Abstract
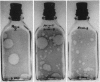
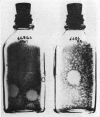
Comparison of the agar overlay technique with the fluid culture technique for isolation and identification of enteroviruses showed the former to be useful: (i) for isolation of enterovirus when the number of virus particles was too small to produce detectable cytopathic effect (CPE) in fluid cultures, (ii) for isolation of echovirus 22 which did not produce detectable CPE in fluid cultures, (iii) as an aid to rapid differentiation of enteroviruses, and (iv) for differentiation of viruses in mixed infections. Nonpolio enterovirus isolation experience in the New Haven area over a 4-year period is presented. It was concluded that the agar overlay technique is both useful and relatively simple for routine examination of clinical specimens in a diagnostic laboratory.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRON A. L., KARZON D. T. Characteristics of ECHO 4 (Shropshire) virus isolated during epidemic of aseptic meningitis. J Immunol. 1961 Nov;87:608–615. [PubMed] [Google Scholar]
- COOPER P. D. The plaque assay of animal viruses. Adv Virus Res. 1961;8:319–378. doi: 10.1016/s0065-3527(08)60689-2. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Production of Plaques in Monolayer Tissue Cultures by Single Particles of an Animal Virus. Proc Natl Acad Sci U S A. 1952 Aug;38(8):747–752. doi: 10.1073/pnas.38.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN R. H., OPTON E. M. Photosensitization of tissue culture cells and its effect on viral plaque formation. Proc Soc Exp Biol Med. 1959 Nov;102:519–521. doi: 10.3181/00379727-102-25303. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. R., TAYLOR R. M. Arthropod-borne virus plaques in agar overlaid tube cultures. Proc Soc Exp Biol Med. 1959 Jun;101(2):257–259. doi: 10.3181/00379727-101-24902. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D. Further studies on characterization and grouping of ECHO viruses. Ann N Y Acad Sci. 1962 Nov 30;101:413–422. doi: 10.1111/j.1749-6632.1962.tb18880.x. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D. The use of agar overlay cultures for detection of new virus isolates. Virology. 1959 Dec;9:717–719. doi: 10.1016/0042-6822(59)90167-9. [DOI] [PubMed] [Google Scholar]
- KARZON D. T., POLLOCK B. F., BARRON A. L. Phase variation in ECHO virus type 6. Virology. 1959 Dec;9:564–576. doi: 10.1016/0042-6822(59)90149-7. [DOI] [PubMed] [Google Scholar]
- PORTERFIELD J. S. Plaque production with yellow fever and related arthropodborne viruses. Nature. 1959 Apr 11;183(4667):1069–1070. doi: 10.1038/1831069b0. [DOI] [PubMed] [Google Scholar]
- SANYAL S. K., MAHDAVY M., GABRIELSON M. O., VIDONE R. A., BROWNE M. J. FATAL MYOCARDITIS IN AN ADOLESCENT CAUSED BY COXSACKIE VIRUS, GROUP B, TYPE FOUR. Pediatrics. 1965 Jan;35:36–41. [PubMed] [Google Scholar]
- WIGAND R., SABIN A. B. Properties of ECHO types 22, 23 and 24 viruses. Arch Gesamte Virusforsch. 1961;11:224–247. doi: 10.1007/BF01241688. [DOI] [PubMed] [Google Scholar]