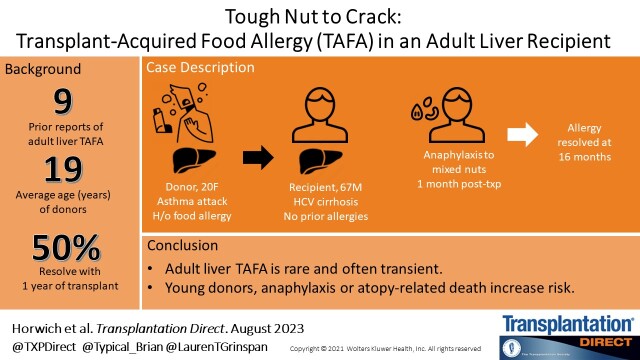
Abstract
Transplant-acquired food allergy (TAFA) is the phenomenon in which a de novo food allergy presents after transplantation.1 Although more widely reported in pediatric literature, liver TAFA remains rare in adults, with only 7 prior reported cases.2–4 The underlying pathophysiologic mechanisms, risk factors, and long-term outcomes of liver TAFA in adults are not well understood. We present a case of TAFA in a patient with no prior allergic history who received a graft from a young donor who died from a reported asthma attack.
CASE DESCRIPTION
A 67-y-old man with history of hepatitis C–associated cirrhosis complicated by ascites and hepatopulmonary syndrome was transplanted with exception points at a Model for End-stage Liver Disease-sodium (MELD-Na) of 28. Postoperative course was complicated by prolonged hypoxic respiratory failure with delayed extubation and mild acute kidney injury. There were no episodes of rejection in the peritransplant period. He was successfully discharged on postoperative day 18 with an immunosuppression regimen of tacrolimus, mycophenolate mofetil, and prednisone.
One month later, he was consuming mixed nuts (cashew, almond, and hazelnut) for the first time since transplantation and developed angioedema of the lips, throat discomfort, generalized pruritus, and abdominal pain. Before transplant, he reported no history of atopy and regularly consumed peanuts, nuts, eggs, wheat, and soy. Family history was relevant for a son with history of atopy and nut allergies. At urgent care, he was treated with diphenhydramine and intramuscular epinephrine with rapid improvement.
Serologic allergy testing for IgE the following day demonstrated sensitization to numerous allergens including peanuts, almond, cashew, soybean, and shrimp. Skin testing could not be performed due to recent diphenhydramine exposure. One month later, skin testing was positive for peanut, pistachio, cashew, walnut, and brazil nut. Serial serologic IgE testing over the following 6 mo demonstrated downtrending levels for all allergens tested (Table 1). Repeat skin testing demonstrated increasing sensitization to peanut, cashew, and pistachio. At 16 mo, the patient no longer has sensitization to peanut. He has tolerated ingestion of pistachio without reaction and passed a supervised oral challenge of cashew. Skin testing for all previously evaluated allergens is now negative.
TABLE 1.
Serologic IgE testing of recipient and donor with corresponding donor immunosuppression regimen
| Recipient | Donor (U/ml) | ||||
|---|---|---|---|---|---|
| 1 mo | 2 mo | 6 mo | 16 mo | At age 2 | |
| Allergen | |||||
| Serology (kU/L) | |||||
| Peanut | 1.72 | 0.74 | 0.56 | <0.10 | >100 |
| Ara H1 | 0.13 | <0.10 | <0.10 | ||
| Ara H2 | 0.24 | 0.16 | <0.10 | ||
| Ara H3 | <0.10 | <0.10 | <0.10 | ||
| Ara H6 | 0.11 | <0.10 | <0.10 | ||
| Ara H8 | <0.10 | <0.10 | <0.10 | ||
| Ara H9 | 0.37 | 0.24 | <0.10 | ||
| Soybean | 0.41 | 16.73 | |||
| Milk | <0.10 | 52.08 | |||
| Wheat | <0.10 | 10.89 | |||
| Egg | <0.10 | 51.59 | |||
| Almond | 0.39 | 0.14 | 0.11 | <0.10 | |
| Brazil Nut | <0.10 | <0.10 | <0.10 | <0.10 | |
| Cashew | 0.20 | 0.19 | 0.14 | ||
| Hazelnut | 0.73 | 0.27 | <0.10 | ||
| Pistachio | 0.54 | 0.38 | 0.14 | 0.12 | |
| Fish and Shellfish | |||||
| Mix | 2.04 | ||||
| Shrimp | 0.96 | ||||
| Crab | 0.15 | ||||
| Lobster | <0.10 | ||||
| Skin (wheal/flare, mm) | |||||
| Peanut | 6/20 | 7/0 | N | ||
| Almond | 2/0 | N | N | ||
| Brazil nut | 3/0 | N | N | ||
| Cashew | 6.5/20 | 12/0 | N | ||
| Hazelnut | 2/0 | 3/0 | N | ||
| Pistachio | 7.5/20 | 10/0 | N | ||
| Walnut | 7/20 | 5/0 | N | ||
| Pecan | 2/0 | N | N | ||
| Histamine | 8/15 | 5/0 | 5/10 | ||
| Immunosuppression | |||||
| Prednisone(mg/d) | 10 | 5 | 5 | – | |
| Tacrolimus dose(mg/12 h) | 2 | 8 | 9 | 4 | |
| Tacrolimus level(ng/mL) | 8.9 | 6.1 | 15.3 | 7.0 | |
Bolded serologies indicate a positive test result.
Due to the recipient’s development of TAFA, the donor’s medical history was reviewed. The donor was a 20-y-old female who presented with severe shortness of breath due to a presumed asthma attack. There was no known preceding food ingestion. Medical history was notable for atopy and allergies to peanuts, chocolate, eggs, wheat, and shellfish. History of tree nut allergy is unknown. Serologic IgE testing at age 2 was positive for peanuts, milk, wheat, pork, and soy (Table 1). Information regarding prior skin testing or IgE serologies at time of death are unavailable. Due to concern for anaphylaxis, epinephrine self-injector was used without response. The donor subsequently developed severe anoxic brain injury with brain death. There is no information available regarding the development of allergy in recipients of other organs from this donor.
DISCUSSION
TAFA is a rare phenomenon with sporadic cases in the adult literature. Although observed in multiple types of solid organ transplantation, it is most frequently seen in liver recipients.1 Onset is generally within 6 mo of transplant, with many experiencing resolution of the allergy within 2 y.2 The first report of liver TAFA was the transfer of a peanut allergy from a deceased donor who suffered anoxic brain injury following an anaphylactic reaction. The recipient, who received a liver and kidney, developed a peanut allergy 3 mo following transplantation.5 Since this seminal case, there have been several other reports of TAFA in adult and pediatric liver recipients including peanuts, soy, wheat, and fish (Table 2).3,6–9
TABLE 2.
Previous reports of transplant-associated food allergy in adult liver transplant recipients
| Citation | Donor age (y), sex | Donor cause of death | Donor culprit allergy | Other atopy, allergic history | Recipient age (y), sex | Time from transplant to allergic event | Trigger allergen | Recipient reaction | Recipient IS | Recipient allergy history | Duration of allergy | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Legendre et al | 22, M | Anaphylaxis | Peanut | – | 35, M | 3 m | Peanut | Anaphylaxis | CsA, AZA, CS | Unknown | >12 w | Kidney-pancreas transfer without TAFA |
| Trotter et al | 15, M | Anaphylaxis | Peanut | – | 28, F | 10 d, 28 d | Peanut | Hives | CsA, CS | None | NS | Heart, kidneys transfer without TAFA |
| Phan et al | 15, M | Anaphylaxis | Peanut | Atopic dermatitis, asthma | 60, M | 25 d | Cashew | Hives | Tacro, AZA, CS | Unknown | >48 w | Kidney, kidney-pancreas transfer without TAFA |
| Vagefi et al | 24, F | Anaphylaxis | Unknown | Nuts, seafood, kiwi, wheat | 54, M | 3 d | Nuts | Throat swelling, dyspnea | Tacro, MMF, CS | None | >4 w | No allergy rechallenge performed |
| Dewatcher et al | 20, M | Traumatic brain injury | – | Atopy, peanut | 62, F | 2 m | Peanut | Anaphylaxis | CsA, MMF, CS | None | 3 m | |
| Aggarwal et al | NS | NS | – | Peanut | 31, F | 4 m | Peanut | Perioral rash, swelling | Tacro, MMF, CS | Atopy | <2 m | Recipient with peanut sensitization, no TAFA |
| Muller et al | “Young”, NS | Anaphylaxis | Peanut | – | NS | 11 d | Peanut | Dyspnea, abdominal pain | NS | Unknown | <9 m | Lung recipients also with peanut TAFA |
| Roberts et al | NS | NS | – | – | NS | NS | Tree nuts, peanuts, soy, fish, seafood | NS | Tacro | NS | NS | Two cases reported |
| Horwich et al | 20, F | Asthma attack | – | Atopy, peanut, egg, wheat, soy, other nut | 67, M | 1 m | Cashew, almond, hazelnut | Anaphylaxis | Tacro, MMF, CS | None | 16 m | Resolution of sensitization and negative oral food challenge |
CS, corticosteroids; CsA, cyclosporine; d, days; IS, immunosuppression; m, months; MMF, mycophenolate; NS, not specified; Tacro, tacrolimus; TAFA, transplant-acquired food allergy; w, week.
Due to the complexity of the post-transplant immunologic environment, the mechanism of TAFA is not well understood. One hypothesis is that allergen-specific immunoglobulin E (IgE) bound to the donor liver is passively transferred during transplantation. When the recipient is subsequently exposed to the allergen, a hypersensitivity reaction occurs.5 This may explain why TAFA is overrepresented in liver transplant recipients and why not all recipients receiving organs from the same donor (eg, kidney and pancreas) develop a TAFA.5 The observation that nearly all adult liver TAFAs are from donors who died of atopy-related causes (eg, anaphylaxis and asthma) further supports this paradigm as they may have higher levels of preformed IgE at time of donation of this large organ. Other hypothesized mechanisms include as follows: (1) transfer of donor allergen-specific T and B cells and (2) preferential selection of Th2 lymphocytes by tacrolimus, promoting an allergic response.10–13
Epidemiologically, one key difference between adult and pediatric liver TAFA is donor allergy status. In children, the majority of TAFA cases received liver grafts from donors with no prior history of allergies.3 In the largest reported cohort of pediatric liver TAFA patients, donor allergy status had no significant association with post-transplant allergy development.2 In contrast, all previously reported cases in the adult liver literature have been recipients whose donors’ cause of death was anaphylaxis or history of prior anaphylaxis.5–7,13,14 Thus, donor allergy status appears to have greater implications in TAFA development in adult liver transplant recipients.
Strikingly, this case and review of prior reports suggests that the age of the donor is likely a significant factor. In all reported cases of adult liver TAFA for which donor information is available, the donors have been adolescents or young adults under the age of 25 y.5–9,13,15 This inverse association between donor age and likelihood of TAFA development has similarly been seen in pediatric cohorts.16 Given the liver’s immunomodulatory function and higher frequency of TAFA in pediatric recipients, it is possible that a younger immune system—whether intra- or extrahepatic—is a driver of TAFA in solid organ transplantation.
Despite the potentially catastrophic consequences of TAFA, there is no consensus regarding risk mitigation strategies in liver recipients. As the prevalence of anaphylaxis continues to rise globally, it is likely that the frequency of liver TAFA will increase.17 Previously, it has been suggested that systematic allergy screening of donors be implemented.11 Other recommendations have included that all potential liver transplant recipients be screened for allergic symptoms pre- and post-transplant.1 Given the uncommonness of adult TAFA, universal screening is unlikely to be practical. The preferred method of screening is also unclear. Prior literature in nontransplant populations suggests that the allergen-specific IgE-to-total IgE ratio is reasonable in predicting reaction to oral food challenge.18 However, serologic testing in the absence of clinical allergic symptoms is not advisable in the post-transplant population, as elevated IgE levels are seen in many individuals receiving tacrolimus-based immunosuppression.19 As allergen sensitization (elevated IgE without allergic reaction upon exposure) is often of little clinical significance, recommending food avoidance based on serologic testing alone may place undue burden on recipients. Based on existing evidence, referral to an experienced allergist should be considered for all liver recipients who received grafts from donors with history of food allergies or atopy.13 This case supports further vigilance in monitoring recipients whose donors died of anaphylaxis or atopy-related condition at age <25.
Once a diagnosis of TAFA has been made in an adult, there are limited data to guide further management. In pediatric cohorts, the majority of acquired allergies self-resolve within several years and may be ameliorated by transitioning immunosuppression from tacrolimus to cyclosporine.2,20 Yet current guidance is that adults with liver TAFA should avoid the offending allergen indefinitely and carry an epinephrine self-injector in case of exposure.1,5,8 Based on this case and several others, a reasonable alternate strategy is careful monitoring with serial IgE serologies and observed food challenge is appropriate in select patients after 1 y. These individuals should routinely follow with an allergist to screen for interval development of new allergies. Recipients and their caregivers must be educated on allergen avoidance, as well as signs and symptoms of an allergic reaction. Elucidation of underlying mechanisms and long-term follow-up of adult liver TAFA is important to further guide donor screening protocols and prospective management of affected recipients.
Footnotes
B.H.H., M.S., A.A., T.D.S., and L.T.G. participated in the writing of the article.
The authors declare no funding or conflicts of interest.
ORCID: 0000-0001-7455-280X.
Data are available from the authors upon request.
Contributor Information
Brian H. Horwich, Email: horwichb@gmail.com.
Maria Shtessel, Email: maria.shtessel@mssm.edu.
Alanna Alvarez, Email: alanna.alvarez@mountsinai.org.
Thomas D. Schiano, Email: thomas.schiano@mountsinai.org.
REFERENCES
- 1.Needham JM, Nicholas SK, Davis CM. Food allergies developing after solid organ transplant. Pediatr Transplant. 2015;19:827–835. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y, Lee YM, Kim MJ, et al. Long-term follow-up of de novo allergy in pediatric liver transplantation—10 yr experience of a single center. Pediatr Transplant. 2013;17:251–255. [DOI] [PubMed] [Google Scholar]
- 3.Boyle RJ, Hardikar W, Tang MLK. The development of food allergy after liver transplantation. Liver Transpl 2005;11:326–330. [DOI] [PubMed] [Google Scholar]
- 4.Topal E, Çatal F, Selimoglu MA, et al. Acquired atopic disease after liver transplantation in children; similarities to and differences from adults: a preliminary study. Eur J Gastroenterol Hepatol. 2014;26:1055–1059. [DOI] [PubMed] [Google Scholar]
- 5.Legendre C, Caillat-Zucman S, Samuel D, et al. Transfer of symptomatic peanut allergy to the recipient of a combined liver-and-kidney transplant. N Engl J Med. 1997;337:822–824. [DOI] [PubMed] [Google Scholar]
- 6.Phan TG, Strasser SI, Koorey D, et al. Passive transfer of nut allergy after liver transplantation. Arch Intern Med. 2003;163:237–239. [DOI] [PubMed] [Google Scholar]
- 7.Vagefi PA, Blazick E, Hamilos D, et al. Transference of food allergy after adult liver transplantation. Transplantation. 2009;87:1426. [DOI] [PubMed] [Google Scholar]
- 8.Dewachter P, Vézinet C, Nicaise-Roland P, et al. Passive transient transfer of peanut allergy by liver transplantation. Am J Transplant 2011;11:1531–1534. [DOI] [PubMed] [Google Scholar]
- 9.Roberts AJ, Lim A, Bishop JR, et al. Atopy and allergy following solid organ transplantation: a 15-year experience. J Paediatr Child Health. 2023;59:537–541. [DOI] [PubMed] [Google Scholar]
- 10.Bhinder S, Heffer MJ, Lee JK, et al. Development of transient peanut allergy following lung transplantation: a case report. Can Respir J. 2011;18:154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins D, Malka-Rais J. Food allergy: transfused and transplanted. Curr Allergy Asthma Rep. 2010;10:250–257. [DOI] [PubMed] [Google Scholar]
- 12.Özdemir O, Arrey-Mensah A, Sorensen RU. Development of multiple food allergies in children taking tacrolimus after heart and liver transplantation. Pediatr Transplant. 2006;10:380–383. [DOI] [PubMed] [Google Scholar]
- 13.Muller YD, Vionnet J, Beyeler F, et al. ; Swiss Transplant Cohort Study. Management of allergy transfer upon solid organ transplantation. Am J Transplant 2020;20:834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal A, Balogun R, Carr TF, et al. Transfer of peanut allergy from donor to recipient after liver transplant. Ann Hepatol. 2019;18:508–513. [DOI] [PubMed] [Google Scholar]
- 15.Trotter JF, Everson GT, Bock SA, et al. Transference of peanut allergy through liver transplantation. Liver Transpl. 2001;7:1088–1089. [DOI] [PubMed] [Google Scholar]
- 16.De Bruyne R, Dullaers M, Van Biervliet S, et al. Post-transplant food allergy in children is associated with liver and not with renal transplantation: a monocentric comparative study. Eur J Pediatr. 2013;172:1069–1075. [DOI] [PubMed] [Google Scholar]
- 17.Turner PJ, Campbell DE, Motosue MS, et al. Global trends in anaphylaxis epidemiology and clinical implications. J Allergy Clin Immunol Pract 2020;8:1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta RS, Lau CH, Hamilton RG, et al. Predicting outcomes of oral food challenges by using the allergen-specific IgE–total IgE ratio. J Allergy Clin Immunol Pract 2014;2:300–305. [DOI] [PubMed] [Google Scholar]
- 19.Granot E, Yakobovich E, Bardenstein R. Tacrolimus immunosuppression: an association with asymptomatic eosinophilia and elevated total and specific IgE levels. Pediatr Transplant. 2006;10:690–693. [DOI] [PubMed] [Google Scholar]
- 20.Maarof G, Krzysiek R, Décline J-L, et al. Management of post–liver transplant–associated IgE-mediated food allergy in children. J Allergy Clin Immunol. 2011;127:1296–1298. [DOI] [PubMed] [Google Scholar]