Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the latest term for steatotic liver disease associated with metabolic syndrome. MASLD is the most common cause of chronic liver disease and is the leading cause of liver-related morbidity and mortality. It is important that all stakeholders be involved in tackling the public health threat of obesity and obesity-related diseases, including MASLD. A simple and clear assessment and referral pathway using non-invasive tests is essential to ensure that patients with severe MASLD are identified and referred to specialist care, while patients with less severe disease remain in primary care, where they are best managed. While lifestyle intervention is the cornerstone of the management of patients with MASLD, cardiovascular disease risk must be properly assessed and managed because cardiovascular disease is the leading cause of mortality. No pharmacological agent has been approved for the treatment of MASLD, but novel anti-hyperglycemic drugs appear to have benefit. Medications used for the treatment of diabetes and other metabolic conditions may need to be adjusted as liver disease progresses to cirrhosis, especially decompensated cirrhosis. Based on non-invasive tests, the concepts of compensated advanced chronic liver disease and clinically significant portal hypertension provide a practical approach to stratifying patients according to the risk of liver-related complications and can help manage such patients. Finally, prevention and management of sarcopenia should be considered in the management of patients with MASLD.
Keywords: Non-alcoholic fatty liver disease, Liver cirrhosis, Portal hypertension
INTRODUCTION
There have been significant developments in the field of non-alcoholic fatty liver disease (NAFLD) in recent years including (1) a change in its nomenclature, (2) a recommendation for screening to identify more severe liver disease among patients with type 2 diabetes mellitus (T2DM), (3) the concept of compensated advanced chronic liver disease (cACLD) and clinically significant portal hypertension (CSPH) diagnosed using non-invasive tests, and (4) recognition of the importance of sarcopenia. This review aims to provide an update on these areas and to highlight some important considerations in terms of lifestyle intervention, cardiovascular disease (CVD) risk reduction, and use of anti-hyperglycemic drugs and other medications for management of metabolic comorbidities, especially in patients with cirrhosis.
NEW NOMENCLATURE
In 1980, Ludwig et al.1 reported a liver condition mimicking alcoholic hepatitis that can progress to cirrhosis in persons who did not consume a significant amount of alcohol. The patients were moderately obese, and many had obesity-related diseases, such as diabetes mellitus. The term non-alcoholic steatohepatitis was coined for this liver condition.1 In 2007, Farrell et al.2 in the Asian-Pacific Working Party for NAFLD proposed the operational definition of NAFLD, which could be diagnosed based on ultrasonography findings, and following exclusion of significant alcohol intake, drugs that can cause hepatic steatosis, and other causes of chronic liver disease. This was followed by a position statement by the European Association for the Study of Liver (EASL) in 2010, which acknowledged that, despite its historic diagnosis by exclusion of other causes of chronic liver disease, the strong association of NAFLD with metabolic syndrome and its co-occurrence with other chronic liver diseases strongly argued for a change in nomenclature.3 The term NAFLD continued to be used in major international guidelines,4-6 but there were signs of an imminent change. For example, EASL used the term primary NAFLD, which was defined as NAFLD-associated with metabolic risk factors, in its 2016 guidelines,5 while the Asian-Pacific Working Party proposed a “positive” definition for NAFLD in its 2017 guidelines.6
In 2020, Eslam et al.7,8 proposed the new term ‘metabolic dysfunction-associated fatty liver disease (MAFLD),’ which could be diagnosed in adults with hepatic steatosis detected by imaging techniques, blood biomarkers, or liver histology, when overweight or obese, or in the presence of T2DM or at least two metabolic risk abnormalities. This was endorsed by the Asian Pacific Association for the Study of the Liver (APASL),9 multiple national societies including the Malaysian Society of Gastroenterology and Hepatology,10,11 and various stakeholders globally.12 In June 2023, a multi-society Delphi consensus statement on a new fatty liver disease nomenclature was published, introducing the term metabolic dysfunction-associated steatotic liver disease (MASLD) and effectively retiring the term NAFLD.13 The terms MAFLD and MASLD have more in common than not and are more appropriate for the condition (Table 1).
Table 1.
The terms MAFLD and MASLD have more in common than not and have achieved the objective of a more appropriate name for the condition8,13
| MAFLD | MASLD | |
|---|---|---|
| Positive diagnostic criteria (i.e., defines what the disease is rather than what it is not) | Yes | Yes |
| Attributes the condition to its etiology | Yes | Yes |
| Criteria | Hepatic steatosis detected either by imaging techniques, blood biomarkers/scores, or liver history, plus (1) Overweight or obese (2) Type 2 diabetes mellitus or (3) Presence of ≥ 2 metabolic risk abnormalities Metabolic risk abnormalities include: (1) Waist circumference ≥ 102/88 cm in Caucasian men and women (or ≥ 90/80 cm in Asian men and women) (2) Blood pressure ≥ 130/85 mmHg or specific drug treatment (3) Plasma triglycerides ≥ 150 mg/dL ( ≥ 1.70 mmol/L) or specific drug treatment (4) Plasma HDL cholesterol < 40 mg/dL (< 1.0 mmol/L) for men and < 50 mg/dL (< 1.3 mmol/L) for women or specific drug treatment (5) Prediabetes (i.e., fasting glucose levels 100–125 mg/dL [5.6–6.9 mmol/L], or 2-hour post-load glucose levels 140–199 mg/dL [7.8–11.0 mmol], or HbA1c 5.7%–6.4%) (6) HOMA-IR ≥ 2.5 (7) Plasma hs-CRP level > 2 mg/L |
Hepatic steatosis detected by imaging or biopsy, plus at least 1 of 5: (1) BMI ≥ 25 kg/m2 ( ≥ 23 kg/m2 in Asian) or waist circumference > 94 cm in men, > 80 cm in women, or ethnicity adjusted (2) Fasting serum glucose ≥ 100 mg/dL ( ≥ 5.6 mmol/L) or 2-hour post-load glucose level ≥ 140 mg/dL ( ≥ 7.8 mmol/L) or HbA1c ≥ 5.7% or on specific drug treatment (3) Blood pressure ≥ 130/85 mmHg or specific drug treatment (4) Plasma triglycerides ≥ 150 mg/dL ( ≥ 1.70 mmol/L) or specific drug treatment (5) Plasma HDL cholesterol < 40 mg/dL (< 1.0 mmol/L) for men and < 50 mg/dL (< 1.3 mmol/L) for women or specific drug treatment |
| Presence of other concomitant liver diseases | Other concomitant liver diseases retain their own term | Falls under a separate group (i.e., MetALD* or other combination etiology) |
*MetALD, i.e., weekly intake 140–350 g for female, 210–420 g for male (average daily 20–50 g for female, 30–60 g for male). MAFLD, metabolic dysfunction-associated fatty liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; HDL, high-density lipoprotein; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostatic model for assessment of insulin resistance; hs-CRP, high sensitivity C-reactive protein; BMI, body mass index; MetALD, MASLD and increased alcohol intake.
EPIDEMIOLOGY, NATURAL HISTORY, AND BURDEN OF DISEASE
MASLD has been estimated to affect 30% of the adult population worldwide, with its prevalence increasing from 22% to 37% from 1991 to 2019.14,15 The increasing prevalence of MASLD parallels the increasing prevalence of obesity and obesity-related diseases. Metabolic dysfunction-associated steatohepatitis (MASH) is the more severe form of MASLD, is defined histologically by the presence of lobular inflammation and hepatocyte ballooning, and is associated with a greater risk of fibrosis progression.16 MASH has been found in 63% of patients with MASLD undergoing liver biopsy in an Asian multi-center cohort.17 Among patients with MASLD without an indication for liver biopsy, the prevalence of MASH is 7%.18 While CVD is the leading cause of mortality in patients with MASLD, those with more severe liver fibrosis are at increased risk of liver-related mortality, with the risk increasing exponentially with fibrosis stage.19 A prospective study on a cohort of patients with MASLD and baseline liver biopsy found the rate of cardiovascular event to be 2.03 per 100 person-years while the rate of liver-related event was 0.43 per 100 person-years. Liver-related events occurred only in patients with advanced liver fibrosis (cumulative incidence 9.1% in patients with advanced liver fibrosis vs. 0% in patients without advanced liver fibrosis), with rates of a liver-related event of 1.47 and 3.85 per 100 person-years among patients with F3 and F4 fibrosis, respectively (F3 is bridging fibrosis on liver biopsy, while F4 is cirrhosis; fibrosis stage ≥F3 is considered advanced liver fibrosis).20
Steatotic liver disease (SLD) contributes significantly to the burden of chronic liver disease. While 62% of patients seen in our liver clinic had MASLD, 47% of patients with other chronic liver diseases had significant hepatic steatosis. Overall, 85% of all patients seen in our liver clinic had significant hepatic steatosis.21 A majority of patients admitted to our ward for cirrhosis and its complications had cryptogenic cirrhosis, which is due to MASLD in most cases.22 Similarly, cryptogenic cirrhosis as the etiology of hepatocellular carcinoma (HCC) cases seen in our center has more than doubled from 16% to 34% (not including 7% with established diagnosis of MASH) between the time periods of 2006–2009 and 2011–2014.23 In the United States, MASH has become one of the leading causes of adult liver transplantation and among patients with HCC undergoing liver transplantation.24,25 The burden of MASLD and its complications, including HCC, is projected to continue to increase in the coming years.26,27
PATIENTS WITH T2DM: AN IMPORTANT TARGET GROUP FOR DETECTION OF ADVANCED LIVER FIBROSIS
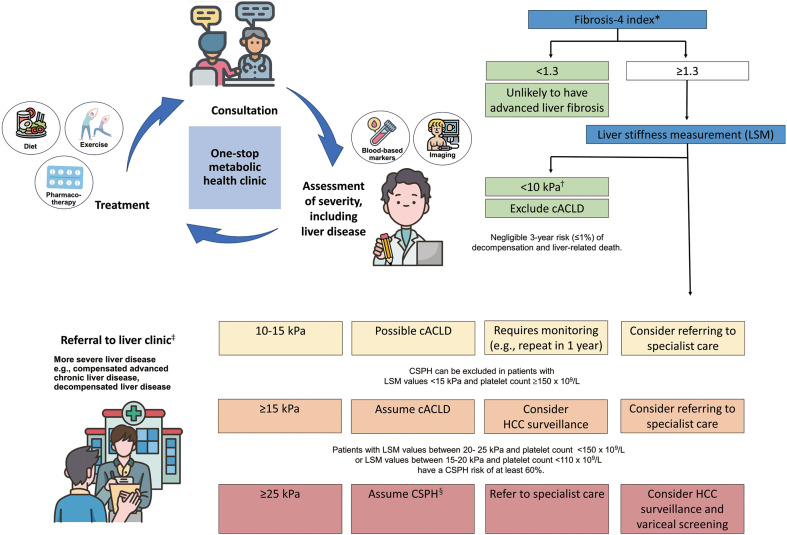
MASLD is prevalent in the general population, with a small yet significant proportion of people having advanced liver fibrosis. For example, in a population-based study in Hong Kong, 27% of participants were found to have MASLD, while 4% had advanced liver fibrosis.28 As the disease is generally asymptomatic until decompensation takes place, many people present for the first time with complications of cirrhosis. A simple and clear assessment and referral pathway is needed to ensure that patients with severe liver disease are referred to specialist care, while those with less severe disease remain in primary care, where they are best managed. In this context, patients with T2DM represent an important target group for detection of advanced liver fibrosis.11,29 The majority of patients with T2DM has SLD, and a substantial proportion of them has advanced liver fibrosis.30 T2DM has been recognized as an independent risk factor for MASH and for advanced liver fibrosis.17 Recent guidelines recommend using simple fibrosis score (e.g., fibrosis-4 index [FIB4]) as an initial test to screen for advanced liver fibrosis.11,31,32 The FIB4 has very good negative predictive value for advanced liver fibrosis, but its positive predictive value is suboptimal.33 Therefore, patients with elevated FIB4 should undergo a second test (e.g., liver stiffness measurement [LSM]) for further risk stratification (see section on ‘Compensated advanced chronic liver disease and clinically significant portal hypertension’ below). The cut-off to prompt referral to specialist care may depend on available resources and local practice in terms of the defined roles of primary and specialist care providers (Fig. 1).11,34
Figure 1.
A simple and clear assessment and referral pathway is essential in streamlining the management of patients with metabolic dysfunction-associated steatotic liver disease, and this depends on available resources and local practice in terms of the defined role between primary and specialist care providers.11,92 *For patients ≥ 65 years old, fibrosis-4 index cut-off 2.0 (instead of 1.3) may be used to improve specificity34; †Sensitivity and negative predictive value > 90%; ‡Patients should be considered for referral to specialist care if they have persistently elevated serum aminotransferase level and/or if the cause of elevated serum aminotransferase level is uncertain; §Specificity and positive predictive value > 90%. cACLD, compensated advanced chronic liver disease; CSPH, clinically significant portal hypertension; HCC, hepatocellular carcinoma.
LIFESTYLE INTERVENTION IS THE CORNERSTONE IN THE MANAGEMENT OF MASLD
Calorie restriction and increased physical activity with resulting weight loss can lead to significant improvements in MASLD. A study on patients with biopsy-proven MASH demonstrated that this could result in resolution of MASH and improvement in liver fibrosis in up to 90% and 45% of patients, respectively.35 Another study showed that lifestyle intervention could result in resolution of SLD in up to 97% of patients.36 The APASL guidelines recommend gradual weight loss (up to 1 kg/wk) through a hypocaloric diet (with 500 to 1,000 kcal deficit) and physical activity (30 min/day of moderate intensity exercise for at least 5 day/week or a total of at least 150 min/wk, or at least 20 min/day of vigorous intensity exercise for at least 3 day/wk or a total of at least 75 min/wk) in the management of MASLD. Lifestyle intervention should be emphasized at all levels of patient care with the goal of improvements in both MASLD and the cardiometabolic and overall health of the individual patient. Weight loss can lead to improvements in blood pressure and glycemic and lipid profiles and reduction in CVD risk, among other benefits.37 There is currently no U.S. Food and Drug Administration (FDA)-approved pharmacological therapy for MASH. However, some commercially available medications may be helpful, including pioglitazone,38,39 glucagon-like peptide-1 receptor agonist (GLP1 RA), and sodium glucose co-transporter-2 (SGLT2) inhibitor (see section on ‘MASLD and anti-hyperglycemic drugs’).
CARDIOVASCULAR DISEASE RISK REDUCTION
As mentioned above, CVD is the leading cause of death in patients with MASLD.40 A recent 2022 American Heart Association (AHA) Scientific Statement recognizes that MASLD is an often-unappreciated independent risk factor for atherosclerotic cardiovascular disease (ASCVD).41 Increased risk of cardiovascular events in patients with MASLD is partially mediated by the strong associations with T2DM and abdominal adiposity. Other proposed mechanisms include increased insulin resistance, pro-inflammatory mediators, pro-atherogenic dyslipidemia, oxidative stress, and hepatokines.42 Weight loss is considered a clinically important target with a positive impact on both MASLD and CVD risk factors (i.e., blood pressure, glucose, and lipid levels).43 In addition to lifestyle interventions (i.e., diet, physical activity, weight management, and smoking cessation), a wide range of proven pharmacological agents is available for CVD risk reduction.44 Treatment targets of CVD risk factors and the recommended pharmacological agents are outlined in Table 2.40,43-50
Table 2.
Cardiovascular disease risk factors and treatment targets in patients with metabolic dysfunction-associated steatotic liver disease40,43-50
| Risk factor | Optimal target | Preferred pharmacotherapeutic agent | Comments |
|---|---|---|---|
| LDL cholesterol | Moderate or intermediate risk* (diabetes alone): < 100 mg/dL (< 2.6 mmol/L) Higher risk (diabetes+multiple other ASCVD risk factors): < 70 mg/dL (< 1.8 mmol/L) High risk (established ASCVD): < 55 mg/dL (< 1.4 mmol/L)† |
1st line: Statin at an appropriate dose/intensity for risk category Moderate intensity: ≥ 30% decrease from baseline e.g., simvastatin 20–40 mg,43,44 atorvastatin 10–20 mg, rosuvastatin 5–10 mg, pravastatin 40–80 mg, lovastatin 40 mg, pitavastatin 2–4 mg High intensity: ≥ 50% decrease from baseline, e.g., rosuvastatin 20–40 mg, atorvastatin 40–80 mg 2nd line: Ezetimibe or PCSK9i can be added if the target is not achieved on maximal tolerated statin dose. |
Safe and may be used if serum aminotransferase level is < 3 × upper limit of normal. All patients at moderate or high risk must start statin regardless of baseline LDL cholesterol level. |
| Triglycerides44 | < 150 mg/dL (< 1.7 mmol/L) | Intensify lifestyle modification and optimize glycemic control | Fibrates do not improve cardiovascular outcomes. |
| BP | Treat if BP ≥ 140/90 mmHg in all patients Treat if BP ≥ 130/80 mmHg in diabetes or ASCVD risk ≥ 10% Aim for BP 120–129/70–79 mmHg |
RAS blockade preferred if albuminuria present or patient has IHD or heart failure. | For elderly patients ( ≥ 65 years) who are nursing home residents with high burden of comorbidity and limited life expectancy, treatment decision should be based on patient preference and team-based assessment of risk vs. benefit. |
| BMI and waist circumference | Ethnicity- and gender-specific targets BMI: < 23 kg/m2 for Asians and < 25 kg/m2 for Caucasians Waist circumference: Asian, < 80 cm (< 85 cm in Korea)48 for female, < 90 cm for male; Caucasian, < 80 cm for female, < 94 cm for male Weight loss ≥ 5% |
Lifestyle modification. Adjunctive GLP1 RA with proven cardiovascular benefit if BMI ≥ 27 kg/m2 with comorbidities or BMI ≥ 30 kg/m2 without comorbidities |
Weight loss ≥ 5% can lower HbA1c and BP and improve lipid profile. Use of GLP1 RA has cardiorenal benefits in patients with diabetes. Bariatric surgery results in sustained weight loss, improves liver histology, and reduces cardiovascular events. |
| HbA1c | < 6.5%‡50 | SGLT2 inhibitor preferred in patients with CVD/heart failure/CKD. GLP1 RA preferred in patients with CVD/albuminuria. |
Pioglitazone improves liver histology in MASLD, lowers glucose, and improves cardiovascular outcomes, but demonstrates safety concerns e.g., heart failure and side effects such as weight gain/edema. SGLT2 inhibitor improves liver fat content and may improve histology in MASLD. GLP1 RA improves liver fat content and histology in MASLD. |
*CVD risk calculated using the American College of Cardiology/American Heart Association ASCVD risk calculator (Risk Estimator Plus), a tool to estimate 10-year risk of a first ASCVD event based on the Pooled Cohort Equations (available online at tools.acc.org/ASCVD-Risk-Estimator-Plus): < 5%, low risk; 5% to < 7.5%, borderline risk; 7.5% to < 20%, moderate/ intermediate risk; and ≥ 20%, high risk; †American Diabetes Association (ADA) 2023 guidelines recommend a target LDL < 55 mg/dL (< 1.4 mmol/L) in patients with diabetes and established ASCVD; however, the American Heart Association 2018 guidelines recommend a target LDL < 70 mg/dL (< 1.8 mmol/L) in this group of patients; ‡HbA1c target should be individualized as recommended by the ADA.
LDL, low-density lipoprotein; ASCVD, atherosclerotic cardiovascular disease; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; BP, blood pressure; RAS, renin-angiotensin system; IHD, ischemic heart disease; BMI, body mass index; GLP1 RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycosylated hemoglobin; SGLT2, sodium glucose cotransporter-2; CVD, cardiovascular disease; CKD, chronic kidney disease; MASLD, metabolic dysfunction-associated steatotic liver disease.
Statins are the first-line therapy to reduce CVD risk by lowering low-density lipoprotein (LDL) cholesterol and reducing high-risk atherosclerotic plaques.45 Statins have been under-utilized in patients with MASLD due to concern of drug-induced liver injury, although they are safe and recommended for both primary and secondary prevention of CVD with robust evidence of reduction in all-cause mortality and cancer mortality in MASLD.42,44,45,51,52 Experimental and epidemiological data indicate that statins could potentially prevent development or progression of HCC, a known complication of MASLD.40,42 Broadly speaking, the decision on statin therapy should be based on (1) whether therapy is for primary or secondary prevention of ASCVD and (2) estimation of the patient’s CVD risk. While several risk estimators have been developed, the AHA recommends the use of the Pooled Cohort Equations, which calculate 10-year CVD risk.44,45 The AHA algorithms provide guidance on not only when to initiate statins, but also the tailoring of intensity (i.e., type and dose) of statin therapy for various at-risk populations. Achieving optimal outcomes based on efficacy data requires (1) aiming for a target LDL cholesterol level or a percentage reduction in LDL cholesterol level from baseline and (2) using a minimum pre-defined dose of a statin with proven improvement in cardiovascular outcome.44,45 The combination of LDL and triglyceride-rich very LDL is more atherogenic than either fraction alone.45 Therefore, it is recommended that triglyceride level be maintained <150 mg/dL (<1.7 mmol/L). Statin therapy can lower both LDL and triglyceride levels; however, the use of fibrates in combination with statins to reduce CVD risk is not recommended.44 Combination treatment of ezetimibe with statin can further decrease LDL and triglyceride levels compared to statin monotherapy and has stronger cardiovascular protective effects in high-risk patients with T2DM.53,54
Lowering blood pressure is also vital to reduce CVD risk. The preferred agents with proven cardiovascular benefits are drugs that block the renin-angiotensin system.44,46 Reducing glycosylated hemoglobin (HbA1c) alone can modestly reduce cardiovascular risk in patients with T2DM, although its benefits are more evident for microvascular complications.44 While HbA1c targets should be individualized, especially in the elderly and those with advanced comorbidities where risk of hypoglycemia is greater, a general target advocated by the American Diabetes Association is HbA1c <7% without significant hypoglycemia. Current guidelines recommend the use of SGLT2 inhibitor and GLP1 RA that can reduce cardiorenal risk in patients with T2DM and established or high-risk ASCVD, heart failure, and/or chronic kidney disease.44 While SGLT2 inhibitors are approved for use in those without diabetes but with ischemic heart disease or heart failure, GLP1 RAs are currently only FDA approved for use in patients with diabetes or obesity. Aspirin is essential for secondary prevention of ASCVD and may be considered for primary prevention in those at high risk of ASCVD.44 Aspirin has even been implicated in improved liver histology in patients with MASLD.42 However, the risk of gastrointestinal bleeding should be discussed as part of a shared decision-making process in the primary prevention group, especially those >70 years old who are at higher risk of bleeding or anemia due to renal disease and complications of liver disease (e.g., portal hypertension).44
MASLD AND ANTI-HYPERGLYCEMIC DRUGS
To date, there are no FDA-approved drugs for the treatment of MASLD and MASH.55,56 Among people with T2DM and MASH, current treatment recommendations are focused on the amelioration of (1) cardiometabolic risk factors (glycemia, blood pressure, lipids, and body weight) and (2) steatohepatitis, especially if there is clinically significant fibrosis (stage ≥F2).55,57,58 Although a liver biopsy is the gold standard to diagnose and stage the severity of liver fibrosis, it is invasive and may not be acceptable or feasible to certain people. Hence, pharmacological therapy can be considered based on (1) elevated FIB4 >1.3, (2) elevated serum aminotransferase level, (3) imaging such as transient elastography and magnetic resonance elastography, and/or (4) plasma biomarkers for liver fibrosis such as the enhanced liver fibrosis test.55,58,59
Among people with T2DM, prediabetes, or obesity, pioglitazone and GLP1 RAs are the two anti-hyperglycemic drugs that have proven efficacy and safety to reverse MASH.55,58 Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces insulin resistance by improving lipid storage/redistribution and glucose utilization.57 It was the first anti-hyperglycemic drug to show efficacy in an early randomized clinical trial (RCT) involving 55 people with prediabetes/T2DM and biopsy-proven MASH.60 In a single-center study involving 101 people with prediabetes/T2DM and biopsy-proven MASH, after a mean follow-up of 36 months, 58% of those treated with pioglitazone 45 mg daily had a reduction of at least two points in NAFLD Activity Score, while 51% reported resolution of MASH (treatment difference: 41% and 32% vs. placebo, respectively).38 Despite a modest weight gain, pioglitazone was associated with sustained improvements in liver fibrosis score, insulin resistance, and hepatic triglyceride contents.38 In a meta-analysis of eight RCTs involving 516 people with biopsy-proven MASH, pioglitazone was associated with 1.6 to 3.2 odds of improved liver fibrosis and MASH resolution.61 However, pioglitazone may be associated with adverse effects including heart failure, fracture, and bladder cancer.55
Given the robust benefits in glycemia, body weight, and other cardiometabolic risk factors, GLP1 RAs have emerged as the key pillars for managing people with T2DM and/or obesity.62 In a phase 2 RCT involving 52 people with body mass index (BMI) ≥25 kg/m2 and biopsy-proven MASH, injectable liraglutide (1.8 mg daily) was significantly associated with a relative risk of 4.3 for resolution of MASH compared with placebo.63 Another phase 2 RCT compared the efficacy and safety of injectable semaglutide at 0.1, 0.2, and 0.4 mg daily (equivalent to 2.4 mg weekly) among 320 people with BMI ≥25 kg/m2 and biopsy-proven MASH.64 The mean percentage of total body weight loss was 13% in the 0.4 mg group, compared with 1% in the placebo group.64 Resolution of MASH was reported in 40% in the 0.1 mg group, 36% in the 0.2 mg group, and 59% in the 0.4 mg group compared with 17% in the placebo group (P<0.001 for semaglutide 0.4 mg vs. placebo).64 Among people with T2DM and/or obesity, a novel GLP1/glucose-dependent insulinotropic polypeptide receptor agonists (i.e., tirzepatide)65 showed superior metabolic efficacy with improved serum aminotransferase level and liver fibrosis score,66 although more evidence of the changes in liver histological features is needed.67
SGLT2 inhibitors, which have proven cardiorenal benefits,62 can be used as adjunctive pharmacological therapy among people with T2DM and MASLD. This is due to improvements in cardiometabolic risk factors and inflammation with reduced hepatic triglyceride contents and serum aminotransferase level.56,68-70 Although there are significant benefits in improving hepatic steatosis assessed by magnetic resonance imaging techniques and non-invasive biomarkers, histological evaluations of liver biopsy after treatment with SGLT2 inhibitors are yet to be reported.56 Given the lack of efficacy data, metformin, dipeptidyl peptidase-4 inhibitors, alpha-glucosidase inhibitors, and insulin are not recommended for the treatment of MASLD. However, these anti-hyperglycemic drugs can be tailored for hyperglycemia among people with T2DM and MASLD. A summary of the effects of anti-hyperglycemic drugs on MASLD can be found in Table 3.55,56,62
Table 3.
Effects of anti-hyperglycemic drugs on metabolic dysfunction-associated steatotic liver disease55,56,62
| Liver parameters | Cardiorenal-metabolic parameters | ||||||
|---|---|---|---|---|---|---|---|
| Serum aminotransferase | Liver fat | Liver fibrosis | MASH resolution | Body weight | Cardiorenal benefits | ||
| Pioglitazone | ↓ | ↓ | ↓ | Yes | ↑ | ↔ | |
| GLP1 RAs | ↓ | ↓ | ↓ | Yes | ↓ | Yes | |
| GLP1/GIP RAs | ↓ | ↓ | Unknown | Unknown | ↓ | Unknown | |
| SGLT2 inhibitors | ↓ | ↓ | Unknown | Unknown | ↓ | Yes | |
| Insulin | ↓ | ↓ | Unknown | Unknown | ↑ | ↔ | |
| Metformin | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| DPP-4 inhibitors | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
MASH, metabolic dysfunction-associated steatohepatitis; GLP1 RA, glucagon-like peptide-1 receptor agonist; GIP RA, glucose-dependent insulinotropic polypeptide receptor agonist; SGLT2, sodium glucose co-transporter-2; DPP-4, dipeptidyl peptidase-4.
MANAGEMENT OF DIABETES AND OTHER METABOLIC COMORBIDITIES IN CIRRHOSIS
The management of T2DM patients with liver cirrhosis has become an increasingly common scenario with the global increase of obesity, diabetes, and MASLD.71,72 There is a clear unmet need in this area, as patients in this population have been rarely studied. Data on glucose monitoring and targets, as well as the use of anti-hyperglycemic drugs in liver cirrhosis, remain sparse. Although diabetes can be diagnosed by conventional criteria of fasting plasma glucose (FPG), 2-hour post prandial glucose after a 75 g oral glucose tolerance test (OGTT), or HbA1c, the accuracy of these methods in liver cirrhosis differs.73,74 The OGTT outperforms the FPG and HbA1c in terms of accuracy in diagnosing new onset diabetes in liver cirrhosis.74 The standard measure of glucose control using HbA1c needs to be used with caution in liver cirrhosis.72 HbA1c does not correlate well with continuous glucose monitoring in patients with decompensated cirrhosis due to changes in erythrocyte turnover, hemolysis caused by splenomegaly, impaired erythropoiesis due to bone marrow suppression, and repeated blood transfusions.75 Other parameters such as fructosamine or glycated albumin may also be less accurate because of hypoalbuminemia. The use of serial monitoring of capillary blood glucose or continuous glucose monitoring methods or flash glucose monitoring are better options to accurately assess glucose control in the setting of decompensated cirrhosis.75-77
The presence of diabetes and poor glucose control is linked to higher risk of decompensation (e.g., hepatic encephalopathy, spontaneous bacterial peritonitis, and variceal bleeding), development of HCC, and reduced survival in patients with liver cirrhosis.78,79 In those with compensated cirrhosis, glycemic targets should be the same as in those without cirrhosis. In decompensated cirrhosis, complications and survival are primarily liver-related; therefore, glycemic targets can be less stringent to prevent hypoglycemia or other undesirable complications from anti-hyperglycemic drugs. To date, there is no evidence for exact measures of glucose targets, but the general consensus is that this needs to be individualized. Commonly used anti-hyperglycemic drugs and their benefits, risks, and safety in liver cirrhosis are summarized in Table 4.61,72,80-86 Most anti-hyperglycemic drugs are contraindicated or need to be used with caution in decompensated cirrhosis. Insulin is the primary treatment of choice in decompensated cirrhosis; however, since insulin is metabolized by hepatic insulinase, the dose may need to be reduced and analogue insulins are preferred to avoid hypoglycemia.72,84-86 Decreased insulin clearance, hyperinsulinemia due to portal circulation stunting in addition to decreased glycogen storage and inadequate gluconeogenesis increases the risk of hypoglycemia. A pre-meal glucose of 100 to 200 mg/dL (5.5 to 11.0 mmol/L) is considered acceptable for those on insulin therapy.72
Table 4.
| Agent | Main site of elimination | Benefits | Major concerns | Compensated cirrhosis, Child-Pugh A | Decompensated cirrhosis, Child-Pugh B | Decompensated cirrhosis, Child-Pugh C |
|---|---|---|---|---|---|---|
| Metformin | Kidney (dose according to renal function) | Possibly reduce HCC risk | Metabolic acidosis | Safe | Contraindicated | Contraindicated |
| Sulphonylureas (only second and third generations) | Kidney/protein-bound | - | Hypoglycemia | Safe | Contraindicated | Contraindicated |
| Glinides* | Liver | - | Hypoglycemia | Caution | Caution | Contraindicated |
| DPP-4 inhibitors† | Kidney | No hypoglycemia risk | Lack of efficacy | Safe | Safe | Contraindicated |
| SGLT2-inhibitors | Liver (small amount by the kidney) | No hypoglycemia risk, benefit in MASLD | Dehydration, hypotension | Safe | Safe | Contraindicated |
| GLP1 RAs (human GLP1-based), e.g., liraglutide, dulaglutide, and semaglutide | Degraded by DPP-4 enzyme | No hypoglycemia risk, benefit in MASLD | Excessive weight loss/malnutrition | Safe | Safe | Contraindicated |
| GLP1 RAs (exendin based), e.g., lixisenatide and exenatide | Kidney | No hypoglycemia risk | Excessive weight loss/malnutrition | Safe | Contraindicated | Contraindicated |
| Acarbose | Gastrointestinal tract | No hypoglycemia risk | Lack of efficacy | Safe | Safe | Contraindicated |
| Pioglitazone | Liver | Benefits NASH without cirrhosis | Fluid retention | Caution | Contraindicated | Contraindicated |
| Insulin (insulin analogues preferred) | Liver | Safe in all degrees of liver cirrhosis | Hypoglycemia | Safe | Safe | Safe |
*Repaglinide and †Vildagliptin are contraindicated in cirrhosis.
HCC, hepatocellular carcinoma; DPP-4, dipeptidyl peptidase-4; SGLT2, sodium glucose co-transporter-2; MASLD, metabolic dysfunction-associated steatotic liver disease; GLP1 RA, glucagon-like peptide-1 receptor agonist; NASH, non-alcoholic steatohepatitis.
As patients with liver cirrhosis develop ascites, their blood pressure often drops. Mineralocorticoid receptor antagonists remain a mainstay of therapy, and loop diuretics may be added if required. Impaired liver function may lead to elevated concentrations of some beta blockers and calcium channel blockers, so doses may need to be reduced.87-89 Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are usually avoided in decompensated cirrhosis as they may further reduce effective arterial volume and renal perfusion.90 Statins are safe in compensated cirrhosis but generally not recommended in decompensated cirrhosis as it is unlikely that there will be benefit from statin at this stage, and there is risk of accumulation of high serum level due to impaired metabolism.91
COMPENSATED ADVANCED CHRONIC LIVER DISEASE AND CLINICALLY SIGNIFICANT PORTAL HYPERTENSION
With increasing utility of both liver biopsy and LSM using vibration-controlled transient elastography (LSM-VCTE), more patients are being diagnosed with advanced liver fibrosis and early cirrhosis. These patients have severe liver disease but are asymptomatic. The term cACLD has been used to describe this group of patients.92 Even though these patients do not manifest clinical decompensation of liver disease, they are at risk of liver-related events, such as index decompensation (ascites or variceal bleed) and HCC.92 It is important to identify these patients so that timely intervention can be administered to attenuate or even reverse disease progression and reduce liver-related events. Patients with LSM-VCTE <10 kPa are unlikely to have cACLD, with a negligible risk of adverse clinical outcomes, while those with LSM-VCTE >15 kPa can be assumed to have cACLD (Fig. 1).34,92,93
Patients with more advanced liver fibrosis can develop CSPH, albeit without any symptomatic manifestation. (1) One such sequalae of CSPH is gastroesophageal varices that require obliteration and initiation of non-selective beta-blocker (NSBB) (i.e., varices needing treatment [VNT]) to prevent bleeding and index decompensation. CSPH is confidently ruled out (>90% sensitivity and negative predictive value) if LSM-VCTE is ≤15 kPa and platelet count is ≥150×109/L; in patients with MASLD, cACLD, and BMI <30 kg/m2, LSM-VCTE ≥25 kPa is sufficient to identify CSPH (specificity and positive predictive value >90%).92 Based on the landmark ANTICIPATE study, patients with LSM values between 20 and 25 kPa and platelet count <150×109/L or LSM-VCTE values between 15 and 20 kPa and platelet count <110×109/L have a CSPH risk of at least 60%.94 However, when the similar cut-off value of LSM-VCTE used in CSPH was applied to patients with BMI ≥30 kg/m2, the specificity and positive predictive values were only 62.8%,94 which implies an overestimation of CSPH in these patients. Hence, a new model (ANTICIPATE-NASH model) to predict CSPH with better accuracy for MASLD patients with BMI ≥30 kg/m2 (which incorporates BMI, LSM-VCTE, and platelet count) has been proposed but requires further validation.94
The current recommendation is that MASLD patients with cACLD do not require variceal screening via endoscopy if LSM-VCTE is <20 kPa and platelet count is >150×109/L,92 where the risk of missing VNT is not more than 5%. LSM-VCTE and platelet count should be monitored annually to initiate variceal surveillance if LSM-VCTE increases to ≥20 kPa or platelet count decreases below 150×109/L.92 An effective strategy to prevent decompensation in patients with CSPH is to initiate treatment with NSBBs (e.g., propranolol, nadolol, or carvedilol). Studies have shown that patients with CSPH and on NSBB have a significantly lower risk of index decompensation,95,96 and this effect is more profound with carvedilol, as it has intrinsic anti-alpha adrenergic vasodilatory effects that provide a greater portal-pressure-reducing effect.92,96 Furthermore, patients who are on NSBB for prevention of decompensation do not need a screening endoscopy for detection of varices since the result will not change management.92,97
Liver cirrhosis is a known risk factor for HCC. Surveillance with 6-monthly ultrasound examination of the liver with or without serum alpha-fetoprotein level is recommended by all major guidelines. A meta-analysis that compared the nature of MASLD-related HCC to HCC due to other etiologies reported that a higher percentage of MASLD-HCC patients were non-cirrhotic (38.5% vs. 14.6%).98 This finding led to a change in recommendation of HCC surveillance, where MASLD patients with advanced fibrosis in EASL guidelines99 or ≥F3 fibrosis with LSM-VCTE ≥16.1 kPa in American Gastroenterological Association guidelines100 may be considered for HCC surveillance. Other than in advanced fibrosis, certain factors in MASLD patients (such as older age, male, obesity, diabetes mellitus, and patatin-like phospholipase domain-containing 3 gene) have been reported to increase the risk of HCC among non-cirrhotic MASLD patients.99-101 Hence, an individualized risk assessment should determine the need for HCC surveillance to improve detection of HCC among non-cirrhotic MASLD patients, but further validation is required.
SARCOPENIA AND MASLD
Sarcopenia is characterized by the loss of muscle mass and muscle strength. In the past few decades, sarcopenia has been described as common among the geriatric population and patients with severe medical illness, including cancer and even liver cirrhosis.102 Of late, there is a growing interest in studies on the association between sarcopenia and MASLD. Notably, MASLD and sarcopenia share similar pathophysiology, especially insulin resistance, physical inactivity, and obesity.103 Sarcopenic obesity is an independent risk factor for MASLD. The risk for MASLD was higher than the presence of obesity only. Similarly, sarcopenia in addition to visceral fat obesity and myosteatosis were significantly associated with MASLD among individuals without obesity.104 In contrast, patients with MASLD were demonstrated to be at increased risk of sarcopenia in a longitudinal study. They had a higher rate of muscle mass loss compared to those without MASLD over 5 years.105 Sarcopenia was also more prevalent among patients with MASH compared to patients with MASLD without MASH and controls (35.0% vs. 17.9% vs. 8.7%, P<0.001).106 In addition, patients with MASLD and sarcopenia were more likely to have significant and advanced liver fibrosis.106,107 Sarcopenia is a strong predictor of mortality and morbidity for MASLD, MASH, and cirrhosis.108,109 Several tools are available to evaluate muscle mass and muscle strength for sarcopenia. The SARC-F questionnaire is a brief self-reported test to detect persons at risk for adverse outcomes from sarcopenia. The screening test encompasses five components: strength, walking with assistance, rising from a chair, climbing stairs, and falling.110 Muscle strength of upper limbs and lower body strength can be assessed using the grip strength measurements and chair stand test, respectively. Skeletal muscle mass can be evaluated by dual-energy X-ray absorptiometry and bioelectrical impedance analysis methods, while computed tomography or magnetic resonance imaging can examine both muscle quantity and quality. The severity of sarcopenia can be measured by evaluating the physical performance of patients, e.g., short physical performance battery, timed-up and go, and 400-m walk tests.102
The main aim of treatment for MASLD is lifestyle modification to achieve weight reduction of 7% to 10%, in particular in those with overweight/obesity. Such a degree of weight loss can lead to MASH resolution and histological improvement (see section on ‘Lifestyle intervention is the cornerstone in the management of MASLD’).5 In contrast, skeletal muscle loss is one of the cardinal features of sarcopenia. Weight loss may lead to further muscle loss if energy restriction is carried out without exercise. The recommended treatment for sarcopenia is resistance exercise and optimizing nutrition intake.111 Increased physical activity and healthy diet have been associated with lower risk of sarcopenia and MASLD and could even reduce the risk of significant liver fibrosis.107,112 Adequate protein intake is advised for patients with sarcopenia, but protein supplementation alone without resistance exercise is ineffective in improving muscle mass and strength.113 Conversely, oral branch-chain amino acid supplementation is particularly beneficial for patients with hepatic encephalopathy and advanced cirrhosis.114 The European Society for Clinical Nutrition and Metabolism recommends the Mediterranean diet for MASH to improve insulin resistance and steatosis.114 Meanwhile, the Mediterranean diet has been shown to have a positive effect on muscle mass and function in sarcopenia.115 Vitamin D supplementation has been reported to improve insulin resistance in MASLD.116 Likewise, vitamin D combined with protein has been shown to improve muscle strength in patients compared to a control with sarcopenia in meta-analysis.117,118
CONCLUSION
The increased understanding of MASLD has brought about a name change to the disease, creating greater awareness. MASLD is the most common cause of chronic liver disease and is the leading cause of liver-related morbidity and mortality. There is no doubt that all stakeholders must be involved in tackling the public health threat of obesity and obesity-related diseases, including MASLD. A simple and clear assessment and referral pathway is essential to ensure that patients with more severe MASLD are identified and referred to specialist care, while patients with less severe disease remain in primary care, where they are best managed. This can serve as a foundation as we refine the shared role between primary and specialist care providers in management of this multi-faceted condition, considering available resources and local practice. Further studies are needed to optimize the management of patients with different disease severity to prevent progression and improve outcomes.
Footnotes
CONFLICTS OF INTEREST
Wah-Kheong Chan has served as a consultant or advisory board member for Roche, Abbvie, Boehringer Ingelheim, and Novo Nordisk and as a speaker for Viatris and Hisky Medical. Lee-Ling Lim reports receiving grants and/or honoraria for consultancy or lectures from Abbott, AstraZeneca, Boehringer Ingelheim, Novartis, Novo Nordisk, Roche, Sanofi, Servier, and Zuellig Pharma. Jeyakantha Ratnasingam has served as a consultant or advisory board member for Novo Nordisk and a speaker for Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Zuellig Pharma, Servier, Roche, Merck, and Abbott. The other authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Study concept and design: WKC; acquisition of data: WKC, KHC, RBR, LLL, JR, and SRV; analysis and interpretation of data: WKC, KHC, RBR, LLL, JR, and SRV; drafting of the manuscript: WKC, KHC, RBR, LLL, JR, and SRV; and critical revision of the manuscript: WKC, KHC, RBR, LLL, JR, and SRV.
REFERENCES
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 2.Farrell GC, Chitturi S, Lau GK, Sollano JD Asia-Pacific Working Party on NAFLD, author. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22:775–7. doi: 10.1111/j.1440-1746.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 3.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO), author EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 7.Eslam M, Sanyal AJ, George J International Consensus Panel, author. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 10.Tan SS, Lee YY, Ali RA, Mustapha F, Chan WK. Endorsing the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6:163. doi: 10.1016/S2468-1253(21)00002-9. [DOI] [PubMed] [Google Scholar]
- 11.Chan WK, Tan SS, Chan SP, Lee YY, Tee HP, Mahadeva S, et al. Malaysian Society of Gastroenterology and Hepatology consensus statement on metabolic dysfunction-associated fatty liver disease. J Gastroenterol Hepatol. 2022;37:795–811. doi: 10.1111/jgh.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7:388–90. doi: 10.1016/S2468-1253(22)00062-0. [DOI] [PubMed] [Google Scholar]
- 13.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023 Jun 24; doi: 10.1097/HEP.0000000000000520. [Epub].https://doi.org/10.1097/HEP.0000000000000520 . [DOI] [PubMed] [Google Scholar]
- 14.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809–17. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2022;31:17–27. doi: 10.7570/jomes22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan WK, Treeprasertsuk S, Imajo K, Nakajima A, Seki Y, Kasama K, et al. Clinical features and treatment of nonalcoholic fatty liver disease across the Asia Pacific region-the GO ASIA initiative. Aliment Pharmacol Ther. 2018;47:816–825. doi: 10.1111/apt.14506. [DOI] [PubMed] [Google Scholar]
- 18.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 19.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan WL, Chong SE, Chang F, Lai LL, Chuah KH, Nik Mustapha NR, et al. Long-term clinical outcomes of adults with metabolic dysfunction-associated fatty liver disease: a single-centre prospective cohort study with baseline liver biopsy. Hepatol Int. 2023;17:870–81. doi: 10.1007/s12072-023-10550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahrani S, Gill SS, Sooi CY, Skantha R, Kumar CV, Limun MF, et al. Frequency of significant steatosis and compensated advanced chronic liver disease among adults with chronic liver disease. J Gastroenterol Hepatol. 2023 Aug 16; doi: 10.1111/jgh.16313. [Epub].https://doi.org/10.1111/jgh.16313 . [DOI] [PubMed] [Google Scholar]
- 22.Mohammed OK, Mahadeva S. Clinical outcomes of cryptogenic compared with non-cryptogenic cirrhosis: a retrospective cohort study. J Gastroenterol Hepatol. 2015;30:1423–8. doi: 10.1111/jgh.12978. [DOI] [PubMed] [Google Scholar]
- 23.Karuthan SR, Koh PS, Chinna K, Chan WK. Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer staging systems for prediction of survival among hepatocellular carcinoma patients. Med J Malaysia. 2021;76:199–204. [PubMed] [Google Scholar]
- 24.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–95. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 26.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 27.Estes C, Chan HL, Chien RN, Chuang WL, Fung J, Goh GB, et al. Modelling NAFLD disease burden in four Asian regions: 2019-2030. Aliment Pharmacol Ther. 2020;51:801–11. doi: 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–15. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 29.Clinical practice guidelines management of type 2 diabetes mellitus. 6th ed. Malaysia Endocrine & Metabolic Society, Ministry of Health Malaysia, Academy of Medicine Malaysia, Diabetes Malaysia, Family Medicine Specialists Association of Malaysia; 2020. [Google Scholar]
- 30.Lai LL, Wan Yusoff WN, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34:1396–403. doi: 10.1111/jgh.14577. [DOI] [PubMed] [Google Scholar]
- 31.European Association for the Study of the Liver, author. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis: 2021 update. J Hepatol. 2021;75:659–89. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan WK, Treeprasertsuk S, Goh GB, Fan JG, Song MJ, Charatcharoenwitthaya P, et al. Optimizing use of nonalcoholic fatty liver disease fibrosis score, fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Clin Gastroenterol Hepatol. 2019;17:2570–80. doi: 10.1016/j.cgh.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 34.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–51. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–78. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–42. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Haase CL, Lopes S, Olsen AH, Satylganova A, Schnecke V, McEwan P. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: evidence from a UK primary care database. Int J Obes (Lond) 2021;45:1249–58. doi: 10.1038/s41366-021-00788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305–15. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 39.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nascimbeni F, Pellegrini E, Lugari S, Mondelli A, Bursi S, Onfiani G, et al. Statins and nonalcoholic fatty liver disease in the era of precision medicine: more friends than foes. Atherosclerosis. 2019;284:66–74. doi: 10.1016/j.atherosclerosis.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 41.Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e168–85. doi: 10.1161/ATV.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 42.Targher G, Corey KE, Byrne CD. NAFLD, and cardiovascular and cardiac diseases: factors influencing risk, prediction and treatment. Diabetes Metab. 2021;47:101215. doi: 10.1016/j.diabet.2020.101215. [DOI] [PubMed] [Google Scholar]
- 43.Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115:1447–63. doi: 10.1016/j.jand.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 44.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 10. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S158–90. doi: 10.2337/dc23-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 47.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 48.Zhou XD, Targher G, Byrne CD, Somers V, Kim SU, Chahal CAA, et al. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol Int. 2023;17:773–91. doi: 10.1007/s12072-023-10543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haam JH, Kim BT, Kim EM, Kwon H, Kang JH, Park JH, et al. Diagnosis of obesity: 2022 update of clinical practice guidelines for obesity by the Korean Society for the Study of Obesity. J Obes Metab Syndr. 2023;32:121–9. doi: 10.7570/jomes23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021;45:461–81. doi: 10.4093/dmj.2021.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng CH, Teng ML, Chew NW, Chan KE, Yong JN, Quek J, et al. Statins decrease overall mortality and cancer related mortality but are underutilized in NAFLD: a longitudinal analysis of 12,538 individuals. Expert Rev Gastroenterol Hepatol. 2022;16:895–901. doi: 10.1080/17474124.2022.2119128. [DOI] [PubMed] [Google Scholar]
- 52.Khoo S, Wong VW, Goh GB, Fan J, Chan WK, Seto WK, et al. Suboptimal treatment of dyslipidemia in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35:320–5. doi: 10.1111/jgh.14794. [DOI] [PubMed] [Google Scholar]
- 53.Cho Y, Rhee H, Kim YE, Lee M, Lee BW, Kang ES, et al. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study) BMC Med. 2022;20:93. doi: 10.1186/s12916-022-02288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohula EA, Morrow DA, Giugliano RP, Blazing MA, He P, Park JG, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911–21. doi: 10.1016/j.jacc.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 55.Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD) Endocr Pract. 2022;28:528–62. doi: 10.1016/j.eprac.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Cariou B. The metabolic triad of non-alcoholic fatty liver disease, visceral adiposity and type 2 diabetes: implications for treatment. Diabetes Obes Metab. 2022;24 Suppl 2:15–27. doi: 10.1111/dom.14651. [DOI] [PubMed] [Google Scholar]
- 57.Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1:312–28. doi: 10.1016/j.jhepr.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40:419–30. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 59.Vali Y, Lee J, Boursier J, Spijker R, Löffler J, Verheij J, et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2020;73:252–62. doi: 10.1016/j.jhep.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 60.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 61.Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177:633–40. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim LL, Chow E, Chan JC. Cardiorenal diseases in type 2 diabetes mellitus: clinical trials and real-world practice. Nat Rev Endocrinol. 2023;19:151–63. doi: 10.1038/s41574-022-00776-2. [DOI] [PubMed] [Google Scholar]
- 63.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 64.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 65.Sinha R, Papamargaritis D, Sargeant JA, Davies MJ. Efficacy and safety of tirzepatide in type 2 diabetes and obesity management. J Obes Metab Syndr. 2023;32:25–45. doi: 10.7570/jomes22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43:1352–5. doi: 10.2337/dc19-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2023;8:179–91. doi: 10.1016/S2468-1253(22)00338-7. [DOI] [PubMed] [Google Scholar]
- 68.Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care. 2020;43:298–305. doi: 10.2337/dc19-0641. [DOI] [PubMed] [Google Scholar]
- 69.Latva-Rasku A, Honka MJ, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care. 2019;42:931–7. doi: 10.2337/dc18-1569. [DOI] [PubMed] [Google Scholar]
- 70.Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:812–21. doi: 10.1111/dom.13584. [DOI] [PubMed] [Google Scholar]
- 71.Wlazlo N, Beijers HJ, Schoon EJ, Sauerwein HP, Stehouwer CD, Bravenboer B. High prevalence of diabetes mellitus in patients with liver cirrhosis. Diabet Med. 2010;27:1308–11. doi: 10.1111/j.1464-5491.2010.03093.x. [DOI] [PubMed] [Google Scholar]
- 72.Boursier J, Anty R, Carette C, Cariou B, Castera L, Caussy C, et al. Management of diabetes mellitus in patients with cirrhosis: an overview and joint statement. Diabetes Metab. 2021;47:101272. doi: 10.1016/j.diabet.2021.101272. [DOI] [PubMed] [Google Scholar]
- 73.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–40. doi: 10.2337/dc23-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.García-Compeán D, Jáquez-Quintana JO, Lavalle-González FJ, Reyes-Cabello E, González-González JA, Muñoz-Espinosa LE, et al. The prevalence and clinical characteristics of glucose metabolism disorders in patients with liver cirrhosis: a prospective study. Ann Hepatol. 2012;11:240–8. doi: 10.1016/S1665-2681(19)31030-0. [DOI] [PubMed] [Google Scholar]
- 75.Addepally NS, George N, Martinez-Macias R, Garcia-Saenz-de-Sicilia M, Kim WR, Duarte-Rojo A. Hemoglobin A1c has suboptimal performance to diagnose and monitor diabetes mellitus in patients with cirrhosis. Dig Dis Sci. 2018;63:3498–508. doi: 10.1007/s10620-018-5265-3. [DOI] [PubMed] [Google Scholar]
- 76.Honda F, Hiramatsu A, Hyogo H, Aikata H, Daijo K, Teraoka Y, et al. Evaluation of glycemic variability in chronic liver disease patients with type 2 diabetes mellitus using continuous glucose monitoring. PLoS One. 2018;13:e0195028. doi: 10.1371/journal.pone.0195028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costa D, Lourenço J, Monteiro AM, Castro B, Oliveira P, Tinoco MC, et al. Clinical performance of flash glucose monitoring system in patients with liver cirrhosis and diabetes mellitus. Sci Rep. 2020;10:7460. doi: 10.1038/s41598-020-64141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goh GB, Pan A, Chow WC, Yuan JM, Koh WP. Association between diabetes mellitus and cirrhosis mortality: the Singapore Chinese Health Study. Liver Int. 2017;37:251–8. doi: 10.1111/liv.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tergast TL, Laser H, Gerbel S, Manns MP, Cornberg M, Maasoumy B. Association between type 2 diabetes mellitus, HbA1c and the risk for spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis and ascites. Clin Transl Gastroenterol. 2018;9:189. doi: 10.1038/s41424-018-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gangopadhyay KK, Singh P. Consensus statement on dose modifications of antidiabetic agents in patients with hepatic impairment. Indian J Endocrinol Metab. 2017;21:341–54. doi: 10.4103/ijem.IJEM_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He YL, Sabo R, Campestrini J, Wang Y, Ligueros-Saylan M, Lasseter KC, et al. The influence of hepatic impairment on the pharmacokinetics of the dipeptidyl peptidase IV (DPP-4) inhibitor vildagliptin. Eur J Clin Pharmacol. 2007;63:677–86. doi: 10.1007/s00228-007-0312-6. [DOI] [PubMed] [Google Scholar]
- 82.Scheen AJ. Pharmacokinetics in patients with chronic liver disease and hepatic safety of incretin-based therapies for the management of type 2 diabetes mellitus. Clin Pharmacokinet. 2014;53:773–85. doi: 10.1007/s40262-014-0157-y. [DOI] [PubMed] [Google Scholar]
- 83.Macha S, Rose P, Mattheus M, Cinca R, Pinnetti S, Broedl UC, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16:118–23. doi: 10.1111/dom.12183. [DOI] [PubMed] [Google Scholar]
- 84.Yen FS, Lai JN, Wei JC, Chiu LT, Hsu CC, Hou MC, et al. Is insulin the preferred treatment in persons with type 2 diabetes and liver cirrhosis? BMC Gastroenterol. 2021;21:263. doi: 10.1186/s12876-021-01773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gentile S, Guarino G, Strollo F, Romano M, Genovese S, Masarone M, et al. Lispro insulin in people with non-alcoholic liver cirrhosis and type 2 diabetes mellitus. Diabetes Res Clin Pract. 2016;113:179–86. doi: 10.1016/j.diabres.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Kannampilly JJ. Role of continuous subcutaneous insulin infusion (insulin pump) in reducing blood glucose in four patients with type 2 diabetes and cirrhosis: a case series. Diabetes Technol Ther. 2010;12:543–5. doi: 10.1089/dia.2009.0166. [DOI] [PubMed] [Google Scholar]
- 87.Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sanchez de la Cuesta F Spanish Collaborative Study Group on Therapeutic Management of Liver Diseases, author. Drug use for non-hepatic associated conditions in patients with liver cirrhosis. Eur J Clin Pharmacol. 2003;59:71–6. doi: 10.1007/s00228-003-0586-2. [DOI] [PubMed] [Google Scholar]
- 88.Franz CC, Egger S, Born C, Rätz Bravo AE, Krähenbühl S. Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur J Clin Pharmacol. 2012;68:179–88. doi: 10.1007/s00228-011-1105-5. [DOI] [PubMed] [Google Scholar]
- 89.Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis: a practical guide. Aliment Pharmacol Ther. 2013;37:1132–56. doi: 10.1111/apt.12324. [DOI] [PubMed] [Google Scholar]
- 90.Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–48. doi: 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- 91.Speliotes EK, Balakrishnan M, Friedman LS, Corey KE. Treatment of dyslipidemia in common liver diseases. Clin Gastroenterol Hepatol. 2018;16:1189–96. doi: 10.1016/j.cgh.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C Baveno VII Faculty, author. Baveno VII: renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mózes FE, Lee JA, Vali Y, Alzoubi O, Staufer K, Trauner M, et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:704–13. doi: 10.1016/S2468-1253(23)00141-3. [DOI] [PubMed] [Google Scholar]
- 94.Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the "Anticipate" study. Hepatology. 2016;64:2173–84. doi: 10.1002/hep.28824. [DOI] [PubMed] [Google Scholar]
- 95.Turco L, Villanueva C, La Mura V, García-Pagán JC, Reiberger T, Genescà J, et al. Lowering portal pressure improves outcomes of patients with cirrhosis, with or without ascites: a meta-analysis. Clin Gastroenterol Hepatol. 2020;18:313–27. doi: 10.1016/j.cgh.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 96.Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. β Blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 97.Dwinata M, Putera DD, Adda'i MF, Hidayat PN, Hasan I. Carvedilol vs endoscopic variceal ligation for primary and secondary prevention of variceal bleeding: systematic review and meta-analysis. World J Hepatol. 2019;11:464–76. doi: 10.4254/wjh.v11.i5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan DJ, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521–30. doi: 10.1016/S1470-2045(22)00078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.European Association for the Study of the Liver, author. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 100.Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158:1822–30. doi: 10.1053/j.gastro.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teng ML, Tan DJH, Ng CH, Huang DQ. Hepatocellular carcinoma surveillance in non-alcoholic fatty liver disease: who and how? Clin Mol Hepatol. 2023;29:404–7. doi: 10.3350/cmh.2023.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwaki M, Kobayashi T, Nogami A, Saito S, Nakajima A, Yoneda M. Impact of sarcopenia on non-alcoholic fatty liver disease. Nutrients. 2023;15:891. doi: 10.3390/nu15040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim HK, Bae SJ, Lee MJ, Kim EH, Park H, Kim HS, et al. Association of visceral fat obesity, sarcopenia, and myosteatosis with non-alcoholic fatty liver disease without obesity. Clin Mol Hepatol. 2023 Jul 5; doi: 10.3350/cmh.2023.0035. [Epub].https://doi.org/10.3350/cmh.2023.0035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sinn DH, Kang D, Kang M, Guallar E, Hong YS, Lee KH, et al. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: a longitudinal cohort study. Hepatology. 2022;76:1746–54. doi: 10.1002/hep.32578. [DOI] [PubMed] [Google Scholar]
- 106.Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–31. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 107.Harring M, Golabi P, Paik JM, Shah D, Racila A, Cable R, et al. Sarcopenia among patients with nonalcoholic fatty liver disease (NAFLD) is associated with advanced fibrosis. Clin Gastroenterol Hepatol. 2023 Feb 26; doi: 10.1016/j.cgh.2023.02.013. [Epub].https://doi.org/10.1016/j.cgh.2023.02.013 . [DOI] [PubMed] [Google Scholar]
- 108.Kim D, Wijarnpreecha K, Sandhu KK, Cholankeril G, Ahmed A. Sarcopenia in nonalcoholic fatty liver disease and all-cause and cause-specific mortality in the United States. Liver Int. 2021;41:1832–40. doi: 10.1111/liv.14852. [DOI] [PubMed] [Google Scholar]
- 109.Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75(Suppl 1):S147–62. doi: 10.1016/j.jhep.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22:1148–61. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 112.Zhao X, Shi X, Gu H, Zhou W, Zhang Q. Association between handgrip strength, nonalcoholic fatty liver disease, advanced hepatic fibrosis and its modifiers: evidence from the NHANES database of the USA. J Gastroenterol Hepatol. 2023 Feb 18; doi: 10.1111/jgh.16150. [Epub]. https://doi.org/10.1111/jgh.16150 . [DOI] [PubMed] [Google Scholar]
- 113.Mertz KH, Reitelseder S, Bechshoeft R, Bulow J, Højfeldt G, Jensen M, et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: a randomized controlled trial. Am J Clin Nutr. 2021;113:790–800. doi: 10.1093/ajcn/nqaa372. [DOI] [PubMed] [Google Scholar]
- 114.Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. 2020;39:3533–62. doi: 10.1016/j.clnu.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Papadopoulou SK, Detopoulou P, Voulgaridou G, Tsoumana D, Spanoudaki M, Sadikou F, et al. Mediterranean diet and sarcopenia features in apparently healthy adults over 65 years: a systematic review. Nutrients. 2023;15:1104. doi: 10.3390/nu15051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sindhughosa DA, Wibawa ID, Mariadi IK, Somayana G. Additional treatment of vitamin D for improvement of insulin resistance in non-alcoholic fatty liver disease patients: a systematic review and meta-analysis. Sci Rep. 2022;12:7716. doi: 10.1038/s41598-022-11950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nasimi N, Sohrabi Z, Nunes EA, Sadeghi E, Jamshidi S, Gholami Z, et al. Whey protein supplementation with or without vitamin D on sarcopenia-related measures: a systematic review and meta-analysis. Adv Nutr. 2023;14:762–73. doi: 10.1016/j.advnut.2023.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gkekas NK, Anagnostis P, Paraschou V, Stamiris D, Dellis S, Kenanidis E, et al. The effect of vitamin D plus protein supplementation on sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Maturitas. 2021;145:56–63. doi: 10.1016/j.maturitas.2021.01.002. [DOI] [PubMed] [Google Scholar]