Abstract
Background
Understanding the financial consequences of endemically prevalent pathogens within the porcine respiratory disease complex (PRDC) and the effects of interventions assists decision-making regarding disease prevention and control. The aim of this systematic review was to identify what economic studies have been carried out on infectious endemic respiratory disease in pigs, what methods are being used, and, when feasible, to identify the economic impacts of PRDC pathogens and the costs and benefits of interventions.
Results
By following the PRISMA method, a total of 58 studies were deemed eligible for the purpose of this systematic review. Twenty-six studies used data derived from European countries, 18 from the US, 6 from Asia, 4 from Oceania, and 4 from other countries, i.e., Canada, Mexico, and Brazil. Main findings from selected publications were: (1) The studies mainly considered endemic scenarios on commercial fattening farms; (2) The porcine reproductive and respiratory syndrome virus was by far the most studied pathogen, followed by Mycoplasma hyopneumoniae, but the absence or presence of other endemic respiratory pathogens was often not verified or accounted for; (3) Most studies calculated the economic impact using primary production data, whereas twelve studies modelled the impact using secondary data only; (4) Seven different economic methods were applied across studies; (5) A large variation exists in the cost and revenue components considered in calculations, with feed costs and reduced carcass value included the most often; (6) The reported median economic impact of one or several co-existing respiratory pathogen(s) ranged from €1.70 to €8.90 per nursery pig, €2.30 to €15.35 per fattening pig, and €100 to €323 per sow per year; and (7) Vaccination was the most studied intervention, and the outcomes of all but three intervention-focused studies were neutral or positive.
Conclusion
The outcomes and discussion from this systematic review provide insight into the studies, their methods, the advantages and limitations of the existing research, and the reported impacts from the endemic respiratory disease complex for pig production systems worldwide. Future research should improve the consistency and comparability of economic assessments by ensuring the inclusion of high impact cost and revenue components and expressing results similarly.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40813-023-00342-w.
Keywords: Systematic review, Economic impact, Respiratory Disease, Pigs
Background
Respiratory disease, referred to as the porcine respiratory disease complex (PRDC) when multiple pathogens and non-infectious factors are involved, is regarded as one of the most serious health problems in contemporary pig production. In Europe, pneumonia and pleuritis are the most frequent lung lesions observed at the slaughterhouse, with prevalence up to 69% and 48%, respectively [1–5]. In the United States, results from the 2012 National Animal Health Monitoring System indicated that respiratory problems were the main cause of deaths in weaned (47.3%) and grower/finisher pigs (75.1%) [6]. Besides increasing mortality, the PRDC is believed to induce production losses through reduced growth rates and a lower feed conversion efficiency [7, 8]. Consequently, respiratory disease remains one of the main reasons for antimicrobial usage in both nursery and growing/finishing pigs [9–11].
The PRDC term was coined to emphasise the complexity of events leading to respiratory disease development, including the involvement of (several) viral and bacterial pathogens as well as environmental, management, and genetic factors [12, 13]. Pathogens involved in the PRDC vary considerably in different countries, regions, and herds over time [14, 15]. The most common primary viral agents include porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus 2 (PCV-2), and swine influenza virus (SIV) [12, 13, 16]. Other primary pathogens such as pseudorabies virus and porcine respiratory coronavirus are reported but they have less impact on today’s porcine health [17]. The bacterial species involved in this disease complex are traditionally distinguished between primary or initiators, such as Mycoplasma (M.) hyopneumoniae, and Actinobacillus (A.) pleuropneumoniae, and secondary or follower pathogens (e.g., Pasteurella multocida, Streptococcus suis and Bordetella bronchiseptica) [12, 13, 16]. The presence of various infectious agents in cases of PRDC leads to complex and potentially synergistic interactions that can increase the severity and duration of clinical signs and lesions, as well as the economic consequences [17].
As economic margins on pig farms are generally small [18], it is valuable to understand costs caused by endemically prevalent individual and co-existing pathogens within the PRDC, as there may be opportunities to increase farm profitability by controlling or preventing these infections. Therefore, estimates of costs and benefits of mitigation measures, can support decision-making regarding disease control at farm, integration system, regional and national levels.
Although one would expect the economic impact of respiratory disease to be well studied for the abovementioned reasons, no review or meta-analysis exists that maps the current state of economic research in this field. The economic implications of pathogens involved in the PRDC are likely to be heavily impacted by the variety in production systems and endemically prevalent strains of different pathogens across countries, as well as by the applied economic methods. These methods are defined by both the type of economic analysis (e.g. basic cost computations, partial budget analysis, cost-benefit analysis) and the cost components considered in this analysis (e.g. labour costs, feed costs, veterinary costs). Thus, the aim of this systematic review was to identify what economic studies have been carried out on infectious endemic respiratory disease in pigs, what economic methods are being used, and, when feasible, to identify the economic impacts of specific or co-existing PRDC pathogens and the costs and benefits of interventions.
Materials and methods
A systematic literature review was conducted to identify relevant economic research on infectious endemic respiratory disease in pigs and related interventions. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines were followed [19], without the use of risk-of-bias analysis (e.g. assessing the selection bias, reporting bias per study).
Literature search
The search for suitable references was conducted in PubMed®, Scopus and CAB Abstracts. We restricted the search to studies published after January 1, 1980, and to peer-reviewed original research in English only. The search strings consisted of three parts (topic, population and focus), which were all required to be present in the title or abstract for a study to be included (for the full search strings, please refer to Supplementary file S1). The terms related to respiratory disease (topic) included terminology for both respiratory disease at syndrome level and for specific respiratory pathogens. The pathogens included were the most common infectious agents within the PRDC that are considered endemic in large parts of the world: the viral agents PRRSV, SIV and PCV-2, and the bacterial agents M. hyopneumoniae and A. pleuropneumoniae. The systematic search was lastly updated on January 23, 2023.
Selection of studies
The abstracts obtained from the search were screened by two independent reviewers (co-authors MB and BGM). Studies were excluded when their main focus was not on respiratory disease in pigs and/or when no mention was made of an impact on either production parameters (e.g. average daily gain, mortality or feed conversion ratio) or on costs or revenues. The two reviewers compared and merged their findings and created a list of studies for the full text review, which was likewise carried out independently by MB and BGM. At this stage, only studies were included when the full text was available, when the report provided a full (e.g., farm budget analysis, cost-benefit analysis) or partial financial evaluation (e.g., cost analysis, basic calculation of medication costs), and when all calculated changes in economic outputs could be attributed to respiratory disease or to the interventions aiming to reduce or control the disease.
In addition, all open-access issues from the Journal of Swine Health and Production (JSHAP) were manually checked, as this journal is not indexed in a number of databases. Studies that met the screening and eligibility criteria were included. Lastly, reference lists and citations of all selected studies were examined for additional studies that met all inclusion criteria (literature snowballing).
Data extraction
Data from the eligible studies were manually extracted by MB and BGM (not independently) and an online spreadsheet for data entry was used. These metadata included study characteristics related to publication (authors, year of publication, country and journal), study focus (syndrome or pathogen level, disease or intervention, unit of interest, farm type and animal age-group), study design (observational, experimental or simulation model) and economic methodology (type of economic evaluation, cost/revenue components, reported economic outcomes and currency). Additionally, we registered the origin of the data used in each study (e.g. primary data collected by the authors, expert opinion, data from scientific literature) and whether the paper mentions the testing of or accounting for the presence of other PRDC pathogens. All collected data are summarized in the text and provided in the Supplementary Files. Where we provide economic outcomes from the included studies, we adjusted the reported study outcomes for inflation using an online tool (https://in2013dollars.com/) and converted the original currency to euros using a currency converter tool (https://cuex.com/en/) (last applied on September 29, 2023). Where applicable, simple calculations were performed to reach a common unit to express the study results, such as the economic impact per fattening pig.
Results
Overview of the included studies
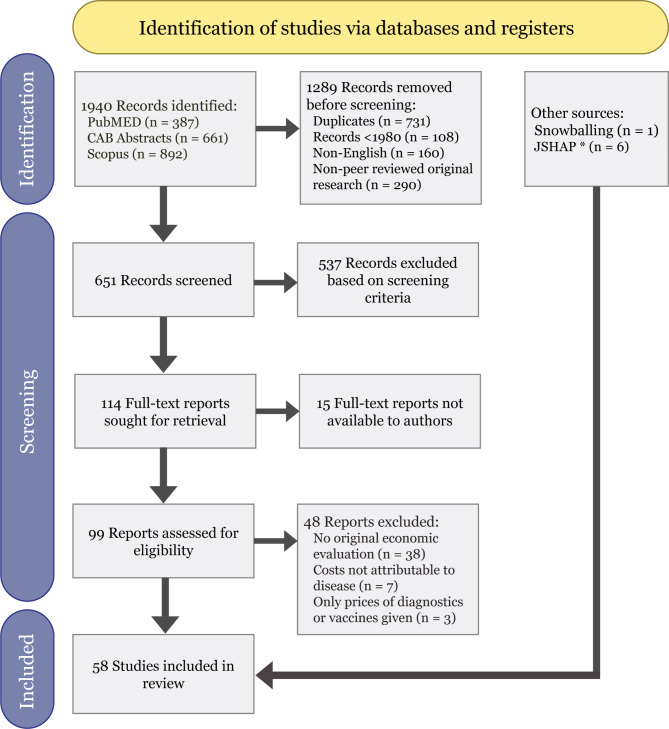
The combination of search terms in the selected databases resulted in 1,940 studies (Fig. 1). In total, 651 non-duplicate citations were screened, and those that did not meet our previously defined screening criteria were excluded, leaving a total of 114 studies. After the final selection, 58 studies were deemed eligible for the purpose of this systematic review, including results from snowballing and JSHAP. The full list of references obtained from the systematic search is available in Supplementary file S2.
Fig. 1.
Flow diagram illustrating the systematic search strategy for identifying relevant studies. *JSHAP = Journal of Swine Health and Production
Characteristics of included studies
Detailed characteristics of the studies included in this review are presented in Tables 1 and 2. Overall, the studies were classified into those focused on the economic impact of the disease (23/58; Table 1) and those assessing economics of interventions to prevent and/or control disease (33/58; Table 2). Two studies analysed both the impact of disease and of interventions [20, 21]. Most intervention-focused studies investigated the effects of vaccination (24/35). Of these studies, seventeen evaluated the costs and benefits of vaccination for a short time period (i.e. in one cycle or one year), while seven evaluated the impact for a longer period. After vaccination, the most studied interventions related to elimination strategies (8/35; i.e. depopulation and repopulation, test and removal, herd closure), for all of which the impacts were studied for a long time period (> 1 year). Other interventions that were studied include animal management-related measures (4/35; no mixing of litters, early weaning, selection of pathogen-free gilts, separate housing), medication (3/35), biosecurity (3/35), alternative diet or feed regimen (2/35), and installation of air filtration systems (1/35). Eight of the intervention-focused studies investigated and compared the effects of several interventions.
Table 1.
Characteristics of studies evaluating the economic impact of disease caused by endemic respiratory pathogens in pigs
| Study | Country | Infection scenario | Infectious agent(s)1 | No. of farms supplying primary production data2 | Stage of production | Unit of analysis | Study design | Data source(s)3 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pointon et al. (1985) | Australia | Endemic/Epidemic | Mhp | 1 | Growing | Pig | Controlled trial | DA; C | [26] |
| Miller and Dorn (1990) | US | Endemic | NS | 13 | Breeding and growing | Pig | Cross-sectional | DA | [65] |
| Brouwer et al. (1994) | Netherlands | Endemic | PRRSV | 91 | Breeding | Sow | Historical control study | DA; C | [66] |
| Christensen (1995) | New Zealand | Endemic | Mhp | 1 | Growing | Percentage point of pneumonia | Cross-sectional | DA | [67] |
| Pejsak and Markowska-Daniel (1997) | Poland | Epidemic | PRRSV | 1 | Breeding and growing | Farm | Historical control study | DA | [68] |
| Garner et al. (2001) | Australia | Endemic/Epidemic | PRRSV | NA | Breeding and growing | Country, region, farm, and pig | Stochastic model | LG | [38] |
| Bennett and IJpelaar (2005) | UK | Endemic | SIV | NA | NR | Country | Deterministic model | LS; E | [69] |
| Losinger (2005) | US | Endemic/Epidemic | App | NR | Growing | Country | Cobb-douglas production model | DA; LS; LG | [28] |
| Neumann et al. (2005) | US | Epidemic | PRRSV | 10 | Breeding and growing | Country and farm | Case-control | DA; LG | [70] |
| Nieuwenhuis et al. (2012)* | Netherlands | Epidemic | PRRSV | 9 | Breeding | Sow | Historical control study | DA | [20] |
| Alarcon, Rushton and Wieland (2013) | UK | Endemic/Epidemic | PCV-2 | 147 | Growing | Country, farm, and pig | Stochastic model | LS; LG; E | [34] |
| Holtkamp et al. (2013) | US | Endemic | PRRSV | 80 breeding farms and 639 groups of growing pigs (No. farms NR) | Breeding and growing | Country, sow, and pig | Cross-sectional | DA; LG | [55] |
| Nathues et al. (2017) | Germany | Endemic | PRRSV | NA | Breeding and growing | Farm, sow, and pig | Stochastic model | LS; LG; E; C | [35] |
| Pham et al. (2017) 4 | Vietnam | Endemic | PRRSV | 162 | Breeding and growing | Farm | Cross-sectional | DA; E; LS; LG | [22] |
| Valdes-Donoso et al. (2018) | US | Epidemic | PRRSV | 16 | Breeding | Farm and sow | Historical control study | DA; LG | [71] |
| Calderón Díaz, Rodrigues da Costa, et al. (2020) | Ireland | Endemic | NS | 56 | Growing | Farm, pig, and Kg of meat | Stochastic model | DA; LS | [37] |
| Calderón Díaz, Fitzgerald, et al. (2020)* | Ireland | Endemic | PRRSV, SIV, Mhp | 56 | Growing | Farm | Stochastic model | DA | [21] |
| Ferraz et al. (2020) | Brazil | Endemic | Mhp | 1 | Growing | Pig | Cross-sectional | DA | [72] |
| Trevisan et al. (2020) | US | Endemic | PRRSV | 20 | Breeding and growing | Pig | Cohort | DA; LG | [73] |
| Paz-Sánchez et al. (2021) | Spain | Endemic | Co-infection | 1 | Growing | Pig | Cross-sectional | DA; LG | [5] |
| Renken et al. (2021) | Germany | Endemic | PRRSV | 21 | Breeding | Farm and sow | Cross-sectional | DA; LS; LG | [39] |
| Kim et al. (2022) | Korea | Epidemic | PRRSV | 1 | Breeding and growing | Sow and pig | Historical control study | DA; LS | [74] |
| Pfuderer et al. (2022) | UK | Endemic | NS | NA | Growing | Pig | Systems dynamics model | DA; LS; LG; A | [27] |
| Trevisi et al. (2022) | Italy | Endemic | PRRSV | NR | Growing | Pig flow and Kg of meat | Cohort | DA | [75] |
| Zhang et al. (2022) | China | Epidemic | PRRSV | 4 | Breeding and growing | Sow and pig | Historical control study | DA; LG | [76] |
* Studies focus on both the economic impact of disease and the effects of interventions to prevent/control disease
1 PRRSV = porcine reproductive and respiratory syndrome virus; PCV-2 = porcine circovirus 2; SIV = swine influenza virus (SIV); Mhp = Mycoplasma hyopneumoniae; App = Actinobacillus pleuropneumoniae; NS = not specified
2 NR = number of farms providing primary data is not reported; NA = no primary data is provided by farms
3 LS = scientific literature; LG = grey literature (e.g. industry reports, websites, proceedings, newsletters, government documents); E = expert(s) opinion; DA = data from authors; C = personal communication; A = author(s) expertise
4 Studies consider economic impact in smallholder farm(s) or research facility settings, whereas all other studies consider only commercial farm(s)
Table 2.
Characteristics of studies evaluating the economic impact of interventions to prevent/control disease caused by endemic respiratory pathogens in pigs
| Study | Country | Infection scenario | Infectious agent(s)1 | Intervention | No. of farms supplying primary production data2 | Stage of production | Unit of analysis | Study design | Data sources3 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Dee (1994) | US | Endemic | Mhp | Medication and early weaning | 2 | Growing | Pig | Historical control study | DA | [77] |
| Dee et al. (1996) | US | Endemic | PRRSV | Nursery depopulation | 5 | Growing | Farm, sow, and pig | Historical control study | DA | [78] |
| Dee et al. (1997) | US | Endemic | PRRSV | Nursery depopulation | 34 | Growing | Sow | Historical control study | DA | [79] |
| Dee and Molitor (1998) | US | Endemic | PRRSV | Elimination (test and removal) | 1 | Breeding | Sow | Case report | DA; A | [32] |
| Maes et al. (1998) | Belgium | Endemic | Mhp | Vaccination | 5 | Growing | Pig | Controlled trial | DA | [42] |
| Pallares et al. (2000) | Spain | Endemic | Mhp | Vaccination | 8 | Growing | Pig | Controlled trial | DA | [43] |
| Kyriakis et al. (2001) | Greece | Endemic | Mhp | Vaccination | 1 | Growing | Pig | Controlled trial | DA | [80] |
| Miller et al. (2001) | US | Endemic | Mhp | Vaccination | NA | Growing | Farm | Deterministic model | LS; A | [81] |
| Pallares et al. (2001) | Spain | Endemic | Mhp | Vaccination | 16 | Growing | Kg of carcass and kg gained in fattening | Controlled trial | DA | [82] |
| Maes et al. (2003) | Belgium | Endemic | Mhp | Vaccination | 14 | Growing | Pig | Controlled trial | DA | [83] |
| Stipkovits et al. (2003) | Hungary | Endemic | Mhp | Vaccination and medication | 1 | Growing | Kg of finishing pig marketed | Controlled trial | DA | [84] |
| Holyoake and Callinan (2006) | Australia | Endemic | Mhp | Vaccination | 3 | Growing | Pig | Controlled trial | DA; LS; A | [85] |
| Schaefer and Morrison (2006) | US | Endemic | PRRSV | Elimination (herd closure) | 15 | Breeding | Herd (farms combined) | Historical control study | DA; A | [86] |
| Rapp-Gabrielson et al. (2007) | US | Endemic | Co-infection | Vaccination | 1 | Growing | Carcass value | Controlled trial | DA; LG | [87] |
| Young et al. (2011) 4 | Canada | Endemic | PCV-2 | Vaccination | 1 | Growing | Pig | Controlled trial | DA; A | [24] |
| Nieuwenhuis et al. (2012)* | Netherlands | Epidemic | PRRSV | Monitoring, vaccination, and eradication | 9 | Breeding | Sow | Historical control study | DA | [20] |
| Alarcon, Rushton, Nathues, et al. (2013) | UK | Endemic | PCV-2 | Vaccination, diets, stocking density, biosecurity, and depopulation-repopulation | 50 | Growing | Farm | Stochastic model | LS; LG; E | [33] |
| Alonso et al. (2013) | US | Endemic | PRRSV | Air filtration | 21 | Breeding | Farm, sow, and pig | Cohort / deterministic model | DA; LS | [30] |
| Zhang et al. (2014) | Vietnam | Endemic/Epidemic | PRRSV | Vaccination | NA | Growing | Country | Deterministic model | DA; LS; A; E | [57] |
| Linhares et al. (2015) | US | Endemic | PRRSV | Vaccination | NA | Breeding | Farm | Deterministic & Stochastic model | DA; LS; A | [44] |
| Ramirez et al. (2015) | US | Endemic | Co-infection | Medication | 4 | Growing | Pig and Kg of body weight | Controlled trial | DA | [40] |
| Stygar et al. (2016) | Finland | Epidemic | App | Cleaning, vaccination, and medication | NA | Growing | Pig space unit | Dynamic programming model | LS; A | [88] |
| Crenshaw et al. (2016)4 | Canada | Endemic | PRRSV | Feed regimen | 1 | Growing | Pig | Controlled trial | DA; A | [25] |
| Kaalberg et al. (2017) | Netherlands | Endemic | Co-infection | Vaccination | 1 | Growing | Pig | Controlled trial | DA; LG | [89] |
| Kim et al. (2017) | Korea | Endemic | PRRSV | Vaccination | 3 | Breeding and growing | Farm | Historical control study | DA; LG | [41] |
| Zhang et al. (2017)4 | Cambodia | Epidemic | PRRSV | Vaccination and biosecurity | NA | Breeding and growing | Farm | Deterministic model | DA; LG; LS | [23] |
| Duivon et al. (2018) | France | Endemic | Co-infection | Vaccination | 1 | Growing | Sow and pig | Controlled trial | DA; LG | [90] |
| Nathues et al. (2018) | Germany | Endemic | PRRSV | Depopulation-repopulation, close and roll-over, vaccination, and biosecurity | NA | Breeding and growing | Farm | Stochastic model | LS; A; E | [36] |
| Silva et al. (2019) | US | Endemic | Mhp | Elimination | 70 | Breeding and growing | Farm, sow, and pig | Cross-sectional/ deterministic model | DA; LS | [31] |
| Calderón Díaz, Fitzgerald, et al. (2020)* | Ireland | Endemic | Co-infection | Vaccination | 56 | Growing | Farm | Stochastic model | DA | [21] |
| Thomann et al. (2020) | Germany | Endemic | PRRSV | Vaccination | NA | Breeding | Country, farm, and sow | Stochastic model | LS; LG; A | [91] |
| Abella et al. (2021) | Spain | Endemic/Epidemic | PRRSV | Animal selection | 1 | Breeding | Sow | Discrete-based event model | DA; LG | [92] |
| Quezada-Fraide et al. (2021) | Mexico | Endemic | PRRSV | Vaccination | 2 | Growing | Pig and day of fattening | Cohort study | DA; A | [93] |
| Jerlström et al. (2022) | Sweden | Endemic | NS | No litter mixing after weaning and separate gilt management | 1 | Growing | Farm | Deterministic & stochastic model | LG; E, C, | [94] |
| Moura et al. (2022) | US | Endemic | PRRSV | Vaccination | 9 | Growing | Experimental group | Controlled trial / deterministic model | DA; LG | [29] |
* Studies focus on both the economic impact of disease and the effects of interventions to prevent/control disease
1 PRRSV = porcine reproductive and respiratory syndrome virus; PCV-2 = porcine circovirus 2; SIV = swine influenza virus (SIV); Mhp = Mycoplasma hyopneumoniae; App = Actinobacillus pleuropneumoniae; NS = not specified
2 NR = number of farms providing primary data is not reported; NA = no primary data is provided by farms
3 LS = scientific literature; LG = grey literature (e.g. industry reports, websites, proceedings, newsletters, government documents); E = expert(s) opinion; DA = data from authors; C = personal communication; A = author(s) expertise
4 Studies consider economic impact in smallholder farm(s) or research facility settings, whereas all other studies consider only commercial farm(s)
The studies were conducted in 23 different countries. Twenty-six studies used data derived from European countries, 18 from the US, 6 from Asia, 4 from Oceania, and 4 from other countries, i.e., Canada, Mexico, and Brazil. Considering the period of 1980 until now, we found that over half of the studies (33/58) were published in the last ten years (2013–2022) and, of those, 61% (20/33) focused on PRRSV. Overall, half of the included studies (29/58) analysed the economic impact of PRRSV associated disease and/or its interventions, followed by M. hyopneumoniae (13/58). For the remaining pathogens the number of indexed studies was low: three for PCV-2, two for A. pleuropneumoniae, and one for SIV. Only in ten of all studies focusing on one specific pathogen, the absence or presence of other specific endemic respiratory pathogens was verified or accounted for. Then, six studies targeted co-infection scenarios (e.g., PRDC). In three of these studies, the co-infection of M. hyopneumoniae and PCV-2 was studied, whereas in the remaining studies different combinations of at least three of the primary pathogens (i.e. PRRSV, SIV, PCV-2, M. hyopneumoniae, A. pleuropneumoniae) were investigated. Lastly, four studies did not specify the respiratory pathogens involved, instead, they assessed the economic impact of lung lesions. Since the pathogens included in the present review are predominantly endemic worldwide, the economic analyses were mainly applied for endemic scenarios, although 24% (14/58) of the studies also included epidemic (i.e., outbreak) episodes in their analyses.
Most of the studies were conducted in commercial herds (54/58), with only two Asian studies of smallholder farms with less than 20 sows or 100 fattening pigs [22, 23] and two studies conducted in research facilities [24, 25]. The number of farms (owned by one or more pig producers) from which primary data were collected on production performance or health ranged from 1 to 162, with a single farm being investigated in 16 of the studies. Studies on the growing phase (33/58), including weaners and fatteners, predominated over the breeding phase (11/58), although several studies assessed economics in both production phases (14/58). Regarding the expression of economic outcomes, 17 different units of analysis were identified (e.g. pig, herd, farm, Kg meat). In 66% (38/58) of the studies, a singular unit was used, whereas the remaining 34% (20/58) used several units to express economic results. The growing pig was the most extensively used unit of analysis (28/58).
Methodology applied in included studies
In most of the disease-focused studies (16/25), an observational study design was used in which data were collected cross-sectionally, longitudinally, or retrospectively, with no intervention except for the collection of the data. Of these observational study designs, the cross-sectional study design (7/16) and the historical control study design (6/16), dominated over cohort (2/16) and case-control (1/16) study designs. Across all disease-focused studies, only one controlled trial was performed [26]. The remaining eight studies calculated economic impacts through modelling (8/25); five models were stochastic, one deterministic, one study described the use of a systems dynamics model [27] and one study applied the Cobb-Douglas production function [28]. In three of the modelling studies, input parameters were based on primary data on production performance or health collected on farms. In the remaining five, only secondary data (from scientific literature, grey literature, expert opinion or personal communication) were used. All modelling studies that used secondary data only, performed sensitivity analysis on uncertain input parameters.
Modelling was part of a large share of intervention-focused research, as 11 studies relied on simulation modelling exclusively. Of these studies, four collected primary production data from farms to be used in their models, whereas seven used secondary data only. As before, the modelling studies that used only secondary data performed sensitivity analysis on uncertain input parameters. Additionally, in three intervention-focused studies, controlled trial [29], cohort [30], or cross-sectional [31] study designs were combined with an economic deterministic model. Furthermore, fourteen studies collected data solely by means of a controlled trial and six by means of observational study designs (five historical control studies and one cohort study). One study, by Dee and Molitor [32], entailed a case report describing the attempt of PRRSV elimination on one farm. For detailed information on the study designs per included study, refer to Tables 1 and 2.
Economic methods that were applied in the eligible studies, ranged from basic cost computations to more comprehensive methods commonly used in animal health economics (Table 3). The most utilised methods were basic cost computations (15/58) and cost analysis (14/58), followed by partial budget analysis (12/58). As expected, methods built for comparing the profitability of on-farm changes, i.e. the partial budget and cost-benefit analysis, were almost exclusively applied in intervention-focused studies. In five modelling studies, multiple economic methods were applied [33–37].
Table 3.
Economic evaluation methods used in the eligible studies
| Method | Description | Number of studies (D|I)* |
|---|---|---|
| Basic computation | Basic calculation of a cost (e.g., total vaccination purchase costs) or of a reduction in revenues (e.g., reduction of number of piglets weaned * sale price per piglet); or adjustment of one value in an external tool. | 5 | 10 |
| Cost analysis | Calculation or estimation of several or total variable costs (including estimation of reduced revenues). | 9 | 5 |
| Margin over specific variable costs | Revenue minus feed and/or veterinary (medication/vaccination) costs. | 0 | 5 |
| Gross margin | Enterprise outputs minus all variable costs1. | 5 | 4 |
| Farm budget | Calculation of the total net profit on a farm, by deducting fixed costs from the gross margin1. | 5 | 2 |
| Partial budget | Determining the change in net profit resulting from a change on a farm. Calculated by identifying revenues foregone, extra costs, additional revenue, and reduced costs1. | 2 | 10 |
| Cost-benefit analysis | Determining the profitability of programs over an extended period of time, by enumerating benefits and costs and applying a discount rate to convert future values to present values. Consequently, the Net Present Value and Benefit-Cost Ratio can be calculated1. | 0 | 2 |
| Other |
Types of economic analysis performed by single studies. 1. Economic welfare analysis 2. Cash flow analysis and decision optimization. |
1. Disease-focused. (28) 2. Intervention- focused. (33) |
* D = Disease-focused, I = Intervention-focused
1 Description derived from Dijkhuizen, Morris and Huirne (95)
Seven studies provided estimates of the economic burden at a national level, of which only two studies included price effects across the industry or looked at changes in consumer and producer surplus due to decreased pork production [28, 38]. The remaining five studies extrapolated farm-level estimates without accounting for additional macro-economic effects. Thus, most studies investigated the financial losses at the farm-level, rather than economic losses. However, to keep the terminology simple, we will keep referring to the calculated impacts as the economic impact.
To calculate the on-farm economic impact, a wide range of cost components were considered across all papers (for detailed information of the components per study, please refer to Supplementary file S3). Studies using the same economic method or focusing on the same disease, often included different cost and revenue components in their calculations (Fig. 2). Overall, the most frequently used cost components were veterinary costs (49/58 studies), feed costs (39/58), and labour costs (26/58); whereas the most frequently used revenue components were reduced carcass value (24/58), fewer growing pigs sold (19/58) and fewer piglets weaned/sold (19/58). The modelling studies that considered the most cost components [33, 36, 37, 39] all reported that feed costs and the reduced revenue from fewer sold piglets or fattening pigs were the costliest components. Although most studies included these components, 19 out of the 58 studies did not consider feed costs, and 24 did not calculate lost revenues due to fewer piglets weaned or fattening pigs sold.
Fig. 2.
Cost and revenue components considered in economic analyses of studies on PRRSV. * Other components include penalties, subsidies/compensation and industry effects
Pathogen specific costs
Despite the variety in units of analysis, the economic outcomes per study could be converted to a common unit for 17 out of 25 disease-focused studies (Fig. 3a-c). This figure serves as an illustration for the range in reported economic impacts, but it should be noted that study outcomes cannot be merged directly due to the variety in study characteristics and methods of calculation.
Fig. 3.
Economic impact of disease caused by endemic respiratory pathogens. The economic impact is expressed in decreased profit (in euros) per sow-year (a), per nursery pig (b), and/or, per fattening pig (c). Circles indicate a single reported outcome, whereas boxplots represent a range of economic outcomes from one study (e.g. when different scenarios with varying disease severity were considered, or when economic losses were reported for multiple farms separately). Reported outcomes were adjusted for inflation up until the year 2023 and converted to euros as a common currency. Studies that are marked with an *, did not include feed costs as a component in their economic analysis
Since most intervention-focused studies analysed the benefits of vaccination, the main economic outcomes for these studies are summarised in Table 4. It is evident from this table that there is no common method for expressing the main economic impact of vaccination. Overall, most of the intervention-focused studies (24/35) reported a positive economic impact due to the implementation of the respective intervention, while three reported a negative impact [21, 32, 40] and four a neutral impact [30, 41–43]. In the remaining four intervention-focused studies, the effects of different interventions were compared with each other rather than with a control group [20, 25, 29, 44]. For all outcomes from both disease-focused and intervention-focused studies in their original currency, please refer to Supplementary file S4.
Table 4.
Economic impact of vaccination against endemic respiratory diseases in pigs
| Study | Economic outcome in euros | Unit | Reference | |
|---|---|---|---|---|
| Co-infections | ||||
| Rapp-Gabrielson et al. (2007)1 | 12.91, 7.82, 9.57 | Increased value per carcass for three different vaccines (compared to control) | [87] | |
| Kaalberg et al. (2017)2 | 3.67 | Benefit per finisher | [89] | |
| Duivon et al. (2018)2 | 2.16 | Benefit per finisher | [90] | |
| Porcine reproductive and respiratory syndrome virus | ||||
| Zhang et al. (2014) | 2.3–4.5 | Benefit-cost ratio | [57] | |
| Moura et al. (2022) | 1.83 | Benefit-cost ratio | [29] | |
| Kim et al. (2017) | Difference in medication costs not significant | [41] | ||
| Linhares et al. (2015) | 32,345 | Difference in opportunity costs between modified-live virus vaccination and field-virus inoculation for a 1,000 sow breeding herd | [44] | |
| Zhang et al. (2017) | 155.20-316.68 | Increased net profits per farm (two-sow breeder; five-pig fattener; single-sow, three-pig farrow-to-finish) | [23] | |
| Thomann et al. (2020) |
1) 211–422 2) 184–335 |
Median annual benefits per sow of (1) vaccinating sows and piglets and (2) Vaccinating only sows | [91] | |
| Quezada-Fraide et al. (2021) | 2.14 | Difference in costs per weaned pig between vaccinating sows and piglets and vaccinating sows only | [93] | |
| Mycoplasma hyopneumoniae | ||||
| Maes et al. (1998) | Difference in curative parental medication costs not significant | [42] | ||
| Pallarés et al. (2000) | Difference in medication costs not significant | [43] | ||
| Kyriakis et al. (2001) | 0.46, 0.36 | Reduced medication cost per pig for two different vaccination schemes (compared to control) | [80] | |
| Stipkovits et al. (2003) |
1) -0.02, -0.06 2) 0.03, 0.08 |
Difference in margin over feed and medication costs per kg of finishing pig marketed for vaccinating (1) once or (2) twice (compared to 2 control groups) | [84] | |
| Maes et al. (2003) | 1.17 | Additional return to labour per pig | [83] | |
| Holyoake and Callinan (2006) | 4.91 | Increase in profit per pig | [85] | |
| Miller et al. (2001) |
1) 4,978 2) 13,056 |
Increased annual profits for farms (1020 fatteners placed) shipping (1) by target weight or (2) on fixed date | [81] | |
| Porcine circovirus 2 | ||||
| Young et al. (2011) | 7.57 | Return on investment from vaccination per pig | [24] | |
| Alarcon, Rushton, Nathues, et al. (2013) |
1) 24,853 2) 97,206 |
Mean expected value of vaccination after 5 years for a (1) moderately affected farm (100 sows), (2) severely affected farm (100 sows) | [33] | |
Reported outcomes were adjusted for inflation up until the year 2023 and converted to euros as a common currency
1 Pigs were vaccinated against Mycoplasma hyopneumoniae
2 Pigs were vaccinated against Mycoplasma hyopneumoniae and porcine circovirus type 2
Discussion
An economic perspective is key to understand the impacts of disease and the intervention options available, and, therefore, to improve decision-making regarding animal health and welfare. This is especially important when endemic diseases are concerned, since their effects are often not easily quantified [45]. The present systematic review is the first in the field aiming to identify the economic impacts of specific or co-existing pathogens involved in the porcine respiratory disease complex (PRDC), and the costs and benefits of interventions. This work additionally reveals the economic evaluation methods that were applied across included studies, including the cost and revenue components that were considered in their calculations.
In an ideal scenario, an estimated disease impact should be completely attributable to the disease that is being analysed. However, often endemic respiratory diseases are multifactorial, and the impact of the disease can be influenced by multiple non-infectious risk factors. In addition, pig herds are often burdened with more than one endemic respiratory disease at the same time under the umbrella of the PRDC [12, 13]. If the whole complex is not carefully studied, this could result in flawed estimates. Consequently, studying the effects of a specific pathogen where multiple disease-causing factors are involved is rather difficult, if not impossible in many cases. Most studies in the present review focused on one respiratory pathogen, and the presence or absence of other pathogen(s) was often not established. Therefore, the reported economic outcomes may not fully be the result of one specific respiratory pathogen only, but will be the product of a complex interaction between infectious agents, management conditions, environment, and genetics [12, 13].
In total, 58 peer-reviewed studies were included within this systematic review. Most of these studies analysed the effects of an intervention, of which nearly half focused on vaccination. With fairly low numbers of studies on PCV-2, A. pleuropneumoniae and SIV, the PRRSV was by far the most studied pathogen, followed by M. hyopneumoniae. However, it should be noted that most studies on PRRSV were from the United States, thus outcomes were based on the effects of PRRSV-2 genotypes, which tend to be considered more virulent than PRRSV-1 ones, predominantly present in Europe [46]. However, others could not confirm that PRRSV-2 genotypes are more virulent than PRRSV-1 [47, 48]. Nevertheless, estimates of PRRSV impact might be overestimated due to the overrepresentation of studies based on PRRSV-2. Although the difference in strain virulence of PRRSV-1 and PRRSV-2 genotypes shows perhaps the most clear difference in disease impact due to differentiated virus species, many studies have shown a variety of genotypes for a respiratory pathogen circulating and evolving within continents, countries, and even within the same swine operation over time [49–52]. The evolution of genotypes may influence not only their virulence, but also their resistance to treatments and vaccine efficacy [53, 54].
An additional factor adding to the variation in economic impact is the variety in production systems and the overall industry structure across countries. Comparing the production losses on a commercial fattening farm in the United States [55] to the losses for a smallholder breeding farm in Vietnam [22] provides an evident example, but even within a continent or country vast differences may exist due to, among others, varying genetics of the pigs (e.g. differing productivity or disease resilience), the internal and external climate, the farm’s biosecurity or health status, access to high quality raw materials and veterinary services, differing target weights for selling and the size of the farm. External factors such as the amount of international import and export and governmental subsidies or other incentives can also lead to differences in economic losses suffered by the industry due to endemic respiratory disease. As this review covers research from a period of nearly 40 years, the evolution of pig production systems and industries regarding these aspects should be considered when drawing conclusions. It should be stressed that, although the described variation may complicate comparing or merging of study outcomes by means of a meta-analysis [56], this variation in research is essential to understand the range in economic impact from endemic respiratory disease at a global level.
When translating production impact into financial consequences, various limitations arise regarding the applied economic methodology. We observed over seven different economic evaluation methods with a large variety in cost and revenue components used to calculate economic outcomes. With the exception of one study [57], in which the farmers’ willingness to pay for a vaccine was estimated, the studies included in this review did not include non-monetary costs (e.g. environmental, social or welfare effects). The methods applied in the eligible studies varied from basic cost calculations to more comprehensive methods such as a farm budget analysis. Even after grouping eligible studies by their applied economic method, it was rare that the same cost and revenue components were used. Although we assume that for most studies, the authors included the components that were most relevant for the specific farms under study, a highly varying level of detail in calculations impacts the comparability of economic outcomes from each study. For instance, while increased feed costs and reduced revenue from fewer weaned or sold pigs were identified as the most important components [33, 36, 37, 39], over a third of all studies did not include one or both components. Although these studies do not provide a specific reason for not including these components, it is recognised that in a number of them calculating the economic impact of a disease or an intervention was not the primary objective. Leaving out these important cost components may, therefore, be suitable for their respective study aims, but referring to the results as true economic impact estimates will lead to biased conclusions and comparisons with other study outcomes, as the total costs are underestimated. Additionally, the amount of feed costs per kg of carcass can differ greatly between countries, especially between continents [58]. This fact additionally holds for revenues per kg of carcass and the costs of medicines and vaccines [58, 59]. Moreover, the prices of feed and raw materials are volatile and particularly rising in Europe during the last few years [60], which further impacts the comparability of economic outcomes estimated during different time periods.
While keeping the differences in economic evaluation methods, their level of detail and the differences in prices across countries and time in mind, most outcomes from the disease-focused studies could be converted to an economic impact in euros per pig, which gives a very rough impression of the range in economic impact of the PRDC syndrome. The median economic impact of one or several co-existing respiratory pathogen(s) as extracted from all studies, ranged from €1.70 to €8.90 per nursery pig, €2.30 to €15.35 per fattening pig, and €100 to €323 per sow per year. Excluding the studies in which feed costs were not considered, increases the minimum reported costs to €2.90 per nursery pig, €2.80 per fattening pig, and €195 per sow per year. Due to the low numbers of studies on pathogens other than PRRSV, the ranges mainly reflect the significant worldwide impact of PRRSV. It is, therefore, unfeasible to compare and rank the various pathogens according to their economic importance. Furthermore, converting absolute economic outcomes to a single currency complicates the interpretability and comparability of the study outcomes, as differences exist in the relative importance of the economic losses suffered by farmers from countries of different income levels. Preferably, outcomes would be reported in a relative manner, such as the percent decrease in profits due to disease. However, most often information on farm profits in a non-diseased scenario is lacking.
Nearly all studies reported neutral or positive impacts of implementing an intervention. This suggests that for a wide range of production systems and disease scenarios, implementing an intervention on a farm with endemic respiratory diseases increases farm profits. There may be an outcome reporting bias, with only the favourable interventions reported that can undermine the validity of systematic reviews [61]. However, we have no evidence that this is the case in our systematic review. Apparently, most studies looked at the effects of vaccination, with very few studies considering long-term sustainable interventions. Where several countries are making efforts to eliminate endemic respiratory diseases completely [62, 63], economic research on long-term interventions (such as improvement of management practices, housing conditions or biosecurity measures) would provide valuable information for countries starting with or expanding the elimination of endemic respiratory pathogens. Besides the low number of studies on an intervention other than vaccination, comparison and ranking of interventions was also made unfeasible by the variation in the expression of results. Future research should use more standardised approaches for economic analyses of interventions with similar outcome metrics. For instance, in human health economics, comparison of control programs is mainly done by determining the cost-effectiveness (e.g. costs per disability-adjusted life-year) or cost-benefit ratio [59]. In the case of interventions requiring a large initial investment, calculations of the payback period or return on investment might be preferred [59].
Although the benefits from a standardised approach seem clear from discussing the limitations in the existing research, developing such an approach poses a challenge. The choice for a specific economic method is often dependent on the data available for the study, as well as the purpose of the study outcomes and the nature of the decision (whether researchers estimate the economic impact at the micro-scale or macro-scale, and for a short- or long-term, etc.). Consequently, the richness in methods could be an advantage, rather than only a limitation, as it will allow better alignment of the studies to the decision process required. It would therefore be of great interest to investigate why different methods or outcomes were chosen over others. Moreover, the industry-level economic burden of respiratory diseases in pigs is not limited to the direct costs, but also includes indirect costs, such as costs suffered by non-affected farms due to biosecurity investments or fluctuations in availability and prices of inputs and outputs. Most studies included in this systematic review focused on farm-level economic impacts, whereas methods well suited to study industry effects, such as the partial equilibrium analysis and econometric models, have not yet been explored. Likewise, economic analyses of the impact of policies to control PRDC pathogens were not found through the search. Therefore, there is currently no clarity on which indirect cost and revenue components from the PRDC seem to be most impactful at industry level. An approach that enhances the understanding of the economic burden of endemic respiratory disease for the entire industry would ideally include a range of economic methods, that captures both the economic impact on the farm, and on the (national or international) industry. Such an approach is being taken by the Global Burden of Animal Diseases programme and is being tested in different parts of the world [62, 63].
Lastly, restricting the review to only peer-reviewed English literature ensures a certain quality of the work but can also narrow the scope of the review and the results. Including “grey” literature during the search, such as conference abstracts and industry reports, would mostly provide additional cost estimations by non-academic organisations or companies. This could assist with reducing publication bias, but it is important to ensure that the study is relevant to the research question and that it is of sufficient quality to be included in the review [64]. In this case, several non-peer-reviewed sources were identified, but oftentimes these entailed works in progress, pilot studies, or works that did not contain adequate or complete information (e.g. explicit information on cost or revenue components). This, together with the fact that searching for abstracts is resource-intensive and availability is usually compromised, advocated for the inclusion of peer-reviewed records only.
In conclusion, respiratory diseases represent a significant economic burden in pig production, as highlighted by the range in economic impact provided in this systematic review. Future research should improve the consistency and comparability of economic assessments by ensuring the inclusion of high impact cost and revenue components and expressing results similarly. Regardless, the outcomes from this systematic review provide insight in the variation in studies, their methods, their advantages and limitations, and the reported impacts from the endemic respiratory disease complex for pig production systems worldwide.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file S1. Terms used to build the full search strings. - File provides a table of the terms that were used in the search for eligible literature
Supplementary file S2. List of eligible studies. - File provides the full list of studies included in the systematic review
Supplementary file S3. Economic methods and cost components per study. - File provides full details on which economic method was applied and which cost components were considered per study
Supplementary file S4. Reported economic outcomes per study. - File provides the economic outcomes in their original valuta as reported in each disease-focused and intervention-focused study. The file additionally includes information on the evaluation period for intervention-focused studies and on whether the economic analysis accounts for the presence/absence of other PRDC pathogens
Acknowledgements
Not applicable.
Abbreviations
- A. pleuropneumoniae
Actinobacillus pleuropneumoniae
- JSHAP
Journal of Swine Health and Production
- M. hyopneumoniae
Mycoplasma hyopneumoniae
- PCV-2
Porcine circovirus 2
- PRDC
Porcine respiratory disease complex
- PRRSV
Porcine reproductive and respiratory syndrome virus
- SIV
Swine influenza virus
Authors’ contributions
Conceptualization: MB, GvS and WS. Literature review and data extraction: MB and BGM. Writing and preparation of draft manuscript: MB and BGM. GvS, WS, JS and JR were involved in discussing draft versions of the manuscript at multiple stages. All authors read, edited and approved the final manuscript.
Funding
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101000494 (DECIDE).
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. All created tables supporting the conclusions of this article are included within the article and its supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Gerdien van Schaik is partly employed at Royal GD; the other authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fraile L, Alegre A, López-Jiménez R, Nofrarías M, Segalés J. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet J. 2010;184(3):326–33. doi: 10.1016/j.tvjl.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Meyns T, Van Steelant J, Rolly E, Dewulf J, Haesebrouck F, Maes D. A cross-sectional study of risk factors associated with pulmonary lesions in pigs at slaughter. Vet J. 2011;187(3):388–92. doi: 10.1016/j.tvjl.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Fablet C, Marois-Créhan C, Simon G, Grasland B, Jestin A, Kobisch M, et al. Infectious agents associated with Respiratory Diseases in 125 farrow-to-finish pig herds: a cross-sectional study. Vet Microbiol. 2012;157(1–2):152–63. doi: 10.1016/j.vetmic.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Merialdi G, Dottori M, Bonilauri P, Luppi A, Gozio S, Pozzi P, et al. Survey of pleuritis and pulmonary lesions in pigs at abattoir with a focus on the extent of the condition and herd risk factors. Vet J. 2012;193(1):234–9. doi: 10.1016/j.tvjl.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Sánchez Y, Herráez P, Quesada-Canales Ó, Poveda CG, Díaz-Delgado J, Quintana-Montesdeoca MP, et al. Assessment of lung Disease in finishing pigs at slaughter: pulmonary lesions and implications on productivity parameters. Animals. 2021;11(12):3604. doi: 10.3390/ani11123604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.USDA. Swine 2012. Part I: Baseline Reference of Swine Health and Management in the United States. 2015.
- 7.Straw BE, Shin SJ, Yeager AE. Effect of Pneumonia on growth rate and feed efficiency of minimal Disease pigs exposed to Actinobacillus pleuropneumoniae and Mycoplasma hyopneumoniae. Prev Vet Med. 1990;9(4):287–94. [Google Scholar]
- 8.Cohen LM, Grøntvedt CA, Klem TB, Gulliksen SM, Ranheim B, Nielsen JP, et al. A descriptive study of acute outbreaks of Respiratory Disease in Norwegian fattening pig herds. Acta Vet Scand. 2020;62(1):1–13. doi: 10.1186/s13028-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarrazin S, Joosten P, Van Gompel L, Luiken RE, Mevius DJ, Wagenaar JA, et al. Quantitative and qualitative analysis of antimicrobial usage patterns in 180 selected farrow-to-finish pig farms from nine European countries based on single batch and purchase data. J Antimicrob Chemother. 2019;74(3):807–16. doi: 10.1093/jac/dky503. [DOI] [PubMed] [Google Scholar]
- 10.Lekagul A, Tangcharoensathien V, Yeung S. Patterns of antibiotic use in global pig production: a systematic review. Veterinary and Animal Science. 2019;7:100058. doi: 10.1016/j.vas.2019.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.USDA. Antimicrobial Use and Stewardship on U.S. Swine Operations., 2017. 2019.
- 12.Brockmeier SL, Halbur PG, Thacker EL. Porcine respiratory disease complex. Polymicrobial diseases. 2002:231 – 58.
- 13.Opriessnig T, Giménez-Lirola L, Halbur P. Polymicrobial Respiratory Disease in pigs. Anim Health Res Reviews. 2011;12(2):133–48. doi: 10.1017/S1466252311000120. [DOI] [PubMed] [Google Scholar]
- 14.Haimi-Hakala M, Hälli O, Laurila T, Raunio-Saarnisto M, Nokireki T, Laine T, et al. Etiology of acute Respiratory Disease in fattening pigs in Finland. Porcine Health Management. 2017;3(1):1–12. doi: 10.1186/s40813-017-0065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin S, Ruan W, Yue H, Tang C, Zhou K, Zhang B. Viral communities associated with porcine Respiratory Disease complex in intensive commercial farms in Sichuan province, China. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-31554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarli G, D’Annunzio G, Gobbo F, Benazzi C, Ostanello F. The role of pathology in the diagnosis of swine Respiratory Disease. Veterinary Sci. 2021;8(11):256. doi: 10.3390/vetsci8110256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saade G, Deblanc C, Bougon J, Marois-Créhan C, Fablet C, Auray G, et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet Res. 2020;51(1):1–19. doi: 10.1186/s13567-020-00807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.n den Broeke A, Leen F, Aluwé M, Van Meensel J, Millet S. The effect of sex and slaughter weight on performance, carcass quality and gross margin, assessed on three commercial pig farms. Animal. 2020;14(7):1546–54. doi: 10.1017/S1751731119003033. [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieuwenhuis N, Duinhof TF, van Nes A. Economic analysis of outbreaks of porcine reproductive and respiratory syndrome virus in nine sow herds. Vet Rec. 2012;170(9):225. doi: 10.1136/vr.100101. [DOI] [PubMed] [Google Scholar]
- 21.Calderón Díaz JA, Fitzgerald RM, Shalloo L, Rodrigues da Costa M, Niemi J, Leonard FC, et al. Financial Analysis of Herd Status and Vaccination practices for Porcine Reproductive and Respiratory Syndrome Virus, Swine Influenza Virus, and Mycoplasma hyopneumoniae in Farrow-to-Finish Pig farms using a Bio-economic Simulation Model. Front Vet Sci. 2020;7:556674. doi: 10.3389/fvets.2020.556674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham HTT, Antoine-Moussiaux N, Grosbois V, Moula N, Truong BD, Phan TD, et al. Financial impacts of Priority Swine Diseases to Pig Farmers in Red River and Mekong River Delta, Vietnam. Transbound Emerg Dis. 2017;64(4):1168–77. doi: 10.1111/tbed.12482. [DOI] [PubMed] [Google Scholar]
- 23.Zhang A, Young JR, Suon S, Ashley K, Windsor PA, Bush RD. Investigating the financial impact of porcine reproductive and respiratory syndrome on smallholder pig farmers in Cambodia. Trop Anim Health Prod. 2017;49(4):791–806. doi: 10.1007/s11250-017-1264-1. [DOI] [PubMed] [Google Scholar]
- 24.Young MG, Cunningham GL, Sanford SE. Circovirus vaccination in pigs with subclinical porcine circovirus type 2 Infection complicated by ileitis. J Swine Health Prod. 2011;19(3):175–80. [Google Scholar]
- 25.Crenshaw JD, Campbell JM, Polo J, Bussières D. Effects of a nursery feed regimen with spray-dried bovine plasma on performance and mortality of weaned pigs positive for porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2016;25(1).
- 26.Pointon AM, Byrt D, Heap P. Effect of enzootic Pneumonia of pigs on growth performance. Aust Vet J. 1985;62(1):13–8. doi: 10.1111/j.1751-0813.1985.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 27.Pfuderer S, Bennett RM, Brown A, Collins LM. A flexible tool for the assessment of the economic cost of pig Disease in growers and finishers at farm level. Prev Vet Med. 2022;208:105757. doi: 10.1016/j.prevetmed.2022.105757. [DOI] [PubMed] [Google Scholar]
- 28.Losinger WC. Economic impacts of reduced pork production associated with the diagnosis of Actinobacillus pleuropneumoniae on grower/finisher swine operations in the United States. Prev Vet Med. 2005;68(2–4):181–93. doi: 10.1016/j.prevetmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Moura CAA, Philips R, Silva GS, Holtkamp DJ, Linhares DCL. Comparison of virus detection, productivity, and economic performance between lots of growing pigs vaccinated with two doses or one dose of PRRS MLV vaccine, under field conditions. Prev Vet Med. 2022;204:105669. doi: 10.1016/j.prevetmed.2022.105669. [DOI] [PubMed] [Google Scholar]
- 30.Alonso C, Davies PR, Polson DD, Dee SA, Lazarus WF. Financial implications of installing air filtration systems to prevent PRRSV Infection in large sow herds. Prev Vet Med. 2013;111(3–4):268–77. doi: 10.1016/j.prevetmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Silva GS, Yeske P, Morrison RB, Linhares DCL. Benefit-cost analysis to estimate the payback time and the economic value of two Mycoplasma hyopneumoniae elimination methods in breeding herds. Prev Vet Med. 2019;168:95–102. doi: 10.1016/j.prevetmed.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Dee SA, Molitor TW. Elimination of porcine reproductive and respiratory syndrome virus using a test and removal process. Vet Rec. 1998;143(17):474–6. doi: 10.1136/vr.143.17.474. [DOI] [PubMed] [Google Scholar]
- 33.Alarcon P, Rushton J, Nathues H, Wieland B. Economic efficiency analysis of different strategies to control post-weaning multi-systemic wasting syndrome and porcine circovirus type 2 subclinical Infection in 3-weekly batch system farms. Prev Vet Med. 2013;110(2):103–18. doi: 10.1016/j.prevetmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarcon P, Rushton J, Wieland B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical Infection in England - an economic Disease model. Prev Vet Med. 2013;110(2):88–102. doi: 10.1016/j.prevetmed.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathues H, Alarcon P, Rushton J, Jolie R, Fiebig K, Jimenez M, et al. Cost of porcine reproductive and respiratory syndrome virus at individual farm level - an economic Disease model. Prev Vet Med. 2017;142:16–29. doi: 10.1016/j.prevetmed.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Nathues H, Alarcon P, Rushton J, Jolie R, Fiebig K, Jimenez M, et al. Modelling the economic efficiency of using different strategies to control Porcine Reproductive & respiratory syndrome at herd level. Prev Vet Med. 2018;152:89–102. doi: 10.1016/j.prevetmed.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Calderón Díaz JA, Rodrigues da Costa M, Shalloo L, Niemi J, Leonard FC, Crespo-Piazuelo D, et al. A bio-economic simulation study on the association between key performance indicators and pluck lesions in Irish farrow-to-finish pig farms. Porcine Health Management. 2020;6(1):1–15. doi: 10.1186/s40813-020-00176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garner MG, Whan IF, Gard GP, Phillips D. The expected economic impact of selected exotic Diseases on the pig industry of Australia. Rev Sci Tech. 2001;20(3):671–85. doi: 10.20506/rst.20.3.1303. [DOI] [PubMed] [Google Scholar]
- 39.Renken C, Nathues C, Swam H, Fiebig K, Weiss C, Eddicks M, et al. Application of an economic calculator to determine the cost of porcine reproductive and respiratory syndrome at farm-level in 21 pig herds in Germany. Porcine Health Manag. 2021;7(1):3. doi: 10.1186/s40813-020-00183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez CR, Harding AL, Forteguerri EB, Aldridge BM, Lowe JF. Limited efficacy of antimicrobial metaphylaxis in finishing pigs: a randomized clinical trial. Prev Vet Med. 2015;121(1–2):176–8. doi: 10.1016/j.prevetmed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Kim JJ, Lee JA, Choi HY, Han JH, Huh W, Pi JH, et al. In vitro and in vivo studies of deglycosylated chimeric porcine reproductive and respiratory syndrome virus as a vaccine candidate and its realistic revenue impact at commercial pig production level. Vaccine. 2017;35(37):4966–73. doi: 10.1016/j.vaccine.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 42.Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Lein A, et al. The effect of vaccination against Mycoplasma hypopneumoniae in pig herds with a continuous production system. Zentralbl Veterinarmed B. 1998;45(8):495–505. doi: 10.1111/j.1439-0450.1998.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 43.Pallares FJ, Gomez S, Ramis G, Seva J, Munoz A. Vaccination against swine enzootic Pneumonia in field conditions: effect on clinical, pathological, zootechnical and economic parameters. Vet Res. 2000;31(6):573–82. doi: 10.1051/vetres:2000141. [DOI] [PubMed] [Google Scholar]
- 44.Linhares DC, Johnson C, Morrison RB. Economic analysis of immunization strategies for PRRS Control [corrected] PLoS ONE. 2015;10(12):e0144265. doi: 10.1371/journal.pone.0144265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Der Hogeveen H. Assessing the economic impact of an endemic Disease: the case of mastitis. Revue Scientifique et Technique (International Office of Epizootics) 2017;36(1):217–26. doi: 10.20506/rst.36.1.2623. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Lobo F, Díez-Fuertes F, Segalés J, García-Artiga C, Simarro I, Castro J, et al. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig Infection model. Vet Microbiol. 2011;154(1–2):58–68. doi: 10.1016/j.vetmic.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Ogno G, Sautter CA, Canelli E, García-Nicolás O, Stadejek T, Martelli P, et al. In vitro characterization of PRRSV isolates with different in vivo virulence using monocyte-derived macrophages. Vet Microbiol. 2019;231:139–46. doi: 10.1016/j.vetmic.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Martín-Valls GE, Cortey M, Allepuz A, Illas F, Tello M, Mateu E. Introduction of a PRRSV-1 strain of increased virulence in a pig production structure in Spain: virus evolution and impact on production. Porcine Health Management. 2023;9(1):1. doi: 10.1186/s40813-022-00298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dos Santos LF, Sreevatsan S, Torremorell M, Moreira MA, Sibila M, Pieters M. Genotype distribution of Mycoplasma hyopneumoniae in swine herds from different geographical regions. Vet Microbiol. 2015;175(2–4):374–81. doi: 10.1016/j.vetmic.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Pantoja LG, Pettit K, Dos Santos LF, Tubbs R, Pieters M. Mycoplasma hyopneumoniae genetic variability within a swine operation. J Vet Diagn Invest. 2016;28(2):175–9. doi: 10.1177/1040638716630767. [DOI] [PubMed] [Google Scholar]
- 51.Xu Q, Zhang Y, Sun W, Chen H, Zhu D, Lu C, et al. Epidemiology and genetic diversity of PCV2 reveals that PCV2e is an emerging genotype in Southern China: a preliminary study. Viruses. 2022;14(4):724. doi: 10.3390/v14040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franzo G, Faustini G, Legnardi M, Cecchinato M, Drigo M, Tucciarone CM. Phylodynamic and phylogeographic reconstruction of porcine reproductive and respiratory syndrome virus (PRRSV) in Europe: patterns and determinants. Transbound Emerg Dis. 2022;69(5):e2175–e84. doi: 10.1111/tbed.14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutiérrez-Martín CB, Del Blanco NG, Blanco M, Navas J, Rodríguez-Ferri EF. Changes in antimicrobial susceptibility of Actinobacillus pleuropneumoniae isolated from pigs in Spain during the last decade. Vet Microbiol. 2006;115(1–3):218–22. doi: 10.1016/j.vetmic.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Meng X. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet Microbiol. 2000;74(4):309–29. doi: 10.1016/S0378-1135(00)00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013;21(2):72–84. [Google Scholar]
- 56.Lean I, Rabiee A, Duffield TF, Dohoo I. Invited review: use of meta-analysis in animal health and reproduction: methods and applications. J Dairy Sci. 2009;92(8):3545–65. doi: 10.3168/jds.2009-2140. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H, Kono H, Kubota S. An Integrated Epidemiological and Economic Analysis of Vaccination against highly pathogenic Porcine Reproductive and Respiratory Syndrome (PRRS) in Thua Thien Hue Province, Vietnam. Asian-Australas J Anim Sci. 2014;27(10):1499–512. doi: 10.5713/ajas.2014.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holtkamp DJ. Benchmarking the profitability of raising pigs: Country comparisons and factors contributing to their relative advantage or disadvantage in a global market – 2020. MSD Animal Health; 2022.
- 59.Rushton J. The economics of animal health and production. Cabi; 2009.
- 60.IFIP. pig333com [Internet]: IFIP Institut du porc. 2023. Available from: https://www.pig333.com/articles/ifip-forecasts-for-the-pig-and-feed-markets-in-2023_18994/.
- 61.Dwan K, Gamble C, Williamson PR, Kirkham JJ, Group RB. Systematic review of the empirical evidence of study publication bias and outcome reporting bias—an updated review. PLoS ONE. 2013;8(7):e66844. doi: 10.1371/journal.pone.0066844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rushton J, Huntington B, Gilbert W, Herrero M, Torgerson PR, Shaw A, et al. Roll-out of the Global Burden of Animal Diseases programme. The Lancet. 2021;397(10279):1045–6. doi: 10.1016/S0140-6736(21)00189-6. [DOI] [PubMed] [Google Scholar]
- 63.Huntington B, Bernardo TM, Bondad-Reantaso M, Bruce M, Devleesschauwer B, Gilbert W, et al. Global Burden of Animal Diseases: a novel approach to understanding and managing Disease in livestock and aquaculture. Volume 40. REVUE SCIENTIFIQUE ET TECHNIQUE-OFFICE INTERNATIONAL DES EPIZOOTIES; 2021. pp. 567–83. 2. [DOI] [PubMed]
- 64.Scherer RW, Saldanha IJ. How should systematic reviewers handle conference abstracts? A view from the trenches. Syst Reviews. 2019;8:1–6. doi: 10.1186/s13643-019-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller GY, Dorn CR. Costs of swine Diseases to producers in Ohio. Prev Vet Med. 1990;8(2–3):183–90. [Google Scholar]
- 66.Brouwer J, Frankena K, de Jong MF, Voets R, Dijkhuizen A, Verheijden J, et al. PRRS: effect on herd performance after initial Infection and risk analysis. Vet Q. 1994;16(2):95–100. doi: 10.1080/01652176.1994.9694427. [DOI] [PubMed] [Google Scholar]
- 67.Christensen NH. Evaluation of the effects of enzootic Pneumonia in pigs on weight gain and days to slaughter under New Zealand conditions. N Z Vet J. 1995;43(4):146–8. doi: 10.1080/00480169.1995.35875. [DOI] [PubMed] [Google Scholar]
- 68.Pejsak Z, Markowska-Daniel I. Losses due to porcine peproductive and respiratory syndrome in a large swine farm. Comp Immunol Microbiol Infect Dis. 1997;20(4):345–52. doi: 10.1016/s0147-9571(97)00010-6. [DOI] [PubMed] [Google Scholar]
- 69.Bennett R, Ijpelaar J. Updated estimates of the costs Associated with Thirty Four Endemic Livestock Diseases in Great Britain: a note. J Agric Econ. 2005;56(1):135–44. [Google Scholar]
- 70.Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005;227(3):385–92. doi: 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- 71.Valdes-Donoso P, Alvarez J, Jarvis LS, Morrison RB, Perez AM. Production losses from an Endemic Animal Disease: Porcine Reproductive and Respiratory Syndrome (PRRS) in Selected Midwest US sow farms. Front Vet Sci. 2018;5:102. doi: 10.3389/fvets.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferraz MES, Almeida HMS, Storino GY, Sonalio K, Souza MR, Moura CAA, et al. Lung consolidation caused by Mycoplasma hyopneumoniae has a negative effect on productive performance and economic revenue in finishing pigs. Prev Vet Med. 2020;182:105091. doi: 10.1016/j.prevetmed.2020.105091. [DOI] [PubMed] [Google Scholar]
- 73.Trevisan G, Robbins RC, Angulo J, Dufresne L, Lopez WA, Macedo N et al. Relationship between weekly porcine reproductive and respiratory syndrome virus exposure in breeding herds and subsequent viral shedding and mortality in the nursery. J Swine Health Prod. 2020;28(5).
- 74.Kim JH, Kim SC, Kim HJ, Jeong CG, Park GS, Choi JS et al. Insight into the Economic effects of a severe Korean PRRSV1 outbreak in a Farrow-to-nursery farm. Anim (Basel). 2022;12(21). [DOI] [PMC free article] [PubMed]
- 75.Trevisi P, Amatucci L, Ruggeri R, Romanelli C, Sandri G, Luise D, et al. Pattern of antibiotic consumption in two Italian production chains differing by the endemic status for Porcine Reproductive and Respiratory Syndrome. Front Vet Sci. 2022;9:840716. doi: 10.3389/fvets.2022.840716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Li Z, Li H, Yang S, Ren F, Bian T, et al. The economic impact of porcine reproductive and respiratory syndrome outbreak in four Chinese farms: based on cost and revenue analysis. Front Vet Sci. 2022;9:1024720. doi: 10.3389/fvets.2022.1024720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dee SA. Apparent prevention of Mycoplasma hyopneumoniae Infection in growing pigs with a low-cost modified medicated-early-weaning program. J Swine Health Prod. 1994;2(6).
- 78.Dee SA, Joo HS, Polson DD. Improved performance of a large pig complex after sequential nursery depopulation. 1996. p. 31–4. [DOI] [PubMed]
- 79.Dee SA, Joo HS, Polson DD, Marsh WE. Evaluation of the effects of nursery depopulation of the profitability of 34 pig farms. Vet Rec. 1997;140(19):498–500. doi: 10.1136/vr.140.19.498. [DOI] [PubMed] [Google Scholar]
- 80.Kyriakis SC, Alexopoulos C, Vlemmas J, Sarris K, Lekkas S, Koutsoviti-Papadopoulou M, et al. Field study on the efficacy of two different vaccination schedules with HYORESP in a Mycoplasma hyopneumoniae-infected commercial pig unit. J Vet Med B Infect Dis Vet Public Health. 2001;48(9):675–84. doi: 10.1046/j.1439-0450.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- 81.Miller GY, Song Y, Bahnson PB. An economic model for estimating batch finishing system profitability with an application in estimating the impact of preventive measures for porcine Respiratory Disease complex. J Swine Health Prod. 2001;9(4):169–77. [Google Scholar]
- 82.Pallares FJ, Gomez S, Munoz A. Evaluation of the zootechnical parameters of vaccinating against swine enzootic Pneumonia under field conditions. Vet Rec. 2001;148(4):104–7. doi: 10.1136/vr.148.4.104. [DOI] [PubMed] [Google Scholar]
- 83.Maes D, Verbeke W, Vicca J, Verdonck M, de Kruif A. Benefit to cost of vaccination against mycoplasma hyopneumoniae in pig herds under Belgian market conditions from 1996 to 2000. Livest Prod Sci. 2003;83(1):85–93. [Google Scholar]
- 84.Stipkovits L, Laky Z, Abonyi T, Siugzdaite J, Szabo I. Reduction of economic losses caused by mycoplasmal Pneumonia of pigs by vaccination with respisure and by Tiamutin treatment. Acta Vet Hung. 2003;51(3):259–71. doi: 10.1556/AVet.51.2003.3.2. [DOI] [PubMed] [Google Scholar]
- 85.Holyoake PK, Callinan APL. How effective is Mycoplasma hyopneumoniae vaccination in pigs less than three weeks of age? J Swine Health Prod. 2006;14(4):189–95. [Google Scholar]
- 86.Schaefer N, Morrison R. Effect on total pigs weaned of herd closure for elimination of porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2006;15(3).
- 87.Rapp-Gabrielson VJ, Hoover T, Sornsen S, Kesl L, Taylor L, Jolie R et al. Effects of Mycoplasma hyopneumoniae vaccination in pigs co-infected with M hyopneumoniae and porcine circovirus type 2. J Swine Health Prod. 2007;16(1).
- 88.Stygar AH, Niemi JK, Oliviero C, Laurila T, Heinonen M. Economic value of mitigating Actinobacillus pleuropneumoniae Infections in pig fattening herds. Agric Syst. 2016;144:113–21. [Google Scholar]
- 89.Kaalberg L, Geurts V, Jolie R. A field efficacy and safety trial in the Netherlands in pigs vaccinated at 3 weeks of age with a ready-to-use porcine circovirus type 2 and Mycoplasma hyopneumoniae combined vaccine. Porcine Health Manag. 2017;3(23):23. doi: 10.1186/s40813-017-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duivon D, Correge I, Hemonic A, Rigaut M, Roudaut D, Jolie R. Field evaluation of piglet vaccination with a Mycoplasma hyopneumoniae bacterin as compared to a ready-to-use product including porcine circovirus 2 and M. Hyopneumoniae in a conventional French farrow-to-finish farm. Porcine Health Manag. 2018;4(4):4. doi: 10.1186/s40813-017-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomann B, Rushton J, Schuepbach-Regula G, Nathues H. Modeling Economic effects of Vaccination against Porcine Reproductive and Respiratory Syndrome: impact of Vaccination Effectiveness, Vaccine Price, and Vaccination Coverage. Front Vet Sci. 2020;7:500. doi: 10.3389/fvets.2020.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abella G, Pages-Bernaus A, Estany J, Pena RN, Fraile L, Pla-Aragones LM. Using PRRSV-Resilient sows improve performance in endemic infected farms with recurrent outbreaks. Anim (Basel) 2021;11(3):1–16. doi: 10.3390/ani11030740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quezada-Fraide EA, Peñuelas-Rivas CG, Moysén-Albarrán FS, Trujillo-Ortega ME, Martínez-Castañeda FE. Productive performance and costs of swine farms with different PRRS virus vaccination protocols. Revista Mexicana De Ciencias Pecuarias. 2021;12(1):205–16. [Google Scholar]
- 94.Jerlström J, Huang W, Ehlorsson C-J, Eriksson I, Reneby A, Comin A. Stochastic partial budget analysis of strategies to reduce the prevalence of lung lesions in finishing pigs at slaughter. 2022. [DOI] [PMC free article] [PubMed]
- 95.Dijkhuizen A, Morris R, Huirne R. Animal health economics: principles and applications. Post Graduate Foundation in Veterinary Science. 1997:25–39.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file S1. Terms used to build the full search strings. - File provides a table of the terms that were used in the search for eligible literature
Supplementary file S2. List of eligible studies. - File provides the full list of studies included in the systematic review
Supplementary file S3. Economic methods and cost components per study. - File provides full details on which economic method was applied and which cost components were considered per study
Supplementary file S4. Reported economic outcomes per study. - File provides the economic outcomes in their original valuta as reported in each disease-focused and intervention-focused study. The file additionally includes information on the evaluation period for intervention-focused studies and on whether the economic analysis accounts for the presence/absence of other PRDC pathogens
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. All created tables supporting the conclusions of this article are included within the article and its supplementary files.