Abstract
Neuromuscular diseases with primary muscle wasting symptoms may also display multi-systemic changes in the body and exhibit secondary pathophysiological alterations in various non-muscle tissues. In some cases, this includes proteome-wide alterations and/or adaptations in the central nervous system. Thus, in order to provide an improved bioanalytical basis for the comprehensive evaluation of animal models that are routinely used in muscle research, this report describes the mass spectrometry-based proteomic characterization of the mouse brain. Crude tissue extracts were examined by bottom-up proteomics and detected 4558 distinct protein species. The detailed analysis of the brain proteome revealed the presence of abundant cellular proteoforms in the neuronal cytoskeleton, as well as various brain region enriched proteins, including markers of the cerebral cortex, cerebellum, hippocampus and the olfactory bulb. Neuroproteomic markers of specific cell types in the brain were identified in association with various types of neurons and glia cells. Markers of subcellular structures were established for the plasmalemma, nucleus, endoplasmic reticulum, mitochondria and other crucial organelles, as well as synaptic components that are involved in presynaptic vesicle docking, neurotransmitter release and synapse remodelling.
Key Words: mass spectrometry, mouse brain, neuromuscular disease, neuroproteomics
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The mouse is one of the most widely used animal models in neuroscience,1 including studies of neuromuscular disorders such as muscular dystrophy,2 or motor neuron disease.3 Many genetic or acquired muscle diseases exhibit besides characteristic symptoms of fiber degeneration and/or contractile dysfunction also body-wide changes that are illustrated by pathophysiological alterations in non-muscular tissues. For example, the highly heterogeneous group of motor neuron diseases displays severe dysfunction of the motor system, but also a variety of extra-motor abnormalities. The most frequently observed adult-onset form of motor neuron disease is amyotrophic lateral sclerosis, which is characterized by progressive muscular weakness.4 Primary abnormalities in an extremely large number of genes have been associated with this disorder. Established animal models of amyotrophic lateral sclerosis include the SOD1 mouse and the wobbler mouse.3 In both patients and animal models of amyotrophic lateral sclerosis, abnormalities are observed in the flow of excitatory signals from neurons in the cortex, brain stem, spinal cord and the neuromuscular junction.5 Thus, in order to better understand the pathobiochemical mechanisms that underlie muscular atrophy due to the degeneration of lower and upper motor neurons, a comprehensive analysis of the peripheral and central nervous system is essential. Proteomics suggests itself as the methodological approach of choice for such a large-scale and high-throughput analysis due to its swift, unbiased and technology-driven nature.6 However, the mass spectrometric survey of the mouse model brain and its pathophysiological involvement in motor neuron disease is ideally based on previously established catalogues of the normal brain proteome.7 This should preferably include the biochemical detection and characterization of brain region enriched markers, cell type specific proteins and the identification of subcellular markers with a distinct function in key organelles of the diverse types of neurons and glial cells.
Another example of a neuromuscular disorder with complex body-wide alterations including abnormal brain functions is Duchenne muscular dystrophy (DMD).8 This X-linked inherited disorder can be clearly defined as a primary muscle wasting disorder but can also be categorized as a multi-systemic disease.9 Dystrophinopathies are due to mutations in the DMD gene that results in the almost complete loss of the membrane cytoskeletal protein dystrophin and a reduced expression of its associated glycoprotein complex in voluntary contractile fibers. This weakens the muscle fiber periphery and renders the sarcolemma more susceptible to contraction-induced rupturing of the surface membrane. Hence, the main pathophysiological hallmarks of X-linked muscular dystrophy are muscle membrane leakage and calcium-related myonecrosis, combined with fat substitution, reactive myofibrosis and chronic inflammation of skeletal muscles.10 Many of these symptoms are also seen in the mdx-type mouse models of dystrophinopathy.2 However, since the extremely large DMD gene has several promoter regions that produce 8 different tissue-specific dystrophin isoforms, mutation-specific alterations in combination with secondary changes can occur in many organ systems besides the skeletal musculature. This may include the liver, kidney, spleen, gastrointestinal tract and the nervous system.11-13 In this context, it is encouraging that the proteomic survey of the dystrophin-deficient brain of the mdx model of Duchenne muscular dystrophy has identified a large number of altered proteins involved in the maintenance of intermediate filaments, membrane repair mechanisms and calcium handling.14
In order to establish the scientific basis for comparative proteomic surveys of the central nervous system in relation to mouse model research of neuromuscular diseases, this report outlines the mass spectrometry-based proteomic characterization of the normal middle-aged mouse brain. The proteomic data of markers of brain regions such as the olfactory bulb, hippocampus, cerebellum, hypothalamus and cerebral cortex, various brain cell types and subcellular structures found in neurons, synapsis and glia cells should be helpful as a reference guide for the comprehensive evaluation of the central nervous system in genetic mouse models that are routinely used in basic and applied myology research.
Materials and Methods
For the proteomic profiling of the mouse brain, general materials and analytical grade reagents were obtained from GE Healthcare (Little Chalfont, Buckinghamshire, UK), Sigma Chemical Company (Dorset, UK) and Bio-Rad Laboratories (Hemel-Hempstead, Hertfordshire, UK). Controlled protein digestion was carried out with sequencing grade-modified trypsin and the proteolytic enzyme Lys-C, in combination with Protease Max Surfactant Trypsin Enhancer, from Promega (Madison, WI, USA). Vivacon 500 (Product number: VN0H22) filter units from Sartorius (Göttingen, Germany) were used for filter-aided sample preparation. Buffers for tissue extraction were supplemented with a protease inhibitor cocktail from Roche Diagnostics (Mannheim, Germany). The protein concentration of extracted samples was determined with the Pierce 660-nm Protein Assay from ThermoFisher Scientific (Dublin, Ireland).
Ethical approval, animal license and animal maintenance
Male wild type C57/BL6 mice were obtained from the Bioresource Unit of the University of Bonn.13 All procedures adhered to German legislation on the use of animals in experimental research (Amt für Umwelt, Verbraucherschutz und Lokale Agenda der Stadt Bonn, North Rhine-Westphalia, Germany). Mice were kept under standard conditions.15 For tissue dissection, protein extraction and subsequent proteomic analyses, 12-months old mice were used. Freshly dissected brain tissue specimens were quick-frozen in liquid nitrogen and transported to Maynooth University in accordance with the Department of Agriculture (animal by-product register number 2016/16 to the Department of Biology, National University of Ireland, Maynooth) on dry ice and stored at -80°C prior to proteomic analysis.14
Preparation of middle-aged mouse brain protein extracts for mass spectrometric analysis
This study was carried out with 12-month old tissue specimens since this represents the fully matured and middle-aged central nervous system.16 Importantly, at this age the proteomic data are suitable for comparative studies of late-onset neuromuscular changes, such as reactive myofibrosis.17 In general, mice of 3-6, 10-15 and 18-24 months of age are considered ‘mature adult’, ‘middle aged’ and ‘old’, respectively. The middle-aged group represents a phase in the life of mice during which early changes due to the natural aging process can be detected. Thus, the murine middle-aged group can be conveniently used in comparative aging studies to evaluate whether an age-associated effect is of an early onset and progressive nature or only seen at much older age.18 Whole brain specimens from wild type (n=6) mice were lysed by homogenization using 4% (w/v) sodium dodecyl sulfate, 0.1M dithiothreitol, 100mM Tris-Cl pH 7.6; supplemented with a protease inhibitor cocktail.19 Following incubation at 95°C for 3 minutes, sonication for 30 seconds and centrifugation at 16,000xg for 5 minutes, the resulting tissue extracts were analyzed by mass spectrometry-based proteomics.20 Peptides were generated by digestion with the proteolytic enzymes LysC and trypsin and then processed using standardized filter-aided sample preparation (FASP).21
Mass spectrometry-based proteomic profiling of the mouse brain
All preparative steps and analytical procedures using label-free liquid chromatography mass spectrometry and data-dependent acquisition methodology were recently described in detail.20 For mass spectrometry, a Thermo UltiMate 3000 nano system was used for reverse-phased capillary high-pressure liquid chromatography, which was directly coupled in-line with a Thermo Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The generated mass spectrometric files were qualitatively analyzed using the UniProtKB-SwissProt database (Mus musculus) with Proteome Discoverer 2.2 using Sequest HT (Thermo Fisher Scientific) and Percolator.19 The following search parameters were employed for protein identification: (i) an allowance of up to two missed cleavages, (ii) MS/MS mass tolerance set to 0.02 Da, (iii) (i) peptide mass tolerance set to 10 ppm, (iv) methionine oxidation set as a variable modification, and (v) carbamido-methylation set as a fixed modification.15 Individual peptides were filtered using a minimum XCorr score of 1.5 for 1, 2.0 for 2, 2.25 for 3 and 2.5 for 4 charge states, with peptide probability set to high confidence. Progenesis QI for Proteomics (version 2.0; Nonlinear Dynamics, a Waters company, Newcastle upon Tyne, UK) was used for quantitative label-free data analysis. Protein identification was carried out with Proteome Discoverer 2.2 using Sequest HT (Thermo Fisher Scientific) and Percolator. Data sets were then imported into Progenesis QI software for further analysis.19 The .raw MS files and the multi-consensus data of this mass spectrometric study have been deposited to the Open Science Framework (OSF) under the title “Proteomic profiling of mouse brain” (https://osf.io/345w8/) with the file name ‘345w8’. Bioinformatic PANTHER analysis of protein families was performed with a publicly available search engine (https://pantherdb.org).22
Results
Mass spectrometric analysis of crude extracts from middle-aged mouse brain
The proteomic analysis of total brain extracts detected 4,558 distinct protein species. Proteins identified with greater than 100 peptides were found to be predominately cytoskeletal proteins. The most abundant brain proteins were identified as the alpha and beta chains of spectrin (Sptan1 gene, Sptbn1 gene), the heavy chain of cytoplasmic dynein-1 (Dync1h1 gene), plectin (Plec gene), ankyrin-2 (Ank2 gene), microtubule-associated proteins 1A and 1B (Map1a gene, Map1b gene) and protein bassoon (Bsn gene). The bioinformatic PANTHER analysis of protein classes in total extracts of mouse brain are presented in Figure 1. Major protein classes include components involved in RNA metabolism, calcium-binding, cell adhesion, cell junction formation, the cellular stress response, chromatin binding, cytoskeletal maintenance, membrane trafficking, enzymatic conversion of metabolites, protein modification, modulation of protein-binding activity, scaffolding and adaptations, translation, transmembrane signaling and transportation.22 The section ‘No Panther category’ consists of protein species without class annotation. These proteins may have unknown functions, have not yet being curated, or no protein class terms describes their function(s).22 A variety of brain region enriched/specific proteins were detected in crude tissue extracts, including generally accepted markers of the cerebral cortex, cerebellum, hippocampus and the olfactory bulb.7,23-25 As a marker of the cerebral cortex, Septin-7 (Sept7 gene) was detected by 24 peptides (63.3% sequence coverage, accession number O55131, molecular mass 101.9 kDa). Protein markers of the cerebellum were established as peripherin (Prph gene) by 6 peptides (13.5% sequence coverage, accession number P15331-3, molecular mass 52.7 kDa) and the metabotropic glutamate receptor 1 (Grm1 gene) by 5 peptides (6.2% sequence coverage, accession number P97772-2, molecular mass 101.6 kDa), as well as cerebellin-1 (Cbln1 gene) by 2 peptides (6.7% sequence coverage, accession number Q9R171, 21.1 kDa molecular mass). The protein fraction derived from the hippocampus was recognized by the presence of neuronal pentraxin-1 (Nptx1 gene) using 10 peptides (27.3% sequence coverage, accession number Q62443, molecular mass 47.1 kDa) and the neuron-specific calcium-binding and calcium-sensing protein hippocalcin (Hpca gene) using 11 peptides (55.4%, accession number P84075, molecular mass 22.4 kDa). An excellent marker of the olfactory bulb is presented by the olfactory marker protein (Omp gene). This protein was detected by 2 peptides (21.5% coverage, accession number Q64288, molecular mass 18.9 kDa).
Proteomic identification of cell type specific proteins in middle-aged mouse brain
The proteomic identification of select mouse brain proteins that are associated with specific cell types using label-free mass spectrometry is listed in Supplemental Table 1. This included the brain/neuron-specific glycolytic enzyme γ-enolase ENO2 and the neuronal beta-3 isoform of tubulin named TUBB3, two well established marker proteins of neurons.7,23-25 Additional neuronal marker were identified as the NeuN/Fox-3 RNA binding protein fox-1 homolog 3, the brain isoform of glycogen phosphorylase, hippocalcin, septin-3, protein kinase C and casein kinase substrate in neurons protein pacsin-1 and calbindin-2. Specialized markers of somato-dendritic neurons, dendritic spines in neocortical pyramidal neurons and cerebellar granule cells were detected in the form of the microtubule-associated protein MAP2, the synaptic Ras GTPase activating protein 1 and the calcium/calmodulin-dependent 3',5'- cyclic nucleotide phosphodiesterase 1A, respectively.7,23-25 As a general marker of glia cells, protein S100-B was determined by proteomic analysis. More cell-specific glia cell markers included the glial fibrillary acidic protein GFAP for astrocytes. Oligodendrocyte-enriched proteins were identified as the myelin-oligodendrocyte glycoprotein MOG, the myelin-associated oligodendrocyte basic protein MOBP and oligodendrocyte-myelin glycoprotein OMG. Myelin sheath and Nodes of Ranvier were recognized by the presence of myelin basic protein MBP and neurofascin, respectively.7,23-25 The mass spectrometric analysis also detected the proteins contactin-1 and contactin-2 as integral parts of neuron-glia interactions zones, and the enzyme gamma-glutamyl-transferase GGT7 as a marker of cerebral capillaries at the blood-brain barrier.
Fig 1.
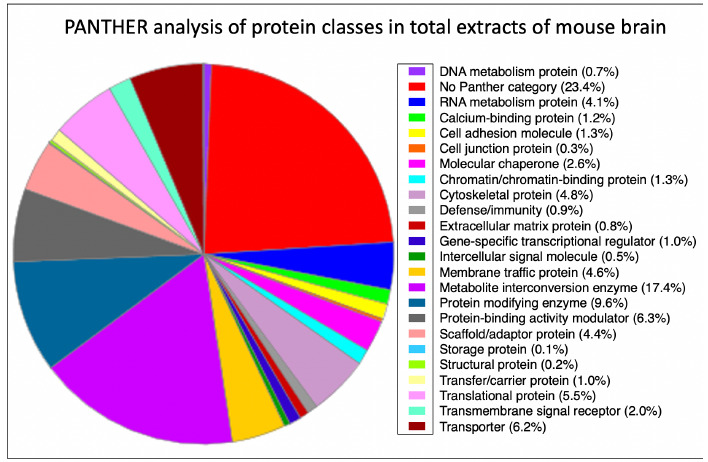
Bioinformatic analysis of the distribution of protein classes in total extracts of mouse brain as determined by mass spectrometric analysis. The pie chart was generated with the help of the PANTHER analysis tool.22
Proteomic identification of subcellular protein markers in middle-aged mouse brain
The proteomic identification of mouse brain associated proteins that are located in distinct subcellular structures and/or are involved in specific cellular processes using label-free mass spectrometry is listed in Supplemental Table 2. The proteomic survey identified markers of the plasma membrane, neuronal cell adhesion processes, the neuronal cytoskeleton, the nucleus, the endoplasmic reticulum, the Golgi apparatus, mitochondria, ribosomes, lysosomes, peroxisome, proteasome, the cytosol, extracellular space and the extracellular matrix. Surface membrane markers included the Na+/K+-ATPase, the plasma membrane Ca2+-ATPase and the neuronal cell adhesion molecules NCAM1, NCAM2, NRCAM, NEGR1, L1CAM AND CADM3.7,23-25 Surface membrane markers involved in glial-guided migration, neurite outgrowth and synaptic plasticity were identified as astrotactin ASTN3, neurotrimin and neuroplastin.
The detection of abundant cytoskeletal proteins that are involved in organizing the cytomatrix included the protein named bassoon, the presynaptic scaffolding protein piccolo PCLO, the major membrane cytoskeletal component spectrin and the cytoskeletal network organizer plectin. In contrast, the membrane cytoskeletal protein dystrophin appears to be of much lower abundance in the brain as compared to skeletal muscles.9 The mass spectrometric survey presented here also covered the main intermediate filament proteins that structurally support the neuronal cytoskeleton of axons, i.e. the light NEFL, medium NEFM and heavy NEFH polypeptides of the neurofilament triplet protein assembly.7,23-25 Microtubules were recognized by the neuronal TUBB3 isoform of tubulin, and the microtubule-associated protein MAP1A and MAP1B. Standard organellar markers included emerin, lamin LMNB2 and histone H4 for the nucleus, the protein ERP29, the Ca2+-ATPase isoform SERCA2 and the RYR2 isoform of the ryanodine receptor Ca2+-release channel for the endoplasmic reticulum, the protein ERGIC1 for the endoplasmic reticulum-Golgi intermediate compartment, and neurocalcin and the general vesicular transport factor USO1 for the Golgi apparatus. Mitochondria were recognized by the organelle-specific metallopeptidase neurolysin, and the various subfractions of mitochondria by the voltage-dependent anion-selective channel protein mt-VDAC1 (outer membrane), NADH dehydrogenase (inner membrane complex I), succinate dehydrogenase (inner membrane complex II), cytochrome b-c1 (inner membrane complex III), cytochrome c oxidase (inner membrane complex IV), ATP synthase (inner membrane complex V) and isocitrate dehydrogenase (mitochondrial matrix).
The mass spectrometric analysis identified 40S ribosomal protein RPSA, lysosome-associated membrane glycoprotein LAMP1, catalase and ubiquitin-conjugating enzyme E2 as markers of ribosomes, lysosomes, peroxisomes and proteasomes, respectively. Cytosolic markers were identified in the context of the glycolytic pathway (phosphofructokinase), metabolite transportation (brain isoform FABP7 of the fatty acid-binding protein), the cellular stress response (the small heat shock protein alpha-B crystallin) and signal transduction (neurochondrin).
Proteins of the extracellular region were covered by the mass spectrometric detection of laminin of the basal lamina, collagen isoform COL-IV of the extracellular matrix, the basement membrane-specific heparan sulfate proteoglycan core protein and neurocan core protein of the proteoglycan matrix, and serum albumin in the extracellular space.
Proteomic identification of synaptic markers in middle-aged mouse brain
The proteomic identification of mouse brain proteins that are associated with the formation, function and maintenance of synapses using label-free mass spectrometry is listed in Supplemental Table 3. This included marker proteins of the presynaptic active zone that are involved in docking of synaptic vesicles at presynaptic active zones, synaptic cell adhesion, formation of synaptic contacts for efficient neurotransmission, synaptic vesicles maintenance, neurotransmitter release, neuronal modulation and synapse remodelling. Mass spectrometry detected neuronal markers that are involved in (i) docking of synaptic vesicles at presynaptic active zones and neurotransmitter exocytosis (syntaxin isoforms STX-1A/1B/6/7/12, and syntaxin-binding protein isoforms STXBP-1/3/5), (ii) synaptic cell adhesion and the formation of synaptic contacts for efficient neurotransmission (neurexin-1/3, neuroligin-1/2/3, neurogranin, and the postsynaptic scaffolding protein PSD-95), (iii) the regulation of synaptic vesicles and neurotransmitter release (synaptosomal-associated proteins SNAP-23 and SNAP-25, vesicle-associated membrane protein VAMP2, synapsin-1/2/3, synaptotagmin-1/2/7, synaptic vesicle glycoprotein 2B, synaptoporin, huntingtin, synaptojanin-1, and synaptogyrin-3), and (iv) neuronal modulation and synapse remodelling (neuronal pentraxin-1, neuronal pentraxin receptor, neurobeachin, neurabin-2, synaptopodin, brain nitric oxide synthase, neuromodulin, neurochondrin, teneurin-4, neuronal membrane glycoproteins M6-a/M6-b, beta-synuclein, and neuronal calcium sensor NCS1).
Discussion
The major aim of this study was to establish useful neuroproteomic markers of the middle-aged mouse brain, which is frequently used in basic and applied studies in neurosciences as a model system. The mass spectrometric survey was carried out with crude brain extracts and identified brain region markers of the cerebellum, hippocampus, the olfactory bulb and the cerebral cortex. In addition, a large number of cell type markers of the brain were detected for the differential analysis of neurons versus glia cells. The proteomic identification of subcellular markers included brain proteins located in the nucleus, endoplasmic reticulum, plasma membrane, mitochondria and other organelles. Importantly, a large number of components that are involved in presynaptic vesicle docking, neuro-transmitter release and synapse remodelling were clearly detected by mass spectrometry. Building on the results of previous cataloguing studies of mouse brain parts and neuronal tissue specimens to establish a brain region and cell type resolved murine brain proteome,7,23-36 this investigation focused on the refined and comprehensive detection of robust proteomic markers of whole mouse brain preparations. Thus, the main objective of this proteomic survey was to establish a proteomic reference map of the middle-aged murine brain using crude protein extracts from small tissue specimens. The biochemical analysis described here employs a minimum of preparative steps for a streamlined proteomic analysis platform that eliminates excessive artefacts for the routine detection of brain proteoforms. The graphical presentations of Figure 2 summarize the distribution of key proteomic markers in specific cell types of the mouse brain and subcellular structures, as well as synaptic processes.
Of special interest is the mass spectrometric identification of isoforms of the neurexin,37 and neuroligin,38 families and the large neurexin-neuroligin complex with its presynaptic linkage to neurotransmitter vesicles and its associations with postsynaptic scaffolds.39 This involves an indirect connection to the postsynaptic density protein PSD-95 and a linkage to the scaffolding protein piccolo PCLO and the cytoskeletal component bassoon on the presynaptic side. The cytomatrix-organising protein bassoon is one of the most abundant brain proteins that was identified by mass spectrometry in this study. Bassoon is intrinsically involved in the regulation of neurotransmitter release at the nerve terminal active zone.40 It has previously been shown by proteomics to be changed in abundance in the brain of the mdx mouse model of dystrophinopathy.14
Previous gel-based and top-down approaches, as well as gel-free and bottom-up proteomic studies, produced large data sets of proteins that are associated with the mouse brain.7,23-36 This included the proteomic establishment of general markers of the olfactory bulb, medulla, midbrain, hypothalamus, hippocampus, cerebellum and cortex using two-dimensional gel electrophoresis,23,27,28 and liquid chromatography,7,25 based protein separation prior to mass spectrometric analysis. A variety of mass spectrometric labelling methods were also used to study the mouse brain proteome.26,29,35,36 Focused cataloguing surveys centered on brain development,27,30 cell type-resolved proteomes,7,35 the composition of the synaptome,32 and regional differences in neuronal versus astrocyte cell populations.36 The application of top-down proteomics, for the detailed identification and characterization of proteoforms41,42 resulted in the establishment of the Mouse Brain Proteoform Atlas.33,34
This report has confirmed many of the detected cellular and subcellular brain markers, and has extended the list of suitable proteoforms that are associated with specific regions, cell types, synaptic processes and subcellular structures in the middle-aged murine brain. The expression of previously established cell specific markers was confirmed in this study, including proteomic markers of neurons such as the light NEFL, medium NEFM and heavy NEFH polypeptides of the neurofilament triplet, as well as the synaptosomal-associated protein SNAP-25.7,25,32 The detection of established synaptic markers included the postsynaptic density protein PSD-95.32 Well-established glia cell markers were confirmed, such as the glial fibrillary acidic protein GFAP for astrocytes, and the myelin-associated proteins MBP and MOG for oligodendrocytes.7,25
Fig 2.
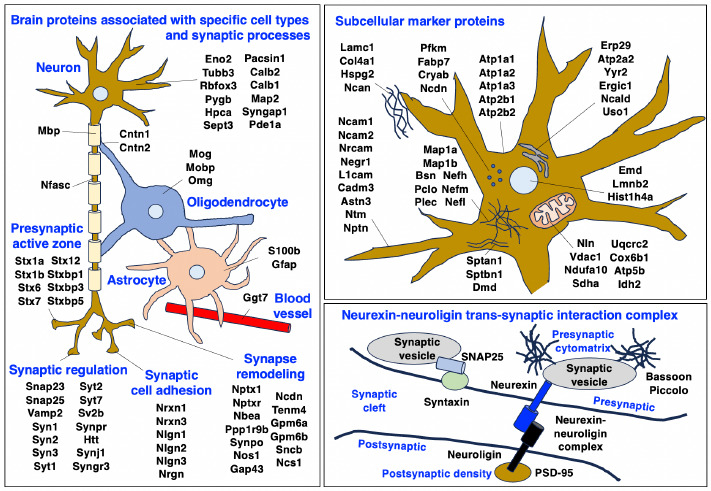
Overview of the distribution of proteomic markers in specific cell types, synaptic processes and subcellular structures in the middle-aged mouse brain. The figure summarizes identified brain proteins that are specifically associated with neurons, neuron-glia contacts, myelin sheets, Nodes of Ranvier, oligodendrocytes, astrocytes, blood vessels, synaptic regulation, synaptic cell adhesion and synapse remodeling, as well as subcellular structures including the plasmalemma, mitochondria, endoplasmic reticulum, nucleus, cytosol, extracellular matrix and the neurexin-neuroligin synapse complex. The names of the abbreviated brain proteins are listed in Supplemental Tables 1-3.
Crucial brain region enriched markers detected in this study included (i) septin of the cerebral cortex, (ii) peripherin, the metabotropic glutamate receptor 1 and cerebellin-1 of the cerebellum, (iii) neuronal pentraxin-1 and the neuron-specific calcium sensor hippocalcin of the hippocampus, and (iv) olfactory marker protein OMP for the olfactory bulb region in the brain.7,23-25 Septin-7 is a cytoskeletal component of the postsynaptic density and exhibits high expression levels in the cerebral cortex. It acts as a GTP-binding protein which polymerizes into heteromeric filaments and interacts with actin and microtubules.43
Crucial cerebellum markers were identified, including peripherin, the metabotropic glutamate receptor 1 and cerebellin-1, which is involved in synapse formation, axon growth and guidance. Peripherin is a major cytoskeletal component and belongs to the desmin-like type III intermediate filament class of proteins that are located in neurons. The peripherin isoform in the cerebellum, which is different from the one present in the photoreceptor outer segments, represents an excellent marker of cerebellar climbing fibers. Mutations in the PRPH gene confer susceptibility to motor neuron disease.44
The metabotropic glutamate receptor is found in dendrites, the soma region, axons and synapses of neurons with a high expression level in the cerebellum.
Neuronal pentraxin-1 is found in axons and synapses and shows high expression levels in the hippocampus, as well as the cerebral cortex. Pentraxin-1 is probably involved in synaptic remodeling mechanisms and was shown to affect the numbers of excitatory synapses via a modulating process of neuronal excitability.45 The olfactory marker protein OMP is a cytoplasmic marker of olfactory receptor neurons in the mature olfactory bulb that plays a key role in odor detection and cellular signaling. OMP sharpens both the response profile to odorants and accelerates the response kinetics to odorants, which is clearly supported by the characterization of OMP-KO mice that have lost the ability to properly respond to distinct odor stimuli.46 The proteomic identification of cell type specific proteins in mouse brain confirmed the presence of general neuronal markers, including the brain/neuron-specific glycolytic enzyme γ-enolase (ENO2) that exhibits neurotrophic and neuroprotective properties, and the neuronal tubulin beta-3 isoform TUBB3/TUJ1 that supports axon guidance and neuron differentiation.7,23-36 The gamma-glutamyl-transferase GGT7 was clearly detected and represents as an established marker of cerebral capillaries at the blood-brain barrier.47
Important proteins that mediate neuron-glia interactions and the organization of axonal domains at Nodes of Ranvier were identified as contactin-1, contactin-2 and neurofascin.7,23-36 Interactions between amyloid precursor protein and contactin are implicated in the pathogenesis of Alzheimer's dementia.48 The presence of established glia markers was confirmed by the mass spectrometric coverage of glial fibrillary acidic protein GFAP, glia-derived nexin, vimentin and the myelin-associated proteins MBP, PLP1, MOG, OMG, MAG and MOBP.7,23-36 Altered GFAP expression levels are widely used as a marker of astrogliosis, but also in the general context of traumatic brain injury, certain psychiatric disorders, neurodegenerative processes and neuroinflammation.49
The field of neuroproteomics, which represents an important sub-discipline of neurochemistry, focuses on the mass spectrometric identification and biochemical characterization of protein species that are critical for the development, maturation and adult functioning of the nervous system.
Proteomic surveys of the central and peripheral nervous system also involve studying the molecular and cellular basis of neurological and neuromuscular disorders. The mass spectrometry-based proteomic profiling of the normal middle-aged mouse brain presented here has identified a large number of brain-associated marker proteins. Since murine brain tissues are frequently used as model systems to study neurological abnormalities in neuromuscular disorders and test novel therapeutic approaches, the identified neuronal and glial proteoforms can now be employed as highly useful biomarkers of the middle-aged central nervous system, brain-specific regions, specific brain-associated cell types and subcellular structures. This includes various protein species that are highly specific for their expression levels in neurons and glia cells in distinct brain regions, such as the cerebral cortex, cerebellum, hippocampus and the olfactory bulb. For the evaluation of cellular interactions, subcellular structures and synaptic systems, this proteomic study has provided detailed information on the identification of key organellar markers and proteins that are located in synaptic vesicles and the neurotransmitter release apparatus.
In conclusion, this report provides the field of experimental myology with an improved set of bioanalytical tools for the detailed characterization of genetic mouse models.
Acknowledgments
We thank Stephan Baader (University of Bonn) for his support of this brain proteomics project.
List of acronyms
- ASTN3
astrotactin 3
- CADM3
cell adhesion molecule 3
- COL-IV
collagen isoform IV
- DMD
Duchenne muscular dystrophy
- ENO2
brain/neuron-specific γ-enolase
- ERGIC1
endoplasmic reticulum-Golgi intermediate compartment protein 1
- ERP29
endoplasmic reticulum resident protein 29
- FABP7
fatty acid binding protein 7
- GFAP
glial fibrillary acidic protein
- GGT7
gamma-glutamyl-transferase 7
- L1CAM
neural cell adhesion molecule L1
- LAMP1
lysosome-associated membrane glycoprotein 1
- LMNB2
lamin B2
- Lys-C
endoproteinase that cleaves on the C-terminal side of lysine residues
- MAP2
microtubule-associated protein 2
- MOBP
myelin-associated oligodendrocyte basic protein
- MOG
myelin-oligodendrocyte glycoprotein
- NCAM 1/2
neural cell adhesion molecule 1/2
- NCS1
neuronal calcium sensor 1
- NEFH
neurofilament, heavy polypeptide
- NEFL
neurofilament, light polypeptide
- NEFM
neurofilament, medium polypeptide
- NEGR1
neuronal growth regulator 1
- NRCAM
neuronal cell adhesion molecule
- OMG
oligodendrocyte-myelin glycoprotein
- OMP
olfactory marker protein
- PCLO
protein piccolo
- RPSA
40S ribosomal protein SA
- RYR2
ryanodine receptor calcium-release channel 2
- SERCA2
endoplasmic reticulum calcium ATPase 2
- SNAP-23/25
synaptosomal-associated proteins 23/25
- STX-1A/1B/6/7/12
syntaxin isoforms 1A/1B/6/7/12
- STXBP-1/3/5
syntaxin-binding protein isoforms 1/3/5
- SOD1
superoxide dismutase 1
- TUBB3
Tubulin isoform beta-3, neuronal
- VAMP
vesicle-associated membrane protein
- VDAC1
voltage-dependent anion-selective channel protein 1
Funding Statement
Funding: This work was supported by the Kathleen Lonsdale Institute for Human Health Research at Maynooth University. The Orbitrap Fusion Tribrid mass spectrometer was funded under a Science Foundation Ireland Infrastructure Award to Dublin City University (SFI 16/RI/3701).
Contributor Information
Paul Dowling, Email: paul.dowling@mu.ie.
Margit Zweyer, Email: margit.zweyer@dzne.de.
Hemmen Sabir, Email: hemmen.sabir@dzne.de.
Michael Henry, Email: michael.henry@dcu.ie.
Paula Meleady, Email: paula.meleady@dcu.ie.
Dieter Swandulla, Email: swandulla@uni-bonn.de.
References
- 1.Navabpour S, Kwapis JL, Jarome TJ. A neuroscientist's guide to transgenic mice and other genetic tools. Neurosci Biobehav Rev. 2020. Jan;108:732-748. doi: 10.1016/j.neubiorev.2019.12.013. Epub 2019 Dec 13. PMID: 31843544; PMCID: PMC8049509. doi: 10.1016/j.neubiorev.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge TA. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J. 2013. Sep;280(17):4177-86. doi: 10.1111/febs.12267. Epub 2013 Apr 22. PMID: 23551987; PMCID: PMC4147949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt-John T. VPS54 and the wobbler mouse. Front Neurosci. 2015. Oct 21;9:381. doi: 10.3389/fnins.2015.00381. PMID: 26539077; PMCID: PMC4612502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RH, Al-Chalabi A. Amyotrophic Lateral Sclerosis. N Engl J Med. 2017. Jul 13;377(2):162-172. doi: 10.1056/NEJMra1603471. PMID: 28700839. [DOI] [PubMed] [Google Scholar]
- 5.Akçimen F, Lopez ER, Landers JE, Nath A, Chiò A, Chia R, Traynor BJ. Amyotrophic lateral sclerosis: translating genetic discoveries into therapies. Nat Rev Genet. 2023. Apr 6. doi: 10.1038/s41576-023-00592-y. Epub ahead of print. PMID: 37024676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capitanio D, Moriggi M, Gelfi C. Mapping the human skeletal muscle proteome: progress and potential. Expert Rev Proteomics. 2017. Sep;14(9):825-839. doi: 10.1080/14789450.2017.1364996. Epub 2017 Aug 14. PMID: 28780899. [DOI] [PubMed] [Google Scholar]
- 7.Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N, Kongi K, Cantuti L, Hanisch UK, Philips MA, Rossner MJ, Mann M, Simons M. Cell type- and brain regionresolved mouse brain proteome. Nat Neurosci. 2015. Dec;18(12):1819-31. doi: 10.1038/nn.4160. Epub 2015 Nov 2. PMID: 26523646; PMCID: PMC7116867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021. Feb 18;7(1):13. doi: 10.1038/s41572-021-00248-3. PMID: 33602943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohlendieck K, Swandulla D. Complexity of skeletal muscle degeneration: multi-systems pathophysiology and organ crosstalk in dystrophinopathy. Pflugers Arch. 2021. Dec;473(12):1813-1839. doi: 10.1007/s00424-021-02623-1. Epub 2021 Sep 22. PMID: 34553265; PMCID: PMC8599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowling P, Gargan S, Swandulla D, Ohlendieck K. Proteomic profiling of impaired excitation-contraction coupling and abnormal calcium handling in muscular dystrophy. Proteomics. 2022. Dec;22(23-24):e2200003. doi: 10.1002/pmic.202200003. Epub 2022 Aug 8. PMID: 35902360; PMCID: PMC10078611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy S, Zweyer M, Henry M, Meleady P, Mundegar RR, Swandulla D, Ohlendieck K. Proteomic profiling of liver tissue from the mdx-4cv mouse model of Duchenne muscular dystrophy. Clin Proteomics. 2018. Oct 29;15:34. doi: 10.1186/s12014-018-9212-2. PMID: 30386187; PMCID: PMC6205794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling P, Zweyer M, Raucamp M, Henry M, Meleady P, Swandulla D, Ohlendieck K. Proteomic and cell biological profiling of the renal phenotype of the mdx-4cv mouse model of Duchenne muscular dystrophy. Eur J Cell Biol. 2020. Jan;99(1):151059. doi: 10.1016/j.ejcb.2019.151059. Epub 2019 Nov 18. PMID: 31776009. [DOI] [PubMed] [Google Scholar]
- 13.Dowling P, Gargan S, Zweyer M, Sabir H, Henry M, Meleady P, Swandulla D, Ohlendieck K. Proteomic profiling of the interface between the stomach wall and the pancreas in dystrophinopathy. Eur J Transl Myol. 2021. Mar 26;31(1):9627. doi: 10.4081/ejtm.2021.9627. PMID: 33709651; PMCID: PMC8056161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy S, Zweyer M, Henry M, Meleady P, Mundegar RR, Swandulla D, Ohlendieck K. Label-free mass spectrometric analysis reveals complex changes in the brain proteome from the mdx-4cv mouse model of Duchenne muscular dystrophy. Clin Proteomics. 2015. Nov 23;12:27. doi: 10.1186/s12014-015-9099-0. PMID: 26604869; PMCID: PMC4657206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy S, Zweyer M, Raucamp M, Henry M, Meleady P, Swandulla D, Ohlendieck K. Proteomic profiling of the mouse diaphragm and refined mass spectrometric analysis of the dystrophic phenotype. J Muscle Res Cell Motil. 2019. Mar;40(1):9-28. doi: 10.1007/s10974-019-09507-z. Epub 2019 Mar 19. PMID: 30888583. [DOI] [PubMed] [Google Scholar]
- 16.Liao CY, Kennedy BK. Mouse models and aging: longevity and progeria. Curr Top Dev Biol. 2014;109:249-85. doi: 10.1016/B978-0-12-397920-9.00003-2. PMID: 24947239. [DOI] [PubMed] [Google Scholar]
- 17.Dowling P, Gargan S, Zweyer M, Swandulla D, Ohlendieck K. Extracellular Matrix Proteomics: The mdx-4cv Mouse Diaphragm as a Surrogate for Studying Myofibrosis in Dystrophinopathy. Biomolecules. 2023. Jul;13,1108. doi: 10.3390/biom13071108. PMID: 37509144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackert-Bicknell CL, Anderson LC, Sheehan S, Hill WG, Chang B, Churchill GA, Chesler EJ, Korstanje R, Peters LL. Aging Research Using Mouse Models. Curr Protoc Mouse Biol. 2015. Jun 1;5(2):95-133. doi: 10.1002/9780470942390.mo140195. PMID: 26069080; PMCID: PMC4590775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowling P, Gargan S, Zweyer M, Henry M, Meleady P, Swandulla D, Ohlendieck K. Proteome-wide Changes in the mdx-4cv Spleen due to Pathophysiological Cross Talk with Dystrophin-Deficient Skeletal Muscle. iScience. 2020. Aug 26;23(9):101500. doi: 10.1016/j.isci.2020.101500. Epub ahead of print. PMID: 32916630; PMCID: PMC7490529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowling P, Gargan S, Zweyer M, Henry M, Meleady P, Swandulla D, Ohlendieck K. Protocol for the Bottom-Up Proteomic Analysis of Mouse Spleen. STAR Protoc. 2020. Dec 3;1(3):100196. doi: 10.1016/j.xpro.2020.100196. PMID: 33377090; PMCID: PMC7757555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiśniewski JR. Filter-Aided Sample Preparation for Proteome Analysis. Methods Mol Biol. 2018;1841:3-10. doi: 10.1007/978-1-4939-8695-8_1. PMID: 30259475. [DOI] [PubMed] [Google Scholar]
- 22.Mi H, Ebert D, Muruganujan A, Mills C, Albou LP, Mushayamaha T, Thomas PD. PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021. Jan 8;49(D1):D394-D403. doi: 10.1093/nar/gkaa1106. PMID: 33290554; PMCID: PMC7778891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taraslia VK, Kouskoukis A, Anagnostopoulos AK, Stravopodis DJ, Margaritis LH, Tsangaris GT. Proteomic analysis of normal murine brain parts. Cancer Genomics Proteomics. 2013. May-Jun;10(3):125-54. PMID: 23741028. [PubMed] [Google Scholar]
- 24.Ghanavatinejad F, Fard Tabrizi ZP, Omidghaemi S, Sharifi E, Møller SG, Jami MS. Protein biomarkers of neural system. J Otol. 2019. Sep;14(3):77-88. doi: 10.1016/j.joto.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korovesi AG, Anagnostopoulos AK, Pierros V, Stravopodis DJ, Tsangaris GT. Normal Mouse Brain Proteome II: Analysis of Brain Regions by High-resolution Mass Spectrometry. Cancer Genomics Proteomics. 2020. Nov-Dec;17(6):757-767. doi: 10.21873/cgp.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishihama Y, Sato T, Tabata T, Miyamoto N, Sagane K, Nagasu T, Oda Y. Quantitative mouse brain proteomics using culture-derived isotope tags as internal standards. Nat Biotechnol. 2005. May;23(5):617-21. doi: 10.1038/nbt1086. Epub 2005 Apr 17. PMID: 15834404. [DOI] [PubMed] [Google Scholar]
- 27.Seefeldt I, Nebrich G, Römer I, Mao L, Klose J. Evaluation of 2-DE protein patterns from pre- and postnatal stages of the mouse brain. Proteomics. 2006. Sep;6(18):4932-9. doi: 10.1002/pmic.200600188. PMID: 16912972. [DOI] [PubMed] [Google Scholar]
- 28.Föcking M, Boersema PJ, O'Donoghue N, Lubec G, Pennington SR, Cotter DR, Dunn MJ. 2-D DIGE as a quantitative tool for investigating the HUPO Brain Proteome Project mouse series. Proteomics. 2006. Sep;6(18):4914-31. doi: 10.1002/pmic.200600269. PMID: 16927420. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Ishihama Y, Oda Y. Quantitative proteomics of mouse brain and specific protein-interaction studies using stable isotope labeling. Methods Mol Biol. 2007;359:53-70. doi: 10.1007/978-1-59745-255-7_4. PMID: 17484110. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Gu Y, Wang L, Hang X, Gao Y, Wang H, Zhang C. HUPO BPP pilot study: a proteomics analysis of the mouse brain of different developmental stages. Proteomics. 2007. Nov;7(21):4008-15. doi: 10.1002/pmic.200700341. PMID: 17922513. [DOI] [PubMed] [Google Scholar]
- 31.Jung SY, Choi JM, Rousseaux MW, Malovannaya A, Kim JJ, Kutzera J, Wang Y, Huang Y, Zhu W, Maity S, Zoghbi HY, Qin J. An Anatomically Resolved Mouse Brain Proteome Reveals Parkinson Disease-relevant Pathways. Mol Cell Proteomics. 2017. Apr;16(4):581-593. doi: 10.1074/mcp.M116.061440. Epub 2017 Feb 2. PMID: 28153913; PMCID: PMC5383780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu F, Cizeron M, Qiu Z, Benavides-Piccione R, Kopanitsa MV, Skene NG, Koniaris B, DeFelipe J, Fransén E, Komiyama NH, Grant SGN. Architecture of the Mouse Brain Synaptome. Neuron. 2018. Aug 22;99(4):781-799.e10. doi: 10.1016/j.neuron.2018.07.007. Epub 2018 Aug 2. PMID: 30078578; PMCID: PMC6117470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis RG, Park HM, Kim K, Greer JB, Fellers RT, LeDuc RD, Romanova EV, Rubakhin SS, Zombeck JA, Wu C, Yau PM, Gao P, van Nispen AJ, Patrie SM, Thomas PM, Sweedler JV, Rhodes JS, Kelleher NL. Top-Down Proteomics Enables Comparative Analysis of Brain Proteoforms Between Mouse Strains. Anal Chem. 2018. Mar 20;90(6):3802-3810. doi: 10.1021/acs.analchem.7b04108. Epub 2018 Feb 26. PMID: 29481055; PMCID: PMC5861018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HM, Satta R, Davis RG, Goo YA, LeDuc RD, Fellers RT, Greer JB, Romanova EV, Rubakhin SS, Tai R, Thomas PM, Sweedler JV, Kelleher NL, Patrie SM, Lasek AW. Multidimensional Top-Down Proteomics of Brain-Region-Specific Mouse Brain Proteoforms Responsive to Cocaine and Estradiol. J Proteome Res. 2019. Nov 1;18(11):3999-4012. doi: 10.1021/acs.jproteome.9b00481. Epub 2019 Oct 2. PMID: 31550894; PMCID: PMC6917473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun X, Sun H, Han X, Chen PC, Jiao Y, Wu Z, Zhang X, Wang Z, Niu M, Yu K, Liu D, Dey KK, Mancieri A, Fu Y, Cho JH, Li Y, Poudel S, Branon TC, Ting AY, Peng J. Deep Single-Cell-Type Proteome Profiling of Mouse Brain by Nonsurgical AAV-Mediated Proximity Labeling. Anal Chem. 2022. Apr 5;94(13):5325-5334. doi: 10.1021/acs.analchem.1c05212. Epub 2022 Mar 22. PMID: 35315655; PMCID: PMC9350993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayaprolu S, Bitarafan S, Santiago JV, Betarbet R, Sunna S, Cheng L, Xiao H, Nelson RS, Kumar P, Bagchi P, Duong DM, Goettemoeller AM, Oláh VJ, Rowan M, Levey AI, Wood LB, Seyfried NT, Rangaraju S. Cell type-specific biotin labeling in vivo resolves regional neuronal and astrocyte proteomic differences in mouse brain. Nat Commun. 2022. May 25;13(1):2927. doi: 10.1038/s41467-022-30623-x. PMID: 35614064; PMCID: PMC9132937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez AM, Traunmüller L, Scheiffele P. Neurexins: molecular codes for shaping neuronal synapses. Nat Rev Neurosci. 2021. Mar;22(3):137-151. doi: 10.1038/s41583-020-00415-7. Epub 2021 Jan 8. PMID: 33420412; PMCID: PMC7612283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Hua F, Yang D, Lin Y, Zhang L, Ying J, Sheng H, Wang X. Roles of neuroligins in central nervous system development: focus on glial neuroligins and neuron neuroligins. J Transl Med. 2022. Sep 10;20(1):418. doi: 10.1186/s12967-022-03625-y. PMID: 36088343; PMCID: PMC9463862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao A, Harms KJ, Craig AM. Neuroligation: building synapses around the neurexin-neuroligin link. Nat Neurosci. 2000. Aug;3(8):747-9. doi: 10.1038/77636. PMID: 10903560. [DOI] [PubMed] [Google Scholar]
- 40.Montenegro-Venegas C, Guhathakurta D, Pina-Fernandez E, Andres-Alonso M, Plattner F, Gundelfinger ED, Fejtova A. Bassoon controls synaptic vesicle release via regulation of presynaptic phosphorylation and cAMP. EMBO Rep. 2022. Aug 3;23(8):e53659. doi: 10.15252/embr.202153659. Epub 2022 Jun 29. PMID: 35766170; PMCID: PMC9346490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith LM, Kelleher NL; Consortium for Top Down Proteomics. Proteoform: a single term describing protein complexity. Nat Methods. 2013. Mar;10(3):186-7. doi: 10.1038/nmeth.2369. PMID: 23443629; PMCID: PMC4114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forgrave LM, Wang M, Yang D, DeMarco ML. Proteoforms and their expanding role in laboratory medicine. Pract Lab Med. 2021. Nov 27;28:e00260. doi:10.1016/j.plabm.2021.e00260. PMID: 34950758; PMCID: PMC8672040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witzmann FA, Arnold RJ, Bai F, Hrncirova P, Kimpel MW, Mechref YS, McBride WJ, Novotny MV, Pedrick NM, Ringham HN, Simon JR. A proteomic survey of rat cerebral cortical synaptosomes. Proteomics. 2005. May;5(8):2177-201. doi: 10.1002/pmic.200401102. PMID: 15852343; PMCID: PMC1472619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Errante L, Tang D, Gardon M, Sekerkova G, Mugnaini E, Shaw G. The intermediate filament protein peripherin is a marker for cerebellar climbing fibres. J Neurocytol. 1998. Feb;27(2):69-84. doi: 10.1023/a:1006991104595. [DOI] [PubMed] [Google Scholar]
- 45.Figueiro-Silva J, Gruart A, Clayton KB, Podlesniy P, Abad MA, Gasull X, Delgado-García JM, Trullas R. Neuronal pentraxin 1 negatively regulates excitatory synapse density and synaptic plasticity. J Neurosci. 2015. Apr 8;35(14):5504-21. doi: 10.1523/JNEUROSCI.2548-14.2015. PMID: 25855168; PMCID: PMC6605318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dibattista M, Al Koborssy D, Genovese F, Reisert J. The functional relevance of olfactory marker protein in the vertebrate olfactory system: a never-ending story. Cell Tissue Res. 2021. Jan;383(1):409-427. doi: 10.1007/s00441-020-03349-9. Epub 2021 Jan 15. PMID: 33447880; PMCID: PMC7878404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu C, Kastin AJ, Ding Y, Pan W. Gamma glutamyl transpeptidase is a dynamic indicator of endothelial response to stroke. Exp Neurol. 2007. Jan;203(1):116-22. doi: 10.1016/j.expneurol.2006.07.023. Epub 2006 Sep 14. PMID: 16973162. [DOI] [PubMed] [Google Scholar]
- 48.Bamford RA, Widagdo J, Takamura N, Eve M, Anggono V, Oguro-Ando A. The Interaction Between Contactin and Amyloid Precursor Protein and Its Role in Alzheimer's Disease. Neuroscience. 2020. Jan 1;424:184-202. doi: 10.1016/j.neuroscience.2019.10.006. Epub 2019 Nov 6. PMID: 31705890. [DOI] [PubMed] [Google Scholar]
- 49.Li D, Liu X, Liu T, Liu H, Tong L, Jia S, Wang YF. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia. 2020. May;68(5):878-897. doi: 10.1002/glia.23734. Epub 2019 Oct 18. PMID: 31626364. [DOI] [PubMed] [Google Scholar]


