Abstract
Benzydamine is an active pharmaceutical compound used in the oral care pharmaceutical preparation as NSAID. Beside from its anti-inflammatory action, benzydamine local application effectively reliefs pain showing analgesic and anaesthetic properties. Benzydamine mechanism of action has been characterized on inflammatory cell types and mediators highlighting its capacity to inhibit pro-inflammatory mediators' synthesis and release. On the other hand, the role of benzydamine as neuronal excitability modulator has not yet fully explored. Thus, we studied benzydamine’s effect over primary cultured DRG nociceptors excitability and after acute and chronic inflammatory sensitization, as a model to evaluate relative nociceptive response. Benzydamine demonstrated to effectively inhibit neuronal basal excitability reducing its firing frequency and increasing rheobase and afterhyperpolarization amplitude. Its effect was time and dose-dependent. At higher doses, benzydamine induced changes in action potential wavelength, decreasing its height and slightly increasing its duration. Moreover, the compound reduced neuronal acute and chronic inflammatory sensitization. It inhibited neuronal excitability mediated either by an inflammatory cocktail, acidic pH or high external KCl. Notably, higher potency was evidenced under inflammatory sensitized conditions. This effect could be explained either by modulation of inflammatory and/or neuronal sensitizing signalling cascades or by direct modulation of proalgesic and action potential firing initiating ion channels. Apparently, the compound inhibited Nav1.8 channel but had no effect over Kv7.2, Kv7.3, TRPV1 and TRPA1. In conclusion, the obtained results strengthen the analgesic and anti-inflammatory effect of benzydamine, highlighting its mode of action on local pain and inflammatory signalling.
Keywords: Benzydamine, inflammation, ion channels, neuronal sensitization, pain
Introduction
Benzydamine (BZ) is an indazole derivative non-steroidal anti-inflammatory drug (NSAID). 1 It is used in the treatment of pain and inflammation of the oropharynx and has demonstrated local anaesthetic and analgesic properties. 2 It has been used in the medication of pharyngitis, gingivitis and oral mucositis caused by radio and chemotherapy in head and neck cancer patients or in post-operative endotracheal intubation sore throat. 3 It has also been exploited given its antiseptic activity to treat oromucosal infections. 4 One of its great advantages is that its topical administration shows highly safe profile without displaying systemic side effects by acting locally. 1 Its topical application has demonstrated a high analgesic and anti-inflammatory activity, higher than ones exerted by other NSAIDs used orally. 5
BZ molecular mechanism is non-specific showing inhibitory effects, potentially acting in a wide range of anti-inflammatory, anaesthetics and analgesic molecular targets.6–8 It is highly effective controlling cytokine release.7–9 Moreover, BZ inhibits reactive oxygen species production, induces a membrane stabilization and decreases vascular permeability, thus demonstrating an anti-oedematous effect.10,11 While its mechanism is deeply characterized on inflammatory mediators, receptors and cell types, its pharmacological action as analgesic has not yet been fully explored.7,8,12 Its relevance on nociceptor activity has not been deeply analysed at functional level on sensory neurons.
Pain regulation depends upon an active crosstalk between nociceptors and the immune system. Noxious stimuli are transduced by voltage- and ligand-gated ion channels, such as Nav1.7-1.9, TRPV1 and TRPA1, which are highly expressed in nociceptor nerve terminals.13,14 The immune cells regulate nociceptor activity through the release of diverse modulators, inducing changes in expression and mobilization, and post-translational ones (phosphorylation, ubiquitination) over ion channels. 15 On the other hand, nociceptors act on immune cells, regulating their function, by the release of neuropeptides and neurotransmitters. In this way, neural signalling contributes to the inflammation development. Interrupting this fundamental dialog between both systems, inevitably lowers peripheral pain inflammatory sensation. 16
Consequently, in order to gather a deeper understanding of BZ action over inflammatory nociception we evaluated its effect on primary culture of neonatal rat DRGs, in inflammation sensitized or control conditions by electrophysiological means, through patch clamp and multielectrode array (MEA).
Material and methods
Animal experimentation
Primary culture of dorsal root ganglia (DRGs) was performed with neonatal Wistar rats obtained from the excess/remains of already isolated sensory neurons from the IDiBE at the University Miguel Hernandez of Elche. Animal procedures were approved by the University Miguel Hernández de Elche Institutional Animal and Ethical Committee in accordance with international guidelines. Animals were kept in a controlled environment (21–23°C, 12 h light/dark cycle), and had food and water available ad libitum.
Primary culture of rat dorsal root ganglia
DRG sensory neurons were isolated from neonatal Wistar rats (3–5 days-old). After extraction, DRGs were located into a petri dish containing ice-cold PBS. Roots were removed and ganglia were digested with 0.25% (w/v) collagenase type-IA in DMEM-GlutaMax media supplemented with 1% penicillin/streptomycin (P/S) for 1 h at 37°C, 5% CO2. After digestion, ganglia were mechanically disrupted using a pipette. Single cell suspension was passed through 100 μm cell strainer and washed with DMEM-GlutaMax media with 10% Fetal Bovine Serum (FBS) and 1% P/S. Cells were seeded on 12 mm ø glass coverslips for patch clamp assays or on 60-electrodes MEA plates, previously treated with poly-L-lysine (8.3 μg/mL) and laminin (5 μg/mL). On the glass coverslips, cells were let sit for 10 min in order to allow attachment to the plate surface and DMEM-GlutaMax media supplemented with 10% FBS, 1% P/S, 50 ng/mL 2.5S murine β-NGF and 1.25 μg/mL cytosine arabinoside was added carefully to each well. On the MEA plates, a 10 µL droplet of concentrated cell suspension was placed just above the electrode array. 30 min after, 0.5 mL complete supplemented media was added. 17 Cells were maintained at 37°C and 5% CO2. All culture procedure was performed in a laminar flow cabinet. Experiments were performed 48 h after cell seeding.
Patch clamp recordings
Whole cell patch clamp recordings in current clamp mode were performed with EPC-10 (HEKA Electronik) and Patchmaster software. Intracellular pipette solution contained in mM: 4 NaCl, 110 K-gluconate, 30 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, 4 ATP-Na, 0.4 GTP-Na, and 10 EGTA, adjusted to pH 7.2 with KOH. Internal solution was filtered and stored at −20°C. External solution contained in mM: 140 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 20 D-Glucose, 20 D-Mannitol, adjusted to pH 7.4 with NaOH. Patch pipettes, prepared from thin-wall borosilicate capillary glass tubing were pulled with a horizontal flaming-brown Micropipette puller model P-97 from Sutter Instrument CO to a final resistance of 2–5 MΩ when filled with internal solution. Neurons were kept at their resting membrane potential (RMP) and stimulated electrically with a steps protocol starting from −40 pA in 10 pA increments. Recordings were acquired at 10 kHz and low-pass filtered at 3 kHz. All procedures were performed at room temperature. Substance application was performed through a gravity driven perfusion system. 18
External solution for recording potassium currents from Kv7.2/7.3 channels contained in mM: 130 Na-gluconate, 20 NaCl, 4 KCl, 1 MgCl2, 1.8 CaCl2, 10 HEPES and 5 D-Glucose, adjusted to pH 7.3 with NaOH. Internal solution contained in mM: 100 K-gluconate, 40 KCl, 1 MgCl2, 10 HEPES and 1 EGTA, adjusted to pH 7.3 with KOH. Human Kv7.2/7.3 currents were registered using IonWorks Quattro. Currents were evoked from a holding potential of −120 mV by a series of five 2500 ms depolarizing pulses with 3000 ms interval. The depolarizing pulses were −100, −80, −60, −30 and 0 mV. This pulse protocol was applied before and after the perfusion of BZ which lasted for 5 min. As a positive control of inhibition linopiridine at 1 and 300 µM was used.
Nav1.8 currents were registered with IonFlux HT. External recording solution contained in mM: 150 NaCl, 4 BaCl2, 1 MgCl2, 1.8 CaCl2, 10 HEPES and 5 D-Glucose, adjusted to pH 7.4 with NaOH. Internal solution contained in mM: 100 CsF, 45 CsCl, 5 NaCl, 10 HEPES and 5 EGTA, adjusted to pH 7.3 with CsOH. Cells were held at −120 mV for 50 ms before stepping to −10 mV for 2 s to completely inactivate the Nav1.8 channels, and stepped back to −120 mV for 10 ms to completely recover from inactivation. Channels bound to drugs never recover from inactivation. Then cells were stepped to −10 mV for 50 ms. Sweep interval was 20 s. Compound was applied for 2 min. As a positive control of inhibition tetracaine at 1, 10, 30 and 100 µM was used.
Calcium flux assay
BZ was assessed as agonist and antagonist of TRPV1 and TRPA1 channels. The agonist assay was conducted on a FLIPRTETRA instrument where BZ, vehicle controls, and reference agonist were added to the assay plate after a fluorescence baseline was established. The agonist assay lasted a total of 3 min and was used to assess BZ’s ability to activate TRPV1 and TRPA1 channels. Upon completion of the agonist assay, the assay plate was removed from the FLIPRTETRA and incubated at 25°C for 10 min. After the incubation period, the assay plate was placed back in the FLIPRTETRA and the antagonist assay was initiated. Using EC80 potency values determined during the agonist assay, all pre-incubated sample compound wells were challenged with EC80 concentration of reference agonist after establishment of a fluorescence baseline. The antagonist assay lasted a total of 3 min and was used to assess BZ’s ability to inhibit TRPV1 and TRPA1 channels. As a positive control capsaicin (1, 5, 15, 50, 150 and 440 nM) and AITC (5, 15, 40, 125 and 375 µM) for activation of TRPV1 and TRPA1 were used, respectively. As positive controls of inhibition capsazepine (0.1, 0.4, 1.1, 3.3, 10 and 30 µM) for TRPV1 and ruthenium red (10, 40, 120, 400, and 1000 nM) for TRPA1 were used.
Multielectrode array recordings
Extracellular electrical activity of primary cultured sensory neurons was explored using 60-multiple electrode planar array MEA chips, with 30 µm diameter electrodes and 200 µm inter-electrode spacing with integrated reference electrode from Multichannel Systems. Recordings were made with the MEA2100-MINI-4X60-SYSTEMS and MultiChannel Systems Experimenter software with a 10 kHz sampling rate. Temperature controller TC02 was used to keep cells at 34.5°C while extracellular solution temperature was maintained at 37°C using PH01 heatable perfusion cannula with temperature sensor, both from Multichannel Systems. Physiological extracellular solution used in cell perfusion was the same as the one used in patch clamp experiments. Neuronal responsiveness was checked at the end of each register by the application of extracellular solution with the same composition as stated previously except for KCl concentration, which was raised to 40 mM.18,19
Substances application
After seeding, control cells were kept for approximately 48 h in DMEM-GlutaMax media supplemented with FBS, P/S, rat β-NGF and cytosine arabinoside prior to register. For patch clamp studies, basal cell state was monitored prior to the instillation of any compound inducing neuronal electric activity by a current injection steps protocol. Then, 1 μM BZ or inflammatory soup (IS) containing 10 μM ATP, 100 nM histamine, 100 nM prostaglandin E2 (PGE2) and 100 nM serotonin (5-HT)20,21 or IS + BZ were applied acutely during 5 min through a constant perfusion. At minute 5, and in presence of the chemical stimuli, cells were stimulated again with the current injection steps protocol. When cells were not stimulated electrically, they were kept at their RMP. RMP, firing frequency, rheobase and afterhyperpolarization (AHP) amplitude data was collected. AHP amplitude was monitored at rheobase elicited firing. For dose-response assays, BZ was used at 0.1, 1, 10 and 30 µM. Action potential heigh and duration data was analysed for cells instilled with 30 µM BZ. Chronic inflammatory condition was simulated keeping cells during 24 h in complete supplemented media containing inflammatory mediators at the concentrations used for acute stimulation. Other chronic conditions consisted in 24 h incubation with 1 µM BZ or IS + BZ. In MEA studies, a more severe inflammation (ISc) was simulated, increasing concentration of inflammatory mediators to 100 μM ATP, 1 μM histamine, 1 μM PGE2 and 1 μM serotonin, compared to patch clamp approach. Neuronal activity was evoked through chemical stimulation by acetylcholine (ACh), ISc, acidic pH or KCl. Application protocols of chemical stimuli consisted in two consecutive pulses (P1 and P2) followed by a KCl pulse, except for KCl stimulation protocol. Before the second pulse of each stimulus, BZ was applied during 1.5 min at 1, 10, 25 and 50 µM.
Data analysis
Patch clamp data were analysed using Patchmaster software. Firing frequency was measured for the current maximising it. Firing frequency at the different time points was always measured at the same injected current for one cell. AHP was determined for rheobase current. For comparison within the same condition, data was normalised to basal values (time 0).
For potassium currents monitoring assays, the peak amplitude of the outward current from the depolarizing pulses was measured before (P1) and after (P2) BZ application. Ratio P2/P1 was calculated.
For sodium currents measurements, the amplitude of the Nav1.8 current was calculated by measuring the difference between the peak inward current on stepping to −10 mV (i.e. peak of the current) and remaining current at the end of the step. The Nav1.8 current was assessed in vehicle control conditions and then at the end of the 2 min BZ application.
MEA data was analysed using MultiChannel Analyzer software. Only those electrodes which responded to KCl were included in the analysis. Raw electrical signal was filtered with a Butterworth high pass filter with a 200 Hz cut off. Action potentials were detected with a threshold of 5 times standard deviation of basal noise for each electrode. Number of action potentials for each applied stimulus time intervals was determined. To compare each electrode, signal ratio of P2/P1 was calculated. Results were normalized to P2/P1 ratio of basal state of each condition and stimulus.
Data is expressed as mean ± standard error of the mean (SEM). Statistical comparison was made using Students’ t test or one-way ANOVA with paired or unpaired measures, depending on the number of groups being compared. One-way ANOVA was followed by Dunnett’s multiple comparison test. Statistical significance was set to p < 0.05. For dose-response curve, experimental data were fitted to the Hill equation with a nonlinear least-square regression algorithm with the GraphPad Prism8 software package. Registered cells or electrodes are represented as points on the graph charts. Exact p-values are shown on the graphs.
Results
Benzydamine inhibits basal neuronal excitability
To explore the analgesic effect of BZ, we tested its capacity to inhibit sensory neurons activity. As shown on Figure 1(a), the instillation during 5 min of 1 µM BZ over DRG neurons in basal state, decreased their action potential (AP) firing elicited by the injection of 50 pA current by 45 ± 10% (p = 0.0001 basal vs 5 min, matched measures, one-way ANOVA, n = 9). Firing frequency decrease was accompanied by a 140 ± 39% increase in rheobase (Figure 1(b), p = 0.0003 basal vs 5 min). Moreover, a significant increase by 25 ± 10% in AHP amplitude was noted after the application of BZ (Figure 1(c), p = 0.014 basal vs 5 min). A dose-response curve reveals an IC50 of 2.4 (95% CI 1.0–6.6) µM (n = 18) for BZ inhibiting neuronal activity (Figure 1(d)). As depicted on Figure 1(e), rheobase increase was also dose-dependent. As reported in Figure 2(a), changes in AP waveform were noticed when using acutely BZ at 30 µM. AP height (Figure 2(b)) and amplitude (Figure 2(c)) were analysed. BZ induces a clear reduction in AP height by 33 ± 7% (p = 0.003, paired Students’ t test, n = 7) and showed a tendency to increase AP duration by 52 ± 32% (p = 0.081). These changes in AP waveform were not observed during the acute application of IS, where both variables were identical to those of basal state (AP height - Figure 2(d), p = 0.153 and AP duration - Figure 2(e), p = 0.161, paired Students’ t test, n = 8). Moreover, RMP of cells instilled with 30 µM BZ significantly moved to more depolarized values (p = 0.012, paired Students’ t test, n = 8), contrary to when the compound was applied at lower concentrations (Figure 2(f), p = 0.687, n = 9).
Figure 1.
Benzydamine decreases basal neuronal activity. Electrophysiological single cell evaluation on neonatal rat DRG neurons. (a) Left–representative register of AP firing tree evoked by 50 pA current injection in basal (black) state and after 5 min instillation of 1 µM BZ (blue). Right - acute application of BZ up to 5 min decreases significantly basal neuronal firing frequency. (b) Left – representative register of injected current needed to elicit one AP in neurons in basal condition and after perfusion of BZ for 5 min. Right - acute application of 1 µM BZ up to 5 min increased significantly rheobase. (c) Left – representative register of AHP amplitude difference between basal and after 5 min acute application of BZ. Right – BZ increases significantly AHP amplitude after 5 min acute instillation. (d) Dose-response curve of BZ was fitted to a Michaelis–Menten Isotherm. The best fit provided an IC50 value of 2.4 (95% CI 1.0–6.6) µM (n = 18). (e) Rheobase increase was also dose-dependent. Each dot in the bar charts accounts for a registered cell. Data in bar charts was normalized to values in basal conditions. Statistical analysis consisted in one-way ANOVA with matched measures and Dunnett’s post hoc test. Comparisons were made with basal condition. Statistical significance was set to p < 0.05, p-value for statistical difference is indicated. All data are expressed as mean ± SEM.
Figure 2.
Benzydamine induces action potential waveform changes at higher concentrations. (a) Representative AP waveform in basal condition and after 30 µM BZ instillation during 5 min over the same cell. AP height and duration (amplitude) span are shown. (b) BZ induces a significant decrease in AP height compared to basal state. (c) BZ shows a tendency to increase AP duration. In some cells AP firing was completely halted. Inflammatory soup application does not induce any change in AP height (d) nor duration (e). (f) 30 µM BZ instillation during 5 min induces a significant depolarization in RMP. This effect was not observed at lower BZ concentrations (g). Each dot in the bar charts accounts for a registered cell. Statistical analysis consisted in paired students’ t test. Statistical significance was set to p < 0.05, p-value for statistical difference is indicated. All data are expressed as mean ± SEM.
Benzydamine reduces neuronal acute and chronic inflammatory sensitization
In order to check if BZ was able to reduce neuronal sensitization induced by inflammatory mediators, we first studied if the selected inflammatory soup (IS) was able to induce an increase in AP firing in an acute application. A clear neuronal excitability was observed after the acute application of IS up to 5 min, traduced in an increased AP firing after current injection (Figure 3(a)). Acute exposure to IS increased firing frequency in 145 ± 58% (p = 0.013 basal vs 5 min, matched measures, one-way ANOVA, n = 9). Simultaneous application of IS + BZ limited neuronal excitability rendering an increment in firing frequency of only 62 ± 32% (p = 0.043 basal vs 5 min, n = 7, Figure 3(b)). Thus, 1 µM BZ tended to limit neuronal firing induced by IS (p = 0.079 IS vs IS + BZ, one-way ANOVA, Figure 3(c)). Later, we studied neuronal response against chronic 24-h maintained inflammation. As shown in Figure 3(d), chronic IS application induced a significant increase in basal neuronal firing of 186 ± 19% (p = 0.041 control vs IS(24 h) - basal, one-way ANOVA). This 24-h maintained inflammation induced a neuronal sensitization plateau (p = 0.366 control vs IS (24 h) - 5’IS) where neuronal activity couldn’t be stimulated much further through IS acute application (p = 0.853 basal vs 5’IS - IS (24)) contrary to IS acute application in control cells (p = 0.006 basal vs 5’IS - control). We then investigated whether chronic 24-h use of 1 µM BZ could protect against acute inflammatory sensitization. As Figure 3(e) shows, chronic application of 1 µM BZ limited neuronal IS-induced excitability (p = 0.007, unpaired Students’ t test, n = 17). Finally, we wanted to know if the use of BZ chronically during the presence of inflammatory mediators could have a similar protective effect against inflammation. Figure 3(f) evidences that the use of BZ has an inhibitory effect of 47 ± 24% over sensory neurons chronically exposed to an inflammatory condition (p = 0.015, unpaired Students’ t test, n = 19).
Figure 3.
Benzydamine reduces neuronal inflammatory sensitization in acute and chronic conditions. (a) Acute instillation of inflammatory soup (IS) up to 5 min induces a significant increase in AP firing. (b) Simultaneous application of IS and 1 µM BZ induces a lower increase in neuronal firing frequency. (c) BZ shows a tendency to decrease neuronal inflammatory sensitization. (d) Chronic application of IS during 24 h induces neuronal sensitization, similarly as an acute application does. Acute application of IS on 24 h already sensitized neurons induces no clear changes in firing frequency. 24-h treatment of cells with 1 µM BZ (e) or IS + BZ (f) shows a protective effect against neuronal activation evoked by a 5 min IS application. Each dot in the bar charts accounts for a registered cell. Statistical analysis consisted in one-way ANOVA with Dunnett’s post hoc test and matched measures for (a) and (b), and unmatched measures for (c) and (d). Data in (e) and (f) was analysed using unpaired student’s t test. Statistical significance was set to p < 0.05, p-value for statistical difference is indicated. All data are expressed as mean ± SEM.
Benzydamine modulatory role over inflammatory pathways
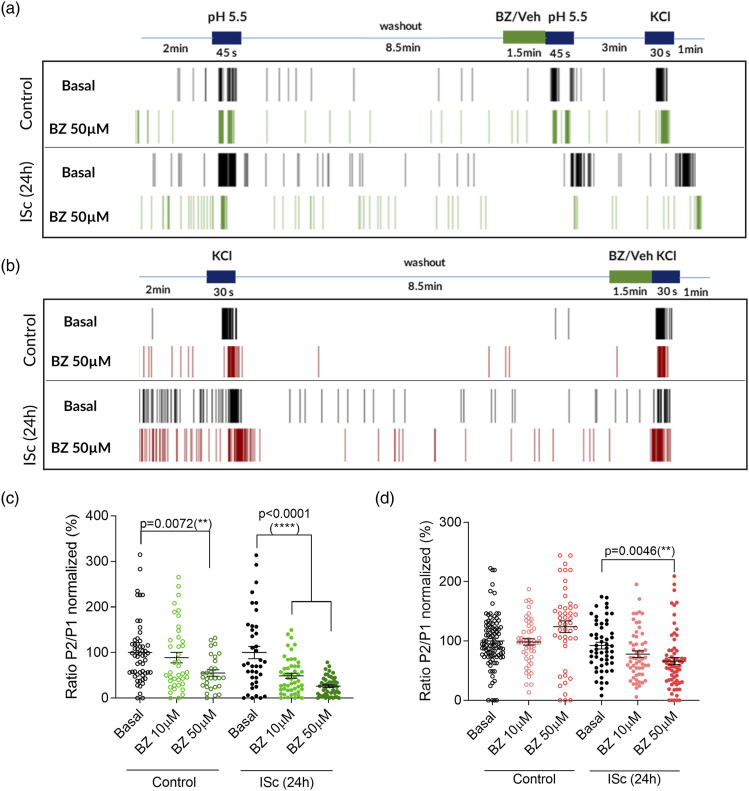
We investigated the role of BZ over neuronal populations subjected to a more severe inflammatory condition increasing the concentration of inflammatory mediators (ISc). Moreover, we wanted to elucidate the implication of BZ in different nociceptive inflammatory pathways, studying the compound’s effect over cholinergic and serotonergic pathways and acidosis. Modulation of elevated extracellular KCl depolarization-induced neuronal activity was also studied as a stimulator of calcium channels activity, signalling and intracellular responses to depolarization. In first place, we checked BZ effect over ACh- and ISc-induced neuronal activity in control and 24-h maintained inflammatory condition on primary culture of neonatal rat DRGs seeded on MEA plates (Figure 4). MEA was used to study the behaviour of BZ over a whole neuronal population with network connections and not only on isolated neuronal cells. This methodology allows the identification of possible synergistic effects between immune and neuronal cells, where these last can be stimulated indirectly by the release of inflammatory mediators produced by glial cells. 1.5 min application of BZ at concentrations ranging from 1 to 50 µM did not induce any change in ACh-elicited neuronal firing neither in control nor inflamed conditions (Figure 4(a) and 4(b)). On the other hand, ISc-induced neuronal activity was inhibited by 55 ± 4% and 72 ± 2% at 25 and 50 µM BZ, respectively, in non-inflamed cells (p < 0.0001 vs basal, one-way ANOVA, n = 60–120). Interestingly, this inhibitory activity was increased in inflamed conditions, being BZ active even at 10 µM (50 ± 6%, p < 0.0001, Figure 4(a) and 4(c)). Secondly, we examined BZ activity upon acidosis-evoked neuronal firing. Again, BZ showed a higher inhibitory effect in inflamed compared to control conditions, being active only at 50 µM in control (45 ± 7% inhibitioin, p = 0.007, one-way ANOVA, n = 35–79) and at 10 and 50 µM after inflammation (51 ± 6% and 74 ± 3% inhibition, respectively, p < 0.0001, Figure 5(a) and 5(c)). Identically, the same higher effect in inflamed conditions was observed for KCl-induced neuronal firing (Figure 5(b) and 5(d)), where 50 µM BZ inhibited AP firing by 28 ± 7% after chronic incubation with inflammatory soup (p = 0.0046 vs basal ISc (24 h)) but showed no effect in control condition.
Figure 4.
Benzydamine reduces neuronal activity upon chronic severe inflammation. Electrophysiological evaluation on neonatal rat DRG neuronal population. (a) Representative raster plot of neuronal AP firing on multielectrode array. Each vertical line denotes a fired AP. Firing was induced by the acute application of stimuli reflected on the protocol. Acetylcholine-(ACh) and inflammatory soup (IS)-evoked firing was monitored simultaneously on the same register protocol. Both stimuli were applied twice on the same register. BZ instillation took place during 1.5 min before the second pulse of each stimulus. Only KCl responsive cells were included. Cells were kept in control or 24-h maintained chronic inflammatory (ISc (24-h)) condition. (b) BZ exerts no effect over ACh-induced firing neither in control nor in inflammatory conditions. (c) In control condition, BZ shows an inhibitory activity upon IS-evoked neuronal firing when applied at 25 and 50 µM, respectively. This inhibition was intensified in chronic inflammatory condition, evidencing an effect at lower concentrations of 10 µM. Data are shown as ratio P2/P1 normalized to mean of basal condition. Each circle represents a responsive electrode. Number of registered electrodes (n) = 60–120. Statistical analysis consisted in one-way ANOVA with Dunnett’s multiple comparison test. Comparisons were made with basal condition. Statistical significance was set to p < 0.05, p-value for statistical difference is indicated. All data are expressed as mean ± SEM.
Figure 5.
Benzydamine reduces acidic pH- and KCl-induced neuronal firing in control condition and chronic severe inflammation. Representative multielectrode array raster plots of AP firing induced by acidic pH (a) and KCl (b) in control and 24-h chronic inflammatory condition. Each vertical line evidences an AP. Both stimuli were applied twice in each protocol. BZ was perfused during 1.5 min before the second pulse of each stimulus. Register protocols ended with a KCl pulse, except in KCl protocol. Only KCl responsive cells were included in the analysis. (c) BZ inhibits acidic pH-induced neuronal firing at 50 µM in control condition but shows a higher inhibitory effect in inflammatory condition, being active even at 10 µM. Number of registered electrodes (n) = 29–56. (d) In control condition, BZ has no effect over KCl-induced neuronal activity, but in inflammatory condition it inhibits neuronal firing, n = 46–96. Data are shown as ratio P2/P1 normalized to mean of basal condition. Each circle represents a responsive electrode. Statistical analysis consisted in one-way ANOVA with Dunnett’s multiple comparison test. Comparisons were made with basal condition. Statistical significance was set to p < 0.05, p-value for statistical difference is indicated. All data are expressed as mean ± SEM.
Benzydamine effect over specific proalgesic ion channels
Finally, given the evidence of possible BZ effect over proalgesic channels, its activity was tested over Kv7.2/7.3, Nav1.8, TRPA1 and TRPV1. The effect of the compound over these targets was studied on cell lines transiently expressing them. BZ effect was explored over both TRP channels as agonist and antagonist. Nevertheless, as shown on Table 1, BZ demonstrated only an inhibitory dose-dependent effect on Nav1.8 sodium currents, exerting no effect over Kv and TRP channels.
Table 1.
Table summarizing the effect of benzydamine over Kv7.2/Kv7.3, Nav1.8, TRPA1 and TRPV1 channels.
| Targeted ion channel | Compound | Concentration (µM) | Percentage inhibition/activation ± SEM |
|---|---|---|---|
| Antagonist Kv7.2/Kv7.3 | Benzydamine | 3.125 | −1.3 ± 1.4 |
| 6.25 | −3.1 ± 0.8 | ||
| 12.5 | 3.3 ± 1.6 | ||
| 25 | 0.1 ± 1.1 | ||
| 50 | 1.0 ± 0.8 | ||
| Linopiridine | 1 | 46.0 ± 2.6**** | |
| 300 | 100.0 ± 0.2**** | ||
| Antagonist Nav1.8 | Benzydamine | 3.125 | 41.3 ± 0.7**** |
| 6.25 | 59.0 ± 1.0**** | ||
| 12.5 | 59.0 ± 0.9**** | ||
| 25 | 70.9 ± 0.9**** | ||
| 50 | 83.5 ± 0.8**** | ||
| Tetracaine | 1 | 42.5 ± 1.3**** | |
| 3 | 49.0 ± 1.3**** | ||
| 10 | 54.9 ± 1.2**** | ||
| 30 | 67.7 ± 1.1**** | ||
| 100 | 89.1 ± 0.6**** | ||
| Agonist TRPA1 | Benzydamine | 4 | 0.0 ± 0.0 |
| 8 | −0.1 ± 0.0 | ||
| 16 | −0.1 ± 0.0 | ||
| 33 | 0.0 ± 0.0 | ||
| 66 | 0.0 ± 0.0 | ||
| AITC | 5 | 0.6 ± 0.4 | |
| 15 | 63.1 ± 0.7**** | ||
| 40 | 82.9 ± 3.3**** | ||
| 125 | 88.3 ± 1.6**** | ||
| 375 | 101.7 ± 3.9**** | ||
| Antagonist TRPA1 | Benzydamine | 3.125 | 6.9 ± 1.4 |
| 6.25 | −6.0 ± 2.6 | ||
| 12.5 | −1.4 ± 3.2 | ||
| 25 | −1.6 ± 2.9 | ||
| 50 | 0.6 ± 2.6 | ||
| Ruthenium red | 0.01 | 7.1 ± 2.8 | |
| 0.04 | 26.8 ± 7.0*** | ||
| 0.12 | 84.7 ± 8.5**** | ||
| 0.40 | 99.5 ± 0.4**** | ||
| 1.00 | 100.0 ± 0.0**** | ||
| Agonist TRPV1 | Benzydamine | 4 | 3.7 ± 1.1 |
| 8 | 1.7 ± 0.6 | ||
| 16 | 0.8 ± 0.6 | ||
| 33 | −0.5 ± 0.3 | ||
| 66 | 3.6 ± 0.8 | ||
| Capsaicin | 1 | 3.3 ± 0.8 | |
| 5 | 10.1 ± 1.8 | ||
| 15 | 43.5 ± 3.7*** | ||
| 50 | 72.3 ± 1.2**** | ||
| 150 | 84.6 ± 1.9**** | ||
| 440 | 101.0 ± 1.5**** | ||
| Antagonist TRPV1 | Benzydamine | 3.125 | −18.7 ± 5.8 |
| 6.25 | −18.8 ± 2.6 | ||
| 12.5 | −13.7 ± 4.2 | ||
| 25 | −12.0 ± 4.6 | ||
| 50 | −7.7 ± 3.7 | ||
| Capsazepine | 0.1 | −0.1 ± 4.0 | |
| 0.4 | 21.3 ± 3.4*** | ||
| 1 | 83.3 ± 1.2**** | ||
| 3 | 94.0 ± 0.6**** | ||
| 10 | 93.1 ± 0.9**** | ||
| 30 | 96.1 ± 0.6**** |
Tested benzydamine concentration is shown for each assay. Significant effects are shown in bold.
Statistical analysis consisted in unpaired one-way ANOVA with Dunnett’s post hoc test. Statistical significance was set to p < 0.05; ***p < 0.001; ****p < 0.0001.
Discussion
In this study the effect of benzydamine was characterized on sensory neurons from rat DRGs aiming to elucidate its analgesic mechanism of action in inflamed condition and its capacity to inhibit peripheral neural sensitization. First, BZ behaviour was studied over basal neural activity stimulated by current injection. Changes in the action potential wave, firing frequency, rheobase, resting potential and other biophysical parameters can help in sorting out the type of ion channel affected by a compound. For instance, 1 µM BZ showed to decrease neural firing frequency in a time and dose-dependent manner. This firing frequency decrease was also supported by a significant increase in rheobase and most importantly an increase in afterhyperpolarization amplitude. No changes in resting membrane potential were observed during and after BZ application. High concentration of BZ (30 µM) revealed other effects not seen at lower doses. Indeed, a substantially reduced overshoot and a slight increase in the action potential duration were noted. These were accompanied by a significant increase in resting membrane potential to more depolarized values at this tested concentration.
As nociceptors express receptors for immune cell-derived mediators, when activated they mediate signalling cascades that change channels gating properties and lead to increased neuronal firing. During inflammation, nociceptors threshold for action potential firing lowers, producing hyperalgesia. In mucosal tissues, it has been shown that there is a tight association between nociceptor terminals and mast cells. Mast cells release diverse cytokines and 5-HT, histamine and nerve growth factor (NGF), inducing this pain sensitization.22,23 PGE2 released by macrophages and neutrophiles also produces the same effect. 24 Extracellular ATP identically modulates pain and inflammation activating directly and indirectly both, nociceptors and immune cells. 25 Acute application of inflammatory soup containing 5-HT, histamine, NGF, PGE2 and ATP showed to increase neuronal firing frequency and sensitized cells when used in a 24-h maintained treatment. The simultaneous acute application of 1 µM BZ and IS, limited slightly this increase in firing frequency, although not as significantly as it did when the compound was incubated during 24 h in presence of inflammatory soup. A possible explanation of this increased inhibitory effect of BZ in chronic conditions could be its accumulation in inflamed tissues, as previously demonstrated. 1
The direct effect of BZ over diverse chemical electrogenic stimuli on neuronal populations was also studied. First of all, a direct inhibition of the synergistic effect of purinergic, histaminergic and serotonergic receptors was observed, when cells were stimulated with concentrated inflammatory soup. This inhibition was increased in 24-h maintained conditions, evidencing not only a direct effect on some of these receptors, but also on sensitized signalling pathways and ion channels by the used inflammatory mediators. For instance, PGE2 sensitizes nociceptors acting on proximal channels and through PKA and PKC signalling. 24 The hyperalgesic effect of PGE2 can be explained given that it enhances TTX resistant sodium currents from Nav1.8 and Nav1.9 and TRPV1 currents. 26 Histamine also increases expression of Nav1.8 channel, 27 TRPA1 28 and TRPV1. 29 Serotonin on the other hand, through PKC activation, increases ASICs neuronal expression. 30 Finally, neuroinflammation produces an endogenous release of ATP, neuropeptides and cytokines, enhancing P2X3 activity. 25 Thus, all inflammatory mediators included in the cocktail produce neural sensitization via proalgesic ion channels. BZ increased inhibition in inflamed conditions could be explained by an effect displayed via proalgesic channels, along with those over inflammatory mediators’ receptors.
It is known that tissue acidosis in inflammation is a common feature.21,31,32 Therefore, looking into a better characterization of BZ pharmacological profile, effect over acidity-induced neuronal activity was also analysed. Again, its effect in inflamed conditions was more significant, supporting BZ inhibition of sensitized acid-sensing ion channels, such as TRPV1, ASICs, P2X 33 or TASK (K2P). 34 On the other hand, BZ was able to reduce neuronal activity upon elevated extracellular KCl-induced depolarization-coupled Ca2+ influx in inflamed conditions, 35 suggesting possible marginal effects over voltage-sensitive Ca2+ channels. 36
This work evidenced BZ implication in modulating probably ion channels which control membrane depolarization (Nav, Kv) and thus action potential initiation and firing or signal transducers (TRP). Thus, testing BZ effect over Kv7.2, Kv7.3, Nav1.8, TRPV1 and TRPA1 channels was considered. The only channel which was affected by BZ was Nav1.8. Its current was inhibited by BZ in a dose-dependent manner. Surprisingly, BZ showed no effect over Kv7.2 and Kv7.3 nor TRPA1 and TRPV1. As the TRP family contains main proalgesic channels, some of which are the highly co-expressed TRPV1 and TRPA1, and as BZ displayed an inhibitory activity over acidity-evoked neuronal firing, we expected that the compound could mediate this inhibitory function through them. Nevertheless, as no direct modulation of TRPV1 nor TRPA1 was observed, acid-induced neuronal firing inhibition through ASICs channels appear as potential BZ target. Moreover, although it was demonstrated that BZ affects sodium currents through Nav1.8, the possibility of modulating other Nav channels such as Nav1.7 or Nav1.9 cannot be ruled out.
Overall, in this study, benzydamine reveals its analgesic effect by directly modulating neuronal excitability and neuronal inflammatory sensitization.
In this respect, novel insights into its peculiar molecular mechanism have been shown, although further investigations are still required to fully understand this molecule. With regards to previous data 6 and with the support of results obtained in the present work, the multitarget effect of benzydamine and its anti-inflammatory/analgesic properties was confirmed and, in line with clinical studies, benzydamine results highlight its pharmacological activities.
Acknowledgements
On behalf of the project, authors thank to Claudio Milanese and Isabella Coletta for their contribution to the research study.
Footnotes
Author contributions: MNK designed and conducted electrophysiological MEA and patch clamp assays, analysed the data, wrote and edited the first and subsequent drafts of the manuscript. AE, MV, LP, GM, LR, SZ, AFM and ID revised and edited the manuscript. MV and ID supervised experiments. ID, MV, LP, GM, LR and SZ conceptualized the project.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Angelini Pharma S.p.A. founded this research. MV, LR and SZ are employees of Angelini Pharma. AFM is founder and shareholder of AntalGenics SL. ID, MNK and AE are employees of Antalgenics. GM and LP are external scientific consultants.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Angelini Pharma SpA.
ORCID iD
Magdalena Nikolaeva-Koleva https://orcid.org/0000-0002-2617-3108
References
- 1.Turnbull R. Benzydamine hydrochloride (tantum) in the management of oral inflammatory conditions. J Can Dent Assoc 1995; 61: 127–134. [PubMed] [Google Scholar]
- 2.Agarwal A, Nath SS, Goswami D, Gupta D, Dhiraaj S, Singh P. An evaluation of the efficacy of aspirin and benzydamine hydrochloride gargle for attenuating postoperative sore throat: a prospective, randomized, single-blind study. Anesth Analg 2006; 103: 1001–1003. [DOI] [PubMed] [Google Scholar]
- 3.Kin-Fong Cheng K, Ka Tsui Yuen J. A pilot study of chlorhexidine and benzydamine oral rinses for the prevention and treatment of irradiation mucositis in patients with head and neck cancer. Cancer Nurs 2006; 29: 423–430. [DOI] [PubMed] [Google Scholar]
- 4.Fanaki N, el-Nakeeb M. Antimicrobial activity of benzydamine, a non-steroid anti-inflammatory agent. J Chemother 1992; 4: 347–352. [DOI] [PubMed] [Google Scholar]
- 5.Cioli V, Corradino C, Scorza Barcellona P. Review of pharmacological data on benzydamine. Int J Tissue React 1985; 7: 205–213. [PubMed] [Google Scholar]
- 6.Mathivanan S, de la Torre-Martinez R, Wolf C, Mangano G, Polenzani L, Milanese C, Ferrer-Montiel A. Effect of econazole and benzydamine on sensory neurons in culture. J Physiol Pharmacol 2016; 67: 851–858. [PubMed] [Google Scholar]
- 7.Sironi M, Pozzi P, Polentarutti N, Benigni F, Coletta I, Guglielmotti A, Milanese C, Ghezzi P, Vecchi A, Pinza M, Mantovani A. Inhibition of inflammatory cytokine production and protection against endotoxin toxicity by benzydamine. Cytokine 1996; 8: 710–716. [DOI] [PubMed] [Google Scholar]
- 8.Sironi M, Massimiliano L, Transidico P, Pinza M, Sozzani S, Mantovani A, Vecchi A. Differential effect of benzydamine on pro- versus anti-inflammatory cytokine production: lack of inhibition of interleukin-10 and interleukin-1 receptor antagonist. Int J Clin Lab Res 2000; 30: 17–19. [DOI] [PubMed] [Google Scholar]
- 9.Riboldi E, Frascaroli G, Transidico P, Luini W, Bernasconi S, Mancini F, Guglielmotti A, Milanese C, Pinza M, Sozzani S, Mantovani A. Benzydamine inhibits monocyte migration and MAPK activation induced by chemotactic agonists. Br J Pharmacol 2003; 140: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertsch S. New aspects on the clinical use of benzydamine. Arzneimittelforschung 1987; 37: 632–634. [PubMed] [Google Scholar]
- 11.Passali D, Arezzo M, De Rose A, De Simone G, Forte G, Jablko-Musial M, Mösges R. Benzydamine hydrochloride for the treatment of sore throat and irritative/inflammatory conditions of the oropharynx: a cross-national survey among pharmacists and general practitioners. BMC Prim Care 2022; 23: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller-Peddinghaus R. New pharmacologic and biochemical findings on the mechanism of action of the non-steroidal antiphlogistic, benzydamine. A synopsis. Arzneimittelforschung 1987; 37: 635–645. [PubMed] [Google Scholar]
- 13.Wood J, Boorman J, Okuse K, Baker M. Voltage-gated sodium channels and pain pathways. J Neurobiol 2004; 61: 55–71. [DOI] [PubMed] [Google Scholar]
- 14.Dib-Hajj S, Waxman S. Translational pain research: lessons from genetics and genomics. Sci Transl Med 2014; 6: 249sr4. [DOI] [PubMed] [Google Scholar]
- 15.von Hehn C, Baron R, Woolf C. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012; 73: 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinho-Ribeiro F, Verri W, Chiu I. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 2017; 38: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devesa I, Ferrándiz-Huertas C, Mathivanan s, Wolf C, Luján R, Changeux J, Ferrer-Montiel A. αCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc Natl Acad Sci U S A 2014; 111: 18345–18350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolaeva-Koleva M, Butron L, González-Rodríguez S, Devesa I, Valente P, Serafini M, Genazzani A, Pirali T, Ballester G, Fernández-Carvajal A, Ferrer-Montiel A. A capsaicinoid-based soft drug, AG1529, for attenuating TRPV1-mediated histaminergic and inflammatory sensory neuron excitability. Sci Rep 2021; 11: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathivanan S, Devesa I, Changeux J, Ferrer-Montiel A. Bradykinin iinduces TRPV1 exocytotic recruitment in peptidergic nociceptors. Front Pharmacol 2016; 7: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidd B, Urban L. Mechanisms of inflammatory pain. Br J Anaesth 2001; 87: 3–11. [DOI] [PubMed] [Google Scholar]
- 21.Steen K, Steen A, Reeh P. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci 1995; 15: 3982–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aich A, Afrin L, Gupta K. Mast cell-mediated mechanisms of nociception. Int J Mol Sci 2015; 16: 29069–29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolf C, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 1997; 121: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira S. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol 1972; 240: 200–203. [DOI] [PubMed] [Google Scholar]
- 25.Fabbretti E. ATP P2X3 receptors and neuronal sensitization. Front Cell Neurosci 2013; 7: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meves H. The action of prostaglandins on ion channels. Curr Neuropharmacol 2006; 4: 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue J, Wang R, Yu J, Tang Y, Hou W, Lou G, Zhang S, Chen Z. Histamine upregulates Nav1.8 expression in primary afferent neurons via H2 receptors: involvement in neuropathic pain. CNS Neurosci Ther 2014; 20: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perin-Martins A, Teixeira J, Tambeli C, Parada C, Fischer L. Mechanisms underlying transient receptor potential ankyrin 1 (TRPA1)-mediated hyperalgesia and edema. J Peripher Nerv Syst 2013; 18: 62–74. [DOI] [PubMed] [Google Scholar]
- 29.Massaad C, Safieh-Garabedian B, Poole S, Atweh S, Jabbur S, Saadé N. Involvement of substance P, CGRP and histamine in the hyperalgesia and cytokine upregulation induced by intraplantar injection of capsaicin in rats. J Neuroimmunol 2004; 153: 171–182. [DOI] [PubMed] [Google Scholar]
- 30.Qiu F, Qiu CY, Liu Y, Wu D, Li J, Hu W. Potentiation of acid-sensing ion channel activity by the activation of 5-HT- receptors in rat dorsal root ganglion neurons. Neuropharmacology 2012; 63: 494–500. [DOI] [PubMed] [Google Scholar]
- 31.Gu Q, Lee L. Acid-sensing ion channels and pain. Pharmaceuticals 2010; 3: 1411–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs). Curr Drug Targets - Inflamm Allergy 2004; 3: 71–79. [DOI] [PubMed] [Google Scholar]
- 33.Stoop R, Surprenant A, North R. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J Neurophysiol 1997; 78: 1837–1840. [DOI] [PubMed] [Google Scholar]
- 34.Morton M, Abohamed A, Sivaprasadarao A, Hunter M. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci U S A 2005; 102: 16102–16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rienecker K, Poston R, Saha R. Merits and limitations of studying neuronal depolarization-dependent processes using elevated external potassium. ASN Neuro 2020; 12: 1759091420974807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roca-Lapirot O, Radwani H, Aby F, Nagy F, Landry M, Fossat P. Calcium signalling through L-type calcium channels: role in pathophysiology of spinal nociceptive transmission. Br J Pharmacol 2018; 175: 2362–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]