ABSTRACT
Klebsiella pneumoniae carbapenemase-2 (KPC-2) presents a clinical threat as this β-lactamase confers resistance to carbapenems. Recent variants of KPC-2 in clinical isolates contribute to concerning resistance phenotypes. Klebsiella pneumoniae expressing KPC-2 D179Y acquired resistance to the ceftazidime/avibactam combination affecting both the β-lactam and the β-lactamase inhibitor yet has lowered minimum inhibitory concentrations for all other β-lactams tested. Furthermore, Klebsiella pneumoniae expressing the KPC-2 D179N variant also manifested resistance to ceftazidime/avibactam yet retained its ability to confer resistance to carbapenems although significantly reduced. This structural study focuses on the inhibition of KPC-2 D179N by avibactam and relebactam and expands our previous analysis that examined ceftazidime resistance conferred by D179N and D179Y variants. Crystal structures of KPC-2 D179N soaked with avibactam and co-crystallized with relebactam were determined. The complex with avibactam reveals avibactam making several hydrogen bonds, including with the deacylation water held in place by Ω loop. These results could explain why the KPC-2 D179Y variant, which has a disordered Ω loop, has a decreased affinity for avibactam. The relebactam KPC-2 D179N complex revealed a new orientation of the diazabicyclooctane (DBO) intermediate with the scaffold piperidine ring rotated ~150° from the standard DBO orientation. The density shows relebactam to be desulfated and present as an imine-hydrolysis intermediate not previously observed. The tetrahedral imine moiety of relebactam interacts with the deacylation water. The rotated relebactam orientation and deacylation water interaction could potentially contribute to KPC-mediated DBO fragmentation. These results elucidate important differences that could aid in the design of novel β-lactamase inhibitors.
KEYWORDS: β-lactamase, antibiotic resistance, protein crystallography, β-lactamase inhibitor
INTRODUCTION
Antimicrobial resistance is one of the most urgent global health problems. A major class of antibiotics are the β-lactam antibiotics (i.e., penicillins, cephalosporins, and carbapenems) (1). These antibiotics inhibit the penicillin-binding protein-mediated crosslinking of peptidoglycan strands in the bacterial cell wall (2), yet resistance against β-lactams is common in large part due to β-lactamases (3, 4). Several of the β-lactamases, in particular KPC- and OXA-type variants, and NDM-1, are clinically problematic as they can inactivate newer β-lactams including later-generation cephalosporins and carbapenems (5 – 7).
To overcome β-lactamase-mediated antibiotic resistance, β-lactamase inhibitors (BLIs) were developed (i.e., clavulanate, sulbactam, tazobactam, and more recently avibactam, relebactam, and vaborbactam) (1). Relebactam and avibactam (Fig. 1) are diazabicyclooctanes (DBOs), whereas vaborbactam is a boronic acid-based inhibitor. These latter three BLIs have potent activity against a wide range of serine β-lactamase, restoring susceptibility of the partner β-lactam (8 – 13).
Fig 1.

The chemical structures of avibactam and relebactam. The unique R1 groups and atom numbering of the scaffold atoms of each inhibitor are indicated.
The clinical efficacy of the older BLIs (clavulanate, sulbactam, and tazobactam) has decreased since they have a relatively narrow β-lactamase inhibitory spectrum phenotype combined with the occurrence of inhibitor-resistant TEM or SHV β-lactamase variants once these mechanism-based inactivators were introduced (14). Resistance is unfortunately also observed for the newer β-lactam/β-lactamase inhibitor combinations, in particular ceftazidime/avibactam (15, 16). Resistance to this latter combination is mainly due to changes related to β-lactamases and factors affecting permeability mediated by porins and efflux pumps (16). Resistance to meropenem/vaborbactam is primarily due to mutations affecting porins, whereas resistance to imipenem/relebactam can be caused by mutations affecting porins and overexpression of certain β-lactamases including KPC-2/-3 (16).
In the case of β-lactamase-mediated resistance to ceftazidime/avibactam, a particularly common site of resistance substitutions is the Ω loop of β-lactamases (16). This Ω loop plays a critical role in the catalytic activity, and in class A, β-lactamases harbor residues E166 and N170 that position the important deacylation water with E166 being the base to prime this water (17 – 20). One often observed altered Ω loop residue is D179 in KPC-2 (21); when changed to Y, this substitution affects both the affinity and turnover of ceftazidime and decreases the affinity for avibactam (22 – 24). We recently structurally characterized the D179Y and D179N KPC-2 variants and observed Ω-loop disorder for the former variant and an ordered yet destabilized and shifted Ω loop for the latter variant (25). We hypothesize that the Ω-loop destabilization/disorder allows the large and relatively rigid ceftazidime to be accommodated in these variants and that ceftazidime can aid in substrate-assisted deacylation (25). The altered loop in D179N KPC-2 (i.e., KPC-51) likely contributes to this variant having mostly wild-type (WT) KPC-2 resistance levels for most β-lactams, yet this variant obtained resistance to ceftazidime/avibactam (26). In contrast, the disordered Ω-loop-containing D179Y KPC-2 variant (substitutions found in KPC-31, -32, -33, -52, -70, and -95) acquired resistance to ceftazidime/avibactam yet has become more susceptible (via lower MICs) to the other β-lactams (including carbapenems) (21, 26 – 31).
Not only the β-lactam but also the inhibitor partner in the β-lactam/β-lactamase combination can be affected by substitutions conferring resistance to ceftazidime/avibactam. Regarding variants at position 179, the D179Y KPC variant causes a 70,000 decrease in acylation rate (k 2/Ki ) by avibactam compared to WT KPC-2 (24); the slower on-rate for avibactam binding to KPC-2 D179Y compared to KPC-2 was also observed by mass spectrometry [<10 s vs >1 min for KPC-2 D179Y (32)]. Due to its less potent inhibition, avibactam could only partially restore microbiological ceftazidime efficacy for this D179Y variant but not to the level of susceptibility (22). In contrast to D179Y, the KPC-2 D179N variant yields a threefold decrease in Ki app for avibactam indicating improved inhibition (33). The opposite effect of these two D179 variants can be explained in that the D179N variant still has its Ω loop ordered although destabilized, whereas the D179Y mutant has a disordered Ω loop; avibactam binds in the vicinity of the Ω loop (25).
Unlike avibactam, meropenem/vaborbactam and imipenem/relebactam have not yet clinically been shown to be negatively affected by the substitutions in the Ω loop of β-lactamases, although they are reported in in vitro mutants (34). This agrees with the observation that the IC50 for vaborbactam inhibition of the D179Y KPC variant only modestly increases (approximately twofold) compared to WT KPC-2 (22). A similar statement can be made for the ability of relebactam to inactivate D179Y (35). Although the inhibition constants were not readily obtained, timed mass spectrometry at a 5-min time point reveals that the acylation of relebactam of D197Y was slower suggesting an increased K i . Regarding relebactam, the KPC-2 D179N variant when expressed confers resistance to imipenem (MIC is 4 µg/mL), yet imipenem susceptibility is restored to 0.5 µg/mL when combined with relebactam (35, 36). Mass spectrometry analysis showed that relebactam forms a stable complex with D179N (35, 36). In a laboratory-generated KPC-3 D179Y variant expressing this β-lactamase in an Escherichia coli host, resistance was not observed for the imipenem/relebactam combination variant as both imipenem and imipenem/relebactam yield MICs in the susceptible range (1 and 0.5 µg/mL, respectively) (36, 37). Despite that the β-lactam-protecting ability of vaborbactam and relebactam seems clinically unaffected by the changes in Ω-loop residues in KPCs, a fourfold increase in imipenem/relebactam MIC was detected in the laboratory-generated KPC-3 L167R Ω-loop variant concomitant with a 76-fold increase in IC50 for relebactam (34).
Residue D179 is known to form a salt bridge interaction with R164, both situated in the neck of the Ω loop, which helps in stabilizing this motif (38, 39). Substitutions at position 179 will disrupt the salt bridge as well as the hydrogen bonding network, therefore, increasing the flexibility of the Ω loop and disordering the protein structure (25, 26, 38). A similar class A β-lactamase enzyme (SHV-1) has also shown flexibility in the Ω loop induced by ligand binding when the D179:R164 salt bridge is disrupted (38).
To gain structural insights into how the newer DBOs are impacted differently by changes in the KPC-2 Ω loop, crystal structures of KPC-2 D179N in complex with avibactam and relebactam were determined. These studies complement and advance our previous structural characterization of the vaborbactam complex with KPC-2 D179N as well as increase our knowledge of DBOs and their mechanism of inactivation (25).
RESULTS AND DISCUSSION
Effect of relebactam and avibactam binding on the thermal stability of WT and D179N KPC-2 β-lactamases
The thermal stability of the KPC-2 D179N and WT KPC-2 β-lactamases was first assessed in the absence and presence of relebactam and avibactam using differential scanning fluorimetry (DSF). The D179N substitution itself significantly decreases the thermal stability of KPC-2, as we noted previously [Fig. 2; (25)]. The melting temperature (Tm ) of KPC-2 D179N in the absence and presence of relebactam and avibactam increases from 43.8°C to 45.0°C and 45.4°C, respectively, indicating a protein stabilizing effect by the BLIs (Fig. 2). For comparison, the Tm of WT KPC-2 in the presence of relebactam and avibactam increased from 54.8°C, for unbound KPC-2, to 59.6°C and 60.2°C, respectively. These data indicate that both relebactam and avibactam bind and stabilize the WT KPC-2 and the KPC-2 D179N variant. However, their thermal stabilizing effect is smaller in the variant compared to that of WT KPC-2. The larger increase in Tm for avibactam compared to relebactam correlates with the fact that IC50 for avibactam inhibition of KPC-2 is 2.3–23-fold lower compared to relebactam (10, 40).
Fig 2.
Differential scanning fluorimetry/thermal shift assay of WT KPC-2 and KPC-2 D179N in the presence or absence of 0.5 mM avibactam or 0.5 mM relebactam.
Crystal structure of avibactam bound to D179N KPC-2 and comparison with that WT KPC-2 complex
The crystal structure of D179N KPC-2 bound to avibactam at 1.28 Å resolution was determined (Fig. 3; Table 1 for refinement statistics). The electron density in the active site reveals that avibactam binds covalently to the catalytic S70 of KPC-2 D179N. The inhibitor forms several hydrogen bonds with the active site residues T216, N132, S130, T235, T237, and three water molecules, including the deacylation water (water 2; Fig. 3B). The binding mode of avibactam and its interactions in KPC-2 D179N is similar to its binding mode in WT KPC-2 (41). One exception is that the sulfate moiety is in a slightly different orientation, with the adjacent nitrogen being closer to the carbonyl carbon for recyclization/deacylation in the WT KPC-2 structure compared to the KPC-2 D179N avibactam complex (Fig. 4). This difference yields an additional hydrogen bond between the oxygen of the sulfate group of avibactam and the side chain hydroxyl of T216 in the KPC-2 D179N complex (24) (Fig. 4). Differences in the orientation of this sulfate group for DBOs bound to β-lactamases have been observed previously (40, 41).
Fig 3.
Avibactam complex crystal structure with KPC-2 D179N. (A) Avibactam (cyan-colored carbons) is covalently bound to S70 in the active site of KPC-2 D179N. The protein is colored green, and the Ω loop is colored yellow. Relevant active site residues are labeled with N179 in bold. (B) Unbiased |Fo|-|Fc| omit map contoured at 3.00 σ showing avibactam covalently bound (zoomed-in view rotated 90° from view in A). The map was obtained after removing avibactam from the model and subsequent 10 cycles of crystallographic refinement prior to the map calculation. (C) Interactions of avibactam in the active site of KPC-2 β-lactamase. Hydrogen bonds are represented by black dashed lines, and water molecules are shown in red spheres. Relevant active site residues are represented in the stick model and are labeled.
TABLE 1.
Data collection and refinement statistics for KPC-2 D179N complexes of avibactam and relebactam
| KPC-2 D179N + avibactam (soaked) | KPC-2 D179N + relebactam (co-crystallized) | |
|---|---|---|
| Wavelength (Å) | 0.92010 | 0.97935 |
| Resolution (Å) | 49.06–1.28 (1.28–1.30) | 29.63–1.26 (1.26–1.29) |
| Space group | P212121 | P212121 |
| Unit cell (Å, °) | 51.45, 66.67, 72.44, 90, 90, 90 | 50.80, 66.23, 72.98, 90, 90, 90 |
| Unique reflections | 60,468 (3,133) | 66,874 (4,781) |
| Multiplicity | 7.4 (7.7) | 13.1 (12.5) |
| Completeness (%) | 92.8 (97.9) | 99.9 (98.4) |
| Mean I/sigma I | 9.4 (0.7) | 21.0 (3.5) |
| R merge | 0.087 (3.026) | 0.068 (0.683) |
| CC1/2 | 0.998 (0.361) | 1.00 (0.931) |
| Refinement | ||
| R-work | 0.167 | 0.134 |
| R free | 0.204 | 0.167 |
| Number of ligand atoms | 17 | 26 |
| Number of water molecules | 235 | 376 |
| Number of protein residues | 263 | 263 |
| r.m.s.d. bond lengths (Å) | 0.012 | 0.013 |
| r.m.s.d. bond angles (°) | 1.60 | 1.63 |
| Ramachandran plot favored (%) | 98.1 | 98.9 |
| Ramachandran plot allowed (%) | 1.9 | 1.2 |
| Ramachandran plot outliers (%) | 0.0 | 0.0 |
Fig 4.
Superimposition of the avibactam-bound structures of KPC-2 D179N and WT KPC-2 β-lactamases. (A) Superimposition showing avibactam-bound active sites of WT KPC-2 (gray color; PDB accession number 4ZBE) (41) and KPC-2 D179N (green color) with avibactam with cyan-colored carbon atoms (avibactam in both structures shown in ball-and-stick). (B) The view is zoomed-out and rotated compared to panel (A) to include the Ω loop (shown in the stick model). The rotations of R164 away and residue D163 toward the site of the D179N mutation are indicated by the red and purple arrows, respectively.
The Ω-loop residues close to the avibactam binding site in the KPC-2 D179N avibactam complex adopt a conformation reminiscent of WT KPC-2 (Fig. 4). In contrast, the Ω-loop region near residue 179 does have conformational differences compared to the WT KPC-2 avibactam complex as a result of the D179N change (Fig. 4). This change leads to loss of the negative charge at residue 179 causing R164 to rotate away, whereas D163 has moved toward N179 to form a hydrogen bond with the nitrogen of the N179 side chain (Fig. 4). These conformational changes are accompanied with backbone shifts as well of the residues near residue 179. These D179N-induced Ω-loop conformational changes were also observed in the apo- and vaborbactam-complexed KPC-2 D179N structures (25). The apo KPC-2 D179N structure has many additional movements in the Ω loop, including near E166 and N170 and the deacylation water, which shift to a mostly WT Ω-loop conformation when bound to avibactam and vaborbactam. We advance that because of the different apo KPC-2 D179N Ω-loop structure that the intact avibactam encounters prior to binding and/or the observed sulfate moiety orientational difference could (in part) explain why KPC-2 D179N has a threefold lower Ki app for avibactam than WT KPC-2 (26). Alternatively, the apparent increase in inhibition can perhaps be explained by the Ω loop, since it is destabilized [this study and reference (25)] and can perhaps slightly shift during some of the acylation and deacylation steps to improve avibactam affinity compared to WT KPC-2. While both D179Y and D179N in KPC-2 contribute to resistance to ceftazidime via inducing disorder and destabilization of the Ω loop, respectively, the effects of those D179 variations on the inhibitor avibactam are not the same as discussed below.
The avibactam complex structure could also explain why, in contrast to the KPC-2 D179N variant, the KPC-2 D179Y variant has a strong negative effect on avibactam affinity [i.e., 70,000 lower acylation rate (k 2/Ki ) (24)]; whereas the affinity for vaborbactam has only decreased twofold (22). The KPC-2 D179Y structure indicated a complete disorder for the Ω loop, including residues E166 and N170 and the deacylation water (25). Since the amide moiety of avibactam makes a 2.9 Å water-mediated interaction with the deacylation water held in place by Ω-loop residues E166 and N170 (water 2 in Fig. 3) and vaborbactam does not (25), the disorder in the Ω loop is anticipated to have a larger effect on avibactam (and possibly relebactam) binding to KPC-2 D179Y compared to vaborbactam. Also, the amide moiety atoms of avibactam are at closer van der Waals distances to N170 compared to vaborbactam as previously noted (i.e., the shortest distance is 3.4 Å for avibactam compared to 3.8 Å for vaborbactam) (25). These water-mediated or direct Ω-loop interaction differences of avibactam and vaborbactam could explain their significant differences in affinity in the KPC-2 D179Y variant. Alternatively, there are larger chemical changes, and thus likely conformational changes during the acylation of avibactam (i.e., bond broken causing loss of the five-membered ring) compared to vaborbactam; the latter involves merely a change from sp 2 to sp 3 of the boron atom. These initial binding steps involving the Michaelis-Menten complex, which are structurally not yet elucidated and could possibly involve some Ω-loop residues or deacylation water, could also be the basis for why KPC-2 D179Y with a disordered Ω loop is more poorly inhibited by avibactam than vaborbactam.
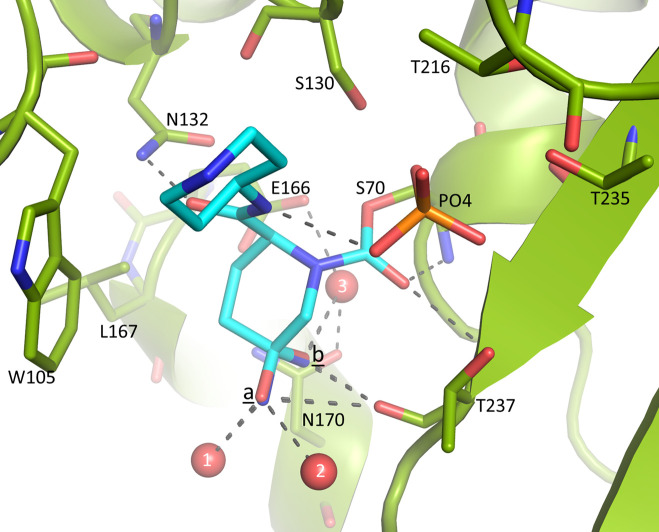
Crystal structure of D179N KPC-2 bound with relebactam
The crystal structure of D179N KPC-2 co-crystallized with relebactam is determined at 1.26 Å resolution (see Table 1 for refinement statistics). The unbiased omit electron density map shows relebactam in the active site covalently bound to the catalytic S70 (Fig. 5). The electron density also indicates that relebactam is desulfated (Fig. 5). Furthermore, the electron density at the main piperidine ring position C5 of relebactam is tetrahedral, indicating an imine hydrolysis intermediate, instead of planar as in the desulfated imine-species (Fig. 5). This planar imine species was observed in other DBO complexes (40, 42). In contrast, the tetrahedral imine hydrolysis intermediate was previously postulated to exist for the DBO avibactam (43) via a general Schiff base hydrolysis mechanism (44). The chirality at the C5 position on the piperidine DBO scaffold ring could neither be definitively assigned via crystallographic refinement and careful B-factor analysis nor via analysis of difference density peak height or difference density shift peaks. Therefore, we modeled the imine hydrolysis intermediate with alternate conformations for the nitrogen and oxygen atom substituents at ring position 5, each at 50% occupancy (at positions labeled a and b, Fig. 5 and 6). The nitrogen or oxygen atom at the b position is hydrogen bonding with the deacylation water (No. 3) and is also situated at 3.0 Å from the backbone carbonyl oxygen of T237. The nitrogen or oxygen in the a position is similarly located at 3.0 Å from the backbone carbonyl oxygen of T237 and is also interacting with two water molecules (No. 1 and No. 2) (Fig. 6).
Fig 5.
Relebactam bound in the active site of KPC-2 D179N. (A) Unbiased omit |Fo|-|Fc| map is obtained after removing relebactam from the model; refinement prior to map calculation to remove phase bias and contour levels is as in Fig. 3. Relebactam is shown in a stick model with cyan carbon atoms; a nearby phosphate ion is labeled “PO4.” The alternate conformations for the oxygen and nitrogen atom substituents attached to the tetrahedral carbon 5 atom (labeled “5”) of the main piperidine ring are labeled “a” and “b.” (B) Same as panel (A) but the view is rotated ~90°.
Fig 6.
Close-up view of relebactam interactions in the active site of KPC-2 D179N. Hydrogen bonds are represented by black dashed lines. Crystallographically observed water molecules interacting with relebactam are shown as red spheres (labeled “1,” “2,” and “3”); the interacting phosphate is labeled “PO4.”
In addition, relebactam makes the following interactions: its carbonyl oxygen makes hydrogen bonds with the oxyanion hole residues (S70 and T237 backbone amide nitrogens), and the amide oxygen makes a hydrogen bond with N132 (Fig. 6). Relebactam also makes hydrophobic interactions with W105 via its piperidine R1 ring, and the scaffold piperidine ring makes hydrophobic interactions with L167. Finally, relebactam also hydrogen bonds with a phosphate ion (an ingredient of crystallization buffer) bound in the active site (Fig. 6). Phosphate ions have been observed at this site in serine β-lactamases [PDBid 2P74 (45)], but so have sulfate ions (40, 42) as sulfate ions are released upon DBO desulfation. We tested refining either a sulfate or phosphate ion at this position, and the former yields negative difference peaks at the sulfur atom, indicating the density corresponds to a phosphate ion (phosphate ions are also at higher concentration due to their presence in the crystallization buffer).
The observation that relebactam bound to KPC-2 D179N is desulfated is not too surprising as it was also observed by protein crystallography and mass spectrometry for WT KPC-2 (46). However, earlier mass spectrometry studies did not observe this including for KPC-2 D179N (10, 36, 42); this is probably due to different experimental conditions and/or mass spectrometry settings.
Structural comparisons of relebactam-bound WT KPC-2 and KPC-2 D179N
A comparison was done between the co-crystallized relebactam KPC-2 D179N structure and the WT KPC-2 relebactam complex; the latter was obtained by soaking the inhibitor for 16 hours (46) (PDB ID: 6QW9) (Fig. 7). The latter complex contains two conformations of relebactam; one conformation represents an acylated relebactam (at 0.65 occupancy, light gray carbon atoms in Fig. 7), and the other one represents a desulfated relebactam as an imine intermediate (at 0.35 occupancy; dark gray carbons in Fig. 7). The carbonyl oxygen is situated in the oxyanion hole in both structures making hydrogen bonds with backbone nitrogens of S70 and T237. One key difference between the relebactam-bound WT and mutant KPC-2 structures is the orientation of the DBO scaffold piperidine ring; this ring is rotated ~150° via a rotation around the C7-N1 bond (Fig. 7). Such a DBO piperidine scaffold ring orientation observed in the KPC-2 D179N complex has not been previously observed to our knowledge. The different relebactam orientation causes the piperidine ring of its R1 group to be on opposite ends of the active site in the two complexes. Remarkably, despite the different piperidine scaffold orientations, the oxygen of relebactam’s amide moiety interacts with N132 in both complexes aided by the rotation around the bond between the amide moiety and the C2 ring atom (Fig. 7).
Fig 7.
Comparison of relebactam-bound WT KPC-2 (PDB ID: 6QW9) and KPC-2 D179N. (A) Superimposition of relebactam in the active site of WT KPC-2 [PDB ID: 6QW9 (46); in gray] and relebactam-bound KPC-2 D179N (green with relebactam shown with cyan carbon atoms). Relebactam is represented in ball-and-stick in both structures. Relebactam in the WT KPC-2 complex is observed as two intermediates, a sulfated acyl-intermediate (light gray carbon atoms) and a desulfated acyl-intermediate (dark gray carbon atoms) with occupancies of 0.65 and 0.35, respectively. The atom numbers for the main scaffold atoms of relebactam are shown in black and gray numbers for the KPC-2 D179N and WT KPC-2 complexes, respectively (atom numbering for the desulfated intermediate in WT KPC-2 is followed by an apostrophe). The two conformations of W105 in the WT KPC-2 complex and the oxygen and nitrogen atoms on the ring of relebactam in the KPC-2 D179N complex are indicated by labels “a” and “b.” The large reorientation of the piperidine ring of relebactam in the WT KPC-2 structure compared to the KPC-2 D179N complex is indicated by the red arrow showing the movement of the piperidine ring carbon “5” atom. The hydrogen bond made by the amide oxygen of relebactam with N132 in both complexes is indicated by a dashed line. The deacylation water held in place by E166 and N170 in the WT KPC-2 and KPC-2 D179N structures is labeled “wtW” and “W 3,” respectively. (B) Zoomed-out view to include the Ω-loop residues. The rotation of R164 away and residue D163 toward the site of the D179N mutation are indicated by the red and purple arrows, respectively.
Another important conformational difference is the geometry around the C5 ring atom when comparing the desulfated relebactam species: it is planar for the 0.35 occupancy relebactam imine species bound to WT KPC-2 yet tetrahedral for relebactam complexed to KPC-2 D179N (Fig. 7). The former structure was obtained by soaking, whereas the latter D179N variant structure was obtained by co-crystallization.
In addition to the above, protein conformational differences in the active site between the KPC-2 D179N and WT KPC-2 relebactam-bound structures are also present. First, W105 is observed in two conformations in the latter, and only one is observed in the KPC-2 D179N complex; this is likely a result of the piperidine ring of its R1 group sterically clashing with that second possible W105 conformation in the KPC-2 D179N complex structure (Fig. 7). Significant shifts are also observed (listed in parenthesis) for residues N132 (1.3 Å), L167 (1.1 Å), N170 (1.1 Å), and the backbone atoms of C238 (1.8 Å; Fig. 7). The deacylation water, held in place by E166 and N170, has also shifted 1.0 Å (Fig. 7). Note, this water is not involved in the reversible deacylation of DBOs as DBO deacylation involves a different mechanism (43) (Fig. 8).
Fig 8.
Schematic of the relebactam inhibition pathway of KPC-2. The relebactam intermediate structure captured by protein crystallography is the imine hydrolysis intermediate. Note that the mass spectrometry accuracy is mentioned to be about 1 Da (43). The different possible states in the relebactam inhibition pathway are labeled A through G.
Regarding changes in the Ω loop, as observed in the avibactam-bound KPC-2 D179N structure, residues R164 and D163 in the Ω loop have shifted due to the D179N mutation; such side chain shifts were also observed for the apo KPC-2 D179N and vaborbactam-bound structures (25) (Fig. 7). Overall, the shifts in the Ω loop are larger in the relebactam KPC-2 D179N complex than the avibactam KPC-2 D179N complex (Fig. 4 and 7). The relebactam KPC-2 D179N complex protein conformation resembles more closely to the apo KPC-2 D179N structure (25) as the root mean square deviation (r.m.s.d.) of 263 Cα atoms is 0.26 Å (25); in contrast, the r.m.s.d. between the relebactam-bound KPC-2 D179N and WT KPC-2 structures is 0.62 Å 263 Cα atoms.
Mechanistic implications of observed imine hydrolysis intermediate for relebactam
Although it was proposed to exist for avibactam (43), the observed imine-hydrolysis intermediate for a DBO has not been crystallographically observed to the best of our knowledge. Based on our structure, we have updated the previous reaction scheme of DBO acylation, deacylation, desulfation, and fragmentation (43) to include intermediate E (for relebactam, see Fig. 8). We emphasize that imine-hydrolysis intermediate E has the same molecular weight (acyl-80) as the postulated hydroxyl-amine intermediate C. This acyl-80 intermediate C was postulated to exist based on mass spectrometry data for both avibactam- and relebactam-bound KPC-2 complexes (43, 46). Intermediate C has never been crystallographically observed, but intermediate E has now been observed and is a relatively stable intermediate as it is stable enough to last the multi-day co-crystallization experiment (this study). It is possible that the mass spectrometry-observed mass for the acyl-80 intermediate is intermediate E instead and that intermediate C is only generated in low quantities or not at all.
The mechanism whereby the imine-hydrolysis intermediate E is generated is an intriguing question and could aid in understanding the DBO desulfation and fragmentation mechanisms. First, the deacylation reaction of the acylated DBO intermediate B is believed to occur with its piperidine scaffold ring in the previously observed conformation (41, 42, 46) allowing for the attack by N6 atom of the C7 atom to re-form the intact DBO A (Fig. 8). Whether the desulfation step leading to intermediate D also occurs involving that same previously observed orientation of the piperidine scaffold ring is not yet known. However, the resulting imine intermediate D is in that same piperidine orientation as observed for both avibactam, relebactam, WCK 4234, WCK 5107, and WCK 5153 (41, 42, 46). It is possible the step between intermediates B and D might adopt a different piperidine ring orientation as it is otherwise difficult to explain why WCK 4234 is the only DBO that does not undergo KPC-mediated desulfation, yet WCK 4234 is observed with that same original DBO orientation that is also conducive to deacylation (42). If a reorientation is required, the more rigid nitrile moiety of WCK 4234 could prevent such a reorientation, thus explaining why it is not desulfated.
The next step leading to the formation of the imine hydrolysis intermediate E involves a water molecule that attacks the carbon atom of the imine moiety such that the C5 ring atom becomes tetrahedral (44) (intermediate E; Fig. 8). The relebactam KPC-2 D179N structure shows that the oxygen and nitrogen atoms attached to the C5 atom make stabilizing interactions including with the deacylation water (Fig. 6). These interactions via this piperidine ring orientation suggest that the deacylation water could play a role in this step going from intermediate D to E, either directly being the water that attacks the carbon of the imine moiety, or indirectly by hydrogen bonding with second water, which is the one that attacks the carbon of the imine moiety. Alternatively, that imine-reacting water could come from the other face of this imine moiety, and the deacylation water instead aids in stabilizing the nitrogen attached (in position b, Fig. 6) to the now tetrahedral C5 atom.
The observed rotated orientation of the DBO scaffold of relebactam in the KPC-2 D179N complex can explain several additional previous observations for relebactam and related DBOs (in addition to what the by mass spectrometry-observed acyl-80 species could be):
Why only the KPC-2-generated oxopiperidine product of avibactam is observed by mass spectrometry (43), which requires first an imine hydrolysis step and then decarboxylation (Fig. 8), and not a piperidin-3-imine product (nor a decarboxylated DBO product that still has the sulfate attached) is still unknown. If the desulfated oxopiperidine intermediate D and F adopted the same conformation as the desulfated imine-DBO conformation (intermediate D) (42, 46) then the catalytic water that leads to decarboxylation and product formation would likely not be influenced by either the nitrogen or oxygen atom attached to the planar C5 atom (i.e., intermediates D and F, respectively): the 4.5 Å distance between N6 and C7 is likely too large to affect the deacylating water, and the N6 atom is also pointing away from the carbonyl carbon bond. However, if the step involving the water attacking the carbon of the imine moiety (from intermediate D to E) can only occur with the DBO in the newly observed DBO scaffold piperidine orientation (Fig. 6) as well as the subsequent steps requiring this piperidine orientation (from intermediate E to F, and then from F to G), it would explain why only oxypiperidine product was observed for avibactam and not a hypothetical piperidin-3-imine product resulting directly from intermediate D (43). As noted above, the deacylation water, held in place by base E166, stabilizes the intermediate E (Fig. 6) and could thus also directly or indirectly assist in the steps from E to F and from F to G.
Why only the KPC enzyme has been shown to desulfate DBOs and not the similar Class A TEM-1 and CTX-M-15 β-lactamases (nor other Class C and D β-lactamases) (43)? One of the major differences between KPC-2, a carbapenemase, and non-carbapenemases TEM-1 and CTX-M-15 is the about 1.5 Å shift in the carbonyl oxygen of the main chain of T237. This shift is due to the presence of a disulfide bond in KPC-2 involving adjacent residue C238 but not in the other two β-lactamases (47). The carbonyl oxygen of T237 is observed to interact with the atoms in both positions a and b of the imine-hydrolysis intermediate (3.0 Å in each instance; Fig. 8). Such a stabilizing interaction would likely not be possible in the TEM-1 or CTX-M-15 β-lactamases.
Why relebactam desulfates slower than avibactam (46)? If the DBO desulfation reaction requires the ~150° rotation of the piperidine DBO scaffold ring, relebactam would be more sterically hindered in the active site compared to avibactam as relebactam has a much larger R1 group (Fig. 1).
Overall, these observations may help us understand how these compounds behave in the bacterial cell and how likely they are to inactivate other β-lactamases.
Conclusions
The structural analysis of the KPC-2 D179N complexes with avibactam and relebactam and comparison with our previously determined complex with vaborbactam (25) yielded important insights into how resistance mutations at this position affect these inhibitors differently. Our studies suggest that the destabilized Ω loop in KPC-2 D179N and the disordered Ω loop in KPC-2 D179Y affect inhibitor potency very differently depending on how the inhibitors interact with the Ω loop including via water-mediated interactions. The difference in the behavior of each inhibitor is in contrast to the β-lactam ceftazidime. In the case of ceftazidime, both KPC-2 D179Y and KPC-2 D179N increase catalytic turnover and lead to ceftazidime resistance in bacteria expressing these variants.
The co-crystallized relebactam KPC-2 D179N complex showed relebactam in an acylated yet desulfated imine-hydrolysis intermediate state. This unique conformation has possible implications in the KPC-2-mediated fragmentation of most DBOs and possibly even explains why KPC-2 is unique in its ability to desulfate DBOs.
Taken together, our analyses help us further explain an important phenotype exhibited by KPC and offer insights on how chemical modification in DBO design has an impact on inactivation chemistry.
MATERIALS AND METHODS
Protein expression and purification
Protein expression and purification using a pET-28a plasmid harboring D179N KPC-2 gene were carried out as previously described (25).
Differential scanning fluorimetry/thermal shift assay
The reaction volume for each DSF experiment was 30 µL containing 8.5 µM protein (KPC-2 or D179N KPC-2) in 50 mM Tris, pH 7, 100 mM NaCl buffer, and 10× SYPRO orange dye (Thermo Fisher), with or without relebactam and avibactam (0.5 mM final concentration). The thermal gradient was ramped up from 25°C to 90°C with 0.2°C/min intervals. The DSF thermal shift assay and fluorescence readout were performed in duplicate on a CFX96 Touch real-time PCR detection system (Bio-Rad), similar to the experiments performed previously (25).
Co-crystallization of KPC-2 D179N with relebactam
The purified KPC-2 D179N protein was concentrated to 17 mg/mL and incubated at room temperature for 30 min with 1 mM relebactam. Then, the KPC-2 D179N relebactam crystals were grown using vapor diffusion at 20°C in the sitting drop method. A volume of 1 µL of protein solution was mixed with 0.5 µL of a reservoir containing 100 mM HEPES pH 8.0 and 0.8 M NaH2PO4, 0.8 M KH2PO4 [condition D11 of MCSG3 crystallization screen (Anatrace, LCC)]. The crystals were cryo-protected in perfluoropolyether and frozen in liquid nitrogen prior to synchrotron data collection.
Crystallization and soaking of KPC-2 D179N with avibactam
Crystals of D179N KPC-2 were grown using the sitting drop method. The protein was concentrated to 16 mg/mL in 10 mM Tris, pH 8.0, 60 mM NaCl, and 10 mM sorbitol; 1 µL of protein was mixed with 1 µL of a reservoir containing 0.1 M sodium acetate pH 6 and 1.25 M ammonium citrate dibasic. The crystals grown from these conditions were used to soak with 1 mM of avibactam for 90 min at room temperature.
Data collection and refinement
X-ray diffraction data for avibactam and relebactam in complex with D179N KPC-2 were collected at the NSLS-II AMX and FMX beamlines, respectively, and processed using XDS (48). The crystal structures were solved via molecular replacement using the program PHASER (49) using chain A of the KPC-2 structure [PDB identifier 2OV5 (47)] as the initial search model. The crystallographic refinement was carried out by using Refmac (50) and model building with Coot (51). Table 1 contains data collection and refinement statistics. Refinement parameter files for relebactam and avibactam were generated using AceDRG (52). Molecular figures were generated using PyMOL 2.4.1. (www.pymol.org). The coordinates and structure factors of the KPC-2 D179N complexes with avibactam and relebactam have been deposited with the Protein Data Bank (PDB with accession numbers 8G2R and 8G2T, respectively).
ACKNOWLEDGMENTS
We thank support personnel at NSLS synchrotron AMX and FMX beamlines for their help with data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs.
The research reported in this publication was supported by a grant (MISP 57394) provided by Merck Sharp & Dohme, Corp., Kenilworth, NJ to R.A.B. This study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award number 1I01B × 001974, to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10.
Contributor Information
F. van den Akker, Email: focco.vandenakker@case.edu.
Laurent Poirel, University of Fribourg, Fribourg, Switzerland .
REFERENCES
- 1. Bush K, Bradford PA. 2019. Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol 17:295–306. doi: 10.1038/s41579-019-0159-8 [DOI] [PubMed] [Google Scholar]
- 2. van Heijenoort J. 2001. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiol 11:25R–36R. doi: 10.1093/glycob/11.3.25r [DOI] [PubMed] [Google Scholar]
- 3. Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. 2018. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 66:1290–1297. doi: 10.1093/cid/cix893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doi Y, Iovleva A, Bonomo RA. 2017. The ecology of extended-spectrum beta-lactamases (ESBLs) in the developed world. J Travel Med 24:S44–S51. doi: 10.1093/jtm/taw102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shapiro AB. 2017. Kinetics of sulbactam hydrolysis by beta-lactamases, and kinetics of beta-lactamase inhibition by sulbactam. Antimicrob Agents Chemother 61:e01612-17. doi: 10.1128/AAC.01612-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nordmann P, Poirel L. 2014. The difficult-to-control spread of carbapenemase producers among enterobacteriaceae worldwide. Clin Microbiol Infect 20:821–830. doi: 10.1111/1469-0691.12719 [DOI] [PubMed] [Google Scholar]
- 7. Papp-Wallace KM, Winkler ML, Taracila MA, Bonomo RA. 2015. Variants of the KPC-2 beta-lactamase which are resistant to inhibition by avibactam. Antimicrob Agents Chemother 59:3710–3717. doi: 10.1128/AAC.04406-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ehmann DE, Jahić H, Ross PL, Gu R-F, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blizzard TA, Chen H, Kim S, Wu J, Bodner R, Gude C, Imbriglio J, Young K, Park Y-W, Ogawa A, Raghoobar S, Hairston N, Painter RE, Wisniewski D, Scapin G, Fitzgerald P, Sharma N, Lu J, Ha S, Hermes J, Hammond ML. 2014. Discovery of MK-7655, a beta-lactamase inhibitor for combination with primaxin(R). Bioorg Med Chem Lett 24:780–785. doi: 10.1016/j.bmcl.2013.12.101 [DOI] [PubMed] [Google Scholar]
- 10. Papp-Wallace KM, Barnes MD, Alsop J, Taracila MA, Bethel CR, Becka SA, van Duin D, Kreiswirth BN, Kaye KS, Bonomo RA. 2018. Relebactam is a potent inhibitor of the KPC-2 beta-lactamase and restores imipenem susceptibility in KPC-producing enterobacteriaceae.. Antimicrob Agents Chemother 62:e00174-18. doi: 10.1128/AAC.00174-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shoulders BR, Casapao AM, Venugopalan V. 2020. An update on existing and emerging data for meropenem-vaborbactam. Clin Ther 42:692–702. doi: 10.1016/j.clinthera.2020.01.023 [DOI] [PubMed] [Google Scholar]
- 12. Iqbal Z, Sun J, Yang H, Ji J, He L, Zhai L, Ji J, Zhou P, Tang D, Mu Y, Wang L, Yang Z. 2022. Recent developments to cope the antibacterial resistance via beta-lactamase inhibition. Molecules 27:3832. doi: 10.3390/molecules27123832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohanty S, Singhal R, Sood S, Dhawan B, Das BK, Kapil A. 2005. Comparative in vitro activity of beta-lactam/beta-lactamase inhibitor combinations against gram negative bacteria. Indian J Med Res 122:425–428. [PubMed] [Google Scholar]
- 14. Cantón R, Morosini MI, de la Maza OMS, de la Pedrosa EGG. 2008. IRT and CMT beta-lactamases and inhibitor resistance. Clin Microbiol Infect 14 Suppl 1:53–62. doi: 10.1111/j.1469-0691.2007.01849.x [DOI] [PubMed] [Google Scholar]
- 15. Hobson CA, Pierrat G, Tenaillon O, Bonacorsi S, Bercot B, Jaouen E, Jacquier H, Birgy A. 2022. Klebsiella pneumoniae carbapenemase variants resistant to ceftazidime-avibactam: an evolutionary overview. Antimicrob Agents Chemother 66:e0044722. doi: 10.1128/aac.00447-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaibani P, Giani T, Bovo F, Lombardo D, Amadesi S, Lazzarotto T, Coppi M, Rossolini GM, Ambretti S. 2022. Resistance to ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam in gram-negative MDR bacilli: molecular mechanisms and susceptibility testing. Antibiotics (Basel) 11:628. doi: 10.3390/antibiotics11050628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pemberton OA, Noor RE, Kumar M V V, Sanishvili R, Kemp MT, Kearns FL, Woodcock HL, Gelis I, Chen Y. 2020. Mechanism of proton transfer in class A β-lactamase catalysis and inhibition by avibactam. Proc Natl Acad Sci U S A 117:5818–5825. doi: 10.1073/pnas.1922203117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egorov A, Rubtsova M, Grigorenko V, Uporov I, Veselovsky A. 2019. The role of the Ω-loop in regulation of the catalytic activity of TEM-type β-lactamases. Biomolecules 9:854. doi: 10.3390/biom9120854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furey IM, Mehta SC, Sankaran B, Hu L, Prasad BVV, Palzkill T. 2021. Local interactions with the Glu166 base and the conformation of an active site loop play key roles in carbapenem hydrolysis by the KPC-2 β-lactamase. J Biol Chem 296:100799. doi: 10.1016/j.jbc.2021.100799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piccirilli A, Cherubini S, Celenza G, Rossolini GM, Brisdelli F, Segatore B, Principe L, Luzzaro F, Andriani L, Amicosante G, Perilli M. 2022. A two amino acid duplication, L167E168, in the Ω-loop drastically decreases carbapenemase activity of KPC-53, a natural class A β-lactamase. Antimicrob Agents Chemother 66:e0240221. doi: 10.1128/aac.02402-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsivkovski R, Lomovskaya O. 2020. Potency of vaborbactam is less affected than that of avibactam in strains producing KPC-2 mutations that confer resistance to ceftazidime-avibactam. Antimicrob Agents Chemother 64:e01936-19. doi: 10.1128/AAC.01936-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shapiro AB, Moussa SH, Carter NM, Gao N, Miller AA. 2021. Ceftazidime-avibactam resistance mutations V240G, D179Y, and D179Y/T243M in KPC-3 β-lactamase do not alter cefpodoxime-ETX1317 susceptibility. ACS Infect Dis 7:79–87. doi: 10.1021/acsinfecdis.0c00575 [DOI] [PubMed] [Google Scholar]
- 24. Compain F, Arthur M. 2017. Impaired inhibition by avibactam and resistance to the ceftazidime-avibactam combination due to the D179Y substitution in the KPC-2 β-lactamase. Antimicrob Agents Chemother 61:e00451-17. doi: 10.1128/AAC.00451-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alsenani TA, Viviani SL, Kumar V, Taracila MA, Bethel CR, Barnes MD, Papp-Wallace KM, Shields RK, Nguyen MH, Clancy CJ, Bonomo RA, van den Akker F. 2022. Structural characterization of the D179N and D179Y variants of KPC-2 β-lactamase: Ω loop destabilization as a mechanism of resistance to ceftazidime/avibactam. Antimicrob Agents Chemother 66:e0241421. doi: 10.1128/aac.02414-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnes MD, Winkler ML, Taracila MA, Page MG, Desarbre E, Kreiswirth BN, Shields RK, Nguyen M-H, Clancy C, Spellberg B, Papp-Wallace KM, Bonomo RA. 2017. Klebsiella pneumoniae carbapenemase-2 (KPC-2), substitutions at ambler position Asp179, and resistance to ceftazidime-avibactam: unique antibiotic-resistant phenotypes emerge from β-lactamase protein engineering. mBio 8:e00528-17. doi: 10.1128/mBio.00528-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. In vitro selection of meropenem resistance among ceftazidime-avibactam-resistant, meropenem-susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother 61:e00079-17. doi: 10.1128/AAC.00079-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giddins MJ, Macesic N, Annavajhala MK, Stump S, Khan S, McConville TH, Mehta M, Gomez-Simmonds A, Uhlemann A-C. 2018. Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother 62:e02101-17. doi: 10.1128/AAC.02101-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaibani P, Campoli C, Lewis RE, Volpe SL, Scaltriti E, Giannella M, Pongolini S, Berlingeri A, Cristini F, Bartoletti M, Tedeschi S, Ambretti S. 2018. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J Antimicrob Chemother 73:1525–1529. doi: 10.1093/jac/dky082 [DOI] [PubMed] [Google Scholar]
- 30. Castanheira M, Arends SJR, Davis AP, Woosley LN, Bhalodi AA, MacVane SH. 2018. Analyses of a ceftazidime-avibactam-resistant Citrobacter freundii isolate carrying blaKPC-2 reveals a heterogenous population and reversible genotype. mSphere 3:e00408-18. doi: 10.1128/mSphere.00408-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wozniak A, Paillavil B, Legarraga P, Zumarán C, Prado S, García P. 2019. Evaluation of a rapid immunochromatographic test for detection of KPC in clinical isolates of enterobacteriaceae and Pseudomonas species. Diagn Microbiol Infect Dis 95:131–133. doi: 10.1016/j.diagmicrobio.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 32. Winkler ML, Rodkey EA, Taracila MA, Drawz SM, Bethel CR, Papp-Wallace KM, Smith KM, Xu Y, Dwulit-Smith JR, Romagnoli C, Caselli E, Prati F, van den Akker F, Bonomo RA. 2013. Design and exploration of novel boronic acid inhibitors reveals important interactions with a clavulanic acid-resistant sulfhydryl-variable (SHV) β-lactamase. J Med Chem 56:1084–1097. doi: 10.1021/jm301490d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Winkler ML, Papp-Wallace KM, Bonomo RA. 2015. Activity of ceftazidime/avibactam against Isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother 70:2279–2286. doi: 10.1093/jac/dkv094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Findlay J, Rens C, Poirel L, Nordmann P. 2022. In vitro mechanisms of resistance development to imipenem-relebactam in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 66:e0091822. doi: 10.1128/aac.00918-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papp-Wallace KM, Barnes MD, Taracila MA, Bethel CR, Rutter JD, Zeiser ET, Young K, Bonomo RA. 2023. The effectiveness of imipenem-relebactam against ceftazidime-avibactam resistant variants of the KPC-2 β-lactamase. Antibiotics (Basel) 12:892. doi: 10.3390/antibiotics12050892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barnes J, Papp-Wallace K, Bonomo RA. 2019. Imipenem/relebactam efficiently inhibits D179 variants of the KPC-2 beta-lactamase. 29th ECCMID, Amsterdam, The Netherlands. [Google Scholar]
- 37. Satapoomin N, Dulyayangkul P, Avison MB. 2022. Klebsiella pneumoniae mutants resistant to ceftazidime-avibactam plus aztreonam, imipenem-relebactam, meropenem-vaborbactam, and cefepime-taniborbactam. Antimicrob Agents Chemother 66:e0217921. doi: 10.1128/aac.02179-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sampson JM, Ke W, Bethel CR, Pagadala SRR, Nottingham MD, Bonomo RA, Buynak JD, van den Akker F. 2011. Ligand-dependent disorder of Ω-loop observed in extended-spectrum SHV-type β-lactamase. Antimicrob Agents Chemother 55:2303–2309. doi: 10.1128/AAC.01360-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levitt PS, Papp-Wallace KM, Taracila MA, Hujer AM, Winkler ML, Smith KM, Xu Y, Harris ME, Bonomo RA. 2012. Exploring the role of a conserved class A residue in the Ω-loop of KPC-2 β-lactamase: a mechanism for ceftazidime hydrolysis. J Biol Chem 287:31783–31793. doi: 10.1074/jbc.M112.348540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tooke CL, Hinchliffe P, Bonomo RA, Schofield CJ, Mulholland AJ, Spencer J. 2021. Natural variants modify Klebsiella pneumoniae carbapenemase (KPC) acyl-enzyme conformational dynamics to extend antibiotic resistance. J Biol Chem 296:100126. doi: 10.1074/jbc.RA120.016461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krishnan NP, Nguyen NQ, Papp-Wallace KM, Bonomo RA, van den Akker F. 2015. Inhibition of Klebsiella β-lactamases (SHV-1 and KPC-2) by avibactam: a structural study. PLoS One 10:e0136813. doi: 10.1371/journal.pone.0136813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Papp-Wallace KM, Nguyen NQ, Jacobs MR, Bethel CR, Barnes MD, Kumar V, Bajaksouzian S, Rudin SD, Rather PN, Bhavsar S, Ravikumar T, Deshpande PK, Patil V, Yeole R, Bhagwat SS, Patel MV, van den Akker F, Bonomo RA. 2018. Strategic approaches to overcome resistance against gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem 61:4067–4086. doi: 10.1021/acs.jmedchem.8b00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ehmann DE, Jahic H, Ross PL, Gu R-F, Hu J, Durand-Réville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cordes EH, Jencks WP. 1963. The mechanism of hydrolysis of schiff bases derived from aliphatic amines. J Am Chem Soc 85:2843–2848. doi: 10.1021/ja00901a037 [DOI] [Google Scholar]
- 45. Chen Y, Bonnet R, Shoichet BK. 2007. The acylation mechanism of CTX-M beta-lactamase at 0.88 a resolution. J Am Chem Soc 129:5378–5380. doi: 10.1021/ja0712064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tooke CL, Hinchliffe P, Lang PA, Mulholland AJ, Brem J, Schofield CJ, Spencer J. 2019. Molecular basis of class A beta-lactamase inhibition by relebactam. Antimicrob Agents Chemother 63:e00564-19. doi: 10.1128/AAC.00564-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ke W, Bethel CR, Thomson JM, Bonomo RA, van den Akker F. 2007. Crystal structure of KPC-2: insights into carbapenemase activity in class A β-lactamases. Biochemistry 46:5732–5740. doi: 10.1021/bi700300u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kabsch W. 2010. Xds. Acta Crystallogr D Biol Crystallogr 66:125–132. doi: 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. doi: 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. 2011. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67:355–367. doi: 10.1107/S0907444911001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi: 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 52. Long F, Nicholls RA, Emsley P, Graǽulis S, Merkys A, Vaitkus A, Murshudov GN. 2017. AceDRG: a stereochemical description generator for ligands. Acta Crystallogr D Struct Biol 73:112–122. doi: 10.1107/S2059798317000067 [DOI] [PMC free article] [PubMed] [Google Scholar]