Abstract
The study aimed to evaluate the effectiveness of radial extracorporeal shock wave therapy (rESWT) and conventional physical therapy (CPT) protocol on the gait pattern in stroke survivors through a new gait analysis technology. Fifteen (n=15) stroke survivors took part in this prospective, observational study and were assessed clinically and through an instrumented treadmill before and after rESWT and CPT. Spasticity grade 95% CI 0.93 (0.79 +/- 1.08), pain intensity 95% CI 1.60 (1.19 +/- 2.01), and clonus score decreased significantly 95% CI 1.13 (0.72 +/- 1.54). The sensorimotor function 95% CI -2.53 (-3.42 +/- 1.65), balance 95% CI -5.67 (-6.64 +/- - 4.69), and gait parameters were enhanced at the end of the program. Step length 95% CI -3.47 (-6.48 +/- 0.46) and step cycle were improved 95% CI -0.09 (-0.17 +/- -0.01), and hip 95% CI -3.90 (-6.92 +/- -0.88), knee 95% CI -2.08 (-3.84 +/- -0.32) and ankle flexion-extension 95% CI -2.08 (-6.64 +/- -4.69) were augmented. Adding the quantitative analysis to the clinical assessment, we gained easy access to track progress and obtained an individualized therapeutic approach for stroke survivors.
Key Words: spasticity, stroke, neurorehabilitation, gait analysis, shock wave therapy
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Stroke is one of the leading causes of long-term disability in adults worldwide with an increasing number of persons affected by this condition.1 Stroke related impairments such as spasticity, muscle weakness, pain, sensorimotor deficits, and poor balance can often lead to a decreased tolerance to daily life activities, autonomy restriction, and sedentary lifestyle.2,3 Gait is a complex task and parameters such as spatiotemporal, kinematic, and kinetic variables are often modified in patients with walking disorders.3 Currently, new technological gait analysis systems offer the possibility of creating a database, reproducible results, and easy access for tracking progress and efficacy of neurorehabilitation programs through telerehabilitation (TR) implementation in the clinical practice, especially since the COVID-19 pandemic context.4,5 Quantitative gait analysis systems include instrumented treadmills as a new alternative to previous overground gait analysis systems, and also promote the assessments and effective therapeutic delivery of care to neurological patients after discharge.3,5,6 Objective, quantitative parameters are needed to ensure good quality in clinical practice since the most commonly used methods for gait analysis were based either on visual observation or questionnaires, which are considered poorly reliable and insufficient methods.7 Currently, the gold standard for measuring and evaluating spatiotemporal and kinematic gait parameters is represented by various 3D motion capture systems.8,9 Poor balance control and gait impairments interfere with standing and walking ability in stroke survivors.2 Gait dynamics changes its pattern especially in stroke survivors due to increased muscle tone and balance impairment. Early detection and assessment of gait anomalies through gait analysis systems evaluate stride length and duration, lower limb range of motion (ROM), limb symmetry or asymmetry, providing objective parameters and ensuring evaluation of progress and recovery during the rehabilitation program.3
Usually, the rehabilitation of post-stroke patients is a long-term process involving multiple assessments. However, during the pandemic of COVID-19, the access to healthcare delivery throughout the world has been significantly impacted creating collateral damages for both, rehabilitation facilities and stroke survivors.
One of the key changes implemented by the health care systems in order to promote rehabilitation continuity, was the rapid expansion of several TR programs and tele-assessments strategies.5,6 Systems such as newly instrumented treadmills for spatiotemporal, kinematic, and kinetic parameters assessment with good levels of agreement, seem a new alternative to previous overground gait analysis systems.3 Integrating clinical assessment and virtual evaluation can easily offer objective data by tracking progress and also give the possibility to a tailored treatment for patients based on their performance at different moments.10
Among the emerging therapies, extracorporeal shock wave therapy (ESWT) is a non-invasive therapeutic intervention used for musculoskeletal disorders, several inflammatory tendon diseases, or spasticity.11 The findings suggest that ESWT may be useful in decreasing plantar flexor muscle tone for adults and children affected by lower limb spasticity.10,12-16 There are some trials which had also showed that radial extracorporeal shockwave therapy (rESWT) was more effective on augmenting the ankle passive range of motion (PROM) compared to focal extracorporeal shockwave therapy (fESWT).13,14 In addition, ESWT proved to be effective not only as treatment for lower limb spasticity, but also for gait pattern since combined with a conventional physical therapy (CPT) program decreased spasticity and improved gait pattern in children with hemiplegic cerebral palsy (CP).12 The findings on ESWT effectiveness for gait pattern improvement could open a new paradigm applicable for the research of this promising, non-invasive therapy already effective for spasticity management.
Added to clinical evaluation, the instrumented treadmills serve both as a tool for gait training and also for recording and measuring spatiotemporal and kinematic parameters, offering objective data for gait analysis, especially after treatment delivery.10,15 Gait analysis through systems such as instrumented treadmills play an important role in clinical practice and clinical trials by assessing gait patterns related to various disorders. This type of analysis through instrumented treadmills is based on measurements which offer the possibility for repeatability. Through the inertial measurement units (IMUs) usage it is granted good validity and reliability for mean spatiotemporal parameters during gait phases.17 Although there are trials validating different types of 3D cameras for gait analysis on the treadmills and overground, only few of them assessed the instrumented treadmills. However, there are gait analysis systems which showed good levels of agreement, and added to the clinical assessment provide the assessor with objective, real-time data.3,10,18-22
Therefore, the aim of this study is to objectively evaluate the effectiveness of rESWT and CPT protocol on the gait pattern in stroke survivors through a new gait analysis technology. This type of technology encompasses spatiotemporal and kinematic parameters, and the findings are correlated with the clinical evaluation, offering a global assessment of gait parameters.
Materials and Methods
Design overview and ethical approval
The present study was a prospective, single-center, observational study performed from March 2021 to August 2022 at the Physical and Rehabilitation Medicine Department, Elias University Emergency Hospital, Bucharest, Romania. The trial was carried out in compliance with the Helsinki Declaration of Ethical Principles for Medical Research, in which human subjects participate, and was approved by the Ethics Committee of the Elias University Emergency Hospital, Bucharest, Romania, Prot. No. 2090/2021. Participants were informed about the objectives of the study and the written informed consent was obtained from all the patients prior to their participation in the study.23
According to the World Health Organization recommendations, the study was prospectively registered in the Register of ClinicalTrials.gov with International Operations under registration number NCT05206240. The study followed the guidelines of STrengthening the Reporting of OBservational studies in Epidemiology (STROBE), the STROBE Statement.24
Patient evaluation was conducted at baseline (T0) and after the end of the CPT protocol and rESWT delivery (T1). The same assessor performed both evaluations. International scales were used for the clinical evaluation and consisted of: Modified Ashworth Scale (MAS), PROM, Visual Analogue Scale (VAS), Clonus score, Fugl-Meyer Assessment for Lower Extremity (FMA-LE), Tinetti Assessment Tool (TAT), Functional Ambulation Categories (FAC), and Timed Up and Go Test (TUG). For the gait analysis there were taken into consideration kinematic and spatiotemporal parameters.
Study participants
Patients admitted to the Physical and Rehabilitation Medicine Department, Elias University Emergency Hospital, Bucharest, Romania were assessed for eligibility. The patients were enrolled in the study if they met the inclusion criteria: 1) suffered from a hemorrhagic or ischemic stroke and they were in the subacute or chronic phase; 2) had no previous stroke history; 3) had lower limb post-stroke spasticity and spasticity grade ≥1 on the MAS; 4) pain intensity measured on the VAS ≥1; 5) ability to stand and walk unassisted for at least 30 seconds; and 6) adult patients (>18 years old). Exclusion criteria consisted of: 1) other neurological, musculoskeletal, orthopedic, or cardiovascular conditions; 2) changes in antispastic medication and dosage, and changes in the analgesic medication in the last month; 3) severe aphasia or inability to understand instructions; 4) severe spasticity grade; 5) myopathy; 6) hemineglect or visual field conditions; 7) anticoagulant medication or any contraindication to receive rESWT, or any contraindication to receive physical therapy sessions.
CPT protocol and rESWT delivery
CPT is defined by the use of various therapies and techniques according to each rehabilitation department or center. Programs and therapies involving verticalization strategies and techniques, ROM exercises, muscle stretching and strengthening exercises, stance and balance training, core stability exercises, gait training, functional training, the use of physical agents and other therapies, and orthoses are part of the CPT protocol along with the occupational therapy sessions, neuropsychology sessions, or speech therapy when indicated. The frequency of the CPT delivery program was 1h/day, 5 days/week during a 10-day rehabilitative program and all the patients followed the same program.
After performing the baseline screening and assessment, the patients considered eligible started the CPT protocol and rESWT delivery. No ultrasonographic guidance was used to detect the rESWT application site. The site for rESWT delivery (Endopuls 811; Enraf Nonius B.V. Vareseweg 127, 3047 AT Rotterdam MedTech, The Netherlands) was the myotendinous junction of the hypertonic triceps surae muscle. The patients completed two rESWT sessions during hospital stay and each session consisted of 2000 shots applied on the myotendinous junction of triceps surae with a frequency of 10 Hz and an energy density of 60 mJ. The therapy delivery did not require any analgesics or local anesthesia. Figure 1 presents the rESWT delivery sessions.
Study participants were assessed at baseline and at the end of the CPT protocol and rESWT delivery by the same assessor, and the adverse events were also monitored during the study. Figure 2 presents the schematic of the CPT protocol and rESWT delivery frequency, time intervals, and the time points of assessments.
Clinical endpoints and evaluation
The clinical outcomes consisted of spasticity grade evaluated through the Modified Ashworth Scale (MAS), PROM measured by a hand-held goniometer, pain intensity ranked on the VAS, Clonus score, the sensorimotor function assessed by FMA-LE, mobility, balance, and gait evaluated through TAT, FAC, and TUG. For the MAS evaluation of the hypertonic triceps surae muscle, patients were laying in supine position with the lower limb in extension, knee fully extended, and stabilized joint. The MAS score ranges from 0 to 4 and it includes a rating of 1+ grade. For the sake of the statistical analysis, a MAS grade 1+ matched 2 points and the grades 2, 3, and 4 were correlated to 3, 4, and 5 points. Pain intensity was measured through the VAS using a 10 cm line (describing pain-free state up to the worst pain possible), and the Clonus score evaluated the number of beats until sustained clonus. FMA-LE consisted of different assessments of sensorimotor function with a maximum score of 28 points. TAT was scored on the patients’ ability to perform specific tasks by using a three-point ordinal scale.
Fig 1.
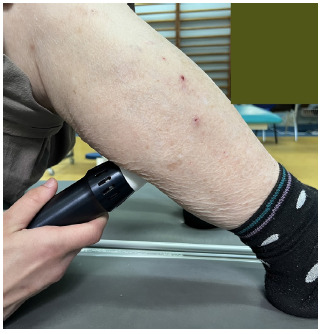
Application of rESWT in a stroke survivor. The original picture was taken by the author E.E. Mihai in the Department of Physical and Rehabilitation Medicine, Elias University Emergency Hospital, Bucharest, using a digital camera.
Fig 2.
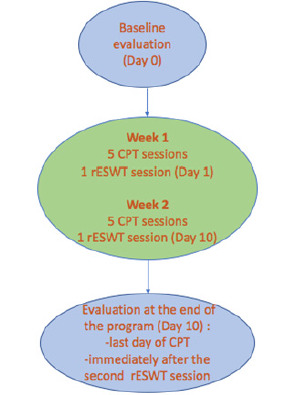
Schematic of the CPT protocol and rESWT delivery
The FAC is a 6-point functional walking test that evaluated the patients’ ambulation ability, and TUG was used as an assessment test to measure the patients’ functional mobility and the risk of falls. The assessment was conducted at baseline and follow-up by the same assessor.
Fig 3.
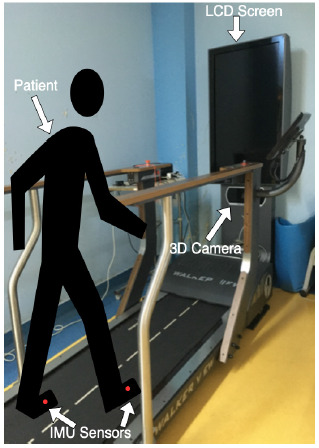
Schematic of the instrumented treadmill Walker View and one participant with IMUs sensors applied on the feet (red dots). Original figure taken by the author E.E. Mihai at the Physical and Rehabilitation Medicine Department of Elias University Emergency Hospital, Bucharest using a digital camera.. Abbreviations: IMUs – inertial measurement units.
Walker View instrumented treadmill gait analysis system
The instrumented treadmill Walker View (TecnoBody®, Bergamo, Italy) was used for quantitative gait analysis, and it consisted of an instrumented belt with eight load cells, and a 3D camera for motion capture (model type Kinect v2, Microsoft). A skilled physical therapist controlled the instrumented tread-mill through a touch screen connected to a personal computer (PC), and a LCD screen displayed real-time visual feedback both for the patient and the physical therapist (Figure 3). The system also displayed on the screen the participants’ avatar in accordance with their appearance, a feature that held their interest and gave them the possibility of further exploring the benefits of real-time visual feedback and virtual reality while using avatars. Since we evaluated post-stroke patients and most of them experienced mild to moderate spasticity and walking difficulties, the speed used was 0.2 km/h (0.05m/s). Also, an experienced physical therapist was always standing next to the patients to ensure safety and to limit any risk of falls.
Spatiotemporal and kinematic parameters
The integrated software of Walker View system performs a real-time analysis of the spatiotemporal parameters (step cycle time, contact time, stance and swing time for foot flexion-extension and pronation-supination, left and right step length) and of the kinematic parameters (trunk flexion-extension, trunk lateral flexion, total ROM, maximum and minimum angle values in the sagittal plane of hip and knee). The knee angle variation in flexion and extension in the sagittal plane could be used to calculate the components of the entire walking cycle.
Spatiotemporal outcome measures consisted of the following variables: walking speed (meters/second), step cycle time (cycles/second), step length (meters), contact time (seconds), stance (degrees), and swing (degrees). Step time represents the time range between the contralateral and the ipsilateral foot contact. Step length is the linear distance between contralateral and ipsilateral foot contact. Stance is described as the entire amount of time the foot is on the ground, and swing represents the amount of time the foot is in the air for foot advancement. Finally, the walking speed is calculated as the stride length divided by the stride time.
Concerning the trunk kinematics, thoracic tilting (trunk flexion-extension) and trunk lateral flexion movements were observed and measured. As for the ROM during stance and swing phase, it was calculated as the average value angle and as the difference between the maximum angle minus the minimum angle. The data was stored on a pdf file and transferred to a PC for further analysis.
Statistical Analysis
Patient characteristics were described as mean and standard deviation (SD) for the continuous data. Baseline mean values and after treatment mean values were calculated and the paired sample t-test was applied to investigate changes of clinical outcomes, spatiotemporal parameters, and kinematic variables. Additionally, 95% confidence intervals (95% CI) were also estimated for every clinical outcome, spatiotemporal and kinematic parameter. The confidence interval does not reflect the variability in the unknown parameter, it rather reflects the amount of random error in the sample. We also considered the presence of distribution of residuals and any influential points. Values of p were also adjusted for multiple comparisons. Pearson’s Correlation Coefficient was used to measure the strength and the relationship between variables.
The Bonferroni correction was also used to avoid type I error since there are multiple outcome measures to be taken into account. A threshold for significance was set for the clinical outcomes (p = 0.006), spatiotemporal, and kinematic parameters (p = 0.003) considering the number of the statistical tests.
The statistical analyses and data processing were computed through Microsoft Excel (Microsoft Excel for Mac, Version 16.38, 2020, Microsoft), GraphPad Software (San Diego, CA, USA), and MATLAB (R2016a, The MathWorks, Inc, Natick, MA, USA), and a p < 0.05 was considered statistically significant.
Results
The enrollment flow chart according to the STROBE guidelines is presented in Figure 4.24 Two hundred and fifty two volunteers were screened, while twenty four of them met the inclusion criteria. Seven participants declined to participate in the study, and other two eligible subjects suffered complications in the early stages of the CPT program. Eventually, 15 patients were included in the study. In the study and final analysis there were enrolled 7 male patients and 8 female patients, with an average age of 60.15 years old, the youngest patient was 41-year-old and the oldest one was 85-year-old. The average time since stroke onset was of 29.01 months, the most recent was 2 months since stroke onset and the latest was 59 months since stroke onset. There were 12 patients diagnosed with ischemic stroke and 3 patients with hemorrhagic stroke based on the cerebral MRI or the CT scan. Also, 5 patients had the right side of the body affected, while 10 patients had the left side affected.
Clinical endpoints
An outline regarding clinical endpoints is provided in Supplementary Table 1. All the parameters showed a statistically significant improvement after the interventions, p < 0.05. For the spasticity grade measured through the MAS there was a statistically significant difference (p < 0.0001) between the mean values of MAS scores obtained at the baseline (T0) and at the post-treatment (T1) evaluation. The MAS score mean values (SD) pre-treatment were 2.07 (0.64), while post-treatment they were 1.15 (0.55), the results indicating that patients experienced significant improvement regarding the spasticity grade. For the ankle PROM it was also found a statistically significant improvement (p < 0.0001), which could be correlated to the decrease in the spasticity grade and consequent gain in terms of amplitude (degrees). Pearson Correlation Coefficient was used to determine the relationship of decreased spasticity grade and augmented PROM, showing good agreement. Concerning the pain intensity measured on the VAS, a statistically significant change was found between baseline and post-treatment comparison (p < 0.0001). Also, the Clonus score showed significant improvement post-treatment, the mean value (SD) decreasing from 1.61 (1.19) to 0.46 (0.77), (p < 0.0001). For the clinical outcome measures the Bonferroni correction was p < 0.0001.
Fig 4.
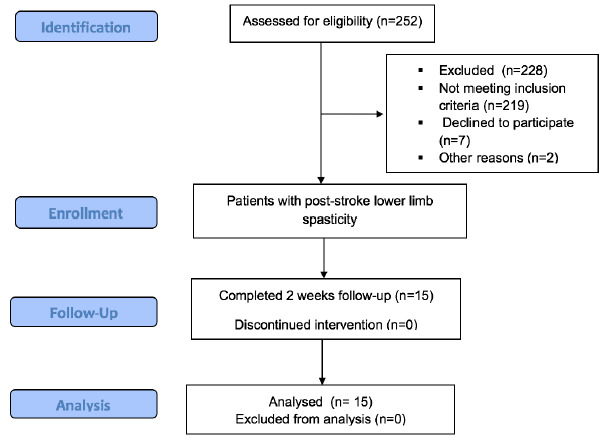
STROBE flow chart of the study.24 Abbreviations: STROBE: STrengthening the Reporting of OBservational studies in Epidemiology.
After applying the correction and considering the number of statistical tests, the threshold for significance was 0.006 for the clinical outcomes. With regard to the lower limb function, balance, and gait there were also found statistically significant improvements of these parameters. FMA-LE scored better results post-treatment, from 20 (1.68) to a mean point (SD) of 22.69 (1.18), (p < 0.0001), 95% CI -2.53 (-3.42-1.65). TAT also yielded significant improvement starting from a mean score (SD) of 18.53 (3.59) pre- treatment to 24.38 (2.75) post-treatment. The mean value (SD) for FAC increased from 5.23 (0.72) pre-treatment to 5.84 (0.37) post-treatment, (p < 0.0001). Improvement for TUG proved to be statistically significant, the meantime decreasing from 28.06 (6.63) pre-treatment to 22.93 (4.96) post-treatment, (p < 0.0001). The results indicated improvement in terms of spasticity grade, clonus, PROM, pain intensity, lower limb function, balance, and gait post-treatment when compared to the baseline assessment. The statistical significance maintained after applying the correction for all the clinical outcomes (p < 0.006).
Kinematic parameters
The kinematic and spatiotemporal parameters evaluated through Walker View system before and after the treatment are presented in Supplementary Table 2. All the parameters have shown a statistically significant change from the baseline except for trunk lateral flexion (p = 0.9), the Bonferroni correction = 0.069. Regarding trunk flexion-extension, it was noted a statistically significant change from the baseline (p = 0.03). Additionally, parameters such as ROM of hip flexion-extension and knee flexion-extension were also evaluated, and both of them yielded augmented results in terms of degrees. Therefore, mean values (SD) for hip flexion-extension ROM augmented from 15.12 (5.19) to 18.85 (6.54) post-treatment, (p = 0.01), while knee flexion-extension increased from 16.14 (8.51) at baseline to 23.47 (9.04) post-treatment, (p = 0.02).
Concerning foot flexion-extension, a statistically significant change was also noted, with a mean value (SD) 10.46 (4.73) pre-treatment and augmenting up to 11.92 (3.93) post-treatment, (p = 0.02). As for foot pronation- supination, it was also found a statistically significant change from 0.84 (2.21) pre-treatment to 1.76 (2.61), (p = 0.03). These results showed significant improvement post-treatment when compared to the baseline evaluation for the kinematic parameters. After applying the Bonferroni correction and considering the number of statistical tests for the kinematic and spatiotemporal parameters, the threshold for significance was set at 0.003.
Spatiotemporal parameters
Regarding the spatiotemporal parameters, there were also noted significant changes regarding step length, step cycle time, stance foot flexion-extension, foot stance pronation- supination, and foot swing flexion-extension. We applied the Bonferroni correction for all the endpoints, the level for significance was set at 0.003 after considering all the statistical tests, and the significance maintained for these outcomes.
The only parameters which showed no significant change from the baseline were the contact time on the affected side and foot swing pronation-supination. The mean value (SD) of step length (m) showed a statistically significant change from the baseline, (p = 0.02), increasing from 0.08 (5.24) to 0.12 (7.25) at the end of was 1.10 (3.92), (p = 0.10). The mean value (SD) for stance foot flexion-extension was -2.18 (2.94) at baseline and -1.14 (3.63) at follow-up, (p = 0.05). Additionally, the mean (SD) for stance foot pronation-supination was 1.9 (2.32) at baseline and 3.23 (3.38) at follow-up, (p = 0.04). As for the swing foot flexion-extension, the mean values (SD) at baseline were -12.86 (5.05) and 13.06 (3.23) post-treatment, (p = 0.05). Concerning the mean values (SD) for swing foot flexion-extension, at baseline the score was -0.18 (3.15), while post-treatment it was 1.10 (3.92), (p = 0.10). The results suggested significant improvement regarding spatiotemporal parameters such as step length, step cycle time, foot stance flexion-extension, foot stance pronation- supination, and foot swing flexion-extension (p < 0.003). Additionally, we checked data for normality using the Jarque-Bera test. Regarding the gait analysis parameters, data for the kinematic parameters were normally distributed, while for the spatiotemporal parameters was non-normally distributed with the median value/IQR of 0.08/2.35 at baseline and 0.12/2.645 at the end of the neurorehabilitation program.
Discussion
The aim of this observational study was to objectively evaluate the effects of rESWT and CPT protocol on the gait pattern, spatiotemporal variables, and kinematic parameters through a new gait analysis system and correlate the findings with the clinical outcomes. The gait pattern of post-stroke patients before and after rESWT delivery and conventional rehabilitation program was assessed through an instrumented treadmill (Walker View) in terms of spatiotemporal and kinematic gait parameters. The usually available instrumented treadmills which are used in practice do not encompass cameras, hence the kinematic gait analysis cannot be performed.3,20,25 Through its software, the system we used, provides the users with a real-time report which encompasses an analysis of spatiotemporal and kinematic parameters for both sides of the body, separately.
The results of this observational study indicated that a 10-day program of combined rESWT and CPT yielded significant improvement especially in terms of spasticity grade and gait pattern.
However, the most significant improvement was achieved for all the clinical endpoints such as spasticity grade, PROM, clonus, pain intensity, lower limb function, and gait (p < 0.006). The results are in agreement with those from studies which assessed the effectiveness of ESWT both in post-stroke adults and children with cerebral palsy (CP) and also found a medium and long-term reduction effect of the muscle tone in spastic muscles.12,26,27 The results of our study presented a significant reduction (p < 0.0001) of the spasticity grade measured on the Modified Ashworth Scale and augmented PROM, (p < 0.0001). These results are also in agreement with those from other trials, which showed that after rESWT delivery a decrease in terms of spasticity grade was shown, with a consequent increase in the ROM, and a significant augmentation of the plantar surface area and peak pressure at the pedobarometric evaluations.26,28 The results of our study are in line with other trials given that clinical and instrumental data supported the effectiveness of ESWT in reducing the grade of muscle spasticity in children with CP, as well as in post-stroke adults.6,12,29 Positive effects of rESWT delivered during a CPT protocol were also noticed for outcomes such as sensorimotor and lower limb function, balance, and gait. A possible explanation for these positive changes after ESWT could be that in children with CP the plantar surface area increased, and consequently, the gait pattern also improved leading to better stability and balance control.12
In our study the decrease in pain intensity and clonus score was statistically significant. The positive effects of ESWT on pain could be explained through this therapy’s efficacy on musculoskeletal diseases for treating pain and decreasing the inflammation, while inducing long-term tissue regeneration, antalgic and anti-inflammatory properties.12,15,16 ESWT initiates mechanical effects, neovascularization, resorption of calcium deposits, changes in the epithelial cell permeability, free radical and NO formation, and also growth factor production.9
To the best of our knowledge, no previous three-dimensional gait analysis study has yet investigated the effects of rESWT delivery combined with CPT in stroke survivors through spatiotemporal parameters and kinematic variables added to the clinical evaluation, aiming to track changes in gait parameters linked to motor recovery. The quantitative analysis offers comparative evaluations throughout the rehabilitation program and TR interventions, and helps to objectively track the patient’s progress. Gait is a very complex process and the role of quantitative gait analysis combined with the clinical judgement opens a new paradigm in discovering and assessing the pathological gait and therefore, the possibility of adapted, tailored interventions, rehabilitation programs, and progress tracking. The validation of different gait analysis technology is becoming an important feature added to the clinical evaluation, showing reliability for both healthy subjects and patients.5,6
According to a recent clinical trial, improved spatiotemporal parameters were linked to improved balance and ambulation within the first 3 months post-stroke.30 Gait speed is a non-specific parameter but it is often associated with various gait parameters, such as step length, cadence, or single support time.30 However, significant improvement of spatiotemporal gait parameters is linked to improved balance and walking ability.
The results from our study yielded significant improvement of spatiotemporal and kinematic parameters (p < 0.003) correlated with the clinical endpoints. Thus, patients could also experience safer and more energy cost-efficient gait. Changes in kinematic and spatiotemporal gait parameters led to changes in balance, and consequently, changes in the gait pattern. A longer amount of time in single support phase corresponding to the affected side may lead to a longer swing phase for the unaffected limb and would also lead to increased step length. It was also suggested that balance improved consequently to asymmetry decrease.30
Results from our study showed that changes in kinematic and spatiotemporal gait parameters are associated with changes in spasticity grade, balance, and gait. Changes in gait speed may be beneficial to monitor the safety and gait‘s cost-efficiency in post-stroke patients. After rESWT delivery and CPT program, the clinical endpoints, spatiotemporal gait parameters and kinematic variables were significantly improved. Correlations between kinematic, spatiotemporal parameters, and clinical out-comes are consistent with improved balance and gait. The more important the decrease in the spasticity grade, the stronger the improvement in spatiotemporal and kinematic parameters was shown, and therefore, safer and better gait capacity was achieved in post-stroke survivors. The instrumented treadmill is a novel tool for gait analysis and even though we did not validate it against other currently used gait measurement units, there are good validity and reliability of IMUs for mean spatiotemporal parameters during walking. The assessment of some of the gait parameters through the gait analysis system was performed in a comparative manner as those from the international scales.17
Some limitations of our study should be mentioned. Firstly, a strong limitation is the lack of a control group. Secondly, not all the mechanisms of action of rESWT on spasticity and gait pattern were entirely determined and further larger, high-quality clinical trials are required. Indeed, there are no specific guidelines or protocols, especially for the lower limb concerning the number of ESWT sessions, intensity, number of pulses, or application site for gait pattern improvement, and therefore, quantifying the effects is still a challenge both in clinical practice and clinical trials. Thirdly, the sample size was not so numerous given the context of COVID-19 pandemic, and this feature could increase the effect of the intervention. Additionally, all the included subjects were recruited from one center i.e. Elias University Emergency Hospital, Bucharest, Romania, and one region of Romania, thus limiting the generalizability of the results obtained. However, the results were consistent with those from the literature, and correlations were performed between the endpoints to avoid type I error. Finally, patients who could not safely ambulate for at least 30 seconds could not be included in the trial, which also limited the number of participants. Despite these limitations, we conclude that our CPT protocol, which includes rESWT, led to beneficial effects on both lower limb spasticity and gait parameters of stroke survivors who participated in our study. A major strength of this study consists of the use of new, valuable tools complementing the clinical evaluation for post-stroke patients, highlighting the importance of tailored, objective assessment, and the possibility of continuous care delivery even after discharge through TR and tele-assessment programs. In current practice, objective, quantitative gait analysis plays a crucial role for the assessment of functionality, treatment implementation, endpoints, and progress tracking during and after the rehabilitation program. Future research must focus on the applicability of such gait analysis systems for TR interventions and tele-assessment after patient discharge and also on repeatability, validity and reliability of the gait analysis system. The data presented in our study suggest that rESWT intervention integrated in a CPT program improved clinical endpoints, spatiotemporal and kinematic parameters. This resulted in decreased spasticity grade, pain intensity, and clonus score and it enhanced sensorimotor outcome and lower limb function, balance, and gait in stroke survivors demonstrating lower limb spasticity. These valuable tools and systems for gait analysis can easily acquire, store and assess complex data and provide the assessor with predictive analysis reports.
However, integrating a gait analysis system within a healthcare service is a challenge and requires multiple steps and key factors, but it is gaining rapid interest since it can change the paradigm in post-stroke gait analysis through the usefulness of quantitative parameters.
These results need to be further confirmed by larger, high-quality clinical trials and future research should further assess the current use of quantitative gait analysis systems to also facilitate easier and more reliable implementation of the TR programs in daily clinical practice. Further understanding of the mechanisms underlying these findings will allow for the development of innovative CPT protocols for the treatment of stroke survivors.
Acknowledgments
The authors thank the volunteers for their cooperation. Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila Bucharest, Romania through the institutional program Publish not Perish.
List of acronyms
- ADL
activities of daily living
- Bonf Corr
Bonferonni correction
- BoNT-A
Botulinum toxin type A
- CI
confidence interval
- COP
center of pressure
- CP
cerebral palsy
- CPT
conventional physical therapy
- ESWT
extracorporeal shock wave therapy
- FAC
functional ambulation categories
- fESWT
focal extracorporeal shockwave therapy
- FMA-LE
Fugl-Meyer assessment for lower extremity
- IMUs
inertial measurement units
- MAS
modified Ashworth scale
- PROM
passive range of motion
- rESWT
radial extracorporeal shock wave therapy
- ROM
range of motion
- SD
standard deviation
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
- TAT
Tinetti assessment tool
- TR
Telerehabilitation
- TUG
timed up and go test
- VAS
visual analogue scale
Funding Statement
Funding: The authors have not received specific funding for this work.
Contributor Information
Jannis Papathanasiou, Email: giannipap@yahoo.co.uk.
Kiril Panayotov, Email: zkm4@abv.bg.
Yana Kashilska, Email: iana_kashilska@abv.bg.
Eugenia Rosulescu, Email: rosulescu.eugenia@ucv.ro.
Calogero Foti, Email: foti@med.uniroma2.it.
Mihai Berteanu, Email: mihai.berteanu@umfcd.ro.
References
- 1.Donkor ES. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res Treat. 2018. Nov 27;2018:3238165. doi: 10.1155/2018/3238165. PMID: 30598741; PMCID: PMC6288566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M, You H, Zhang H, Zhao W, Han T, Liu J, Jiang S, Feng X. Effects of visual feedback balance training with the Pro-kin system on walking and self-care abilities in stroke patients. Medicine (Baltimore). 2020. Sep 25;99(39):e22425. doi: 10.1097/MD.0000000000022425. PMID: 32991477; PMCID: PMC7523840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bravi M, Massaroni C, Santacaterina F, Di Tocco J, Schena E, Sterzi S, Bressi F, Miccinilli S. Validity Analysis of WalkerViewTM Instrumented Treadmill for Measuring Spatiotemporal and Kinematic Gait Parameters. Sensors (Basel). 2021. Jul 14;21(14):4795. doi: 10.3390/s21144795. PMID: 34300534; PMCID: PMC8309770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan DM, Khandoker AH, Wasti SA, Ismail Ibrahim Ismail Alali S, Jelinek HF, Khalaf K. Assessment Methods of Post-stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front Neurol. 2021. Jun 8;12:650024. doi: 10.3389/fneur.2021.650024. PMID: 34168608; PMCID: PMC8217618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papathanasiou J, Kashilska Y, Bozov H, Petrov I, Masiero S. The outbreak of the SARS-CoV-2 Omicron variant make imperative the adoption of telerehabilitation in the Bulgarian health care system. Eur J Transl Myol. 2022. Feb 2;32(1):10355. doi: 10.4081/ejtm.2022.10355. PMID: 35107088; PMCID: PMC8992671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mihai EE, Popescu MN, Beiu C, Gheorghe L, Berteanu M. Tele-Rehabilitation Strategies for a Patient With Post-stroke Spasticity: A Powerful Tool Amid the COVID-19 Pandemic. Cureus. 2021. Nov 2;13(11):e19201. doi: 10.7759/cureus.19201. PMID: 34877194; PMCID: PMC8642141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindemann U. Spatiotemporal gait analysis of older persons in clinical practice and research : Which parameters are relevant? Z Gerontol Geriatr. 2020. Mar;53(2):171-178. English. doi: 10.1007/s00391-019-01520-8. Epub 2019 Feb 15. PMID: 30770991. [DOI] [PubMed] [Google Scholar]
- 8.Ziagkas E, Loukovitis A, Zekakos DX, Chau TD, Petrelis A, Grouios G. A Novel Tool for Gait Analysis: Validation Study of the Smart Insole PODOSmart®. Sensors (Basel). 2021. Sep 6;21(17):5972. doi: 10.3390/s21175972. PMID: 34502861; PMCID: PMC8434608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang M, Awrejcewicz J, Fekete G, Ren F, Gu Y. Using Gold-standard Gait Analysis Methods to Assess Experience Effects on Lower-limb Mechanics During Moderate High-heeled Jogging and Running. J Vis Exp. 2017. Sep 14;(127):55714. doi: 10.3791/55714. PMID: 28994758; PMCID: PMC5752245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihai EE, Berteanu M. Early Individualized Approach for a Patient with Spasticity of Stroke Origin. Curr Health Sci J. 2021. Oct-Dec;47(4):608-611. doi: 10.12865/CHSJ.47.04.20. Epub 2021 Dec 31. PMID: 35444829; PMCID: PMC8987471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maccarone MC. Extracorporeal Shock Wave Therapy (ESWT). In: Papathanasiou J, Panayotov K, eds. Essentials of Physical and Rehabilitation Medicine for undergraduate medical students. Ruse, BG: Avangard Print; 2023. pp 135-142. [Google Scholar]
- 12.El-Shamy SM, Eid MA, El-Banna MF. Effect of extracorporeal shock wave therapy on gait pattern in hemiplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil. 2014. Dec;93(12):1065-72. doi: 10.1097/PHM.000000 0000000133. PMID: 24879552. [DOI] [PubMed] [Google Scholar]
- 13.Mihai EE, Popescu MN, Iliescu AN, Berteanu M. A systematic review on extracorporeal shock wave therapy and botulinum toxin for spasticity treatment: a comparison on efficacy. Eur J Phys Rehabil Med. 2022. Aug;58(4):565-574. doi: 10.23736/S1973-9087.22.07136-2. Epub 2022 Apr 12. PMID: 35412036; PMCID: PMC9980509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radinmehr H, Ansari NN, Naghdi S, Tabatabaei A, Moghimi E. Comparison of Therapeutic Ultrasound and Radial Shock Wave Therapy in the Treatment of Plantar Flexor Spasticity After Stroke: A Prospective, Single-blind, Randomized Clinical Trial. J Stroke Cerebrovasc Dis. 2019. Jun;28(6):1546-1554. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.008. Epub 2019 Mar 30. PMID: 30935809. [DOI] [PubMed] [Google Scholar]
- 15.Mihai EE, Dumitru L, Mihai IV, Berteanu M. Long-Term Efficacy of Extracorporeal Shock Wave Therapy on Lower Limb Post-Stroke Spasticity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2020. Dec 29;10(1):86. doi: 10.3390/jcm10010086. PMID: 33383655; PMCID: PMC7795167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang E, Lew HL, Özçakar L, Wu CH. Recent Advances in the Treatment of Spasticity: Extracorporeal Shock Wave Therapy. J Clin Med. 2021. Oct 14;10(20):4723. doi: 10.3390/jcm10204723. PMID: 34682846; PMCID: PMC8539559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobsar D, Charlton JM, Tse CTF, Esculier JF, Graffos A, Krowchuk NM, Thatcher D, Hunt MA. Validity and reliability of wearable inertial sensors in healthy adult walking: a systematic review and meta-analysis. J Neuroeng Rehabil. 2020. May 11;17(1):62. doi: 10.1186/s12984-020-00685-3. PMID: 32393301; PMCID: PMC7216606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltoukhy M, Oh J, Kuenze C, Signorile J. Improved kinect-based spatiotemporal and kinematic treadmill gait assessment. Gait Posture. 2017. Jan;51:77-83. doi: 10.1016/j.gaitpost.2016.10.001. Epub 2016 Oct 4. PMID: 27721202. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, McGorry RW, Chou LS, Lin JH, Chang CC. Accuracy of the Microsoft Kinect for measuring gait parameters during treadmill walking. Gait Posture. 2015. Jul;42(2):145-51. doi: 10.1016/j.gaitpost.2015.05.002. Epub 2015 May 14. PMID: 26002604. [DOI] [PubMed] [Google Scholar]
- 20.McSweeney SC, Reed LF, Wearing SC. Reliability and minimum detectable change of measures of gait in children during walking and running on an instrumented treadmill. Gait Posture. 2020. Jan;75:105-108. doi: 10.1016/j.gaitpost.2019.10. 004. Epub 2019 Oct 7. PMID: 31648119. [DOI] [PubMed] [Google Scholar]
- 21.Mentiplay BF, Perraton LG, Bower KJ, Pua YH, McGaw R, Heywood S, Clark RA. Gait assessment using the Microsoft Xbox One Kinect: Concurrent validity and inter-day reliability of spatiotemporal and kinematic variables. J Biomech. 2015. Jul 16;48(10):2166-70. doi: 10.1016/j.jbiomech.2015.05.021. Epub 2015 May 28. PMID: 26065332. [DOI] [PubMed] [Google Scholar]
- 22.Clark RA, Bower KJ, Mentiplay BF, Paterson K, Pua YH. Concurrent validity of the Microsoft Kinect for assessment of spatiotemporal gait variables. J Biomech. 2013. Oct 18;46(15):2722-5. doi: 10.1016/j.jbiomech.2013.08.011. Epub 2013 Aug 26. PMID: 24016679. [DOI] [PubMed] [Google Scholar]
- 23.Mavrov M. The law institute of patient’s informed consent. 1st ed. Stovi Group Publishers:2018. [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014. Dec;12(12):1495-9. doi: 10.1016/j.ijsu.2014.07.013. Epub 2014 Jul 18. PMID: 25046131.25046131 [Google Scholar]
- 25.Reed LF, Urry SR, Wearing SC. Reliability of spatiotemporal and kinetic gait parameters determined by a new instrumented treadmill system. BMC Musculoskelet Disord. 2013. Aug 21;14:249. doi: 10.1186/1471-2474-14-249. PMID: 23964707; PMCID: PMC3766030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amelio E, Manganotti P. Effect of shock wave stimulation on hypertonic plantar flexor muscles in patients with cerebral palsy: a placebo-controlled study. J Rehabil Med. 2010. Apr;42(4):339-43. doi: 10.2340/16501977-0522. PMID: 20358168. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Wang G, Wang B. Rehabilitation treatment of spastic cerebral palsy with radial extracorporeal shock wave therapy and rehabilitation therapy. Medicine (Baltimore). 2018. Dec;97(51):e13828. doi: 10.1097/MD.0000000000013828. PMID: 30572548; PMCID: PMC6320024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal X, Morral A, Costa L, Tur M. Radial extracorporeal shock wave therapy (rESWT) in the treatment of spasticity in cerebral palsy: a randomized, placebo-controlled clinical trial. NeuroRehabilitation. 2011;29(4):413-9. doi: 10.3233/NRE-2011-0720. PMID: 22207070. [DOI] [PubMed] [Google Scholar]
- 29.Mihai EE, Mihai IV, Berteanu M. Effectiveness of Radial Extracorporeal Shock Wave Therapy and Visual Feedback Balance Training on Lower Limb Post-Stroke Spasticity, Trunk Performance, and Balance: A Randomized Controlled Trial. J Clin Med. 2021. Dec 28;11(1):147. doi: 10.3390/jcm11010147. PMID: 35011889; PMCID: PMC8745149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norvang OP, Askim T, Egerton T, Dahl AE, Thingstad P. Associations between changes in gait parameters, balance, and walking capacity during the first 3 months after stroke: a prospective observational study. Physiother Theory Pract. 2022. Apr;38(4):534-542. doi: 10.1080/09593985.2020. 1771802. Epub 2020 Jun 10. PMID: 32569492. [DOI] [PubMed] [Google Scholar]


