Abstract
L-749,345 is a new parenteral carbapenem with a very long half-life similar to that of ceftriaxone. The aim of the present study was to investigate different pharmacodynamic parameters of L-749,345 in comparison with those of ceftriaxone and imipenem. The following studies were performed: (i) comparative studies of the MICs of L-749,345, imipenem, and ceftriaxone for 70 strains of gram-positive and gram-negative bacteria; (ii) comparative studies of the rate of killing of gram-positive and gram-negative bacteria by L-749,345, imipenem, and ceftriaxone; (iii) studies of the postantibiotic effects of L-749,345, imipenem, and ceftriaxone; and (iv) studies of the postantibiotic sub-MIC effects of L-749,345, imipenem, and ceftriaxone. Significantly lower MICs of L-749,345 compared with those of ceftriaxone were found for all gram-negative organisms except Haemophilus influenzae. The MICs of L-749,345 were similar to those of imipenem for all organisms except Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus, for which the MICs of L-749,345 were higher. A concentration-dependent killing of methicillin-resistant S. aureus but not methicillin-susceptible strains was noted for both L-749,345 and imipenem. All three of the investigated drugs exhibited a postantibiotic effect against the gram-positive strains but exhibited no postantibiotic effect against the gram-negative strains.
The carbapenems are broad-spectrum agents with excellent in vitro activities against gram-positive and gram-negative bacteria including strictly anaerobic bacteria. Imipenem and meropenem, which are registered on the market, are both highly resistant to hydrolysis by β-lactamases, with a few exceptions, such as the β-lactamases of Stenotrophomonas maltophilia and some Aeromonas strains (6, 7, 11). The carbapenems have been shown to exhibit a postantibiotic effect (PAE) against gram-positive bacteria and also against some gram-negative strains (3, 4, 12, 16, 19). Imipenem is less stable than meropenem against human dehydropeptidase and must therefore be administered with cilastatin. Both drugs have half-lives of approximately 1 h (1).
L-749,345 is a new parenteral broad-spectrum carbapenem that is resistant to most β-lactamases and which is also stable against human dehydropeptidase. Pharmacokinetic studies with rats and rhesus monkeys indicate that L-749,345 has a long half-life comparable to that of ceftriaxone. The level of protein binding of L-749,345 in human plasma is estimated to be 95% (10a).
The aim of the present study was to compare the in vitro pharmacodynamic properties of L-749,345 with those of imipenem and ceftriaxone. In the study the following experiments were performed: (i) comparative studies of the MICs of L-749,345, imipenem, and ceftriaxone for reference strains and clinical isolates of gram-positive and gram-negative bacteria; (ii) comparative studies of the rate and extent of killing of reference strains of methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) and reference strains of the family Enterobacteriaceae by L-749,345, imipenem, and ceftriaxone at five different concentrations (L-749,345 and ceftriaxone were also investigated against three clinical isolates of MSSA, MRSA, and Enterobacter cloacae); and (iii) studies of the PAEs and (iv) studies of the postantibiotic sub-MIC effects (PA-SME) of L-749,345, imipenem, and ceftriaxone against gram-positive and gram-negative strains.
(This material was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996.)
MATERIALS AND METHODS
Antibiotics.
L-749,345 and imipenem were provided by Merck Sharp & Dohme, Starnberg, Germany, and ceftriaxone was provided by Roche, Stockholm, Sweden. The antibiotics were obtained as reference powders with known potencies. L-749,345 was diluted in distilled water, and ceftriaxone was diluted in Sörensens buffer (pH 7.0). Dilutions were made on the same day that the experiments were performed.
Bacterial strains and media.
The strains used in the MIC studies are listed in Table 1. The strains used in the second study were S. aureus ATCC 29213 (MSSA) and three clinical isolates of the same species (strains 2005, 1003, 3028), S. aureus Col 1841 (MRSA) and three clinical isolates of MRSA (strains 6010, 2007, 1011), and E. cloacae EN 20 and three clinical isolates of E. cloacae (strains 1012, 4006, 3025). The strains used in the third and fourth studies were S. aureus ATCC 29213, Streptococcus pneumoniae ATCC 6306, Haemophilus influenzae NTCC (National Type Culture Collection) 8468, Escherichia coli ATCC 25922, and E. cloacae EN 20. The clinical strains were obtained from the Clinical Microbiological Laboratory, Uppsala, Sweden. All the gram-negative strains except H. influenzae were grown in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with 50 mg of Ca2+ per liter and 25 mg of Mg2+ per liter for 6 h at 37°C, yielding an initial inoculum of approximately 109 CFU/ml. H. influenzae was cultured in Progressive Diagnostics Manufacturers broth (Biodisk, Solna, Sweden) supplemented with 30 mg of hemin per liter and 1% IsoVitaleX for 6 h at 37°C, resulting in approximately 109 CFU/ml. The gram-positive strains were grown in Todd-Hewitt broth for 6 h at 37°C, resulting in approximately 109 CFU/ml.
TABLE 1.
MICs
| Strain | MIC (mg/liter)
|
||
|---|---|---|---|
| L-749,345 | Imipenem | Ceftriaxone | |
| MSSA | |||
| ATCC 29213 | 0.5 | 0.016 | 4 |
| 3028 | 1.0 | 0.016 | 16 |
| 2005 | 1.0 | 0.03 | 8 |
| 1050 | 2.0 | 0.03 | 16 |
| 1003 | 1.0 | 0.03 | 8 |
| MRSA | |||
| Col 1841 | 8 | 0.5 | >256 |
| 1011 | 2 | 0.03 | 16 |
| 555 | 8 | 0.25 | 128 |
| 6010 | 4 | 0.06 | 64 |
| 2007 | 4 | 0.06 | 32 |
| Penicillin-sensitive S. pneumoniae | |||
| 5018 | 0.03 | 0.008 | 0.06 |
| 1041 | 0.03 | 0.016 | 0.03 |
| 6306 | 0.03 | 0.008 | 0.016 |
| 3021 | 0.06 | 0.008 | 0.06 |
| 2054 | 0.06 | 0.03 | 0.016 |
| Penicillin-resistant S. pneumoniae | |||
| 2151 | 2 | 0.5 | 2 |
| 40932 | 2 | 0.5 | 4 |
| 1020 | 2 | 0.5 | 2 |
| 1046 | 1 | 0.25 | 2 |
| 2003 | 8 | 1 | 1 |
| Non-β-lactamase-producing H. influenzae | |||
| NTCC 8468 | 1 | 0.5 | 0.008 |
| 1076 | 1 | 1 | 0.008 |
| 2050 | 0.06 | 0.5 | 0.008 |
| 5056 | 0.5 | 0.5 | 0.004 |
| 5069 | 1 | 0.5 | 0.004 |
| β-Lactamase-producing H. influenzae | |||
| 2045 | 0.06 | 1 | 0.008 |
| 3029 | 0.06 | 0.5 | 0.004 |
| 5044 | 0.06 | 0.5 | 0.004 |
| 1041 | 0.06 | 0.5 | 0.008 |
| 2012 | 0.06 | 0.5 | 0.004 |
| Ampicillin-resistant E. coli | |||
| ATCC 25922 | 0.016 | 0.25 | 0.125 |
| 1058 | 0.008 | 0.5 | 0.06 |
| 3003 | 0.016 | 0.25 | 0.06 |
| 3004 | 0.08 | 0.25 | 0.06 |
| 3054 | 0.125 | 0.5 | 0.06 |
| Ampicillin-sensitive E. coli | |||
| 2093 | 0.008 | 0.13 | 0.06 |
| 6023 | 0.008 | 0.25 | 0.125 |
| 2089 | 0.008 | 0.25 | 0.06 |
| 2084 | 0.008 | 0.13 | 0.06 |
| 1004 | 0.008 | 0.13 | 0.06 |
| K. pneumoniae | |||
| ATCC 29665 | 0.008 | 1 | 0.06 |
| 1028 | 0.008 | 0.25 | 0.13 |
| 2029 | 0.016 | 0.5 | 0.06 |
| 4058 | 0.016 | 0.5 | 0.06 |
| 4001 | 0.008 | 0.5 | 0.06 |
| Ceftazidime-sensitive E. cloacae | |||
| 1013 | 0.125 | 0.5 | 64 |
| 5009 | 0.016 | 1 | 1 |
| 6001 | 0.125 | 0.25 | 0.5 |
| 5028 | 0.06 | 1 | 0.13 |
| 4002 | 0.5 | 1 | 8 |
| Ceftazidime-resistant E. cloacae | |||
| 3025 | 0.5 | 0.5 | >256 |
| 1012 | 1 | 0.5 | >256 |
| 4006 | 0.5 | 0.25 | >256 |
| Ceftazidime-resistant Enterobacter aerogenes | |||
| EN 20 | 0.5 | 0.25 | >256 |
| EN 22 | 0.5 | 0.25 | >256 |
| B 3025 | 2 | 0.5 | 128 |
| S. marcescens | |||
| 14 | 0.03 | 1 | 0.5 |
| 40 | 0.03 | 8 | 4 |
| 47 | 0.06 | 2 | 1 |
| 6003 | 0.03 | 4 | 2 |
| 2048 | 0.03 | 2 | 0.5 |
| Citrobacter freundii | |||
| 7014 | 0.008 | 0.25 | 0.06 |
| 4013 | 0.03 | 4 | 2 |
| 2016 | 0.13 | 0.5 | 128 |
| 2001 | 0.03 | 1 | 0.13 |
| 3005 | 0.008 | 1 | 2 |
| P. aeruginosa | |||
| ATCC 27853 | 16 | 2 | 32 |
| 2057 | 16 | 2 | 32 |
| 5003 | 4 | 1 | 32 |
| 2060 | 8 | 1 | 16 |
| 5007 | 8 | 1 | 128 |
Determination of MICs.
The MICs for the investigated strains were determined in fluid media by a macrodilution technique in triplicate on different occasions by the methods recommended by the National Committee for Clinical Laboratory Standards. Twofold serial dilutions of the antibiotics were added to broth, and the broth was inoculated with a final inoculum of approximately 105 CFU of the test strain per ml and incubated at 37°C for 24 h. The MIC was defined as the lowest concentration of the antibiotic allowing no visible growth. At the end of the experiments in which S. aureus ATCC 29213 and S. aureus Col 1841 were exposed to different concentrations of imipenem, the MICs for bacteria exposed to 4 and 16× the MIC, respectively, were reinvestigated by the same method described above.
Determination of the rate and extent of killing at different concentrations.
In the second study, different concentrations of L-749,345, imipenem, and ceftriaxone were used. Tubes containing 4 ml of the same media described above to which one of the antibiotics had been added at 2, 4, 8, 16, and 32× the MIC were inoculated with a suspension of the test strain, giving a final bacterial count of approximately 5 × 105 CFU/ml. The tubes were incubated at 37°C, and samples were withdrawn at 0, 3, 6, 9, 12, and 24 h and, if necessary, diluted in phosphate-buffered saline. Three dilutions of each sample were spread onto blood agar plates (Columbia agar base with 5% horse blood), the plates were incubated at 37°C, and the colonies were counted after 24 h. Only the colonies on plates with 50 to 500 colonies were counted. The activity of L-749,345 was tested against MSSA ATCC 29213, 2005, 1003, and 3028; MRSA Col 1841, 6010, 2007, and 1011; and E. cloacae EN 20, 1012, 4006, and 3025. The activity of imipenem was tested against MSSA ATCC 29213, MRSA Col 1841, and E. cloacae EN 20. Due to very high MICs for MRSA and E. cloacae, the activity of ceftriaxone was investigated only against MSSA. Three experiments were performed for each of the reference strains, and one experiment was performed for each of the clinical strains.
Determination of the PAEs of L-749,345, imipenem, and ceftriaxone in the BioScreen C.
The PAEs of L-749,345, imipenem, and ceftriaxone against S. aureus ATCC 29213, S. pneumoniae ATCC 6306, H. influenzae NTCC 8468, and E. coli ATCC 25922 were investigated. The PAEs of L-749,345 and imipenem against E. cloacae EN 20 were also studied. All antibiotic-bacterium combinations were investigated in triplicate. After incubation for 6 h at 37°C, all strains were diluted 1:10 in order to obtain an inoculum of approximately 5 × 107 CFU/ml at the beginning of the experiments. The strains were then exposed to 10× the MIC of the antibiotic for 2 h at 37°C. To eliminate the antibiotic, the cultures were washed three times, after each wash centrifuged at 1,400 × g for 5 min. Depending on the rate of killing, some of the cultures were thereafter diluted 1:10. The unexposed control strains were washed similarly but were also diluted 10−2, 10−3, 10−4, and 10−5 in order to obtain an inoculum as close to that of the exposed strains as possible. Both the exposed strains and the different dilutions of the controls were then diluted 1:10 and inoculated in microtiter wells of 400 μl and incubated in the BioScreen C (Lab Systems). BioScreen C is a computerized incubator which provides automatic serial dilutions of bacteria and antibiotics. It also incubates the bacteria, measures growth continuously by vertical photometry (optical density) at a wavelength of 540 nm, processes the data, and provides a printout of the results. Earlier experimental studies have revealed that the shape of the control growth curves were independent of the inoculum and that 1 log10 difference in the initial inoculum corresponded to a constant delay in growth. A control curve for each strain and experiment could therefore be constructed by interpolation of the curve for the controls with the growth curve for an inoculum nearest that of the experimental culture. The PAE was calculated as the difference in time for the exposed cultures and the corresponding control to grow up to a defined point (A50) on the absorbance curve, where A50 was defined as 50% of the maximal absorbance of the control. A50 was chosen, since this point represented growth of approximately 1 log10 CFU (9, 16, 19). Since killing cannot be measured in BioScreen C, viable counts were used to measure the initial level of killing of the exposed cultures and after the washing. Viable counts of the controls were also measured at the start of the experiments and before and after washing at 2 h.
Determination of the PA-SMEs of L-749,345, imipenem, and ceftriaxone.
The PA-SMEs of L-749,345, imipenem, and ceftriaxone for the same strains used in the third experiment were determined on three different occasions. The postantibiotic phase was induced as described above, and the controls were diluted (10−2, 10−3, 10−4, 10−5) in the same way as in the PAE experiments. Viable counts were also used as described above. The strains in the postantibiotic phase and the different dilutions of the controls were then exposed to 0.1, 0.2, 0.3, 0.4, and 0.5× the MICs of L-749,345, imipenem, and ceftriaxone and were incubated in BioScreen C. Growth curves were monitored automatically for 20 h. The PA-SME was defined as the difference in time for the cultures in the postantibiotic phase and then later exposed to the drugs at sub-MICs and the corresponding controls with the same initial inoculum as the preexposed culture to reach the A50 (defined as described above) (9, 16, 19).
RESULTS
MICs.
The MICs of L-749,345, imipenem, and ceftriaxone for the strains studied are presented in Table 1. L-749,345 had the lowest MICs for E. coli (both TEM-1-producing and non-TEM-1-producing strains), Klebsiella pneumoniae, E. cloacae (ceftazidime susceptible), Serratia marcescens, and Citrobacter freundii. Imipenem was 4- to 8-fold more active than L-749,345 against Pseudomonas aeruginosa and E. cloacae (ceftazidime resistant) and was 100-fold more active than L-749,345 against MSSA. However, L-749,345 was fourfold more active than ceftriaxone against MSSA. For MRSA, L-749,345 MICs ranged from 2 to 8 mg/liter, values which were lower than those of ceftriaxone but higher than those of imipenem. The MICs of all drugs for penicillin-sensitive S. pneumoniae were similar, but imipenem had the lowest MICs for penicillin-resistant S. pneumoniae. Ceftriaxone had the lowest MICs for both β-lactamase-producing and non-β-lactamase-producing strains of H. influenzae.
Rate and extent of killing at different concentrations.
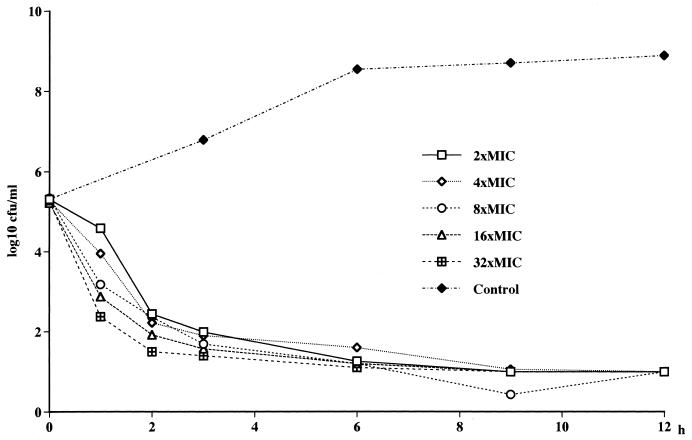
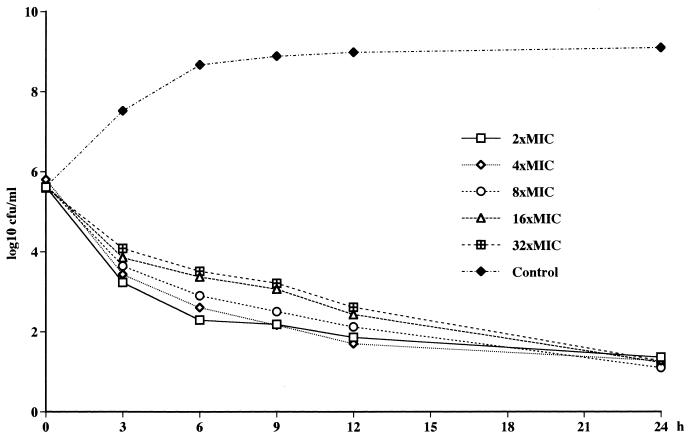
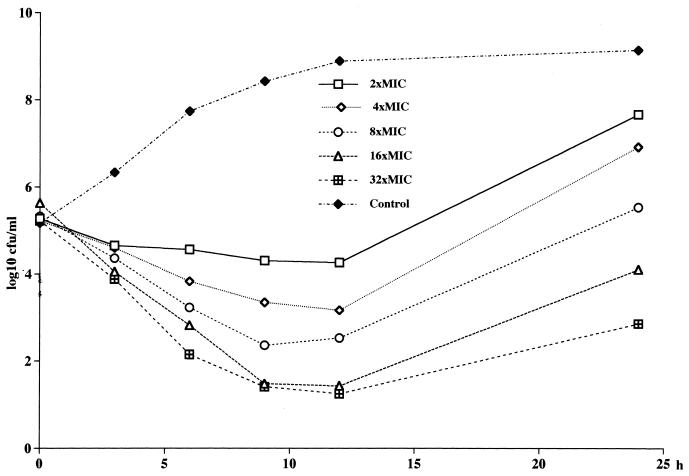
No concentration-dependent killing of MSSA and E. cloacae by L-749,345, imipenem, and ceftriaxone was noted (Fig. 1). A tendency toward a paradoxical effect against MSSA could be noted, with the fastest killing occurring at the lowest concentration (Fig. 2). Against the MRSA, 3 to 4 log10 CFU better killing was noted for both L-749,345 and imipenem at 32× the MIC compared to that at 2× the MIC (Fig. 3). At 24 h regrowth was seen for three of the four strains tested with L-749,345 and for the MRSA strain tested with imipenem. At the end of the experiments, determination of the MICs of imipenem for S. aureus Col 1841 revealed the emergence of resistant strains, with an increase in the MICs from 0.5 to 64 mg/liter. When S. aureus ATCC 29213 was investigated in the same manner, no increase in MICs was seen.
FIG. 1.
Killing curves for L-749,345 at different concentrations against E. cloacae EN 20.
FIG. 2.
Killing curves for L-749,345 at different concentrations against MSSA ATCC 29213.
FIG. 3.
Killing curves for L-749,345 at different concentrations against MRSA Col 1841.
PAEs and PA-SMEs of L-749,345, imipenem, and ceftriaxone.
The PAEs and PA-SMEs of L-749,345, ceftriaxone, and imipenem are presented in Tables 2, 3, and 4, respectively. L-749,345 and imipenem had almost identical PAEs. L-749,345, imipenem, and ceftriaxone had similar PAEs against S. pneumoniae (2.4, 2.4, and 2.6 h, respectively) but no PAE or a negative PAE against the gram-negative strains. L-749,345 and imipenem had slightly longer PAEs against S. aureus compared with that of ceftriaxone. The PA-SMEs of L-749,345 at 0.3× the MIC were 2.9 h against S. aureus, 6.9 h against S. pneumoniae and H. influenzae, 1.0 h against E. coli, and 5.0 h against E. cloacae. Imipenem had PA-SMEs similar to those of L-749,345 with the exception of that against E. coli; for imipenem at 0.3× the MIC the PA-SME was substantially longer (13.9 h) than that of L-749,345. Ceftriaxone had PA-SMEs against S. aureus and S. pneumoniae similar to those of L-749,345 but had no PA-SME against H. influenzae.
TABLE 2.
PAEs and PA-SMEs of L-749,345a
| Strain | PAE (h) | PA-SME (h) of L-749,345 at the following multiples of the MIC:
|
||||
|---|---|---|---|---|---|---|
| 0.1× | 0.2× | 0.3× | 0.4× | 0.5× | ||
| S. aureus ATCC 29231 | 1.5 (1.4–1.6) | 2.0 (1.9–2.1) | 2.4 (2.3–2.6) | 2.9 (2.9–3.0) | 3.6 (3.4–4.1) | 4.2 (4.1–4.3) |
| S. pneumoniae ATCC 6306 | 2.4 (2.1–2.6) | 3.5 (3.0–3.9) | 4.3 (4.1–4.6) | 6.9 (5.4–9.0) | >15.7 (>15.7) | >15.7 (>15.7) |
| H. influenzae NTCC 8468 | 0 (0) | 1.8 (0.0–1.9) | 3.7 (3.1–4.0) | 6.9 (6.6–7.1) | 10.1 (9.6–10.7) | 11.7 (11.4–12.4) |
| E. coli ATCC 25922 | −0.3 (−0.1–−0.4) | 0.2 (0–0.3) | 0.6 (0.4–0.9) | 1.0 (0.9–1.3) | 1.6 (1.4–2.0) | 3.1 (2.3–3.6) |
| E. cloacae EN 20 | 0.3 (0–0.7) | 1.6 (1.4–2.0) | 3.3 (3.0–3.4) | 5.0 (4.0–6.4) | 6.5 (5.3–7.9) | 7.6 (5.3–9.0) |
Values are means (ranges).
TABLE 3.
PAEs and PA-SMEs of ceftriaxonea
| Strain | PAE (h) | PA-SME (h) of ceftriaxone at the following multiples of the MIC:
|
||||
|---|---|---|---|---|---|---|
| 0.1× | 0.2× | 0.3× | 0.4× | 0.5× | ||
| S. aureus ATCC 29231 | 0.9 (0.7–1.0) | 3.0 (2.7–3.3) | 4.8 (4.1–5.6) | 7.2 (6.1–8.5) | (>15.9–>16.1) | (>15.9–>16.1) |
| S. pneumoniae ATCC 6306 | 2.6 (2.3–2.9) | 2.9 (2.6–3.3) | 4.2 (3.7–4.9) | 6.2 (5.7–6.7) | (>14.3–>15) | (>14.3–>15) |
| H. influenzae NTCC 8468 | −0.8 (−0.6–−1.0) | −0.6 (−0.3–−1.0) | −0.4 (−0.3–−0.6) | −0.3 (−0.3) | 0.4 (−0.3–−1.0) | 3.8 (0.6–4.4) |
| E. coli ATCC 25922 | −2.1 (−2.0–−2.1) | −2.1 (−2.0–−2.1) | −1.9 (−1.7–−2.1) | −1.6 (−1.2–−2.1) | −1.3 (−0.7–−1.6) | 7.1 (5.3–8.3) |
Values are means (ranges).
TABLE 4.
PAEs and PA-SMEs of imipenema
| Strain | PAE (h) | PA-SME (h) of imipenem at the following multiples of the MIC:
|
||||
|---|---|---|---|---|---|---|
| 0.1× | 0.2× | 0.3× | 0.4× | 0.5× | ||
| S. aureus ATCC 29231 | 1.3 (1.1–1.6) | 2.1 (2.0–2.3) | 3.2 (3.0–3.5) | 5.1 (4.7–5.5) | 8.3 (8.1–8.5) | >14.8 (>14.8) |
| S. pneumoniae ATCC 6306 | 2.4 (2.1–2.7) | 3.1 (2.9–3.3) | 3.8 (3.4–4.1) | 5.2 (4.7–5.7) | 6.4 (5.7–7.0) | 7.6 (6.7–8.4) |
| H. influenzae NTCC 8468 | −0.5 (−1.4–0) | 6.4 (2.9–8.7) | 9.9 (8.7–10.7) | >10.7 (>10.7) | >10.7 (>10.7) | >10.7 (>10.7) |
| E. coli ATCC 25922 | −0.9 (−0.8–−1.0) | −0.2 (−1.0–−0.6) | 7.9 (7.1–8.1) | 13.9 (13.5–14.3) | >15 (>15) | >15 (>15) |
| E. cloacae EN 20 | 0.3 (0.2–0.4) | 0.5 (0.5–0.5) | 0.8 (0.7–0.8) | 1.8 (1.6–2.0) | 4.1 (3.8–4.4) | 6.2 (5.5–6.8) |
Values are means (ranges).
DISCUSSION
Several pharmacodynamic parameters such as the MICs, the rate and extent of bacterial killing, and PAEs have been recognized as important factors that may influence the optimal antibiotic dosing regimens (4, 8, 21, 23). In the present study, we have studied the pharmacodynamic parameters mentioned above for L-749,345, imipenem, and ceftriaxone. In comparison with the MICs of ceftriaxone, the MICs of L-749,345 for all the gram-negative strains except H. influenzae were lower. Compared with the MICs of imipenem, the MICs of L-749,345 for P. aeruginosa, MSSA, MRSA, and penicillin-resistant strains of S. pneumoniae were higher. Ceftazidime-resistant strains of E. cloacae were sensitive to both L-749,345 and imipenem but were highly resistant to ceftriaxone. A pronounced concentration-dependent killing was seen against the reference strain of MRSA for both L-749,345 and imipenem, and for L-749,345 this was also seen when clinical strains were tested. In contrast, no concentration-dependent killing of the methicillin-sensitive strains was seen for any of the three drugs tested. The MICs for S. aureus Col 1841 after exposure to imipenem for 24 h revealed the emergence of resistant strains, probably due to the presence of a heterogeneous population. When S. aureus ATCC 29213 was investigated in the same manner, no increases in the MICs were seen, which implies that this strain is homogeneously sensitive to the β-lactams investigated. No concentration-dependent killing of E. cloacae was seen for L-749,345 or imipenem.
A pharmacodynamic factor that has attracted great interest during the last 10 years is the PAE, i.e., the inhibition of bacterial growth after a short exposure to antibiotics (2, 4, 5, 10, 22, 24). In general, all β-lactam antibiotics except carbapenems induce a PAE against gram-positive bacteria but no PAE or a negative PAE against gram-negative bacteria; carbapenems may produce a PAE against some gram-negative organisms, depending on the method used (3, 4, 12, 13, 16, 19). The negative PAEs occasionally seen have been explained by the formation of filamentous bacteria during the exposure to the antibiotic. At the time of drug removal and in the postantibiotic phase, cell division occurs, yielding a more rapid increase in the number of bacterial cells than the rate for the control culture (4). Why the carbapenems produce a PAE against some gram-negative bacteria is not clear. It has, however, been speculated that, at least with imipenem, its failure to bind to PBP 3 would prevent the formation of filamentous bacteria, and because of this a short PAE may be seen against some bacterial species. In this study, the PAEs of L-749,345, imipenem, and ceftriaxone were investigated by the optical density method. All three antibiotics produced a PAE against H. influenzae and E. coli but no PAE or a negative PAE against the gram-negative strains; the two carbapenems, however, produced a short PAE against E. cloacae. The PAE results are in agreement with those from earlier studies of imipenem, meropenem, and BO-2727 (12, 16, 19).
When the PAE is determined, the bacteria are exposed to the antibiotic for a limited period of time at a given constant concentration, followed by removal of the antibiotic. This is in contrast to the clinical situation, in which the bacteria are exposed to suprainhibitory concentrations followed by subinhibitory levels (sub-MICs). We have earlier studied the influence of the effect of sub-MICs on bacteria in the postantibiotic phase and have found a very long delay in bacterial regrowth for many antibiotic classes and different bacterial species (14, 15, 17–20). In the present study, we showed that L-749,345 at 0.3× the MIC produces a PA-SME of 5 to 7 h against S. pneumoniae, H. influenzae, and E. cloacae. Shorter PA-SMEs were noted against S. aureus and E. coli. The relatively long PA-SME of L-749,345 at 0.3× the MIC against H. influenzae, even though it had no PAE, is explained by the direct effects of subinhibitory concentrations (sub-MIC effect [SME]) (17, 18). The SME of L-749,345 at 0.3× the MIC was 5.7 h. Imipenem had similar PA-SMEs against S. pneumoniae and H. influenzae but longer values against S. aureus and E. coli compared to those of L-749,345. Ceftriaxone had a PA-SME of 5 to 7 h against S. aureus and S. pneumoniae but a short PA-SME against H. influenzae.
In conclusion, L-749,345 is a new carbapenem with pharmacodynamic properties similar to those of other carbapenems, e.g., fast but non-concentration-dependent killing of gram-negative bacteria; a slower, almost paradoxical killing of S. aureus MSSA; and a concentration-dependent killing of MRSA. The MICs of L-749,345 for all of the gram-negative strains tested except P. aeruginosa were lower than those of imipenem. However, imipenem had more favorable activity against the gram-positive strains. In comparison with the MICs of ceftriaxone, L-749,345 had significantly lower MICs for all gram-negative strains investigated except H. influenzae. All three antibiotics investigated in the present study, L-749,345, imipenem, and ceftriaxone, induced a PAE against the gram-positive strains but not against the gram-negative strains; the two carbapenems, however, produced a short PAE against E. cloacae.
ACKNOWLEDGMENT
This study was supported by a grant from Merck & Co., Rahway, N.J.
REFERENCES
- 1.Buckley M M, Brogden R N, Barradell L B, Goa K L. Imipenem/cilastatin. A reappraisal of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1992;4:408–444. doi: 10.2165/00003495-199244030-00008. [DOI] [PubMed] [Google Scholar]
- 2.Bundtzen R W, Gerber A U, Cohn D L, Craig W A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981;3:28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Bustamente C I, Drusano G L, Tatem B A, Standiford H C. Postantibiotic effects of imipenem on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;5:678–682. doi: 10.1128/aac.26.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig W A, Gudmundsson S. The postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 296–329. [Google Scholar]
- 5.Fantin B, Ebert S, Leggett J, Vogelman B, Craig W A. Factors affecting duration of in-vivo postantibiotic effect for aminoglycosides against gram-negative bacilli. J Antimicrob Chemother. 1990;27:829–836. doi: 10.1093/jac/27.6.829. [DOI] [PubMed] [Google Scholar]
- 6.Jones, R. N., A. L. Barry, and C. Thornsberry. 1989. In-vitro studies of meropenem. J. Antimicrob. Chemother. 24(Suppl. A):9–29. [DOI] [PubMed]
- 7.Labia, R., A. Morand, and M. Guionie. 1986. β-Lactamase stability of imipenem. J. Antimicrob. Chemother. 18(Suppl. E):1–8. [DOI] [PubMed]
- 8.Leggett J E, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig W A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989;159:281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- 9.Löwdin E, Odenholt-Tornqvist I, Cars O. A new method to determine postantibiotic effect and effects of subinhibitory antibiotic concentrations. Antimicrob Agents Chemother. 1993;37:2200–2205. doi: 10.1128/aac.37.10.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald P J, Craig W A, Kunin C M. Persistent effects of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977;135:217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- 10a.Merck & Co. Data on file.
- 11.Moellering, R. C., Jr., G. M. Eliopoulos, and D. E. Sentochnil. 1989. The carbapenems: new broad spectrum β-lactam antibiotics. J. Antimicrob. Chemother. 24(Suppl. A):1–7. [DOI] [PubMed]
- 12.Nadler, H. I., D. H. Pitkin, and W. Sheikh. 1989. The postantibiotic effect of meropenem and imipenem on selected bacteria. J. Antimicrob. Chemother. 24(Suppl. A):225–231. [DOI] [PubMed]
- 13.Odenholt I, Isaksson B, Nilsson L, Cars O. Postantibiotic and bactericidal effect of imipenem against Pseudomonas aeruginosa. Eur J Clin Bacteriol Infect Dis. 1989;8:136–141. doi: 10.1007/BF01963897. [DOI] [PubMed] [Google Scholar]
- 14.Odenholt I, Holm S E, Cars O. Effects of benzylpenicillin on group A β-hemolytic streptococci during the postantibiotic phase in vitro. J Antimicrob Chemother. 1989;24:147–156. doi: 10.1093/jac/24.2.147. [DOI] [PubMed] [Google Scholar]
- 15.Odenholt I, Holm S E, Cars O. Effects of supra- and sub-MIC benzylpenicillin concentrations on group A β-hemolytic streptococci during the postantibiotic phase in vivo. J Antimicrob Chemother. 1990;26:193–201. doi: 10.1093/jac/26.2.193. [DOI] [PubMed] [Google Scholar]
- 16.Odenholt I, Löwdin E, Cars O. Comparative in vitro pharmacodynamics of BO-2727, meropenem and imipenem against gram-positive and gram-negative bacteria. Clin Microbiol Infect. 1997;3:73–81. doi: 10.1111/j.1469-0691.1997.tb00254.x. [DOI] [PubMed] [Google Scholar]
- 17.Odenholt-Tornqvist I, Löwdin E, Cars O. Pharmacodynamic effects of subinhibitory concentrations of β-lactam antibiotics in vitro. Antimicrob Agents Chemother. 1991;35:1834–1839. doi: 10.1128/aac.35.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odenholt-Tornqvist I, Löwdin E, Cars O. Postantibiotic sub-MIC effects of vancomycin, roxithromycin, sparfloxacin, and amikacin. Antimicrob Agents Chemother. 1992;36:1852–1858. doi: 10.1128/aac.36.9.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odenholt-Tornqvist I. Studies on the postantibiotic effect and the postantibiotic sub-MIC effect of meropenem. J Antimicrob Chemother. 1993;31:881–892. doi: 10.1093/jac/31.6.881. [DOI] [PubMed] [Google Scholar]
- 20.Odenholt-Tornqvist I, Löwdin E, Cars O. Postantibiotic effects and postantibiotic sub-MIC effects of roxithromycin, clarithromycin, and azithromycin on respiratory tract pathogens. Antimicrob Agents Chemother. 1995;39:221–226. doi: 10.1128/aac.39.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogelman B, Craig W A. Kinetics of antimicrobial activity. J Pediatr. 1986;108:835–840. doi: 10.1016/s0022-3476(86)80754-5. [DOI] [PubMed] [Google Scholar]
- 22.Vogelman B, Gudmundsson S, Turnidge J, Leggett J, Craig W A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988;157:287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- 23.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 24.Wilson D A, Rolinson G N. The recovery period following exposure of bacteria to penicillin. Chemotherapy (Basel) 1979;25:14–22. doi: 10.1159/000237817. [DOI] [PubMed] [Google Scholar]