Summary
Background
School-based targeted preventive chemotherapy (PC), the main strategy for soil-transmitted helminths (STH) control, excludes other at-risk populations including adults and preschool children. Mass drug administration (MDA), covering all age groups, would bring additional health benefits but also requires greater investment. This cost survey and cost-effectiveness analysis compared MDA with school-based targeted PC for STH control in Dak Lak, Vietnam, where STH are endemic.
Methods
A cost survey was conducted in 2020 to estimate the total and per person economic and financial cost of each strategy. Monte Carlo simulation accounted for uncertainty in cost estimates. The primary effectiveness measure was hookworm-related disability-adjusted life years (DALYs) averted, and secondary measures were hookworm infection-years averted and moderate-to-heavy intensity hookworm infection-years averted. A Markov model was used to determine the incremental cost-effectiveness ratio (ICER) of MDA compared to school-based targeted PC using a government payer perspective and a ten-year time horizon. One-way and probabilistic sensitivity analyses (PSA) were performed. Costs are reported in 2020 USD ($).
Findings
The economic cost per person was $0.27 for MDA and $0.43 for school-based targeted PC. MDA in Dak Lak will cost $472,000 per year, while school-based targeted PC will cost $117,000. Over 10 years, MDA is estimated to avert an additional 121,465 DALYs; 4,019,262 hookworm infection-years, and 765,844 moderate-to-heavy intensity hookworm infection-years compared to school-based targeted PC. The ICER was $28.55 per DALY averted; $0.87 per hookworm infection-years averted, and $4.54 per moderate-to-heavy intensity hookworm infection-years averted. MDA was cost-effective in all PSA iterations.
Interpretation
In areas where hookworm predominates and adults suffer a significant burden of infection, MDA is cost effective compared to school based targeted PC and is the best strategy to achieve global targets.
Funding
The project was funded by the National Health and Medical Research Council (NHMRC) of Australia (Project Grant APP1139561) and JPCDT was supported by a UNSW Scientia PhD Scholarship.
Keywords: Soil-transmitted helminth infections, Preventive chemotherapy, School-based targeted preventive chemotherapy, Mass drug administration, Cost survey, Cost-effectiveness analysis
Research in context.
Evidence before this study
We reviewed the literature for cost or cost-effectiveness analyses of preventive chemotherapy (PC) for soil-transmitted helminths (STH) control published after year 2000. Twenty-two studies reported cost of school-based targeted PC or mass drug administration (MDA) for STH control with or without integration with PC for other Neglected Tropical Diseases (NTDs). Of those, 11 reported costs for STH control alone, 4 included both MDA and school-based targeted PC; and only one, conducted as part of a multi-country trial in India, Malawi, and Benin, compared the cost of school-based targeted PC and MDA for STH control alone. On the other hand, nine cost-effectiveness analyses involved school-based targeted PC or MDA for STH with or without integration with PC for other NTDs. Three of these, all using mathematical modelling approaches, examined the cost-effectiveness of MDA compared to school-based targeted PC, and none used primary cost or effectiveness data. Only one estimated the incremental cost-effectiveness ratio specific for STH. There are no published cost or cost-effectiveness analysis studies relative to Southeast Asian countries.
Added value of this study
Our study showed that MDA is a cost-effective alternative to school-based targeted PC that will avert more disability-adjusted life years (DALYs). This study adds to the lack of literature providing and comparing disaggregated costs of MDA and school-based targeted PC in Southeast Asia, which could allow regional comparisons. We provide the first cost estimates of MDA and school-based targeted PC for STH control alone which accounted for uncertainty through a Monte Carlo simulation. Our cost-effectiveness analysis is the first of its kind in comparing the two strategies for STH control utilising primary cost and effectiveness data. We also provide the first budget estimate for provincial program in Dak Lak. Using DALYs averted as the primary outcome allows comparison with future cost-effectiveness analyses even for interventions targeting other infections. Our sensitivity analysis examines a wider range of variables that have not typically been accounted for in past cost-effectiveness analyses, including disability weights and PC coverage.
Implications of all the available evidence
In areas where hookworm predominates and there is a high STH burden in adults, our results suggest that MDA should be used to avert more DALYs and to help attain key global targets.
Introduction
Soil-transmitted helminths (STH) which include Ascaris lumbricoides, Trichuris trichiura, and the hookworm species Necator americanus, Ancylostoma duodenale, and Ancylostoma ceylanicum; affect about 1.5 billion people in 2016.1 Moderate-to-heavy intensity infections are associated with anaemia and poor growth, particularly in children and women of reproductive age, and overall accounted for 3.5 million disability-adjusted life years (DALYS) lost in 2016.1
The World Health Organisation (WHO) has set a target for achieving and maintaining the elimination of STH morbidity in children by 2030, with the number of countries with a prevalence of moderate-to-heavy intensity STH in children of <2% as the indicator.1 The main recommended strategy to achieve this target is school-based targeted preventive chemotherapy (PC), where children attending primary school (typically 5–12 years of age) are administered anthelmintics without prior diagnosis, annually or biannually depending on STH prevalence.1 While effective in controlling morbidity in schoolchildren,2 this approach has limited impact on STH transmission, particularly in settings where hookworm predominates because large infection reservoirs exist in adults.3, 4, 5
Mass drug administration (MDA), a PC approach where everyone above one year of age receives treatment, can lead to greater overall STH prevalence reduction,6,7 including among schoolchildren8; and to a greater reduction in the prevalence of moderate-to-heavy intensity infections in children, when compared to school-based targeted PC.9 MDA, however, is more costly overall as additional resources are required to reach the entire population.10
Cost-effectiveness analysis is a useful tool for deciding whether MDA should be implemented to better control STH. It evaluates the trade-off between the costs and benefits of interventions, usually presented as the incremental cost-effectiveness ratio (ICER).11 There is limited data on the costs and cost-effectiveness of MDA compared to school-based targeted PC for STH control. There were only three studies,12, 13, 14 all based on modelling, that examined the cost-effectiveness of MDA compared to school-based targeted PC. One examined the relative increase in cost-effectiveness of MDA for hookworm control using an “arbitrary” cost of school-based targeted PC and MDA but did not calculate an ICER.14 Of the other two studies,12,13 both related to integrated STH and schistosomiasis PC programs, one based on Cote D’Ivoire12 and the other on Sub-Saharan Africa,13 only one provided an ICER estimate specific to STH.13
The only study that presented an ICER specific to STH found that MDA was cost-effective in areas with at least 60% STH prevalence at the community level.13 Cost estimates used for MDA were extrapolated from school-based targeted PC data, with the total per person treated cost being higher for MDA than for school-based targeted PC due to increased logistical costs of community delivery and remuneration of community health workers.12,13 However, cost surveys of PC for STH and schistosomiasis in Niger15 and Yemen16 found that the cost per person was lower for MDA than for school-based targeted PC largely due to economies of scale associated with the higher number of people treated. On the other hand, a cost survey which was part of a multi-country trial in India, Benin, and Malawi found that the cost per person was higher for MDA than for school-based targeted PC in India and Malawi but not in Benin.10 However, this study also found that when “supportive” activities designed to optimise PC coverage were excluded, such as supervision from a non-governmental organisation, the cost per person was lower for MDA than for school-based targeted PC in all countries.10 Real-world cost survey data and effectiveness data derived from experimental epidemiological studies are required to provide accurate evidence to determine if MDA is cost-effective compared to school-based targeted PC for STH control.17
Here we report findings from a cost survey and a cost-effectiveness analysis comparing school-based targeted PC and MDA in Dak Lak province in Vietnam, where since 2007, a school-based targeted PC program targeting schoolchildren attending primary schools, usually aged 6–11 years old, has been operating. Treatment regimens changed from biannual to annual in 2019 (Dinh Ng-Nguyen, personal communication). In 2019, the prevalence of N. americanus infection, the dominant STH in Dak Lak was 13.7% among schoolchildren.18 The province was also the site for the CoDe-STH trial, the first randomised controlled trial comparing the impact of community-wide MDA and school-based targeted PC on STH infections in schoolchildren.9 To our knowledge, this is the first cost analysis comparing the economic and financial costs of MDA and school-based targeted PC for STH control in Southeast Asia. This is also the first cost-effectiveness analysis that utilises primary data from a cost survey and effectiveness data from a trial in the same setting to compare these two strategies.
Methods
Cost survey and analysis
Interviewer administered questionnaires were developed to collect information on cost, resource use, and time spent on the implementation of annual school-based targeted PC and MDA programs using albendazole (400 mg), which were assumed to be donated for school-age children and purchased for other population groups. Costs were categorised according to the components of PC implementation7,10,19: albendazole procurement (which includes its purchase, transport, and storage), training, community sensitisation, albendazole administration, and PC monitoring and evaluation (Appendix p 2). The questionnaires were translated from English to Vietnamese and were pre-tested and revised with the research assistants who conducted the interviews. Three districts (out of the 13 total) were selected to reflect the different administrative settings: a city (Buon Ma Thuot), a town (Buon Ho), and a rural district (Lak). From each district selected, a village was purposively selected in consultation with the local expert (NND), ensuring these were also study sites of the CoDe-STH trial and that the interviewees were also aware of how MDA was implemented. From each selected village, a health worker and a medical teacher were interviewed. Staff from the Ministry of Health and the Ministry of Education and Training who are in charge of the STH control program at the provincial and district levels were also interviewed (Appendix p 18). Survey responses were translated from Vietnamese to English and entered into a Microsoft Excel spreadsheet. Data from the survey were complemented by online sources where additional information was required, and the average and range were compiled for each item (Appendix pp 3–9). All equipment were costed using straight-line depreciation with an expected life of 10 years. Prices in Vietnamese Dong (VND) were adjusted if required to 2020 using the consumer price index20; and converted to United States Dollars ($) at a rate of $1 = VND 22,750.21
Based on the cost survey, the annual economic and financial cost of implementing MDA and school-based targeted PC were estimated. Financial cost is the dollar amount paid in a financial transaction, while economic cost is the total cost of resources used including both financial costs and opportunity costs.22 Opportunity costs correspond to the monetary value of donated resources including time worked.22 Resource savings associated with reductions in STH cases was not considered due to a lack of data on STH morbidity-related health service utilisation.
A cost model was developed in TreeAge Pro Healthcare (TreeAge Software, LLC) (Appendix p 19). The model had five branches, corresponding to the main cost categories, and respective sub-categories. The model was independently populated with economic and financial cost data with cost subcategories assumed to follow a triangular distribution. The cost per person for MDA was calculated by dividing the total cost by 1,886,230, which was the estimated population of Dak Lak in 2020.23 For school-based targeted PC, the total cost was divided by 283,123, which was the estimated population of schoolchildren aged 5–14 years old.23,24 To improve cost estimate uncertainties Monte Carlo simulations (10,000 iterations) were used to provide the mean and 95% credible interval (CI) of the total and per person economic and financial cost of each strategy. 95% CI is the range where the results of 95% of the iterations fall. All total costs were rounded to the nearest thousand dollars for ease of reporting.
Effectiveness measures and cost-effectiveness analysis
The primary measure of effectiveness was hookworm-related disability-adjusted life years (DALYs) averted. DALYs are a summary measure of the number of years lived with a disability and the years of life lost to due to premature death, with one DALY being the loss of one year of healthy life.25 We calculated DALYs by multiplying the number of years lived with light, moderate, or heavy intensity hookworm infections by the appropriate disability weight for hookworm infection and anaemia. The disability weight of anaemia associated with hookworm infection was obtained by multiplying the disability weight of anaemia due to hookworm “disease”26 by the probability of anaemia developing from a case of hookworm infection (Appendix p 10, p 17).27 Secondary measures of effectiveness were hookworm infection-years averted and moderate-to-heavy intensity hookworm infection-years averted.
Inputs for the Markov model related to the impact of school-based targeted PC and MDA on STH infections were based on results from the CoDe-STH trial summarised below.9 This two-arm cluster randomised controlled trial was conducted in 64 primary schools (32 schools per arm) in 13 districts of Dak Lak.28 In the “school arm”, school-based targeted PC was administered to all children attending the schools. In the “community arm”, in addition to school-based targeted PC, MDA was implemented in hamlets surrounding the selected school. In those hamlets, treatment was offered to all residents over one year except women in their first trimester of pregnancy, and children who reported already receiving albendazole at school. A single dose of albendazole 400 mg was used in both arms. STH prevalence and intensity was measured in schoolchildren in grades one to four (6–11 years old) at baseline (November 2019) and 12 months following PC, using quantitative real-time PCR. Baseline and follow-up infection status data were obtained from a total of 3659 schoolchildren in the school arm and 3717 schoolchildren in the community arm.9
A Markov model was developed using TreeAge Pro Healthcare (TreeAge Software, LLC) to simulate the costs and effectiveness of MDA compared to school-based targeted PC over 10 years (Appendix p 20). The population of Dak Lak was divided into three groups: preschool (≤5 years old), schoolchildren (6–11 years old), and adolescents and adults (≥12 years old). At baseline, individuals were assigned to one of the four health states based on hookworm infection intensity; no infection (0 eggs per gram), light (1–1999 eggs per gram), moderate (2000–3999 eggs per gram), or heavy intensity infection (>4000 eggs per gram).29 The distribution of schoolchildren by infection status was derived by multiplying the baseline prevalence from the CoDe-STH trial18 by the population size of Dak Lak in 202023 and the proportion of school-age children in the population.24 A similar approach was used for preschool, adolescents, and adults; taking into account the distribution of infection across each age group found in a 2020 community survey (Susana Vaz Nery, personal communication). PC coverage was obtained from the national program for school-based targeted PC (Dinh Ng-Nguyen, personal communication) and from the CoDe-STH trial for MDA.9 For each one-year cycle, based on the probability of receiving albendazole and transition probabilities, individuals could stay in the same state or transition to another state. Transition probabilities were based on the impact of one round of school-based targeted PC or MDA on infection status and intensity of infection one year after albendazole delivery observed in the CoDe-STH trial.9,30 The model assumed that without treatment, the intensity of infection would not change and that the transition probabilities do not change over time. The parameter values used for the Markov model and the data sources are summarised in Table 1 and in Appendix p 11. Half-cycle correction was used. The cost-effectiveness analysis was conducted from the perspective of the government as payer, where only the expenses to be incurred by the government were considered. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) were followed (Appendix p 12).22
Table 1.
Markov model estimates and sources.
| Variable | Base case value | Low and high values | Distribution | Source |
|---|---|---|---|---|
| Costs ($)a | Gamma | |||
| Per treatment cost of MDA | 0.27 | 0.22–0.31 | Cost analysis (2020) | |
| Per treatment cost of school-based targeted PC | 0.43 | 0.29–0.60 | ||
| Cost of drug | 0.04 | 30 | ||
| Health states and disability weights for hookworm infectionb | Triangular | 25,26 | ||
| Children | ||||
| Light intensity infection | 0.005 | 0.004–0.046 | ||
| Moderate intensity infection | 0.095 | 0.005–0.056 | ||
| Heavy intensity infection | 0.036 | 0.020–0.101 | ||
| No hookworm | 0.000 | |||
| Adults | ||||
| Light intensity infection | 0.025 | 0.001–0.053 | ||
| Moderate intensity infection | 0.032 | 0.003–0.056 | ||
| Heavy intensity infection | 0.063 | 0.017–0.101 | ||
| No hookworm | 0.000 | |||
| Population description | Dirichlet | 8,22,23 | ||
| Preschool children | ||||
| No hookworm infection | 138,274 | |||
| Light intensity hookworm infection | 6777 | |||
| Moderate intensity hookworm infection | 0 | |||
| Heavy intensity hookworm infection | 0 | |||
| Schoolchildren | ||||
| No hookworm infection | 243,514 | |||
| Light intensity hookworm infection | 29,700 | |||
| Moderate intensity hookworm infection | 4785 | |||
| Heavy intensity hookworm infection | 5125 | |||
| Adults | ||||
| No hookworm infection | 885,143 | |||
| Light intensity infection | 474,135 | |||
| Moderate intensity hookworm infection | 48,291 | |||
| Heavy intensity hookworm infection | 50,487 | |||
| PC coverage | Triangular | |||
| School-based targeted PC–school children | 0.98 | 0.75–1.00 | NND | |
| School-based targeted PC–adults, adolescents, and preschoolc | N/A | |||
| MDA–school children | 0.95 | 0.75–1.00 | 8 | |
| MDA–adults, adolescents, and preschool | 0.90 | 0.75–1.00 | 8 | |
| Transition probabilities–annual | Dirichlet | |||
| No treatmentd | 0.000 | |||
| School-based targeted PC | 8 | |||
| From no infection | ||||
| To no infection | 0.974 | |||
| To light intensity infection | 0.022 | |||
| To moderate intensity infection | 0.002 | |||
| To heavy intensity infection | 0.002 | |||
| From light intensity infection | ||||
| To no infection | 0.490 | |||
| To light intensity infection | 0.457 | |||
| To moderate intensity infection | 0.036 | |||
| To heavy intensity infection | 0.016 | |||
| From moderate intensity infection | ||||
| To no infection | 0.306 | |||
| To light intensity infection | 0.500 | |||
| To moderate intensity infection | 0.083 | |||
| To heavy intensity infection | 0.111 | |||
| From heavy intensity infection | ||||
| To no infection | 0.267 | |||
| To light intensity infection | 0.311 | |||
| To moderate intensity infection | 0.133 | |||
| To heavy intensity infection | 0.289 | |||
| Mass drug administration | 8 | |||
| From no infection | ||||
| To no infection | 0.973 | |||
| To light intensity infection | 0.027 | |||
| To moderate intensity infection | 0.000 | |||
| To heavy intensity infection | 0.000 | |||
| From light intensity infection | ||||
| To no infection | 0.668 | |||
| To light intensity infection | 0.310 | |||
| To moderate intensity infection | 0.021 | |||
| To heavy intensity infection | 0.000 | |||
| From moderate intensity infection | ||||
| To no infection | 0.611 | |||
| To light intensity infection | 0.333 | |||
| To moderate intensity infection | 0.028 | |||
| To heavy intensity infection | 0.028 | |||
| From heavy intensity infection | ||||
| To no infection | 0.583 | |||
| To light intensity infection | 0.250 | |||
| To moderate intensity infection | 0.083 | |||
| To heavy intensity infection | 0.083 | |||
MDA, mass drug administration; PC, preventive chemotherapy; NND, Nguyen Ngoc Dinh personal communication.
All hookworm infections are associated with morbidity due to anaemia, and heavy intensity infection has additional disease-specific morbidity. Disability weight of anaemia was obtained by multiplying the disability weight of anaemia due to hookworm disease25 by the probability of anaemia developing from a case of hookworm infection.26
Not treated.
Assumed to not change status.
Costs and outcomes were discounted at 3% per annum.31 Disability weights were not age-weighted, following WHO recommendations.25
Incremental cost-effectiveness ratios (ICERs) were calculated to compare the relative cost-effectiveness of the intervention (MDA) based on the formula: (Cost MDA–Cost school-based targeted PC)/(Effectiveness MDA–Effectiveness school-based targeted PC).11 MDA is considered cost-effective if the ICER is below the midpoint of the cost-effectiveness threshold range based on opportunity cost and adjusted to inflation (2020), estimated to be $689 (range $176–$1201) per DALY averted in Vietnam.32
Sensitivity analysis
One-way sensitivity analysis was conducted using the parameter ranges for Markov model (Table 1) to identify variables affecting the ICER. Probabilistic sensitivity analysis using Monte Carlo simulation with 10,000 iterations was conducted to assess the robustness of the cost-effectiveness analysis. Dirichlet distribution was used for the transition probabilities and initial distribution of the population by infection status, gamma distribution for costs, and triangular distribution for disability weights and PC coverage. Scenarios explored in the sensitivity analysis included undiscounted costs and outcomes and paying the minimum monthly wage, VND 3,250,000 (range VND 3,070,000–3,430,00) or $143 (range $135–$151) to village health workers instead of monthly allowances.
Budget impact analysis
The budget impact analysis provided estimates of the financial cost to the government to implement annual MDA in Dak Lak province over five years, instead of school-based targeted PC. The costs were not discounted.33
Ethical considerations
This study has received ethics approval from the Human Research Ethics Advisory Panel at the University of New South Wales (HC200538) and Tay Nguyen University (1804-QĐ-ĐHTN-TCCB).
Role of funding source
The funders of the study had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the manuscript for publication.
Results
Cost of annual implementation of MDA and school-based targeted PC
A total of 13 respondents participated in the cost survey: eight staff in charge of the STH control program in the Ministry of Health and the Ministry of Education and Training at provincial and district levels; and three village health workers and two medical teachers in the selected villages (Appendix p 18). The cost and resource use values for school-based targeted PC and for MDA are shown in Appendix pp 3–9. The average and the range for each cost subcategory are separated into economic costs (Appendix p 13) and financial costs (Appendix p 14).
Based on Monte Carlo simulation, the economic cost of implementing MDA is $472,000 (95% CI: $387,000–$561,000) or $0.27 per person targeted (95% CI: $0.22–$0.31) (Table 2). The economic cost of implementing school-based targeted PC is $117,000 (95% CI: $91,000–$146,000) with a cost per person of $0.43 (95% CI: $0.29–$0.60) (Table 2). The total cost over ten years for MDA is $4,534,000 and for school-based targeted PC is $1,061,000 (Appendix p 21). The total annual financial cost of MDA is $424,000 (95% CI: $345,000–$507,000), with a cost per person of $0.23 (95% CI: $0.16–$0.30). The total annual financial cost of school-based targeted PC is $91,000 (95% CI: $70,000–$114,000) with a cost per person of $0.32 (95% CI: $0.21–$0.48) (Table 2).
Table 2.
Modelled mean economic and financial cost (USD) and 95% credible intervals (CIs) of school-based targeted preventive chemotherapy and mass drug administration in Dak Lak, Vietnam based on Monte Carlo simulation.a
| Cost categories | Economic cost |
Financial cost |
||||||
|---|---|---|---|---|---|---|---|---|
| Mass drug administration |
School-based targeted preventive chemotherapy |
Mass drug administration |
School-based targeted preventive chemotherapy |
|||||
| Mean (%) | 95% CI | Mean (%) | 95% CI | Mean (%) | 95% CI | Mean (%) | 95% CI | |
| Albendazole procurement | 74,000 (16) | 47,000–103,000 | 13,000 (11) | 9000–18,000 | 63,000 (15) | 33,000–94,000 | 2000 (2) | 1000–4000 |
| Purchases | 71,000 (15) | 45,000–98,000 | 10,000 (9) | 7000–14,000 | 61,000 (14) | 32,000–90,000 | N/A | N/A |
| Transport | 2000 (<1) | 1000–3000 | 2000 (2) | 1000–3000 | 2000 (<1) | 1000–4000 | 2000 (2) | 1000–4000 |
| Storage | 1000 (<1) | 1000–2000 | 1000 (1) | 1000–1000 | N/A | N/A | N/A | N/A |
| Training | 29,000 (6) | 14,000–46,000 | 6000 (5) | 4000–9000 | 29,000 (7) | 13,000–48,000 | 7000 (8) | 3000–10,000 |
| Participants’ allowance and kits | 28,000 (6) | 14,000–45,000 | 6000 (5) | 4000–8000 | 28,000 (7) | 13,000–47,000 | 6000 (7) | 3000–9000 |
| Training course | 1000 (<1) | <1000–1000 | 1000 (1) | <1000–1000 | 1000 (<1) | <1000–1000 | 1000 (1) | <1000–1000 |
| Community Sensitisation | 143,000 (30) | 109,000–176,000 | 38,000 (32) | 25,000–49,000 | 132,000 (31) | 97,000–168,000 | 27,000 (30) | 19,000–35,000 |
| Flyers | 62,000 (13) | 46,000–78,000 | 22,000 (19) | 16,000–27,000 | 62,000 (15) | 42,000–83,000 | 22,000 (24) | 15,000–29,000 |
| Posters | 70,000 (15) | 58,000–82,000 | 5000 (4) | 4000–6000 | 70,000 (17) | 55,000–85,000 | 5000 (6) | 4000–6000 |
| Loudspeaker | 11,000 (2) | 5000–16,000 | 11,000 (9) | 5000–16,000 | N/A | N/A | N/A | N/A |
| Albendazole administration | 223,000 (47) | 137,000–320,000 | 58,000 (50) | 29,000–89,000 | 222,000 (52) | 133,000–320,000 | 58,000 (64) | 28,000–90,000 |
| Personnel | 192,000 (41) | 118,000–274,000 | 47,000 (40) | 23,000–74,000 | 192,000 (45) | 118,000–273,000 | 47,000 (52) | 23,000–74,000 |
| Supplies | 14,000 (3) | 9000–20,000 | 7000 (6) | 5000–8000 | 14,000 (3) | 8000–21,000 | 7000 (8) | 5000–9000 |
| Equipment | 1000 (<1) | 1000–2000 | <1000 (<1) | <1000–<1000 | N/A | N/A | N/A | N/A |
| Transportation of personnel | 16,000 (3) | 9000–24,000 | 4000 (3) | 1000–7000 | 16,000 (4) | 7000–26,000 | 4000 (4) | <1000–7000 |
| PC monitoring and evaluation | 31,000 (7) | 23,000–37,000 | 8000 (7) | 6000–9000 | 4000 (1) | 3000–4000 | 2000 (2) | 2000–2000 |
| PC day monitoring | 1000 (<1) | <1000–<1000 | 1000 (<1) | <1000–1000 | <1000 (<1) | <1000–<1000 | <1000 (<1) | <1000–<1000 |
| Submission of reports | 28,000 (6) | 21,000–35,000 | 5000 (4) | 4000–7000 | 2000 (<1) | 1000–2000 | <1000 (<1) | <1000–<1000 |
| Parasitological assessment | 2000 (<1) | 2000–2000 | 2000 (2) | 2000–2000 | 2000 (<1) | 2000–2000 | 2000 (2) | 2000–2000 |
| Total cost | 472,000 | 387,000–561,000 | 117,000 | 91,000–146,000 | 424,000 | 345,000–507,000 | 91,000 | 70,000–114,000 |
| Cost per person | 0.27 | 0.22–0.31 | 0.43 | 0.29–0.60 | 0.23 | 0.16–0.30 | 0.32 | 0.21–0.48 |
95% CI, 95% credible interval.
Values based on the Monte Carlo simulation conducted using data obtained from the cost survey. The total cost is the mean of the 10,000 iterations of the total cost (and not the total of the means of each sub-categories); Costs in VND adjusted to 2020 using consumer price index19; and converted to $ (rate: $1 = VND 22,750).20
The cost of albendazole administration, which included the personnel, supplies, equipment, and transportation of personnel required to administer albendazole, accounted for most of the costs of both strategies (economic cost: 47% for MDA and 50% for school-based targeted PC; financial cost: 52% for MDA and 64% for school-based targeted PC). Several other cost categories were similar in both strategies including community sensitisation, (30% and 31% for economic and financial costs of MDA, respectively, versus 32% and 30% for economic and financial costs of school-based targeted PC, respectively), training (6% for economic cost and 7% for financial cost of MDA versus 5% for economic cost and 8% for financial cost of school-based targeted PC) and PC monitoring and evaluation (7% for economic cost and 1% for financial cost of MDA and 7% for economic cost and 2% for financial cost of school-based targeted PC) (Table 2, Appendix pp 22–23). Albendazole procurement contributed slightly more to the total economic and financial cost for MDA (16% for economic cost and 15% for financial cost) compared to school-based targeted PC (11% for economic cost and 2% for financial cost).
Effectiveness and cost-effectiveness
Over a 10-year period, MDA in Dak Lak would reduce the overall prevalence of hookworm infections from 32.6% to 4.2% (Fig. 1A1); while with school-based targeted PC it would remain almost unchanged at 31.4% (Fig. 1A1), with the prevalence in schoolchildren reduced from 14.0% to 5.9% in both strategies (Fig. 1A3). Additionally, MDA would reduce the prevalence of moderate-to-heavy intensity hookworm infections from 5.7% to 0.3%; compared to a small reduction to 5.4% with school-based targeted PC (Fig. 1B1). Prevalence of moderate-to-heavy intensity infections in schoolchildren would decrease from 3.5% to 0.25% with MDA; versus from 3.5% to 1.2% with school-based targeted PC (Fig. 1B3). After two years of MDA, overall prevalence of moderate-to-heavy infections in the population would decrease to less than 2% (1.0%) (Fig. 1B1). The modelling results confirm the findings of the CoDe-STH trial,9 where the prevalence of moderate-to-heavy intensity infections in schoolchildren was reduced to less than 2% after one round of MDA, but not after one round of school-based targeted PC (Fig. 1B3).
Fig. 1.
Prevalence of hookworm infections (A), prevalence of moderate-heavy intensity hookworm infections (B), and DALY averted (C) per year for MDA (♦) and school-based targeted preventive chemotherapy (▪) and DALYs averted by MDA relative to school targeted PC (•, D) in overall population (1), preschool (2), schoolchildren (3), and adolescents/adults (4).
The model estimated that of the 44,825 DALYs lost with MDA, 40,402 DALYs would be in adults; and of the 166,290 DALYs lost with school-based targeted PC, 160,307 DALYs would be in adults (Fig. 1C). Over 10 years, MDA would avert 121,465 DALYs compared to school-based targeted PC (Fig. 1D, Appendix pp 24–25). Implementation of MDA would also avert 4,019,262 hookworm infection-years and 765,844 moderate-to-heavy intensity hookworm infection-years compared to school-based targeted PC (Appendix pp 26–27).
Over ten years, the ICERs for MDA (compared to school-based targeted PC) were $28.55 (95% CI: $20.51–$53.43) per DALY averted, $0.87 (95% CI: $0.74–$1.00) per hookworm infection-years averted, and $4.54 (95% CI: $3.90–$5.32) per moderate-to-heavy intensity hookworm infection-years averted (Table 3).
Table 3.
Total costs ($), effectiveness, and incremental cost-effectiveness ratio (ICER).
| Strategy | Total cost | Effectiveness |
||
|---|---|---|---|---|
| DALYsa | Hookworm infection-years | MHI hookworm infection-years | ||
| School-based targeted PC | 1,061,000 | 166,290 | 5,645,040 | 970,054 |
| Mass drug administration | 4,534,000 | 44,825 | 1,631,100 | 204,968 |
| Difference between the two strategies | 3,473,000 | 121,465 | 4,013,939 | 765,086 |
| ICER 95% CI | 28.55 (20.51–53.43) | 0.87 (0.74–1.00) | 4.54 (3.90–5.32) | |
DALY, disability-adjusted life years; MHI, moderate-to-heavy intensity; % CI, 95% credible interval.
DALYs lost for each strategy and DALYs averted for the difference between strategies.
Sensitivity analysis
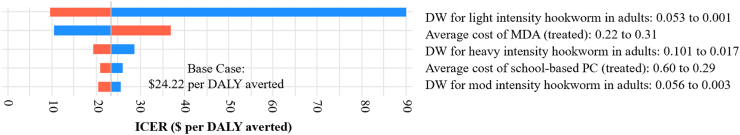
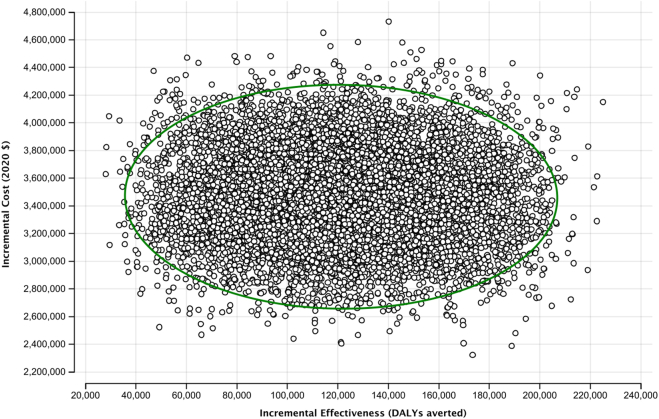
The one-way sensitivity analysis showed that the variables contributing the most to the uncertainty of the results were disability weight per light intensity hookworm infection in adults, the cost per person treated for MDA, and disability weight per heavy intensity hookworm infection in adults (Fig. 2). None of the variables tested in one-way sensitivity analysis have sufficient uncertainty that, alone, could make MDA not cost-effective. Based on a threshold of $689 per DALY averted, MDA would be considered cost-effective up to a cost of $6.83 per person; and at the lower range of the threshold ($176 per DALY averted), remaining cost-effective up to $1.61 per person. The probabilistic sensitivity analysis showed that MDA is cost-effective compared to school-based targeted PC in all iterations (Fig. 3).
Fig. 2.
Tornado diagram showing results from one-way sensitivity analysis and key variables influencing the results. None of the variables are close to the inflation-adjusted (2020) cost-effectiveness threshold in Vietnam estimated to be $689 ($176–$1201) per DALY averted.33 DW, disability weight; MDA, mass drug administration; PC, preventive chemotherapy.
Fig. 3.
Results from probabilistic sensitivity analysis. Each dot on the scatterplot indicates a cost and effectiveness point estimate generated from 10,000 iterations. All the iterations showed that MDA is cost-effective, with the cost-effectiveness threshold beyond the upper left of the plot. The ellipse showing the 95% credible interval.
If the costs and outcomes are undiscounted, the ICERs decrease from $28.55 to $27.49 per DALY averted, from $0.87 to $0.83 per hookworm infection-years averted, and from $4.54 to $4.36 per moderate-to-heavy intensity hookworm infection-years averted.
Currently village health workers receive a monthly allowance between $4.40 and $13.19. If the national monthly minimum wage of VND 3,070,000–VND 3,430,000 ($135–$151) was assumed for village health workers instead of monthly allowances, MDA in Dak Lak would cost $645,000 per year ($426,000–$896,000) (Appendix pp 15,28), with an economic cost per person of $0.36 for MDA, representing a one-third increase from the base case. In this scenario, the ICER increased from $24.22 to $33.47 per DALY averted, from $0.87 to $1.19 per hookworm infection-years averted, and from $4.54 to $6.25 per moderate-to-heavy intensity hookworm infection-years averted. MDA was cost-effective in all probabilistic sensitivity analysis iterations.
Budget impact–financial costs
If the Vietnam Ministry of Health implemented MDA instead of school-based targeted PC based on current spending, the total cost over five years would be $2,172,000 (95% CI: $1,493,000–$2,872,000) compared to current spending of $504,000 ($380,000–$646,000) for school-based targeted PC, and thus MDA would increase spending by more than four times. This would require an increase on current expenditure by $1,667,000 (95% CI: $1,372,000–1,969,000) which equates to $333,000 annually. Implementing MDA for five years would avert 66,954 DALYs; 2,011,642 hookworm infection-years; and 387,732 moderate-to-heavy intensity hookworm infection-years compared to school-based targeted PC.
Discussion
We present the findings of the first cost analysis in Southeast Asia and the first cost-effectiveness analysis worldwide directly comparing MDA to school-based targeted PC for STH control based on primary data for both cost and effectiveness estimates. Our study found that, in Dak Lak, the economic cost per person was lower for MDA than school-based targeted PC, and over ten years the ICER of $24.22 (95% CI: $17.40–$45.28) per DALY averted for MDA compared to school-based targeted PC was cost-effective based on a cost-effectiveness threshold in Vietnam of $689 per DALY averted.32
The lower cost per person for MDA than for school-based targeted PC is likely due to economies of scale where MDA covers more than six times the people treated by school-based targeted PC.19 Other studies also found that the cost per person is lower for MDA than for school-based targeted PC.10,15,16 To allow comparison with our results, the average cost per person for MDA and for school-based targeted PC reported in published studies were adjusted to 2020 prices and all values were converted to international dollars (int. $). Our range of economic cost per person for MDA (int. $0.67-int. $0.94) is comparable to available estimates (int. $0.82-int. $0.90).7,15,16 The same is true for our range of economic cost per person for school-based targeted PC (int. $1.15- int. $2.64) versus other available estimates (int. $0.91-int. $1.97).15,16,34, 35, 36 When looking at the main contributors to economic costs, we found that albendazole administration contributes approximately half the total cost in both strategies, due mainly to staff costs, similar to what was observed by others.10 This also highlights the importance in taking caution in comparing cost data, and the need for more standard and comparable cost categories for PC.
The ICER in our study of $24.22 per DALY averted is less than what was reported in the only other published cost-effectiveness analysis comparing MDA to school-based targeted PC for STH control alone, which reported an ICER of $1050 per DALY averted in a modelled 5000-people community in sub-Saharan Africa with a 60% STH prevalence and 75% PC coverage.13 This difference in ICER estimates may be due to the higher relative STH prevalence in schoolchildren than adults and similar prevalence across STH species, whereas in Dak Lak, hookworm predominates and at baseline only 7% of the hookworm infections were in schoolchildren (Susana Vaz Nery, personal communication). Additionally, in the sub-Saharan study, it was estimated that 25% of the population were schoolchildren, compared to 15% in Dak Lak. Thus, greater benefits are realised in treating schoolchildren than adults in the sub-Saharan study. Furthermore, the sub-Saharan study used the 2010 Global Burden of Disease Study disability weights as opposed to the 2019 edition possibly resulting in a lower disability weight per STH infection.26,37 Lastly, the cost-effectiveness analysis in sub-Saharan Africa was not completely based on primary cost data and estimated that the cost per person for MDA was higher than that for school-based targeted PC.13 Other studies have reported lower ICERs than the one found in sub-Saharan Africa but these examined the cost-effectiveness of PC for STH integrated with PC for other NTDs,2,12,35,38 Indeed, integrating interventions could be more cost-effective as morbidity from multiple conditions are being targeted by the same PC program.
A limitation of this study is that we were unable to estimate the cost of advocacy, planning, and data management which we could not ascertain from our questionnaires but is likely similar for both strategies. Additionally, we were unable to assess treatment costs associated with anaemia; and did not include wasting as a sequelae of hookworm infection, which may have underestimated morbidity associated with anaemia. Our approach was similar to a global hookworm burden study which also did not include wasting.27 We only included hookworm infections, and thus the results are not generalisable in areas other STH species dominate. We also used the total number of school-age children instead of including only those who were enrolled. The Markov model used, which is a static model, has some limitations: firstly, that the model assumes individuals stay in a health state for the entire year (cycle length), which therefore underestimates the DALYs averted by MDA in preschool, adolescents, and adults because DALYs are averted before the end of the year; secondly, the impact of treatment on other individuals was not incorporated which would underestimate the impact of transmission-reducing interventions, particularly MDA; and thirdly, all-cause mortality was not considered in the model. Furthermore, transition probabilities for all age groups were derived from a trial that only measured the impact of PC in schoolchildren.
This study adds real world cost data to the limited literature comparing costs of MDA and school-based targeted PC. This is the first costing study in Southeast Asia and the first globally to account for uncertainty in cost estimates by categories through Monte Carlo simulation, with the multi-country study being the only other study comparing the cost of school-based targeted and MDA for STH control alone.10 The disaggregated cost estimates for implementing school-based targeted PC and MDA in Dak Lak can be used to provide cost components in other public health interventions and may be useful for regional comparisons. This study is also the first cost-effectiveness analysis that uses primary data for both cost and effectiveness measures, with only one other study providing ICER estimates for PC for STH but relying on secondary cost and effectiveness inputs.13 Additionally, it considered uncertainties comprehensively by examining cost, disability weights, PC coverage, transition probabilities and baseline hookworm prevalence in the sensitivity analysis.
In summary, in Dak Lak and in areas where hookworm predominates and the burden of STH in adults is considerable, MDA is a cost-effective strategy that averts more DALYs than school-based targeted PC. This study provides much needed evidence to guide global STH control program towards accelerating progress in meeting the global targets.
Contributors
John Paul Caesar delos Trinos–Conceptualization, Project administration, Methodology, Investigation, Data curation, Formal Analysis, Visualization, Writing—original draft.
Dinh Ng-Nguyen–Methodology, Investigation, Validation, Writing—review & editing.
Luc E. Coffeng–Supervision, Conceptualization, Methodology, Investigation, Validation, Visualization, Writing—review & editing.
Clare E.F. Dyer–Project administration, Investigation, Writing—review & editing.
Naomi Clarke–Project administration, Investigation, Writing—review & editing.
Rebecca Traub–Funding acquisition, Resources, Methodology, Writing—review & editing.
Kate Halton Funding acquisition, Methodology, Writing—review & editing.
Virginia Wiseman–Supervision, Conceptualization, Funding acquisition, Methodology, Writing—review & editing.
Caroline Watts–Supervision, Conceptualization, Methodology, Investigation, Validation, Writing—review & editing.
Susana Vaz Nery–Supervision, Conceptualization, Funding acquisition, Resources, Methodology, Investigation, Validation, Writing—review & editing.
Data sharing statement
The data analysed in this study are provided in the article or supplementary materials. Further inquiries should be directed to the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgements
The authors acknowledge the research assistants in Tay Nguyen University, namely Van Trong Nguyen, Van Thai Nguyen, and Ngoc Hieu Nguyen for their valuable contribution in collecting the data in Vietnam, as well as staff of Ministry of Health and Ministry of Education and Training, health workers, and teachers who responded to the surveys.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100913.
Appendix A. Supplementary data
References
- 1.World Health Organisation Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. 2020. https://www.who.int/publications/i/item/9789240010352 [cited 2023 25 May]. Available from:
- 2.Miguel E., Kremer M. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72(1):159–217. [Google Scholar]
- 3.Anderson R.M., Truscott J.E., Pullan R.L., Brooker S.J., Hollingsworth T.D. How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis. 2013;7(2) doi: 10.1371/journal.pntd.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffeng L.E., Bakker R., Montresor A., de Vlas S.J. Feasibility of controlling hookworm infection through preventive chemotherapy: a simulation study using the individual-based WORMSIM modelling framework. Parasit Vectors. 2015;8(1):541. doi: 10.1186/s13071-015-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bethony J., Chen J., Lin S., et al. Emerging patterns of hookworm infection: influence of aging on the intensity of necator infection in Hainan province, people’s Republic of China. Clin Infect Dis. 2002;35(11):1336–1344. doi: 10.1086/344268. [DOI] [PubMed] [Google Scholar]
- 6.Clarke N.E., Clements A.C.A., Amaral S., et al. (S)WASH-D for Worms: a pilot study investigating the differential impact of school- versus community-based integrated control programs for soil-transmitted helminths. PLoS Negl Trop Dis. 2018;12(5) doi: 10.1371/journal.pntd.0006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullan R.L., Halliday K.E., Oswald W.E., et al. Effects, equity, and cost of school-based and community-wide treatment strategies for soil-transmitted helminths in Kenya: a cluster-randomised controlled trial. Lancet. 2019;393(10185):2039–2050. doi: 10.1016/S0140-6736(18)32591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke N.E., Clements A.C.A., Doi S.A., et al. Differential effect of mass deworming and targeted deworming for soil-transmitted helminth control in children: a systematic review and meta-analysis. Lancet. 2017;389(10066):287–297. doi: 10.1016/S0140-6736(16)32123-7. [DOI] [PubMed] [Google Scholar]
- 9.Dyer Clare E.F., Ng-Nguyen Dinh, E Clarke Naomi, et al. 2023. Community-wide deworming leads to a lower burden of STH infection in school-aged children compared with school-based deworming alone: results from the Community Deworming against Soil-Transmitted Helminths (CoDe-STH) trial Lancet Glob Health [under review] [Google Scholar]
- 10.Morozoff C., Avokpaho E., Puthupalayam Kaliappan S., et al. Costs of community-wide mass drug administration and school-based deworming for soil-transmitted helminths: evidence from a randomised controlled trial in Benin, India and Malawi. BMJ Open. 2022;12(7) doi: 10.1136/bmjopen-2021-059565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drummond M. Fourth edition ed. Oxford University Press; Oxford, United Kingdom: 2015. Methods for the economic evaluation of health care programmes. [Google Scholar]
- 12.Lo N.C., Bogoch I.I., Blackburn B.G., et al. Comparison of community-wide, integrated mass drug administration strategies for schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. Lancet Glob Health. 2015;3(10):e629–e638. doi: 10.1016/S2214-109X(15)00047-9. [DOI] [PubMed] [Google Scholar]
- 13.Lo N.C., Lai Y.-S., Karagiannis-Voules D.-A., et al. Assessment of global guidelines for preventive chemotherapy against schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. Lancet Infect Dis. 2016;16(9):1065–1075. doi: 10.1016/S1473-3099(16)30073-1. [DOI] [PubMed] [Google Scholar]
- 14.Turner H.C., Truscott J.E., Bettis A.A., et al. An economic evaluation of expanding hookworm control strategies to target the whole community. Parasites Vectors. 2015;8(1):570. doi: 10.1186/s13071-015-1187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie J., Garba A., Oliva E.B., et al. Schistosomiasis and soil-transmitted helminth control in Niger: cost effectiveness of school based and community distributed mass drug administration [corrected] PLoS Negl Trop Dis. 2011;5(10):e1326. doi: 10.1371/journal.pntd.0001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshish A., AlKohlani A., Hamed A., et al. Towards nationwide control of schistosomiasis in Yemen: a pilot project to expand treatment to the whole community. Trans R Soc Trop Med Hyg. 2011;105(11):617–627. doi: 10.1016/j.trstmh.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Turner H.C., Truscott J.E., Hollingsworth T.D., Bettis A.A., Brooker S.J., Anderson R.M. Cost and cost-effectiveness of soil-transmitted helminth treatment programmes: systematic review and research needs. Parasit Vectors. 2015;8(1):355. doi: 10.1186/s13071-015-0885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angus Hughes D.N.-N., Clarke Naomi E., Dyer Clare, et al. 2023. Risk factors for soil-transmitted helminth infection amongst school children in Dak Lak province, Vietnam: a cross-sectional prevalence survey using quantitative PCR Parasit Vectors. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner H.C., Truscott J.E., Fleming F.M., Hollingsworth T.D., Brooker S.J., Anderson R.M. Cost-effectiveness of scaling up mass drug administration for the control of soil-transmitted helminths: a comparison of cost function and constant costs analyses. Lancet Infect Dis. 2016;16(7):838–846. doi: 10.1016/S1473-3099(15)00268-6. [DOI] [PubMed] [Google Scholar]
- 20.The World Bank Inflation, consumer prices (annual %) - Vietnam 2023. https://data.worldbank.org/indicator/FP.CPI.TOTL.ZG?locations=VN [cited 2023 February 23]. Available from:
- 21.US dollar to Vietnamese Dong exchange rate chart: XE historical currency exchange rates chart 2023. https://www.xe.com/currencycharts/?from=USD&to=VND&view=10Y XE. [cited 2023 February 21]. Available from:
- 22.Husereau D., Drummond M., Petrou S., et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1–e5. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Dak Lak Peoples’ Commitee Dak Lak’s population ranks first in the Central Highlands 2019. 2019. https://daklak.gov.vn/web/english/-/dak-lak-s-population-ranks-first-in-the-central-highlands [cited 2022 December 30]. Available from:
- 24.United Nations - Department of Economic and Social Affairs - Population Division World population prospects 2022 online edition 2022. https://population.un.org/wpp/ [cited 2023 February 21]. Available from:
- 25.World Health Organisation WHO methods and data sources for global burden of disease estimates 2000-2019. 2019. https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/ghe2019_daly-methods.pdf?sfvrsn=31b25009_7 [cited 2022 December 30]. Available from:
- 26.Global Burden of Disease Collaborative Network/Institute for Health Metrics and Evaluation (IHME) Global burden of disease study 2019 disability weights. Seattle, United States. 2019. http://ghdx.healthdata.org/record/ihme-data/gbd-2019-disability-weights [cited 2022 December 30]. Available from:
- 27.Bartsch S.M., Hotez P.J., Asti L., et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10(9) doi: 10.1371/journal.pntd.0004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke N.E., Ng-Nguyen D., Traub R.J., et al. A cluster-randomised controlled trial comparing school and community-based deworming for soil transmitted helminth control in school-age children: the CoDe-STH trial protocol. BMC Infect Dis. 2019;19(1):822. doi: 10.1186/s12879-019-4449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organisation Helminth control in school-age children 2011. https://apps.who.int/iris/handle/10665/44671 [cited 2022 December 30]. Available from:
- 30.Montresor A., Gabrielli A.F., Yajima A., et al. Markov model to forecast the change in prevalence of soil-transmitted helminths during a control programme: a case study in Vietnam. Trans R Soc Trop Med Hyg. 2013;107(5):313–318. doi: 10.1093/trstmh/trt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson T., Sculpher M.J., Claxton K., et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health. 2016;19(8):921–928. doi: 10.1016/j.jval.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Woods B., Revill P., Sculpher M., Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–935. doi: 10.1016/j.jval.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan S.D., Mauskopf J.A., Augustovski F., et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17(1):5–14. doi: 10.1016/j.jval.2013.08.2291. [DOI] [PubMed] [Google Scholar]
- 34.Bartsch S.M., Hotez P.J., Hertenstein D.L., et al. Modeling the economic and epidemiologic impact of hookworm vaccine and mass drug administration (MDA) in Brazil, a high transmission setting. Vaccine. 2016;34(19):2197–2206. doi: 10.1016/j.vaccine.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooker S., Kabatereine N.B., Fleming F., Devlin N. Cost and cost-effectiveness of nationwide school-based helminth control in Uganda: intra-country variation and effects of scaling-up. Health Policy Plan. 2008;23(1):24–35. doi: 10.1093/heapol/czm041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boselli G., Yajima A., Aratchige P.E., et al. Integration of deworming into an existing immunisation and vitamin A supplementation campaign is a highly effective approach to maximise health benefits with minimal cost in Lao PDR. Int Health. 2011;3(4):240–245. doi: 10.1016/j.inhe.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner H.C., Wilma A.S., Anthony W.S., et al. Are current preventive chemotherapy strategies for controlling and eliminating neglected tropical diseases cost-effective? BMJ Glob Health. 2021;6(8) doi: 10.1136/bmjgh-2021-005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Neve J.-W., Andriantavison R.L., Croke K., et al. Health, financial, and education gains of investing in preventive chemotherapy for schistosomiasis, soil-transmitted helminthiases, and lymphatic filariasis in Madagascar: a modeling study. PLoS Negl Trop Dis. 2018;12(12) doi: 10.1371/journal.pntd.0007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.