Abstract
Antibiotics affect microbial diversity in the gut, leading to dysbiosis and impaired immunity. However, the impact of antibiotics on microbial communities at other sites, such as vagina is less understood. It is also not clear if changes induced by antibiotics in both microbiomes affect the development of cervical cancer. In this study we utilized the murine model to evaluate these questions. We show that oral application of broad-spectrum antibiotics in mice changed not only diversity, but composition and sharing of gut and vaginal microbiomes in mice and influenced cervical cancer development in an orthotopic tumor model. Antibiotics decreased richness and diversity indexes in the gut but increased them in the vagina. Some beneficial taxa, such as Bacteroides, Ruminococcaceae and Lachnospiraceae increased their abundance in the vagina while other pathogenic species, such as Proteobacteria, were decreased. As a result of the changes, mice with greater richness and diversity of the vaginal microbiome after antibiotics exposure were less likely developed tumors. No association between richness and diversity of the gut microbiome and tumor development was identified.
Keywords: antibiotics, microbial diversity, vagina, mouse model, association network
Introduction
Antibiotics are the most commonly prescribed medications. They have profound negative effects on microbial diversity and composition in the gut (1–3) and associate with poor survival in cancer patients and with their response to therapy (4–6). Several studies have also associated recurrent exposure to antibiotics with higher rates of developing different malignancies (7,8). The effect of antibiotic exposure on risk of gynecological malignancies, however, is not clear (9). The exposure associated with a decreased risk of cervical cancer, but no association was found for ovarian or uterine cancer. The difference may be due to specific etiology of the cervical cancers that are known to be promoted by the chronic human papillomavirus (HPV) infection.
HPV infection is common, but only ~10% of HPV infected individuals develop persistent HPV associated lesions, which may include dysplasia or cancer (10). Emerging evidence suggest that structural changes of the vaginal microbiome, from dominated by Lactobacillus species to dominated by anaerobic bacteria, play a crucial role in the progression of HPV infection into cervical cancer (11,12). A complex interplay between the type of antibiotics (AB) exposure, vaginal microbiota, and human papillomavirus (HPV) infection may affect cervical cancer development (13–15). Changes in structure of the microbiome can clear the HPV infection, or, vice versa, lead to infection and dysbiosis. An antiviral activity of a strain of Bifidobacterium adolescentis was demonstrated, for example, for HPV16 (16) and of some other vaginal bacteria that may be used for treatment of the infection as probiotics (17). Vice versa, bacterial dysbiosis in the vagina has a strong negative effect on beneficial bacteria, such as Lactobacillus crispatus, and leads to outgrowth of anaerobic non-Lactobacilli species and to different pathological conditions, such as bacterial vaginosis that associates with cervical intraephitelial neoplasia, a precursor of the cervical cancer (18,19). Multiple factors including ethnicity, sexual behavior, hygiene, pregnancy and alcohol use affect structure of bacterial communities in the vagina leading to outgrowth of vaginosis associated species or those that suppress pathogens and exert anticancer effect in human (17,20–22).
Most studies of oral AB application focus on changes of the bacterial communities in the gut. Women on antibiotics are usually excluded from studies of the microbiome in cervical cancer (18). It is known, however, that local bacterial communities can be affected by antibiotics. Studies have explored effects of antibiotics on respiratory (23), skin (24) and vaginal microbiomes (14). In the last study, mice treated with oral antibiotics experienced dysbiosis and imbalance of vaginal commensal bacteria that made the mice more susceptible to mucosal HSV-2 infection (25). To our knowledge, none of the studies explored the effect of antibiotics on associations between the bacterial communities in the gut and local bacterial communities. In addition, although murine models are heavily utilized to investigate the microbiomes and cancer development, the effect of antibiotic exposure on gut and tumor microbiome in commonly used orthotopic murine cancer models is poorly understood. It is not clear if negative effects of the exposure on microbial diversity and composition in the gut are mirrored in the vagina and how the changes effect the cervical cancer development. Specific bacteria in the gut/vagina that facilitate or suppress the tumor development in the presence of the AB exposure are not known either. Comprehensive answers to the question will help to optimize treatment of patients not only with ABs, but also with probiotics, a promising novel therapy against bacterial vaginosis and vaginal dysbiosis (11).
In this study we use the mouse model to answer the questions. Namely we explore the relationship between gut and vaginal microbiomes in AB treated and not treated mice and show how changes in the relationship contribute to development of the cervical cancer. We also identify specific bacterial species that promote or suppress tumor development in the model.
Materials and Methods
Study Design
Two cohorts of mice were treated with and without antibiotics. The first cohort was used as the discovery dataset and is comprised of 52 mice; 20 treated with AB and 32 untreated mice. The second cohort (n=30, 15 AB-treated and 15 untreated) was used for validation. Design of the experiment was similar in each cohort (Fig. 1A). Mice, strain C57Bl/6, were treated with a broad-spectrum antibiotic cocktail including vancomycin, imipenem/cilastatin and neomycin (AB) continuously in drinking water starting three weeks prior to tumor inoculation. This antibiotic regimen has been used previously for cancer studies of decreased microbiome diversity in murine models (Iida et al 2013. Three weeks after treatment (before tumor challenge) paired samples were collected from vagina (Puritan sterile polyester tipped applicator swabs) and gut (fecal pellets).
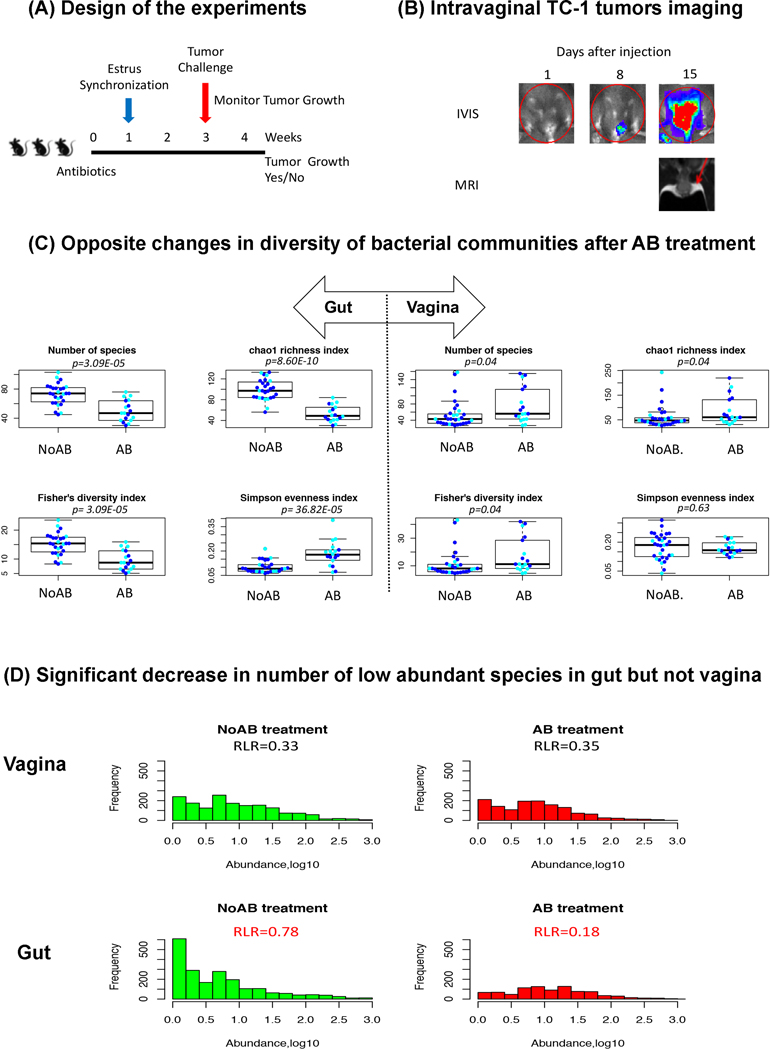
Fig. 1.
Effect of antibiotics on bacterial diversity in the vagina versus gut.
A, Design of the experiment. B, Imaging of intravaginal TC-1 tumors following injection, IVIS imaging was performed on days 1, 5, 8, 12 and 15 following intraperitoneal injection of coelentrazine, red circle demonstrates the region of interest for measuring photons/second using bioluminescent imaging to quantitate tumor burden, T2 weighted MRI was performed on day 15 with red arrow pointing to tumor mass. C, Opposite changes in diversity of bacterial communities after AB treatment in gut and in vagina. D, Changes in distribution of species with different abundances in the gut and in the vagina after AB treatment, RLR is the ratio of low abundant species (log10 of the abundance < 0.2) to the rest.
Estrous synchronization was initiated on day 1 by injecting all mice subcutaneously with 0.1ug of estradiol diluted in peanut oil. On day 2, mice were then injected subcutaneously with 2mg of medroxyprogesterone acetate. On day 6, a cytology check was performed by swabbing the vaginal canal and applying to a microscope slide to confirm synchronization.
Each mouse was categorized according to development of tumor growth (Yes or No). A syngeneic orthotopic tumor model using E6/7 expressing TC-1 tumors cells administered intravaginally (Bartkowiak et al 2015, Lin et al 1996) was employed for the categorization. The TC-1 tumor cell line is derived from a murine fibroblast line transformed with the HPV tumor antigens. The tumor growth was monitored using In Vivo Imaging System (IVIS) (Fig. 1B).
16S sequencing
16S rRNA gene sequencing was performed by the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine as described before (5). The generated tables of Operational Taxonomic Units (OTUs) for discovery and validation cohorts are available as Supplementary Tables S1 and S2 respectively. The complete pair of gut and vaginal 16S samples were not available for each mouse. We had to discount some samples because of low number of reads. Supplementary Table S3 provides information on gut and vaginal 16S samples used in the analysis for each cohort as well as other metadata.
Bacterial community structure
We use 3 groups of diversity indexes to characterize structure of the microbiomes in the gut and in the vaginal samples. They include (i) 2 indexes of richness: observed (number of species) and chao1; (ii) 5 indexes of evenness: Camargo’s evenness, Simpson’s evenness, Pielou’s evenness, also known as Shannon evenness, Smith and Wilson’s Evar index (evar) and Bulla’s index; and (iii) 5 indexes of biodiversity: Inverse Simpson diversity, Gini-Simpson diversity, Shannon diversity, and Fisher alpha diversity. The calculations were done using functions diversity(), richness(), and evenness() implemented in the R library “microbiome”. The ratio of low abundance species to the rest (RLR) was used to characterize the enrichment of microbiome with low abundant species. The ratio was calculated using the eq. RLR = (Number of low abundant species (Abndance,log10 < 0.2)/(Number of the rest species).
Gut and vaginal microbiomes relationship
The relationship was studied using Anets (association networks) for putative species or OTUs (Operational Taxonomic Units) (26). The following parameters were used to build the network in the discovery cohort: the number of co-occurred items is 2, the low limit for association between a pair of OTUs is 0.3. The limit for association was set to 0.6 in the validation cohort. The limits were inferred from the tradeoff between the number of associated nodes in the network and number of clusters. The networks were further explored building heatmaps of microbiomes in AB and in NoAB mice. The gut and vaginal samples (horizontal line) in the heatmap were clustered separately and then sorted by their richness, from high to low, in each environment. The OTUs (vertical line) were clustered by Markov Clustering (MCL).
Associations of specific bacteria with AB treatment and the tumor growth
LEfSe (Linear discriminant analysis Effect Size) software (27) implemented by the Galaxy Project was used to find OTUs (taxa) differentiating microbial communities in the gut/vagina of AB and NoAB treated mice, as well as mice with and without tumor growth. Default parameters were used.
Results
Effect of antibiotics on cervical cancer development
Overall, there was no significant difference in the rate of tumor development between mice treated with or without antibiotics. In the discovery cohort, 11/ 20 (55%) of AB mice developed tumors and only 9/32 (28%) of NoAB mice (fisher’s exact test p=0.08). In the validation cohort, the rate of development was higher overall with 12/15 (80%) of AB mice and 14/15 (93%) of no AB mice developed tumors.
Effect of antibiotics on bacterial diversity in the vagina versus gut
Similar to previous observations, oral AB exposure significantly decreased gut richness and diversity and increased evenness in the discovery cohort (Fig. 1C). Opposite changes of the indexes were observed in vaginal microbiomes. Namely, while richness and diversity indexes decreased in the gut, they increased in the vagina. As a result of the changes, the gut community were richer and more diverse than vaginal community in NoAB mice, and less rich and diverse than vaginal community in AB mice (Fig. S1). The reverse changes of the diversity indexes between gut and vagina were confirmed using the validation cohort (Fig. S2 and Fig. S3); although statistically significant changes were observed only in the vaginal community.
Considering a significant increase in the evenness of the gut bacteria after the AB exposure, we investigated whether antibiotics specifically depleted rare low abundance species. Indeed, the distribution of gut species by abundances was significantly affected by AB exposure in the discovery cohort (Fig. 1D), revealing a decrease in low abundance species. The Ratio of Low abundant species to the Rest (RLR) decreased from 0.78 to 0.18 in the gut, while no changes in the distribution was observed in the vagina. Although the validation cohort didn’t show significant changes in the distribution of species by abundances (Fig. S4), the RLR was also decreased after AB exposure, from 0.93 to 0.88.
Differentially abundant taxa between AB and NoAB mice in the gut and vagina
The reverse changes in structure of the gut and vaginal microbiomes after AB exposure were consistent with the reverse changes in differentially abundant taxa (AB treated vs NoAB treated mice) in each of the microbiomes (Fig 2A and 2B). Namely, almost half of the taxa (48%) found to be enriched (LDA score>2) in the vagina after AB treatment were found to be significantly depleted after the treatment in the gut. In contrast, the taxa depleted in the vagina after the treatment were significantly enriched in the gut. Only one class, Coriobacteriia, showed depletion after the treatment in both the gut and in the vagina. Two families, Prevotellacea and Alcaligenaceae, specifically the Parasutterella genus of the latter family, showed an enrichment after the treatment. This genus has been linked to lack of diversity in gut (28).
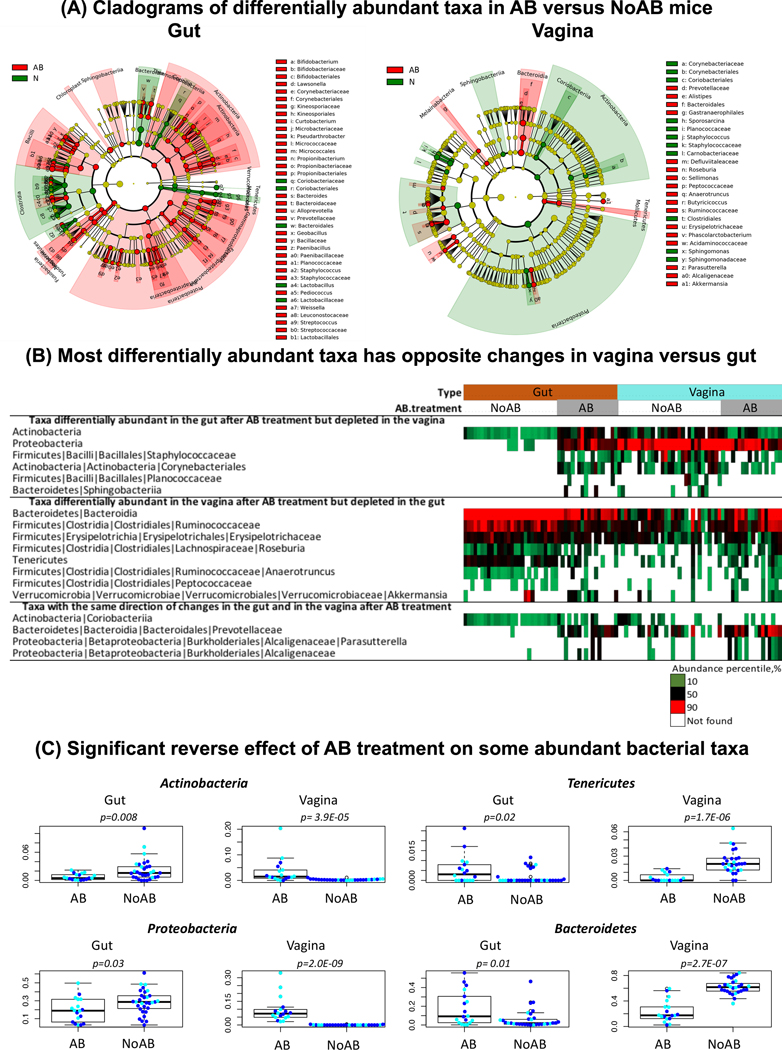
Fig 2.
Differentially abundant taxa identified by LEfSe between AB and NoAB mice in the gut and in the vagina. A, Cladograms of differentially abundant taxa in AB versus NoAB mice. B, most differentially abundant taxa has opposite changes in vagina versus gut. C, Opposite effect of AB treatment on some abundant bacterial taxa in the gut versus vagina.
The Fig. 2B provides a summary of those differentially abundant taxa that show reverse changes in the vagina versus gut after AB treatment. Two phyla (Fig. 2C), Actinobacteria and Proteobacteria show significant enrichment in the gut of AB vs NoAB-treated mice, but significant depletion in their vagina. In contrast, 2 phyla, Tenericutes and Bacteroidetes, were significantly depleted in the gut, but enriched in the vagina. Ruminococcaceae, Erysipelotrichaceae, Roseburia, Anaerotruncus, and Akkermansia were also depleted in the gut of AB vs NoAB mice but enriched in their vagina following antibiotic treatment. Staphylococcaceae and Corynebacteriales show an opposite pattern of enrichment in the gut and depletion in the vagina.
These reverse changes (the gut versus vagina) were confirmed in the validation cohort (Fig. S5). According to LefSe analysis of the cohort, there were 28 taxa significantly enriched in vaginas of AB vs NoAB mice and 5 of the taxa (18%) were significantly depleted in the gut. Only one taxon (3.5%) were also enriched in the gut. Only 2 taxa were depleted in the vagina after AB treatment vs NoAB, although 1 of them showed reverse changes in the gut. Similar to the discovery cohort, phylum Tenericutes was significantly depleted in the gut after AB treatment (Mann-Whitney p-value= 0.004), but enriched in the vagina (Mann-Whitney p-value= 0.001) (Fig. S6). The phylum Bacteroidetes was also significantly depleted in the gut, although no significant changes were observed in vagina. Actinobacteria and Proteobacteria significantly enriched in the gut of AB versus NoAB mice in the discovery cohort, had similar significant changes in the validation cohort, but not in the vagina.
Gut-vaginal microbiomes relationship in AB vs NoAB-treated mice
Considering the reverse effect of the AB treatment on bacterial communities in the gut and in the vagina, we hypothesized that antibiotic treatment may affect associations between local and gut communities. To test this, we created association networks of OTUs in AB and NoAB mice, clustered the network and then visualized it as a heatmap (Fig 3).
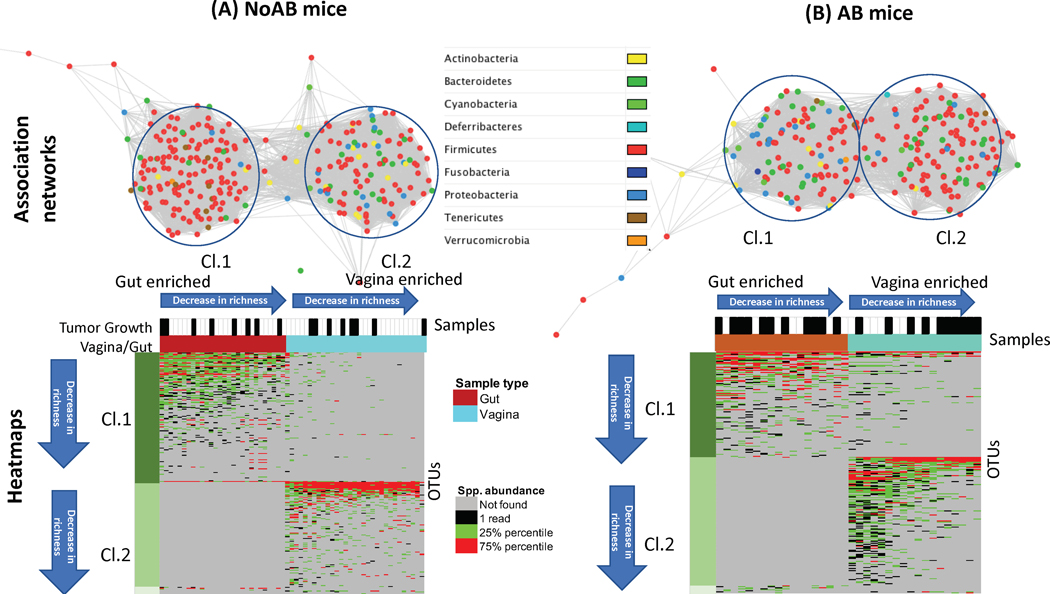
Fig. 3.
Gut-vaginal microbiomes relationship in AB vs NoAB-treated mice. A, Association networks of bacterial species in terms of similarity of co-occurrence profiles of the OTUs in the studied samples and identified clusters of the OTUs. B, Heatmaps of OTU abundances in the gut and in the vagina. Samples in each cluster sorted by species richness, from high to low. OTUs in each cluster sorted by number of samples where OTU is found, from high to low.
The association networks of OTUs (Fig. 3A and 3B) shows that NoAB mice have more species in the gut than in the vagina, while AB mice have significantly more species in the vagina than in the gut. Importantly, gut and vaginal microbiomes are more strongly linked in AB mice and have significantly more associations when compared with NoAB mice. The taxonomic architecture is also affected by AB exposure in both microbiomes.
According to the heatmaps, the gut enriched species (Cl.1) were essentially more often shared with vagina than vagina-enriched species (CL.2) were shared with the gut. Namely, 48% (AB mice) and 45% (NoAB mice) of the gut enriched species were shared with vagina and less than 3% of species enriched in the vagina, were shared with the gut. Interestingly, the AB mice shared the gut species more often than NoAB mice, likely because of the negative effect of AB on the gut environment. Similar observations were made in the validation cohort (Fig. S7).
Vaginal microbiome and tumor development
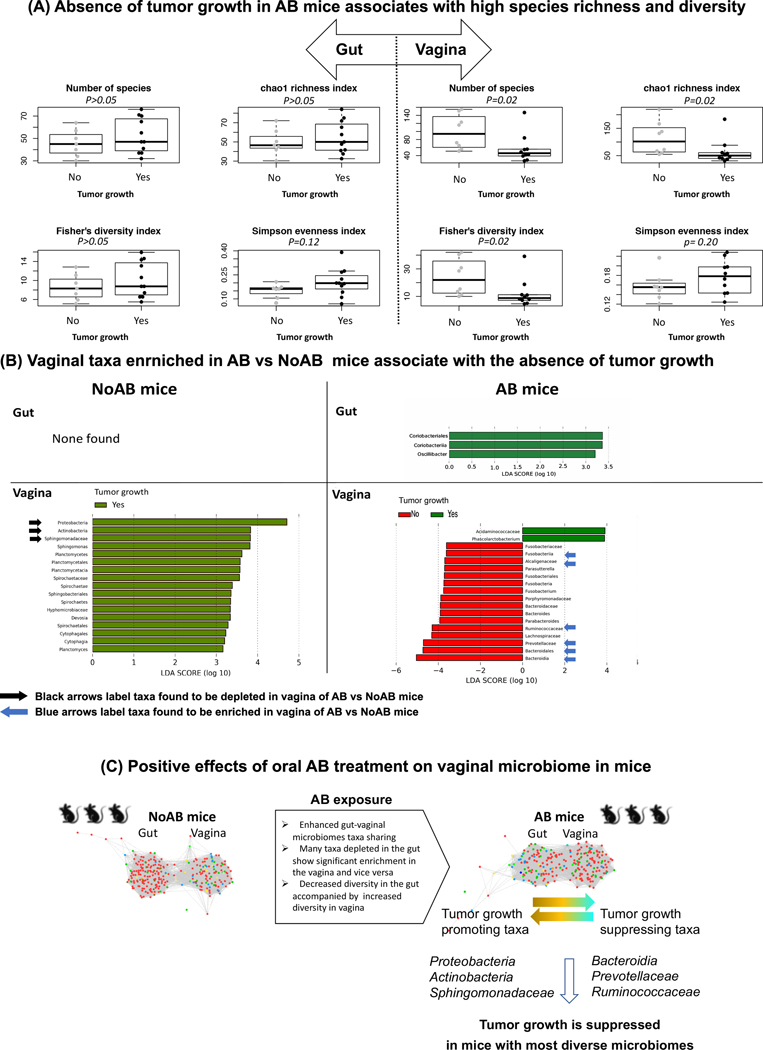
According to the heatmap of species abundances in AB mice (Fig. 3B, Cl.2), 6 AB mice with lowest richness in the vagina developed the tumor, while only 4 out of 12 mice that have greater species richness. This indicates significant association of the high species richness in the vagina of AB mice with the absence of tumor growth (the Fisher’s exact test p-value is 0.01). No association of the tumor growth with the species richness was found in NoAB mice (Fig. 3A).
Because almost all mice in the validation cohort developed tumors, we could not validate the observed association. The validation cohort, however, clearly confirmed changes in relationships between the gut and the vaginal microbiomes (Fig. S7). The AB mice (Fig. S7A) had significantly more associations and sharing between gut and vaginal species than NoAB (Fig. S7B) mice; AB and NoAB mice also had significant differences in taxonomic compositions in the gut and vaginal microbiomes.
Association of the gut and vaginal microbiomes with the tumor growth
We confirmed the association of the tumor growth with the species richness in the AB mice by comparing indexes of diversity in the mice that develop tumor with those mice that don’t develop. We found that not only richness, but also diversity of the vaginal microbiome significantly associated with the tumor growth (Fig. 4A), but the association was found only in AB mice. No associations were detected between the gut microbiome and the tumor growth, either in AB or in NoAB mice (Fig. S8). We also didn’t identify any taxa in the gut of NoAB mice that associate significantly, positively or negatively, with the tumor growth (Fig. 4B). And only 2 taxa, Oscillibacter and Coriobacteriales, negatively associated with the tumor development in AB mice. Essentially more taxa associated with the tumor growth or no growth were found in the vagina. in AB mice, almost all the vaginal taxa associated negatively with the tumor development; the taxa included Fusobacteria, Bacteroidia, such as Prevotellaceae, Parabacteroides, and Bacteroides, as well as Alcaligenaceae, Lachnospiraceae, and Ruminococcaceae. Importantly, 33% of the taxa, such as Bacteroidia, Bacteroidales, Prevotellaceae, Ruminococcaceae, Parasutterella, and Alcaligenaceae were also found to be significantly enriched in vagina after AB exposure, which means that many taxa that increased abundances in vagina after AB treatment are actually tumor suppressing.
Fig. 4.
Association of vaginal microbiomes with the tumor growth. A, Absence of tumor growth in AB mice associates with high species richness and diversity. B, Tumor promoting and tumor suppressive taxa enriched in mice treated and not treated by antibiotics. C, Effects of oral AB treatment on relationships between gut and vaginal microbiomes and cervical cancer development.
Discussion
Results of the study reveal that oral AB exposure of mice can exert a profound effect not only on their gut microbiome, but also on the vaginal microbiome. Although decreased microbial diversity in the gut after AB administration is well documented, it was not shown before that negative changes induced in the gut by AB are accompanied by positive changes in vaginal microbiomes (Table S4). The positive effects include an increase in richness and diversity of species, as well as positive structural taxonomic changes of the vaginal microbiome (Fig. 4C). At the phylum level we observe a significant decrease of Actinobacteria and Proteobacteria in vagina of AB treated mice, although both phyla are increased in their gut. The opposite changes were found for Tenericutes and Bacteroidetes. Proteobacteria is comprised of many known human pathogens associated with intestinal diseases and an inflammatory phenotype; it is emerged as “a possible microbial signature of disease” (29). An association of the phylum with post-menopausal and HIV positive states was also reported in human (30). An increase in Actinobacteria and decline in Bacteroidetes were also linked to dysbiosis and to development of inflammatory bowel disease in human (31).
Importantly, the observed positive changes in the vagina of mice associated with frequency of the cervical cancer development when the mice were administered intravaginally with TC-1 tumor cells. Mice with high microbial diversity in vagina were able to suppress the development of tumor. We find that many taxa that decrease abundance after AB exposure were also tumor suppressive and had significant negative association with the tumor development. Some of these tumor suppressive species, such as Prevotella and Bacteroides, are anaerobic bacteria. In human, the species have been linked to bacterial vaginosis, characterized by decline in number of Lactobacillus spp. and an increase in other anaerobic bacteria (22). The composition of microbiomes in mice, however, is different from human, especially at lower taxonomic levels, and many bacterial genera and species including Lactobacillus spp. that are present in human gut may be even not detected in mice and vice versa (32).
The present study doesn’t identify biological mechanism underlying the reverse changes in species diversity and abundances between the gut and vaginal microbiomes after AB exposure. However, the association networks and heatmaps of OTUs in AB and NoAB mice clearly show an increased sharing of gut taxa with vaginal microbiome after the exposure. One potential mechanism of the sharing can be the movement of bacteria within genital and gastrointestinal tracts of the mice. The movement can be active or with body fluids. Many bacteria are motile and have morphology and apparatus supporting the active swimming (33). Motility is also a known factor promoting active segregation and spatial exclusion of species leading to increased diversity (34). Colonization of mice vagina by species externally from fecal pellets is another potential mechanism. Additional studies are necessary to specify mechanisms for sharing of bacterial cells between the body sites.
Although the validation cohort in the study confirmed the sharing of bacterial taxa and reverse changes in species diversity and abundance between gut and vagina, we were not able to verify effects of the positive changes on the tumor development because most mice in the validation cohort developed tumors. We believe that one of the reasons may be technical. We had increased experience in tumor implantation by the time the validation cohort was treated. The other reason may be biological. Although all mice used in the study were ordered from Jackson Laboratories, the mice for each of the cohorts were ordered at different times; they were not from the same batch. This resulted in the baseline microbiome differences between the discovery and validation cohort. NoAB Mice in the validation cohort had richer microbial community in the gut in terms of number of identified OTUs (Mann-Whitney test p-value = 0.02). It may be a reason why the effect of AB exposure in the validation cohort on the rare species in the gut microbiome was not as pronounced as in the discovery cohort. Indeed, the frequency distribution of species abundances in the gut of mice in the validation cohort was almost the same in AB and NoAB mice, while in the discovery cohort, the distribution revealed a dramatic decrease of rare species after AB exposure. The baseline taxonomic organization of microbiomes was also different between the cohorts. It was more complex in mice of the validation cohort, especially in the vagina (Fig. S7A). The microbiome included a cluster of OTUs enriched with Proteobacteria species. The cluster was comprised of 46% of the species, while the remaining clusters had only from 0 to 6%. Species of Proteobacteria are known by their inflammatory phenotype (29) discussed above, therefore the enrichment might contribute to high rate of the tumor development in the validation cohort. Despite these differences in the baseline microbiome, we identified many commonalities in the response to antibiotics, including increased sharing of gut and vaginal microbiomes in mice treated with antibiotics and shifts in specific organisms.
Additionally, the murine model used in the study may be not perfect in assessing the effect of AB on the tumor development. The immune environment at the tumor initiation stage was not studied using the model. It is not clear how an increase in the immunity resulting from an increase in microbial diversity can be translated into a probability of the tumor development. According to our previous study (35), the TC-1 tumors may be considered as hot because of high infiltration of cytotoxic T-cells. The cells may suppress the tumor development after the growth has been initiated. Another immunosuppressive mechanism may operate at the tumor initiation stage if a mouse has been developed immunity to the HPV tumor antigens. Future studies could more specifically address the role of microbiome in producing mucosal immunity in this model using different approaches.
Results of the study suggest that a strong negative effect of oral AB exposure in mice on the gut microbiome associates with some positive effects on their vaginal microbiome (Fig. 4C). These positive effects are mediated by sharing of species between microbiomes and may contribute to a decreased risk of cervical cancer development after AB exposure in a human population-based case-control study (9). The revealed tight and complex association between the gut and vaginal microbiomes should be taken into consideration when the microbiomes are modulated via probiotics and when antibiotic murine models are used for mechanistic microbiome studies of human cancer (36), particularly in studying mucosal cancer sites other than the gut.
Supplementary Material
Acknowledgments
This work was supported in part by the Radiological Society of North America (RSNA) Resident/Fellow Award (LEC), ASCO Young Investigator Award (LEC), National Institutes of Health (NIH) through MD Anderson’s Cancer Center Support Grant P30CA016672 and The University of Texas MD Anderson Cancer Center HPV-related Cancers Moonshot (LEC, AHK). The human subjects who participated in this study are gratefully acknowledged, in addition to our clinical research team.
Footnotes
Conflict of Interest
None declared
References
- 1.Neuman H, Forsythe P, Uzan A, Avni O, Koren O. Antibiotics in early life: dysbiosis and the damage done. Fems Microbiol Rev 2018;42(4):489–99 doi 10.1093/femsre/fuy018. [DOI] [PubMed] [Google Scholar]
- 2.Zeineldin M, Aldridge B, Lowe J. Antimicrobial Effects on Swine Gastrointestinal Microbiota and Their Accompanying Antibiotic Resistome. Front Microbiol 2019;10 doi ARTN 1035 10.3389/fmicb.2019.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin YX, Wu Y, Zeng ZY, Jin CY, Wu SS, Wang YY, et al. From the Cover: Exposure to Oral Antibiotics Induces Gut Microbiota Dysbiosis Associated with Lipid Metabolism Dysfunction and Low-Grade Inflammation in Mice. Toxicol Sci 2016;154(1):140–52 doi 10.1093/toxsci/kfw150. [DOI] [PubMed] [Google Scholar]
- 4.Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019;8(4) doi ARTN e1568812 10.1080/2162402X.2019.1568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359(6371):97–103 doi 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359(6371):91-+ doi 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 7.Tamim HM, Hanley JA, Hajeer AH, Boivin JF, Collet JP. Risk of breast cancer in relation to antibiotic use. Pharmacoepidem Dr S 2008;17(2):144–50 doi 10.1002/pds.1512. [DOI] [PubMed] [Google Scholar]
- 8.Boursi B, Mamtani R, Haynes K, Yang YX. Recurrent antibiotic exposure may promote cancer formation - Another step in understanding the role of the human microbiota? Eur J Cancer 2015;51(17):2655–64 doi 10.1016/j.ejca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamim HM, Musallam KM, Al Kadri HMF, Boivin JF, Collet JP. Antibiotic use and risk of gynecological cancer. Eur J Obstet Gyn R B 2011;159(2):388–93 doi 10.1016/j.ejogrb.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189(1):12–9 doi Doi . [DOI] [PubMed] [Google Scholar]
- 11.Laniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol 2020. doi 10.1038/s41585-020-0286-z. [DOI] [PMC free article] [PubMed]
- 12.Chase D, Goulder A, Zenhausern F, Monk B, Herbst-Kralovetz M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol Oncol 2015;138(1):190–200 doi 10.1016/j.ygyno.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012;37(1):158–70 doi 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh JE, Kim BC, Chang DH, Kwon M, Lee SY, Kang D, et al. Dysbiosis-induced IL-33 contributes to impaired antiviral immunity in the genital mucosa. Proc Natl Acad Sci U S A 2016;113(6):E762–71 doi 10.1073/pnas.1518589113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thackray LB, Handley SA, Gorman MJ, Poddar S, Bagadia P, Briseno CG, et al. Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. Cell Rep 2018;22(13):3440–53 e6 doi 10.1016/j.celrep.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha MK, Lee DK, An HM, Lee SW, Shin SH, Kwon JH, et al. Antiviral activity of Bifidobacterium adolescentis SPM1005-A on human papillomavirus type 16. Bmc Med 2012;10:72 doi 10.1186/1741-7015-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pourmollaei S, Barzegari A, Farshbaf-Khalili A, Nouri M, Fattahi A, Shahnazi M, et al. Anticancer effect of bacteria on cervical cancer: Molecular aspects and therapeutic implications. Life Sci 2020;246:117413 doi 10.1016/j.lfs.2020.117413. [DOI] [PubMed] [Google Scholar]
- 18.Wiik J, Sengpiel V, Kyrgiou M, Nilsson S, Mitra A, Tanbo T, et al. Cervical microbiota in women with cervical intra-epithelial neoplasia, prior to and after local excisional treatment, a Norwegian cohort study. BMC Womens Health 2019;19(1):30 doi 10.1186/s12905-019-0727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG 2020;127(2):171–80 doi 10.1111/1471-0528.15854. [DOI] [PubMed] [Google Scholar]
- 20.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014;160(Pt 10):2272–82 doi 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasquez A, Jakobsson T, Ahrne S, Forsum U, Molin G. Vaginal lactobacillus flora of healthy Swedish women. J Clin Microbiol 2002;40(8):2746–9 doi 10.1128/jcm.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrientos-Duran A, Fuentes-Lopez A, de Salazar A, Plaza-Diaz J, Garcia F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020;12(2) doi 10.3390/nu12020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity 2012;37(1):158–70 doi 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012;337(6098):1115–9 doi DOI 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh JE, Kim BC, Chang DH, Kwon M, Lee SY, Kang D, et al. Dysbiosis-induced IL-33 contributes to impaired antiviral immunity in the genital mucosa. P Natl Acad Sci USA 2016;113(6):E762–E71 doi 10.1073/pnas.1518589113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karpinets TV, Gopalakrishnan V, Wargo J, Futreal AP, Schadt CW, Zhang JH. Linking Associations of Rare Low-Abundance Species to Their Environments by Association Networks. Front Microbiol 2018;9 doi ARTN 297 10.3389/fmicb.2018.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12(6):R60 doi 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiodini RJ, Dowd SE, Chamberlin WM, Galandiuk S, Davis B, Glassing A. Microbial Population Differentials between Mucosal and Submucosal Intestinal Tissues in Advanced Crohn’s Disease of the Ileum. Plos One 2015;10(7) doi ARTN e0134382 10.1371/journal.pone.0134382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int 2017. doi Artn 9351507 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed]
- 30.Dareng EO, Ma B, Famooto AO, Adebamowo SN, Offiong RA, Olaniyan O, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect 2016;144(1):123–37 doi 10.1017/S0950268815000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toor D, Wasson MK, Kumar P, Karthikeyan G, Kaushik NK, Goel C, et al. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int J Mol Sci 2019;20(10) doi ARTN 2432 10.3390/ijms20102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech 2015;8(1):1–16 doi 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg HC. Motile behavior of bacteria. Phys Today 2000;53(1):24–9 doi Doi 10.1063/1.882934. [DOI] [Google Scholar]
- 34.Gude S, Pince E, Taute KM, Seinen AB, Shimizu TS, Tans SJ. Bacterial coexistence driven by motility and spatial competition. Nature 2020;578(7796):588-+ doi 10.1038/s41586-020-2033-2. [DOI] [PubMed] [Google Scholar]
- 35.Bartkowiak T, Singh S, Yang GJ, Galvan G, Haria D, Ai M, et al. Unique potential of 4–1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. P Natl Acad Sci USA 2015;112(38):E5290–E9 doi 10.1073/pnas.1514418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy EA, King KY, Baldridge MT. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front Physiol 2018;9:1534 doi 10.3389/fphys.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.