | This paper | N/A |
| pISRE-luc | Unterholzner et al., 2011130 | N/A |
| pNF-κB-luc | Unterholzner et al., 2011130 | N/A |
| pAP-1-luc | Unterholzner et al., 2011130 | N/A |
| pIFN-β-luc | Unterholzner et al., 2011130 | N/A |
| pcDNA3.1-GFP-FLAG | This paper | N/A |
| pcDNA3.1-TOMM20-FLAG | This paper | N/A |
| Software and algorithms | ||
| Prism version 7.0c for Mac OS X | GraphPad software | N/A |
| MATLAB software | MathWorks | N/A |
| SnapGene | GSL Biotech LLC | N/A |
| Image Studio | LI-COR | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Andrew Firth (aef24@cam.ac.uk).
Materials availability
Plasmids generated in this study have been deposited to Addgene (pCAG-ORF3cHA: ID 208653; pCAG-ORF3cStrep: ID 208728; pL3cSGRLuc: ID 208729).
Experimental model and study participant details
Vero, HEK293T, A549 and HeLa cell lines were purchased from the American Type Culture Collection (ATCC). Vero cells (ATCC, CRL-1586) are from a female African green monkey (Cercopithecus aethiops). HEK293T cells (ATCC, CRL-3216) are female in origin. A549 cells (ATCC, CCL-185) are male in origin. HeLa (ATCC, CCL-2) are female in origin.
Vero, HEK293T, A549 cells and HeLa cells were maintained in high-glucose Dulbecco's-modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, 100 μg/ml streptomycin, 1 mM nonessential amino acids, 25 mM HEPES, and 1% L-glutamine (complete DMEM) in a humidified incubator at 37°C with 5% CO2. Cell lines were not authenticated internally. All cells were routinely tested and confirmed to be free of mycoplasma (MycoAlert™ PLUS Assay, Lonza).
Method details
Cell culture and transfections
A549 cells stably expressing ACE2 and TMPRSS2 (A549 +ACE2 +TMPRSS2) were kindly provided by Prof. Massimo Palmarini (University of Glasgow)129 and were maintained in the above-described medium, with 2 mg/mL G418 and 200 μg/mL hygromycin B.
For DNA transfections, cells were seeded in 6-well plates at 30–40% confluency the day prior to transfection. Four μg of pure plasmid DNA was transfected with 30 μl of Lipofectamine 2000 (Invitrogen) in a total volume of 1 ml serum-free OptiMem (Invitrogen) according to manufacturer's instructions. At 3 h post-transfection, 1.5 ml of DMEM with 10% FBS was added to each well. Cells were harvested 24 h post-transfection.
HEK293T-Flp-In™ T-Rex™ (HEK293T) cells were maintained and cultured in DMEM media (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% sterile filtered FBS (Sigma, Saint Louis, MO, USA) at 37°C with 5% CO2. For all the mitochondrial import experiments, HEK293T cells were freshly plated three days before to reach 80% confluency.
Viruses
SARS-CoV-2 strains BetaCoV/Australia/VIC01/2020 (B lineage),131 BetaCoV/England/ MIG457/2020 (B.1.1.7), and delta variants ASL516 (B.1.617.2-POR1) and ASL517 (B.1.617.2-POR2) were obtained from Porton Down, UK Health Security Agency, and propagated in Vero +TMPRSS2 +hACE2 cells under BSL-3 conditions. SARS-CoV-2 delta variant (GISAID accession number: EPI_ISL_1731019) was kindly provided by Prof. Wendy Barclay. SARS-CoV-2 (Wuhan) (Genbank Accession MW041156.1) was isolated at the University of Bristol.
Generation of ORF3c expression constructs
The coding sequence of ORF3c fused with Strep or HA coding sequences (either N- or C- terminal) was amplified via PCR and each amplicon was inserted into the pCAG-PM vector132,133 using AflII and PacI restriction sites. A GGSGGS linker was used in these cases. The resulting plasmids were confirmed by Sanger sequencing (University of Cambridge, Department of Biochemistry DNA Sequencing Facility).
OPG2-tagged ORF3c plasmids were generated by site-directed mutagenesis (Stratagene QuikChange, Agilent Technologies) and confirmed by DNA sequencing (GATC, Eurofins Genomics). To improve the signal intensity of in vitro synthesised radiolabelled proteins, linear DNA templates containing an additional five methionine residues (5M) at the C terminus were generated by PCR, and linear DNA templates were transcribed into mRNA using T7 polymerase (Promega).
FLAG-tagged and untagged ORF3c expression vectors were constructed by PCR amplification from pCAG.ORF3a and inserted into pCAG-FLAG or pcDNA6B-FLAG. FLAG-tagged ORF3c mutant Q5Y was prepared by site-directed mutagenesis using a commercial kit (New England Biolabs, E0554S).
Additional plasmids
Dual-luciferase reporter assay plasmids containing ISRE, NF-κB, AP-1 or IFN-β responsive promoters driving FLuc expression, and the plasmid expressing RLuc were described.130 The ISG56.1 reporter plasmid was kindly provided by Dr Ganes Sen, Cleveland Clinic, Cleveland, OH. Vectors expressing MAVS, TBK1, TRIFΔRIP and IRF3-5D were described.130 Vectors expressing RIG-I-CARD and MDA5 were provided by Dr Carlos Maluquer de Motes (School of Biosciences and Medicine, University of Surrey, UK) and Prof. Richard Randall (School of Biology, University of St Andrews, UK), respectively. Plasmids expressing MAVS mutants were generated by site-directed mutagenesis. SARS-CoV-2 vectors expressing ORF3a, NSP6, NSP9, NSP12 and empty vector (EV) pCAG.FLAG were kindly provided by Dr Peihui Wang, Shandong University, China.134 PGAM5-FLAG-pcDNA3.1 and TOMM70A-FLAG-pcDNA3.1 constructs were purchased directly from GenScript (GenScript, Netherlands).
Generation of an inducible ORF3c-expressing cell line
Inducible A549 cells expressing ORF3c were prepared by lentivirus transduction. The lentivirus preparation and infection have been described.135 In brief, A549 cells were transduced with a lentivirus vector expressing a tetracycline repressor (TetR) linked to a nuclear localisation signal (NLS) and enhanced green fluorescent protein (EGFPnlsTetR). The transduced cells were selected with medium supplemented with 0.5 mg/mL G418, and then the EGFPnlsTetR positive cells were sorted by fluorescence activated cell sorting (FACS). The sorted cells were transduced with a second lentivirus vector expressing FLAG-tagged ORF3c. Transduced cells were then selected by medium supplemented with 500 ng/mL puromycin. The lentivirus vectors pLKO.neo.EGFPnls-TetR and pLKO.DCMV-TetO.mcs were kind gifts from Prof. Roger Everett, MRC University of Glasgow Centre for Virus Research, Glasgow, UK. The pLKO.DCMV.TetO.ORF3c plasmid was constructed in the G.L.S. laboratory.
Reporter gene assays
HEK293T cells were seeded in 96-well plates with 3 × 105 cells per well. After overnight incubation, cells were transfected with 100 ng/well of FLuc plasmids (pISRE-luc, pNF-κB-luc, pAP-1-luc, pIFN-β-luc or pISG56.1-luc), 10 ng/well of RLuc plasmid, and the indicated viral protein expression vectors. At 24 h post-transfection, the cells were stimulated as follows: 6 h stimulation of 1000 unit/ml IFN-ɑ (Peprotech, 300-02AA) for pISRE-luc, 6 h stimulation of 20 ng/ml TNF-ɑ (Peprotech, 300-01A) for pNF-κB-luc, 24 h infection of SeV (Cantell Strain, Licence No. ITIMP17.0612A) for pISG56.1-luc and pIFN β-luc, and 24 h stimulation of 10 ng/ml PMA (Sigma-Aldrich, P8139) for pAP-1-luc. Stimulated, or infected, cells were lysed with 50 μL/well of passive lysis buffer (Promega) and kept at −20°C. To measure luciferase activity, 50 μL FLuc reagent, or 50 μL RLuc reagent was added to 8 μL cell lysate. The luminescent value of the lysate was measured with a microplate reader (BMG Labtech). Relative luminescent values were calculated by normalisation of the FLuc value to the RLuc value, for each well. Sendai virus (SeV) used in this study was kindly provided by Professor Steve Goodbourn, St George’s Hospital Medical School, University of London. At least 3 biological replicates were conducted, with 3 technical replicate wells, for each experiment.
qRT-PCR analysis
Inducible A549 cells expressing ORF3c-FLAG or EV were seeded in triplicate at 4 × 105 cells per well on 24-well plates. The next day, the cells were mock-induced or induced with 100 ng/mL doxycycline overnight. Then, the cells were infected with SeV (40 HAV/mL) for 6 h to stimulate IFN-β transcription. In other experiments, HEK293T cells were transfected with either EV or increasing amounts of ORF3c-FLAG. At 24 h post transfection, the cells were starved with DMEM without serum for 3 h and then stimulated with TNF-α (20 ng/mL) for 4 h to activate the NF-κB pathway. The cells were harvested and total RNA was extracted using RNeasy Mini Kit (Qiagen, 74106). Thereafter, 500 ng RNA was used in cDNA synthesis using SuperScript III reverse transcriptase (Invitrogen, 18080093). The expression of genes of interest was analysed by qPCR. Each cDNA sample was duplicated in the reaction, using SYBR Green Master Mix (Thermo Fisher Scientific, 4309155). A real-time PCR system (Thermo Fisher Scientific) was used to determine each reaction cycle threshold. Amplification of the investigated genes was normalised to GAPDH amplification. Fold induction of each gene was calculated relative to unstimulated control of each condition.
ELISA quantification of IFN-β production
Inducible A549 cells possessing ORF3c-FLAG or EV were seeded in triplicate at 4 × 105 cells per well on 24-well plates. The next day, the cells were mock-induced or induced with 100 ng/mL doxycycline overnight. The cells were then infected with SeV for 16 h to stimulate IFN-β production. The supernatant was harvested and clarified. A commercial ELISA kit was used according to the manufacturer’s protocol to quantify IFN-β levels in the supernatant (Human IFN-β Quantikine ELISA kit, Bio-Techne). A standard curve was generated with each assay using the provided control protein (R2 ≥ 0.99). Samples were diluted to ensure all measurements fell within the linear range of the standards.
Cytochrome c release assay
Cytosolic cytochrome c levels were quantified using a commercial kit (Cytochrome c Release Assay Kit, Abcam). Briefly, 5 × 107 HEK293T cells were transfected with the plasmids of interest. Cells were harvested at 24 h post transfection. The cytosolic fraction was separated from a mitochondrial-enriched fraction using a Dounce homogeniser. The cystolic fraction was probed for cytochrome c, GAPDH and both HA- and FLAG-tagged proteins by immunoblot. Cytochrome c levels were quantified, normalised to GAPDH and then normalised to the respective EV sample.
Live cell measurement of caspase 3/7 cleavage
HEK293T cells were seeded into 96-well plates at 3 × 105 cells per well. The next day, cells were transfected with the plasmids of interest in triplicate and the Incucyte caspase 3/7 green dye was added (1:1000 dilution) (Sartorius, catalogue 4440). Green fluorescent objects, indicative of cells undergoing caspase-3/7-mediated apoptosis, were counted for 48 h post transfection with a live cell analysis system (Incucyte SX5, Sartorius, 10× objective). The mean number of green positive cells per image was calculated from four non-overlapping fluorescent images. A fixed threshold of 3.0 GCU (green calibrated fluorescent units) was applied to all datasets, which resulted in zero artefacts (zero green units) in a dye-free control well. 1 μM staurosporine (STS) was included as a positive control to induce apoptosis in all cells.
Caspase inhibitor treatments
HEK293T cells were seeded into 24-well plates at 1 × 106 cells per well. Cells were transfected with either FLAG-MAVS and EV, or FLAG-MAVS and ORF3c-HA. After removal of the transfection complexes, a range of caspase inhibitors or control compounds were added: ABT-737 (20 μM) (Merck), Z-VAD (40 μM), Z-VEID-FMK (38 μM) (Sigma Aldrich), Caspase-3/7 Inhibitor I (125 μM) (Merck), Ac-FLTD-CMK (50 μM) (MedChem Express), Z-LEHD-FMK (43 μM) (Sigma Aldrich) or Z-DEVD-FMK (90 μM) (Santa Cruz). Pure DMSO was included (final concentration 0.5%) as an additional control as this was the highest level used in the drug treatments. Lysates were harvested 24 h post transfection and probed for the proteins of interest by immunoblot.
Leaky scanning assays
A plasmid was engineered in which a T7 promoter sequence preceded the first 77 nucleotides (the leader sequence) of the ORF3a subgenomic RNA. This was followed by the ORF3a coding region, up to but excluding the stop codon of ORF3c. This was followed by a foot and mouth disease virus 2A peptide sequence and then a RLuc ORF. The RLuc was in-frame with the AUG (start) codon of either ORF3a or ORF3c; the plasmids were termed pL_3a_SGRluc and pL_3c_SGRluc respectively. These plasmids were linearised with FastDigest AanI (Invitrogen) and RNA was in vitro transcribed using the T7 mMessage kit (Invitrogen) and terminated with DNase treatment. RNA was purified with a silica-gel based membrane method (RNA Clean and Concentrator, Zymo Research) and the integrity was confirmed by gel electrophoresis. FLuc RNA was used as a transfection control. This RNA was generated by T7 in vitro transcription, using an in-house FLuc plasmid linearised with BamHI as described.136
RNA transfections were conducted in triplicate in 96-well plates. Vero cells were seeded the day prior at 40% confluency (1 × 105 cells per well). One hundred and fifty nanograms of L3a_SGRluc RNA (or that of a derived mutant) plus 30 ng of FLuc RNA was transfected into each well using 1 μl of Lipofectamine 2000, according to the manufacturer’s instructions, in a total volume of 40 μl of sera-free OptiMem. At 3 h post-transfection, 60 μl of DMEM with 10% FBS was added to each well. Cells were harvested at 20 h post-transfection with passive lysis buffer (Promega) and a dual luciferase detection assay was conducted upon the lysates (Promega). At least 3 biological replicates were conducted, with 3 technical replicate wells, for each experiment.
To quantify the translation efficiency, the RLuc counts were first divided by the FLuc counts to normalise for transfection efficiency. The RLuc / FLuc values for each RNA were then divided by the corresponding value for the ORF3a-2A-RLuc RNA, i.e. relative translation efficiency of RNAx = [RLuc/FLuc]RNAx / [RLuc/FLuc]ORF3a-2A-RLuc.
SDS-PAGE and immunoblots
Transfected cells were lysed in either RIPA buffer or PBS with 0.5% NP40, supplemented with protease inhibitors. Lysates were analysed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using Tris-Glycine, at the percentage polyacrylamide suitable for resolution of the protein of interest. Precast Novex 10–20% tricine protein gels (Thermofisher Scientific) were used to resolve proteins under 10 kDa. Proteins were transferred to 0.2 μm nitrocellulose membranes and blocked with a commercial blocking buffer (LI-COR) before being probed with the relevant primary and secondary antibodies. Membranes were imaged using an Odyssey CLx imaging system (LI-COR). Primary antibodies used in this study were anti-FLAG (1:1000, rabbit polyclonal, Merck, F7425), anti-HA (1:500, mouse monoclonal, BioLegend, 901501), anti-actin (1:1000, rabbit polyclonal, Sigma Aldrich, A2066), anti-ORF7a (1:500, sheep polyclonal, University of Dundee, MRC PPU) and anti-ORF3a (1:500, sheep polyclonal, University of Dundee, MRC PPU). Secondary antibodies used in this study were anti-mouse IgG (1:10,000, donkey, LI-COR, 926-68072), anti-mouse IgG (1:10,000, goat, LI-COR, 926-32210), anti-rabbit IgG (1:10,000, donkey, LI-COR, 926-68073), anti-mouse IgG (1:10,000, goat, LI-COR, 926-32211) and anti-goat IgG (1:10,000, donkey, LI-COR, 926-68074).
Subcellular fractionation
Vero cells were transfected with a range of plasmids as described above. At 24 h post-transfection, monolayers were washed in phosphate-buffered saline (PBS) and subject to subcellular fractionation using a commercially available kit (Thermofisher Scientific) according to the manufacturer’s instructions. An input sample (10%) was retained for analysis prior to the first lysis step. Target-specific antibodies were anti-HA (Abcam, ab20084, 1:1000), anti-alpha-tubulin (Abcam, ab15568, 1:1000), anti-VDAC1 (Abcam, ab14734, 1:1000), and anti-lamin A/C (Abcam, ab133256, 1:1000).
Immunoprecipitation
To detect protein-protein interactions, HEK293T cells (3 × 106 cells per plate) were transfected with 2 μg of FLAG-tagged GFP, MAVS, TOMM70, TOMM20 or PGAM5 plasmids and 2 μg of ORF3c-HA expressing plasmid. At 48 h post transfection, transfected cells were washed with ice-cold PBS and lysed with cell lysis buffer (200 mM NaCl, 50 mM Tris, 1% NP40, pH 7.0) supplemented with cOmplete Mini EDTA-free protease inhibitor cocktail (Roche, 11836170001). The lysates were cleared by centrifugation at 13,000 rpm, and the FLAG-tagged proteins were immunoprecipitated with anti-FLAG agarose (A2220, Merck). Input samples were collected as described above.
Confocal microscopy
Vero cells were transfected transiently with ORF3c plasmids (described above). At 24 h post-transfection, cells were trypsinised and re-seeded upon sterile 13 mm glass coverslips. At 48 h post-transfection, the cell culture medium was removed and coverslips were washed once with PBS prior to fixation with 4% PFA/PBS.
For MitoTracker staining, the stock solution of MitoTracker® Red CM-H2XRos (Invitrogen, M7513) was prepared with DMSO to a concentration of 1 mM. Before staining live cells on coverslips, MitoTracker was diluted to 100 nM with pre-warmed DMEM without serum. The cells were washed twice with pre-warmed DMEM to remove serum and then incubated with MitoTracker containing medium at 37°C for 1 h before fixation.
For SARS-CoV-2 infections, A549 +ACE2 +TMPRSS2 cells were grown on glass-bottomed 24-well plates (MatTek, P24G-0-13-F). Cells were infected at an MOI of 1 and placed on a rocker at room temperature for 2 h. The inocula were removed and replaced with full medium. At 24 h post infection plates were submerged in 4% PFA/PBS for 20 min to fix cells.
Free aldehydes were quenched with 15 mM glycine/PBS before cells were permeabilised with 0.1% saponin/PBS. Blocking and subsequent steps were performed using 1% BSA/0.01% saponin/PBS. Coverslips were inverted into droplets of primary antibody for 1 h, before being washed three times with PBS. Coverslips were then placed onto a second droplet containing fluorophore-conjugated secondary antibody for 45 min. Coverslips were washed three times before being mounted on to glass slides using ProLong Gold Antifade mountant containing DAPI (Invitrogen, P36931). Cells were imaged using a LSM700 confocal microscope (63x/1.4 NA oil immersion objective; ZEISS).
Primary antibodies used were anti-Tom20 (1:200, mouse monoclonal, Abcam, ab283317), anti-Tom70 (1:500, rabbit polyclonal, Proteintech, 14528-1-AP), anti-Calnexin, (1:250, rabbit monoclonal, Cell Signalling Technology, C5C9), anti-RCAS1 (1:250, rabbit monoclonal, Cell Signalling Technology, D2B6N), anti-Syntaxin 6 (1:250, rabbit monoclonal, Cell Signalling Technology, C34B2), anti-alpha-tubulin (1:500, mouse monoclonal, ProteinTech, 66031-1), anti-EEA1 (1:500, mouse monoclonal, BD Biosciences, 14/EEA1), anti-CD63 (1:400, mouse monoclonal, BioLegend, H5C6), anti-LAMP1 (1:400, mouse monoclonal, BioLegend, H4A3), anti-TGN46 (1:500, rabbit polyclonal, Abcam, ab50595), anti-HA (1:500, rabbit polyclonal, Cell Signalling Technology, C29F4), anti-HA (1:500, rat monoclonal, Roche, 3F10), anti-OPG2 (1:100, mouse monoclonal137), StrepMAB-Classic (1:500, mouse monoclonal, IBA lifesciences, 2-1507-001), anti-SARS-CoV-2 Spike (1:100, mouse monoclonal, GeneTex, 1A9), anti-MAVS (1:100, rabbit polyclonal, Cell Signalling Technology, 3993), anti-ORF3a (1:100, sheep polyclonal, University of Dundee, MRC PPU), anti-HA (1:500, mouse monoclonal, BioLegend, 901501), anti-FLAG (1:500, rabbit polyclonal, Sigma, F2555).
Secondary antibodies used in this study were Alexa Fluor 488 goat anti-rat IgG (1:400, Invitrogen, A-11006), Alexa Fluor 488 goat anti-mouse IgG (1:400, Invitrogen, A-11001), Alexa Fluor 488 donkey anti-sheep IgG (1:400, Invitrogen, A-11015), Alexa Fluor Plus 555 goat anti-rabbit IgG (1:400, Invitrogen, A32732).
Electron microscopy
Cells were seeded upon plastic Thermanox coverslips in 24-well plates. Following infection, plates containing infected cells (see virus infections methods) were submerged in 2% PFA / 2.5% glutaraldehyde / 0.1 M cacodylate buffer, pH 7.4. Cells were washed with 0.1 M cacodylate buffer before being stained using 1% osmium tetroxide:1.5% potassium ferrocyanide for 1 h, and staining was further enhanced with UA-Zero (Agar Scientific) for 30 min. Cells were washed, dehydrated and infiltrated with Epoxy propane (CY212 Epoxy resin:propylene oxide) before being embedded in Epoxy resin. Epoxy was polymerised at 65°C overnight before Thermanox coverslips were removed using a heat-block. Seventy nm sections were cut using a Diatome diamond knife mounted to an ultramicrotome. Ultrathin sections were stained with lead citrate. An FEI Tecnai transmission electron microscope at an operating voltage of 80 kV was used to visualise samples.
Semi-permeabilised (SP) cell preparation
HeLa cells (human epithelial cervix carcinoma cells, mycoplasma-free), as described,53,54,138 were provided by Martin Lowe (University of Manchester) and were cultured in DMEM supplemented with 10% (v/v) FBS (Gibco, 10500-064) and maintained in a 5% CO2 humidified incubator at 37°C. Cells were seeded at 1 × 106 per 10 cm2 dish and, once ∼80% confluent, cells were semi-permeabilised using digitonin (Calbiochem) and endogenous mRNA was removed by treatment with 0.2 U Nuclease S7 Micrococcal nuclease, from Staphylococcus aureus (Sigma-Aldrich, 10107921001) and 1 mM CaCl2 as described.53,54 After quenching by the addition of EGTA to 4 mM final concentration, SP cells were resuspended in an appropriate volume of KHM buffer (110 mM KOAc, 2 mM Mg(OAc)2, 20 mM HEPES-KOH pH 7.2) to give a suspension of 3 × 106 SP cells/mL as determined by trypan blue staining (Sigma-Aldrich, T8154). Freshly prepared SP cells were then included in translation master mixes such that each translation reaction contained 2 × 105 cells/mL.
In vitro ER import assays
Translation and membrane insertion assays, supplemented with nuclease-treated canine pancreatic microsomes (from stock with OD280 = 44/mL) or SP HeLa cells, were performed in nuclease-treated rabbit reticulocyte lysate (Promega) as described.53,54 Briefly, in the presence of EasyTag EXPRESS 35S Protein Labelling Mix containing [35S] methionine (Perkin Elmer) (0.533 MBq; 30.15 TBq/mmol), 25 μM amino acids minus methionine (Promega), 6.5% (v/v) ER-derived microsomes or SP cells and 10% (v/v) of in vitro transcribed ORF3c mRNA (∼1 μg/μL) encoding the relevant ORF3c precursor protein. All translation reactions (30 μL) were performed at 30°C for 1 h and finished by incubating with 0.1 mM puromycin for 10 min at 30°C to ensure translation termination and the ribosomal release of newly synthesised proteins prior to analysis.
Analysis of radiolabelled products synthesised in ER import assays
Following puromycin treatment, microsomal or SP cell membrane associated fractions were recovered by centrifugation through an 80 μL high-salt cushion [0.75 M sucrose, 0.5 M KOAc, 5 mM Mg(OAc)2 and 50 mM HEPES-KOH, pH 7.9] at 100,000 g for 10 min at 4°C. Then, the pellet was suspended in SDS sample buffer and, where indicated, samples were treated with 1000 U of endoglycosidase Hf (New England Biolabs, P0703S). To confirm association of ORF3c with the ER membrane, microsomal membrane-associated fractions were resuspended in KHM buffer (20 μL) and subjected to a protease protection assay using trypsin (1 μg/mL) with and without 0.1% Triton X-100 or sodium carbonate extraction (0.1 M Na2CO3, pH 11.3) as described,53 prior to suspension in SDS sample buffer. All samples were solubilised for 12 h at 37°C prior to resolution by SDS–PAGE (16% PAGE, 120 V, 120 min). Gels were fixed for 5 min (20% MeOH, 10% AcOH), dried for 2 h at 65°C, and radiolabelled products were visualised using a Typhoon FLA-700 (GE Healthcare) following exposure to a phosphorimaging plate for 24–72 h.
Mitochondria isolation
HEK293T cells were harvested in PBS, washed, and resuspended in 1X THE buffer (300 mM trehalose, 10 mM KCl, 10 mM HEPES, 1 mM EDTA, and 2 mM PMSF). Homogenization was performed in a PTFE pestle/glass Potter-Elvehjem at 700 rpm. The resulting cell lysate was centrifuged at 400 x g for 10 min at 4°C, followed by a second centrifugation step at 800 x g for 10 min at 4°C to remove unbroken cells. To sediment mitochondria, the supernatant was centrifuged at 10,000 x g for 10 min at 4°C. The mitochondrial sediment was washed with THE buffer and resuspended in the same buffer. The concentration of mitochondria was determined using a standard Bradford assay.
In vitro import of [35S]ORF3c-5M into isolated mitochondria
The ORF3c gene was amplified by PCR, adding five methionine codons to the C terminus (ORF3c-5M). The corresponding mRNA was prepared using the SP6 mMESSAGE mMACHINE kit (Invitrogen) according to the manufacturer’s specifications. Radiolabelled [35S]ORF3c-5M was synthesised in vitro in Flexi Rabbit Reticulocyte Lysate system (Promega) in the presence of 0.5 mM of KCl, 0.5 mM MgAc and 200 ng of ORF3c mRNA. For protein import, 100 μg of mitochondria resuspended in import buffer (250 mM sucrose, 80 mM potassium acetate, 5 mM magnesium acetate, 5 mM methionine, 10 mM sodium succinate, and 20 mM HEPES pH 7.4) with 1 mg/ml final concentration were used per reaction. [35S]ORF3c-5M protein was added at 5% (v/v) to the mitochondrial suspension and incubated at 37°C for 30 min under mild agitation (450 rpm). After import, mitochondria were sedimented at 10,000 x g for 10 min at 4°C and washed with HS buffer (500 mM sucrose and 20 mM HEPES/KOH pH 7.4).
Proteinase K accessibility assay
After [35S]ORF3c-5M import, mitochondria were resuspended in TBS (intact mitochondria) or TBS + 1% Triton X-100 (lysed mitochondria), followed by digestion with proteinase K (PK) (10 μg/mL final concentration) for 10 min on ice. Next, PK was inhibited with 2 mM PMSF. Intact mitochondria were then washed. Proteins were precipitated with trichloroacetic acid (TCA). Samples were analysed by Tris/tricine SDS-PAGE. Proteins were transferred onto PVDF membranes. The [35S]ORF3c-5M protein signal was detected by digital autoradiography and mitochondrial proteins were immunodetected.
Chemical extraction of mitochondrial membranes
After [35S]ORF3c-5M import, mitochondria were incubated in 100 mM sodium carbonate buffer (pH 11.5) or in 1% Triton X-100 (in TBS) at 1 mg/mL final concentration for 20 min on ice. Insoluble material was sedimented at 100,000 x g for 1 h at 4°C. Pellet and soluble fractions were collected, and the protein was precipitated with TCA. Proteins were resolved on urea SDS-PAGE, transferred to PVDF membranes, and analysed for the [35S]ORF3c-5M protein by digital autoradiography and mitochondrial proteins by immunodetection.
Immuno-isolation of mitochondria after ORF3c import
After import of [35S]ORF3c-5M, mitochondria were re-isolated with an anti-TOM22 mitochondria isolation kit (Miltenyi Biotec). In brief, resuspended mitochondria were incubated with anti-TOM22 Microbeads for 1 h with end-to-end mixing at 4°C. Beads were then collected through a MACS column placed in a magnetic MACS separator. The column was then washed with resuspension buffer to remove non-specific binding. After removing the column from the magnetic MACS separator, the beads were recovered in the resuspension buffer and treated with TCA. Samples were separated on Tris/tricine SDS-PAGE, transferred onto PVDF membranes, and analysed by autoradiography to detect [35S]ORF3c-5M protein and immunodetection for the required proteins.
SILAC immunoprecipitation experiment
Vero cells were grown in high glucose DMEM lacking arginine and lysine (Gibco), with 10% dialysed (7 kDa MWCO) FBS and supplemented with light (R0K0), medium (R6K4) or heavy (R10K8) stable isotope labelled arginine and lysine. Cells were maintained in labelled medium for 6 passages before transfection and immunoprecipitation. Labels were switched for the different replicates to control for any impact of the different culture media on cell growth/gene expression as follows: replicate 1 (L: ORF3c-HA, M: Control), replicate 2 (M: ORF3c-HA, H: Control) and replicate 3 (H: ORF3c-HA, L: Control).
Vero cells were transfected with ORF3c-HA as described above. At 24 h post-transfection, monolayers were washed in ice-cold PBS and co-immunoprecipitation of the HA-tagged protein and interacting proteins was performed using a commercially available kit (HA Tag Magnetic IP/Co-IP kit, Pierce). An input sample (10%) was retained for analysis by immunoblot prior to binding the lysates to the magnetic anti-HA beads. After removing the remains of the wash buffer (third and final wash), samples were heated to 95°C for 5 min in buffer consisting of 200 mM HEPES pH 8, 1% SDS and 1% NP40. Beads were collected by centrifugation and the supernatant was retained. Equal volumes of the control and ORF3c-HA samples for each replicate were then combined and the samples were reduced, alkylated and trypsin-digested using the SP3 method.139
LC-MS/MS analysis was conducted on a Dionex 3000 coupled in line to a Q-Exactive-HF mass spectrometer. Digests were loaded onto a trap column (Acclaim PepMap 100, 2 cm × 75 μm inner diameter, C18, 3 μm, 100 Å) at 5 μL per min in 0.1% (v/v) TFA and 2% (v/v) acetonitrile. After 3 min, the trap column was set in line with an analytical column (Easy-Spray PepMap® RSLC 15 μm × 50 cm inner diameter, C18, 2 μm, 100 Å) (Dionex). Peptides were loaded in 0.1% (v/v) formic acid and eluted with a linear gradient of 3.8–50% buffer B (HPLC grade acetonitrile 80% (v/v) with 0.1% (v/v) formic acid) over 95 min at 300 nL per min, followed by a washing step (5 min at 99% solvent B) and an equilibration step (25 min at 3.8% solvent). The Q-Exactive-HF was operated in data-dependent mode with survey scans acquired at a resolution of 60,000 at 200 m/z over a scan range of 350–2000 m/z. The top 16 most abundant ions with charge states +2 to +5 from the survey scan were selected for MS2 analysis at 60,000 m/z resolution with an isolation window of 0.7 m/z, with a (N)CE of 30%. The maximum injection times were 100 and 90 ms for MS1 and MS2, respectively, and AGC targets were 3e6 and 1e5, respectively. Dynamic exclusion (20 s) was enabled.
Data analysis was conducted in MaxQuant 1.6.7.0.140 Options were set at default unless specified. Multiplicity was set at 3, with Arg6 and Lys4 set as ‘medium’ labels and Arg10 and Lys8 set as ‘heavy’ labels. Digestion was Trypsin/P, permitting up to two missed cleavages. Oxidation (M) and N-terminal protein acetylation were selected as variable modifications, and carbamidomethylation as a fixed modification. Under instrument settings, intensity was set to ‘total sum’. Fasta files containing the Uniprot African Green Monkey proteome (Chlorocebus sabeus, 19,223 entries, downloaded 16 May 2020) and a custom .fasta file containing ORF3c-HA protein sequences were used for the search databases. The proteomics data generated in this study have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE repository (accession PXD037765).
Downstream data analysis was conducted in Matlab R2019a from the MaxQuant proteinGroups output file. Reverse database hits and common contaminants (MaxQuant contaminant list) were removed. Within each replicate, data were first normalised for equal protein loading based on the median SILAC intensity of shared proteins. Rows with >3 missing values were removed. Missing data were imputed (knn), and data were converted to HA-3C/HA-only ratios for each replicate, and then re-normalised across the three replicates by dividing by column median. A one-sample t-test was used to determine statistical significance. Multiple hypothesis testing was controlled using the approach of Storey, 2002,141 with all meeting a FDR < 0.05. The Matlab scripts used for all data processing and figure generation with this data are available from the Emmott lab Github page at: https://github.com/emmottlab/sars2_3c/.
Virus infections
To prepare lysates for immunoblots, A549 +ACE2 +TMPRSS2 cells were infected with SARS-CoV-2 (Wuhan) [GenBankAcc: MW041156.1] or delta (B.1.617.2) variant [GISAID accession number: EPI_ISL_1731019, kindly provided by Prof. Wendy Barclay, Imperial College London] at a MOI of 2, or mock infected. At 24 h post-infection, cells were lysed directly with 2x SDS sample buffer and heated to 95°C for 10 min. Different virus stocks were used for each biological repeat. To prepare samples for electron microscopy, A549 +ACE2 +TMPRSS2 cells were grown on 13-mm Thermanox (plastic) coverslips (Nunc) in the wells of a 24-well plate. Cells were infected at an MOI of 1 and placed on a rocker at room temperature for 2 h. The inocula were removed and replaced with full medium and cells were returned to incubators for 24 h, at which point the plate was prepared for EM (see electron microscopy methods).
Identification of sarbecovirus ORF3c variants
To identify sequences with coverage of the ORF3a region, NCBI online tblastn142 was used on 11 Oct 2022, using the SARS-CoV-2 ORF3a protein (GenBank: YP_009724391.1) as query and the NCBI nr/nt database as subject, with organism taxonomy limited to Coronaviridae (taxid:11118) excluding SARS-CoV-2 (taxid:2697049), word size = 2, max target sequences = 1000, no low-complexity masking, and other parameters as defaults. The highest e-value of the 417 returned sequences was 8 × 10−4. The coordinates of tblastn matches on the subject sequences were extended to maximal stop-codon-to-stop-codon open reading frames, which were extracted, translated, and aligned as amino acid sequences with MUSCLE v3.8.31.143 The amino acid alignment was used to guide alignment of the nucleotide sequences (EMBOSS:tranalign144) and the nucleotide sequences were 5′-truncated to the conserved ORF3a AUG initiation site. Sequences with defective or truncated ORF3a due to incomplete sequencing, frame-disrupting insertion/deletion errors, or other inconsistencies were discarded. An ORF homologous to SARS-CoV-2 ORF3c was not identified in the following sequences: MZ293757 (Hipposideros bat coronavirus, unclassified Coronaviridae member), and HQ166910 and NC_025217/KF636752 (subgenus Hibecovirus) and these sequences were therefore discarded. All remaining sequences had ≥62.6% amino acid identity to SARS-CoV-2 in ORF3a and may be regarded as members of subgenus Sarbecovirus, whereas the four discarded highly divergent non-sarbecovirus sequences had ≤27.1% amino acid identity to SARS-CoV-2 in ORF3a.
For the remaining 394 sequences, the ORF3c amino acid sequences were determined based on the conserved initiation site. In one (MG772933) ORF3c began with a GUG instead of an AUG codon. ORF3c was truncated in eight sequences. Five of these (EU371560, EU371561, EU371562, EU371563 and EU371564; ORF3c truncated to MLLLQVLFMLQQ) form a discrete subclade of SARS-CoV-1 viruses, but the sequences lack metadata and their provenance is unclear; nonetheless their ORF3a proteins have 99.3% amino acid identity to other SARS-CoV-1 isolates that have intact ORF3c. Another one (FJ882963; ORF3c truncated to MLLLQVLFML) also has an ORF3a protein with 99.3% amino acid identity to other SARS-CoV-1 isolates with intact ORF3c. Although these eight SARS-CoV-1 sequences were found to be ORF3c-defective, 184 other SARS-CoV-1 sequences had an ORF3c amino acid sequence that was identical to that of the SARS-CoV-1 reference sequence. In contrast, the remaining two sequences with truncated ORF3c (shown in Figure S1B) represent distinct sarbecovirus lineages.
ORF3c sequences with 100% amino acid identity to ORF3c of either the SARS-CoV-1 or SARS-CoV-2 NCBI reference sequences (NC_004718 and NC_045512, respectively) were discarded. Furthermore, four additional sequences (KF514407, AY463059, AY463060 and HG994853) with ≥99% amino acid identity to NC_004718 or NC_045512 in ORF3a were also discarded. In general, sequences with <99% identity in ORF3a were associated with non-human hosts and – where they had a different ORF3c amino acid sequence from NC_004718 and NC_045512 – were retained. Lab mutant recombinant sequences MT782114 and MT782115 were also removed. The remaining 190 ORF3c amino acid sequences represented 53 unique sequences (Figure S1A). ORF3c sequences were clustered with BLASTCLUST with a 90% identity threshold (-p T -L 0.95 -b T -S 90), resulting in seven clusters, and a representative sequence (SARS-CoV-1, SARS-CoV-2, or the most abundant unique sequence in the cluster) was chosen for Figure 1A.
Analysis of SARS-CoV-2 ORF3c sequences
SARS-CoV-2 genome sequences were downloaded from epicov.org,80 most recently on 10 Oct 2022. The findings of this study are based on sequence and metadata associated with 13,467,316 sequences available on GISAID via gisaid.org/EPI_SET_221101zc. All genome sequences and associated metadata in this dataset are published in GISAID’s EpiCoV database. To view the contributors of each individual sequence with details such as accession number, virus name, collection date, originating lab and submitting lab and the list of authors, visit https://doi.org/10.55876/gis8.221101zc.
To identify and extract ORF3c sequences, we searched for exact matches to any of seven 18-nt seed sequence queries spanning the region from the start of the ORF3a sgmRNA transcription regulatory sequence (TRS) to the nucleotide 5′-adjacent to the ORF3c AUG initiation codon, where each seed sequence had a 9-nt overlap with the previous seed sequence (viz. ACGAACTTATGGATTTGT, ..., AGCAAGGTGAAATCAAGG). We then extracted the 200 nt downstream from the 3′-most matched seed, removed sequences with any "N"s (i.e. ambiguous nucleotides) in this region, trimmed the 5′ ends to the start of ORF3c, truncated the 3′ end at 126 nt downstream to encompass the 41 sense codons and the stop codon of ORF3c, leaving 12,906,225 sequences covering the ORF3c region. These were translated and the number of occurrences of each unique 42-mer (amino acids + stop codons) were enumerated. Any sequence with fewer than 12,906 occurrences (i.e. 0.1% of total) were discarded, leaving 11 unique 42-mers. Five of these were variants of the PTC mutant where the different 42-mers give rise to a single translatable peptide MLLL, and therefore their occurrence counts (4,110,409, 21,375, 17,287, 15,798 and 14,876) were summed. The remaining seven variants are shown in Figure S6B. Collection date and country metadata were extracted from the sequence records and used to produce Figures 7D and 7E.
Quantification and statistical analysis
Three independent experimental replicates were performed for all experiments unless otherwise stated. The applied statistical tests are indicated in the figure legends, where n = number of independent biological repeats. For all tests, p values less than or equal to 0.05 were deemed significant. All statistical analyses were performed using GraphPad Prism version 7.0c for Mac OS X (GraphPad software, USA).
Acknowledgments
The authors would like to thank Dr James Hastie and the staff at the MRC Protein Phosphorylation and Ubiquitylation Unit, University of Dundee, for providing anti-3c custom sheep antibodies. We would also like to thank the UK Health Security Agency for providing virus isolates. We gratefully acknowledge all data contributors, i.e., the Authors and their Originating laboratories responsible for obtaining the specimens, and their Submitting laboratories for generating the genetic sequence and metadata and sharing via the GISAID Initiative, on which some of this research is based. The graphical abstract was created with BioRender.com.
A.E.F and H.S. were supported by Wellcome Trust Senior Research Fellowships (106207/Z/14/Z, 220814/Z/20/Z) and a European Research Council grant (646891). S.H. was supported by a Wellcome Trust Investigator Award in Science (204957/Z/16/Z). J.R.E. was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (216370/Z/19/Z). G.L.S. was supported by a Wellcome Trust Principal Research Fellowship (090315). A.D.D. and D.A.M. were supported by UK Research and Innovation/Medical Research Council grant MR/W005611/1/ and the United States Food and Drug Administration (contract 5F40120C00085). V.L. was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (220620/Z/20/Z) and an Isaac Newton Trust/Wellcome Trust ISSF/University of Cambridge Joint Research Grant. Y.L. was supported by the Wellcome Trust and the Isaac Newton Trust. H.A.M. was supported by The Master’s Fund of Selwyn College, University of Cambridge. S.K.T. was supported by a Department of Pathology, University of Cambridge PhD studentship. Y.A. was supported by a Lister Institute Summer Studentship. E.E. was supported by an Academy of Medical Sciences Springboard Award (SBF006/1008) which is supported by the British Heart Foundation, Diabetes UK, the Global Challenges Research Fund, the Government Department for Business, Energy and Industrial Strategy and the Wellcome Trust, and by Medical Research Council grant (MR/X000885/1). A.V., L.D.C.Z., and P.R. were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC 2067/1- 390729940), SFB1190 (projects P13, P.R.) and the Max Planck Society (P.R.). For the purpose of open access, the authors have applied a CC BY public copyright licence to any Author Accepted article version arising from this submission.
Author contributions
H.S., Y.L., A.E.F., G.L.S., J.R.E., S.H., and P.R. designed the study and experiments. H.S., Y.L., S.O’K., G.W.C., Y.A., R.M., J.R.E., E.E., S.K.N., N.L., H.A.M., A.V., L.D.C.Z, D.A.M., and A.D.D. performed experiments. H.S., Y.L., S.H., J.R.E., E.E., V.L., I.J., and P.R. provided expertise on data analysis, interpretation and methodology. A.E.F. performed bioinformatic analyses. H.S., Y.L., and S.O’K. wrote the article. All authors edited and discussed the article. A.E.F., E.E., G.L.S., and J.L.H. secured funding.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse and equitable conduct of research.
Published: September 28, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108080.
Contributor Information
Hazel Stewart, Email: hs623@cam.ac.uk.
James R. Edgar, Email: je333@cam.ac.uk.
Geoffrey L. Smith, Email: geoffrey.smith@path.ox.ac.uk.
Andrew E. Firth, Email: aef24@cam.ac.uk.
Supplemental information
Data and code availability
-
•
This paper analyses existing, publicly available data. These accession numbers for the datasets are listed in the key resources table. Proteomics data have been deposited at the ProteomeXchange Consortium via the PRIDE repository and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- 1.Finkel Y., Mizrahi O., Nachshon A., Weingarten-Gabbay S., Morgenstern D., Yahalom-Ronen Y., Tamir H., Achdout H., Stein D., Israeli O., et al. The coding capacity of SARS-CoV-2. Nature. 2021;589:125–130. doi: 10.1038/s41586-020-2739-1. [DOI] [PubMed] [Google Scholar]
- 2.Jungreis I., Sealfon R., Kellis M. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes. Nat. Commun. 2021;12:2642. doi: 10.1038/s41467-021-22905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson C.W., Ardern Z., Goldberg T.L., Meng C., Kuo C.H., Ludwig C., Kolokotronis S.O., Wei X. Dynamically evolving novel overlapping gene as a factor in the SARS-CoV-2 pandemic. Elife. 2020;9 doi: 10.7554/eLife.59633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavesi A. New insights into the evolutionary features of viral overlapping genes by discriminant analysis. Virology. 2020;546:51–66. doi: 10.1016/j.virol.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y., Sauter D., Gifford R.J., USFQ-COVID19 Consortium. Nakagawa S., Sato K. SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel C.J., Mayer C., Poch O., Thompson J.D. Characterization of accessory genes in coronavirus genomes. Virol. J. 2020;17:131. doi: 10.1186/s12985-020-01402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The Architecture of SARS-CoV-2 Transcriptome. Cell. 2020;181:914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson A.D., Williamson M.K., Lewis S., Shoemark D., Carroll M.W., Heesom K.J., Zambon M., Ellis J., Lewis P.A., Hiscox J.A., Matthews D.A. Characterisation of the transcriptome and proteome of SARS-CoV-2 reveals a cell passage induced in-frame deletion of the furin-like cleavage site from the spike glycoprotein. Genome Med. 2020;12:68. doi: 10.1186/s13073-020-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., Cinatl J., Münch C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firth A.E. A putative new SARS-CoV protein, 3c, encoded in an ORF overlapping ORF3a. J. Gen. Virol. 2020;101:1085–1089. doi: 10.1099/jgv.0.001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cagliani R., Forni D., Clerici M., Sironi M. Coding potential and sequence conservation of SARS-CoV-2 and related animal viruses. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jungreis I., Nelson C.W., Ardern Z., Finkel Y., Krogan N.J., Sato K., Ziebuhr J., Stern-Ginossar N., Pavesi A., Firth A.E., et al. Conflicting and ambiguous names of overlapping ORFs in the SARS-CoV-2 genome: A homology-based resolution. Virology. 2021;558:145–151. doi: 10.1016/j.virol.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaune D., Hul V., Karlsson E.A., Hassanin A., Ou T.P., Baidaliuk A., Gámbaro F., Prot M., Tu V.T., Chea S., et al. A novel SARS-CoV-2 related coronavirus in bats from Cambodia. Nat. Commun. 2021;12:6563. doi: 10.1038/s41467-021-26809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H., Ji J., Chen X., Bi Y., Li J., Wang Q., Hu T., Song H., Zhao R., Chen Y., et al. Identification of novel bat coronaviruses sheds light on the evolutionary origins of SARS-CoV-2 and related viruses. Cell. 2021;184:4380–4391.e14. doi: 10.1016/j.cell.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacharapluesadee S., Tan C.W., Maneeorn P., Duengkae P., Zhu F., Joyjinda Y., Kaewpom T., Chia W.N., Ampoot W., Lim B.L., et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021;12:972. doi: 10.1038/s41467-021-21240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami S., Kitamura T., Suzuki J., Sato R., Aoi T., Fujii M., Matsugo H., Kamiki H., Ishida H., Takenaka-Uema A., et al. Detection and Characterization of Bat Sarbecovirus Phylogenetically Related to SARS-CoV-2, Japan. Emerg. Infect. Dis. 2020;26:3025–3029. doi: 10.3201/eid2612.203386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia H., Cao Z., Xie X., Zhang X., Chen J.Y.C., Wang H., Menachery V.D., Rajsbaum R., Shi P.Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y., Michel H.A., Wang P.-H., Smith G.L. Manipulation of innate immune signaling pathways by SARS-CoV-2 non-structural proteins. Front. Microbiol. 2022;13:1027015. doi: 10.3389/fmicb.2022.1027015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J.S., Shin E.C. The type I interferon response in COVID-19: implications for treatment. Nat. Rev. Immunol. 2020;20:585–586. doi: 10.1038/s41577-020-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Cui S., Eisenächer K., Kirchhofer A., Brzózka K., Lammens A., Lammens K., Fujita T., Conzelmann K.K., Krug A., Hopfner K.P. The C-terminal regulatory domain is the RNA 5'-triphosphate sensor of RIG-I. Mol. Cell. 2008;29:169–179. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Pichlmair A., Schulz O., Tan C.P., Rehwinkel J., Kato H., Takeuchi O., Akira S., Way M., Schiavo G., Reis e Sousa C. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J. Virol. 2009;83:10761–10769. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S., et al. Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reikine S., Nguyen J.B., Modis Y. Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Front. Immunol. 2014;5:342. doi: 10.3389/fimmu.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wathelet M.G., Lin C.H., Parekh B.S., Ronco L.V., Howley P.M., Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 28.Käll L., Krogh A., Sonnhammer E.L.L. Advantages of combined transmembrane topology and signal peptide prediction--the Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Li W., Liu S., Xu J. RaptorX-Property: a web server for protein structure property prediction. Nucleic Acids Res. 2016;44:W430–W435. doi: 10.1093/nar/gkw306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaecher S.R., Mackenzie J.M., Pekosz A. The ORF7b protein of severe acute respiratory syndrome coronavirus (SARS-CoV) is expressed in virus-infected cells and incorporated into SARS-CoV particles. J. Virol. 2007;81:718–731. doi: 10.1128/JVI.01691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu K., Zheng B.J., Zeng R., Lu W., Lin Y.P., Xue L., Li L., Yang L.L., Xu C., Dai J., et al. Severe acute respiratory syndrome coronavirus accessory protein 9b is a virion-associated protein. Virology. 2009;388:279–285. doi: 10.1016/j.virol.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Firth A.E., Brierley I. Non-canonical translation in RNA viruses. J. Gen. Virol. 2012;93:1385–1409. doi: 10.1099/vir.0.042499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 34.Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. 2013;4 doi: 10.1128/mBio.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santerre M., Arjona S.P., Allen C.N., Shcherbik N., Sawaya B.E. Why do SARS-CoV-2 NSPs rush to the ER? J. Neurol. 2021;268:2013–2022. doi: 10.1007/s00415-020-10197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Netland J., Ferraro D., Pewe L., Olivares H., Gallagher T., Perlman S. Enhancement of murine coronavirus replication by severe acute respiratory syndrome coronavirus protein 6 requires the N-terminal hydrophobic region but not C-terminal sorting motifs. J. Virol. 2007;81:11520–11525. doi: 10.1128/JVI.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frieman M., Yount B., Heise M., Kopecky-Bromberg S.A., Palese P., Baric R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007;81:9812–9824. doi: 10.1128/JVI.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson C.A., Pekosz A., Lee C.A., Diamond M.S., Fremont D.H. Structure and intracellular targeting of the SARS-coronavirus Orf7a accessory protein. Structure. 2005;13:75–85. doi: 10.1016/j.str.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaecher S.R., Diamond M.S., Pekosz A. The transmembrane domain of the severe acute respiratory syndrome coronavirus ORF7b protein is necessary and sufficient for its retention in the Golgi complex. J. Virol. 2008;82:9477–9491. doi: 10.1128/JVI.00784-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier C., Aricescu A.R., Assenberg R., Aplin R.T., Gilbert R.J.C., Grimes J.M., Stuart D.I. The crystal structure of ORF-9b, a lipid binding protein from the SARS coronavirus. Structure. 2006;14:1157–1165. doi: 10.1016/j.str.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi C.S., Qi H.Y., Boularan C., Huang N.N., Abu-Asab M., Shelhamer J.H., Kehrl J.H. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 2014;193:3080–3089. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh S., Dellibovi-Ragheb T.A., Kerviel A., Pak E., Qiu Q., Fisher M., Takvorian P.M., Bleck C., Hsu V.W., Fehr A.R., et al. beta-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell. 2020;183:1520–1535.e14. doi: 10.1016/j.cell.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padhan K., Tanwar C., Hussain A., Hui P.Y., Lee M.Y., Cheung C.Y., Peiris J.S.M., Jameel S. Severe acute respiratory syndrome coronavirus Orf3a protein interacts with caveolin. J. Gen. Virol. 2007;88:3067–3077. doi: 10.1099/vir.0.82856-0. [DOI] [PubMed] [Google Scholar]
- 44.Tan Y.J., Teng E., Shen S., Tan T.H.P., Goh P.Y., Fielding B.C., Ooi E.E., Tan H.C., Lim S.G., Hong W. A novel severe acute respiratory syndrome coronavirus protein, U274, is transported to the cell surface and undergoes endocytosis. J. Virol. 2004;78:6723–6734. doi: 10.1128/JVI.78.13.6723-6734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kemper C., Habib S.J., Engl G., Heckmeyer P., Dimmer K.S., Rapaport D. Integration of tail-anchored proteins into the mitochondrial outer membrane does not require any known import components. J. Cell Sci. 2008;121:1990–1998. doi: 10.1242/jcs.024034. [DOI] [PubMed] [Google Scholar]
- 47.Chio U.S., Cho H., Shan S.O. Mechanisms of Tail-Anchored Membrane Protein Targeting and Insertion. Annu. Rev. Cell Dev. Biol. 2017;33:417–438. doi: 10.1146/annurev-cellbio-100616-060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knoops K., Kikkert M., Worm S.H.E.v.d., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snijder E.J., Limpens R.W.A.L., de Wilde A.H., de Jong A.W.M., Zevenhoven-Dobbe J.C., Maier H.J., Faas F.F.G.A., Koster A.J., Bárcena M. A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A., Weber F., Kochs G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Figueiredo Costa B., Cassella P., Colombo S.F., Borgese N. Discrimination between the endoplasmic reticulum and mitochondria by spontaneously inserting tail-anchored proteins. Traffic. 2018;19:182–197. doi: 10.1111/tra.12550. [DOI] [PubMed] [Google Scholar]
- 52.Costello J.L., Castro I.G., Camões F., Schrader T.A., McNeall D., Yang J., Giannopoulou E.A., Gomes S., Pogenberg V., Bonekamp N.A., et al. Predicting the targeting of tail-anchored proteins to subcellular compartments in mammalian cells. J. Cell Sci. 2017;130:1675–1687. doi: 10.1242/jcs.200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Keefe S., Roboti P., Duah K.B., Zong G., Schneider H., Shi W.Q., High S. Ipomoeassin-F inhibits the in vitro biogenesis of the SARS-CoV-2 spike protein and its host cell membrane receptor. J. Cell Sci. 2021;134 doi: 10.1242/jcs.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Keefe S., Zong G., Duah K.B., Andrews L.E., Shi W.Q., High S. An alternative pathway for membrane protein biogenesis at the endoplasmic reticulum. Commun. Biol. 2021;4:828. doi: 10.1038/s42003-021-02363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson R., Allen A.J., Oliver J., Brookman J.L., High S., Bulleid N.J. The translocation, folding, assembly and redox-dependent degradation of secretory and membrane proteins in semi-permeabilized mammalian cells. Biochem. J. 1995;307(Pt 3):679–687. doi: 10.1042/bj3070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farkas Á., Bohnsack K.E. Capture and delivery of tail-anchored proteins to the endoplasmic reticulum. J. Cell Biol. 2021;220 doi: 10.1083/jcb.202105004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu Y.Q., Zielinska M., Li W., Bernkopf D.B., Heilingloh C.S., Neurath M.F., Becker C. PGAM5-MAVS interaction regulates TBK1/IRF3 dependent antiviral responses. Sci. Rep. 2020;10:8323. doi: 10.1038/s41598-020-65155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Z., Zheng H., Li H., Chen Y., Hou D., Fan Q., Song J., Guo L., Liu L. The expression of IFN-beta is suppressed by the viral 3D polymerase via its impact on PGAM5 expression during enterovirus D68 infection. Virus Res. 2021;304 doi: 10.1016/j.virusres.2021.198549. [DOI] [PubMed] [Google Scholar]
- 59.Nieva J.L., Madan V., Carrasco L. Viroporins: structure and biological functions. Nat. Rev. Microbiol. 2012;10:563–574. doi: 10.1038/nrmicro2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z., Jiang H., Chen S., Du F., Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 61.Lo S.C., Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp. Cell Res. 2008;314:1789–1803. doi: 10.1016/j.yexcr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma K., Zhang Z., Chang R., Cheng H., Mu C., Zhao T., Chen L., Zhang C., Luo Q., Lin J., et al. Dynamic PGAM5 multimers dephosphorylate BCL-xL or FUNDC1 to regulate mitochondrial and cellular fate. Cell Death Differ. 2020;27:1036–1051. doi: 10.1038/s41418-019-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaikuad A., Filippakopoulos P., Marcsisin S.R., Picaud S., Schröder M., Sekine S., Ichijo H., Engen J.R., Takeda K., Knapp S. Structures of PGAM5 Provide Insight into Active Site Plasticity and Multimeric Assembly. Structure. 2017;25:1089–1099.e3. doi: 10.1016/j.str.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruiz K., Thaker T.M., Agnew C., Miller-Vedam L., Trenker R., Herrera C., Ingaramo M., Toso D., Frost A., Jura N. Functional role of PGAM5 multimeric assemblies and their polymerization into filaments. Nat. Commun. 2019;10:531. doi: 10.1038/s41467-019-08393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seth R.B., Sun L., Ea C.K., Chen Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Hou F., Sun L., Zheng H., Skaug B., Jiang Q.X., Chen Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H., Zheng H., Zhu J., Dong Q., Wang J., Fan H., Chen Y., Zhang X., Han X., Li Q., et al. Ubiquitin-Modified Proteome of SARS-CoV-2-Infected Host Cells Reveals Insights into Virus-Host Interaction and Pathogenesis. J. Proteome Res. 2021;20:2224–2239. doi: 10.1021/acs.jproteome.0c00758. [DOI] [PubMed] [Google Scholar]
- 68.Yuen C.K., Lam J.Y., Wong W.M., Mak L.F., Wang X., Chu H., Cai J.P., Jin D.Y., To K.K.W., Chan J.F.W., et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microb. Infect. 2020;9:1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J.Y., Liao C.H., Wang Q., Tan Y.J., Luo R., Qiu Y., Ge X.Y. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y., Zhuang M.W., Han L., Zhang J., Nan M.L., Zhan P., Kang D., Liu X., Gao C., Wang P.H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct. Targeted Ther. 2020;5:299. doi: 10.1038/s41392-020-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J., Shi Y., Pan X., Wu S., Hou R., Zhang Y., Zhong T., Tang H., Du W., Wang L., et al. SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li A., Zhao K., Zhang B., Hua R., Fang Y., Jiang W., Zhang J., Hui L., Zheng Y., Li Y., et al. SARS-CoV-2 NSP12 Protein Is Not an Interferon-beta Antagonist. J. Virol. 2021;95 doi: 10.1128/JVI.00747-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clément J.F., Bibeau-Poirier A., Gravel S.P., Grandvaux N., Bonneil E., Thibault P., Meloche S., Servant M.J. Phosphorylation of IRF-3 on Ser 339 generates a hyperactive form of IRF-3 through regulation of dimerization and CBP association. J. Virol. 2008;82:3984–3996. doi: 10.1128/JVI.02526-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pertel T., Hausmann S., Morger D., Züger S., Guerra J., Lascano J., Reinhard C., Santoni F.A., Uchil P.D., Chatel L., et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472:361–365. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ning X., Wang Y., Jing M., Sha M., Lv M., Gao P., Zhang R., Huang X., Feng J.M., Jiang Z. Apoptotic Caspases Suppress Type I Interferon Production via the Cleavage of cGAS, MAVS, and IRF3. Mol. Cell. 2019;74:19–31.e7. doi: 10.1016/j.molcel.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 76.Li X.D., Sun L., Seth R.B., Pineda G., Chen Z.J. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. USA. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng H., Sander A.L., Moreira-Soto A., Yamane D., Drexler J.F., Lemon S.M. Hepatovirus 3ABC proteases and evolution of mitochondrial antiviral signaling protein (MAVS) J. Hepatol. 2019;71:25–34. doi: 10.1016/j.jhep.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng B., Abdullahi A., Ferreira I.A.T.M., Goonawardane N., Saito A., Kimura I., Yamasoba D., Gerber P.P., Fatihi S., Rathore S., et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603:706–714. doi: 10.1038/s41586-022-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob. Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sekine S., Kanamaru Y., Koike M., Nishihara A., Okada M., Kinoshita H., Kamiyama M., Maruyama J., Uchiyama Y., Ishihara N., et al. Rhomboid protease PARL mediates the mitochondrial membrane potential loss-induced cleavage of PGAM5. J. Biol. Chem. 2012;287:34635–34645. doi: 10.1074/jbc.M112.357509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamaguchi A., Ishikawa H., Furuoka M., Yokozeki M., Matsuda N., Tanimura S., Takeda K. Cleaved PGAM5 is released from mitochondria depending on proteasome-mediated rupture of the outer mitochondrial membrane during mitophagy. J. Biochem. 2019;165:19–25. doi: 10.1093/jb/mvy077. [DOI] [PubMed] [Google Scholar]
- 83.Takeda K., Komuro Y., Hayakawa T., Oguchi H., Ishida Y., Murakami S., Noguchi T., Kinoshita H., Sekine Y., Iemura S.i., et al. Mitochondrial phosphoglycerate mutase 5 uses alternate catalytic activity as a protein serine/threonine phosphatase to activate ASK1. Proc. Natl. Acad. Sci. USA. 2009;106:12301–12305. doi: 10.1073/pnas.0901823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng M., Lin N., Dong D., Ma J., Su J., Sun L. PGAM5: A crucial role in mitochondrial dynamics and programmed cell death. Eur. J. Cell Biol. 2021;100 doi: 10.1016/j.ejcb.2020.151144. [DOI] [PubMed] [Google Scholar]
- 85.Lin H.Y., Lai R.H., Lin S.T., Lin R.C., Wang M.J., Lin C.C., Lee H.C., Wang F.F., Chen J.Y. Suppressor of cytokine signaling 6 (SOCS6) promotes mitochondrial fission via regulating DRP1 translocation. Cell Death Differ. 2013;20:139–153. doi: 10.1038/cdd.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He G.W., Günther C., Kremer A.E., Thonn V., Amann K., Poremba C., Neurath M.F., Wirtz S., Becker C. PGAM5-mediated programmed necrosis of hepatocytes drives acute liver injury. Gut. 2017;66:716–723. doi: 10.1136/gutjnl-2015-311247. [DOI] [PubMed] [Google Scholar]
- 87.Moriwaki K., Farias Luz N., Balaji S., De Rosa M.J., O'Donnell C.L., Gough P.J., Bertin J., Welsh R.M., Chan F.K.M. The Mitochondrial Phosphatase PGAM5 Is Dispensable for Necroptosis but Promotes Inflammasome Activation in Macrophages. J. Immunol. 2016;196:407–415. doi: 10.4049/jimmunol.1501662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishida Y., Sekine Y., Oguchi H., Chihara T., Miura M., Ichijo H., Takeda K. Prevention of apoptosis by mitochondrial phosphatase PGAM5 in the mushroom body is crucial for heat shock resistance in Drosophila melanogaster. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu W., Jing L., Wang Q., Lin C.C., Chen X., Diao J., Liu Y., Sun X. Bax-PGAM5L-Drp1 complex is required for intrinsic apoptosis execution. Oncotarget. 2015;6:30017–30034. doi: 10.18632/oncotarget.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu H., Xue D., Chen G., Han Z., Huang L., Zhu C., Wang X., Jin H., Wang J., Zhu Y., et al. The BCL2L1 and PGAM5 axis defines hypoxia-induced receptor-mediated mitophagy. Autophagy. 2014;10:1712–1725. doi: 10.4161/auto.29568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhuang M., Guan S., Wang H., Burlingame A.L., Wells J.A. Substrates of IAP ubiquitin ligases identified with a designed orthogonal E3 ligase, the NEDDylator. Mol. Cell. 2013;49:273–282. doi: 10.1016/j.molcel.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen G., Han Z., Feng D., Chen Y., Chen L., Wu H., Huang L., Zhou C., Cai X., Fu C., et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol. Cell. 2014;54:362–377. doi: 10.1016/j.molcel.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 93.Park Y.S., Choi S.E., Koh H.C. PGAM5 regulates PINK1/Parkin-mediated mitophagy via DRP1 in CCCP-induced mitochondrial dysfunction. Toxicol. Lett. 2018;284:120–128. doi: 10.1016/j.toxlet.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 94.Lu W., Karuppagounder S.S., Springer D.A., Allen M.D., Zheng L., Chao B., Zhang Y., Dawson V.L., Dawson T.M., Lenardo M. Genetic deficiency of the mitochondrial protein PGAM5 causes a Parkinson's-like movement disorder. Nat. Commun. 2014;5:4930. doi: 10.1038/ncomms5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ganzleben I., He G.W., Günther C., Prigge E.S., Richter K., Rieker R.J., Mougiakakos D., Neurath M.F., Becker C. PGAM5 is a key driver of mitochondrial dysfunction in experimental lung fibrosis. Cell. Mol. Life Sci. 2019;76:4783–4794. doi: 10.1007/s00018-019-03133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Padhan K., Minakshi R., Towheed M.A.B., Jameel S. Severe acute respiratory syndrome coronavirus 3a protein activates the mitochondrial death pathway through p38 MAP kinase activation. J. Gen. Virol. 2008;89:1960–1969. doi: 10.1099/vir.0.83665-0. [DOI] [PubMed] [Google Scholar]
- 97.Ren Y., Shu T., Wu D., Mu J., Wang C., Huang M., Han Y., Zhang X.Y., Zhou W., Qiu Y., Zhou X. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 2020;17:881–883. doi: 10.1038/s41423-020-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernkopf D.B., Jalal K., Brückner M., Knaup K.X., Gentzel M., Schambony A., Behrens J. Pgam5 released from damaged mitochondria induces mitochondrial biogenesis via Wnt signaling. J. Cell Biol. 2018;217:1383–1394. doi: 10.1083/jcb.201708191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mozzi A., Oldani M., Forcella M.E., Vantaggiato C., Cappelletti G., Pontremoli C., Valenti F., Forni D., Saresella M., Biasin M., et al. SARS-CoV-2 ORF3c impairs mitochondrial respiratory metabolism, oxidative stress, and autophagic flux. iScience. 2023;26 doi: 10.1016/j.isci.2023.107118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miorin L., Kehrer T., Sanchez-Aparicio M.T., Zhang K., Cohen P., Patel R.S., Cupic A., Makio T., Mei M., Moreno E., et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl. Acad. Sci. USA. 2020;117:28344–28354. doi: 10.1073/pnas.2016650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oh S.J., Shin O.S. SARS-CoV-2 Nucleocapsid Protein Targets RIG-I-Like Receptor Pathways to Inhibit the Induction of Interferon Response. Cells. 2021;10:530. doi: 10.3390/cells10030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen K., Xiao F., Hu D., Ge W., Tian M., Wang W., Pan P., Wu K., Wu J. SARS-CoV-2 Nucleocapsid Protein Interacts with RIG-I and Represses RIG-Mediated IFN-beta Production. Viruses. 2020;13:47. doi: 10.3390/v13010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fung S.Y., Siu K.L., Lin H., Yeung M.L., Jin D.Y. SARS-CoV-2 main protease suppresses type I interferon production by preventing nuclear translocation of phosphorylated IRF3. Int. J. Biol. Sci. 2021;17:1547–1554. doi: 10.7150/ijbs.59943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y., Qin C., Rao Y., Ngo C., Feng J.J., Zhao J., Zhang S., Wang T.Y., Carriere J., Savas A.C., et al. SARS-CoV-2 Nsp5 Demonstrates Two Distinct Mechanisms Targeting RIG-I and MAVS To Evade the Innate Immune Response. mBio. 2021;12 doi: 10.1128/mBio.02335-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menachery V.D., Yount B.L., Jr., Josset L., Gralinski L.E., Scobey T., Agnihothram S., Katze M.G., Baric R.S. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2'-o-methyltransferase activity. J. Virol. 2014;88:4251–4264. doi: 10.1128/JVI.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsu J.C.C., Laurent-Rolle M., Pawlak J.B., Wilen C.B., Cresswell P. Translational shutdown and evasion of the innate immune response by SARS-CoV-2 NSP14 protein. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2101161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar A., Ishida R., Strilets T., Cole J., Lopez-Orozco J., Fayad N., Felix-Lopez A., Elaish M., Evseev D., Magor K.E., et al. SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors. J. Virol. 2021;95 doi: 10.1128/JVI.00266-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thorne L.G., Bouhaddou M., Reuschl A.K., Zuliani-Alvarez L., Polacco B., Pelin A., Batra J., Whelan M.V.X., Hosmillo M., Fossati A., et al. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature. 2022;602:487–495. doi: 10.1038/s41586-021-04352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gordon D.E., Hiatt J., Bouhaddou M., Rezelj V.V., Ulferts S., Braberg H., Jureka A.S., Obernier K., Guo J.Z., Batra J., et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370 doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X., Hou P., Ma W., Wang X., Wang H., Yu Z., Chang H., Wang T., Jin S., Wang X., et al. SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell. Mol. Immunol. 2022;19:67–78. doi: 10.1038/s41423-021-00807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang H.W., Zhang H.N., Meng Q.F., Xie J., Li Y., Chen H., Zheng Y.X., Wang X.N., Qi H., Zhang J., et al. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell. Mol. Immunol. 2020;17:998–1000. doi: 10.1038/s41423-020-0514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu X.Y., Wei B., Shi H.X., Shan Y.F., Wang C. Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res. 2010;20:994–1011. doi: 10.1038/cr.2010.103. [DOI] [PubMed] [Google Scholar]
- 114.Wei B., Cui Y., Huang Y., Liu H., Li L., Li M., Ruan K.C., Zhou Q., Wang C. Tom70 mediates Sendai virus-induced apoptosis on mitochondria. J. Virol. 2015;89:3804–3818. doi: 10.1128/JVI.02959-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Edmonson A.M., Mayfield D.K., Vervoort V., DuPont B.R., Argyropoulos G. Characterization of a human import component of the mitochondrial outer membrane. Cell Commun. Adhes. 2002;9:15–27. doi: 10.1080/15419060212186. [DOI] [PubMed] [Google Scholar]
- 116.Crozier T.W.M., Greenwood E.J.D., Williamson J.C., Guo W., Porter L.M., Gabaev I., Teixeira-Silva A., Grice G.L., Wickenhagen A., Stanton R.J., et al. Quantitative proteomic analysis of SARS-CoV-2 infection of primary human airway ciliated cells and lung epithelial cells demonstrates the effectiveness of SARS-CoV-2 innate immune evasion [version 1; peer review: awaiting peer review] Wellcome Open Res. 2022;7:224. doi: 10.12688/wellcomeopenres.17946.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perrin-Cocon L., Diaz O., Jacquemin C., Barthel V., Ogire E., Ramière C., André P., Lotteau V., Vidalain P.O. The current landscape of coronavirus-host protein-protein interactions. J. Transl. Med. 2020;18:319. doi: 10.1186/s12967-020-02480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu Y.Z., Wang S.Y., Zheng Z.Q., Yi H., Li W.W., Xu Z.S., Wang Y.Y. SARS-CoV-2 membrane glycoprotein M antagonizes the MAVS-mediated innate antiviral response. Cell. Mol. Immunol. 2021;18:613–620. doi: 10.1038/s41423-020-00571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yin X., Riva L., Pu Y., Martin-Sancho L., Kanamune J., Yamamoto Y., Sakai K., Gotoh S., Miorin L., De Jesus P.D., et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rebendenne A., Valadão A.L.C., Tauziet M., Maarifi G., Bonaventure B., McKellar J., Planès R., Nisole S., Arnaud-Arnould M., Moncorgé O., Goujon C. SARS-CoV-2 triggers an MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells. J. Virol. 2021;95 doi: 10.1128/JVI.02415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamada T., Sato S., Sotoyama Y., Orba Y., Sawa H., Yamauchi H., Sasaki M., Takaoka A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021;22:820–828. doi: 10.1038/s41590-021-00942-0. [DOI] [PubMed] [Google Scholar]
- 122.Yang D.M., Geng T.T., Harrison A.G., Wang P.H. Differential roles of RIG-I like receptors in SARS-CoV-2 infection. Mil. Med. Res. 2021;8:49. doi: 10.1186/s40779-021-00340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meyer B., Chiaravalli J., Gellenoncourt S., Brownridge P., Bryne D.P., Daly L.A., Grauslys A., Walter M., Agou F., Chakrabarti L.A., et al. Characterising proteolysis during SARS-CoV-2 infection identifies viral cleavage sites and cellular targets with therapeutic potential. Nat. Commun. 2021;12:5553. doi: 10.1038/s41467-021-25796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weingarten-Gabbay S., Chen D.Y., Sarkizova S., Taylor H.B., Gentili M., Pearlman L.R., Bauer M.R., Rice C.M., Clauser K.R., Hacohen N., et al. The HLA-II immunopeptidome of SARS-CoV-2. bioRxiv. 2023 doi: 10.1101/2023.05.26.542482. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Osada N., Kohara A., Yamaji T., Hirayama N., Kasai F., Sekizuka T., Kuroda M., Hanada K. The genome landscape of the african green monkey kidney-derived vero cell line. DNA Res. 2014;21:673–683. doi: 10.1093/dnares/dsu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yount B., Roberts R.S., Sims A.C., Deming D., Frieman M.B., Sparks J., Denison M.R., Davis N., Baric R.S. Severe acute respiratory syndrome coronavirus group-specific open reading frames encode nonessential functions for replication in cell cultures and mice. J. Virol. 2005;79:14909–14922. doi: 10.1128/JVI.79.23.14909-14922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Castaño-Rodriguez C., Honrubia J.M., Gutiérrez-Álvarez J., DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeño J.M., Regla-Nava J.A., Fernandez-Delgado R., Verdia-Báguena C., Queralt-Martín M., et al. Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis. mBio. 2018;9 doi: 10.1128/mBio.02325-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Freundt E.C., Yu L., Goldsmith C.S., Welsh S., Cheng A., Yount B., Liu W., Frieman M.B., Buchholz U.J., Screaton G.R., et al. The open reading frame 3a protein of severe acute respiratory syndrome-associated coronavirus promotes membrane rearrangement and cell death. J. Virol. 2010;84:1097–1109. doi: 10.1128/JVI.01662-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rihn S.J., Merits A., Bakshi S., Turnbull M.L., Wickenhagen A., Alexander A.J.T., Baillie C., Brennan B., Brown F., Brunker K., et al. A plasmid DNA-launched SARS-CoV-2 reverse genetics system and coronavirus toolkit for COVID-19 research. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Unterholzner L., Sumner R.P., Baran M., Ren H., Mansur D.S., Bourke N.M., Randow F., Smith G.L., Bowie A.G. Vaccinia virus protein C6 is a virulence factor that binds TBK-1 adaptor proteins and inhibits activation of IRF3 and IRF7. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caly L., Druce J., Roberts J., Bond K., Tran T., Kostecki R., Yoga Y., Naughton W., Taiaroa G., Seemann T., et al. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med. J. Aust. 2020;212:459–462. doi: 10.5694/mja2.50569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lulla V., Lulla A., Wernike K., Aebischer A., Beer M., Roy P. Assembly of Replication-Incompetent African Horse Sickness Virus Particles: Rational Design of Vaccines for All Serotypes. J. Virol. 2016;90:7405–7414. doi: 10.1128/JVI.00548-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lulla V., Dinan A.M., Hosmillo M., Chaudhry Y., Sherry L., Irigoyen N., Nayak K.M., Stonehouse N.J., Zilbauer M., Goodfellow I., Firth A.E. An upstream protein-coding region in enteroviruses modulates virus infection in gut epithelial cells. Nat. Microbiol. 2019;4:280–292. doi: 10.1038/s41564-018-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang J., Cruz-Cosme R., Zhuang M.W., Liu D., Liu Y., Teng S., Wang P.H., Tang Q. A systemic and molecular study of subcellular localization of SARS-CoV-2 proteins. Signal Transduct. Targeted Ther. 2020;5:269. doi: 10.1038/s41392-020-00372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Soday L., Lu Y., Albarnaz J.D., Davies C.T.R., Antrobus R., Smith G.L., Weekes M.P. Quantitative Temporal Proteomic Analysis of Vaccinia Virus Infection Reveals Regulation of Histone Deacetylases by an Interferon Antagonist. Cell Rep. 2019;27:1920–1933.e7. doi: 10.1016/j.celrep.2019.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lulla V., Firth A.E. A hidden gene in astroviruses encodes a viroporin. Nat. Commun. 2020;11:4070. doi: 10.1038/s41467-020-17906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McKenna M., Simmonds R.E., High S. Mechanistic insights into the inhibition of Sec61-dependent co- and post-translational translocation by mycolactone. J. Cell Sci. 2016;129:1404–1415. doi: 10.1242/jcs.182352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roboti P., Sato K., Lowe M. The golgin GMAP-210 is required for efficient membrane trafficking in the early secretory pathway. J. Cell Sci. 2015;128:1595–1606. doi: 10.1242/jcs.166710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hughes C.S., Moggridge S., Müller T., Sorensen P.H., Morin G.B., Krijgsveld J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 140.Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 141.Storey J.D. A Direct Approach to False Discovery Rates. J. Roy. Stat. Soc. B. 2002;64:479–498. [Google Scholar]
- 142.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 143.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rice P., Longden I., Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper analyses existing, publicly available data. These accession numbers for the datasets are listed in the key resources table. Proteomics data have been deposited at the ProteomeXchange Consortium via the PRIDE repository and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.