Abstract
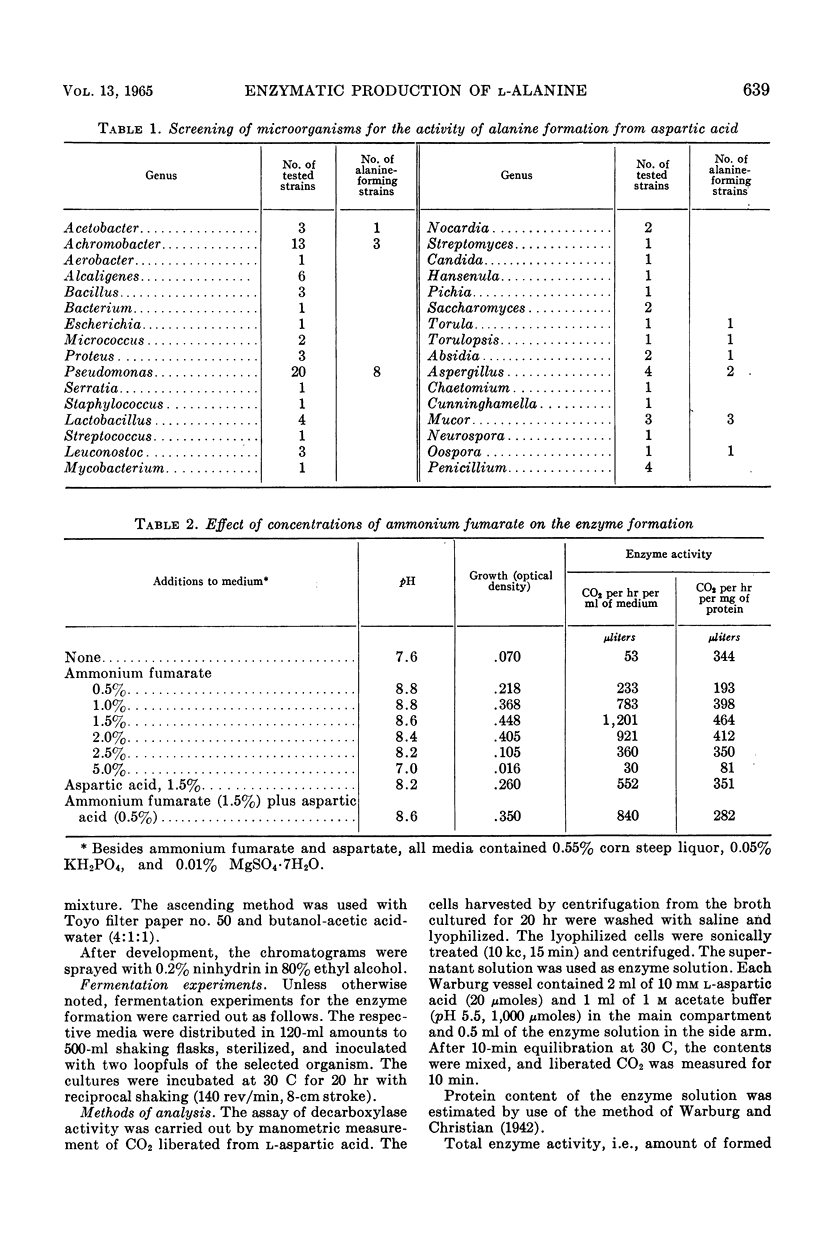
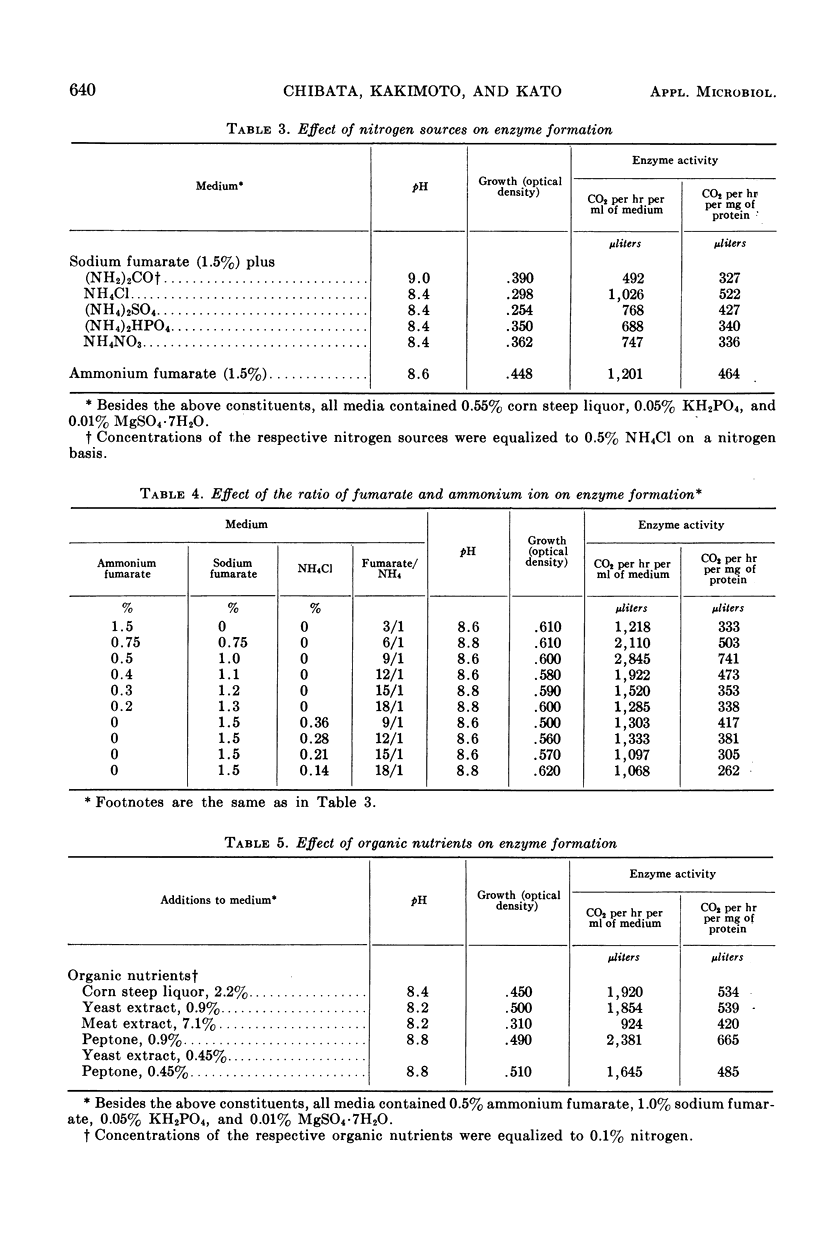
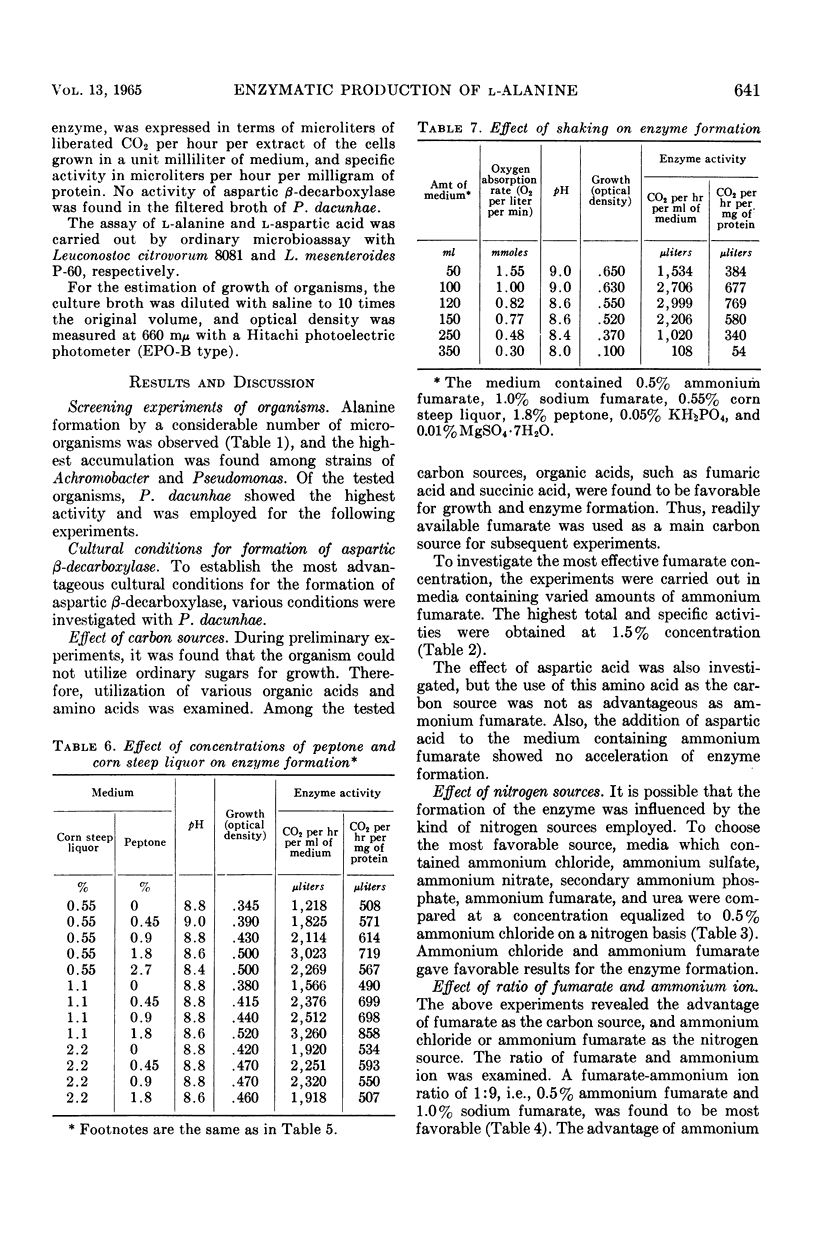
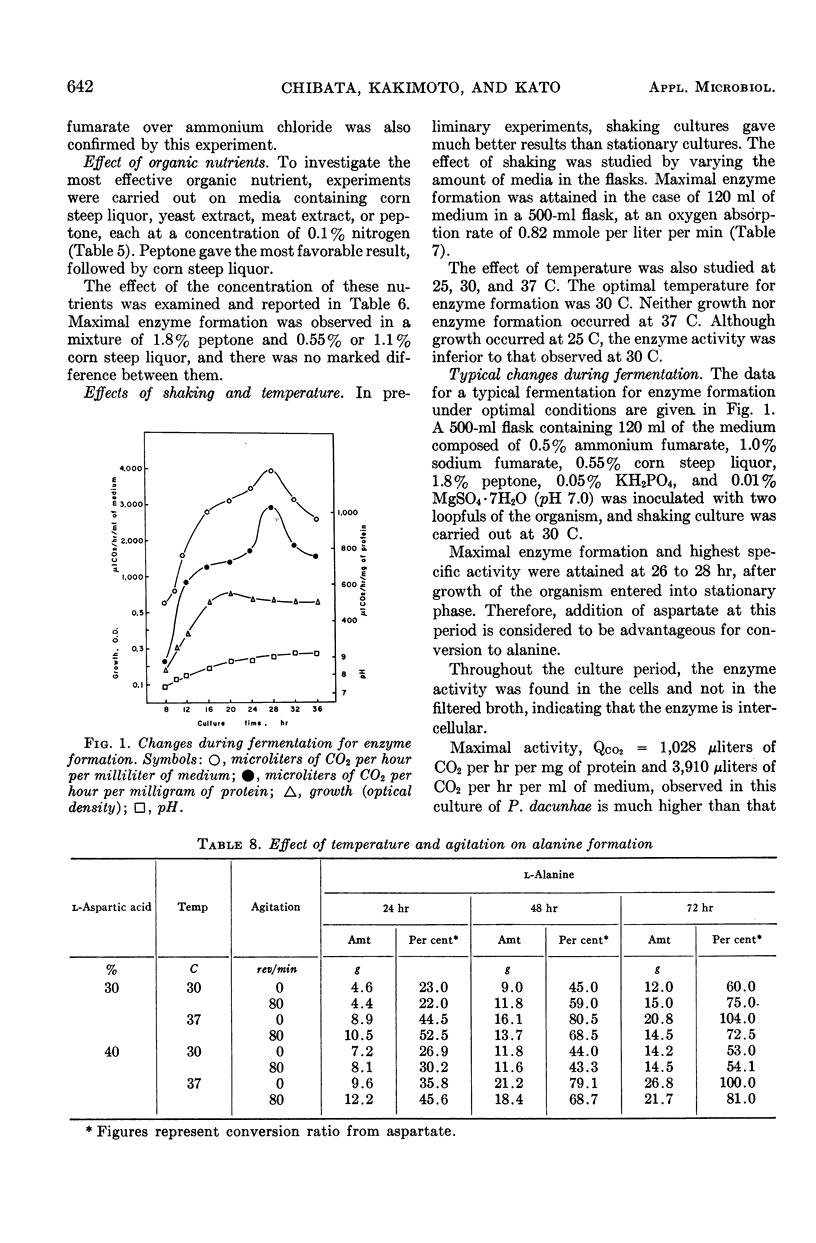
To establish an advantageous method for the production of l-alanine, a procedure was studied for converting l-aspartic acid to l-alanine by microbial l-aspartic β-decarboxylase. A number of organisms were screened to test their ability to form and accumulate alanine from aspartic acid. Pseudomonas dacunhae was selected as the most advantageous organism. With this organism, enzyme activity as high as 3,910 μliters of CO2 per hr per ml of medium could be produced by shaking the culture at 30 C in the medium containing ammonium fumarate, sodium fumarate, corn steep liquor, peptone, and inorganic salts. For the enzymatic conversion of l-aspartic acid to l-alanine, the culture broth was employed as the enzyme source. A large amount of l-aspartic acid (as much as 40% of the broth) was converted stoichiometrically to alanine in 72 hr at 37 C. Furthermore, appropriate addition of a surface-active agent to the reaction mixture was found to be highly effective in shortening the time required for the conversion. Accumulated l-alanine was readily isolated in pure form by ordinary procedures with ion-exchange resins. Yields of isolated l-alanine of over 90% from l-aspartic acid were easily attainable.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CATTANEO-LACOMBE J., SENEZ J. C., BEAUMONT P. Sur la purification de la 4-aspartique décarboxylase de Desulfovibrio desulfuricans. Biochim Biophys Acta. 1958 Dec;30(3):458–465. doi: 10.1016/0006-3002(58)90090-8. [DOI] [PubMed] [Google Scholar]
- CHIBATA I., TOSA T., SANO R. A NEW MOULD D-AMINO-ACID OXIDASE. Nature. 1964 Nov 14;204:684–685. doi: 10.1038/204684b0. [DOI] [PubMed] [Google Scholar]
- COOKSEY K. E., RAINBOW C. Metabolic patterns in acetic acid bacteria. J Gen Microbiol. 1962 Jan;27:135–142. doi: 10.1099/00221287-27-1-135. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. Studies on the aspartic decarboxylase of Nocardia globerula. Biochem J. 1958 Feb;68(2):221–225. doi: 10.1042/bj0680221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A., SOBER H. A., TICE S. V. Enzymatic decarboxylation of aspartic acid to alpha-alanine. J Biol Chem. 1951 Apr;189(2):577–590. [PubMed] [Google Scholar]
- NOVOGRODSKY A., MEISTER A. CONTROL OF ASPARTATE BETA-DECARBOXYLASE ACTIVITY BY TRANSAMINATION. J Biol Chem. 1964 Mar;239:879–888. [PubMed] [Google Scholar]
- NOVOGRODSKY A., NISHIMURA J. S., MEISTER A. Transamination and beta-decarboxylation of aspartate catalyzed by the same pyridoxal phosphate-enzyme. J Biol Chem. 1963 May;238:1903–1905. [PubMed] [Google Scholar]
- SEAMAN G. R. Amino acid decarboxylases in a pseudomonad. J Bacteriol. 1960 Dec;80:830–836. doi: 10.1128/jb.80.6.830-836.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENEZ J. C., CATTANEO-LACOMBE J. Transformation de l'acide L-aspartique en alpha-alanine par des extraits de Desulfovibrio desulfuricans. C R Hebd Seances Acad Sci. 1956 Feb 13;242(7):941–943. [PubMed] [Google Scholar]
- WILSON E. M. Crystalline L-aspartate 4-carboxy-lyase. Biochim Biophys Acta. 1963 Feb 12;67:345–348. doi: 10.1016/0006-3002(63)91840-7. [DOI] [PubMed] [Google Scholar]
- WILSON E. M., KORNBERG H. L. PROPERTIES OF CRYSTALLINE L-ASPARTATE 4-CARBOXY-LYASE FROM ACHROMOBACTER SP. Biochem J. 1963 Sep;88:578–587. doi: 10.1042/bj0880578. [DOI] [PMC free article] [PubMed] [Google Scholar]


