Abstract
Bisphenol A (BPA) is an established environmental endocrine disruptor and can interfere with the development of female germ cells. However, the underlying mechanisms are still unclear. We investigated the effects of BPA on granulosa cell development and meiosis of oocytes using in vitro culture system of mouse preantral follicles. Preantral follicles from D14 mouse ovary were treated with 10 μg/mL BPA in vitro for 11 days. The adherent area of follicles was measured. On D11, cumulus cell expansion was observed. The meiosis recovery rate was calculated. Western blot detected P53, proliferating cell nuclear antigen (PCNA), estrogen receptor α (ERα), and cyclin B1. ELISA measured estrogen and progesterone levels. Immunofluorescence detected Cx37 on oocyte membrane. Gap junction communication was assessed. We found that BPA significantly promoted the expressions of PCNA and ERα in granulosa cells and the secretion of estrogen and progesterone by granulosa cells on D10 and significantly increased the attachment area of the follicles on D8 and D10. However, it reduced the expansion of cumulus cells, Cx37 expression, and the gap junction communication between cumulus cells and oocytes on D11. BPA promoted the recovery of oocytes from meiosis, interrupted the expression of cyclin B1 protein in arrested germinal vesicle breakdown (GVBD) oocytes, and reduced the in vitro maturation rate of oocytes. These GVBD oocytes were live without apoptosis or death. Conclusively, BPA disturbs the development of granulosa cells and the meiosis progression of oocytes by decreasing gap junction communication between oocytes and the granulosa cells as well as regulating cyclin B1 expression in GVBD oocytes.
Keywords: Bisphenol A, granulosa cell, oocyte, preantral follicle, meiosis
Impact Statement
We investigated the effects of bisphenol A (BPA) on granulosa cell development and meiosis of oocytes using in vitro culture system of mouse preantral follicles. The results showed that BPA promoted the development of granulosa cells in the late stage of follicular development; increased the expressions of proliferating cell nuclear antigen (PCNA) and estrogen receptor α (ERα) on granulosa cells and the secretion of estrogen and progesterone but reduced the expansion of cumulus cells around cell-oocyte complexes (COCs), Cx37 expression, and the gap junction communication between cumulus cells and oocytes; promoted the recovery of oocyte meiosis; and led to the developmental arrest of oocytes after germinal vesicle breakdown (GVBD), which may be related to the abnormal expression of cyclin B1. This study more systematically clarified the adverse effects of BPA as an environmental estrogen-like chemical on the development of granulosa cells and oocytes.
Introduction
Bisphenol A (BPA) is one of the most widely used industrial compounds in the world. It is contained in mineral water bottles, medical equipment, and food packaging. BPA can be released from food packaging to food or beverages. Different concentrations of BPA have been found in human urine, blood, follicular fluid, and amniotic fluid.1–4 The structure of BPA is similar to estradiol and diethylstilbestrol. It is an estrogen-like endocrine disruptor and can act as estrogen and antagonize androgens in the body.5,6 Clinical study has found that the high concentration of BPA in the urine of infertile women is clearly related to the decrease in the number of primordial follicles. 7 BPA can also interfere with the synthesis and secretion of hormones, and cause embryonic development failure.8–10 Therefore, the effect of BPA on female germ cells has received more and more attention.
The effects of BPA on ovarian development have been investigated in vivo. Because normal ovarian follicle development and sex hormone secretion are necessary for female fertility, 11 these in vivo studies have focused on the effects of BPA on ovarian follicle number and sex hormone secretion.12–14 Rivera et al. 12 found that intraperitoneal injection of 50 μg/kg bw/d of BPA in lambs 30 days after birth significantly reduced the reserve of primordial follicles and increased the number of multioocyte and atretic follicles. Rodríguez et al. 13 reported that intraperitoneal injection of 20 mg/kg bw/d of BPA significantly reduced the number of primordial follicles in eight-day-old rats. After exposure of neonatal mice and adult rats to BPA, the serum estrogen levels were significantly increased. Patel et al. 14 revealed that different concentrations of BPA had different effects on ovarian follicle numbers and sex hormone levels at different ovarian developmental stages. However, the mechanisms underlying the effects of BPA on ovarian/follicle development need to be further clarified by in vitro experiments.
BPA can also affect the development and function of granulosa cells. For example, Pogrmic-Majkic et al. 15 found that BPA significantly increased the expression of STAR mRNA and increased the synthesis and secretion of progesterone in human granulosa cells. Lee et al. 16 found that BPA reduced the secretion of 17β-estrogen by rat granulosa cells. However, in the in vitro culture system of pig cumulus cell-oocyte complexes (COCs), Song et al. 17 reported that BPA significantly increased the secretion of 17β-estrogen by granulosa cells and promoted the proliferation of granulosa cells. Huang et al. 18 found that BPA induced KGN (a human granulosa-like tumor cell line) cell apoptosis by activating the reactive oxygen species/Ca2+-apoptosis signal-regulating kinase-c-Jun N-terminal protein kinase (ROS/Ca2+-ASK-JNK) pathway. Xu et al. 19 reported that BPA induced apoptosis and G2-to-M arrest of murine ovarian granulosa cells. Different effects of BPA on the development and function of granulosa cells have been found in different species, even in the same species, using different experimental model systems.
BPA can reduce the expression of connexin 43 (Cx43) on human granulosa cells through the estrogen receptor (ER) and mitogen-activated protein kinase (MAPK) pathway, thereby reducing the gap junction between granulosa cells. 20 During the culture of mouse COCs, it is found that BPA did not affect the expression of gap junction protein Cx37. 21 However, during rat COCs culture, BPA changed the expression of gap junction genes (Gja1 and Gja4) and transfer rate. 22 Thus, the effects of BPA on the gap junction between granulosa cells or between cumulus cells and oocytes and on the development of oocytes are still inconclusive.
At present, studies on the mechanism of BPA’s adverse effects have been carried out in the in vitro cultured granulosa cells, oocytes, or COCs15–18,20–22 However, since the development of oocytes in follicle is inseparable from granulosa cells, the results obtained in these previous studies may have a certain degree of one-sidedness. Granulosa cells and oocytes form a functional unit in the follicle. They communicate with each other through gap junctions. Oocytes can affect the development of granulosa cells, and granulosa cells can also affect the development of oocytes. In vivo, BPA affects the development of granulosa cells through follicles but does not directly affect granulosa cells and oocytes. Therefore, in order to better simulate the developmental environment of oocytes and granulosa cells and to more accurately explore the effect of BPA on the development of female germ cells, we used preantral follicles in this study and cultured them in vitro for 11 days. The dose of BPA was determined at 10 μg/mL according to a previous study. 23 The effects of 10 μg/mL BPA on the development of granulosa cells around oocytes, the expansion of cumulus cells, the secretion of estrogen and progesterone, gap junction communication, and oocyte development were evaluated. Our findings may provide stronger evidence for understanding the effects and mechanisms of BPA on granulosa cell development and oocyte meiosis.
Materials and methods
Experimental animals
Female mice of Kunming strain (n = 90; 14-day old) were purchased from Yisi Experimental Animal Technology Co., Ltd. (Changchun, China). They were housed in a temperature-controlled room (21 ± 2°C) with a light cycle of 12 h light/12 h dark and free access to food and water. The study was carried out in compliance with the Animal Research: Reporting in Vivo Experiments guidelines. All animal experimental procedures were approved by the Ethics Committee of Jilin Medical University.
In vitro culture and treatment of mouse preantral follicles
Kunming female mice were sacrificed by cervical dislocation, and their bilateral ovaries were quickly isolated and incubated with L-15 working solution (containing 10% fetal bovine serum [FBS]) (11415114, Gibco, Carlsbad, CA, USA). Follicles were mechanically separated under a stereoscope. 23 According to the previously described grading standard, 24 grade 4 or 5a follicles were selected, that is, preantral follicles containing 2–4 layers of granulosa cells. The selected preantral follicles were cultured in minimum essential medium-alpha (α-MEM) culture medium (12571063, Gibco, Carlsbad, CA, USA), which contained 5% FBS (10091155, Gibco), 1% insulin-transferrin-selenium (ITS) (41400045, Gibco, Carlsbad, CA, USA), and 0.1 IU/mL recombinant follicle stimulating hormone (r-FSH) (Gonal-F, Merk Serono, Darmstadt, Germany), for 11 days at 37°C and 5% CO2, in the presence of 10 µg/mL of BPA (239658, Sigma, St. Louis, MO, USA) (BPA group), or 0.1% dimethyl sulfoxide (DMSO) (D2650, Sigma, St. Louis, MO, USA) (DMSO Group). The preantral follicles without treatment served as normal control. The dose of BPA has been confirmed to have germ cell toxicity. 23 The medium was changed semi-quantitatively every other day.
On the 10th day, 10 COCs were collected from follicles of each group. The remaining follicles were continuously cultured in the α-MEM culture medium containing 2.5 U/mL human chorionic gonadotropin (hCG) (Livzon Pharmaceutical Factory, Zhuhai, China). After 14–16 h (on the 11th day), COCs were obtained from mature follicles, and the extent of cumulus cell expansion was compared with the COCs obtained from D10. Then, the cumulus cells around the oocytes were removed by hyaluronidase. The follicles at various developmental stages, and the meiosis and maturity of the oocytes were observed and recorded under Olympus IX-83. The diameter of oocytes was measured, and the attachment area of the follicles on the petri dish was analyzed by Image J software. The development rate of oocytes in each stage was calculated as the ratio of the number of oocytes at this stage to the total number of living oocytes at all stages.
Mitochondrial membrane potential assay
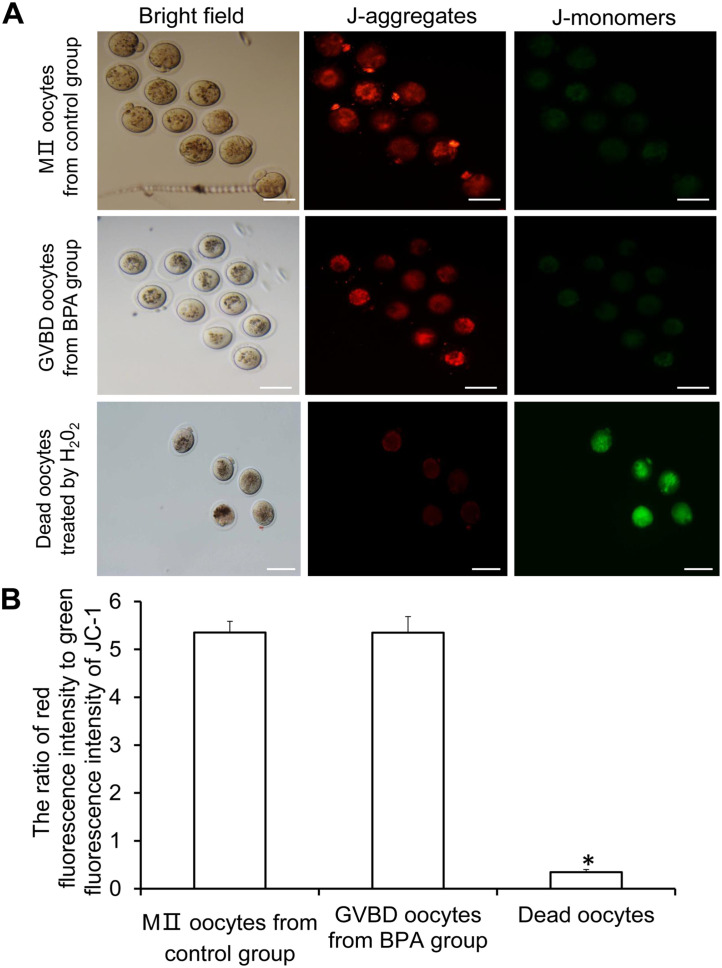
MII oocytes from control group, germinal vesicle breakdown (GVBD) oocytes from BPA group, and dead oocytes treated with 100 µM H2O2 for 30 min were washed three times with phosphate-buffered saline (PBS), and then incubated with a culture medium containing 0.5 µmol/L JC-1 (Invitrogen, Grand Island, NY, USA) at 37°C and 5% CO2 in an incubator for 30 min, as previously described. 25 When the mitochondrial membrane potential is normal, JC-1 could enter the mitochondria and gather in the mitochondrial matrix to form J-aggregates, producing red fluorescence; if there is cell apoptosis with decreased or lost mitochondrial membrane potential, there would be J-monomers in the cytoplasm, exhibiting green fluorescence. The oocytes were observed under the Olympus IX-83 fluorescence microscope. The membrane potential of mitochondria was evaluated by the ratio of red fluorescence intensity to green fluorescence intensity.
Oocyte viability assessment
MII oocytes from control group, GVBD oocytes from BPA group, and dead oocytes treated with 100 µM H2O2 for 30 min were incubated with 10 µg/mL propidium iodide (PI) and 2 µM Calcein acetoxymethyl ester (Calcein AM) (C2012, Beyotime, Shanghai, China) at 37°C for 15 min, as described previously. 26 After washing three times with PBS, the fluorescence of the oocytes was observed using the Olympus IX-73 fluorescence microscope. Oocytes with green fluorescence in the cytoplasm were considered alive, and those with red fluorescence in the nucleus were considered dead.
Western blotting analysis
In each group, granulosa cells were obtained from 20 follicles on the 4th or 10th day of in vitro culture, and the protein was extracted with radio immunoprecipitation assay buffer (RIPA) (containing 1% phenylmethanesulfonyl fluoride [PMSF]) (P0013B and P1006, Beyotime, Shanghai, China). Meanwhile, 60 GVBD oocytes were obtained from the COCs discharged from D11 of each group and subjected to lysis with protein lysis buffer (Laemmli sample buffer + β-mercaptoethanol + protease inhibitor) at room temperature. The proteins were transferred to the polyvinylidene fluoride (PVDF) membrane. After blocking with 5% skimmed milk for 1 h, the membrane was probed with anti-PCNA (proliferating cell nuclear antigen) (ab92552, Abcam, Cambridge, UK), anti-P53 (ab26, Abcam, Cambridge, UK), anti-ERα (ab32063, Abcam, Cambridge, UK), anti-cyclin B1 (ab72, Abcam, Cambridge, UK), and anti-β-actin (ab8226, Abcam, Cambridge, UK) primary antibodies overnight at 4°C. After washing the membrane with phosphate buffered saline with Tween 20 (PBST), the membrane was incubated in goat anti-rabbit horseradish peroxidase (HRP)-labeled secondary antibody (31210, Thermo Scientific Pierce, Waltham, MA, USA) for 2 h at room temperature, and then subjected to enhanced chemiluminescence color development. 23 The ChemiDOC XRS+ imaging system (Bio-Rad Laboratories, Hercules, CA, USA) was used to scan the film. Image J software was used to analyze the relative expression levels of PCNA, P53, ERα, and cyclin B1.
ELISA assay
The follicle culture medium on the 10th day was collected for analyzing estrogen and progesterone levels, which were measured with ELISA kits (BPE20376 and BPE20381, Shanghai Lengton Bioscience Co., Ltd., Shanghai, China) according to the instructions. Briefly, the samples and detection reagents were added to a 96-well plate and incubated at 37°C for 30 min. After washing, the chromogenic solution was added and incubated at 37°C for 10 min in the dark. 23 The absorbance value OD450 was measured with a SpectraMax Absorbance Reader (Molecular Devices, Sunnyvale, CA, USA).
Immunofluorescence
After the oocytes were fixed in 4% paraformaldehyde for 0.5 h, they were permeabilized in PBS containing 1% Triton-X100 for 0.5 h. After washing with PBS twice, blocking with 1% BSA for 1 h was performed. Then, the oocytes were incubated with rabbit anti-Cx37 antibody (bs-4067R, Bioss, Beijing, China) at 37°C for 1 h. After washing again, the oocytes were incubated with goat anti-rabbit fluorescein isothiocyanate (FITC)-labeled secondary antibody at 37°C for 1 h. Then, the nucleus was stained with 5 μg/mL Hoechst 33342 at room temperature for 10 min. 26 Finally, the oocytes were mounted on the glass slides and observed under a laser confocal microscope (FV3000, Olympus, Tokyo, Japan). Image J software analyzed the average fluorescence intensity of Cx37 protein in oocytes, and 10 oocytes of each group were analyzed.
Communication analysis of gap junction
We analyzed the gap junction communication of cumulus cells and oocytes using the method described by Sasseville’ et al., 27 with slight modifications. In detail, the COCs were incubated with Calcein AM-PBS (C2012, Beyotime, Shanghai, China) for 15 min to transfer Calcein AM from cumulus cells to oocytes through gap junctions. 26 Then, the cumulus cells around the oocytes were digested and removed with 0.1% hyaluronidase. The oocytes were observed under Olympus IX-83. Image J software analyzed the average fluorescence intensity of Calcein AM in oocytes. Ten COCs in each group were analyzed, and the experiment was repeated three times.
Statistical analysis
All data are expressed as mean ± standard deviation and analyzed by SPSS 17.0 statistical software. One-way analysis of variance (ANOVA) followed by least significant difference (LSD) post doc was used for multigroup comparison. P < 0.05 suggested significant difference.
Results
BPA promotes the development of granulosa cells in the late stage of follicular development
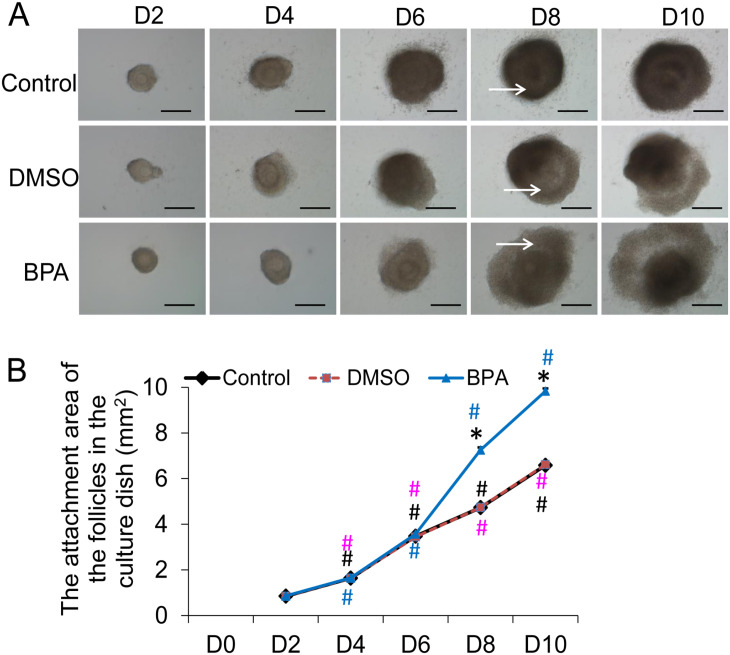
Preantral follicles with a diameter of 110–130 µm were isolated from the ovary of 14-day-old mouse and cultured in vitro for 11 days. The developmental status of the follicles was observed on Day 2 (D2), Day 4 (D4), Day 6 (D6), Day 8 (D8), and Day 10 (D10) (Figure 1(A)). On D2, the outer cells of the follicles began to grow outward. With the development of follicular granulosa cells, the volume of the follicle gradually increased and the follicular cavity with follicular fluid appeared in the follicle on D8. The attachment area of the follicles in the petri dish was measured (Figure 1(B)). The results showed that within each group, the attachment area of follicles on D4, D6, D8, and D10 increased significantly with prolonged culture time (P < 0.05, Figure 1(B)). Compared with the normal group, 0.1% DMSO had no significant effect on the attachment area of the follicles at each time point (P > 0.05, Figure 1(B)). The attachment area of the D2, D4, and D6 follicles in the 10 μg/mL BPA group was not significantly different than that of the normal follicles (P > 0.05). However, the attachment area of D8 and D10 follicles was significantly larger than that of the normal group and the DMSO group (P < 0.05). Thus, 10 µg/mL BPA significantly promoted the development of granulosa cells starting from D8.
Figure 1.
The in vitro development of follicles at each stage and the adherence area on the bottom of the petri dish. (A) In vitro development of follicles at each stage (scale bar = 40 μm). The white arrow indicates the follicular cavity. (B) The adherent area of follicles in each stage at the bottom of the petri dish (mm2) (N = 10).
Compared with control, *P < 0.05. Within control group, D4 versus D2, D6 versus D8, and D10 versus D8, #P < 0.05 (black). Within DMSO group, D4 versus D2, D6 versus D8, and D10 versus D8, #P < 0.05 (pink). Within BPA group, D4 versus D2, D6 versus D8, and D10 versus D8, #P < 0.05 (blue).
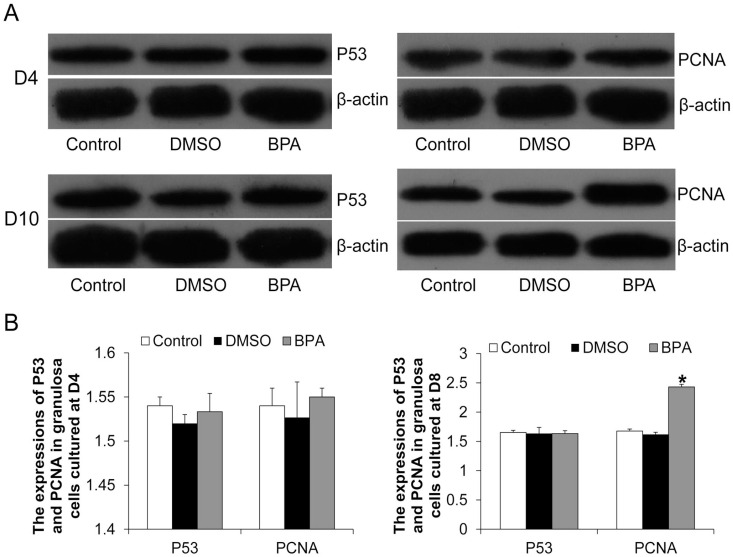
The granulosa cells of D4 and D10 follicles were collected, and Western blotting analysis of P53 and PCNA was performed (Figure 2(A)). Compared with the control group and the DMSO group, 10 µg/mL of BPA had no significant effect on P53 expression in D4 and D10 granulosa cells (P > 0.05, Figure 2(B)) and on PCNA expression in D4 granulosa cells (P > 0.05, Figure 2(B)). However, BPA significantly increased PCNA expression in D10 granulosa cells (P < 0.05, Figure 2(B)), suggesting that it may promote proliferation of granulosa cells.
Figure 2.
The expression of P53 and PCNA in granulosa cells. (A) Western blotting detected the expression of P53 and PCNA in D4 and D10 follicular granulosa cells. Representative results were shown. (B) Image J software analyzed the relative expression levels of P53 and PCNA.
Compared with control, *P < 0.05.
BPA improves the secretion of estrogen and progesterone and the expression of ERα by granulosa cells
The levels of estrogen and progesterone secreted by D10 follicles were measured by ELISA. The results showed that compared with the control group and the DMSO group, the levels of estrogen and progesterone in the culture medium of the 10 µg/mL BPA group were significantly increased (P < 0.05, Table 1), indicating the promotive effect of the 10 µg/mL BPA on secretion of estrogen and progesterone by granulosa cells.
Table 1.
Estrogen and progesterone secreted by follicles on day 10.
| Group | No. of mice | The number of replicates | Estrogen (ng/L) | Coefficient of variability (%) | Progesterone (ng/mL) | Coefficient of variability (%) |
|---|---|---|---|---|---|---|
| Control | 9 | 3 | 21.65 ± 2.73 | 12.61 | 0.64 ± 0.09 | 14.06 |
| DMSO | 9 | 3 | 21.88 ± 2.82 | 12.89 | 0.65 ± 0.09 | 13.85 |
| 10 μg/mL BPA | 9 | 3 | 42.60 ± 2.85* | 6.69 | 0.86 ± 0.07* | 8.14 |
DMSO: dimethyl sulfoxide; BPA: bisphenol A.
Compared with control group, *P < 0.05.
Western blotting analysis revealed that compared with the control group and the DMSO group, the expression of ERα in granulosa cells in the BPA group was significantly increased (Figure 3(A) and (B)). This demonstrates that BPA could stimulate the expression of ERα in granulosa cells in late follicular development.
Figure 3.
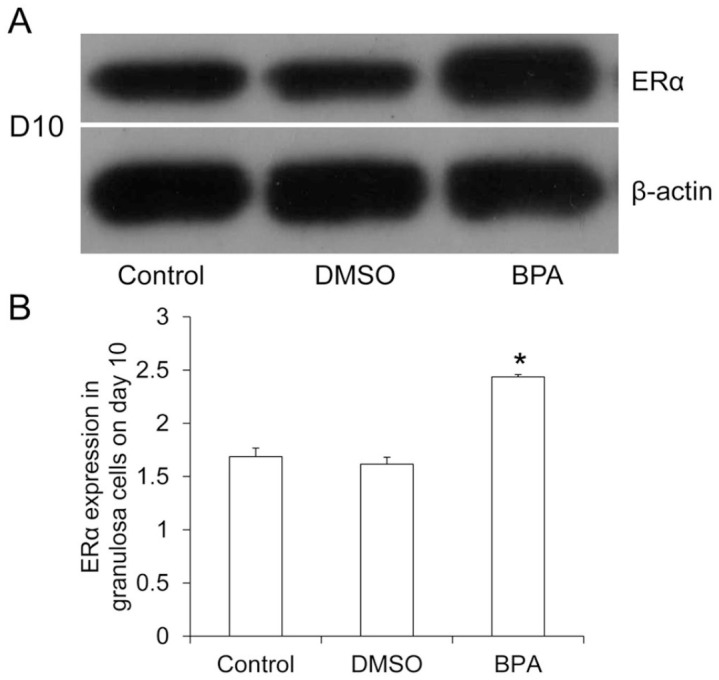
Expression of ERα in D10 follicular granulosa cells. (A) Western blotting analyzed ERα expression in D10 follicular granulosa cells. Representative results were shown. (B) Image J software analyzed the relative expression level of ERα.
Compared with control, *P < 0.05.
BPA reduces the expansion of cumulus cells
The COCs were collected from antral follicles cultured on D10 and D11 for comparing cumulus cell expansion. As shown in Figure 4, the cumulus cells of COCs on D10 were poorly expanded, while the COCs on D11 had expanded cumulus cells under the action of hCG. The percentage of well and poorly expanded cumulus cells by COCs of D11 were calculated (Table 2). It was found that in control and DMSO groups, the percentage of COCs with poorly expanded cumulus cells was significantly lower than that with well-expanded cumulus cells (P < 0.05, Table 2). However, there was no significant difference in BPA group. For comparison among different groups, the percentage of COCs with well-expanded cumulus cells in the BPA group was significantly lower than that of the control group and the DMSO group, but the percentage of COCs with poorly expanded cumulus cells was significantly higher in the BPA group (P < 0.05, Table 2). Therefore, BPA could reduce the expansion of cumulus cells.
Figure 4.
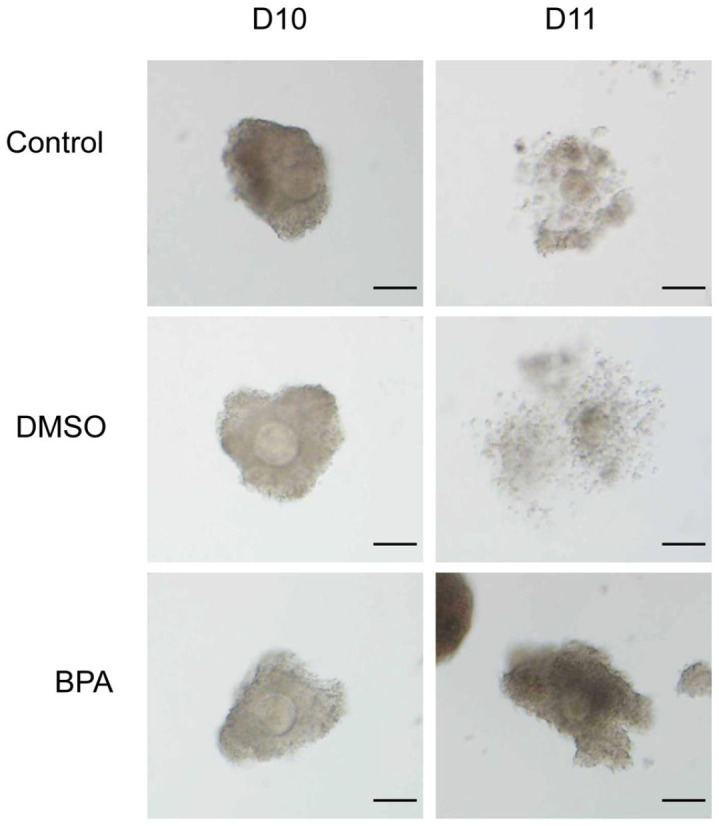
COCs on D10 and D11 of each group cultured in vitro.
Scale bar = 80 µm.
Table 2.
Ratio of COCs with well-expanded and poorly expanded cumulus cells on D11.
| Group | The total number of COCs | The total number of COCs with well-expanded cumulus cells (%) | The total number of COCs with poorly expanded cumulus cells (%) |
|---|---|---|---|
| Control | 42 | 39 (92.76 ± 1.04) | 3 (7.24 ± 1.04)# |
| DMSO | 50 | 46 (92.11 ± 2.83) | 4 (7.89 ± 2.83)# |
| 10 μg/mL BPA | 40 | 21 (52.60 ± 6.12)* | 19 (47.40 ± 6.12)* |
COCs: cumulus cell-oocyte complexes; DMSO: dimethyl sulfoxide; BPA: bisphenol A.
Compared with control group, *P < 0.05. Compared within group (total number of COCs with well-expanded cumulus cells versus total number of COCs with poorly expanded cumulus cells), #P < 0.05.
BPA promotes the recovery of oocytes meiosis but inhibits the development of GVBD oocytes
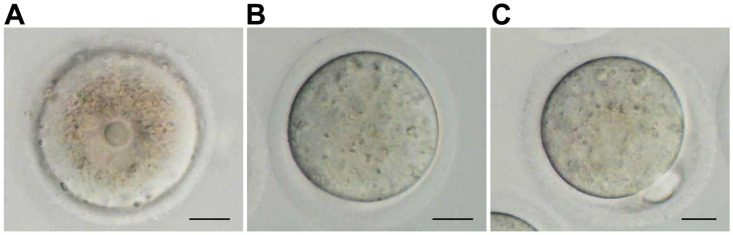
The COCs were collected from antral follicles on D11 of culture. After the cumulus cells were removed, the meiosis recovery and maturation of the oocytes were evaluated. Oocytes with germinal vesicle (GV) were GV-stage oocytes (Figure 5(A)); oocytes with GV disappeared but not discharged from the first polar body were GVBD oocytes (Figure 5(B)); and oocytes with the first polar body were mature oocytes, also called MII oocytes (Figure 5(C)). Both GVBD oocytes and MII-stage oocytes were oocytes with recovered meiosis. GVBD oocytes were oocytes with developmental arrest after meiosis recovery. The development rate of mouse oocytes in each stage was assessed. As shown in Table 2, in the control group, DMSO group, and BPA group, the proportion of oocytes with recovered meiosis was significantly higher than that of MII-stage oocytes, but the proportion of GV-stage oocytes was significantly lower than that of MII-stage oocytes (P < 0.05). The proportion of MII-stage oocytes in the control group and DMSO group was significantly higher than that of GVBD oocytes, while the proportion of MII-stage oocytes in BPA group was significantly lower than that of GVBD oocytes (P < 0.05). This suggests that BPA causes developmental arrest after meiotic recovery of oocytes. Compared with the control group and the DMSO group, 10 µg/mL of BPA significantly reduced the percentages of GV-stage oocytes and mature oocytes (P < 0.05, Table 3). However, it significantly increased the percentage of GVBD oocytes and the recovery rate of meiosis (P < 0.05, Table 3). These data reveal that BPA could promote the recovery of oocytes from meiosis but inhibit the maturation of oocytes.
Figure 5.
Mouse oocytes at different development stages. (A) GV-stage oocytes. There were obvious vesicles in the middle of the oocytes (scale bar = 20 μm). (B) Oocytes after GVBD. The vesicles disappeared, but the oocytes did not discharge the first polar body (scale bar = 20 μm). (C) MII-stage oocytes.
The oocytes had the first polar body and were also called mature oocytes (scale bar = 20 μm).
Table 3.
In vitro development rate of mouse oocytes.
| Group | The total number of live oocytes | The number of GV-stage oocytes (%) | The number of GVBD-stage oocytes (%) | The number of MII-stage oocytes (%) | The number of meiosis recovery oocytes (%) |
|---|---|---|---|---|---|
| Control | 216 | 24 (10.59 ± 1.44) # | 17 (8.31 ± 1.48) # | 175 (81.03 ± 2.93) | 192 (89.41 ± 1.44) # |
| DMSO | 215 | 25 (11.44 ± 1.55) # | 20 (9.48 ± 1.63) # | 170 (79.08 ± 2.66) | 190 (88.56 ± 1.55) # |
| 10 μg/mL BPA | 247 | 18 (7.31 ± 1.24) # * | 122 (49.23 ± 3.81) # * | 107 (43.46 ± 2.86)* | 229 (92.69 ± 1.24) # * |
GV: germinal vesicle; GVBD: germinal vesicle breakdown; DMSO: dimethyl sulfoxide; BPA: bisphenol A.
Compared with control group, *P < 0.05. Compared with the number of MII-stage oocytes within each group, #P < 0.05.
BPA does not change the size of oocytes, nor does it induce apoptosis and death of GVBD oocytes
At 14–16 h after hCG injection, the diameters of GV-stage oocytes, GVBD-stage oocytes, and MII-stage oocytes in each group were measured. There was no significant difference in the diameter of different development stages in each group and among the three groups (control group, DMSO group, and BPA group) (P > 0.05, Figure 6). Thus, BPA did not significantly affect the size of oocytes.
Figure 6.
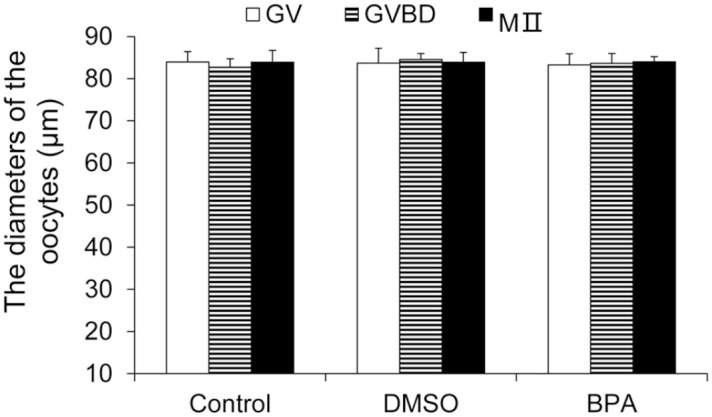
The diameter of mouse oocytes at different development stages of the BPA group (μm).
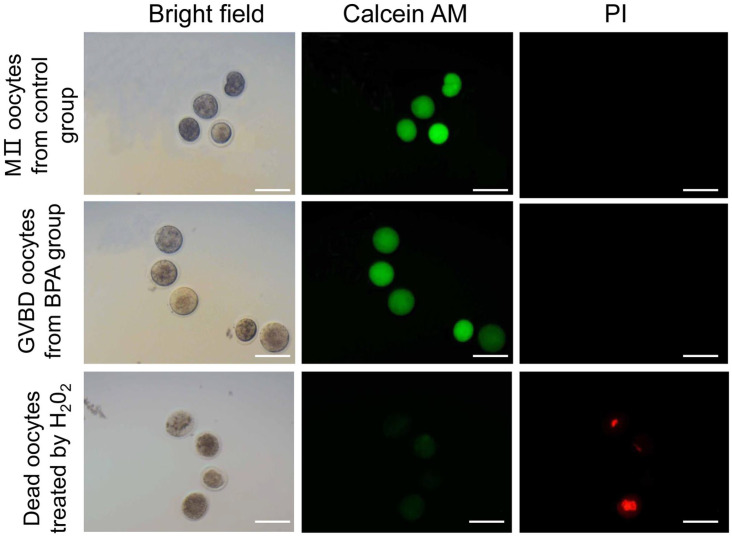
The staining with Calcein AM and PI was used to determine whether the oocytes are alive or dead. As shown in Figure 7, the green fluorescence of the cytoplasm of dead oocytes was very weak, and the nucleus showed red fluorescence. The cytoplasm of GVBD oocytes after treatment by BPA and MII oocytes in control group showed strong green fluorescence, while there was no red fluorescence in the nucleus, suggesting that these oocytes were alive. Thus, BPA did not cause the death of GVBD oocytes.
Figure 7.
The live and dead oocytes in BPA group.
Calcein AM and PI were used to stain oocytes. Oocytes with green fluorescence in the cytoplasm were considered alive, and those with red fluorescence in the nucleus were considered dead. Scale bar = 100 µm
The JC-1 was used to detect the mitochondrial membrane potential of GVBD oocytes in BPA group and MII-stage oocytes in control group. As shown in Figure 8(A), GVBD oocytes in BPA group and MII-stage oocytes in control group showed strong red fluorescence while weak green fluorescence (Figure 8(A)). Statistically, there was no significant difference in the ratio of red fluorescence to green fluorescence between GVBD oocytes in BPA group and MII-stage oocytes in control group (P > 0.05, Figure 8(B)), indicating that there was no abnormality in the mitochondria membrane potential of GVBD oocytes in BPA group. Thus, BPA did not induce the apoptosis of GVBD oocytes.
Figure 8.
Mitochondrial membrane potential of oocytes after GVBD and MII-stage oocytes in the BPA group. (A) The representative images of JC-1 staining. J-aggregates produce red fluorescence while J-monomers exhibit green fluorescence. Scale bar = 80 µm. (B) Ratio of red fluorescence intensity to green fluorescence intensity of JC-1.
Compared with GVBD and MII, *P < 0.05.
BPA reduces the gap junction communication between cumulus cells and oocytes and the expression of Cx37 on the oocytes
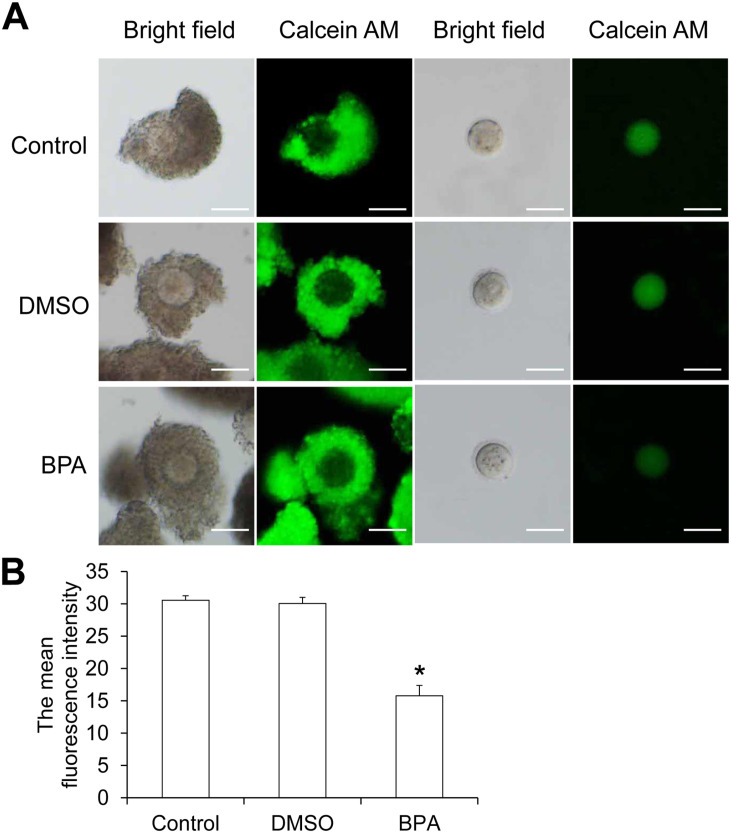
To determine the degree of gap junction communication between cumulus cells and oocytes, the collected COCs were incubated with Calcein AM for 15 min, and the fluorescence intensity of Calcein AM was detected (Figure 9(A)). The green fluorescence intensity in the oocytes of the BPA group was significantly reduced than the control group and the DMSO group (Figure 9(B)). Thus, 10 µg/mL BPA significantly reduced the ability of gap junction communication between cumulus cells and oocytes.
Figure 9.
Gap junction communication between cumulus cells and oocytes assessed by Calcein AM. (A) COCs were incubated with Calcein AM for 10 min or 20 min. After removing the cumulus cells, the green fluorescence of Calcein AM transferred to oocytes through gap junctions was detected. Scale bar = 80 µm. (B) The average fluorescence intensity of Calcein AM in oocytes measured using Image J image analysis (N = 10).
Compared with control, *P < 0.05.
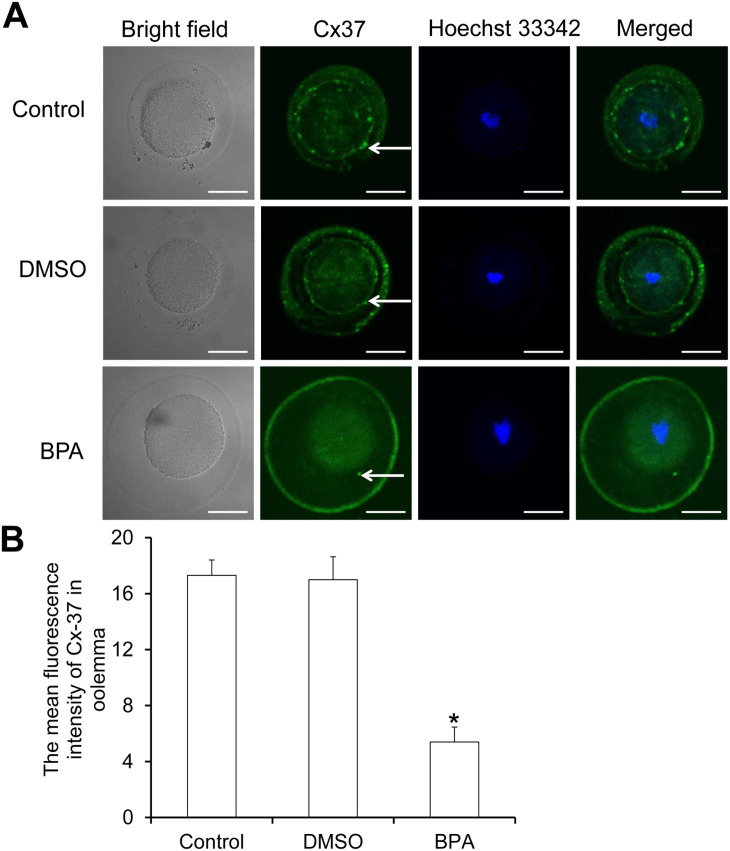
The collected oocytes were subjected to immunofluorescence staining of Cx37 protein (Figure 10(A)). Cx37 protein is the main structural protein of the gap junction between cumulus cells and oocytes. We observed that it was expressed on the oocyte membrane (Figure 10(A)). Compared with the control group and the DMSO group, the average fluorescence intensity of Cx37 on the oocyte membrane of the BPA group was significantly reduced (Figure 10(B)). This may be the main reason for the decrease of gap junction communication between cumulus cells and oocytes.
Figure 10.
The expression of Cx37 on the oocytes of each group. (A) Immunofluorescence labeling of Cx37. Cx37 on the oocyte membrane shows green fluorescence, and the oocyte nucleus shows blue fluorescence. Scale bar = 40 µm. (B) The average fluorescence intensity of Cx37 detected with Image J software (N = 10).
Compared with control, *P < 0.05.
BPA increases the expression of cyclin B1 protein in arrested GVBD oocytes
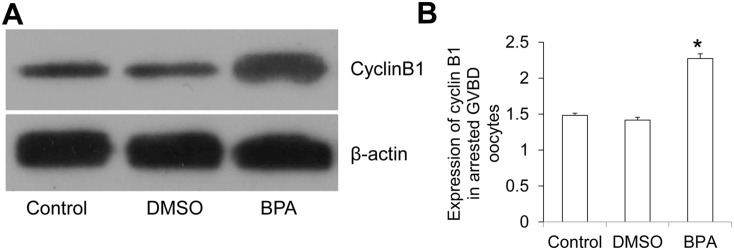
The arrested GVBD oocytes were collected 14–16 h after hCG injection and subjected to Western blotting analysis of cyclin B1. The representative Western blotting results were shown in Figure 11(A). Statistically, compared with the control group and the DMSO group, there was significantly higher expression of cyclin B1 in the arrested GVBD oocytes in the BPA group (Figure 11(B)). The abnormal increase of cyclin B1 may affect the procession of GVBD to maturation of the oocytes.
Figure 11.
Expression of cyclin B1 in GVBD oocytes. (A) The expression of cyclin B1 in GVBD oocytes was measured with Western blotting. (B) Image J software assessed the relative expression level of cyclin B1.
Compared with control, *P < 0.05.
Discussion
The adverse effects of BPA are caused by its chronic accumulation. 28 At present, most studies on the adverse effects of BPA on female reproductive cells were conducted with separated granulosa cells, oocytes, or COCs of the ovary. 29 The study system cannot better simulate the process of human exposure to BPA. Therefore, in this study, preantral follicles were isolated and cultured in vitro for 11 days in the presence of BPA. We found that BPA significantly promoted the development of granulosa cells in the late stage of follicular development, increased the secretion of estrogen and progesterone and the expression of ERα by granulosa cells, and, promoted oocyte meiosis recovery. However, BPA reduced the expansion rate of COCs cumulus cells and gap junction communication, induced the developmental arrest of the oocytes after GVBD, and reduced the maturation rate of the oocytes. GVBD oocytes treated by BPA were live oocytes with normal diameters and normal mitochondrial membrane potential. In addition, the developmental arrest of oocytes after GVBD caused by BPA may be related to the reduction of the gap junction communication and the abnormal expression of cyclin B1 in oocytes after GVBD.
Granulosa cells, as important constituents of follicles, can secrete estrogen and progesterone to participate in the maintenance of follicular development, oocyte maturation, female reproductive cycle, fertilization, and so on. 30 As an environmental estrogen-like chemical, BPA can disrupt the secretion of estrogen and progesterone.17,18 Consistently, our results showed that BPA (10 µg/mL) significantly increased the secretion of estrogen and progesterone by granulosa cells. Our previous research also found that BPA increased the expression of CYP19A1 and HSD17B by granulosa cells, 23 which are important enzymes that regulate estrogen synthesis. Castro et al. 31 also found that BPA increased the secretion of estrogen by increasing the expression of aromatase in the rat ovary. Pogrmic-Majkic et al. 15 reported that BPA promoted the synthesis of progesterone by human granulosa cells through the peroxisome proliferator-activated receptor γ (PPARγ)-dependent epidermal growth factor receptor (EGFR) and ERK1/2 pathway, but it reduced the expression of CYP19A1 mRNA and the synthesis of estrogen. Qi et al. 32 believed that BPA might reduce the synthesis of progesterone by human granulosa cells by up-regulating the expression of ABAC1. BPA may affect the synthesis and secretion of estrogen and progesterone by granulosa cells through different signaling pathways, and this effect may be related to its concentration, species, and experimental model.
Estrogen can inhibit the apoptosis and promote the growth and differentiation of granulosa cells. 17 As an environmental estrogen-like chemical, 10 µM BPA significantly promoted the proliferation of granulosa cells and attenuated their apoptosis by improving estrogen synthesis and estrogen-mediated signaling pathways. 17 Similarly, our study found that BPA significantly increased the expression of PCNA in granulosa cells and promoted the development of granulosa cells in the late stage of follicular development. Studies have found that BPA interferes with cell development and function mainly through regulating the expression of ER and its downstream signaling pathways.33–37 Bruno et al. 34 found that BPA exposure activated ERα and ERβ in the spleen at 24 h after infection and phosphorylated ERα and ERβ during myocarditis. BPA and its alternatives act as ERα agonists to activate downstream ER signaling 35 and induce tumor-like outgrowths in female transgenic mice. 36 However, the suppressive effects of BPA on the expression levels of sex steroid receptors (ERα and ERβ) in the rat uterus were observed. 37 In this study, we found that BPA promoted the expression of ERα in granulosa cells on day 11 of the culture. BPA may have different effects on ER expression and function due to the differences in cell sensitivity to BPA. Studies18,38,39 have found that BPA can bind to G protein-coupled estrogen receptor (GPER) in addition to ERα and ERβ. In the human granulosa cell line KGN, BPA could induce granulosa cell apoptosis by binding to GPER and activating the ROS/Ca2+-ASK1-JNK pathway. 18 In the mouse spermatocyte cell line GC-2, BPA could bind to a membrane ER GPR30, up-regulating c-Fos gene expression and down-regulating Cyclin D1 gene expression, thereby inhibiting growth and inducing apoptosis of spermatocyte. 39 BPA not only affects the expression of ERs, but also has different effects on the growth of different types of cells through different pathways by binding to different ERs.18,38,39 Furthermore, studies are needed to investigate the regulation of BPA on different types of ERs and their downstream signaling pathways in granulosa cells.
In the follicles, the cumulus cells can nourish the oocytes via the gap junctions and can regulate the arrest or recovery of the oocyte meiosis. BPA can reduce the expansion of cumulus cells and affect the maturation quality of oocytes; 21 meanwhile, it can reduce the gap communication between cumulus cells and oocytes, resulting in decreased cyclic adenosine monophosphate (cAMP) and/or cyclic guanosine monophosphate (cGMP) in oocytes, leading to premature meiosis and GVBD. 21 In this study, we found that BPA reduced the expansion of mouse COCs cumulus cells and the expression of gap junction protein Cx37, and reduced the transmission of Calcein AM between cumulus cells and oocytes through gap junctions. In addition, we found that BPA significantly increased the percentage of GVBD oocytes and the recovery rate of oocytes from meiosis, but reduced the rate of in vitro maturation of oocytes. Lin et al. 20 also found that BPA reduced the expression of Cx43 on granulosa cells through ER and MAPK pathways. Campen et al. 22 found that BPA accelerated the recovery of oocyte meiosis by changing the expression of gap junction proteins and the communication ability of gap junctions. Jiao et al. 40 showed that BHPF (9,9-bis(3,4-dihydroxyphenyl)fluorene), a substitute for BPA, had similar adverse effects as BPA, which inhibited the expansion of cumulus cells, reduced the excretion rate of the first polar body, and suppressed oocyte maturation. The down regulation of gap junction intercellular communication by single nucleotide polymorphisms (SNPs) in cardiomyocytes is mediated through down regulation of Cx43 and upregulation of P-Cx43. SNPs-induced apoptosis through the disrupted functional gap junction was correlated with abnormal expressions of the proteins involved in the mitochondrial pathway-related apoptosis such as Bcl-2/Bax, cytochrome C, Caspase-9, and Caspase-3. 41 In the present study, BPA decreased Cx37 expression and reduced Gap junction intercellular communication between oocytes and cumulus cells. However, it was found that GVBD oocytes in BPA group had the same diameter and mitochondrial membrane potential as MII oocytes in control group. GVBD oocytes after 14–16 h of hCG were arrested without apoptosis and death. BPA caused oocyte developmental arrest at the GVBD stage and significantly reduced the oocyte maturation rate. However, the mitochondrial membrane potential of the developmentally arrested oocytes was normal. Thus, the decrease of gap junction intercellular communication by BPA is likely to disturb the normal progression of the oocyte cell cycle.
The occurrence of GVBD is regulated by maturation promoting factor (MPF), composed of cyclin B1 and cyclin-dependent kinase 1, which regulates the procession of oocytes to resume meiosis.42,43 When the MPF reaches its peak, the oocyte develops GVBD or progresses to the MI stage. 44 Soon after the occurrence of GVBD, cyclin B degradation will occur, MPF will be inactivated, and the oocyte will progress from M phase to late phase. 45 The abnormal degradation and accumulation of cyclin B1 can cause defective anaphase-promoting complex/cyclosome activation and the arrest of oocytes in anaphase. 43 During the maturation of oocytes, cumulus cells will transmit cyclic nucleotides to oocytes through gap junction, thus affecting the expression of cyclin B1 and MPF activity in oocytes and altering the procession of oocyte meiosis. 46 Based on these findings and our results that BPA decreased Cx37 expression, reduced Gap junction intercellular communication between oocytes and cumulus cells and cause the developmental arrest of oocytes after GVBD, we speculate that BPA might also affect the expression of cyclin B1. To verify this, we detected the expression of cyclin B1 in the oocytes that were developmentally arrested after GVBD and were unable to discharge the first polar body. The results showed that the expression of cyclin B1 in the arrested GVBD oocytes of the BPA group was significantly higher than that of the control group. The developmental arrest of oocytes after GVBD caused by BPA may be related to the abnormal expression of cyclin B1. Oocyte development arrest at the GVBD stage will lead to follicular atresia and the follicles cannot mature, rupture, or ovulate, resulting in female infertility.47,48
In summary, the in vitro culture system of preantral follicles in this study could maintain the connection between granulosa cells and oocytes, and more closely simulate the in vivo development of oocytes and granulosa cells. Our findings demonstrate that BPA promoted the development of granulosa cells in the late stage of follicular development; increased the expressions of PCNA and ERα on granulosa cells and the secretion of estrogen and progesterone but reduced the expansion of cumulus cells around COCs, Cx37 expression, and the gap junction communication between cumulus cells and oocytes; promoted the recovery of oocyte meiosis; and led to the developmental arrest of oocytes after GVBD, which may be related to the abnormal expression of cyclin B1. This study more systematically clarified the adverse effects of BPA as an environmental estrogen-like chemical on the development of granulosa cells and oocytes. These adverse effects caused by BPA will lead to follicular atresia, affect the production of mature oocytes, and cause female infertility. These findings suggest that we should speed up the development of BPA substitutes that have no adverse effects on reproductive capacity, thus avoiding the female reproduction damage caused by BPA.
Footnotes
Authors’ Contributions: XP designed the study. YL, SL, FG, ZP, JZ, SL, and DL collected and analyzed the data. YL wrote the paper. XP collected the funds and revised the paper. All authors have read and approved the submitted manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Scientific and Technological Research Project of Jilin Province (grant number 20200201132JC), the Hygiene and Health Research Project of Jilin Province for the training of high-tech youth talents (grant number 2018Q047), the Scientific and Technological Innovation Project of Jilin City (grant numbers 201831721 and 20190403224), the “Thirteenth Five-Year” Scientific and Technological Research Projects of Education Department of Jilin Province (grant numbers JJKH20191065KJ and JJKH20200454KJ), Traditional Chinese Medicine Science and Technology Development Program of Shandong Province (grant number 2019-0463), and Undergraduate Training Programs for Innovation and Entrepreneurship of Jilin Province (grant numbers 201913706031 and 202013706019).
ORCID iD: Xiaoyan Pan  https://orcid.org/0000-0001-7357-090X
https://orcid.org/0000-0001-7357-090X
References
- 1. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 2008;116:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takeuchi T, Tsutsumi O. Serum bisphenol A concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun 2002;291:76–8 [DOI] [PubMed] [Google Scholar]
- 3. Ye X, Kuklenyik Z, Needham LL, Calafat AM. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2006;831:110–5 [DOI] [PubMed] [Google Scholar]
- 4. Suteau V, Briet C, Lebeault M, Gourdin L, Henrion D, Rodien P, Munier M. Human amniotic fluid-based exposure levels of phthalates and bisphenol A mixture reduce INSL3/RXFP2 signaling. Environ Int 2020;138:105585. [DOI] [PubMed] [Google Scholar]
- 5. Santoro A, Chianese R, Troisi J, Richards S, Nori SL, Fasano S, Guida M, Plunk E, Viggiano A, Pierantoni R, Meccariello R. Neuro-toxic and reproductive effects of BPA. Curr Neuropharmacol 2019;17:1109–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma Y, Liu H, Wu J, Yuan L, Wang Y, Du X, Wang R, Marwa PW, Petlulu P, Chen X, Zhang H. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ Res 2019;176:108575. [DOI] [PubMed] [Google Scholar]
- 7. Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, Hauser R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol 2013;42:224–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J. Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: a literature review. Nutrients 2020;12:532–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu X, Wang Z, Liu F. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 2021;262:127880. [DOI] [PubMed] [Google Scholar]
- 10. Mao J, Jain A, Denslow ND, Nouri MZ, Chen S, Wang T, Zhu N, Koh J, Sarma SJ, Sumner BW, Lei Z, Sumner LW, Bivens NJ, Roberts RM, Tuteja G, Rosenfeld CS. Bisphenol A and bisphenol S disruptions of the mouse placenta and potential effects on the placenta-brain axis. Proc Natl Acad Sci U S A 2020;117:4642–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991;124:43–101 [DOI] [PubMed] [Google Scholar]
- 12. Rivera OE, Varayoud J, Rodriguez HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol 2011;32:304–12 [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez HA, Santambrosio N, Santamaría CG, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol 2010;30:550–7 [DOI] [PubMed] [Google Scholar]
- 14. Patel S, Brehm E, Gao L, Rattan S, Ziv-Gal A, Flaws JA. Bisphenol A exposure, ovarian follicle numbers, and female sex steroid hormone levels: results from a CLARITY-BPA study. Endocrinology 2017;158:1727–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pogrmic-Majkic K, Samardzija Nenadov D, Fa S, Stanic B, Trninic Pjevic A, Andric N. BPA activates EGFR and ERK1/2 through PPARγ to increase expression of steroidogenic acute regulatory protein in human cumulus granulosa cells. Chemosphere 2019;229:60–7 [DOI] [PubMed] [Google Scholar]
- 16. Lee CT, Wang JY, Chou KY, Hsu MI. 1,25-dihydroxyvitamin D3 modulates the effects of sublethal BPA on mitochondrial function via activating PI3K-Akt pathway and 17beta-estradiol secretion in rat granulosa cells. J Steroid Biochem Mol Biol 2019;185:200–11 [DOI] [PubMed] [Google Scholar]
- 17. Song D, Wu G, Wei Q, Shi F. Bisphenol A attenuates thyroxine-induced apoptosis in ovarian granulosa cells of pigs. Reprod Domest Anim 2019;54:864–72 [DOI] [PubMed] [Google Scholar]
- 18. Huang M, Huang M, Li X, Liu S, Fu L, Jiang X, Yang M. Bisphenol A induces apoptosis through GPER-dependent activation of the ROS/Ca(2+)-ASK1-JNK pathway in human granulosa cell line KGN. Ecotoxicol Environ Saf 2021;208:111429. [DOI] [PubMed] [Google Scholar]
- 19. Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, Tsutsumi O. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun 2002;292:456–62 [DOI] [PubMed] [Google Scholar]
- 20. Lin TC, Wang KH, Chuang KH, Kao AP, Kuo TC. Downregulation of gap junctional intercellular communication and connexin 43 expression by bisphenol A in human granulosa cells. Biotechnol Appl Biochem 2021;68:676–82 [DOI] [PubMed] [Google Scholar]
- 21. Acuna-Hernandez DG, Arreola-Mendoza L, Santacruz-Marquez R, Garcia-Zepeda SP, Parra-Forero LY, Olivares-Reyes JA, Hernandez-Ochoa I. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol Appl Pharmacol 2018;344:13–22 [DOI] [PubMed] [Google Scholar]
- 22. Campen KA, McNatty KP, Pitman JL. A protective role of cumulus cells after short-term exposure of rat cumulus cell-oocyte complexes to lifestyle or environmental contaminants. Reprod Toxicol 2017;69:19–33 [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Jiang SW, Wang L, Sun Y, Xu F, He H, Wang S, Zhang Z, Pan X. Interfering effects of bisphenol A on in vitro growth of preantral follicles and maturation of oocyes. Clin Chim Acta 2018;485:119–25 [DOI] [PubMed] [Google Scholar]
- 24. Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 1968;17:555–7 [DOI] [PubMed] [Google Scholar]
- 25. Al-Zubaidi U, Liu J, Cinar O, Robker RL, Adhikari D, Carroll J. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol Hum Reprod 2019;25:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ratchford AM, Esguerra CR, Moley KH. Decreased oocyte-granulosa cell gap junction communication and connexin expression in a type 1 diabetic mouse model. Mol Endocrinol 2008;22:2643–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sasseville’ M, Gagnon MC, Guillemette C, Sullivan R, Gilchrist RB, Richard FJ. Regulation of gap junctions in porcine cumulus-oocyte complexes: contributions of granulosa cell contact, gonadotropins, and lipid rafts. Mol Endocrinol 2009;23:700–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wazir U, Mokbel K. Bisphenol A: a concise review of literature and a discussion of health and regulatory implications. In Vivo 2019;33:1421–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siracusa JS, Yin L, Measel E, Liang S, Yu X. Effects of bisphenol A and its analogs on reproductive health: a mini review. Reprod Toxicol 2018;79:96–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kordus RJ, LaVoie HA. Granulosa cell biomarkers to predict pregnancy in ART: pieces to solve the puzzle. Reproduction 2017;153:R69–83 [DOI] [PubMed] [Google Scholar]
- 31. Castro B, Sanchez P, Torres JM, Ortega E. Effects of perinatal exposure to bisphenol A on the intraprostatic levels of aromatase and 5α-reductase isozymes in juvenile rats. Food Chem Toxicol 2018;115:20–5 [DOI] [PubMed] [Google Scholar]
- 32. Qi J, Liu L, Yang J, Gao X, Zhang W. Bisphenol A decreases progesterone synthesis in human ovarian granulosa cells. Birth Defects Res 2020;112:1843–9 [DOI] [PubMed] [Google Scholar]
- 33. Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, Bertrand-Michel J, Pineau T, Guillou H, Martin PG, Mselli-Lakhal L. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 2012;55:395–407 [DOI] [PubMed] [Google Scholar]
- 34. Bruno KA, Mathews JE, Yang AL, Frisancho JA, Scott AJ, Greyner HD, Molina FA, Greenaway MS, Cooper GM, Bucek A, Morales-Lara AC, Hill AR, Mease AA, Di Florio DN, Sousou JM, Coronado AC, Stafford AR, Fairweather D. BPA alters estrogen receptor expression in the heart after viral infection activating cardiac mast cells and T cells leading to perimyocarditis and fibrosis. Front Endocrinol 2019;10:598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mesnage R, Phedonos A, Arno M, Balu S, Corton JC, Antoniou MN. Editor’s highlight: transcriptome profiling reveals bisphenol A alternatives activate estrogen receptor alpha in human breast cancer cells. Toxicol Sci 2017;158:431–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sekar TV, Foygel K, Massoud TF, Gambhir SS, Paulmurugan R. A transgenic mouse model expressing an ERα folding biosensor reveals the effects of bisphenol A on estrogen receptor signaling. Sci Rep 2016;6:34788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zaid SSM, Othman S, Kassim NM. Protective role of Mas Cotek (Ficus deltoidea) against the toxic effects of bisphenol A on morphology and sex steroid receptor expression in the rat uterus. Biomed Pharmacother 2021;140:111757. [DOI] [PubMed] [Google Scholar]
- 38. Watson CS, Jeng YJ, Guptarak J. Endocrine disruption via estrogen receptors that participate in nongenomic signaling pathways. J Steroid Biochem Mol Biol 2011;127:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang C, Zhang J, Li Q, Zhang T, Deng Z, Lian J, Jia D, Li R, Zheng T, Ding X, Yang F, Ma C, Wang R, Zhang W, Guo Wen J. Low concentration of BPA induces mice spermatocytes apoptosis via GPR30. Oncotarget 2017;8:49005–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiao X, Ding Z, Meng F, Zhang X, Wang Y, Chen F, Duan Z, Wu D, Zhang S, Miao Y, Huo L. The toxic effects of Fluorene-9-bisphenol on porcine oocyte in vitro maturation. Environ Toxicol 2020;35:152–8 [DOI] [PubMed] [Google Scholar]
- 41. Du ZJ, Cui GQ, Zhang J, Liu XM, Zhang ZH, Jia Q, Ng JC, Peng C, Bo CX, Shao H. Inhibition of gap junction intercellular communication is involved in silica nanoparticles-induced H9c2 cardiomyocytes apoptosis via the mitochondrial pathway. Int J Nanomedicine 2017;12:2179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yi ZY, Meng TG, Ma XS, Li J, Zhang CH, Ouyang YC, Schatten H, Qiao J, Sun QY, Qian WP. CDC6 regulates both G2/M transition and metaphase-to-anaphase transition during the first meiosis of mouse oocytes. J Cell Physiol 2020;235:5541–54 [DOI] [PubMed] [Google Scholar]
- 43. Karasu ME, Bouftas N, Keeney S, Wassmann K. Cyclin B3 promotes anaphase I onset in oocyte meiosis. J Cell Biol 2019;218:1265–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bai GY, Choe MH, Kim JS, Oh JS. Mis12 controls cyclin B1 stabilization via Cdc14B-mediated APC/C(Cdh1) regulation during meiotic G2/M transition in mouse oocytes. Development 2020;147:322–41 [DOI] [PubMed] [Google Scholar]
- 45. Sun J, Guo Y, Zhang Q, Bu S, Li B, Wang Q, Lai D. Chronic restraint stress disturbs meiotic resumption through APC/C-mediated cyclin B1 excessive degradation in mouse oocytes. Cell Cycle 2018;17:1591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tiwari M, Chaube SK. Carbenoxolone reduces cyclic nucleotides level, destabilizes maturation promoting factor and induces meiotic exit from diplotene arrest in rat cumulus oocytes complexes cultured in vitro. Biomed Pharmacother 2017;94:219–30 [DOI] [PubMed] [Google Scholar]
- 47. Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu Rev Physiol 2017;79:237–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jessus C, Munro C, Houliston E. Managing the oocyte meiotic arrest-lessons from frogs and jellyfish. Cells 2020;9:1150–86 [DOI] [PMC free article] [PubMed] [Google Scholar]