Summary
Background
Single low doses of primaquine, when added to artemisinin-based combination therapy, might prevent transmission of Plasmodium falciparum malaria to mosquitoes. We aimed to establish the activity and safety of four low doses of primaquine combined with dihydroartemisinin–piperaquine in male patients in Mali.
Methods
In this phase 2, single-blind, dose-ranging, adaptive randomised trial, we enrolled boys and men with uncomplicated P falciparum malaria at the Malaria Research and Training Centre (MRTC) field site in Ouelessebougou, Mali. All participants were confirmed positive carriers of gametocytes through microscopy and had normal function of glucose-6-phosphate dehydrogenase (G6PD) on colorimetric quantification. In the first phase, participants were randomly assigned (1:1:1) to one of three primaquine doses: 0 mg/kg (control), 0·125 mg/kg, and 0·5 mg/kg. Randomisation was done with a computer-generated randomisation list (in block sizes of six) and concealed with sealed, opaque envelopes. In the second phase, different participants were sequentially assigned (1:1) to 0·25 mg/kg primaquine or 0·0625 mg/kg primaquine. Primaquine tablets were dissolved into a solution and administered orally in a single dose. Participants were also given a 3 day course of dihydroartemisinin–piperaquine, administered by weight (320 mg dihydroartemisinin and 40 mg piperaquine per tablet). Outcome assessors were masked to treatment allocation, but participants were permitted to find out group assignment. Infectivity was assessed through membranefeeding assays, which were optimised through the beginning part of phase one. The primary efficacy endpoint was the mean within-person percentage change in mosquito infectivity 2 days after primaquine treatment in participants who completed the study after optimisation of the infectivity assay, had both a pre-treatment infectivity measurement and at least one follow-up infectivity measurement, and who were given the correct primaquine dose. The safety endpoint was the mean within-person change in haemoglobin concentration during 28 days of study follow-up in participants with at least one follow-up visit. This study is registered with ClinicalTrials.gov, number NCT01743820.
Findings
Between Jan 2, 2013, and Nov 27, 2014, we enrolled 81 participants. In the primary analysis sample (n=71), participants in the 0·25 mg/kg primaquine dose group (n=15) and 0·5 mg/kg primaquine dose group (n=14) had significantly lower mean within-person reductions in infectivity at day 2—92·6% (95% CI 78·3–100; p=0·0014) for the 0·25 mg/kg group; and 75·0% (45·7–100; p=0·014) for the 0·5 mg/kg primaquine group—compared with those in the control group (n=14; 11·3% [−27·4 to 50·0]). Reductions were not significantly different from control for participants assigned to the 0·0625 mg/kg dose group (n=16; 41·9% [1·4–82·5]; p=0·16) and the 0·125 mg/kg dose group (n=12; 54·9% [13·4–96·3]; p=0·096). No clinically meaningful or statistically significant drops in haemoglobin were recorded in any individual in the haemoglobin analysis (n=70) during follow-up. No serious adverse events were reported and adverse events did not differ between treatment groups.
Interpretation
A single dose of 0·25 mg/kg primaquine, given alongside dihydroartemisinin–piperaquine, was safe and efficacious for the prevention of P falciparum malaria transmission in boys and men who are not deficient in G6PD. Future studies should assess the safety of single-dose primaquine in G6PD-deficient individuals to define the therapeutic range of primaquine to enable the safe roll-out of community interventions with primaquine.
Funding
Bill & Melinda Gates Foundation.
Introduction
In the past decade, great progress has been made in the control of malaria through widespread access to and use of artemisinin-based combination therapy and insecticide-treated bednets.1 Although malaria transmission has been substantially reduced,2 elimination is unlikely to be achieved with further scale-up of conventional methods in most settings in Africa.3 New tools and strategies to reduce malaria transmission are necessary to maintain the gains of malaria control and push towards elimination.4
In settings of low transmission and drug resistance, WHO5 recommends single doses of 0·25 mg/kg of primaquine as an addition to standard artemisinin-based combination therapy to prevent onward transmission of Plasmodium falciparum infections to anopheline mosquitoes. Primaquine, an 8-aminoquinoline, has a unique drug action against mature P falciparum gametocytes, the sexual stages of the parasite responsible for onward transmission.5 However, the widespread use of primaquine is hindered by safety concerns of dose-dependent haemolysis in people who are deficient in glucose-6-phosphate dehydrogenase (G6PD). G6PD deficiency is the most common inherited enzyme deficiency globally and is estimated at a community prevalence of 10–15% in much of malaria-endemic Africa, rising to much higher levels in certain specific communities.6
Although recommendations are available, no formal dose-finding experiments have been done with a standardised infectivity assay to assess single doses of primaquine alongside artemisinin-based combination therapy. Recommendations are based on a review of historical infectivity trials7 from 158 individual gametocytaemic individuals treated with plasmoquine or primaquine in different studies with different vectors and different drug exposures. WHO’s recommendation was not supported by the statement “Strong recommendation, high quality evidence” typical of recommendations for antimalarial use, and national malaria control programme managers expressed reluctance to implement this policy until modern data were available.
There have been no formal assessments of the efficacy of single low doses of primaquine in reducing mosquito infection rates when added to artemisinin-based therapy. The first formal primaquine dose-finding study concluded that low densities of P falciparum gametocytes detected by molecular methods persist after single low doses of primaquine. That study did not test the new WHO recommended low dose, but concluded that 0·10 mg/kg was inferior to the standard 0·75 mg/kg primaquine dose when given in combination with artemether–lumefantrine.8 Importantly, to our knowledge, the infectivity of low-density gametocytes after primaquine in combination with an artemisinin-based combination therapy has not been established. Molecular gametocyte detection methods could lead to false conclusions about residual transmission potential if these tools detect non-transmissible gametocytes.9 Determination of the lowest efficacious dose of primaquine is of great importance to reduce the risk of dose-dependent haemolysis.10 In this study we aimed to establish activity for different doses of primaquine through repeated assessments of human infectivity to mosquitoes alongside sensitive molecular gametocyte detection and quantification.
Methods
Study design and participants
We did a single-blind, dose-ranging, adaptive randomised, phase 2 trial of single low doses of primaquine in boys and men infected with P falciparum malaria. The protocol is available online. This study was done in two phases (details in appendix p 1). In the first phase, we optimised the membrane feeding assay and assessed three primaquine dose groups. The data safety monitoring committee and study team then reviewed the infectivity and safety results from the first phase and selected two additional primaquine dose groups to be tested sequentially in the second phase. We recruited participants from the town of Oulessebougou and its surrounding villages in Mali, at the Clinical Research Centre of the Malaria Research and Training Centre (MRTC) of the University of Bamako, Mali. Malaria transmission is high and seasonal in this setting—an incidence rate of about two episodes per year in children younger than age 5 years.11
Inclusion criteria included male sex (to reduce the risk of haemolysis through incorrect classification of G6PD status, which more commonly occurs in heterozygote female individuals), age of 5–50 years, having at least two P falciparum gametocytes per 500 white blood cells on thick film microscopy (corresponding to ≥32 gametocytes per μL assuming 8000 white blood cells per μL of blood), a haemoglobin concentration of 80 g/L or higher, and a normal G6PD test result via colorimetric quantification (OSMMR2000-D G-6-PD, R&D Diagnostics, Papagos, Greece). We excluded participants who had taken malaria drugs within 7 days of screening, had known allergies to the study drugs, had serious or chronic illness, or had diagnosed cardiac arrhythmias. All participants older than 18 years provided written informed consent before screening and enrolment. We required parental consent for participants younger than 18 years, with children aged 12–17 years also providing assent for inclusion.
Participants were compensated for time and travel. The study was approved by the Ethics Committee of the Malaria Research and Training Centre Faculty of Medicine, Pharmacy and Dentistry of the University of Science, Techniques and Technologies of Bamako, and the Committee on Human Research at the University of California, San Francisco (UCSF).
Randomisation and masking
Treatment allocations were randomly generated and assigned to a list of prespecified participant identifier (ID) numbers by a UCSF investigator using a computer program (Excel). This investigator created sealed, opaque envelopes with participant ID number on the outside and treatment allocation on the inside of the envelope. Enrolled participants were sequentially assigned a participant ID number. The site pharmacist in Mali opened the sealed envelope corresponding to the participant ID number and provided the allocated treatment regimen. In the first phase, participants were randomly assigned (1:1:1) into three primaquine dose groups—0 mg/kg (control), 0·125 mg/kg, and 0·5 mg/kg—in block sizes of six. In the second phase, participants were sequentially assigned (1:1) and enrolled first into 0·25 mg/kg primaquine group and then into the 0·0625 mg/kg primaquine group when full. Block size was concealed from investigators.
Study investigators assessing outcomes were masked to treatment group assignment. Participants were allowed to ask the study physician which treatment they received and so were not masked to allocation. Additionally, participants could have recognised the presence of primaquine through the bitter taste of the active pharmaceutical ingredient.
Procedures
After collection of day 0 samples, the study pharmacist provided primaquine (Sanofi, Laval, QC, Canada) through crushing 15 mg primaquine tablets and dissolving them in 15 mL of drinking water. Primaquine was administered to the nearest mL, as described elsewhere.8 Each participant received an oral dose of primaquine according to their group assignment after a fatty snack (biscuits) to prevent gastrointestinal side-effects. All participants were given the first tablet of a 3 day course of dihydroartemisinin–piperaquine (Eurartesim, Sigma-Tau, Italy). Dihydroartemisinin–piperaquine (320 mg and 40 mg per tablet) was given as per manufacturer’s guidelines once a day for 3 days with the following considerations for weight: 13 kg to <24 kg one tablet; 24 kg to <36 kg two tablets; 36 kg to <75 kg three tablets; and 75–100 kg four tablets. All treatment doses were directly observed by the study pharmacist and his assistant.
Participants attended follow-up appointments at the clinic at 2 h, 6 h, 12 h, and 1 day, 2 days, 3 days, 7 days, 14 days, and 28 days after the start of treatment. Mosquito infectivity was measured at day 0 (pre-treatment), day 1, day 2, and day 7. Venous blood was collected in heparin tubes kept at 37°C and placed in a membrane feeding system within 1 min of being collected. Three cups of 30 Anopheles gambiae mosquitoes each (90 mosquitoes in total except at day 0 when 180 mosquitoes were used) were fed the participant’s blood for 15–20 min with methods previously described.12 Blood-fed mosquitoes were then transported the next day to the insectary in Bamako, Mali, where they were kept until dissection on day 7 post-feeding and examination for oocysts in 1% mercurochrome. The presence of oocysts was confirmed by a second microscopist. Mosquito infectivity from the person to the mosquito was defined as the proportion of dissected mosquitoes with oocysts. Additionally, participants were classified as infectious (yes vs no) if at least one dissected mosquito had at least one oocyst.
Haemoglobin was measured before treatment (day 0) and at each follow-up visit (days 1, 2, 3, 7, 14, and 28) using HemoCue (AB Leo Diagnostics, Helsingborg, Sweden). Adverse events were assessed actively during follow-up visits (days 1, 2, 3, 7, 14, and 28) and passively through the availability of study clinicians 24 h per day, 7 days per week, without charge during the follow-up period, including free transportation if needed. Adverse events were graded by severity (mild, grade 1; moderate, grade 2; or severe, grade 3, in intensity, and whether they were considered serious by the treating physician) and categorised by their relationship with the study drugs (unrelated or probably not, possibly, probably, or definitely related). Additionally, we recorded the duration of adverse event, any actions taken, and outcome of adverse events.
Gametocyte density measurements were done on day 0 (pre-treatment), 2 h, 6 h, and 12 h after treatment, and on days 1, 2, 3, 7, 14, and 28. Blood slides stained with Giemsa were double read by expert research micro-scopists over 500 fields for quantification of gametocytes and asexual stages. 100 μL of whole blood was collected in L6 buffer (Severn Biotech, Kidderminster, UK), total nucleic acid was extracted using a MagNAPure LC automatic extractor (Total Nucleic Acid Isolation Kit–High Performance; Roche Applied Science, Indianapolis, IN, USA) followed by RQ1 DNaseI digest (Promega, Sunnyvale, CA, USA), cDNA synthesis (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Foster City, CA, USA), and molecular quantification of gametocytes using Pfs25 mRNA quantitative real-time (qRT) PCR, as described elsewhere.13 Gametocyte concentration was estimated using a reference trendline of cultured mature stage V gametocytes (NF54 strain14) that was serially diluted using tenfold dilutions from 106 to 10 gametocytes per mL.
Genomic DNA available from total nucleic acids extracted for gametocyte quantification were used for genotyping cytochrome P450 2D6 (CYP2D6). Samples from all participants were tested, and participants with a sufficient amount and quality of DNA were genotyped for CYP2D6 *2, *3, *4, *6, *7, *8, *9, *10, *11, *15, *17, *18, *19, *20, *29, *40, and *41 alleles using Openarray technology on a QuantStudio 12K Flex RT PCR system (Life Technologies, Carlsbad, CA, USA). The CYP2D6 copy number was determined with a TaqMan copy number assay targeting intron 2 on the QuantStudio 12K Flex system, and CYP2D6 metaboliser status was inferred from the genotypes using both classic and activity score methods.15,16
Outcomes
The primary efficacy outcome was the mean within-person percentage change in mosquito infectivity per group, 2 days after primaquine administration. Prespecified secondary efficacy endpoints were the presence of oocysts in mosquito at other timepoints (day 1 and day 7), the proportion of individuals infecting at least one mosquito (at all timepoints), gametocyte prevalence, and density determined microscopically and by molecular methods, and asexual parasite prevalence and density. Safety outcomes were adverse events and haemolysis, for which we calculated the average within-person change in haemoglobin, the proportion of participants with a 25% or greater drop in haemoglobin, and the proportion of participants with a haemoglobin level of 80 g/L or lower during follow-up. As a prespecified secondary analysis, we aimed to explore the genetics of cytochrome P450, the enzyme responsible for the activation of primaquine to its active metabolite for the cure of vivax malaria.
Statistical analysis
Sample size calculations assumed that the proportion of people that would infect at least one mosquito was 0·80, and that on average the proportion of mosquitoes that would become infected was 0·25. We originally calculated that we would need 30 participants per group to have 80% power to detect a 90% reduction in the number of mosquitoes with oocysts after treatment with a 0·05 significance. However after assay optimisation we revisited the sample size calculation and found that, after allowing for 10% loss to follow-up, we needed 15 individuals per group.
For the primary outcome we calculated the average within-person percent change in infectivity and accompanying 95% CI for each treatment group. To make pairwise comparisons of the change in infectivity and change in haemoglobin concentration after treatment (control vs each treatment group) we used the one-sided non-parametric Wilcoxon rank-sum test. Additionally, we used the one-sided exact probability test to compare the proportion of infectious individuals in each treatment group to the control group at each visit, and the one-sided non-parametric Wilcoxon rank-sum test to compare the median proportion of mosquitoes infected in each treatment group to the median proportion of mosquitoes infected in the control group at each visit.
qRT-PCR gametocyte density and prevalence at individual treatment days were compared between individual primaquine groups and the control group using linear (log10 density) and logistic (prevalence) regression models with treatment group and baseline qRT-PCR gametocyte density as independent variables. qRT-PCR gametocyte density was used to generate the area under the curve (AUC) of gametocyte density versus time, which was compared between the treatment and control groups as described previously17 with log10 transformed AUC as dependent variable in a linear regression model with treatment group and baseline qRT-PCR gametocyte density as independent variables. The association between the proportion of infected mosquitoes and qRT-PCR gametocyte density was determined by Spearman correlation coefficient before and after treatment.
Analyses of gametocytes were done among all enrolled participants, excluding those who violated the protocol. Analysis of the change in infectivity (primary endpoint) included only patients who completed the study after optimisation of the infectivity assay, who had a pre-treatment infectivity measurement and at least one follow-up infectivity measurement, and who were given the correct primaquine dose (primary infectivity analysis sample). In addition, we did a change in infectivity analysis including only participants who infected at least one mosquito pre-treatment. Analyses of adverse events and the change in haemoglobin (safely endpoint) after treatment were restricted to participants with a pre-treatment measurement and at least one follow-up visit (total enrolled sample with a follow-up visit). Analyses were done with Stata version 12.1.
A data safety monitoring committee oversaw the study, and the study was monitored by an external clinical trials monitoring group, Agence Africaine de Recherche en Santé Humaine. Dakar, Senegal. The study was registered with ClinicalTrials.gov, number NCT01743820.
Role of the funding source
The funder of the study had no role in the data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the publication.
Results
We screened 1485 individuals, enrolling 81 participants into the study between Jan 19, 2013, and Dec 20, 2014 (figure 1, table 1). Of those screened, most were not eligible due to the absence of gametocytes assessed by microscopy (n=1159). 77 participants (95%) were retained through the day 28 follow-up visit; no participants were lost to follow-up.
Figure 1: Trial profile.
WBC=white blood cells. G6PD=glucose-6-phosphate dehydrogenase. AE=adverse event.
Table 1:
Baseline characteristics of participants overall and by primaquine dose group
| Overall (n=81) | Control group (0 mg/kg; n=16) |
0·0625 mg/kg group (n=16) |
0·125 mg/kg group (n=17) |
0·25 mg/kg group (n=15) |
0·5 mg/kg group (n=17) |
|
|---|---|---|---|---|---|---|
| Age (years) | 12 (7–25) | 22 (7–30) | 10 (8–11) | 22 (7–32) | 10 (7–16) | 18 (8–30) |
| Haemoglobin (g/L) | 124 (112–138) | 130 (110–139) | 120 (108–129) | 135 (111–144) | 119 (112–132) | 122 (113–142) |
| Gametocyte density by microscopy (per μL) | 64 (32–720) | 64 (32–144) | 96 (48–576) | 80 (32–320) | 144 (48–720) | 48 (32–192) |
| Asexual parasite prevalence by microscopy | 54 (67%) | 8 (50%) | 11 (69%) | 14 (82%) | 8 (53%) | 13 (77%) |
| Asexual parasite density by microscopy (per μL)* | 376 (64–2128) | 176 (40–3840) | 1088 (144–2832) | 216 (48–1264) | 1656 (432–9808) | 144 (32–1792) |
| Symptomatic malaria† | 6 (7%) | 0 | 3 (19%) | 1 (6%) | 0 | 2 (12%) |
Data are median (IQR) or n (%). * Median parasite densities were calculated for parasite-positive individuals only. † Temperature ≥37·5°C and parasitaemic.
In phase one (Jan 2, 2013, to Feb 22, 2014), 16 participants were randomly assigned to the control group, with 17 randomly assigned to the 0·125 mg/kg primaquine group and 17 to the 0·5 mg/kg primaquine group. One participant assigned to the 0·125 mg/kg primaquine group was inadvertently given 0·375mg/kg. This error was discovered at the day 0 visit, and the participant was retained in follow-up through day 28 and included in the haemoglobin and adverse event analysis but excluded from the change in infectivity analyses. In phase two (Aug 5, 2014, to Nov 28, 2014), 15 patients were sequentially allocated to the 0·25 primaquine group and then 16 were sequentially allocated to the 0·0625 mg/kg primaquine group.
The study was halted on Feb 13, 2013, after eight participants had been recruited because pre-treatment infection rates in the mosquitoes were unacceptably low. Assay optimisation and protocol amendments were completed and the study restarted enrolment on the Sept 17, 2013 (appendix p 1). After optimisation 79% (58 of 73) of pre-treatment membrane feeding experiments resulted in at least one infected mosquito, and 24% (1763 of 7301) of mosquitoes became infected. Overall, we used 32 040 mosquitoes in feeding experiments, of which 24009 (75%) survived to be dissected on day 7, resulting in 2043 infection-positive results (appendix p 2).
The primary analysis of the change in infectivity was done in the 71 participants in the primary analysis sample. We recorded significantly reduced mean within-person infectivity on day 2 in participants assigned to the 0·25 mg/kg (92· 6% [95% CI 78· 3–100; p=0·0014) and the 0·5 mg/kg groups (75·0% [45·7–100]; p=0·014) compared with the control group (11·3% [−27·4 to 50·0]; table 2). Among individuals who were infectious before treatment (infected at least one mosquito; subgroup sample [n=57]), within-person infectivity after treatment was largely reduced and significantly differed from controls at day 2 in those assigned to the 0·125 mg/kg, 0·25 mg/kg, and 0·5 mg/kg groups (table 2).
Table 2:
Average within-person percentage reduction in mosquito infectivity at day 1, 2, and 7 after treatment
| Reduction at day 1 | Reduction at day 2 | Reduction at day 7 | |
|---|---|---|---|
| Population assessed for infectivity (n=71) | |||
| Control group | 16·2% (14·7 to 46·9); ref | 11·3% (−27·4 to 50·0); ref | 45·8% (11·9 to 82·2); ref |
| 0·0625 mg/kg group | 38·2% (7·4 to 68·9); p=0·17 | 41·9% (1·4 to 82·5); p=0·16 | 77·9% (55·1 to 100); p=0·050 |
| 0·125 mg/kg group | 37·9% (−6·7 to 82·6); p=0·19 | 54·9% (13·4 to 96·3); p=0·096 | 66·7% (35·4 to 97·9); p=0·25 |
| 0·25 mg/kg group | 80·9% (59·4 to 100); p=0·0036 | 92·6% (78·3 to 100); p=0·0014 | 93·3% (79·0 to 100); p=0·0099 |
| 0·5 mg/kg group | 78·5% (54·0 to 100); p=0·0040 | 75·0% (45·7 to 100); p=0·014 | 81·5% (59·6 to 100); p=0·049 |
| Participants who were infectious before treatment (n=57) | |||
| Control group | 22·6% (−22·4 to 67·6); ref | 27·5% (−22·7 to 77·7); ref | 66·1% (22·7 to 100); ref |
| 0·0625 mg/kg group | 47·0% (10·2 to 83·6); p=0·18 | 59·3% (16·9 to 100); p=0·18 | 97·4% (91·7 to 100); p=0·052 |
| 0·125 mg/kg group | 69·4% (21·4 to 100); p=0·057 | 94·9% (84·6 to 100); p=0·045 | 100% (100 to 100); p=0·142 |
| 0·25 mg/kg group | 87·8% (70·4 to 100); p=0·014 | 99·2% (97·6 to 100); p=0·0082 | 100% (100 to 100); p=0·052 |
| 0·5 mg/kg group | 91·5% (73·4 to 100); p=0·0064 | 87·6% (60·2 to 100); p=0·030 | 95·1% (84·2 to 100); p=0·11 |
Data are mean (95% CI), unless stated otherwise. Reduction in infectivity measured as the average change in proportion of dissected mosquitoes with oocysts.
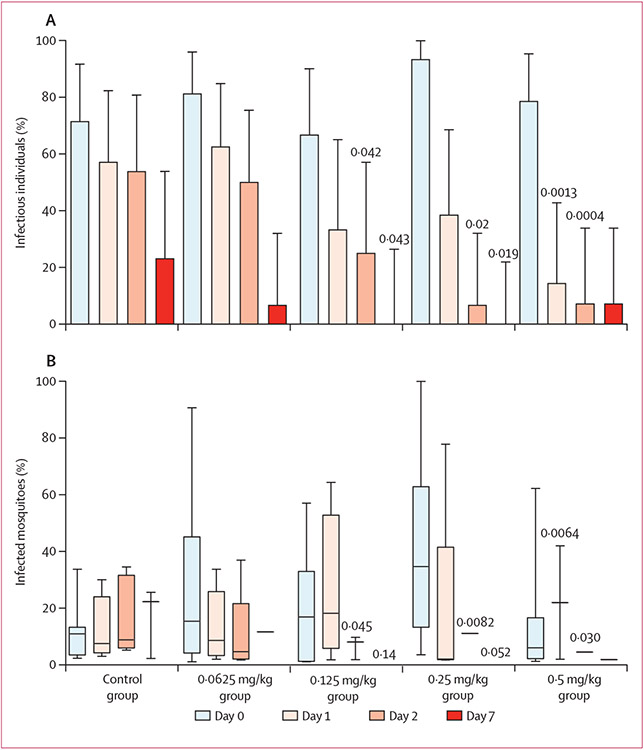
The proportion of individuals that infected at least one mosquito 2 days after treatment was significantly reduced in the 0·125 mg/kg, 0·25 mg/kg, and 0·5 mg/kg dose groups (figure 2A; appendix p 2). Analysis of the median number of mosquitoes infected at each timepoint by dose group showed significantly fewer mosquitoes becoming infected on day 2 in dose groups 0·125 mg/kg, 0·25 mg/kg, and 0·5 mg/kg compared with control group (figure 2B; appendix p 2). No participants in either the 0·125 mg/kg group or the 0·25 mg/kg group infected mosquitoes at day 7, and one participant in the 0·5 mg/kg primaquine group infected a single mosquito on day 7 after treatment.
Figure 2: Proportion (95% CI) of individuals who infected at least one mosquito (A) and proportion (median [line], IQR [box], and range [whisker]) of mosquitoes infected by these 57 individuals (B), by group and visit.
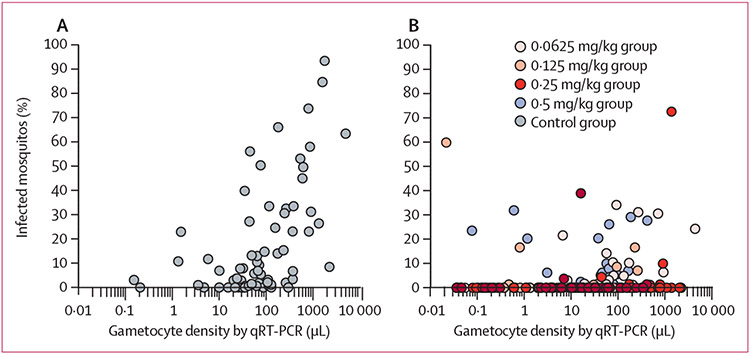
All study participants who received the correct dose of primaquine (n=80) were gametocyte positive on Pfs25 mRNA qRT-PCR at enrolment, with a significantly higher gametocyte density at enrolment in the 0·25 mg/kg primaquine group compared with the control group (appendix p 3; p=0·013). After adjustment for baseline gametocyte density, gametocyte prevalence was significantly lower in the 0·5 mg/kg primaquine group than the control group on day 7, day 14, and day 28 (figure 3A). On day 28, gametocyte prevalence was lower in all participants in primaquine dose groups compared with the control group (figure 3A). Gametocyte density decreased during follow-up for all treatment groups (appendix p 3); the density of gametocytes in gametocyte-positive individuals was significantly lower in groups assigned to primaquine doses of 0·125 mg/kg and lower from day 3 onwards, after adjusting for baseline density (figure 3B; appendix p 3). The AUC of gametocyte density over time was significantly lower in all primaquine groups compared with the control group, after adjustment for baseline density (p≤0·027 for 0·125 mg/kg vs control; appendix p 3). Before treatment, Pfs25 mRNA qRT-PCR gametocyte density was strongly associated with mosquito infection rates (n=73; Spearman correlation coefficient 0·56, 95% CI 0·44–0·66; p<0·0001; figure 4, appendix p 3). This association was no longer apparent on day 1 (n=71; r=0·07, 95% CI −0·17 to 0·30; p=0·57), day 2 (n=71; r=0·17, 95% CI −0·07 to 0·39; p=0·16), or day 7 (n=46; r=0·15, 95% CI −0·15 to 0·42; p=0·34) after initiation of treatment (figure 4, appendix p 3).
Figure 3: Gametocyte prevalence (A) and density (B) by Pfs25mRNA qRT PCR by visit and treatment group.
Gametocyte prevalence was zero in the 0·5 mg/kg group at day 14 and day 28
Figure 4:
The association between gametocyte density and infectivity pre-treatment (A) and post-treatment (B)
Analyses of change in haemoglobin and of adverse events were restricted to participants with at least one follow-up visit (n=79). Overall median haemoglobin concentration on day 0 was 124 g/L (range 86–162), on day 7 was 116 g/L (range 85–179), and on day 28 was 123 g/L (range 102–173). Within-person changes in haemoglobin concentration were not significant in any of the treatment groups compared with the control group at any timepoint (appendix p 4). During follow-up, no participants had a haemoglobin concentration lower than 80 g/L or had a 25% or greater drop in haemoglobin after treatment.
42 (53%) participants had an adverse event during follow-up (table 3). The number of participants that had an adverse event did not significantly differ between groups (Fisher’s exact test, p=0·48). Most adverse events (69 [87%]) were mild (grade 1), with ten (13%) of moderate severity (grade 2). No adverse events resulted in stopping study treatment or study participation. One participant in the 0·5 mg/kg group reported transitory dark urine (mild, grade 1) on day 1, which was considered unrelated to study drug because this participant’s haemoglobin concentration was 142 g/L on day 0 and remained higher than this concentration until day 28. One participant in the control group reported shortness of breath (mild, grade 1), which was resolved and considered unrelated to study treatment. There were no severe adverse events or serious adverse events detected during the study, and all adverse events resolved during study follow-up.
Table 3:
Adverse events
| Control group (n=15) |
0·0625 mg/kg group (n=16) |
0·125 mg/kg group (n=16) |
0·25 mg/kg group (n=15) |
0·5 mg/kg group (n=17) |
|
|---|---|---|---|---|---|
| Participants with an adverse event | 6 (40%) | 9 (56%) | 10 (63%) | 6 (40%) | 11 (65%) |
| Number of adverse events | 20 | 13 | 16 | 10 | 20 |
| Number drug related* | 3 | 0 | 1 | 0 | 0 |
| Headache | |||||
| Mild | 2 | 4 | 1 | 2 | 3 |
| Moderate | 2 | 0 | 1 | 1 | 0 |
| Abdominal pain | |||||
| Mild | 2 | 2 | 2 | 1 | 1 |
| Fever | |||||
| Mild | 0 | 3 | 0 | 3 | 1 |
| Loss of appetite | |||||
| Mild | 3 | 0 | 2 | 0 | 1 |
| Fatigue | |||||
| Mild | 2 | 0 | 2 | 0 | 1 |
| Moderate | 0 | 0 | 1 | 0 | 0 |
| Fatigue | |||||
| Mild | 1 | 0 | 0 | 1 | 3 |
| Nausea | |||||
| Mild | 1 | 0 | 2 | 0 | 2 |
| Runny nose | |||||
| Mild | 1 | 0 | 2 | 1 | 0 |
| Dizziness | |||||
| Mild | 1 | 1 | 0 | 1 | 0 |
| Muscle aches | |||||
| Mild | 1 | 0 | 1 | 0 | 1 |
| Diarrhoea | |||||
| Mild | 0 | 1 | 0 | 0 | 1 |
| Respiratory infection | |||||
| Mild | 0 | 1 | 1 | 0 | 0 |
| Back pain | |||||
| Mild | 0 | 0 | 0 | 0 | 1 |
| Moderate | 2 | 0 | 0 | 0 | 0 |
| Burning with urination | |||||
| Mild | 0 | 0 | 0 | 0 | 1 |
| Pain with urination | |||||
| Mild | 0 | 0 | 0 | 0 | 1 |
| Dark urine | |||||
| Mild | 0 | 0 | 0 | 0 | 1 |
| Rhinobronchitis | |||||
| Mild | 0 | 0 | 1 | 0 | 0 |
| Shortness of breath | |||||
| Mild | 1 | 0 | 0 | 0 | 0 |
| Vomiting | |||||
| Mild | 0 | 1 | 0 | 0 | 0 |
| Whitlow | |||||
| Moderate | 0 | 0 | 0 | 1 | |
| Joint osteoarthritis in the legs | |||||
| Moderate | 1 | 0 | 0 | 0 | 0 |
There were no severe or serious adverse events. All events were self-reported. Mild events were defined as those causing no or mlnlmal Interference with usual social and functional activities; moderate events as those causing greater than minimaI interference; and severe events as those causing inability to perform usual social and functional activities. * Defined as probably or definitely related to study treatment.
We successfully genotyped CYP2D6 DNA for 43 (53%) of 81 participants.18 Two participants were poor metabolisers (one in the control group and one in the 0·0625 mg/kg group) and four were intermediate metabolisers by both conventional phenotype inference. Five were intermediate metabolisers by the conventional method, but extensive metabolisers by activity score, 30 were extensive metabolisers by both the conventional method and activity score, and two were ultra-rapid metabolisers (one in the 0·0625 mg/kg group and one in the 0·25 mg/kg group). CYP2D6 typing failed for the participant who received 0·5 mg/kg primaquine and was infectious on day 7. CYP2D6 genotypes did not differ significantly between treatment groups (Fisher’s exact test, p=0·35).
Discussion
We show that although gametocytes persist on molecular qRT PCR, malaria infectivity is reduced by greater than 90% on day 2 and day 7 after one 0·25 mg/kg primaquine dose and artemisinin-based combination therapy. Among participants that infected mosquitoes pretreatment, we saw a greater than 90% reduction in infectivity with primaquine doses of 0·125 mg/kg or higher by day 2 and in all groups given single low doses of primaquine by day 7. We observed no serious adverse events or evidence of haemolysis in our study population of G6PD-functional boys and men.
Treatment with artemisinin-based combination therapy results in a rapid reduction in P falciparum gametocyte carriage and transmissibility. In our study, 79% of all participants with microscopically detectable gametocytes infected at least one mosquito before treatment, similar to findings from a meta-analysis on membrane feeding assays.19 With dihydroartemisinin–piperaquine treatment alone (ie, the control group), this proportion was reduced to 54% by day 2 and 23% by day 7, representing reductions in infectivity of 27% and 66%, respectively (figure 2A). The residual transmission after dihydroartemisinin–piperaquine treatment has been observed before20 and is partly explained by the limited effect of the artemisinin component against mature (stage V) gametocytes that were present before treatment.21 Single low doses of primaquine greater than 0·125 mg/kg potentiate the transmission-reducing effect of dihydroartemisinin–piperaquine. Only one individual was infectious on day 7 after primaquine treatment among those given doses of primaquine of 0·125 mg/kg or higher and this person infected only one mosquito. Our findings of near complete prevention of transmission in all individuals given 0·125 mg/kg of primaquine or higher, when given in combination with dihydroartemisinin–piperaquine, are consistent with historical data in which primaquine was administered in conjunction with other antimalarial drugs.7 Future studies should explore whether the transmission-blocking effects of single low doses of primaquine depend on the type of artemisinin-based combination therapy they are co-administered with.
Gametocytes were detected in all treatment groups after treatment. Notably, the presence of gametocytes and gametocyte density were not associated with infectivity after treatment. Recent studies on the efficacy of single low doses of primaquine frequently used gametocyte prevalence as the primary endpoint as a surrogate for infectivity.22,23 In our study population most individuals in the control group retained low densities of gametocytes measured by Pfs25 mRNA qRT PCR until the end of follow-up on day 28. Although primaquine shows a dose-dependent effect on gametocyte prevalence, there was a clear discordance between gametocyte prevalence and density estimates and the likelihood of mosquito infection after treatment. Before treatment, gametocyte densities were strongly associated with the proportion of infected mosquitoes, but this association was lost during days post-treatment. Older studies had similar findings with gametocytes being detectable by microscopy for several days after primaquine but failed to infect mosquitoes.21 Our results support the view that available tests to assess gametocyte density cannot be used to estimate post-treatment infectivity.
The gametocyte density and infectivity mismatch shown in our findings might be explained by preferential measurement of female gametocytes in our methods. Pfs25 mRNA transcripts, which we used to measure gametocytes in this study, could be female specific.9,24 Recently, primaquine was postulated to disproportionally kill male gametocytes9 and thereby prevent transmission that depends on the presence of both male and female gametocytes in permissive concentrations. Methylene blue, another antigametocyte drug, is more potent against male gametocytes than against female gametocytes.25 It is conceivable that our quantification of gametocytes is female-biased and becomes progressively more so after treatment. This could explain the absence of mosquito infectivity at later timepoints after primaquine when male gametocytes might have been effectively cleared. We tested samples with a new qRT PCR that detects male Pfs230p transcripts;24 however, the transcript levels of Pfs230p are much lower than those for Pfs2514 and we were unable to detect male gametocytes in most baseline samples (data not shown). Future studies should establish whether primaquine has a higher potency against male gametocytes and is a potential explanation for our findings of gametocytes being present, yet non-infective. Findings from our study leave unaddressed the question of whether there is a differential effect of antimalarial treatment on male and female gametocytes. Until better indicators of gametocyte infectivity become available (which will probably consist of sensitive, sex-specific, quantitative molecular gametocyte detection methods), the transmission-blocking properties of antimalarial drugs can only be assessed by mosquito feeding assays.5,7
The labour intensiveness of membrane feeding assays limits the number of sites at which trials with a transmission endpoint can be done and limits the study size that can be used. Although our study had a relatively small sample size, our findings more than double the available evidence on single low doses of primaquine. Available recommendations are based on retrospective analysis of nine studies and a total of 65 individuals who received single low doses of primaquine in combination with an artemisinin-based combination therapy.7 The present study includes 24009 mosquito observations from 284 experiments before and at three timepoints after single low doses of primaquine. Infectivity in our study was maximised through enrolment of high-density gametocyte carriers that are highly infectious compared with average malaria-infected individuals who have much lower gametocyte densities and are considerably less infectious.26 In individuals with low gametocyte densities, who comprise a considerable proportion of the human infectious reservoir for malaria,27 single low doses of primaquine are likely to be even more efficacious than we reported for our study population. A fraction of gametocyte carriers are not infectious to mosquitoes.19 In our study, 14 (20%) of 71 participants were not measured to have infectivity pre-treatment and 11 (17%) of them never infected mosquitoes during the whole study period. This failure to infect mosquitoes despite relatively high gametocyte densities could be caused by gametocyte fitness or host factors such as naturally acquired immune responses to gametocyte antigens that could reduce transmission efficiency, as well as limitations of the membrane feeding assay.19 These factors are unlikely to have affected our comparisons of within-person changes in infectivity or comparisons of dose groups.
Our adaptive study design resulted in two phases of enrolment, the second of which was not randomised. We consider it unlikely that this shortcoming has affected our conclusions on the transmission-blocking properties of the different doses of primaquine since our main analysis was based on within-person changes in infectivity; other comparisons between treatment groups were adjusted for baseline infectivity or baseline gametocyte density, where appropriate.
Residual transmission after single low doses of primaquine is of particular importance considering possible interindividual variation in primaquine metabolism.18 In our study, we successfully genotyped 53% of participants for cytochrome P450 2D6 (CYP2D6). This low success rate is a result of the availability of small volume blood samples that were collected for gametocyte detection and not for detailed human genetic analyses. One single participant received the highest dose of single low doses of primaquine tested (0·5 mg/kg) yet remained infectious, infecting one mosquito on day 7. CYP2D6 typing failed for this individual; whether this participant was a CYP2D6 poor metaboliser is therefore unknown.
This study was carried out in a relatively small population of men and boys with normal functioning G6PD in Mali. Thus, we are unable to generalise the findings to young children, G6PD-deficient people, women, and other African populations or people residing outside sub-Saharan Africa, in whom G6PD variants that are associated with more severe G6PD enzyme deficiency might occur. We chose boys and men with normal G6PD function with haemoglobin concentrations 80 g/L before treatment because their risk of haemolysis after exposure to single low doses of primaquine would be low. A previous study in Uganda showed a transient reduction in haemoglobin concentrations in individuals who were genotypically G6PD-deficient but were screened as having normal functioning G6PD by fluorescence spot test.28 We did not observe any significant decreases in haemoglobin or increases in adverse events in primaquine groups compared with the control group. Future studies should address the safety profile of single low doses of primaquine in larger populations that include G6PD-deficient participants to inform the highest tolerable dose without previous testing for G6PD deficiency. WHO recommends use of 0·25 mg/kg primaquine without previous G6PD testing in areas of low transmission or areas threatened by artemisinin resistance.7 However, most antimalarial drugs are dispensed based on age-based categories, removing the need to weigh patients before treatment. If single low doses of primaquine are to be rolled out, similar categories will be needed to define a range of dosing that is both safe and efficacious.10 A second more challenging safety issue is the safety of primaquine in pregnancy.29 This issue might better be assessed through active pharmacovigilance of the use of single low doses of primaquine in programme use29 or safety assessments of mass drug administration studies in which early stage pregnancies might be inadvertently treated and later followed-up to note pregnancy outcomes.30
Our findings support WHO’s recommendation for the addition of one-time primaquine at a dose of 0·25 mg/kg in combination with dihydroartemisin–piperaquine for prevention of P falciparum transmission. In community treatment campaigns, a simplified delivery of single low doses of primaquine might involve age-based dosing to replace the current weight-based recommended dose regimen. This regimen could result in relative over-dosing and under-dosing of fractions of the population. In this context, our study provides valuable information on the dose of 0·125 mg/kg. Although not significant in the primary analysis, in secondary analyses the 0·125mg/kg dose showed potent and significant efficacy in preventing and reducing transmission to mosquitoes by day 2.
Further studies to assess the highest-tolerable single primaquine dose in G6PD-deficient individuals are needed to define the therapeutic range for simplified delivery of the intervention. Community studies can then be done to show the impact of single low doses of primaquine on malaria transmission in populations that might benefit from this intervention, including in areas of low malaria transmission intensity.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed on Sept 18, 2015, with the terms “primaquine” and “malaria, falciparum”, “primaquine” and “gametocyte” or “primaquine” and “transmission”. We identified no randomised controlled trials of single low doses of primaquine with endpoints of gametocyte or transmission endpoints published after the dose-finding study by Eziefula and colleagues (2014). In that study, one low dose of primaquine was added to artemether–lumefantrine combination therapy with investigators measuring duration of Plasmodium falciparum gametocytaemia with Pfs25 mRNA quantitative nucleic acid sequence-based amplification (QT-NASBA) as the primary outcome measure for efficacy. The authors concluded that single low-dose primaquine at 0·4 mg/kg had similar gametocytocidal efficacy compared with 0·75 mg/kg, the single primaquine dose recommended by WHO for P falciparum until 2012, whereas they found a dose of 0·1 mg/kg did not show non-inferiority. However, no assessments of transmissibility of gametocytes were done, which was highlighted as an important limitation. The best available evidence on the transmission-blocking effects of primaquine was based on infectivity data from 158 individual gametocytaemic individuals treated with plasmoquine or primaquine in different studies with different vectors and different drug exposures. A pooled analysis of these data supported the use of primaquine at a single dose lower than 0·75mg/kg.
Added value of this study
To our knowledge, our study is the first dose-finding study of single low-dose primaquine that uses mosquito feeding assays, a resource-intensive but necessary method to obtain conclusive infectivity assessments. In individuals with normal glucose-6-phosphate dehydrogenase (G6PD) enzyme function, doses of primaquine of 0·25 mg/kg and 0·5 mg/kg resulted in significant decreases in infectivity to mosquitoes on day 2. Our findings support WHO’s recommendation for single-dose primaquine at 0·25 mg/kg to prevent malaria transmission along with artemisinin-based combination therapy dihydroartemisinin–piperaquine.
Implications of all the available evidence
Single low doses of primaquine should be rolled out, especially in areas where national malaria control programme managers have requested that contemporary dose-finding studies be carried out. Studies are warranted on the safety of single low doses of primaquine in individuals who are deficient in G6PD.
Acknowledgments
The trial was funded by the Bill & Melinda Gates Foundation (OPP1013179). AD received support from the government of Mali and different grants including from the Bill & Melinda Gates Foundation (OPP1013179); the UK Medical Research Council (MRC; grant MR/K0073191/1 to London School of Hygiene & Tropical Medicine), and the Partnerships for Enhanced Engagement in Research (PEER) Health (grant AID-OAA-A-11-00012). JH receives salary support from the President’s Malaria Initiative. CM is supported through grants from the National Institutes of Health USA. TB received support through grants from the Bill & Melinda Gates Foundation (AFIRM OPP1034789) and a fellowship from the European Research Council (ERC-2014-StG 639776). All other authors (JMB, HD, IB, AM, HMS, KS, FK, SK, SFT, IC, EP, KL, HP, MN, FN, and RG) were supported by the Bill & Melinda Gates Foundation (OPP1013179). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. We thank the data safety monitoring committee (Phillip Rosenthal, Kevin Baird, and Diadier Diallo); the hard work of the insectary, laboratory, and field staff; and the participants, the population, the members of community health association, and the staff of the health centres in Ouelessebougou for their cooperation throughout the study. We thank Sanofi for their kind donation of primaquine.
Footnotes
Declaration of interests
CM reports grants from NIH, during the conduct of the study. JMB reports grants from Bill & Melinda Gates Foundation, during the conduct of the study. IC reports grants from Bill & Melinda Gates Foundation. All other authors declare no competing interests.
Contributor Information
Alassane Dicko, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Joelle M Brown, Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA, USA.
Halimatou Diawara, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Ibrahima Baber, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Almahamoudou Mahamar, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Harouna M Soumare, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Koualy Sanogo, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Fanta Koita, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Sekouba Keita, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Sekou F Traore, Malaria Research and Training Centre, Faculty of Pharmacy and Faculty of Medicine and Dentistry, University of Science, Techniques and Technologies of Bamako, Bamako, Mali.
Ingrid Chen, Global Health Group, Malaria Elimination Initiative University of California San Francisco, San Francisco, CA, USA.
Eugenie Poirot, Department of Epidemiology and Biostatistics University of California San Francisco, San Francisco, CA, USA; Global Health Group, Malaria Elimination Initiative University of California San Francisco, San Francisco, CA, USA.
jimee Hwang, Global Health Group, Malaria Elimination Initiative University of California San Francisco, San Francisco, CA, USA; Malaria Branch, Centers for Disease Control and Prevention, Atlanta, GA, USA; President’s Malaria Initiative, Washington, DC, USA.
Charles McCulloch, Department of Epidemiology and Biostatistics University of California San Francisco, San Francisco, CA, USA.
Kjerstin Lanke, Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, Netherlands.
Helmi Pett, Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, Netherlands; Department of Clinical Pharmacology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Mikko Niemi, Department of Clinical Pharmacology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
François Nosten, Centre for Tropical Medicine, Nuffield Department of Medicine, University of Oxford, Oxford, UK; Shoklo Malaria Research Unit, Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Mae Sot, Thailand.
Teun Bousema, Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, Netherlands; Department of Immunology & Infection, London School of Hygiene & Tropical Medicine, London, UK.
Roly Gosling, Department of Epidemiology and Biostatistics University of California San Francisco, San Francisco, CA, USA; Global Health Group, Malaria Elimination Initiative University of California San Francisco, San Francisco, CA, USA.
References
- 1.WHO. World Malaria Report. Geneva: World Health Organization, 2014. [Google Scholar]
- 2.Noor AM, Kinyoki DK, Mundia CW, et al. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000–10: a spatial and temporal analysis of transmission intensity. Lancet 2014; 383: 1739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin JT, Hollingsworth TD, Okell LC, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med 2010; 7: e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter C, Sturrock HJ, Hsiang MS, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 2013; 382: 900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Evidence review group on the safety and efficacy of gametocytocidal doses of primaquine for Plasmodium falciparum malaria. Geneva: World Health Organization, 2012. [Google Scholar]
- 6.Ruwende C, Khoo SC, Snow RW, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 1995; 376: 246–49. [DOI] [PubMed] [Google Scholar]
- 7.White NJ, Qiao LG, Qi G, Luzzatto L. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar J 2012; 11: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eziefula AC BT, Yeung S, Kamya M, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis 2014; 14: 130–39. [DOI] [PubMed] [Google Scholar]
- 9.White NJ, Ashley EA, Recht J, et al. Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar J 2014; 13: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen I, Poirot E, Newman M, et al. An assessment of the supply, programmatic use, and regulatory issues of single low-dose primaquine as a Plasmodium falciparum gametocytocide for sub-Saharan Africa. Malar J 2015; 14: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dicko A, Diallo AI, Tembine I, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in mali: a randomised, double-blind, placebo-controlled trial. PLoS Med 2011; 8: e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouedraogo AL, Guelbeogo WM, Cohuet A, et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. Malaria World J 2013; 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wampfler R, Mwingira F, Javati S, et al. Strategies for detection of Plasmodium species gametocytes. PLoS One 2013; 8: e76316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider P, Wolters L, Schoone G, et al. Real-time nucleic acid sequence-based amplification is more convenient than real-time pcr for quantification of Plasmodium falciparum. J Clin Microbiol 2005; 43: 402–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol 2004; 369: 23–37. [DOI] [PubMed] [Google Scholar]
- 16.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 2008; 83: 234–42. [DOI] [PubMed] [Google Scholar]
- 17.Shekalaghe S, Drakeley C, Gosling R, et al. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One 2007. 10: e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett JW, Pybus BS, Anjali Y, Tosh D, Sousa JC. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 2013; 269: 1381–82. [DOI] [PubMed] [Google Scholar]
- 19.Bousema T, Dinglasan RR, Morlais I, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One 2012; 7: e42821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawa P, Shekalaghe SA, Drakeley CJ, et al. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: a randomized trial. J Infect Dis 2013; 207: 1637–45. [DOI] [PubMed] [Google Scholar]
- 21.White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis 2013; 13: 175–81. [DOI] [PubMed] [Google Scholar]
- 22.Sutanto I, Suprijanto S, Kosasih A, et al. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in South sumatra, Western indonesia: an open-label, randomized, controlled trial. Clin Infect Dis 2013; 56: 685–93. [DOI] [PubMed] [Google Scholar]
- 23.Smithuis F, Kyaw MK, Phe O, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 2010; 10: 673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider P, Reece SE, van Schaijk BC, et al. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol Biochem Parasitol 2015; 199: 29–33. [DOI] [PubMed] [Google Scholar]
- 25.Delves MJ, Ruecker A, Straschil U, et al. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 2013; 57: 3268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 2014; 12: 833–40. [DOI] [PubMed] [Google Scholar]
- 27.Ouedraogo AL, Gonçalves BP, Gneme A, et al. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 2015; published online July 3. DOI: 10.1093/infdis/jiv370. [DOI] [PubMed] [Google Scholar]
- 28.Eziefula AC, Pett H, Grignard L, et al. Glucose-6-phosphate dehydrogenase status and risk of hemolysis in Plasmodium falciparum-infected African children receiving single-dose primaquine. Antimicrob Agents Chemother 2014; 58: 4971–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen IT, Gosling RD. Targeting Plasmodium falciparum with primaquine: same efficacy, improved safety with a lower dose? Expert Rev Clin Pharm 2014; 7: 681–86. [DOI] [PubMed] [Google Scholar]
- 30.Deen JL, von Seidlein L, Pinder M, Walraven GE, Greenwood BM. The safety of the combination artesunate and pyrimethamine-sulfadoxine given during pregnancy. Trans R Soc Trop Med Hyg 2001; 95: 424–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.