Abstract
Immune-competent ICR and BALB/c athymic (nude) mice were infected intravenously with Histoplasma capsulatum and treated with either fluconazole or nikkomycin Z or 5% dextrose (controls). In immune-competent ICR mice, fluconazole and nikkomycin Z both prolonged survival when given at 5 mg/kg of body weight twice daily. When administered in doses as low as 2.5 mg/kg twice daily, nikkomycin Z reduced fungal counts in both the spleen and liver. When both drugs were combined, there was no antagonism, and in combined therapy spleen and liver counts were reduced more than for either drug alone. However, nikkomycin Z had no effect on brain fungal burden. In nude mice fluconazole and nikkomycin Z had an additive effect in prolongation of survival and reduction of liver and spleen burden. Nikkomycin Z is well tolerated, is at least as effective as fluconazole, and may interact beneficially with fluconazole for treatment of murine histoplasmosis.
Chitin is an important component of fungal cell walls and helps confer structural rigidity. Fungi contain multiple chitin synthase (CS) enzymes (CSI, CSII, and CSIII, etc.) which contribute to the synthesis of different parts of the fungal cell wall. Some, such as CSII and CSIII, apparently play no role in the virulence of Candida albicans, but others, such as CSI, may be more important (10). Chitin is not present in mammalian cells, and CS inhibition is thus a desirable fungal-specific target for antimycotic therapy. Nikkomycin Z is a nucleoside dipeptide which acts by competitive inhibition of fungal CS enzymes, thus compromising the contribution of chitin to the fungal cell walls (7). Nikkomycin Z has been found to be effective in vitro against Coccidioides immitis, a fungal pathogen very rich in chitin. Nikkomycin Z has also been effective as a single agent in mice infected with C. immitis (9). Nikkomycin Z has been less effective when used in mice infected with C. albicans, a fungus with relatively less chitin in the cell wall. However, in murine candidiasis there appear to be additive effects in vitro and in vivo when nikkomycin Z is combined with fluconazole (4, 8). In the present studies we have examined the effects of nikkomycin Z alone or combined with fluconazole in the treatment of murine histoplasmosis.
MATERIALS AND METHODS
Pathogen.
Histoplasma capsulatum, clinical isolate no. 93-255, was obtained from the Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, Tex. The fungus was maintained in the yeast phase at 37°C on brain heart infusion agar with 10% sheep blood. For studies fungal colonies were scraped off the agar, washed three times in sterile saline, and resuspended in sterile saline. Clumps of yeast cells were disrupted by agitation and then counted in a hemacytometer. Viable inocula were determined by ethidium bromide staining and were confirmed at usually >90% viability by quantitative cultures. All counts are expressed as CFU of viable organisms. The MIC for this isolate, determined according to the National Committee for Clinical Laboratory Standards macrobroth method adjusted for H. capsulatum (7 days incubation), was 4.0 μg/ml for fluconazole and 0.5 μg/ml for nikkomycin Z (11).
Mouse model.
Outbred male ICR mice, approximately 30 g each, were obtained from Harlan Sprague Laboratories. Six-week-old nude mice were obtained from our breeding colony, a barrier facility in which mice are maintained in a specific-pathogen-free environment and monitored regularly for mouse hepatitis virus, Sendai virus, Mycoplasma spp., and bacterial pathogens. ICR mice were allowed to acclimate to our facility for 3 days. Mice were then infected intravenously with 0.2 ml of sterile saline containing H. capsulatum. Two days were allowed for the infection to become established in ICR mice, and 1 day was allowed for infection of nude mice. Treatment was then begun by gavage with 0.2-ml volumes containing either 5% dextrose, fluconazole at 5, 10, or 25 mg/kg of body weight/dose in Noble agar, or nikkomycin Z in various doses dissolved in 5% dextrose. The doses of nikkomycin Z initially chosen were adapted from other studies (8, 9). Fluconazole was prepared once for each study, and nikkomycin Z was prepared every 4 days. Because of the rapid clearance of nikkomycin Z and fluconazole in mice, in all experiments groups of mice were treated twice daily. For survival studies groups of 10 mice were treated for 10 days after infection and were observed through day 30. For tissue counts groups of 7 or 10 mice were treated for 7 days and were sacrificed the next day. Livers, spleens, and brains were removed aseptically and homogenized and serial dilutions were plated on brain heart infusion agar with 10% sheep blood. These were incubated for 2 weeks at 37°C, and yeast colonies were counted in serial 10-fold dilution cultures. Our minimal detectable count was 18 CFU per g.
Analysis.
The log rank and Wilcoxon tests of life tables were used for the analysis of survival data, and Sidak’s multiple comparison test or Dunnett’s one- tailed t test was used for analysis of tissue counts. A P value of <0.01 to 0.001 was required for significance, depending on the number of comparisons.
RESULTS
In the initial two studies of immune-competent mice a large inoculum of 1.7 × 107 (experiment 1) or 6.4 × 107 (experiment 2) CFU per mouse was used. Table 1 presents survival data for ICR mice. In experiments 1 and 2 most mice succumbed by day 8, and there was no protection conferred by nikkomycin Z at a dose of 2.5, 5, or 10 mg/kg. Fluconazole was modestly protective in experiment 2. In the follow-up experiment 3, a lower infecting dose of 106 CFU was used. Fluconazole and nikkomycin Z at doses of >2.5 mg/kg prolonged survival significantly compared with that of controls. With a slightly higher infecting dose of 1.7 × 106 CFU (experiment 4), and the nikkomycin Z dose raised to 3 mg/kg, only the combination of nikkomycin Z and fluconazole prolonged survival significantly over that of controls. These studies suggest a strong inoculum effect on the dose response curve of nikkomycin Z, with diminished efficacy at fungal inocula of more than 106 CFU. Table 2 shows the results of two studies of tissue burden in ICR mice. In experiment 5 (infecting dose of 2.3 × 105 CFU/mouse), nikkomycin Z, at doses from 2.5 to 25 mg/kg, but not fluconazole, significantly reduced the fungal burden in the spleen and liver but not in the brain. In experiment 6, nikkomycin Z at doses of 5 and 25 mg/kg significantly reduced counts in the spleen and liver but not in the brain. Fluconazole reduced counts only in the liver. In the combination study with nikkomycin Z at 25 mg/kg and fluconazole, the combined therapy reduced liver and spleen counts more than fluconazole alone but not more than nikkomycin Z. However, in the spleen, the combination was superior to either drug used alone.
TABLE 1.
Survival of mice after infection with H. capsulatum and treatment with nikkomycin Z, fluconazole, or both drugs
| Expt | Inoculum | Druga | Dose (mg/kg)b | Mean survival ±SE (days) |
|---|---|---|---|---|
| 1 (n = 10/group) | 6.4 × 107 | None | None | 5.3 ± 0.5 |
| Nik Z | 2.5 | 4.3 ± 0.4 | ||
| Nik Z | 10 | 4.3 ± 0.5 | ||
| Flu | 5 | 5.3 ± 0.6 | ||
| 2 (n = 10/group) | 1.7 × 107 | None | None | 4.6 ± 0.3 |
| Nik Z | 2.5 | 8.1 ± 2.5 | ||
| Flu | 5.0* | 8.0 ± 0.7 | ||
| 3 (n = 20/group) | 1 × 106 | None | None | 8.0 ± 2.0 |
| Nik Z | 100* | 26 ± 2.4 | ||
| Nik Z | 25* | 24 ± 2.3 | ||
| Nik Z | 5* | 22 ± 2.7 | ||
| Nik Z | 2.5* | 21 ± 2.6 | ||
| Flu | 5* | 24 ± 2.4 | ||
| 4 | 1.7 × 106 | None | None | 9 ± 1.7 |
| Nik Z | 3 | 16 ± 3.8 | ||
| Flu | 5 | 21 ± 4.3 | ||
| Nik Z + Flu | 3 + 5* | 26 ± 3.7 |
Nik Z, nikkomycin Z; Flu, fluconazole.
*, survival significantly longer than controls.
TABLE 2.
Tissue burden in mice infected with H. capsulatum and treated with nikkomycin Z, fluconazole, or both drugs
| Expt | Inoculum (CFU) | Drugb | Dose (mg/kg) | Tissue burden (105 CFU/g) ina:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spleen
|
Liver
|
Brain
|
||||||||||
| Mean | Lower CI | Upper CI | Mean | Lower CI | Upper CI | Mean | Lower CI | Upper CI | ||||
| 5 (n = 7/group) | 2.3 × 105 | None | 1.98 | 1.01 | 4.95 | 1.28 | 6.05 | 28.06 | 0.05 | 0.03 | 0.14 | |
| Nik Z | 2.5 | 0.22* | 0.06 | 0.61 | 2.12* | 1.14 | 5.27 | 0.06 | 0.03 | 0.05 | ||
| 5 | 0.11* | 0.06 | 0.27 | 1.89* | 1.01 | 4.69 | ||||||
| 10 | 0.12* | 0.06 | 0.30 | 2.64* | 1.42 | 6.57 | 0.02 | 0.01 | 0.05 | |||
| 25 | 0.07* | 0.04 | 0.19 | 0.71* | 0.38 | 1.77 | 0.13 | 0.07 | 0.33 | |||
| Flu | 5 | 3.72 | 1.99 | 9.22 | 8.46 | 4.53 | 21.03 | 0.07 | 0.04 | 0.18 | ||
| 6 (n = 7/group) | 7 × 105 | None | 10.89 | 5.84 | 27.09 | 60.26 | 32.30 | 149.90 | 0.64 | 0.34 | 1.59 | |
| Nik Z | 5 | 1.96* | 1.05 | 4.87 | 6.97* | 3.74 | 17.34 | 0.38 | 0.20 | 0.94 | ||
| 25 | 0.44* | 0.24 | 1.10 | 10.16* | 5.44 | 25.26 | 0.61 | 0.33 | 1.53 | |||
| Flu | 5 | 4.08 | 2.19 | 10.15 | 19.43* | 10.41 | 48.33 | 0.50 | 0.27 | 1.26 | ||
| Nik Z + Flu | 5 + 5 | 0.50** | 0.30 | 1.21 | 2.79* | 1.50 | 6.95 | 0.33 | 0.18 | 0.82 | ||
| 5 + 25 | 0.37* | 0.20 | 0.92 | 2.87* | 1.54 | 7.14 | ||||||
Lower CI, lower 95% confidence interval; Upper CI, upper 95% confidence interval. *, significantly different from control (none) (P < 0.05); **, significantly different from control (P < 0.01).
Nik Z, nikkomycin Z; Flu, fluconazole.
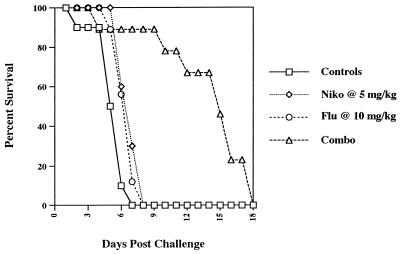
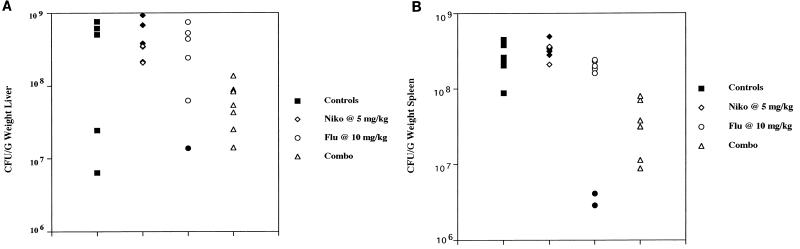
Additional studies were conducted with nude mice. Nikkomycin Z at 5 mg/kg/dose was evaluated alone and in combination with fluconazole at 5 mg/kg/dose. In a survival study with nikkomycin Z and/or fluconazole, only the combination prolonged survival, and that only modestly over controls (data not shown). In a follow-up study (Fig. 1) groups of 10 nude mice infected with 7 × 106 H. capsulatum and treated with nikkomycin Z at 5 mg/kg/dose and fluconazole at 10 mg/kg/dose showed markedly prolonged survival over the survival of mice treated with each drug used alone. In studies of tissue burden in which the fluconazole and nikkomycin Z doses were 5 mg/kg, there was a minimal but significant benefit of the combination in reducing spleen tissue counts and no benefit in reducing liver counts (data not shown). Nude mice were then infected with H. capsulatum at 1.5 × 106 CFU/mouse (Fig. 2) and the fluconazole dose was raised to 10 mg/kg. In the liver (Fig. 2A) neither drug given alone reduced tissue burden, but the combination significantly reduced the median tissue count by 1 log. In the spleen (Fig. 2B) neither nikkomycin Z nor fluconazole alone were effective, but the combination significantly reduced counts below those of controls and nikkomycin Z alone. No regimen significantly reduced brain tissue counts (data not shown).
FIG. 1.
Survival of groups of 10 nude mice after infection with 7 × 106 H. capsulatum and treatment from day 1 through 10 with 5% glucose (control), nikkomycin Z (5 mg/kg/dose), fluconazole (10 mg/kg/dose), or both drugs.
FIG. 2.
Tissue burden in the livers and spleens of groups of 7 nude mice infected with 1.4 × 106 CFU/mouse and treated with the same drug doses as detailed in the legend for Fig. 1. Mice were sacrificed on day 8. Solid symbols indicate counts in mice which succumbed before day 8. (A) Liver counts. Liver cultures of one fluconazole recipient and one nikkomycin Z recipient were contaminated and unreadable; (B) spleen counts.
DISCUSSION
Chitin, along with glucans, is a crucial component of fungal cell walls. There are at least three CS enzymes in Saccharomyces cereviesiae, each of which is related to the synthesis of the structure of a different part of the cell wall (1). CSI is involved with budding. CSII is engaged with the determination of cell morphology, septation, and separation, while CSIII generates 90% of the cell wall chitin. The cell wall is formed largely of chitin and β-glucans and serves to maintain the structural integrity of the cell and is a potential barrier for excluding some substances. Inhibition of one CS enzyme is not lethal, but the blocking of all three is lethal for S. cerevisiae (1, 2). Nikkomycins are highly potent in vitro. They inhibit CSI and CSII (1) and more recently have been found to inhibit CSIII as well (5). However, our information on their in vivo activities is much less complete. Nikkomycins have somewhat more limited effects on intact fungal cells. This may result in part from the different amounts of chitin in cells and/or from the variable access of these compounds to the fungal cell membrane, which is the site of the CSs (9). It is not yet clear which CS enzyme(s) is targeted in H. capsulatum.
After intravenous infection in mice, H. capsulatum disseminates widely, producing by 9 to 15 days maximum tissue counts of 5 × 105 to 5 × 106 CFU/g of liver, 5 × 104 to 5 × 105 CFU/g of spleen, and 5 × 102 to 5 × 104 CFU/g of brain. Nikkomycin Z at a dosage of as little as 2.5 mg/kg twice daily reduced counts in the liver and spleen but not in the brain. In vivo growth inhibition by nikkomycin Z was measured by both prolongation of survival and by reduction of liver and spleen counts. Brain counts were not affected. It is possible that nikkomycin Z may benefit mice by reducing the extracerebral burden of H. capsulatum but permit multiplication in a protected site such as the central nervous system.
In immune-competent mice CD4-mediated defenses, including interferon gamma and tumor necrosis factor alpha, control the infection in cerebral and extracerebral sites (12). Infection in mice reaches a peak intensity between 1 and 2 weeks after infection, after which time surviving mice have a rapid improvement caused by mobilization of cell-mediated immune defenses (3, 6). However, nude mice (and patients with AIDS, with somewhat similar defects in immune response) have progressive infection and ultimately die with widespread dissemination, including meningoencephalitis (13–15). It is not clear whether nikkomycin Z will be as effective in immune-suppressed mice (or AIDS patients) as with immunocompetent hosts. Also, the pharmacokinetics of nikkomycin Z indicate rapid clearance in mice, with a peak concentration of 320 μg/ml after a 100-mg/kg dose but clearance with a half-life of between 10 min and 1 h (9).
In vivo, no antifungal drug is completely fungicidal, and benefits are lower in immune-compromised subjects. These subjects, such as patients with AIDS, have high fungal burdens and few host defenses to combat mycoses. In these patients combination therapy might have more effect than any one drug. The additive effect of nikkomycin Z and fluconazole in nude mice encourages evaluation of combined therapy in immune-depressed patients such as those with AIDS.
Nikkomycin Z is under Phase I investigation for treatment of coccidioidomycosis and may be useful in other mycoses. Nikkomycin Z also has potential for favorable interaction with drugs such as the echinocandins, which inhibit β-1,3-glucan synthesis in the cell wall (2). Therefore, it is possible that nikkomycin Z may be most effective when used with other antifungals, such as triazoles or papulocandins, for the treatment of histoplasmosis. The present study suggests that this drug is a candidate for further development.
ACKNOWLEDGMENTS
This study was supported by a grant from Shaman Pharmaceuticals.
We thank Renkai Li for performing antifungal susceptibility tests.
REFERENCES
- 1.Bulawa C E. Genetics and molecular biology of chitin synthesis in fungi. Annu Rev Microbiol. 1993;47:505–534. doi: 10.1146/annurev.mi.47.100193.002445. [DOI] [PubMed] [Google Scholar]
- 2.Debono M, Gordee R S. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 3.Deepe G S., Jr The immune response to Histoplasma capsulatum: unearthing its secrets. J Lab Clin Med. 1994;123:201–205. [PubMed] [Google Scholar]
- 4.Flores M E, Hector R F. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro activity of the antifungal nikkomycin Z (SP-920704) in combination with fluconazole or itraconazole vs. yeasts, abstr. F190; p. 133. [Google Scholar]
- 5.Gaughran J P, Lai M H, Kirsch D R, Silverman S J. Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vitro and in vivo. J Bacteriol. 1994;176:5857–5860. doi: 10.1128/jb.176.18.5857-5860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez A M, Bullock W E, Taylor C L, Deepe G S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988;56:1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hector R F. Compounds active against cell walls of medically important fungi. Clin Microbiol Rev. 1993;6:1–21. doi: 10.1128/cmr.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hector R F, Schaller K. Positive interaction of nikkomycins and azoles against Candida albicans in vitro and in vivo. Antimicrob Agents Chemother. 1992;36:1284–1289. doi: 10.1128/aac.36.6.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hector R F, Zimmer B L, Pappagianis D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob Agents Chemother. 1990;34:587–593. doi: 10.1128/aac.34.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mio T, Yabe T, Sudoh M, Satoh Y, Nakajima T, Arisawa M, Yamada-Okabe H. Role of three chitin synthase genes in the growth of Candida albicans. J Bacteriol. 1996;178:2416–2419. doi: 10.1128/jb.178.8.2416-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeasts: proposed standard M27-P. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 12.Smith J G, Magee D M, Williams D W, Graybill J R. Tumor necrosis factor-α plays a role in host defense against Histoplasma capsulatum. J Infect Dis. 1990;162:1349–1353. doi: 10.1093/infdis/162.6.1349. [DOI] [PubMed] [Google Scholar]
- 13.Wheat L J, Connolly-Springfield P A, Backer R L, Curfman M F, Eads M E, Israel K, Norris S A, Webb D, Zeckel M L. Disseminated histoplasmosis in the acquired immunodeficiency syndrome: clinical findings, diagnosis and treatment and review of the literature. Medicine. 1990;69:361–374. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Wheat L J, Slama T G, Zeckel M L. Histoplasmosis in the acquired immune deficiency syndrome. Am J Med. 1985;78:203–210. doi: 10.1016/0002-9343(85)90427-9. [DOI] [PubMed] [Google Scholar]
- 15.Williams D M, Graybill J R, Drutz D J. Experimental chemotherapy of histoplasmosis in nude mice. Am Rev Respir Dis. 1979;120:837–842. doi: 10.1164/arrd.1979.120.4.837. [DOI] [PubMed] [Google Scholar]