Abstract
Objective
To compare the long term efficacy and safety of rosuvastatin with atorvastatin treatment in adults with coronary artery disease.
Design
Randomised, open label, multicentre trial.
Setting
12 hospitals in South Korea, September 2016 to November 2019.
Participants
4400 adults (age ≥19 years) with coronary artery disease.
Interventions
Participants were assigned to receive either rosuvastatin (n=2204) or atorvastatin (n=2196) using 2×2 factorial randomisation.
Main outcome measures
The primary outcome was a three year composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation. Secondary outcomes were safety endpoints: new onset diabetes mellitus; hospital admissions due to heart failure; deep vein thrombosis or pulmonary thromboembolism; endovascular revascularisation for peripheral artery disease; aortic intervention or surgery; end stage kidney disease; discontinuation of study drugs owing to intolerance; cataract surgery; and a composite of laboratory detected abnormalities.
Results
4341 of the 4400 participants (98.7%) completed the trial. Mean daily dose of study drugs was 17.1 mg (standard deviation (SD) 5.2 mg) in the rosuvastatin group and 36.0 (12.8) mg in the atorvastatin group at three years (P<0.001). The primary outcome occurred in 189 participants (8.7%) in the rosuvastatin group and 178 (8.2%) in the atorvastatin group (hazard ratio 1.06, 95% confidence interval 0.86 to 1.30; P=0.58). The mean low density lipoprotein (LDL) cholesterol level during treatment was 1.8 mmol/L (SD 0.5 mmol/L) in the rosuvastatin group and 1.9 (0.5) mmol/L in the atorvastatin group (P<0.001). The rosuvastatin group had a higher incidence of new onset diabetes mellitus requiring initiation of antidiabetics (7.2% v 5.3%; hazard ratio 1.39, 95% confidence interval 1.03 to 1.87; P=0.03) and cataract surgery (2.5% v 1.5%; 1.66, 1.07 to 2.58; P=0.02). Other safety endpoints did not differ between the two groups.
Conclusions
In adults with coronary artery disease, rosuvastatin and atorvastatin showed comparable efficacy for the composite outcome of all cause death, myocardial infarction, stroke, or any coronary revascularisation at three years. Rosuvastatin was associated with lower LDL cholesterol levels but a higher risk of new onset diabetes mellitus requiring antidiabetics and cataract surgery compared with atorvastatin.
Trial registration
ClinicalTrials.gov NCT02579499.
Introduction
Intensive reduction in low density lipoprotein (LDL) cholesterol levels is recommended in people with coronary artery disease, who are regarded as being at high risk or very high risk of future atherosclerotic cardiovascular events.1 2 Among the various lipid lowering drugs available, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) are the cornerstone of treatment, and high intensity statins are generally the choice for LDL cholesterol lowering treatment in people with coronary artery disease.1 2 Doctors make decisions not only about statin intensity (high, moderate, or low) but also about statin type; however, although previous studies have evaluated clinical outcomes according to different intensities of statins for managing dyslipidaemia in people with coronary artery disease, clinical trials have not sufficiently evaluated the effects of different types of statins.4 5 6 Furthermore, few randomised clinical trials have directly compared the long term clinical outcomes of the two most potent statins—rosuvastatin and atorvastatin—in people with coronary artery disease.
In addition to statins’ efficacy in reducing LDL cholesterol levels and the risk of future adverse cardiovascular events, safety concerns, including statin related adverse effects and intolerance, should also be considered in real world practice.7 8 9 10 Statin associated muscle symptoms and other concerning statin related adverse effects on glucose homeostasis or hepatic or renal function are more common with high potency statins than with low potency statins.7 8 9 11 However, although various statin related adverse effects have been reported, it is not clear whether the adverse effects are due to the drug itself or to drug class effects.7 8 9 We therefore conducted the LODESTAR (Low-Density Lipoprotein Cholesterol-Targeting Statin Therapy Versus Intensity-Based Statin Therapy in Patients With Coronary Artery Disease) trial, a multicentre, randomised trial for the management of dyslipidaemia in people with coronary artery disease. This secondary analysis of the LODESTAR trial focused on the efficacy and safety of rosuvastatin versus atorvastatin treatment over three years in people with coronary artery disease.
Methods
Study design and population
The LODESTAR trial, conducted at 12 centres in South Korea, was an investigator initiated, prospective, multicentre, randomised, open label trial using 2×2 factorial randomisation.3 The trial evaluated statin intensity strategy and statin type for managing dyslipidaemia in adults (age ≥19 years) with coronary artery disease. The study design, protocol, and rationale for the LODESTAR trial are described in detail elsewhere.3 Adults with clinically diagnosed coronary artery disease, including stable ischaemic heart disease and acute coronary syndrome (unstable angina and acute myocardial infarction), who required statin treatment to lower their LDL cholesterol levels were eligible to participate in the trial.3 Supplementary table S1 shows the inclusion and exclusion criteria. All participants provided written informed consent before participation in the trial. Study coordination, data management, and site management were performed at the Cardiovascular Research Centre (Seoul, South Korea). Those designated to monitor the trial reviewed the data twice a year for accuracy and completeness and ensured adherence to the protocol. A data and safety monitoring board of independent doctors oversaw the safety of the study. These doctors acted in an advisory capability to check on safety of the participants, evaluate study progress, and review the study process.
Randomisation
Participants were assigned to treatments using a 2×2 factorial randomisation. The factors were statin intensity strategy strategy versus high intensity statin strategy) and statin type (rosuvastatin versus atorvastatin).3 Eligible participants underwent randomisation using an interactive web response permuted block randomisation procedure (mixed blocks of 4 or 6) at each participating site, stratified by baseline LDL cholesterol levels of 2.6 mmol/L, acute coronary syndrome, and the presence of diabetes mellitus.3 Participants were randomly assigned to receive a statin using either a treat-to-target strategy or a high intensity statin strategy; participants were also randomly assigned to receive either rosuvastatin or atorvastatin.3 The investigators and participants were blinded to the randomisation sequence. The results of the analysis of the treat-to-target strategy using titrated intensity statin treatment to reach a target LDL cholesterol level of 1.3-1.8 mmol/L versus high intensity statin strategy without a target goal were recently reported.3
Study procedures
Adherence to the assigned statin type (rosuvastatin or atorvastatin) was strongly recommended during the entire follow-up period. The intensity of statin treatment was classified on the basis of the 2013 American College of Cardiology/American Heart Association guidelines on the management of dyslipidaemia.3 12 In each statin type group, the intensity of statin was titrated or maintained following the assigned statin intensity strategy, and the principles for titration or maintenance were identical for both groups.3 Briefly, in the group assigned to receive the treat-to-target strategy, statin naïve participants were started on moderate intensity statin treatment (rosuvastatin 10 mg or atorvastatin 20 mg), and those already using a statin received a corresponding intensity of rosuvastatin or atorvastatin based on their LDL cholesterol levels at randomisation (equivalent intensity for those with LDL cholesterol levels <1.8 mmol/L or an up-titrated intensity for those with LDL cholesterol levels ≥1.8 mmol/L).3 During follow-up, we titrated statin intensity based on the obtained LDL cholesterol levels: up-titration for those whose LDL cholesterol levels were ≥1.8 mmol/L, maintenance of the same intensity without titration for those whose LDL cholesterol levels were ≥1.3 mmol/L and <1.8 mmol/L, and down-titration for those whose LDL cholesterol levels were <1.3 mmol/L.3 For participants assigned to receive the high intensity statin strategy, high intensity statin treatment (rosuvastatin 20 mg or atorvastatin 40 mg) was initiated and maintained irrespective of patients’ LDL cholesterol levels at randomisation and follow-up.3 Adding non-statin agents, such as the cholesterol absorption inhibitor ezetimibe, was strongly not recommended to focus on data for statin treatment and to prevent confounding.3 Data on the use of the study drugs were collected from doctors’ records of prescriptions, and drug adherence was measured by participants’ self-reported pill count.3 For other medical treatments, guideline directed treatment was strongly recommended, and modification of risk factors, including blood pressure or glucose control, weight reduction, exercise, dietary changes, and smoking cessation, was also encouraged.3
Follow-up visits to assess general health status, use of study drugs, and the occurrence of study outcomes or adverse events took place at six weeks and 3, 6, 12, 24, and 36 months after study initiation.3 To confirm the obtained LDL cholesterol levels and monitor statin related adverse effects, serial follow-up of patients’ lipid profiles (total cholesterol, LDL cholesterol, high density lipoprotein cholesterol, and triglyceride levels), aspartate aminotransferase, alanine aminotransferase, creatine kinase, and creatinine levels were performed at six weeks and 12, 24, and 36 months.3 Serial follow-up of plasma glucose and haemoglobin A1c levels was carried out at 12, 24, and 36 months.3
Study outcomes and definitions
The primary outcome was major adverse cardiac and cerebrovascular events, defined as a composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation within three years.3 Death was classified as cardiovascular death and non-cardiovascular death. Cardiovascular death was defined as death from myocardial infarction, heart failure, stroke, cardiovascular procedures or haemorrhage, sudden cardiac death, and any case of death in which a cardiovascular cause could not be excluded, as adjudicated by a clinical endpoints committee.13 Myocardial infarction was defined on the basis of symptoms, changes on electrocardiography, or abnormal findings on imaging studies, combined with an increase in the creatine kinase myocardial band fraction above the upper limit of normal or an increase in troponin T or troponin I level >99th centile of the upper limit of normal.14 Stroke was defined as an acute cerebrovascular event resulting in a neurological deficit at >24 hours or the presence of acute infarction noted by imaging studies.15 Any coronary revascularisation included percutaneous coronary intervention and coronary artery bypass graft surgery, and clinically indicated revascularisation was defined as a diameter stenosis ≥50% on invasive coronary angiography with ischaemic symptoms or signs, or as a percentage diameter stenosis ≥70% even in the absence of symptoms or signs.13 Staged coronary revascularisations planned at randomisation were not considered as adverse events.3
The secondary outcomes were new onset diabetes mellitus; hospital admissions due to heart failure; deep vein thrombosis or pulmonary thromboembolism; endovascular revascularisation for peripheral artery disease; aortic intervention or surgery; end stage kidney disease; discontinuation of study drugs owing to intolerance; cataract surgery; and a composite of laboratory detected abnormalities.3 New onset diabetes mellitus was defined as a fasting plasma glucose level ≥7.0 mmol/L or new initiation of antidiabetics.11 16 A post hoc analysis of the trial database was performed to identify and include participants with a haemoglobin A1c level ≥6.5% during the study period as having new onset diabetes mellitus.16 17 The supplementary methods section provides definitions of the other secondary outcomes. An independent clinical endpoint committee blinded to the treatment assignments and primary results of the trial adjudicated both the primary and the secondary outcomes.3
Statistical analysis
The sample size estimation for the LODESTAR trial was performed on the basis of the primary objective of the study: to compare the treat-to-target strategy with the high intensity statin strategy for the occurrence of the primary outcome, a composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation within three years.3 Additional details about the sample size estimation are published elsewhere.3 The sample size estimation was not performed for comparing the different types of statin. This study focused on the clinical outcomes at three years between rosuvastatin and atorvastatin treatment, and the analysis of this study was performed using an intention-to-treat approach, with all participants randomly assigned to a treatment group. Sensitivity analyses were performed in the per protocol population after excluding participants who did not receive the assigned treatment (participants who discontinued statin treatment or those who did not receive the assigned statin type).
Categorical variables are reported as number (percentage), and continuous variables are reported as mean (standard deviation (SD) or median (interquartile range)), depending on distribution. Time-to-event curves were plotted using a Kaplan-Meier survival analysis from the time of randomisation to the occurrence of the first event of interest during follow-up, and the event rates between the two groups were compared using log rank tests. Hazard ratios with 95% confidence intervals were estimated using a Cox regression analysis. To assess whether treatment effects (rosuvastatin versus atorvastatin) differed according to statin intensity strategy (treat-to-target strategy versus high intensity statin strategy), P values for interaction between statin type and statin intensity strategy were calculated using Cox proportional hazard regression model. Additional subgroup analyses were performed according to age, sex, body mass index, presence of diabetes mellitus, hypertension, chronic kidney disease, clinical presentation at randomisation, previous percutaneous coronary intervention, use of ezetimibe before randomisation, and baseline LDL cholesterol levels. To evaluate the association between new onset diabetes mellitus as a time dependent variable and the primary outcome, a time dependent Cox regression analysis was performed. No imputation was used to infer missing values, and those with missing data for primary or secondary outcomes were censored at the time of withdrawal of consent or loss to follow-up. All tests were two sided, and P<0.05 was considered to indicate statistical significance, with no adjustment for multiple comparisons. Statistical analyses were performed using IBM SPSS, version 25.0 (IBM, Chicago, IL) and R 3.5.3 software (R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
Although our study dealt with an important area for public health and was of interest to patients given the high percentage of people worldwide who take statins, patient and public involvement and training could not be managed for this study without additional funding, particularly as the training would have needed to be done across the 12 sites and coordinated. As a result, no patients or members of the public were directly involved in setting the research question or developing plans for the design or implementation of the study or in the interpretation or writing up of the results.
Results
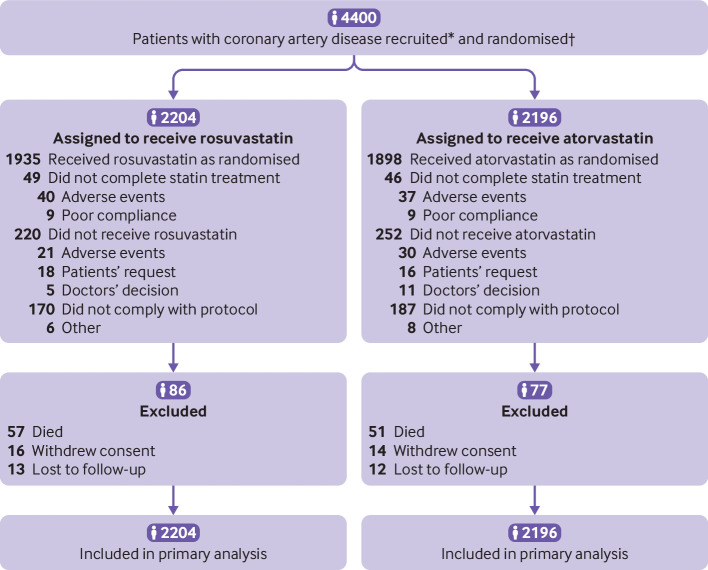
Between September 2016 and November 2019, 4400 adults with coronary artery disease were randomly assigned to receive either rosuvastatin (n=2204) or atorvastatin (n=2196) (fig 1). Supplementary table S2 lists the reasons for withdrawal of consent and death. The baseline characteristics of the participants were well balanced between the two groups (table 1). Mean age was 65 years (SD 10 years) and overall 27.9% were women, 33.4% had diabetes mellitus, 55.8% had undergone percutaneous coronary intervention, and 74.3% had received their initial diagnosis or coronary revascularisation more than one year previously. In the rosuvastatin group, 93.9% of patients were taking the assigned statin type at six weeks, 93.3% at three months, 93.4% at six months, 93.4% at one year, 92.0% at two years, and 91.1% at three years; the corresponding rates in the atorvastatin group were 93.8%, 92.6%, 92.5%, 92.5%, 91.3%, and 89.5%, respectively (see supplementary table S3). Although the use of a high intensity statin did not differ between the two groups at six weeks, three and six months, or one year, it was lower in the rosuvastatin group than atorvastatin group at two years (71.9% v 74.7%; P=0.04) and three years (70.9% v 74.0%; P=0.02) (see supplementary tables S3 and S4). The mean daily dose at three years was 17.1 mg (SD 5.2 mg) in the rosuvastatin group and 36.0 (12.8) mg in the atorvastatin group (P<0.001). The use of ezetimibe was lower in the rosuvastatin group than atorvastatin group from three months (all P<0.05) (see supplementary tables S3 and S5). Supplementary table S6 presents the use of other cardiovascular drugs.
Fig 1.
Flow of participants through study. *Data on screening were not collected. †Randomisation was stratified by baseline low density lipoprotein cholesterol levels ≥2.6 mmol/L, acute coronary syndrome, and presence of diabetes mellitus
Table 1.
Baseline characteristics. Values are number (percentage) unless stated otherwise
| Characteristics | Rosuvastatin group (n=2204) | Atorvastatin group (n=2196) |
|---|---|---|
| Mean (SD) age (years) | 65 (10) | 65 (10) |
| Women | 602 (27.3) | 626 (28.5) |
| Mean (SD) weight (kg) | 67 (11) | 67 (10) |
| Mean (SD) height (cm) | 164 (8) | 165 (8) |
| Mean (SD) body mass index | 24.8 (3.0) | 24.7 (2.8) |
| Current smoker | 291 (13.2) | 312 (14.2) |
| Comorbidities | ||
| Hypertension | 1498 (68.0) | 1439 (65.5) |
| Diabetes mellitus | 725 (32.9) | 743 (33.8) |
| Diabetes mellitus: insulin treatment | 83 (3.8) | 79 (3.6) |
| Chronic kidney disease* | 149 (6.8) | 170 (7.7) |
| End stage kidney disease: receiving dialysis | 14 (0.6) | 15 (0.7) |
| Mean (SD) eGFR (mL/min/1.73 m2) | 88.1 (17.4) | 87.9 (17.9) |
| Medical history | ||
| Percutaneous coronary intervention | 1258 (57.1) | 1199 (54.6) |
| Coronary artery bypass grafting surgery | 167 (7.6) | 167 (7.6) |
| Stroke | 140 (6.4) | 123 (5.6) |
| Clinical presentation at randomisation: | ||
| Acute myocardial infarction <1 year | 175 (7.9) | 163 (7.4) |
| Unstable angina or revascularisation <1 year | 404 (18.3) | 384 (17.5) |
| Myocardial infarction >1 year ago | 322 (14.6) | 353 (16.1) |
| Unstable angina or revascularisation >1 year ago | 906 (41.1) | 878 (40.0) |
| Detection of asymptomatic CAD at screening | 397 (18.0) | 418 (19.0) |
| Lipid lowering treatment before randomisation | ||
| Statin intensity: | ||
| High | 533 (24.2) | 572 (26.0) |
| Moderate | 1277 (57.9) | 1247 (56.8) |
| Low | 43 (2.0) | 50 (2.3) |
| None | 351 (15.9) | 327 (14.9) |
| Ezetimibe | 259 (11.8) | 220 (10.0) |
| Mean (SD) lipids (mmol/L) | ||
| LDL cholesterol | 2.2 (0.9) | 2.3 (0.8) |
| HDL cholesterol | 1.2 (0.3) | 1.2 (0.3) |
| Total cholesterol | 4.1 (1.0) | 4.1 (0.9) |
| Triglycerides | 1.6 (0.9) | 1.6 (0.9) |
CAD=coronary artery disease; eGFR=estimated glomerular filtration rate; HDL=high density lipoprotein; LDL=low density lipoprotein; SD=standard deviation.
Defined as eGFR <60 mL/min/1.73 m2 of body surface area.
Clinical efficacy and LDL cholesterol levels
The median follow-up duration was 3 years (interquartile range 3-3 years), and 4341 participants (98.7%) completed the clinical follow-up at three years (table 2). The primary outcome occurred in 189 participants (8.7%) in the rosuvastatin group and 178 (8.2%) in the atorvastatin group (hazard ratio 1.06, 95% confidence interval 0.86 to 1.30; P=0.58) (fig 2). All cause death occurred in 57 participants (2.6%) in the rosuvastatin group and 51 (2.3%) in the atorvastatin group (1.12, 0.77 to 1.63; P=0.57) (table 2). Myocardial infarction was observed in 34 (1.5%) and 26 (1.2%) participants, respectively (1.27, 0.76 to 2.12; P=0.37). The occurrence of stroke did not differ between the two groups (1.1% v 0.9%; 1.20, 0.66 to 2.17; P=0.55). Any coronary revascularisation occurred in 115 (5.3%) and 111 (5.2%) participants, respectively (1.03, 0.80 to 1.34; P=0.81). These findings were consistent in the per protocol population (see supplementary table S7).
Table 2.
Primary and secondary outcomes at three years in adults assigned to receive rosuvastatin or atorvastatin.* Values are number (percentage) unless stated otherwise
| Outcome | Rosuvastatin group (n=2204) | Atorvastatin group (n=2196) | Absolute difference (95% CI) | Hazard ratio (95% CI) | P value† |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Death, myocardial infarction, stroke, or any coronary revascularisation | 189 (8.7) | 178 (8.2) | 0.5 (−1.2 to 2.1) | 1.06 (0.86 to 1.30) | 0.58 |
| Components of primary outcome | |||||
| Death: | 57 (2.6) | 51 (2.3) | 0.3 (−0.7 to 1.2) | 1.12 (0.77 to 1.63) | 0.57 |
| Cardiac death (No) | 14 | 15 | |||
| Myocardial infarction | 34 (1.5) | 26 (1.2) | 0.3 (−0.4 to 1.0) | 1.27 (0.76 to 2.12) | 0.37 |
| Stroke: | 24 (1.1) | 20 (0.9) | 0.2 (−0.4 to 0.8) | 1.20 (0.66 to 2.17) | 0.55 |
| Ischaemic (No) | 16 | 16 | |||
| Haemorrhagic (No) | 8 | 4 | |||
| Coronary revascularisation‡ | 115 (5.3) | 111 (5.2) | 0.2 (−1.2 to 1.5) | 1.03 (0.80 to 1.34) | 0.81 |
| Secondary outcomes | |||||
| New onset diabetes mellitus | 152 (7.1) | 119 (5.5) | 1.5 (0.1 to 3.0) | 1.29 (1.01 to 1.63) | 0.04 |
| New onset diabetes mellitus among participants without diabetes mellitus at baseline § | 152/1479 (10.4) | 119/1453 (8.4) | 2.1 (−0.0 to 4.2) | 1.26 (0.99 to 1.60) | 0.06 |
| Initiation of antidiabetics among participants without diabetes mellitus at baseline § | 104/1479 (7.2) | 74/1453 (5.3) | 2.0 (0.2 to 3.7) | 1.39 (1.03 to 1.87) | 0.03 |
| Hospital admission due to heart failure | 12 (0.6) | 8 (0.4) | 0.2 (−0.2 to 0.6) | 1.50 (0.61 to 3.66) | 0.37 |
| Deep vein thrombosis or pulmonary embolism: | 7 (0.3) | 2 (0.1) | 0.2 (−0.0 to 0.5) | 3.50 (0.73 to 16.84) | 0.10 |
| Deep vein thrombosis (No) | 5 | 2 | |||
| Pulmonary embolism (No) | 3 | 0 | |||
| Peripheral artery revascularisation | 12 (0.5) | 17 (0.8) | −0.3 (−0.8 to 0.2) | 0.65 (0.30 to 1.38) | 0.25 |
| Aortic intervention or surgery: | 3 (0.1) | 2 (0.1) | 0.0 (−0.2 to 0.3) | 1.50 (0.25 to 8.94) | 0.66 |
| Endovascular treatment (No) | 3 | 0 | |||
| Surgical treatment (No) | 0 | 2 | |||
| End stage kidney disease | 9 (0.4) | 4 (0.2) | 0.2 (−0.1 to 0.6) | 2.25 (0.69 to 7.30) | 0.17 |
| Discontinuation of statin treatment | 40 (1.8) | 37 (1.7) | 0.1 (−0.7 to 0.9) | 1.08 (0.69 to 1.69) | 0.74 |
| Cataract surgery | 53 (2.5) | 32 (1.5) | 1.0 (1.4 to 1.8) | 1.66 (1.07 to 2.58) | 0.02 |
| Composite of laboratory detected abnormalities¶: | 26 (1.2) | 22 (1.0) | 0.2 (−0.4 to 0.8) | 1.24 (0.70 to 2.20) | 0.47 |
| Increase in aminotransferase (No) | 10 | 10 | |||
| Increase in creatine kinase (No) | 5 | 6 | |||
| Increase in creatinine (No) | 11 | 7 |
CI=confidence interval.
Primary and secondary outcomes were evaluated in the intention-to-treat population three years after randomisation. The listed percentages were estimated using the Kaplan-Meier method, so values might not calculate mathematically.
Calculated using log rank test.
All coronary revascularisations were clinically indicated by a diameter stenosis ≥50% on invasive coronary angiography with ischaemic symptoms or signs or ≥70% even in the absence of symptoms or signs.
Data are number of patients/total number of patients (%).
An increase in aminotransferase level was defined as more than baseline level and >3 times the upper reference limit; an increase in creatine kinase level was defined as more than baseline level and >5 times the upper reference limit; and an increase in creatinine level was defined as >50% increase from baseline and greater than the upper reference limit.
Fig 2.
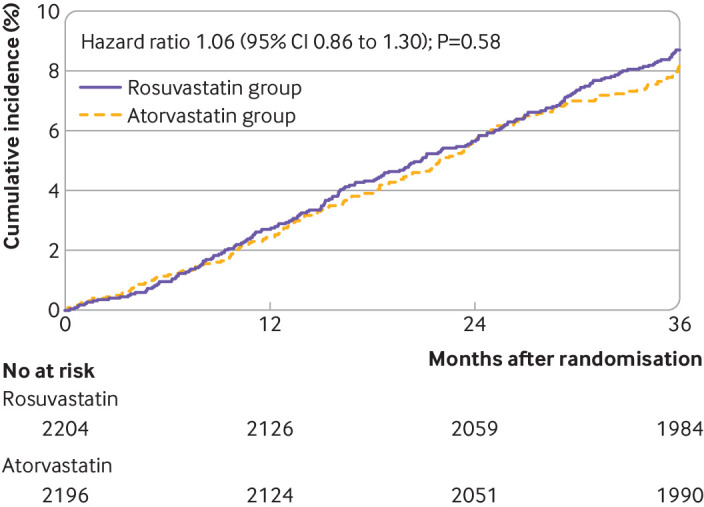
Kaplan-Meier survival (time-to-event) curves for primary outcome (all cause death, myocardial infarction, stroke, or any coronary revascularisation) in adults assigned to rosuvastatin or atorvastatin. CI=confidence interval
Figure 3 shows the serial changes in LDL cholesterol levels during the study period (also see supplementary table S8). The mean LDL cholesterol level during the overall study period was 1.8 mmol/L (SD 0.5 mmol/L) in the rosuvastatin group and 1.9 (0.5) mmol/L in the atorvastatin group (P<0.001). The mean LDL cholesterol levels were consistently lower in the rosuvastatin group than atorvastatin group (1.7 v 1.8 mmol/L at six weeks, three months, six months, one year, two years, and three years; all P<0.001) (fig 3). The proportion of participants with LDL cholesterol levels <1.8 mmol/L was also consistently higher in the rosuvastatin group than atorvastatin group: at six weeks (62.9% v 54.6%; P<0.001), three months (66.7% v 58.8%; P=0.02), six months (64.3% v 53.1%; P<0.001), one year (61.5% v 53.1%; P<0.001), two years (64.0% v 57.2%; P<0.001), and three years (62.5% v 55.2%; P<0.001) (see supplementary fig S1). Supplementary table S8 presents serial changes in the other lipid profiles.
Fig 3.
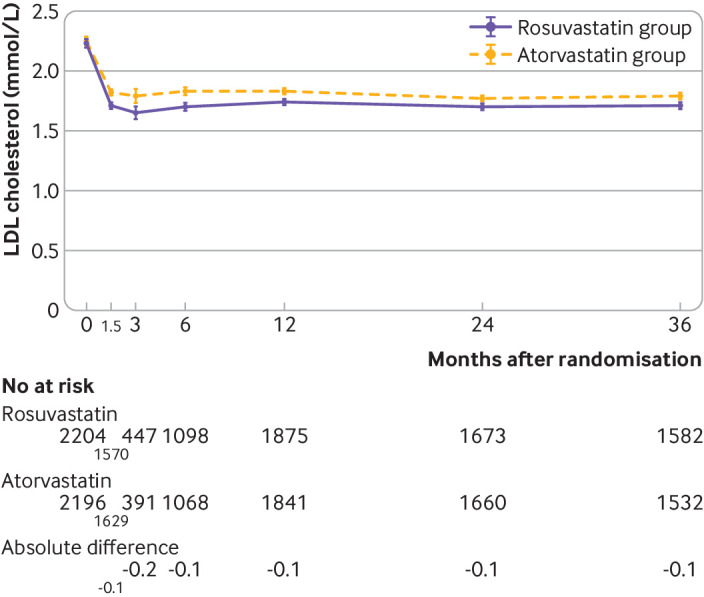
Serial mean values for LDL cholesterol over time in participants assigned to rosuvastatin or atorvastatin. The whiskers indicate 95% confidence intervals. The absolute difference (mmol/L) in LDL cholesterol levels between the two groups is presented under the graph. All differences were significant (P<0.001). LDL=low density lipoprotein
Clinical safety
More participants in the rosuvastatin group than atorvastatin group developed new onset diabetes mellitus (7.1% v 5.5%; hazard ratio 1.29, 95% confidence interval 1.01 to 1.63; P=0.04) and underwent cataract surgery (2.5% v 1.5%; 1.66, 1.07 to 2.58; P=0.02) (table 2 and supplementary fig S2). Among participants without diabetes mellitus at baseline, the rosuvastatin group had a higher incidence of new onset diabetes mellitus requiring initiation of antidiabetics (7.2% v 5.3%; 1.39, 1.03 to 1.87; P=0.03) and a trend towards a higher incidence of new onset diabetes mellitus (10.4% v 8.4%; 1.26, 0.99 to 1.60; P=0.06) (table 2). The other secondary outcomes did not differ between the two groups. These findings were consistent in the per protocol population (see supplementary table S7). Supplementary table S9 lists the reasons for discontinuation of statin treatment.
Additional analyses
In a post hoc analysis using a definition of new onset diabetes mellitus that incorporated a haemoglobin A1c level ≥6.5% during the study period, the incidence of new onset diabetes mellitus was still higher in the rosuvastatin group than atorvastatin group (9.5% v 7.7%; 1.25, 1.02 to 1.53; P=0.03).
No significant interaction occurred between statin type and statin intensity strategy for the primary outcome (P=0.77 for interaction) (supplementary table S10). Supplementary fig S3 shows the results of the subgroup analyses for the primary outcome. The effect of rosuvastatin treatment versus atorvastatin treatment was consistent for the primary outcome across all subgroups.
New onset diabetes mellitus as a time dependent variable was not associated with increased risk of the primary outcome (hazard ratio 1.16, 95% confidence interval 0.51 to 2.64; P=0.73) and no significant interaction occurred between statin type and new onset diabetes mellitus for the primary outcome (P=0.08 for interaction).
Discussion
The main findings of this secondary analysis of the randomised LODESTAR trial comparing clinical outcomes over three years between rosuvastatin and atorvastatin treatment in adults with coronary artery disease were that the risk of a three year composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation did not differ between the two groups; rosuvastatin treatment resulted in lower LDL cholesterol levels and a higher proportion of participants achieving LDL cholesterol levels <1.8 mmol/L throughout the study period, compared with atorvastatin treatment; and rosuvastatin treatment was associated with a higher incidence of new onset diabetes mellitus requiring initiation of antidiabetics and cataract surgery than atorvastatin treatment.
In clinical practice, appropriate decisions for statin type as well as statin intensity are important—however, only rosuvastatin and atorvastatin can offer both the high intensity and moderate intensity statin treatment usually required by people with coronary artery disease to intensively lower their LDL cholesterol levels.1 2 3 12 The clinical benefits of using either of these two potent statins in people with coronary artery disease have been shown in previous studies.4 5 18 However, to our knowledge, only the SATURN (Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin) trial directly compared the effects of rosuvastatin and atorvastatin treatment in people with coronary artery disease.19 Among people with coronary artery disease who were randomised to either rosuvastatin 40 mg or atorvastatin 80 mg in that study, the primary outcome—the change in intravascular ultrasound defined percentage atheroma volume at 104 weeks—did not differ between the two groups (−0.99% v −1.22%, P=0.17).19 In the secondary outcomes of that study, the occurrence of a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, arterial revascularisation, or admission to hospital for unstable angina did not differ between the groups (7.5% v 7.1%), although the rosuvastatin group had lower LDL cholesterol levels than the atorvastatin group (1.6 v 1.8 mmol/L; P<0.001).19 However, the SATURN trial primarily evaluated the effects of the highest doses of rosuvastatin and atorvastatin on the progression of coronary atherosclerosis by means of intravascular ultrasonography, rather than clinical outcomes, and it included fewer participants (n=1039) and a shorter follow-up time (two years) than the current study. Our randomised study, however, compared the effects of rosuvastatin and atorvastatin treatment in terms of a composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation in 4400 patients with coronary artery disease during three years of follow-up. The results show that rosuvastatin was associated with greater efficacy in reducing LDL cholesterol levels throughout the study period, which is in line with a previous meta-analysis showing the superiority of rosuvastatin over atorvastatin in lowering LDL cholesterol levels.20 This difference in the LDL cholesterol lowering capacity might have contributed to the higher use of a high intensity statin (from two years) and ezetimibe (from three months) in the atorvastatin group. Although the difference between these two potent statins is unclear, factors such as their bonding capacity to HMG-CoA reductase and plasma half-life might have contributed to the difference in LDL cholesterol lowering capacity.21 22 23 Although both rosuvastatin and atorvastatin have greater bonding capacity to HMG-CoA reductase than other statin types, rosuvastatin has the greatest bonding interaction with HMG-CoA reductase.21 22 23 In addition, rosuvastatin has a longer plasma half-life than atorvastatin (19 hours v 15 hours).23 Nevertheless, in this study, the pronounced reduction in LDL cholesterol levels with rosuvastatin did not translate into incremental benefit in reducing three year composite outcomes, as in the SATURN trial.19 In fact, in both trials, the rate of composite clinical outcomes was numerically higher in the rosuvastatin group than atorvastatin group.19 That finding could be due to the low between group difference in reduction of LDL cholesterol levels, as well as the difference in pharmacological properties between the two statins. Whereas lipophilic statins such as atorvastatin can cross cellular membranes through passive diffusion and are therefore widely distributed in different tissues, hydrophilic statins such as rosuvastatin are more liver selective owing to the active carrier mediated uptake mechanism, and thus they are more limited in their ability to have additional effects beyond cholesterol lowering (pleiotropic effects) in extrahepatic tissues.23 24 In addition, the atorvastatin group’s higher use of ezetimibe, which can not only reduce LDL cholesterol levels but also inhibit platelet aggregation and activation, reduce oxidative stress, and accelerate plaque regression, could be another explanation of our findings.25 26 Further study is, however, required before any causative effect can be established or rebutted.
Although reducing LDL cholesterol levels and the risk for future adverse cardiovascular events is the primary aim of statin treatment in people with coronary artery disease, safety is also a major concern for long term statin treatment.7 8 9 JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) was the first randomised trial to report an increase in new onset diabetes mellitus among participants receiving statin treatment.27 Among participants who were randomised to either rosuvastatin 20 mg or placebo, a 0.6% higher incidence of new onset diabetes mellitus was noted in those receiving rosuvastatin.27 This finding was also confirmed in a meta-analysis, which showed that statin treatment was associated with a 9% increased risk of new onset diabetes mellitus.28 Whether statin related new onset diabetes mellitus is a drug or a drug class effect remains controversial, however, and no head-to-head comparisons between the two most potent statins (rosuvastatin and atorvastatin) regarding new onset diabetes mellitus have been conducted previously. In this study, a higher incidence of new onset diabetes mellitus was shown for rosuvastatin than for atorvastatin. Even though the mechanisms of statin treatment and new onset diabetes mellitus are not yet fully understood, a meta-analysis of genetic data from 223 463 individuals showed that the association could be related to the lowered activity of HMG-CoA reductase, the target of statin treatment.7 9 29 Two single nucleotide polymorphisms (rs17238484-G and rs12916-T) in the HMG-CoA reductase gene reduced LDL cholesterol levels by 0.1 mmol/L and increased the risk of new onset diabetes mellitus by 2% and 6%, respectively.29 Insofar as the risk of new onset diabetes mellitus is related to the degree to which HMG-CoA reductase activity is inhibited, rosuvastatin, which has greater bonding interaction with HMG-CoA reductase than atorvastatin, could be expected to be associated with the higher risk of new onset diabetes mellitus shown in this study.21 22 30 However, the higher incidence of new onset diabetes mellitus did not translate into higher risk of the primary outcome, and the use of ezetimibe was lower in the rosuvastatin group. Further studies evaluating the association between statin type, new onset diabetes mellitus, and future cardiovascular events, as well as those evaluating the effects of ezetimibe on new onset diabetes mellitus are required.
In this study, the incidence of cataract surgery differed according to statin type. Although statins’ antioxidant and anti-inflammatory effects on the lens are expected to slow the aging process of the lens nucleus and epithelium, a concern has been that statin treatment could increase the risk of cataracts based on the hypothesis that statins inhibit proper epithelial cell development within the crystalline lens, where cholesterol biosynthesis is critical to maintain transparency and structure of the lens.9 31 A possible association between statin treatment and cataracts was shown in previous studies.32 33 In this study, 1.9% of patients underwent cataract surgery during the median follow-up of 3.0 years, which is in line with the findings of the HOPE (Heart Outcomes Prevention Evaluation)-3 trial, which showed that 3.8% of patients receiving statin treatment underwent cataract surgery during a median follow-up of 5.6 years.33 Consistently, compared with atorvastatin, the incidence of cataract surgery with rosuvastatin was 1.0% higher. The greater LDL cholesterol lowering capacity of rosuvastatin might have prevented epithelial cell development within the crystalline lens. Therefore, when using rosuvastatin over atorvastatin as a statin regimen in people with coronary artery disease, a greater reduction in LDL cholesterol levels can be expected; however, meticulous monitoring and appropriate lifestyle interventions should be considered to mitigate the risk of new onset diabetes mellitus or cataracts. To determine whether the increase in new onset diabetes mellitus and cataract surgery is directly related to the statin treatment, the underpinning mechanism for these relations and the possible mechanism for a drug effect still require further investigations.
Limitations of this study
This study has several limitations. Firstly, although a 2×2 factorial randomisation was prespecified, no a priori sample size estimation was performed on the basis of testing the different statin types. At the time of the trial design, data were limited to provide evidence for the sample size estimation based on statin type. Secondly, this was an open label trial. However, an independent clinical endpoint committee blinded to the treatment assignments adjudicated all clinical outcomes. Thirdly, the comparison of individual components of the primary outcome was hampered by the small number of events. Fourthly, the initial definition for new onset diabetes mellitus did not include haemoglobin A1c levels. However, a post hoc analysis using a definition of new onset diabetes mellitus that incorporated the haemoglobin A1c level showed consistent results. Fifthly, regular ophthalmological examinations for the detection of cataracts were not specified in the protocol. Sixthly, only Asian participants were included in this trial. Finally, the study period was three years, which may have been relatively short to find longer term effects of two statin types. Therefore, our findings should be interpreted with caution, and further dedicated investigation with longer follow-up is warranted.
Conclusions
In people with coronary artery disease, rosuvastatin and atorvastatin treatment showed comparable efficacy in terms of the composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation within three years. Rosuvastatin treatment was associated with lower LDL cholesterol levels, but it also carried a higher risk of new onset diabetes mellitus requiring antidiabetics and cataract surgery, compared with atorvastatin treatment.
What is already known on this topic
Low density lipoprotein (LDL) cholesterol lowering capacity varies by statin type
The comparative long term efficacy and safety between two potent statins (rosuvastatin and atorvastatin) in people with coronary artery disease are unclear
What this study adds
In people with coronary artery disease, rosuvastatin and atorvastatin showed comparable efficacy in terms of a composite of all cause death, myocardial infarction, stroke, or any coronary revascularisation within three years
Rosuvastatin was associated with greater efficacy in reducing LDL cholesterol levels, but it incurred a higher risk of new onset diabetes mellitus requiring antidiabetics and cataract surgery than atorvastatin
Acknowledgments
Affiliations of the LODESTAR investigators: Myeong-Ki Hong, Donghoon Choi, Young-Guk Ko, Byeong-Keuk Kim, Jung-Sun Kim, Chul-Min Ahn, Sung-Jin Hong, Seung-Jun Lee, Yong-Joon Lee (Severance Hospital, Yonsei University College of Medicine, Seoul, Korea); Bum-Kee Hong, Hyuck Moon Kwon, Jong-Youn Kim, Pil Ki Min, Young Won Yoon, Byoung Kwon Lee, Se-Joong Rim, Eui-Young Choi (Gangnam Severance Hospital, Seoul, Korea); Woong Chol Kang, Pyung Chun Oh (Gachon University College of Medicine, Incheon, Korea); Jong-Young Lee (Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea); Jin-Bae Lee, Kee Sik Kim, Ji Yong Choi, Jae Kean Ryu, Seung Pyo Hong, Chang Yeon Kim (Daegu Catholic University Medical Center, Daegu, Korea); Tae-Hyun Yang, Hyung-Jin Cho (Inje University Busan Paik Hospital, Busan, Korea); Junghan Yoon, Min-Soo Ahn, Sung Gyun Ahn, Jun-Won Lee, Jung-Woo Son (Wonju Severance Christian Hospital, Wonju, Korea); Yangsoo Jang (CHA University College of Medicine, Seongnam, Korea); Hyuck-Jun Yoon, Cheol Hyun Lee, Jongmin Hwang, Yun-Kyeong Cho, Seung-Ho Hur, Seongwook Han, Chang-Wook Nam, Hyoungseop Kim, Hyoung-Seob Park, In-Cheol Kim (Keimyung University Dongsan Medical Center, Daegu, Korea); Yun-Hyeong Cho, Hyeon-Ju Jeong, Jin-Ho Kim, Chewan Lim, Yongsung Suh, Eui Seok Hwang, Ji Hyun Lee (Myongji Hospital, Hanyang University College of Medicine, Ilsan, Korean); Sung Yun Lee, Sung Uk Kwon (Inje University Ilsan Paik Hospital, Ilsan, Korea); Song-Yi Kim (Jeju National University Hospital, Jeju, Korea); Keun-Ho Park, Hyun Kuk Kim (Chosun University Hospital, Gwangju, Korea).
Web extra.
Extra material supplied by authors
Supplementary information: Supplementary methods, tables S1-S10, and figures S1-S3
Contributors: Y-JL and S-JH are joint first authors and contributed equally to this work. S-JH, B-KK, and M-KH designed the study. Y-JL, S-JH, and M-KH participated in the final analyses and data interpretation. All authors participated in the enrolment of participants, performed clinical follow-up, and revised the draft critically for important intellectual content. This report was drafted by Y-JL, S-JH, B-KK, and M-KH. All authors approved the final version of the manuscript and ensured that the accuracy and integrity of all parts of the work have been appropriately investigated and resolved. All authors had full access to all the data in the study and share final responsibility for the decision to submit for publication. B-KK and M-KH are the guarantors. B-KK is the co-corresponding author and can be reached at kimbk@yuhs.ac. The corresponding authors (B-KK and M-KH) attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by Sam Jin Pharmaceutical, Seoul, Korea, and Chong Kun Dang Pharmaceutical, Seoul, Korea, and supported by the Cardiovascular Research Centre, Seoul, Korea. The funders had no role in considering the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: the study was funded by a grant from Sam Jin Pharmaceutical and Chong Kun Dang Pharmaceutical. M-KH has received speaker’s fees from Medtronic, Edward Lifesciences, and Viatris Korea, and institutional research grants from Sam Jin Pharmaceutical and Chong Kun Dang Pharmaceutical; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The manuscript’s guarantors (B-KK and M-KH) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Dissemination to participants and related patient and public communities: There are no plans to communicate the results to the study participants. Our research findings will be disseminated to the public and healthcare professionals through press releases, interviews with local and national media, social media posts on Twitter, Facebook, and academic conferences.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: LODESTAR investigators, Myeong-Ki Hong, Donghoon Choi, Young-Guk Ko, Byeong-Keuk Kim, Jung-Sun Kim, Chul-Min Ahn, Sung-Jin Hong, Seung-Jun Lee, Yong-Joon Lee, Bum-Kee Hong, Hyuck Moon Kwon, Jong-Youn Kim, Pil Ki Min, Young Won Yoon, Byoung Kwon Lee, Se-Joong Rim, Eui-Young Choi, Woong Chol Kang, Pyung Chun Oh, Jong-Young Lee, Jin-Bae Lee, Kee Sik Kim, Ji Yong Choi, Jae Kean Ryu, Seung Pyo Hong, Chang Yeon Kim, Tae-Hyun Yang, Hyung-Jin Cho, Junghan Yoon, Min-Soo Ahn, Sung Gyun Ahn, Jun-Won Lee, Jung-Woo Son, Yangsoo Jang, Hyuck-Jun Yoon, Cheol Hyun Lee, Jongmin Hwang, Yun-Kyeong Cho, Seung-Ho Hur, Seongwook Han, Chang-Wook Nam, Hyoungseop Kim, Hyoung-Seob Park, In-Cheol Kim, Yun-Hyeong Cho, Hyeon-Ju Jeong, Jin-Ho Kim, Chewan Lim, Yongsung Suh, Eui Seok Hwang, Ji Hyun Lee, Sung Yun Lee, Sung Uk Kwon, Song-Yi Kim, Keun-Ho Park, and Hyun Kuk Kim
Ethics statements
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the local institutional review boards or ethics committees of all participating centres (Yonsei University Health System, Institutional Review Board, 4-2015-0713).
Data availability statement
Data will be shared by the corresponding author upon reasonable request.
References
- 1. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019;73:e285-350. 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 2. Mach F, Baigent C, Catapano AL, et al. ESC Scientific Document Group . 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 3. Hong SJ, Lee YJ, Lee SJ, et al. LODESTAR Investigators . Treat-to-target or high-intensity statin in patients with coronary artery disease: a randomized clinical trial. JAMA 2023;329:1078-87. 10.1001/jama.2023.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LaRosa JC, Grundy SM, Waters DD, et al. Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35. 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 5. Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504. 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 6. Armitage J, Bowman L, Wallendszus K, et al. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group . Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 2010;376:1658-69. 10.1016/S0140-6736(10)60310-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson PD, Panza G, Zaleski A, Taylor B. Statin-Associated Side Effects. J Am Coll Cardiol 2016;67:2395-410. 10.1016/j.jacc.2016.02.071 [DOI] [PubMed] [Google Scholar]
- 8. Stroes ES, Thompson PD, Corsini A, et al. European Atherosclerosis Society Consensus Panel . Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society consensus panel statement on assessment, aetiology and management. Eur Heart J 2015;36:1012-22. 10.1093/eurheartj/ehv043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mach F, Ray KK, Wiklund O, et al. European Atherosclerosis Society Consensus Panel . Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J 2018;39:2526-39. 10.1093/eurheartj/ehy182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serban MC, Colantonio LD, Manthripragada AD, et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol 2017;69:1386-95. 10.1016/j.jacc.2016.12.036 [DOI] [PubMed] [Google Scholar]
- 11. Dormuth CR, Filion KB, Paterson JM, et al. Canadian Network for Observational Drug Effect Studies Investigators . Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ 2014;348:g3244. 10.1136/bmj.g3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889-934. 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 13. Hicks KA, Tcheng JE, Bozkurt B, et al. American College of Cardiology. American Heart Association . 2014 ACC/AHA Key data elements and definitions for cardiovascular endpoint events in clinical trials: A report of the American College of Cardiology/American Heart Association task force on clinical data standards (Writing committee to develop cardiovascular endpoints data standards). Circulation 2015;132:302-61. 10.1161/CIR.0000000000000156 [DOI] [PubMed] [Google Scholar]
- 14. Thygesen K, Alpert JS, Jaffe AS, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 15. Sacco RL, Kasner SE, Broderick JP, et al. American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia. Council on Cardiovascular Radiology and Intervention. Council on Cardiovascular and Stroke Nursing. Council on Epidemiology and Prevention. Council on Peripheral Vascular Disease. Council on Nutrition, Physical Activity and Metabolism . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064-89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association . (2) Classification and diagnosis of diabetes. Diabetes Care 2015;38(Suppl):S8-16. 10.2337/dc15-S005 [DOI] [PubMed] [Google Scholar]
- 17. Lee YJ, Cho JY, You SC, et al. Moderate-intensity statin with ezetimibe vs. high-intensity statin in patients with diabetes and atherosclerotic cardiovascular disease in the RACING trial. Eur Heart J 2023;44:972-83. 10.1093/eurheartj/ehac709. [DOI] [PubMed] [Google Scholar]
- 18. Kim BK, Hong SJ, Lee YJ, et al. RACING investigators . Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet 2022;400:380-90. 10.1016/S0140-6736(22)00916-3 [DOI] [PubMed] [Google Scholar]
- 19. Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078-87. 10.1056/NEJMoa1110874 [DOI] [PubMed] [Google Scholar]
- 20. Nicholls SJ, Brandrup-Wognsen G, Palmer M, Barter PJ. Meta-analysis of comparative efficacy of increasing dose of Atorvastatin versus Rosuvastatin versus Simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am J Cardiol 2010;105:69-76. 10.1016/j.amjcard.2009.08.651 [DOI] [PubMed] [Google Scholar]
- 21. Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005;45:89-118. 10.1146/annurev.pharmtox.45.120403.095748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001;292:1160-4. 10.1126/science.1059344 [DOI] [PubMed] [Google Scholar]
- 23. Luvai A, Mbagaya W, Hall AS, Barth JH. Rosuvastatin: a review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin Med Insights Cardiol 2012;6:17-33. 10.4137/CMC.S4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cortese F, Gesualdo M, Cortese A, et al. Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol Res 2016;107:1-18. 10.1016/j.phrs.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 25. Cannon CP, Blazing MA, Giugliano RP, et al. IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387-97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 26. Giugliano RP, Cannon CP, Blazing MA, et al. IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators . Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: Results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 2018;137:1571-82. 10.1161/CIRCULATIONAHA.117.030950 [DOI] [PubMed] [Google Scholar]
- 27. Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195-207. 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 28. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735-42. 10.1016/S0140-6736(09)61965-6 [DOI] [PubMed] [Google Scholar]
- 29. Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. DIAGRAM Consortium. MAGIC Consortium. InterAct Consortium . HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015;385:351-61. 10.1016/S0140-6736(14)61183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Betteridge DJ, Carmena R. The diabetogenic action of statins - mechanisms and clinical implications. Nat Rev Endocrinol 2016;12:99-110. 10.1038/nrendo.2015.194 [DOI] [PubMed] [Google Scholar]
- 31. Desai CS, Martin SS, Blumenthal RS. Non-cardiovascular effects associated with statins. BMJ 2014;349:g3743. 10.1136/bmj.g3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leuschen J, Mortensen EM, Frei CR, Mansi EA, Panday V, Mansi I. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol 2013;131:1427-34. 10.1001/jamaophthalmol.2013.4575 [DOI] [PubMed] [Google Scholar]
- 33. Yusuf S, Bosch J, Dagenais G, et al. HOPE-3 Investigators . Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021-31. 10.1056/NEJMoa1600176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Supplementary methods, tables S1-S10, and figures S1-S3
Data Availability Statement
Data will be shared by the corresponding author upon reasonable request.