Abstract
Preterm birth worldwide remains a significant cause of neonatal morbidity and mortality, yet the exact mechanisms of preterm parturition remain unclear. Preterm birth is not a single condition, but rather a syndrome with a multifactorial etiology. This multifactorial nature explains why individual predictive measures for preterm birth have had limited sensitivity and specificity. One proposed pathway for preterm birth is via placentally synthesized corticotrophin-releasing hormone (CRH). CRH is a peptide hormone that increases exponentially in pregnancy and has been implicated in preterm birth because of its endocrine, autocrine, and paracrine roles. CRH has actions that increase placental production of estriol and of the transcription factor nuclear factor-κB, that likely play a key role in activating the myometrium. CRH has been proposed as part of a placental clock, with early activation of placental production resulting in preterm birth. This article will review the current understanding of preterm birth, CRH as an initiator of human parturition, and the evidence regarding the use of CRH in the prediction of preterm birth.
Keywords: preterm birth, corticotrophin-releasing hormone, parturition, placental clock
Preterm birth is defined as birth occurring before 37 completed weeks of gestational age. Preterm birth affects families substantially both in the United States and worldwide, accounting for approximately 10.09% and an estimated 11% of births, respectively (1, 2). Rates of preterm birth in most countries have gradually risen over the past decade (3). Preterm birth is the leading cause of childhood mortality, accounting for approximately 1 million childhood deaths every year (2). Long-term studies have shown that preterm birth carries deleterious effects into adult life, including effects on cardiovascular and neurodevelopmental health (4). Enhanced understanding of the pathologic mechanisms by which preterm birth occurs is required to improve the prediction and prevention of preterm delivery. In this review, we will outline the current understanding of preterm birth mechanisms and specifically the role of corticotrophin-releasing hormone (CRH) as a potential pathologic mechanism for preterm birth.
Preterm Birth Principles
Clinically preterm birth is the result of 3 scenarios: (1) spontaneous preterm labor, (2) spontaneous preterm prelabor rupture of membranes, and (3) delivery due to a maternal or fetal indication, otherwise referred to as indicated. National statistics often do not separate these clinical scenarios, though they are important from an etiologic perspective. The relative proportions are often quoted as 70% spontaneous preterm births (preterm labor or premature rupture of membranes) and 30% indicated, though there are large variations among regions and countries (5). In higher income countries, preterm rates appear to be increasing, though methodological considerations must be accounted for given enhanced reporting, reductions in thresholds of viability, and increased multiple birth rates (5, 6).
Gestational age subdivisions of preterm birth have been defined by various organizations. The American College of Obstetricians and Gynecologists defines preterm birth occurring between 34 and 36 completed weeks as late preterm and before 34 weeks as early preterm (7). The World Health Organization defines birth before 28 completed weeks as extremely preterm, 28 to 32 completed weeks as very preterm, and from 32 to 37 weeks as moderate to late preterm (8). These definitions seek to convey the degree of prematurity, as morbidity is gestational-age dependent. Short-term morbidity of late preterm infants includes temperature instability, respiratory distress, apnea, hypoglycemia, seizures, jaundice, kernicterus, feeding difficulties, and periventricular leukomalacia (9). Longer-term effects of late preterm birth on motor skills, cognition, and behavior have been observed (10). Increasing morbidity is observed in extremely preterm infants, and includes considerable respiratory distress requiring ventilatory support, increased rates of interventricular hemorrhage with associated moderate to severe neurodevelopmental impairment, and significant behavioral sequalae (9). Infant mortality is increased across all categories of preterm birth. In the United States, 66% of infant deaths occur in those born preterm (11).
Preterm Birth as a Syndrome
The physiologic pathways that lead to parturition at term remain incompletely understood. At present, preterm birth appears to be not a single pathway or condition, but rather a syndrome (12). That is to say that there are multiple pathologic etiologies that may or may not share common pathways with parturition at term and result in preterm birth.
Events necessary for parturition at term include increasing myometrial contractility, cervical remodeling, and rupture of membranes. Myometrial quiescence is favored through the majority of gestation due to the actions of progesterone, which antagonizes the nuclear factor-κB (NF-κB) pathway and suppresses expression of proinflammatory cytokines (eg, interleukin-1 [IL-1], IL-6, IL-8) (13). At term progesterone action is reduced by several different mechanisms. Progesterone action during pregnancy is mediated by the progesterone receptor subtype B. Within the myometrium, subtype B inhibits expression of contraction-associated proteins and induces the transcription factor zinc finger E-box-binding homeobox 1 (ZEB1), which in turn acts to inhibit contraction-associated proteins and inhibit expression of progesterone-metabolizing enzymes (eg, 20 alpha-hydroxysteroid dehydrogenase) (14). At term inflammatory signal–regulated phosphorylation of progesterone receptor A antagonizes the action of the B subtype and creates a functional progesterone withdrawal (15, 16). While progesterone action is reducing at term, synthesis of active forms of the estrogen receptor 1 is increasing concurrently with increasing plasma estriol concentrations (17, 18). Estrogens in turn promote myometrial changes conducive for contractions as well as cervical ripening (19). Recent transcriptome analysis suggests that the myometrial changes in progesterone receptors, progesterone metabolism, and estrogen action are downstream from increases in the transcription factor NF-κB, which in turn may be driven by increasing CRH concentrations, as within the brain CRH has been shown to stimulate NF-κB activity (20). The exact cell types within myometrial tissue that mediate these changes are not yet clear, although work on single-cell transcriptomics will likely resolve this uncertainty (21). Recent work suggests that the term transformation of myometrium occurs by the reorganization of gene transcription within topologically associated domains, that is, groups of genes that are coregulated and coexpressed to generate a change in the tissue phenotype (22). This new knowledge regarding term labor provides a necessary framework to understand preterm labor in which the mechanisms of premature uterine activation, cervical remodeling, and membrane rupture may be different (23).
Cervical remodeling for dilation is mediated by changes in the structure and mechanical function of the extracellular matrix (24). Through pregnancy the replacement of fibrillar collagen containing strong cross-links with collagen containing weak cross-links is achieved in the progesterone-dominant phase termed cervical softening (25). Loss of progesterone function in late pregnancy during the cervical ripening phase promotes the influx of tissue monocytes into the cervix with activation of proinflammatory and tissue repair macrophages evident in the postpartum repair of the cervix (26). Similar to the myometrium, changes in progesterone receptor isoforms and increased metabolism of progesterone in late pregnancy contribute to cervical dilation necessary for parturition (27).
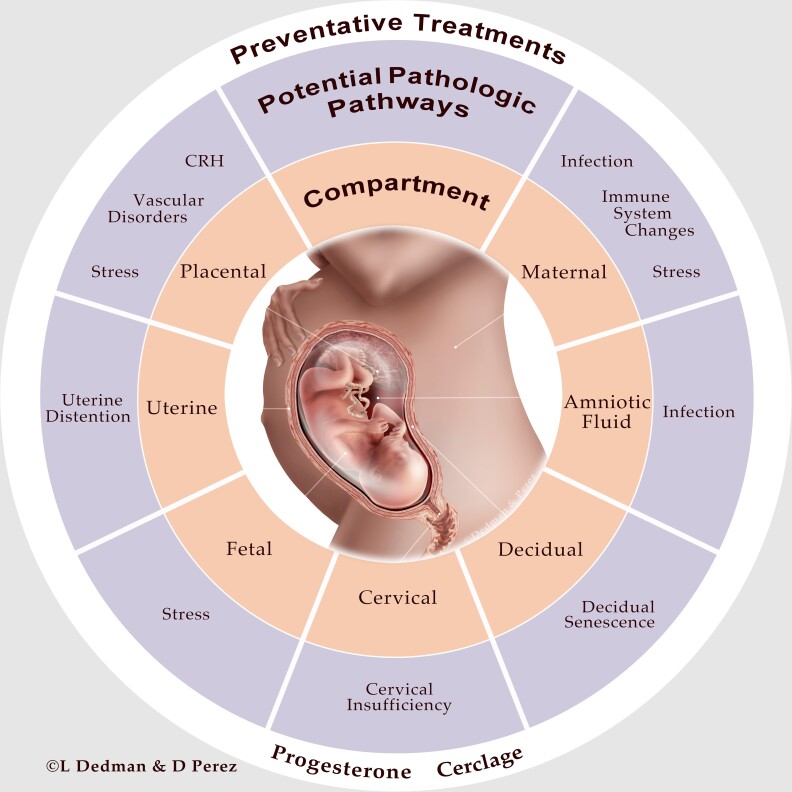
Membrane rupture results through the dynamic interactions with the decidua from either increased expression of inflammatory cytokines (eg, tumor necrosis factor-α and IL-1) or thrombin activation (decidual bleeding/abruption) associated with changes in activity of matrix metalloproteases and tissue inhibitors of matrix metalloproteases, increased granulocyte-macrophage colony-stimulating factor activity, dissolution of cellular cements such as fibronectin, and apoptosis (28–30). Again, these changes may be downstream from NF-κB activation and NF-κB has been emphasized as a target for novel tocolytics (31). Pathologic disruption of these 3 processes can occur at multiple levels—maternal, uterine (eg, overdistention from polyhydramnios, multifetal gestation), placental, decidual and/or cervical and via multiple pathways—stress, infection, vascular disorders, and/or altered immune tolerance (Fig. 1) (12, 32). Genetic and environmental factors also likely play a role.
Figure 1.
Potential compartments and pathologic pathway(s) involved in preterm birth. Preventive treatment(s) of preterm birth are also shown.
Intra-amniotic infection is one mechanism that has been causally linked to spontaneous preterm birth (33). Bacteria may gain access to the amniotic cavity via ascending infection, transplacental passage, retrograde seeding from the fallopian tubes, or introduction via invasive procedures (eg, amniocentesis). Ascending infection is the most common route of entry, wherein microorganisms that colonize the cervix, decidua, and membranes may then enter the amniotic sac (32). Most studies have defined intra-amniotic infection as those with a positive amniotic fluid culture for a pathologic microorganism, rather than “clinical chorioamnionitis,” or the clinical syndrome associated with bacterial invasion of the amniotic cavity (34). Based on this definition, colonization with an infectious agent has been detected in 25% to 40% of all preterm births (35), though this number may be limited by culture techniques (36). The mechanisms by which infection leads to preterm birth are related to the innate immune system—with activation of pattern recognition receptors that elicit inflammatory cascades that stimulate the production of prostaglandins and matrix-degrading enzymes (35, 37). Transcriptomic analysis of myometrium from individuals with clinical chorioamnionitis show marked upregulation of many inflammation-related genes, but even those without evidence of chorioamnionitis still show an immune signature suggesting that immunological activation is common to preterm labor of differing etiologies (38). Another mechanism linked to spontaneous preterm birth is decidual activation by thrombin (decidual bleeding/abruption) (30, 39).
Prediction of Preterm Birth
Numerous tests and monitoring modalities have been proposed as markers for preterm birth risk due to known association. These include cervical length assessment (40, 41); biomarkers such as fetal fibronectin (42), phosphorylated insulin-like growth factor–binding protein 1 (43), and placental alpha microglobulin 1 (44); and uterine monitoring (45). However, there is no single test that can accurately predict a women's risk for preterm birth. At present prediction of preterm birth is primarily based on a summative clinical picture determined by a patient's risk factors. Several risk factors have been identified. A prior history of preterm birth remains the most important. A prior history of preterm birth, however, predicts only approximately 10% of preterm births. In the United States persistent racial disparities have been observed in risk for preterm birth, with higher rates observed in non-Hispanic Black women compared to White and Hispanic women (1). Pregnancy factors such as a threatened abortion, fetal abnormalities, multifetal gestation, polyhydramnios, short cervical length, and short or long interpregnancy interval are known to increase the risk of preterm birth (32). Additional contributing factors for preterm birth risk include lifestyle (cigarette and illicit drug use, prepregnancy body mass index), genetic, infectious (mycoplasma, bacterial vaginosis), and periodontal disease (32). Given the multiple pathologic etiologies of preterm birth, it is worth noting that it is unlikely that a single test will predict all preterm birth, but rather a test may be designed to target the specific etiology of preterm birth, as we will review with CRH in later sections.
Prevention of Preterm Birth
Three treatments are recommended strategies by the Society for Maternal-Fetal Medicine and American College of Obstetricians and Gynecologists for the prevention of preterm birth in selected populations: vaginal progesterone, intramuscular progesterone, and cervical cerclage (7). Progesterone supplementation is given on the premise that withdrawal in mammals is a parturition-triggering event and that supplementation during pregnancy may prolong uterine quiescence. Data on the ability of progesterone to prevent preterm birth have been inconsistent in clinical trials (46–52), and there remains a dearth of information to support its mechanistic benefit in humans. Vaginal progesterone is recommended in women with a singleton gestation without a prior history of preterm birth but with a short cervix (< 25 mm); either vaginal or intramuscular progesterone can be considered in women with a singleton gestation and a prior history of preterm birth (7). Cervical cerclage is a treatment for the subset of women identified to have cervical dysfunction consistent with cervical insufficiency or a short cervix (< 25 mm) and a prior history of preterm birth (53). Serial cervical length screening is recommended in women with a singleton gestation and a prior history of preterm birth because of the potential for treatment with cerclage (7). In addition, modification of known risk factors (eg, cigarette and illicit drug use, low prepregnancy weight) can contribute to preterm birth risk reduction.
Present Preterm Birth Needs
There are many gaps in the knowledge of prediction and prevention of preterm birth. With progesterone there remains a need to better understand mechanistically its function to better identify those women who would benefit from supplementation across various indications. Moving beyond progesterone is the next step. There is a current need to identify alternative pathologic pathways, determine rationales for identifying populations at risk of preterm birth based on pathology, and then develop novel strategies for the prevention of preterm birth. One such pathway is CRH.
Corticotrophin-Releasing Hormone in Humans
CRH is a 41 amino acid peptide hormone synthesized in the hypothalamus that regulates the secretion of the anterior pituitary adrenocorticotropin (ACTH) secretion in response to stress (54). Outside pregnancy, CRH is barely detectable in the human circulation. However, in pregnancy, placental synthesis of CRH leads to exponential increases in the maternal circulation, the amniotic fluid, and the fetal circulation (55, 56). CRH has a known specific circulating binding protein (CRH-BP) (57). When CRH levels are high in late gestation, the saturated binding protein dimerizes and is cleared, leading to increased free, biologically active CRH, which is potentially able to contribute to triggering parturition (58–60).
Transcriptional and Epigenetic Regulation of the CRH Gene
Transcriptional and epigenetic regulation of the CRH gene varies by species and tissue type. Placental expression of CRH within the syncytiotrophoblast is specific to human and anthropoid primates. In New and Old World monkeys, placental CRH production peaks in mid-gestation (61, 62), and only in the apes, including humans, does placental CRH production increase exponentially across pregnancy, peaking at term (63). The single-copy CRH gene is also expressed in the hypothalamus, where it is under negative feedback control by glucocorticoids and is a key regulator of the stress response (64). In contrast, placental expression of CRH is under positive feedback by glucocorticoids and by cyclic adenosine monophosphate (cAMP), leading to the exponential increase in expression across gestation. The regulatory elements of the CRH gene in humans is shown in Fig. 2. The placenta-specific expression of CRH in humans and anthropoid primates is attributed to the presence of the retroviral long terminal repeats (LTRs) of the transposon-like human element 1B (THE1B) in the upstream region of the human CRH gene (65). The LTR element of THE1B acts as an enhancer for the CRH gene. Transgenic expression of the human CRH gene with the LTR of THE1B in mice delayed parturition by 15 hours and induced the expression of human CRH in mouse placenta (66). Deletion of the LTR of THE1B in transgenic mice returned the mice to normal parturition and stopped human CRH production in the mouse placenta (66). The human CRH gene promoter has 2 cAMP response elements (CRE), a consensus CRE, and a caudal-type homeobox response element (CDXRE) (67). The CRH promoter also has a negative glucocorticoid response element (nGRE), through which glucocorticoids suppress CRH production in the hypothalamus. However, King et al (67) identified a particular section of the CRH promoter (−213 to −99 base pairs) mediating glucocorticoid stimulation of CRH within the syncytiotrophoblast. The CRH promoter also has an NF-κB2 enhancer site, and chromatin immunoprecipitation showed that this enhancer is associated with RelB and P52 in trophoblast, which are part of the noncanonical pathway that can be upregulated in the placenta and myometrium in preterm birth (68). This enhancer interaction with RelB and P52 is increased by glucocorticoids and decreased by progesterone (68). Glucocorticoids have also been shown to enhance CRH expression by acetylating H3K9 histone in primary trophoblast culture (69), while cAMP stimulates the expression of CRH messenger RNA (mRNA) by increasing histone-3, lysine-4 trimethylation (H3K4me3), and histone-4 acetylation (acH4) (70). In normal pregnancies the mRNA for CRH is upregulated in the placenta after the fifth week of gestation (71).
Figure 2.
Regulatory elements in the CRH gene in humans. An endogenous retrovirus long-term repeat enhancer sequence (ERV-LTR), NF-κB enhancer sequence, negative glucocorticoid response element (nGRE), cAMP response element (CRE), caudal-type homeobox response element (CDXRE), and a TATA element are present in the promoter region of the human CRH gene.
CRH Downstream Effects in Pregnancy
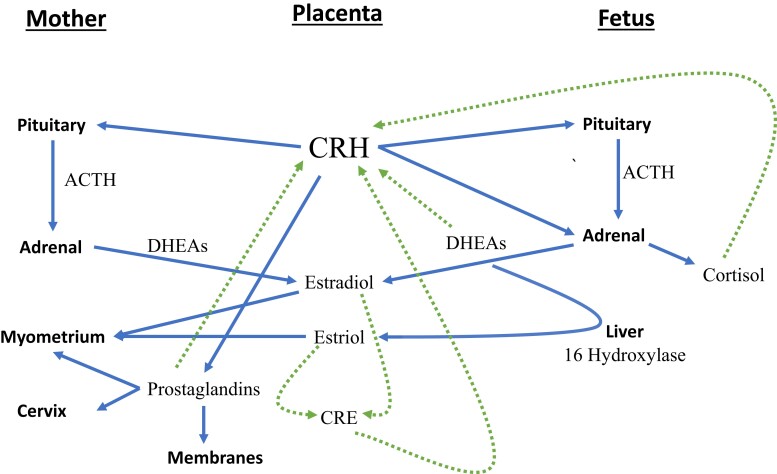
The effect of CRH expression in pregnancy is tissue specific based on in vivo and in vitro studies. In the maternal pituitary, CRH has a stimulatory effect on ACTH, β-endorphin, and cortisol production that is subsequently tempered by desensitization of the pituitary receptor to CRH in late pregnancy (Fig. 3) (72–74). In the myometrium, CRH potentiates the action of uterotonics such as oxytocin and prostaglandin E2 (PGE2) (75–78). Work by Grammatopoulos and Hillhouse elucidated that there are several isoforms of the CRH receptor in the myometrium (79). When nearing parturition, there appears to be a shift in isoforms that reduces cAMP signaling pathways promoting relaxation, with a consequent increase in contractility (80, 81). In the membranes and amniotic compartment, CRH increases synthesis of prostaglandins (PGE2 and PGF2α), promoting cervical ripening and uterine contractions (82). Additionally, prostaglandins stimulate further CRH secretion, creating a positive feedback loop in the placenta (82, 83). In the maternal circulation, CRH stimulates an increase in IL-6 from peripheral blood monocular cells (84). These cells migrate to the placenta and cervix and are notably increased in parturition and infection (84). IL-6 along with IL-1 also increase placental CRH production as another positive feedback loop (85).
Figure 3.
CRH pathways and feedback loops in the maternal, placental, and fetal compartments. Solid lines represent potential roles for CRH in parturition. Dashed lines represent positive feedback loops that could lead to progressive amplification of CRH. Adapted from Herrera 2021.
In the fetal circulation, by mid-trimester CRH stimulates ACTH in the fetal pituitary that may increase cortisol and dehydroepiandrosterone sulfate (DHEA-S) production (86). CRH also directly stimulates cortisol production from the fetal adrenals (87). Cortisol in turn has a direct effect on fetal lung maturity, as indicated by increased indices of fetal lung maturity at birth in women with higher CRH levels (88). Fetal cortisol positively feeds back to the placenta, unlike the hypothalamus, promoting increased placental CRH production (see Fig. 2) (89, 90). CRH directly stimulates DHEA-S production in the fetal zone of the fetal adrenal (91, 92). Fetal DHEA-S in turn is the major substrate for placental production of estriol, after 16 hydroxylation in the fetal liver. Estriol is then released into the maternal bloodstream, where it can promote labor via actions on the myometrium, cervix, and fetal membranes. Again, like cortisol, DHEA-S also positively feeds back to increase CRH secretion (93). Estrogen and progesterone have been shown to modulate placental CRH production through a separate CRE in the CRH promoter (see Fig. 2) (94–96). The multiple positive feedback loops of CRH allow progressive amplification of CRH until parturition occurs (see Fig. 3) (97).
CRH and Preterm Birth
Multiple cross-sectional studies reported that high concentrations of CRH are associated with preterm delivery (98–101). This led McLean et al to conduct a prospective study in 1995 that introduced the idea of CRH as a placental clock (102). CRH was found to increase exponentially in the maternal plasma throughout pregnancy. Controlling for gestational age compared to women who delivered at term, CRH levels were higher in those women who experienced preterm birth and lower in those with postterm delivery. These differences were apparent as early as 16 to 18 weeks of gestation. There was no difference in CRH-BP concentrations among women in the 3 populations. From these findings, CRH was proposed as a placental clock—earlier elevated levels of CRH sufficient to saturate CRH-BP in the maternal bloodstream may result in increased bioavailable CRH capable of initiating parturition. Within this framework, parturition is suggested to result from processes beginning before the mid-trimester and at a rate that could be inferred from observation of CRH levels, enabling preterm birth prediction. Following the work of McLean and colleagues, several studies (103–112) confirmed the association of higher second-trimester CRH with preterm birth.
However, a few studies failed to observe an association between maternal plasma CRH concentrations and preterm birth (113, 114). There are 2 explanations for these disparate data. One, technical considerations have led to difficulty in comparison of published results. Over the years differences have been reported in plasma processing, extraction technique, and the method of measurement of CRH (115). Extraction is necessary as CRH is bound by plasma proteins, including CRH-BP, and the degree of binding is highly variable between individuals (116). Failure to use an extraction technique can lead to erroneous results (97, 113). The second issue has to do with heterogeneity of populations. CRH is altered by race and ethnicity, with non-Hispanic Black (111) and Hispanic (117) women found to have lower CRH levels compared to non-Hispanic White women, though women delivering preterm still demonstrate a high CRH level for their racial and ethnic group (111).
Despite the association of increased CRH and preterm birth, in an unselected population the predictive capability of CRH alone is low. Promisingly, a recent study did find that CRH was increased in women who experienced recurrent preterm birth as compared to women who had a prior preterm birth and subsequent term birth (97). This suggests CRH does specify a population predisposed to preterm birth. That is to say that CRH plays a clear role in normal pregnancy physiology that can be altered to form a pathophysiologic process in a subset of women that results in preterm birth.
Pathologic Interactions of CRH
Placental CRH has been found to be elevated in the setting of preterm prelabor rupture of membranes with chorioamnionitis, suggesting that placental expression of CRH is activated by stress-related pathways that are active in infective processes (118). In twin gestations, CRH is elevated to more than 3 times a singleton gestation; however, preterm birth does not always occur. Variability in ratios of progesterone, estriol, and estradiol may explain this nuance (119). CRH is also elevated in the setting of many indicated preterm births, including fetal growth restriction (112, 120), pregnancy-induced hypertension (110, 121, 122), and gestational diabetes (122). In these cases, CRH elevation may be a compensatory mechanism as a result of stress-related pathways independent of infection status, as induction of these stress-related pathways can influence early brain development (123).
Future Potential of CRH
There is convincing evidence that early increases in plasma CRH correlate with an increased risk of preterm birth. However, the specific threshold at which substantial risk develops has not yet been defined. As a result of racial and ethnic variation, the establishment of large-scale data sets would be necessary for comparative analysis. In addition, any future data must be obtained in a standardized fashion for analysis. Given proven reproducibility of results, this would be via an extraction technique followed by radioimmunoassay (97). If the target population can be identified for diagnostic purposes, the next step would be the potential for therapeutic intervention. A CRH receptor antagonist is then an attractive option for the prevention of preterm birth. Both peptide and nonpeptide antagonists have been developed (124, 125). In sheep CRH antagonism was found to significantly delay delivery (126). Development of CRH antagonism for application in human pregnancy is promising provided diagnostic identification can be performed. Moving beyond CRH, patient-specific genomic profiling may also be another area of benefit, as recent research shows RNA profiling may indicate those at risk for preterm delivery (127).
Summary
In summary, comprehensive understanding of preterm birth must recognize the multifactorial etiology and contributing factors of this clinical syndrome. Current prediction and prevention of preterm birth are limited by our lack of pathophysiologic understanding. One potential pathologic pathway resulting in preterm birth is placentally produced CRH. Despite strong evidence for its biologic plausibility and preterm birth association, CRH measurement for clinical prediction remains problematic as a clear threshold for use has not been established.
Acknowledgments
The authors wish to thank Mala Mahendroo for her thoughtful input in the revision of the manuscript and Lee Dedman and Dayna Perez for their creative design of Fig. 1.
Abbreviations
- ACTH
adrenocorticotropin
- cAMP
cyclic adenosine monophosphate
- CRE
cAMP response element
- CRH
corticotrophin-releasing hormone
- CRH-BP
CRH binding protein
- DHEA-S
dehydroepiandrosterone sulfate
- IL
interleukin
- LTR
long terminal repeat
- mRNA
messenger RNA
- NF-κB
nuclear factor κB
- THE1B
transposon-like human element 1B
Contributor Information
Christina L Herrera, Department of Obstetrics & Gynecology, University of Texas Southwestern Medical Center, Dallas, Texas 75390-9032, USA.
Kaushik Maiti, Mothers and Babies Research Centre, Hunter Medical Research Institute, University of Newcastle, Newcastle, New South Wales 2305, Australia.
Roger Smith, Mothers and Babies Research Centre, Hunter Medical Research Institute, University of Newcastle, Newcastle, New South Wales 2305, Australia.
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the present study.
References
- 1. Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2020. National Center for Health Statistics; 2022. [PubMed] [Google Scholar]
- 2. Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. 2020;150(1):31–33. [DOI] [PubMed] [Google Scholar]
- 3. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crump C. An overview of adult health outcomes after preterm birth. Early Hum Dev. 2020;150:105187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. [DOI] [PubMed] [Google Scholar]
- 6. Bonet M, Cuttini M, Piedvache A, et al. ; MOSAIC and EPICE Research Groups . Changes in management policies for extremely preterm births and neonatal outcomes from 2003 to 2012: two population-based studies in ten European regions. BJOG. 2017;124(10):1595–1604. [DOI] [PubMed] [Google Scholar]
- 7. American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138(2):e65–e90. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . Preterm birth. Published 2018. Accessed June 26, 2022. https://www.who.int/news-room/fact-sheets/detail/preterm-birth
- 9. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. [DOI] [PubMed] [Google Scholar]
- 10. Huddy CL, Johnson A, Hope PL. Educational and behavioural problems in babies of 32-35 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;85(1):F23–F28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ely DM, Driscoll AK. Infant Mortality in the United States, 2019: Data From the Period Linked Birth/Infant Death File. National Center for Health Statistics; 2021. [PubMed] [Google Scholar]
- 12. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Renthal NE, Williams KC, Mendelson CR. MicroRNAs—mediators of myometrial contractility during pregnancy and labour. Nat Rev Endocrinol. 2013;9(7):391–401. [DOI] [PubMed] [Google Scholar]
- 14. Mesiano S. Progesterone withdrawal and parturition. J Steroid Biochem Mol Biol. 2022;224:106177. [DOI] [PubMed] [Google Scholar]
- 15. Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab. 2004;89(2):1010–1013. [DOI] [PubMed] [Google Scholar]
- 16. Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun. 2016;7:11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith R, Butler T, Chan EC. Do estrogen receptor variants explain the enigma of human birth? EBioMedicine. 2019;39:25–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anamthathmakula P, Kyathanahalli C, Ingles J, Hassan SS, Condon JC, Jeyasuria P. Estrogen receptor alpha isoform ERdelta7 in myometrium modulates uterine quiescence during pregnancy. EBioMedicine. 2019;39:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vannuccini S, Bocchi C, Severi FM, Challis JR, Petraglia F. Endocrinology of human parturition. Ann Endocrinol (Paris). 2016;77(2):105–113. [DOI] [PubMed] [Google Scholar]
- 20. Battaglia CR, Cursano S, Calzia E, Catanese A, Boeckers TM. Corticotropin-releasing hormone (CRH) alters mitochondrial morphology and function by activating the NF-kB-DRP1 axis in hippocampal neurons. Cell Death Dis. 2020;11(11):1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pique-Regi R, Romero R, Garcia-Flores V, et al. A single-cell atlas of the myometrium in human parturition. JCI Insight. 2022;7(5):e153921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tyagi S, Chan EC, Barker D, et al. Transcriptomic analysis reveals myometrial topologically associated domains linked to the onset of human term labour. Mol Hum Reprod. 2022;28(3):gaac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phung J, Wang CA, Reeders J, et al. Preterm labor is a distinct process from term labor following computational analysis of human myometrium. Am J Obstet Gynecol. 2022;226(1):106.e101–106.e116. [DOI] [PubMed] [Google Scholar]
- 24. Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143(4):429–438. [DOI] [PubMed] [Google Scholar]
- 25. Yoshida K, Jiang H, Kim M, et al. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS One. 2014;9(11):e112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182(5):2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tripathy S, Nallasamy S, Mahendroo M. Progesterone and its receptor signaling in cervical remodeling: mechanisms of physiological actions and therapeutic implications. J Steroid Biochem Mol Biol. 2022;223:106137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):467–478. [DOI] [PubMed] [Google Scholar]
- 29. Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27(11-12):1037–1051. [DOI] [PubMed] [Google Scholar]
- 30. Kumar D, Moore RM, Mercer BM, Mansour JM, Moore JJ. Mechanism of human fetal membrane biomechanical weakening, rupture and potential targets for therapeutic intervention. Obstet Gynecol Clin North Am. 2020;47(4):523–544. [DOI] [PubMed] [Google Scholar]
- 31. Sykes L, MacIntyre DA, Teoh TG, Bennett PR. Anti-inflammatory prostaglandins for the prevention of preterm labour. Reproduction. 2014;148(2):R29–R40. [DOI] [PubMed] [Google Scholar]
- 32. Cunningham FG, Leveno KJ, Dashe JS, Hoffman BL, Spong CY, Casey BM, eds. Preterm birth. In: Williams Obstetrics. 26th ed. McGraw Hill; 2022. [Google Scholar]
- 33. Romero R, Gómez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15:41–56. [DOI] [PubMed] [Google Scholar]
- 34. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145(1):1–8. [DOI] [PubMed] [Google Scholar]
- 35. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. Accessed June 26, 2022. https://obgyn-mhmedical-com.foyer.swmed.edu/content.aspx?bookid=2977§ionid=263821201 [DOI] [PubMed] [Google Scholar]
- 36. Relman DA, Falkow S. Identification of uncultured microorganisms: expanding the spectrum of characterized microbial pathogens. Infect Agents Dis. 1992;1(5):245–253. [PubMed] [Google Scholar]
- 37. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phung J, Wang C, Reeders J, et al. Preterm labor with and without chorioamnionitis is associated with activation of myometrial inflammatory networks: a comprehensive transcriptomic analysis. Am J Obstet Gynecol. Published online August 21, 2022. doi: 10.1016/j.ajog.2022.08.036 [DOI] [PubMed] [Google Scholar]
- 39. Sinkey RG, Guzeloglu-Kayisli O, Arlier S, et al. Thrombin-induced decidual colony-stimulating factor-2 promotes abruption-related preterm birth by weakening fetal membranes. Am J Pathol. 2020;190(2):388–399. [DOI] [PubMed] [Google Scholar]
- 40. Esplin MS, Elovitz MA, Iams JD, et al. Predictive accuracy of serial transvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567–572. [DOI] [PubMed] [Google Scholar]
- 42. Berghella V, Saccone G. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst Rev. 2019;7(7):CD006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conde-Agudelo A, Romero R. Cervical phosphorylated insulin-like growth factor binding protein-1 test for the prediction of preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(1):57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pirjani R, Moini A, Almasi-Hashiani A, et al. Placental alpha microglobulin-1 (PartoSure) test for the prediction of preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021;34(20):3445–3457. [DOI] [PubMed] [Google Scholar]
- 45. Iams JD, Newman RB, Thom EA, et al. Frequency of uterine contractions and the risk of spontaneous preterm delivery. N Engl J Med. 2002;346(4):250–255. [DOI] [PubMed] [Google Scholar]
- 46. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening Group . Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469. [DOI] [PubMed] [Google Scholar]
- 47. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. [DOI] [PubMed] [Google Scholar]
- 48. Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37(2):127–136. [DOI] [PubMed] [Google Scholar]
- 49. Hassan SS, Romero R, Vidyadhari D, et al. ; PREGNANT Trial . Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Norman JE, Marlow N, Messow CM, et al. ; OPPTIMUM Study Group . Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet. 2016;387(10033):2106–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nelson DB, McIntire DD, McDonald J, Gard J, Turrichi P, Leveno KJ. 17-alpha Hydroxyprogesterone caproate did not reduce the rate of recurrent preterm birth in a prospective cohort study. Am J Obstet Gynecol. 2017;216(6):600.e1–600.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. EPPPIC Group . Evaluating progestogens for preventing preterm birth international collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397(10280):1183–1194. [DOI] [PubMed] [Google Scholar]
- 53. ACOG Practice bulletin No. 142: cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 Pt 1):372–379. [DOI] [PubMed] [Google Scholar]
- 54. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. [DOI] [PubMed] [Google Scholar]
- 55. Sasaki A, Shinkawa O, Margioris AN, et al. Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor, and delivery. J Clin Endocrinol Metab. 1987;64(2):224–229. [DOI] [PubMed] [Google Scholar]
- 56. Campbell EA, Linton EA, Wolfe CD, Scraggs PR, Jones MT, Lowry PJ. Plasma corticotropin-releasing hormone concentrations during pregnancy and parturition. J Clin Endocrinol Metab. 1987;64(5):1054–1059. [DOI] [PubMed] [Google Scholar]
- 57. Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349(6308):423–426. [DOI] [PubMed] [Google Scholar]
- 58. Woods RJ, Kennedy KM, Gibbins JM, Behan D, Vale W, Lowry PJ. Corticotropin-releasing factor binding protein dimerizes after association with ligand. Endocrinology. 1994;135(2):768–773. [DOI] [PubMed] [Google Scholar]
- 59. Behan DP, Khongsaly O, Liu XJ, et al. Measurement of corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in human plasma by two-site enzyme-linked immunoabsorbant assay. J Clin Endocrinol Metab. 1996;81(7):2579–2586. [DOI] [PubMed] [Google Scholar]
- 60. Linton EA, Perkins AV, Woods RJ, et al. Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab. 1993;76(1):260–262. [DOI] [PubMed] [Google Scholar]
- 61. Power ML, Bowman ME, Smith R, et al. Pattern of maternal serum corticotropin-releasing hormone concentration during pregnancy in the common marmoset (Callithrix jacchus). Am J Primatol. 2006;68(2):181–188. [DOI] [PubMed] [Google Scholar]
- 62. Smith R, Chan EC, Bowman ME, Harewood WJ, Phippard AF. Corticotropin-releasing hormone in baboon pregnancy. J Clin Endocrinol Metab. 1993;76(4):1063–1068. [DOI] [PubMed] [Google Scholar]
- 63. Smith R, Wickings EJ, Bowman ME, et al. Corticotropin-releasing hormone in chimpanzee and gorilla pregnancies. J Clin Endocrinol Metab. 1999;84(8):2820–2825. [DOI] [PubMed] [Google Scholar]
- 64. Sharma D, Bhave S, Gregg E, Uht R. Dexamethasone induces a putative repressor complex and chromatin modifications in the CRH promoter. Mol Endocrinol. 2013;27(7):1142–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chuong EB. The placenta goes viral: retroviruses control gene expression in pregnancy. PLoS Biol. 2018;16(10):e3000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dunn-Fletcher CE, Muglia LM, Pavlicev M, et al. Anthropoid primate-specific retroviral element THE1B controls expression of CRH in placenta and alters gestation length. PLoS Biol. 2018;16(9):e2006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. King BR, Smith R, Nicholson RC. Novel glucocorticoid and cAMP interactions on the CRH gene promoter. Mol Cell Endocrinol. 2002;194(1-2):19–28. [DOI] [PubMed] [Google Scholar]
- 68. Wang B, Parobchak N, Rosen T. Relb/NF-κB2 regulates corticotropin-releasing hormone in the human placenta. Mol Endocrinol. 2012;26(8):1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Di Stefano V, Wang B, Parobchak N, Roche N, Rosen T. Relb/p52-mediated NF-κB signaling alters histone acetylation to increase the abundance of corticotropin-releasing hormone in human placenta. Sci Signal. 2015;8(391):ra85. [DOI] [PubMed] [Google Scholar]
- 70. Pan X, Bowman M, Scott RJ, Fitter J, Smith R, Zakar T. Promoter methylation pattern controls corticotropin releasing hormone gene activity in human trophoblasts. PLoS One. 2017;12(2):e0170671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Frim DM, Emanuel RL, Robinson BG, Smas CM, Adler GK, Majzoub JA. Characterization and gestational regulation of corticotropin-releasing hormone messenger RNA in human placenta. J Clin Invest. 1988;82(1):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith R, Cubis J, Brinsmead M, et al. Mood changes, obstetric experience and alterations in plasma cortisol, beta-endorphin and corticotrophin releasing hormone during pregnancy and the puerperium. J Psychosom Res. 1990;34(1):53–69. [DOI] [PubMed] [Google Scholar]
- 73. Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf). 1990;33(1):99–106. [DOI] [PubMed] [Google Scholar]
- 74. Thomson M, Chan EC, Falconer J, et al. Desensitization of superfused isolated ovine anterior pituitary cells to human corticotropin-releasing factor. J Neuroendocrinol. 1990;2(2):181–187. [DOI] [PubMed] [Google Scholar]
- 75. Quartero HW, Fry CH. Placental corticotrophin releasing factor may modulate human parturition. Placenta. 1989;10(5):439–443. [DOI] [PubMed] [Google Scholar]
- 76. Benedetto C, Petraglia F, Marozio L, et al. Corticotropin-releasing hormone increases prostaglandin F2 alpha activity on human myometrium in vitro. Am J Obstet Gynecol. 1994;171(1):126–131. [DOI] [PubMed] [Google Scholar]
- 77. Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;354(9189):1546–1549. [DOI] [PubMed] [Google Scholar]
- 78. Linton EA, Woodman JR, Asboth G, Glynn BP, Plested CP, Bernal AL. Corticotrophin releasing hormone: its potential for a role in human myometrium. Exp Physiol. 2001;86(2):273–281. [DOI] [PubMed] [Google Scholar]
- 79. Grammatopoulos D, Thompson S, Hillhouse EW. The human myometrium expresses multiple isoforms of the corticotropin-releasing hormone receptor. J Clin Endocrinol Metab. 1995;80(8):2388–2393. [DOI] [PubMed] [Google Scholar]
- 80. Grammatopoulos DK. The role of CRH receptors and their agonists in myometrial contractility and quiescence during pregnancy and labour. Front Biosci. 2007;12(1):561–571. [DOI] [PubMed] [Google Scholar]
- 81. Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19(2):474–490. [DOI] [PubMed] [Google Scholar]
- 82. Petraglia F, Florio P, Nappi C, Genazzani AR. Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17(2):156–186. [DOI] [PubMed] [Google Scholar]
- 83. Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160(1):247–251. [DOI] [PubMed] [Google Scholar]
- 84. Angioni S, Petraglia F, Gallinelli A, et al. Corticotropin-releasing hormone modulates cytokines release in cultured human peripheral blood mononuclear cells. Life Sci. 1993;53(23):1735–1742. [DOI] [PubMed] [Google Scholar]
- 85. Petraglia F, Garuti GC, De Ramundo B, Angioni S, Genazzani AR, Bilezikjian LM. Mechanism of action of interleukin-1 beta in increasing corticotropin-releasing factor and adrenocorticotropin hormone release from cultured human placental cells. Am J Obstet Gynecol. 1990;163(4 Pt 1):1307–1312. [DOI] [PubMed] [Google Scholar]
- 86. Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987;328(6132):717–719. [DOI] [PubMed] [Google Scholar]
- 87. Sirianni R, Rehman KS, Carr BR, Parker CR, Rainey WE. Corticotropin-releasing hormone directly stimulates cortisol and the cortisol biosynthetic pathway in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90(1):279–285. [DOI] [PubMed] [Google Scholar]
- 88. Laatikainen TJ, Raisanen IJ, Salminen KR. Corticotropin-releasing hormone in amniotic fluid during gestation and labor and in relation to fetal lung maturation. Am J Obstet Gynecol. 1988;159(4):891–895. [DOI] [PubMed] [Google Scholar]
- 89. Robinson BG, Emanuel RL, Frim DM, Majzoub JA. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci U S A. 1988;85(14):5244–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jones SA, Brooks AN, Challis JR. Steroids modulate corticotropin-releasing hormone production in human fetal membranes and placenta. J Clin Endocrinol Metab. 1989;68(4):825–830. [DOI] [PubMed] [Google Scholar]
- 91. Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83(8):2916–2920. [DOI] [PubMed] [Google Scholar]
- 92. Sirianni R, Mayhew BA, Carr BR, Parker CR, Rainey WE. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90(9):5393–5400. [DOI] [PubMed] [Google Scholar]
- 93. Challis JR, Hooper S. Birth: outcome of a positive cascade. Baillieres Clin Endocrinol Metab. 1989;3(3):781–793. [DOI] [PubMed] [Google Scholar]
- 94. Ni X, Nicholson RC, King BR, Chan EC, Read MA, Smith R. Estrogen represses whereas the estrogen-antagonist ICI 182780 stimulates placental CRH gene expression. J Clin Endocrinol Metab. 2002;87(8):3774–3778. [DOI] [PubMed] [Google Scholar]
- 95. Ni X, Hou Y, Yang R, Tang X, Smith R, Nicholson RC. Progesterone receptors A and B differentially modulate corticotropin-releasing hormone gene expression through a cAMP regulatory element. Cell Mol Life Sci. 2004;61(9):1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (CRH) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3',5'-cyclic adenosine 5'-monophosphate regulatory region of the proximal CRH promoter. Endocrinology. 2008;149(1):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Herrera CL, Bowman ME, McIntire DD, Nelson DB, Smith R. Revisiting the placental clock: early corticotrophin-releasing hormone rise in recurrent preterm birth. PLoS One. 2021;16(9):e0257422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wolfe CD, Patel SP, Linton EA, et al. Plasma corticotrophin-releasing factor (CRF) in abnormal pregnancy. Br J Obstet Gynaecol. 1988;95(10):1003–1006. [DOI] [PubMed] [Google Scholar]
- 99. Warren WB, Patrick SL, Goland RS. Elevated maternal plasma corticotropin-releasing hormone levels in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1992;166(4):1198–1204; discussion 1204-1207. [DOI] [PubMed] [Google Scholar]
- 100. Stalla GK, Bost H, Stalla J, et al. Human corticotropin-releasing hormone during pregnancy. Gynecol Endocrinol. 1989;3(1):1–10. [DOI] [PubMed] [Google Scholar]
- 101. McLean M, Thompson D, Zhang HP, Brinsmead M, Smith R. Corticotrophin-releasing hormone and beta-endorphin in labour. Eur J Endocrinol. 1994;131(2):167–172. [DOI] [PubMed] [Google Scholar]
- 102. McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–463. [DOI] [PubMed] [Google Scholar]
- 103. Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 Pt 3):S257–S263. [DOI] [PubMed] [Google Scholar]
- 104. Leung TN, Chung TK, Madsen G, McLean M, Chang AM, Smith R. Elevated mid-trimester maternal corticotrophin-releasing hormone levels in pregnancies that delivered before 34 weeks. Br J Obstet Gynaecol. 1999;106(10):1041–1046. [DOI] [PubMed] [Google Scholar]
- 105. Ellis MJ, Livesey JH, Inder WJ, Prickett TC, Reid R. Plasma corticotropin-releasing hormone and unconjugated estriol in human pregnancy: gestational patterns and ability to predict preterm delivery. Am J Obstet Gynecol. 2002;186(1):94–99. [DOI] [PubMed] [Google Scholar]
- 106. Inder WJ, Prickett TC, Ellis MJ, et al. The utility of plasma CRH as a predictor of preterm delivery. J Clin Endocrinol Metab. 2001;86(12):5706–5710. [DOI] [PubMed] [Google Scholar]
- 107. Moawad AH, Goldenberg RL, Mercer B, et al. ; NICHD MFMU Network . The Preterm Prediction Study: the value of serum alkaline phosphatase, alpha-fetoprotein, plasma corticotropin-releasing hormone, and other serum markers for the prediction of spontaneous preterm birth. Am J Obstet Gynecol. 2002;186(5):990–996. [DOI] [PubMed] [Google Scholar]
- 108. Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179(4):1079–1085. [DOI] [PubMed] [Google Scholar]
- 109. McLean M, Bisits A, Davies J, et al. Predicting risk of preterm delivery by second-trimester measurement of maternal plasma corticotropin-releasing hormone and alpha-fetoprotein concentrations. Am J Obstet Gynecol. 1999;181(1):207–215. [DOI] [PubMed] [Google Scholar]
- 110. Hobel CJ, Arora CP, Korst LM. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Ann N Y Acad Sci. 1999;897:54–65. [DOI] [PubMed] [Google Scholar]
- 111. Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97(5 Pt 1):657–663. [DOI] [PubMed] [Google Scholar]
- 112. Wadhwa PD, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–1069. [DOI] [PubMed] [Google Scholar]
- 113. Sibai B, Meis PJ, Klebanoff M, et al. Plasma CRH measurement at 16 to 20 weeks’ gestation does not predict preterm delivery in women at high-risk for preterm delivery. Am J Obstet Gynecol. 2005;193(3 Pt 2):1181–1186. [DOI] [PubMed] [Google Scholar]
- 114. Berkowitz GS, Lapinski RH, Lockwood CJ, Florio P, Blackmore-Prince C, Petraglia F. Corticotropin-releasing factor and its binding protein: maternal serum levels in term and preterm deliveries. Am J Obstet Gynecol. 1996;174(5):1477–1483. [DOI] [PubMed] [Google Scholar]
- 115. Latendresse G, Ruiz RJ. Bioassay research methodology: measuring CRH in pregnancy. Biol Res Nurs. 2008;10(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Linton EA, Perkins AV, Hagan P, et al. Corticotrophin-releasing hormone (CRH)-binding protein interference with CRH antibody binding: implications for direct CRH immunoassay. J Endocrinol. 1995;146(1):45–53. [DOI] [PubMed] [Google Scholar]
- 117. Siler-Khodr TM, Forthman G, Khodr C, Matyszczyk S, Khodr Z, Khodr G. Maternal serum corticotropin-releasing hormone at midgestation in Hispanic and White women. Obstet Gynecol. 2003;101(3):557–564. [DOI] [PubMed] [Google Scholar]
- 118. Torricelli M, Novembri R, Bloise E, De Bonis M, Challis JR, Petraglia F. Changes in placental CRH, urocortins, and CRH-receptor mRNA expression associated with preterm delivery and chorioamnionitis. J Clin Endocrinol Metabol. 2011;96(2):534–540. [DOI] [PubMed] [Google Scholar]
- 119. Smith R, Smith JI, Shen X, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metabol. 2009;94(6):2066–2074. [DOI] [PubMed] [Google Scholar]
- 120. Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J Clin Endocrinol Metabol. 1993;77(5):1174–1179. [DOI] [PubMed] [Google Scholar]
- 121. Goland RS, Tropper PJ, Warren WB, Stark RI, Jozak SM, Conwell IM. Concentrations of corticotrophin-releasing hormone in the umbilical-cord blood of pregnancies complicated by pre-eclampsia. Reprod Fertil Dev. 1995;7(5):1227–1230. [DOI] [PubMed] [Google Scholar]
- 122. Steine IM, LeWinn KZ, Lisha N, et al. Maternal exposure to childhood traumatic events, but not multi-domain psychosocial stressors, predict placental corticotrophin releasing hormone across pregnancy. Soc Sci Med. 2020;266:113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27(2):309–318. [DOI] [PubMed] [Google Scholar]
- 124. Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 2003;9(1):57–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zoumakis E, Chrousos GP. Corticotropin-releasing hormone receptor antagonists: an update. Endocr Dev. 2010;17:36–43. [DOI] [PubMed] [Google Scholar]
- 126. Chan EC, Falconer J, Madsen G, et al. A corticotropin-releasing hormone type I receptor antagonist delays parturition in sheep. Endocrinology. 1998;139(7):3357–3360. [DOI] [PubMed] [Google Scholar]
- 127. Camunas-Soler J, Gee EPS, Reddy M, et al. Predictive RNA profiles for early and very early spontaneous preterm birth. Am J Obstet Gynecol. 2022;227(1):72.e1–72.e16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the present study.