Abstract
The in vivo activities of amoxicillin and amoxicillin-clavulanate against 17 strains of Streptococcus pneumoniae with penicillin MICs of 0.12–8.0 mg/liter were assessed in a cyclophosphamide-induced neutropenic murine thigh infection model. Renal impairment was produced by administration of uranyl nitrate to prolong the amoxicillin half-life in the mice from 21 to 65 min, simulating human pharmacokinetics. Two hours after thigh infection with 105 to 106 CFU, groups of mice were treated with 7 mg of amoxicillin per kg of body weight alone or combined with clavulanate (ratio, 4:1) every 8 h for 1 and 4 days. There was an excellent correlation between the MIC of amoxicillin (0.03 to 5.6 mg/liter) and (i) the change in log10 CFU/thigh at 24 h and (ii) survival after 4 days of therapy. Organisms for which MICs were 2 mg/liter or less were killed at 1.4 to 4.2 and 1.6 to 4.1 log10 CFU/thigh at 24 h by amoxicillin and amoxicillin-clavulanate, respectively. The four strains for which MICs were >4 mg/liter grew 0.2 to 2.6 and 0.6 to 2.3 logs at 24 h despite therapy with amoxicillin and amoxicillin-clavulanate, respectively. Infection was uniformly fatal by 72 h in untreated mice. Amoxicillin therapy resulted in no mortality with organisms for which MICs were 1 mg/liter or less, 20 to 40% mortality with organisms for which MICs were 2 mg/liter, and 80 to 100% mortality with organisms for which MICs were 4.0–5.6 mg/liter. Lower and higher doses (0.5, 2, and 20 mg/kg) of amoxicillin were studied against organisms for which MICs were near the breakpoint. These studies demonstrate that a reduction of 1 log10 or greater in CFU/thigh at 24 h is consistently observed when amoxicillin levels exceed the MIC for 25 to 30% of the dosing interval. These studies would support amoxicillin (and amoxicillin-clavulanate) MIC breakpoints of 1 mg/liter for susceptible, 2 mg/liter for intermediate, and 4 mg/liter for resistant strains of S. pneumoniae.
Infections caused by Streptococcus pneumoniae with reduced susceptibility to beta-lactam antimicrobial agents are an increasingly encountered problem in clinical practice. The incidence of infection with penicillin-resistant pneumococci now exceeds 30% in most areas of the United States (22). There have been reports of failure of conventional antimicrobial regimens in acute otitis media and meningitis caused by highly penicillin-resistant pneumococci (2, 3, 11, 19, 22). These therapeutic failures suggest that the current standards for predicting effective therapy of infection with these organisms may need to be altered.
Amoxicillin and amoxicillin-clavulanate are orally administered beta-lactams, with thrice daily dosing in humans commonly used to treat infections caused by S. pneumoniae. Although these agents have the lowest MICs for S. pneumoniae among currently available oral beta-lactams, the precise MIC breakpoint separating effective and noneffective in vivo activity is currently unknown. The use of animal models has become integral in the evaluation of the therapeutic efficacies of antimicrobial agents. Animal models can describe the time course of in vivo antibiotic therapy and dose-response relationships and determine the influence of antimicrobial agent resistance in vitro on various in vivo outcomes observed during the treatment of these infections (5, 10, 13, 24).
The goals of these studies were (i) to stimulate the human pharmacokinetic profiles of amoxicillin and amoxicillin-clavulanate in the murine infection model in order to appropriately compare these results to those that might be expected in humans and (ii) to characterize the in vivo activities of amoxicillin and amoxicillin-clavulanate against strains of S. pneumoniae for which penicillin and amoxicillin MICs vary. By observing efficacy against organisms with a range of in vitro activity, we hoped to identify in vivo MIC breakpoints of amoxicillin and amoxicillin-clavulanate for S. pneumoniae. These studies would also enable us to determine if clavulanate enhances the in vivo activity against strains of S. pneumoniae with various susceptibilities to penicillin.
(Part of this research was presented at the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., September 1995.)
MATERIALS AND METHODS
Bacteria, media, and antibiotics.
S. pneumoniae ATCC 10813 and 49619 and 15 clinical isolates (provided by Fred Tenover of the Centers for Disease Control and Prevention and Gary Doern of the University of Iowa) were used for these experiments. Organisms were grown, subcultured, and quantified in Mueller-Hinton broth (MHB) (Difco Laboratories, Detroit, Mich.) and on sheep blood agar plates (Remel, Milwaukee, Wis.). Amoxicillin and amoxicillin-clavulanate were provided by SmithKline Beecham, Philadelphia, Pa.
In vitro studies.
MICs of amoxicillin and penicillin for the various isolates were determined by standard National Committee for Clinical Laboratory Standards (NCCLS) microdilution methods with geometric two-fold serial dilutions in cation-adjusted MHB supplemented with 3% lysed horse erythrocytes. Each well contained 100 μl (50 μl of inoculum plus 50 μl of drug-containing MHB). The final inoculum was 1 × 105 to 5 × 105. MIC endpoints were determined by visual inspection after CO2 incubation (Forma Scientific Incubator, Marietta, Ohio) at 37°C for 18 to 24 h. Final results were expressed as geometric means of two to four determinations.
Mouse preparation and infection.
Six-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 23 to 27 g (Harlan Sprague-Dawley, Madison, Wis.) were rendered neutropenic (neutrophils, <100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before experimental infection. Previous studies have shown that this regimen produces neutropenia in this model for 5 days (6). Renal impairment was produced by one intraperitoneal injection of uranyl nitrate (10 mg/kg) 3 days before therapy with an antimicrobial agent. This intervention causes a reversible acute tubular necrosis and a subsequent significant stable reduction in renal function for at least 7 days (6, 14, and data not shown). Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance of 0.3 at 580 nm (Spectronic 88; Bausch and Lomb, Rochester, N.Y.). After a 1:10 dilution into fresh MHB, bacterial counts of the inoculum ranged from 105 to 106 CFU/ml. Thigh infections with each of the S. pneumoniae strains were produced by injection of 0.1 ml of inoculum into each of the ether-anesthetized mice 2 h before therapy with an antimicrobial agent was begun.
Treatment with an antimicrobial agent.
Mice were treated with amoxicillin administered by subcutaneous injection every 8 h at a dose of 7 mg/kg. Lower doses (0.5 and 2 mg/kg) and a higher dose (20 mg/kg) were used with some strains (673, 145, 1293, 1329, and 1285) to vary the duration of time that serum levels exceeded the MICs for the infecting strains. Amoxicillin-clavulanate was administered subcutaneously every 8 h at a dose of 7 mg of amoxicillin per kg and 1.75 mg of clavulanic acid per kg. These dosing intervals were chosen to simulate one of the commonly used regimens in humans. Control animals received subcutaneous injections of 0.85% saline. Antibiotic treatment was administered in 0.2-ml volumes 2 h after thigh infection for a total duration of 24 h. Pairs of mice were used for each dosing regimen and sacrificed at the end of therapy. Untreated control mice were sacrificed just after thigh infection (n = 1), just before drug treatment (n = 2), and after 24 h (n = 2).
After sacrifice, thighs were removed and homogenized (Polytron tissue homogenizer; Kinematica, Lucerne, Switzerland) in 10 ml of iced 0.85% saline. Ten-microliter aliquots of four serial 10-fold dilutions were plated on sheep blood agar for CFU determinations. Efficacy in these studies was calculated by subtracting the log10 CFU per thigh of each mouse at the end of therapy with an antimicrobial agent from the mean log10 CFU per thigh of control mice just before therapy (0 h). The lower limit of detection in the antibiotic-treated mice was 102 CFU/thigh.
Other groups of five mice were similarly infected with a subset of 13 strains, including penicillin-susceptible, -intermediate, and -resistant pneumococci, for evaluation of survival after 96 h of therapy. Mice received either a regimen with amoxicillin (7 mg/kg) or amoxicillin-clavulanate (7/1.75 mg/kg) administered at 8-h intervals, with a total of 12 doses.
Drug pharmacokinetics.
Single-dose serum pharmacokinetic studies were performed with thigh-infected mice given amoxicillin at doses of 7, 20, and 48 mg/kg. For each of the doses examined, one or two groups of three to four mice with normal and impaired (uranyl nitrate) renal function were sampled four times at 15- to 60-min intervals. Blood was obtained by retro-orbital puncture with heparinized capillary tubes (Fischer Scientific Co., Pittsburgh, Pa.) in ether-anesthetized mice. Following collection, the samples were centrifuged (model MB; International Equipment Co.) for 3 to 5 min at 10,000 × g, and drug levels in plasma were determined by agar-well microbiologic assay with standard media and by standard methods, with Staphylococcus aureus ATCC 6538P as the test organism. Standard samples were prepared in pooled normal mouse serum. Pharmacokinetic constants, including elimination rate constant, half-life, volume of distribution, area under the concentration-time curve (AUC), and peak level, were calculated with a one-compartment model with zero-order absorption and first-order elimination by nonlinear squares techniques (MINSQ; Micromath Inc., Salt Lake City, Utah).
Statistical analysis.
A Mann-Whitney test was used to compare differences in in vivo activity of amoxicillin-clavulanate between each of the strains. A sigmoid dose-effect model (Emax model) was used to characterize in vivo antimicrobial activity. The model is derived from the Hill equation E = (Emax × TN)/(P50N + TN), where E is the observed effect (change in log10 CFU per thigh compared with untreated controls at 24 h or percent mortality), T is the percentage of time serum levels were above the MIC, Emax is the maximum effect, P50 is the time above MIC needed to achieve 50% of the maximum effect, and N is the slope of the response relationship (16). Emax, P50, and N were calculated by nonlinear least-squares regression (MINSQ). R2 (the coefficient of determination) was used to estimate the fraction of the total variance in the effect of the antimicrobial agent that could be attributed to its linear regression with the pharmacokinetic parameter.
RESULTS
MIC determinations.
The MICs of amoxicillin and penicillin G for the 17 study strains are shown in Table 1. The MICs varied 500-fold for amoxicillin and 1,000-fold for penicillin. In general, AUCs for amoxicillin were similar or two- to fourfold lower than AUCs for penicillin G. Based on NCCLS breakpoints, the organisms studied included one strain susceptible to penicillin (MIC, 0.1 mg/liter), eight strains with intermediate susceptibility (MICs, 0.1 to 1.0 mg/liter), and eight strains resistant to penicillin (MICs, >2.0 mg/liter).
TABLE 1.
MICs of amoxicillin and penicillin G for strains of S. pneumoniae
| Organism | Amoxicillin MIC (mg/liter) | Penicillin G MIC (mg/liter) |
|---|---|---|
| S. pneumoniae ATCC 10813 | 0.016 | 0.008 |
| S. pneumoniae R20 | 0.031 | 0.125 |
| S. pneumoniae 135 | 0.062 | 0.125 |
| S. pneumoniae 131 | 0.062 | 0.25 |
| S. pneumoniae 1396 | 0.25 | 0.5 |
| S. pneumoniae 1199 | 0.25 | 1.0 |
| S. pneumoniae R3 | 0.5 | 0.5 |
| S. pneumoniae 1325 | 0.5 | 2.0 |
| S. pneumoniae 1285 | 0.5 | 0.5 |
| S. pneumoniae 1020 | 1.0 | 2.0 |
| S. pneumoniae 1293 | 2.0 | 2.0 |
| S. pneumoniae 138 | 2.0 | 2.0 |
| S. pneumoniae 1329 | 4.0 | 2.0 |
| S. pneumoniae 146 | 4.0 | 4.0 |
| S. pneumoniae 145 | 4.0 | 4.0 |
| S. pneumoniae 673 | 5.6 | 8.0 |
Pharmacokinetics.
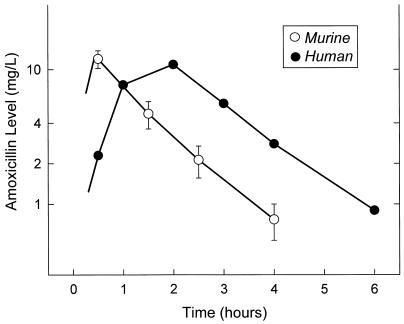
Pharmacokinetic profiles for amoxicillin following doses of 7, 20, and 48 mg/kg resulted in peak/dose ratios of 0.7 ± 0.3 (mean ± standard deviation) and 1.5 ± 0.4 and elimination half-lives of 21 ± 3 and 65 ± 13 min in mice with normal and impaired renal function, respectively. The peak level was twofold higher in renally impaired mice compared to that in mice with normal renal function. The elimination half-life was increased 3.1-fold in the renally impaired mice, approximating that observed in humans with normal renal function. The serum concentrations following a subcutaneous dose of 7 mg of amoxicillin per kg in renally impaired mice and a 500-mg oral dose in normal human volunteers are shown in Fig. 1. Although both doses produced similar mean peak levels (12.0 mg/liter in mice and 10.9 mg/liter in humans), peak levels occurred much earlier in the murine model. Since the rates of elimination were very similar in mice and humans, the duration of time that drug levels in serum would exceed the MIC would be 1 to 1.5 h longer in humans than in the murine model.
FIG. 1.
Serum concentrations of amoxicillin in renal-impaired mice and human volunteers.
Organism growth in thighs.
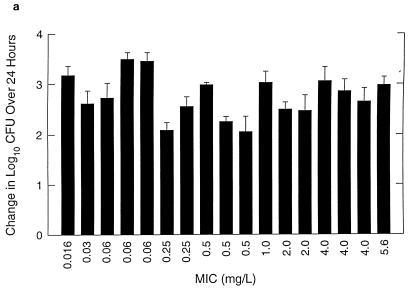
Organisms exhibited logarithmic growth prior to therapy in all control experiments. Bacterial numbers at the start of therapy ranged from 6.03 to 7.46 log10 CFU per thigh. Control growth over 24 h for each organism studied is shown in Fig. 2a. All organisms grew at 2.06 to 3.35 log10 CFU per thigh over 24 h in untreated control animals.
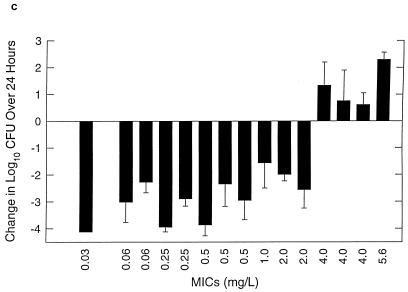
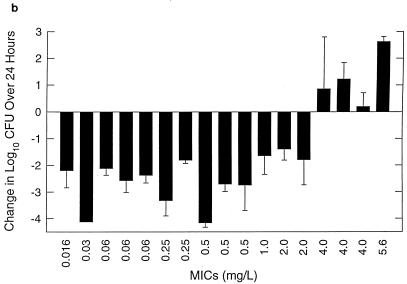
FIG. 2.
Control growth (a) and effect of amoxicillin at 7 mg/kg/8 h (b) and amoxicillin-clavulanate at 7/1.75 mg/kg/8 h (c) against multiple strains of S. pneumoniae in thighs of neutropenic mice over 24 h. Values represent means ± standard deviations for 4 to 8 thighs.
In vivo efficacy.
Organism growth (positive numbers) or killing (negative numbers) following 24 h of therapy with amoxicillin (7 mg/kg) and amoxicillin-clavulanate (7/1.75 mg/kg) every 8 h is shown in Fig. 2b and c, respectively. Two of the most amoxicillin-susceptible strains were not studied with amoxicillin-clavulanate. The differences between the extent of killing for amoxicillin and amoxicillin-clavulanate at the end of 24 h of therapy were small and insignificant (P = 0.06 to 1.0). In fact, the two strains (138 and 1285) for which differences were closest to being significant (P = 0.06) gave better results with amoxicillin, not amoxicillin-clavulanate. The magnitude of killing ranged from 1.5 to 4.0 log10 CFU per thigh. The extent of organism killing with both drugs was dependent on the MICs for the organisms. Significant killing was observed with all organisms for which MICs were 2 mg/liter or less, while growth or minimal net change in bacterial numbers was observed with organisms for which MICs were 4 mg/liter or greater.
Mortality following 4 days of antimicrobial therapy with amoxicillin (7 mg/kg/8 h) and amoxicillin-clavulanate (7/1.75 mg/kg/8 h) is shown in Table 2. Mortality was 100% within 3 days in untreated controls for all strains. The MICs for the various study organisms were predictive of efficacy when mortality was used as an endpoint as well. All animals that were infected with organisms for which MICs were 1 mg/liter or less survived. With the two strains for which MICs were 2 mg/liter, 20 to 40% mortality was observed with amoxicillin, but no mortality was observed with amoxicillin-clavulanate. In contrast, mortality was 80 to 100% in mice infected with organisms for which MICs were 4 mg/liter or greater.
TABLE 2.
Relationship between MIC for S. pneumoniae and mortality after four days of therapy
| Strain | MIC (mg/liter) | Mortality (%) for
|
|
|---|---|---|---|
| Amoxicillin | Amoxicillin-Clavulanate | ||
| ATCC 10813 | 0.01 | 0 | |
| R20 | 0.03 | 0 | 0 |
| 131 | 0.06 | 0 | |
| 1396 | 0.25 | 0 | 0 |
| R3 | 0.5 | 0 | 0 |
| 1325 | 0.5 | 0 | 0 |
| 1020 | 1.0 | 0 | 0 |
| 1293 | 2.0 | 20 | 0 |
| 138 | 2.0 | 40 | 0 |
| 1329 | 4.0 | 80 | 80 |
| 146 | 4.0 | 100 | 100 |
| 145 | 4.0 | 100 | 80 |
| 673 | 5.6 | 80 | 100 |
Correlation between in vivo efficacy and duration of time serum levels exceeds the MIC.
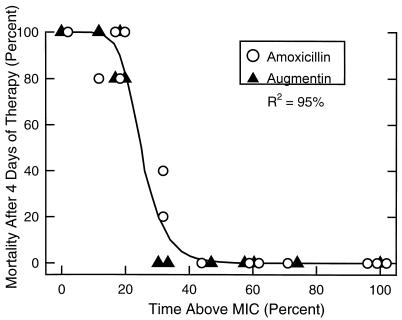
As shown in Fig. 3, there was an excellent correlation between mortality after 4 days of therapy and the duration of time that serum levels exceeded the MIC for both amoxicillin and amoxicillin-clavulanate (R2 = 95%). The highest mortality rates, 80 to 100%, were seen when serum levels exceeded the MIC for less than 20% of the dosing interval. Maximal survival was approached when serum levels exceeded the MIC for 40% of the 8-h dosing interval, or approximately 3 h per dose.
FIG. 3.
Relationship between mortality and duration of time that serum levels exceed the MIC following doses of amoxicillin at 2, 7, and 20 mg/kg and amoxicillin-clavulanate at 7 mg/kg every 8 h. Each value represents the mean for two thighs.
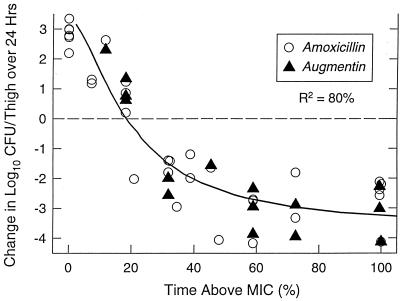
The correlation between therapeutic efficacy, measured by killing or growth of bacteria at 24 h, and the duration of time that serum levels exceeded the MICs of amoxicillin and amoxicillin-clavulanate against the various organisms is shown in Fig. 4. The graph also includes data from the study of additional dosage regimens. Organisms that were not killed at 7 mg/kg/8 h were killed at dosage regimens of 20 mg/kg/8 h. Organisms that were killed at 7 mg/kg/8 h exhibited growth when the dose was reduced to 2.0 or 0.5 mg/kg/8 h. An excellent correlation between in vivo antimicrobial activity and time above MIC was obtained (R2 = 80%). A static effect or no net growth was demonstrated when the time above MIC was 20 to 30% of the dosing interval. Maximum in vivo killing was observed with time above MICs of 50 to 60% of the 8-h dosing interval.
FIG. 4.
Relationship between change in log10 CFU/thigh over 24 h and duration of time that serum levels exceed the MIC following doses of 2, 7, and 20 mg of amoxicillin per kg every 8 h and doses of 7 mg of amoxicillin-clavulanate per kg every 8 h. Each value represents the mean for two thighs.
DISCUSSION
Escalating resistance to antimicrobial agents seen in clinical isolates of S. pneumoniae has reduced the efficacy of many oral drugs used to treat respiratory infections such as otitis media (2–4). Assessing the utility of different antimicrobial agents and respective dosing regimens against resistant pneumococci has thus become a pressing priority. The use of animal models to predict effective dosing regimens in humans is one approach to this problem (5, 7). Despite the many positive aspects of animal models, several criticisms, such as altered pharmacokinetics in animals, have precluded direct application of results to clinical practice in humans. By altering the renal kinetics of amoxicillin in the murine thigh infection model, we have simulated the serum concentration time course of this agent observed in humans. Doses of 7 mg/kg produced a kinetic elimination profile similar to that seen with a 500-mg oral dose in humans with normal renal function. However, subcutaneous absorption of amoxicillin in mice was faster than gastrointestinal absorption of the drug in humans. This resulted in similar peak concentrations but a shorter time above MIC in the mice than would be observed in humans.
Prior experiments in a murine infection model have shown that the in vivo efficacies of antimicrobial agents correlate closely with their respective in vitro activities (6, 20, 24). Several clinical studies have demonstrated a similar relationship between MICs and in vivo outcomes (1, 17). In our infection experiments, the in vivo efficacies of amoxicillin and amoxicillin-clavulanate were inversely related to the MICs for the infecting organisms over a wide range of susceptibilities, including penicillin-resistant organisms. At a dose of 7 mg/kg, bacterial killing was observed for organisms for which MICs were as high as 2 mg/liter. A recent clinical trial of augmentin in acute otitis media demonstrated equal efficacies in patients infected with penicillin-susceptible, -intermediate and resistant strains of S. pneumoniae (15). The highest MIC of amoxicillin observed in this study was 2 mg/liter.
Several experimental models have proven that therapeutic efficacy for the beta-lactam class of antibiotics correlates best with the duration of time that serum levels exceed the MIC for the infecting organism (8, 9, 12). With the penicillins, bacteriostatic efficacy has been achieved in the thigh infection studies when the magnitude of this parameter reaches 25 to 30%, while maximal bacteriologic efficacy and survival are predictably seen when serum levels remain above the MIC for 40 to 50% of the dosing interval (8). We found that the percentage of time above the MIC required for utility of amoxicillin and amoxicillin-clavulanate did not differ for penicillin-susceptible and -resistant strains of S. pneumoniae. Again, bacteriologic efficacy and survival were maximal when serum levels exceeded the MIC for 40 to 50% of the 8-h dosing interval.
Determination of MIC breakpoints of amoxicillin and amoxicillin-clavulanate in humans can be estimated by calculating the highest drug concentration that is maintained in serum for at least 50% of the dosing interval with standard oral doses. Following a 250- and a 500-mg dose of amoxicillin in normal volunteers, serum levels exceed 1.0 and 2.0 mg/liter, respectively, for at least 50% of the dosing interval. NCCLS-approved MIC breakpoints for these agents are currently 0.5 mg/liter for susceptible, 1.0 mg/liter for intermediate, and 2.0 mg/liter for resistant strains. Based on the simulation of human pharmacokinetics in this animal model, estimated breakpoints of amoxicillin and amoxicillin-clavulanate for S. pneumoniae would be 1 dilution higher, at 1.0 mg/liter for susceptible, 2.0 mg/liter for intermediate, and 4.0 mg/liter for resistant strains. If the recently suggested dosage escalation to 875 mg twice daily is considered, serum levels would remain above a MIC of 1 mg/liter for an adequate percentage of the 12-h dosing interval, making the above-mentioned breakpoints acceptable for this regimen as well. Our results, even in the presence of profound neutropenia and the high clinical success with amoxicillin-clavulanate against strains of S. pneumoniae for which amoxicillin MICs are as high as 2 mg/liter, support a reevaluation of current breakpoints.
ACKNOWLEDGMENTS
We thank Fred Tenover of the CDC and Gary Doern of the University of Iowa for providing the clinical isolates used in these experiments.
This work was supported by a grant from SmithKline Beecham, Philadelphia, Pa.
REFERENCES
- 1.Abboud M F, Waisbren B A. Correlation between in vitro studies and response to antibiotic therapy in staphylococci bacteremia. Am J Med. 1959;104:226–233. doi: 10.1001/archinte.1959.00270080052006. [DOI] [PubMed] [Google Scholar]
- 2.Asensi F, Perez-Tamarit D, Otero M C, et al. Imipenem-cilastin therapy in a child with meningitis caused by a multiply resistant pneumococcus. Pediatr Infect Dis J. 1989;163:895. doi: 10.1097/00006454-198912000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Barry B, Gehanno P, Bluman M, Boucot I. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Clinical outcome of acute otitis media due to Streptococcus pneumoniae with decreased susceptibility to penicillin, abstr. 1373; p. 370. [Google Scholar]
- 4.Block S, Hedrick J, Harrison C, et al. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Penicillin-resistant S. pneumoniae in acute otitis media, abstr. 1046; p. 311. [Google Scholar]
- 5.Craig, W. 1993. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. 1):55–57. [DOI] [PubMed]
- 6.Craig, W. A., J. Redington, and S. C. Ebert. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl. C):29–40. [DOI] [PubMed]
- 7.Craig W A, Andes D R. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Craig W A, Ebert S, Wantanabe Y. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Differences in time above MIC required for efficacy of beta-lactams in animal models, abstr. 86; p. 135. [Google Scholar]
- 9.Craig W A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22:89–93. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 10.Dagan R, Abramason O, Leibovitz E, Greenberg D, Lang R, Goshen S, Yagupsky P, Leiberman A, Fliss D M. Bacteriologic response to oral cephalosporins: are established susceptibility breakpoints appropriate in the case of acute otitis media? J Infect Dis. 1997;176:1253–1259. doi: 10.1086/514120. [DOI] [PubMed] [Google Scholar]
- 11.Friedland I R, Shelton S, Paris M, et al. Dilemmas in diagnosis and management of cephalosporin-resistant Streptococcus pneumoniae meningitis. Pediatr Infect Dis J. 1993;12:196–200. doi: 10.1097/00006454-199303000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Frimodt-Moller N, Bentzon M W, Thomsen V F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986;154:511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- 13.Gehanno P, Lenoir G, Berche P. In vivo correlates for Streptococcus pneumoniae penicillin resistance in acute otitis media. Antimicrob Agents Chemother. 1994;39:271–272. doi: 10.1128/aac.39.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacomini K, Roberts S M, Levy G. Evaluation of methods for producing renal dysfunction in rats. J Pharm Sci. 1981;70:117–121. doi: 10.1002/jps.2600700202. [DOI] [PubMed] [Google Scholar]
- 15.Hoberman A, Paradise J L, Block S, Burch D J, Jacobs M R, Balanescu M I. Efficacy of amoxicillin-clavulanate for acute otitis media: relation to Streptococcus pneumoniae susceptibility. Pediatr Infect Dis J. 1996;15:955–962. doi: 10.1097/00006454-199610000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Holford N H, Sheiner L B. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Pharmacokinetics. 1981;6:429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe H J, Biddle J W, Thornsberry C, Johnson R E, Kaufman R E, Reynolds G H, Wiesner P J the Cooperative Study Group. National gonorrhea therapy monitoring study. In vitro antibiotic susceptibility and its correlation with treatment results. N Engl J Med. 1976;294:5–9. doi: 10.1056/NEJM197601012940102. [DOI] [PubMed] [Google Scholar]
- 18.Klassen M, Edberg S C. Measurement of antibiotics in human body fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: Williams and Wilkins Co.; 1996. pp. 230–295. [Google Scholar]
- 19.Kleiman M D, Wienbery G A, Reynolds J K, Allen S D. Meningitis with beta-lactam resistant Streptococcus pneumoniae: the need for early repeat lumbar puncture. Pediatr Infect Dis J. 1993;12:782–784. doi: 10.1097/00006454-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Leggett J E, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Maftie H, Craig W A. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989;159:281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- 21.Moonsammy G, Tucher R, Rosen R, Drehobl M the LRTI/UTI Collaborative Study Group. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Improved safety profile of a new amoxicillin/clavulanate (A/C) adult BID formulation compared with the standard A/C TID formulation, abstr. LM51; p. 290. [Google Scholar]
- 22.Thornsberry C, Ogilvie P, Kahn J, Mauriz Y. Program and abstracts of the 35th Annual Meeting of the Infectious Diseases Society of America. Calif: San Francisco; 1997. Surveillance of antimicrobial resistance in S. pneumoniae and H. influenzae and M. catarrhalis in the U.S. during the 1996–97 respiratory season, abstr. 53; p. 82. [Google Scholar]
- 23.Todd P A, Benfield P. Amoxicillin/clavulanic acid: an update of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1990;39:264–307. doi: 10.2165/00003495-199039020-00008. [DOI] [PubMed] [Google Scholar]
- 24.Vogleman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]