Abstract
9-[2-(R)-(Phosphonomethoxy)propyl]adenine (PMPA) is a nucleotide analogue with potent antiretroviral activity in vitro and in simian models. A randomized, double-blind, placebo-controlled, dose-escalation clinical trial of intravenous PMPA monotherapy was conducted in 20 human immunodeficiency virus (HIV)-infected adults with CD4 cell counts of ≥200 cells/mm3 and plasma HIV RNA levels of ≥10,000 copies/ml. Two dose levels were evaluated (1 and 3 mg/kg of body weight/day). Ten subjects were enrolled at each dose level (eight randomized to receive PMPA and two randomized to receive placebo). On day 1, a single dose of PMPA or placebo was administered by intravenous infusion. Beginning on study day 8, PMPA or placebo was administered once daily for an additional 7 consecutive days. All subjects tolerated dosing without significant adverse events. Mean peak serum PMPA concentrations were 2.7 ± 0.9 and 9.1 ± 2.1 μg/ml in the 1- and 3-mg/kg cohorts, respectively. Serum concentrations declined in a biexponential fashion, with a terminal half-life of 4 to 8 h. At 3 mg/kg/day, a single infusion of PMPA resulted in a 0.4 log10 median decline in plasma HIV RNA by study day 8. Following 7 consecutive days of study drug administration thereafter, the median changes in plasma HIV RNA from baseline were −1.1, −0.6, and 0.1 log10 in the 3-mg/kg/day, 1-mg/kg/day, and placebo dose groups, respectively. Following the final dose in the 3-mg/kg/day cohort, the reduction in HIV RNA was sustained for 7 days before returning toward baseline. Further studies evaluating an oral prodrug of PMPA are under way.
Nucleoside analogues, such as zidovudine and d4T, require intracellular phosphorylation to an active triphosphate metabolite. The expression of the intracellular nucleoside kinase responsible for the initial phosphorylation is low in resting lymphocytes and macrophages/monocytes, thus limiting the activity of such nucleoside analogues in these important cellular targets of human immunodeficiency virus (HIV) infection (2, 7, 10). In contrast, nucleoside monophosphate (nucleotide) analogues do not require intracellular phosphorylation by the nucleoside kinase and thus may possess greater activity in a broader range of cell types (3).
9-[2-(R)-(Phosphonomethoxy)propyl]adenine (PMPA) is a nucleotide analogue with potent antiretroviral activity. In a 4-week study of adult macaques chronically infected with simian immunodeficiency virus (SIV), once-daily subcutaneous injections of PMPA (30 to 75 mg/kg of body weight/day) resulted in a decline in plasma SIV RNA levels from a baseline of 2 to 3 log10 copies/ml (12). With continued dosing, this effect was sustained for greater than 1 year (14). In macaques, 4 weeks of once-daily subcutaneous injections of PMPA (30 mg/kg/day) initiated as late as 24 h after intravenous inoculation of SIV prevented infection (13).
In preclinical toxicity studies, PMPA at effective doses appeared to be safe. Nephrotoxicity, characterized by proximal convoluted tubule degeneration, was the principal toxicity when PMPA was infused at very high doses (>75 mg/kg/day). In vivo and in vitro, the active metabolite of PMPA has an intracellular half-life of 12 to 15 h in activated lymphocytes and 33 to 50 h in resting lymphocytes, thus suggesting that once-a-day dosing regimens may be possible (11). Finally, preliminary data indicate that PMPA does not select for high-level resistance during in vitro propagation of the HIV in the presence of the drug (4). Considering these favorable preclinical results, a randomized, double-blind, placebo-controlled, dose-escalation clinical trial of intravenous PMPA monotherapy was initiated in 20 HIV-infected adults.
(Part of this research was presented at the International Conference on Antiviral Research, Atlanta, Ga., April 1997, and at the Sixth European Conference on the Clinical Aspects and Treatment of HIV Infection, Hamburg, Germany, October 1997.)
MATERIALS AND METHODS
Study design.
Eligible subjects were men or women aged 18 to 60 years with documented HIV infection, plasma HIV RNA levels of ≥10,000 copies/ml, and CD4 cell counts of ≥200 cells/mm3. Other inclusion criteria included serum creatinine concentrations of ≤1.5 mg/dl, calculated creatinine clearance (CLCR) of ≥50 ml/min (determined by the Cockcroft-Gault formula), ≤1+ proteinuria, total bilirubin concentrations of ≤1.5 mg/dl, hepatic transaminases of ≤3 times the upper limit of normal, absolute neutrophil counts of ≥1,000 cells/mm3, platelet counts of ≥75,000/mm3, hemoglobin concentrations of ≥9.0 g/dl, and prothrombin times of <1.2 times the upper limit of normal. A negative serum pregnancy test was required at the time of enrollment for women of childbearing age. Subjects with positive serum tests for hepatitis B surface antigen were excluded, as were those with active, serious infections.
Subjects were required to discontinue all antiretroviral therapies for 2 weeks prior to randomization and until after the study day 28 visit. Subjects receiving ongoing therapy with any of the following agents were excluded: aminoglycoside antibiotics, diuretics, itraconazole, fluconazole, ketoconazole, isoniazid, rifampin, rifabutin, clarithromycin, azithromycin, systemic chemotherapeutic agents, systemic corticosteroids, and any investigational agent.
A total of 20 subjects were enrolled and randomized in a 4:1 ratio to receive intravenous PMPA or placebo. Randomization was performed centrally by a computer-generated random number program that assigned patient numbers within each dose cohort to active drug or placebo treatment in blocks of five patients. The first 10 subjects enrolled received 1 mg of PMPA/kg or an equivalent volume of placebo. After a full safety and pharmacokinetic analysis of the first cohort was completed, the second 10 subjects were enrolled and received 3 mg of PMPA/kg or an equivalent volume of placebo. At each dose level, 8 subjects were randomized to receive PMPA and 2 were randomized to receive placebo.
PMPA (in normal saline) or placebo (normal saline) was supplied by Gilead Sciences, Inc., and administered by intravenous infusion into a peripheral vein over a 1-hour period. Study medication was administered as a single intravenous infusion over 60 min on day 1 of the study period, followed by a 7-day washout period. Beginning on day 8, study medication was administered once daily as an intravenous infusion for seven consecutive days (i.e., days 8 to 14).
Safety and efficacy analyses.
Subjects underwent a qualifying physical examination 24 h prior to the first dose and had directed regular physical examinations through study day 42. Assessments of serum chemistries, hematology, coagulation parameters, and urinalyses were performed regularly during study drug administration and on days 21, 28, and 42 after study drug discontinuation.
Blood and plasma samples for T-cell subset analysis (CD4 and CD8) and HIV RNA assays were obtained prior to the dose on days 1, 2, 4, 8, and 11 and immediately after the day 14 dose. Final plasma HIV RNA determinations were performed on days 21 and 28. For HIV RNA analysis, plasma was obtained by centrifugation within 4 h of blood collection and stored at −70°C. Samples were shipped to a central laboratory and analyzed as one batch by reverse transcriptase quantitative polymerase chain reaction assay (Amplicor; Roche Diagnostics Inc.; lower limit of quantification, 400 copies/ml).
Symptoms, signs, and laboratory abnormalities were recorded with a graded toxicity scale based on the AIDS Clinical Trials Group’s Table for Grading Severity of Adult Adverse Events. In general, mild symptoms were classified as grade I, moderate symptoms as grade II, severe symptoms as grade III, and life-threatening symptoms as grade IV. The relationship of an adverse event to the study medication was assessed by the treating investigator and was done without knowledge of treatment assignment to PMPA or placebo. Subjects with grade III or IV events thought by the investigator to be drug related were removed from study. For grade III or IV events not considered to be study drug related, the drug was withheld until the event resolved. At the discretion of the investigators, subjects with grade II events could remain on the study drug.
Pharmacokinetic analyses.
Prior to the first dose, subjects were admitted to an inpatient research center. From midnight prior to the first dose until 2 h after the first dose, subjects were maintained in a fasting state (except water ad lib). On day 1, serum for pharmacokinetic analysis was obtained at 0 h (predose), 0.5 h (mid-infusion), and 1 h (end of infusion). Thereafter, serum was obtained at hours 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24. A similar inpatient pharmacokinetic assessment was performed following the final dose (day 14). At each time point, approximately 10 ml of blood was obtained; serum was separated and stored at −20°C.
Serum and urine samples were analyzed for PMPA concentrations by validated high-performance liquid chromatographic assays involving precolumn derivatization and fluorescence detection. The serum method was linear over the range 25 to 1,000 ng/ml, and the limit of quantitation was 25 ng/ml. The intraday precision and accuracy (percent deviation from nominal) were <5.9 and <5.2%, respectively. The urine method was linear over the range 1 to 20 μg/ml, and the limit of quantitation was 1 μg/ml. The intraday precision and accuracy levels (percent deviation from nominal) were <3.5 and <0.7%, respectively.
The pharmacokinetic parameters for intravenous PMPA were assessed by application of the nonlinear curve fitting software package PCNONLIN (version 4.2; Statistical Consultants, Inc., Lexington, Ky.) by using noncompartmental methods. A minimum of three data points were used to determine the terminal phase. Additional parameters were calculated manually. Total serum clearance (CL) was calculated as the dose/area under the concentration-time curve from 0 h to infinity (AUC0–∞). The steady state volume of distribution (VSS) was calculated as mean residence time × CL. Urine samples were collected for 72 h following the start of the PMPA infusion. The concentration of PMPA in serum at the end of the 72-h urine collection period (C72) was calculated as Clast × e[−beta × (72 − tlast)], where Clast is the last quantifiable concentration of PMPA in serum and tlast is the time of the last quantifiable concentration of PMPA in serum. The area under the serum concentration-time curve up to the end of the urine collection period, AUC0–72, was calculated as AUC0–∞ − (C72/beta). The renal clearance (CLR) of PMPA following the first intravenous administration was calculated as (U0–72 × 1,000)/(AUC0–24 × Wt) where U0–72 is the total milligrams of PMPA excreted in the urine during the entire urine collection and Wt is the body weight of the patient in kilograms. The corresponding CLR on day 14 of the study was calculated as (U0–24 × 1,000)/(AUCSS × Wt), where AUCSS is the steady-state AUC. The cumulative amount of PMPA excreted at the end of each urine collection period, U0–t, was calculated as the sum of the amounts excreted in all previous collection periods. The cumulative percentage of the dose excreted at the end of each collection period was calculated as 100 × (U0–t/dose).
Statistical analyses.
The distribution of all summary data for each analyzed variable was assessed for normality. Statistical analyses were performed for comparisons of the pooled placebo group to each active drug group. All P values were two sided. The significance level of all tests was set at 5%. Baseline characteristics were compared among dose groups by analysis of variance for continuous variables and Pearson’s chi-square test for categorical variables. Statistical comparisons of changes in plasma HIV RNA levels through the completion of the study period were assessed by absolute change from baseline. Changes in CD4 cell counts and plasma HIV RNA levels between the placebo and each active dose group were compared by the Wilcoxon rank-sum test.
RESULTS
Baseline characteristics.
Between October 1996 and January 1997, 20 HIV-infected subjects were enrolled (10 at the 1-mg/kg dose level and 10 at the 3-mg/kg dose level, with 2 subjects in each dosing group receiving placebo). The baseline characteristics in the placebo group and the two treatment groups are shown in Table 1. Seventeen (85%) of the subjects were male. Each of the three female subjects in the study was randomized to receive placebo. The median age of the study subjects was 39 years. The median CD4 cell count at entry for the entire study group was 363 cells/mm3 (range, 133 to 1,259), while the median baseline plasma HIV RNA level was 4.7 log10 copies/ml (range, 3.5 to 5.6) (Table 1).
TABLE 1.
Baseline characteristics of study subjects
| Dosage of PMPA received (no. of subjects) | Median age of dosage group subjects (yr) | No. of males in dosage group | CD4 cells (median no. of cells/mm3) | HIV RNA (median log10 copies/ml) | No. of subjects in group who had undergone prior anti- retroviral therapy |
|---|---|---|---|---|---|
| None (placebo) (4) | 39 | 1 | 804 | 4.3 | 0 |
| 1 mg/kg/day (8) | 39 | 8 | 496 | 4.7 | 4 |
| 3 mg/kg/day (8) | 38 | 8 | 239 | 5.1 | 4 |
Pharmacokinetics.
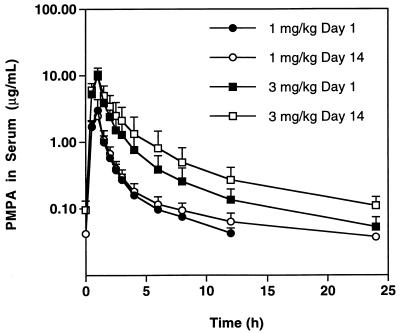
Pharmacokinetic data from both dose groups are outlined in Table 2 and Fig. 1. For the first dose cohort (1 mg/kg/day), a single infusion of PMPA resulted in a maximum serum concentration (Cmax) of 2.7 ± 0.9 μg/ml. Serum concentrations declined in an apparently biexponential fashion with a terminal half-life of 4.5 ± 0.6 h. All subjects that were given a single 1-mg/kg dose had detectable levels of PMPA 12 h later. Administration of a single 3-mg/kg dose resulted in a serum Cmax of 9.1 ± 2.1 μg/ml. Serum PMPA concentrations declined in a biexponential manner with a terminal half-life of 7.1 ± 1.3 h. All subjects in the higher dose cohort displayed quantifiable serum levels for up to 24 h after the first dose. The apparent difference in half-life at the 1.0- and 3.0-mg/kg dose levels may be artifactual, resulting from the constraints of the bioanalytical method (serum concentrations were not quantifiable beyond 12 h postdosing at the 1.0-mg/kg dose).
TABLE 2.
Pharmacokinetic resultsa
| Dosage of PMPA received (no. of subjects) | Weight (kg) | Cmax (μg/ml) | AUC0–∞ (μg · h/ml) | AUCSS (μg · h/ml) | Beta (h − 1) | Half-life (h) | CL (ml/h/kg) | MRTb (h) | VSS (ml/kg) | CLCR (ml/h/kg) | CLR (ml/h/kg) | % of dose recovered in 72 h | % of dose recovered in 24 h |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg/kg/day (8) | |||||||||||||
| Day 1 | 81.1 (22.0) | 2.71 (0.870) | 4.41 (0.930) | 0.158 (0.023) | 4.46 (0.610) | 237 (56.3) | 3.29 (0.501) | 797 (290) | 80.7 (13.5) | 161 (60.6) | 66.4 (15.8) | ||
| Day 14 | 81.7 (21.8) | 2.50 (0.523) | 4.84 (1.11) | 0.116 (0.043) | 6.64 (2.10) | 217 (55.8) | 3.74 (0.678) | 813 (266) | 81.4 (14.9) | 158 (53.7) | 72.4 (14.4) | ||
| 3 mg/kg/day (7)c | |||||||||||||
| Day 1 | 71.7 (11.8) | 9.11 (2.07) | 16.6 (6.05) | 0.101 (0.018) | 7.07 (1.26) | 203 (71.9) | 4.21 (0.60) | 853 (328) | 82.1 (15.5) | 164 (51.0) | 81.7 (13.3) | ||
| Day 14 | 70.5 (10.1) | 9.83 (1.95) | 22.5 (9.75) | 0.089 (0.023) | 8.14 (1.78) | 153 (56.5) | 4.00 (0.57) | 586 (146) | 75.2 (18.5) | 123 (51.7) | 78.4 (10.7) |
Data are mean (SD) values.
MRT, mean residence time.
Data omitted for one subject who received the first infusion over 45 min.
FIG. 1.
Mean concentrations of PMPA in serum. Mean concentrations, with standard deviations of PMPA in serum following daily intravenous infusions of PMPA.
The mean AUC after single infusions was dose proportional (Table 2). On day 1, the clearance of intravenous PMPA was 237 ± 56 ml/h/kg for the 1-mg/kg dose and 203 ± 72 ml/h/kg for the 3-mg/kg dose group. At baseline, calculated CLCR for all subjects was 81.2 ± 12.3 ml/h/kg. Assuming that PMPA was eliminated unchanged in urine (as demonstrated in animal studies), the total body clearance of PMPA reflects the CLR of drug. Therefore, CLR of PMPA greatly exceeded the glomerular filtration, suggesting active tubular secretion of PMPA by the kidney.
After 7 days of continuous daily dosing at 3.0 mg/kg, there was an apparent decrease in the clearance of PMPA. Clearance of PMPA was 203 ± 72 ml/h/kg on day 1 and 153 ± 57 ml/h/kg on day 14 (P = 0.018, Wilcoxon signed rank test). The mean decrease in PMPA clearance was approximately 24% (range, 8.4 to 38.1%). The calculated CLCR for patients in the 3-mg/kg dose cohort was determined to be 81.2 ± 14.9 ml/h/kg on day 1 and 75.8 ± 18.2 ml/h/kg on day 14 (P = 0.018, Wilcoxon signed rank test).
Safety and tolerability.
All 20 subjects completed dosing as planned. All patients reported at least one adverse event during the study period; 14 patients (70%) had at least one adverse event that was felt to be possibly or probably related to study drug administration. No life-threatening events occurred during the study. Events graded as moderate or severe in toxicity and considered possibly or probably related to the study drug occurred in four patients and included headache, fatigue, proteinuria, neutropenia, and abdominal pain. These were all graded moderate events with the exception of the proteinuria and neutropenia, which were each graded severe and which occurred in a single placebo recipient. Distribution of the events relative to dose group is presented in Table 3. There was no apparent relationship between event frequency or severity and dose.
TABLE 3.
Mild-to-severe adverse events possibly or probably related to study drug
| Dosage of PMPA received | No. of occurrences of:
|
||||
|---|---|---|---|---|---|
| Headache | Fatigue | Proteinuria | Neutro- penia | Abdominal pain | |
| None (placebo) | 0 | 0 | 1 | 1 | 0 |
| 1 mg/kg/day | 0 | 0 | 0 | 0 | 1 |
| 3 mg/kg/day | 1 | 1 | 0 | 0 | 0 |
| Total | 1 | 1 | 1 | 1 | 1 |
T-cell subsets.
After completion of dosing with PMPA (i.e., study day 14), there was a median increase in CD4 cell count from baseline of 27 and 32 cells/mm3 in subjects in the 1- and 3-mg/kg dose groups, respectively, compared to a median decrease of 65 cells in placebo recipients. This difference between the active and placebo groups did not reach statistical significance (P = 0.2 for the 1-mg/kg-dose group versus a placebo; P = 0.2 for the 3-mg/kg-dose group versus a placebo; Wilcoxon rank-sum tests).
HIV RNA levels.
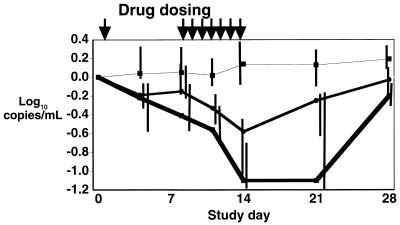
After a single intravenous infusion of PMPA, there was a moderate decrease in plasma HIV RNA levels at day 4 (median log10 declines of −0.2 and −0.2 copies/ml in the 1- and 3-mg/kg dose groups, respectively; see Fig. 2). At day 8, just prior to initiation of continued daily dosing, there was a median decrease from baseline of 0.15 log10 copies/ml in the 1-mg/kg treatment group, and a median decrease of 0.4 log10 copies/ml in the 3-mg/kg treatment group, compared to a median increase of 0.05 log10 copies/ml in the placebo group (P = 0.4 and P = 0.07 for the 1- and 3-mg/kg groups versus a placebo, respectively).
FIG. 2.
Median log10 change in plasma HIV-1 RNA levels from that at baseline. Top line (with boxes), placebo; middle line (with circles), 1-mg/kg/day dose; bottom line (with boxes), 3-mg/kg/day dose. Vertical bars represent values from quartile 1 to quartile 3 for each dosing cohort.
After completion of 7 consecutive days of intravenous PMPA dosing (study days 8 to 14), the median log10 changes in plasma HIV RNA from baseline were 0.1, −0.6, and −1.1 copies/ml in the placebo group and 1.0- and 3.0-mg/kg/day dose groups, respectively (P = 0.009 for the 1.0-mg/kg-dose group versus a placebo and P = 0.03 for the 3.0-mg/kg-dose group versus a placebo). The median reduction in plasma HIV RNA levels was not significantly different between the two active treatment groups (P = 0.4).
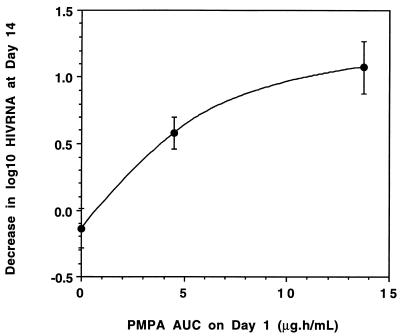
Seven days after PMPA was discontinued (study day 21), the median plasma HIV RNA level began to return towards baseline in the 1-mg/kg-dose group. However, in the 3-mg/kg-dose group, the median log10 plasma HIV RNA level remained stable at 1.1 copies/ml below baseline on day 21, eventually returning to near baseline by day 28 (Fig. 2). As illustrated in Fig. 3, there appeared to be a correlation between the antiviral response after 7 consecutive days of dosing (i.e., the median decrease in plasma HIV RNA on study day 14) and the exposure to PMPA (i.e., the median AUC on study day 1).
FIG. 3.
Median AUC on day 1 versus median antiviral response on day 14. Median AUC on day 1 for placebo, 1-mg/kg/day, and 3-mg/kg/day dose groups versus the median decrease in log10 plasma HIV RNA levels from baseline on day 14. Vertical bars represent the standard error.
DISCUSSION
In this phase I/II blinded multicenter clinical trial, short-term use of the nucleotide analogue PMPA administered intravenously to HIV-infected subjects was safe, well tolerated, and effective in reducing plasma HIV RNA levels.
In preclinical studies, administration of PMPA as monotherapy was associated with a decline in plasma SIV RNA levels in chronically infected macaques of >2 log10 copies/ml (12, 14). The clinical data generated by this first study of PMPA in humans demonstrated a dose-dependent, statistically significant decrease in plasma HIV RNA levels when PMPA was administered eight times over a 2-week period. At the higher dose studied (3 mg/kg/day), median plasma HIV RNA levels declined by greater than 90% (median log10 decrease, 1.1 copies/ml). This magnitude of the antiviral effect is comparable to that observed after limited dosing of other potent antiretroviral therapies, including HIV-1 protease inhibitors (5, 8). Accruing data suggest that declines in plasma HIV RNA levels are highly predictive of clinical outcome (9).
At both doses of PMPA studied, viral load continuously decreased during the PMPA 7-day consecutive treatment period (days 8 to 14). Since the nadir was not clearly reached, a more potent effect may be obtained with continued administration of PMPA. Moreover, although statistical significance was not achieved, there appeared to be an anti-HIV effect seen following a single dose of PMPA (median decline in plasma HIV RNA levels at 3 mg/kg, 0.4 log10 copies/ml). This effect persisted for up to 7 days after a single infusion. Similarly, when PMPA (3 mg/kg/day) was discontinued (after 7 days of continuous dosing), HIV RNA suppression persisted for up to 1 week. The sustained depression in plasma HIV RNA observed after drug discontinuation is consistent with the prolonged intracellular half-life of the intracellular phosphorylated drug, which has been found to be 12 to 50 h in activated and resting peripheral blood lymphocytes, respectively, in vivo (11).
PMPA administered parenterally was well tolerated in this study. All subjects completed dosing without interruptions. The most frequent adverse events were mild and transient and included headache, dizziness, fatigue, and nausea. The only moderate (grade II) adverse events thought to be related to PMPA were transient episodes of nausea, fatigue, and abdominal pain, each occurring in one patient. All symptoms resolved despite continuous administration of drug. No clinically significant laboratory abnormalities were noted during the trial.
There was a significant increase in the AUC of PMPA in serum after 14 days of dosing at 3.0 mg/kg/day. A possible explanation is that clearance of PMPA decreased over the course of the study. An alternative explanation, however, is that of gradual accumulation of a fraction of the dose due to a slowly equilibrating compartment with a long half-life. Analysis of the serum data for 3.0 mg of PMPA/day with an open three-compartment model with multiple dosing (data not shown) gave a good fit of the data with a terminal half-life of 31 h. This compartment may represent the prolonged half-life of the intracellular phosphorylated drug, as discussed above.
In studies to date, selection of resistance mutations in the reverse transcriptase gene in vitro has been relatively difficult, and high-level resistance has not been observed (4). Similarly, PMPA did not select for high-level viral resistance in SIV-infected macaques (14). Since the emergence of resistance to PMPA was not analyzed in the present study due to the short duration of dosing, future studies will need to confirm a low propensity for development of resistance during more prolonged dosing.
In summary, in this phase I/II clinical study, intravenous PMPA appeared to be safe and well tolerated. Parenterally administered PMPA resulted in a significant reduction in plasma HIV RNA levels, even after a single infusion. An oral prodrug of PMPA, bis(isopropyloxymethylcarbonyl) PMPA, has been developed (1, 11) and is currently under clinical evaluation (6). The greater lipophilicity of the oral prodrug than the PMPA the parent compound has been shown in preclinical models to result in a greater ability to cross cellular membranes and therefore enhances oral bioavailability. Future plans are to evaluate the safety and efficacy of long-term dosing of PMPA when administered orally.
ACKNOWLEDGMENT
This study was supported by Gilead Sciences, Inc.
REFERENCES
- 1.Arimilli M N, Kim C U, Dougherty J, Mulato A, Oliyai R, Shaw J P, Crundy K U, Bischofberger N. Synthesis, in vitro biological evaluation and oral bioavailability of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) prodrugs. Antivir Chem Chemother. 1997;8:557–564. [Google Scholar]
- 2.Balzarini J, Perno C, Schols D, De Clercq E. Activity of acyclic phosphonate analogues against human immunodeficiency virus in monocytes/macrophages and peripheral blood lymphocytes. Biochem Biophys Res Commun. 1991;178:329–335. doi: 10.1016/0006-291x(91)91818-w. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini J, Zhang H, Herdewijn P, Johns D, De Clercq E. Intracellular metabolism and mechanism of antiretrovirus action of 9-(2-phosphonylmethoxyethyl)adenine, a potent anti-human immunodeficiency virus compound. Proc Natl Acad Sci USA. 1991;88:1499–1503. doi: 10.1073/pnas.88.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherrington J M, Chandok R, Mulato A S, Lamy P D, Mitsuya H, Wainberg M. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. 1997. In vitro selection and characterization of HIV-1 variants with reduced susceptibility to PMPA, abstr. I-113; p. 264. Toronto, Ontario, Canada. [Google Scholar]
- 5.Danner S A, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V, Gil Aguado A, Garci de Lomas J, Delgado R, Borleffs J C C, Hsu A, Valdes J M, Boucher C A B, Cooper D A. A short term study of the safety, pharmacokinetics and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 6.Deeks S G, Barditch-Crovo P, Lietman P S, Collier A, Safrin S, Coleman R, Cundy K C, Kahn J O. Program and abstracts of the Fifth Conference on Retroviruses and Opportunistic Infections. 1998. The safety and efficacy of PMPA prodrug monotherapy: preliminary results of a phase I/II dose-escalation study, abstr. LB8. Chicago, Ill. [Google Scholar]
- 7.Gao W Y, Shirasaka T, Johns D G, Broder S, Mitsutya H. Differential phosphorylation of azidothymidine, dideoxycytidine and dideoxyinosine in resting and activated peripheral blood nuclear cells. J Clin Investig. 1993;91:2326–2333. doi: 10.1172/JCI116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 9.Katzenstein D A, Hammer S M, Hughes M D, Gundacker H, Jackson J B, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japour A, Merigan T C, Hirsch M S. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. N Engl J Med. 1996;335:1091–1098. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 10.Poli G, Orenstein J M, Kinter A, Folks T M, Fauci A S. Interferon-a but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989;244:575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- 11.Robbins B L, Srinivas R V, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42:612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai, C.-C., K. E. Follis, T. W. Beck, A. Sabo, N. Bischofberger, and P. J. Dailey. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy in chronic SIV infection in macaques. AIDS Res. Hum. Retroviruses 13:707–711. [DOI] [PubMed]
- 13.Tsai C-C, Follis K, Sabo A, Beck T, Grant R, Bischofberger N, Benveniste R, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 14.van Rompay K K A, Cherrington J M, Marthas M, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bischofberger N, Pedersen N. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]