Abstract
Intramuscular hemangiomas are uncommon benign endotheliomas that typically occur in the trunk and limbs. Head and neck involvement is relatively infrequent, with the masseter muscle being the most commonly affected site. We present a rare case of intramuscular hemangiomas arising from the semispinalis muscle. A 31-year-old male presented with a painless swelling in the left upper neck region, gradually increasing in size over the past year. Imaging studies revealed a well-defined mass originating from the semispinalis muscle. Surgical excision was performed successfully, and histological examination confirmed the diagnosis of a mixed intramuscular hemangioma. The patient remained recurrence-free during the 2-year follow-up period. Intramuscular hemangiomas in the posterior neck muscles are rare, with only a few reported cases. Wide surgical resection with control of feeding vessels is the optimal treatment, and follow-up is recommended to monitor for local recurrence. This case report highlights the clinical presentation, diagnostic challenges, and successful surgical management of intramuscular hemangiomas in a unique location, emphasizing the importance of accurate diagnosis and appropriate treatment of this rare tumor.
Keywords: Hemangioma, neck mass, imaging, surgery
Introduction
Intramuscular hemangiomas (IMHs) are rare benign endotheliomas. They account for less than 1% of the hemangioma and occur usually in the trunk and limbs. 1 The head and neck location is relatively infrequent, accounting for 14%–21% of all IMH. 1 Within these areas, the masseter muscle is the most commonly affected site followed by the trapezius and temporalis muscles. 2 The most effective imaging technique for assessing IMH is magnetic resonance imaging. 3 Tailoring the treatment of IMH should be individualized according to factors such as patient age, location of the lesion, accessibility, size, rate of growth, and aesthetic factors.
We report a rare case of a IMH arising from the semispinalis muscle.
Case report
A 31-year-old man presented with a 2-year history of a painless left upper neck region swelling that had a gradual growth in size over the last year.
Physical examination revealed a soft mass on the area of the paraspinal muscles of the neck’s upper part, measuring 8 cm × 6 cm in size, not adhering to the surrounding tissues, nonpulsatile, and with a normal overlying skin. The mass did not increase in volume in the declivity position. There were no associated cervical nodes or definite neurological deficits.
The computed tomography (CT) demonstrated a round-shaped and well-defined mass of the left semispinalis muscle with moderate and heterogeneous enhancement (Figure 1).
Figure 1.
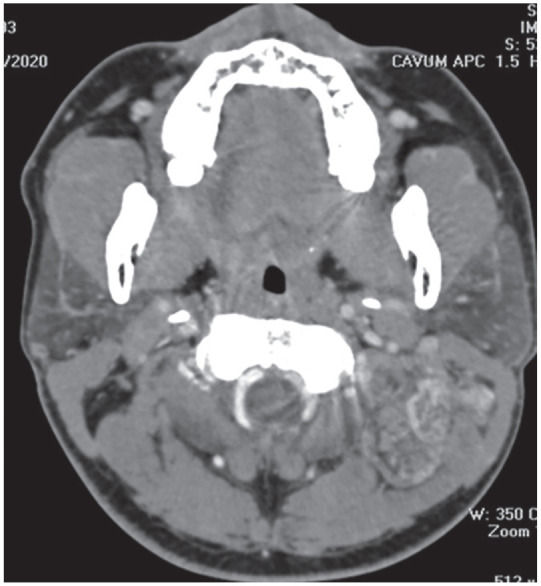
Axial CT contrast scan showed a, well-defined mass with moderate and heterogeneous enhancement in the left semispinalis without calcifications.
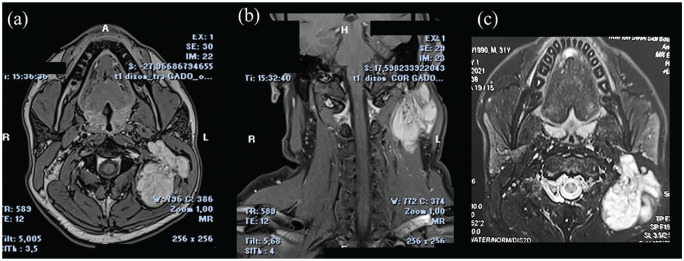
A magnetic resonance imaging (MRI) showed a well-defined, lobulated mass originating of the left semispinalis muscle, pushing the sternocleidomastoid, splenius, and trapezius muscles outward. This mass was hypo-intense on T1-weighted images, high intense on T2 and T2 Fat-sat weighted images, and intense and heterogeneous enhancement on contrast-enhanced T1-weighted images (Figure 2). The mass was 64 mm × 54 mm × 41 mm in size.
Figure 2.
MRI showing an intramuscular lobulated and well-defined mass involving the left semispinalis muscle. The mass appears hyperintense on T2 Fat-Sat images (a,b) and demonstrates intense and heterogeneous enhancement on gadolinium-enhanced T1-weighted images (c) without any serpiginous flow voids.
A surgery was performed through a vertical L-shaped lateral skin incision. The tumor was deeply situated in relation to the posterior muscles and firmly attached to them, without evidence of invasion into the surrounding bony structures. After carefully dissecting the spinal accessory nerve and the feeder vessels, the mass was successfully excised, along with a portion of the adjacent muscles.
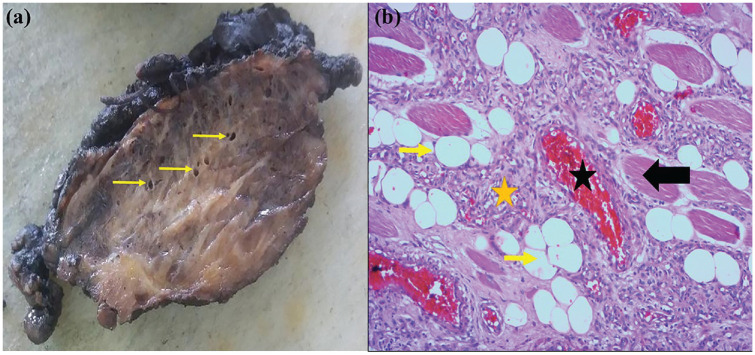
The macroscopic mass examination showed a nodular tumor tissue with a purplish-brownish appearance, small-sized vascular channels, and intervening yellow fat (Figure 3). The histological examination found a mixture of large, dilated, medium, and small vascular channels that were trapped between skeletal muscle fibers with admixed adipose tissue (Figure 4). The mixed-type intramuscular hemangioma was confirmed.
Figure 3.
Intramuscular hemangioma. (a) Grossly, the tumor has a heterogenous aspect with purple hemorrhagic areas, yellowish areas, and medium-sized vessels (yellow arrows). (b) Microscopically, the tumor shows skeletal muscle (black arrow) infiltrated by vascular channels, often small sized, (yellow asterisk), sometimes larger (black asterisk), filled with red blood cells. Note the presence of entrapped fat tissue (yellow arrow) (HE × 100).
Figure 4.
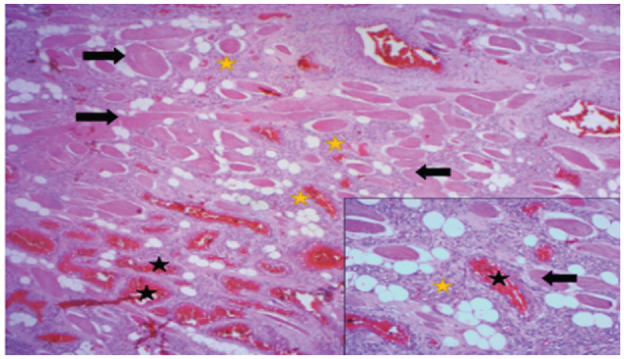
Striated muscle tissue (black arrows) dissociated by vascular proliferation, often capillary (yellow stars), sometimes cavernous (black stars), and gorged with red blood cells (HE × 25). At high magnification, vascular structures are bordered by turgid endothelial cells with no atypia or mitosis (HE × 100).
The patient presented limited neck mobility for 10 days postoperatively, which recovered without motor sequelae.
The patient remained free of any recurrence during the 2-year follow-up period.
Discussion
The masseter muscle (36%), trapezius muscle (12%), sternocleidomastoid muscle (10%), and temporalis muscles (8%) are the most commonly affected sites for the formation of intramuscular hemangioma (IMH) in the head and neck. 4 The occurrence of IMH in the posterior area is rare, and only a few cases have been documented. These cases include involvement of the sternocleidomastoid muscle, 4 the splenius capitis muscle, 3 and the semispinalis capitis muscle. 5 We report the second case of semispinalis muscle hemangioma.
The exact etiopathogenesis of IMH remains unclear; however, several theories have been proposed, including congenital factors, hormonal imbalances, and trauma. These hypotheses attempt to explain the origins and development of intramuscular hemangiomas, but further research is needed to establish a definitive cause. 6 Allen and Enzinger subdivided IMH according to vessel size as capillary hemangioma, cavernous hemangioma, and mixed hemangioma. 7 For head and neck IMH, the capillary hemangioma are the most frequent and accounts for 68%, while the cavernous hemangioma accounts for 26%, and the mixed-type hemangioma accounts for 5%. 8 In our case, we identified a mixed-type hemangioma.
More than 90% of cases are misdiagnosed before surgery. 9 It may be explained by the lack of specific symptoms, the variability of presentations, and the unreliability of the clinical exam. It usually presents as a slow growing, mobile, and painless mass.
Radiological findings can be valuable, particularly in cases of cavernous hemangioma, as they often reveal characteristic features such as tortuous vascular spaces, thrombus formation, and the presence of phleboliths (15%–25%). 10 The MRI is the gold standard preoperative diagnostic tool. Hemangiomas are generally iso-intense to muscle on T1-weighted images and hyperintense on T2-weighted images. 11 MRI offers superior soft tissue characterization, enabling a more precise delineation of tumor components and assessment of the extent of local involvement compared to CT. It can show focal heterogeneities which represent areas of thrombosis, fibrosis, or calcification. 8
CT imaging lacks specificity for this particular tumor; however, it can be useful in primarily assessing potential bone involvement and associated damage. 12
Several factors can guide the therapeutic decision-making process, including the location, size of the IMH, growth rate, vascularity, and the presence of cosmetic deformity. The optimal treatment is wide resection, including the cuff of the surrounding muscles, and the control of the feeding vessels to prevent recurrence.5,8 In large-sized IMH, preoperative embolization of feeding arteries may be necessary to reduce the risk of bleeding. It is important to notice that embolization alone is considered inappropriate therapy without surgical resection.10,11 In our case, embolization was not necessary.
Other therapeutic options are available, including sclerotherapy, corticosteroids, cryotherapy, laser-based therapies, and radiation therapies. These modalities may be considered for cases involving giant intramuscular hemangiomas, aiming to reduce the size of the larger lesion and mitigate functional and cosmetic impairments.10,11 Alternatively, these therapeutic options can be regarded as palliative measures for IMH, affecting vital organs such as the tongue, where extensive or complete surgical removal may result in undue postoperative morbidity. In such cases, these interventions serve to alleviate symptoms and improve the quality of life. 10 These treatments can lead to the regression of mass with a relief of pain and compression symptoms.
The local recurrence rates following surgery were estimated to be 28% for the mixed type, 20% for the capillary type, and 9% for the cavernous type.5,13 It may be a result of an incomplete surgery.10,14 Clinical and radiological follow-up is recommended for the initial 2 years to detect any potential local recurrence. 15 In our case, there was no recurrence observed within the 2 years following complete surgery. Spontaneous regression or malignant transformation of the tumor has not been reported.5,8
Conclusion
IMH is a rare benign tumor. This case report highlights an instance of IMH affecting the posterior neck muscles. The clinical presentation is nonspecific, and imaging findings often indicate its vascular nature. Surgical intervention involving complete removal was performed.
Acknowledgments
We are grateful to the medical personnel who were caring for the patient.
Footnotes
Data availability statements: All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Moncef Sellami  https://orcid.org/0000-0003-2409-3996
https://orcid.org/0000-0003-2409-3996
References
- 1. Garefis K, Nikolaidis V, Kipriotou A, et al. A rare clinical report of intramuscular hemangioma of the middle scalene muscle. Ear Nose Throat J 2023; 102: 359–361. [DOI] [PubMed] [Google Scholar]
- 2. Bucci T, De Giulio F, Romano A, et al. Cavernous haemangioma of the temporalis muscle: case report and review of the literature. Acta Otorhinolaryngol Ital 2008; 28: 83–86. [PMC free article] [PubMed] [Google Scholar]
- 3. Maharjan S, Hona A, Karki S. A rare case of intramuscular hemangioma of splenius capitis: a case report. Ann Med Surg 2023; 85: 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhan FH, Jian XC, Shen ZH. Giant hemangioma of the sternocleidomastoid muscle: report of a case. J Oral Maxillofac Surg 1997; 55: 190–193. [DOI] [PubMed] [Google Scholar]
- 5. Jang K-M, Park S-W, Kim Y-B. An intramuscular hemangioma at the cervical muscle: a case report. Korean J Spine 2015; 12: 196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scott JE. Haemangiomata in skeletal muscle. Br J Surg 1957; 44: 496–501. [DOI] [PubMed] [Google Scholar]
- 7. Allen PW, Enzinger FM. Hemangioma of skeletal muscle. An analysis of 89 cases. Cancer 1972; 29: 8–22. [DOI] [PubMed] [Google Scholar]
- 8. Yu D, Choi JH, Jeon I. Intramuscular hemangioma with hemorrhagic transformation arising from paraspinal muscles of posterior neck. Medicine (Baltimore) 2020; 99: e21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Odabasi AO, Metin KK, Mutlu C, et al. Intramuscular hemangioma of the masseter muscle. Eur Arch Otorhinolaryngol 1999; 256: 366–369. [DOI] [PubMed] [Google Scholar]
- 10. Giudice M, Piazza C, Bolzoni A, et al. Head and neck intramuscular haemangioma: report of two cases with unusual localization. Eur Arch Otorhinolaryngol 2003; 260: 498–501. [DOI] [PubMed] [Google Scholar]
- 11. Sayad Z, Dani B, Benazzou S, et al. An unusual location of a cavernous hemangioma: a case report. Pan Afr Med J 2021; 39: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cui B, Wang D-H, Wang G-J, et al. Cavernous hemangiomas of the temporalis muscle with prominent formation of phleboliths: case report and review of the literature. Medicine (Baltimore) 2017; 96: e8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolf GT, Daniel F, Krause CJ, et al. Intramuscular hemangioma of the head and neck. Laryngoscope 1985; 95: 210–213. [DOI] [PubMed] [Google Scholar]
- 14. Lee DY, Kang SH, Kim JH, et al. Survival and recurrence of resectable tongue cancer: resection margin cutoff value by T classification. Head Neck 2018; 40: 283–291. [DOI] [PubMed] [Google Scholar]
- 15. Cappabianca P, Cirillo S, de Divitiis E, et al. Hemangioma of the temporal muscle. Head Neck 1996; 18: 197–200. [DOI] [PubMed] [Google Scholar]