ABSTRACT
Background and Objectives:
We aimed to investigate whether the lumbar paraspinal muscle/fat ratio influences the outcomes of patients who had simple decompressive surgeries for lumbar disc herniation (LDH) or lumbar spinal stenosis. We also wanted to see if the spinopelvic parameters change with surgery and whether this change influences the outcomes.
Materials and Methods:
This was a prospective study on patients with lumbar spinal stenosis (20 patients) and LDH (20 patients) who underwent simple discectomy or decompressive surgery between November 2021 and May 2022. Visual Analog Scale (VAS) for back and leg pain, Oswestry Disability Index, and Japanese Orthopedic Association (JOA) score were performed before and 3 months after surgery. Spinopelvic parameters were measured on whole spine radiographs before and 3 months after surgery. On axial magnetic resonance images, paraspinal muscle volume and muscle/fat ratios were calculated. All data were statistically analyzed with SPSS program.
Results:
There was a significant improvement in VAS, Oswestry, and JOA scores after surgery. We observed that more preoperative paraspinal muscle mass was positively correlated with lumbar lordosis (LL) and negatively correlated with sagittal vertical axis (SVA), VAS leg scores, and Oswestry scores. Furthermore, we observed a positive correlation between preoperative SVA and VAS leg scores.
Conclusion:
Despite limited number of patients, and shorter follow-ups, this prospective study demonstrates a correlation among the lumbar paraspinal muscle/fat ratio, preoperative/postoperative spinopelvic parameters, and surgical outcomes. Increased paraspinal muscle ratio was correlated with lower SVA values and increased LL; lower VAS leg scores; higher Oswestry scores which reflects better surgical outcomes.
Keywords: Lumbar disc herniation, lumbar stenosis, paraspinal muscle atrophy, sagittal balance, Visual Analog Scale
INTRODUCTION
Low back pain (LBP) is a common condition and 65%–85% of general population experience LBP once in their life.[1] Lumbar disc herniation (LDH) is one of the most common causes of LBP and leads to leg pain (sciatic) along with LBP.[2] Lumbar spinal canal stenosis (LSS) is another cause of LBP which frequently seen among elderly. Its main manifestation is leg pain, neurogenic claudication.Bending forward widens the spinal canal and can alleviate symptoms which may cause sagittal imbalance.[3] Sagittal spinal balance has been defined as the presence of lumbar lordosis (LL) and thoracic kyphosis (TK) in equilibrium. Restoring the sagittal spinal balance has been associated with pain relief after spine surgery in several diseases.[4] In addition, paraspinal muscle atrophy and fatty infiltration of paraspinal muscles may also correlate with LBP.[5] Erector spinae (ES) and lumbar multifidus (LM) are two important muscles of lower back which have a crucial role in maintaining spinal stability.[6] Therefore, evaluating these muscle groups is vital to understand the effect of paraspinal muscles on LBP. Magnetic resonance imaging (MRI) is commonly used in the assessment of the lumbar spinal anatomy and pathology and provides quantitative and qualitative measurements such as fatty infiltration, muscle size (cross sectional area or volume), and muscle/fat ratio.[1]
The aim of our prospective clinical study is to investigate whether the lumbar paraspinal muscle/fat ratio influences the outcomes of patients who had simple decompressive surgeries for LDH or lumbar spinal stenosis. We also wanted to see if the spinopelvic parameters change with surgery and whether this change influences the outcomes. In order to obtain more precise results, measurements of lumbar paraspinal muscle were performed using the three-dimensional (3D) slicer program on lumbar MRI images.[7]
Our hypotheses for this study were as follows: (a) patients with lumbar spinal stenosis are expected to have worse spinopelvic parameters than patients with LDH, (b) patients with lumbar spinal stenosis are expected to have worse paravertebral muscle mass and more atrophic muscles than patients with LDH, (c) the preoperative spinopelvic parameters are expected to be abnormal in preoperative period and improve postoperatively, and (d) patients with less lumbar paravertebral muscle mass and more fat are expected to have a worse outcome.
MATERIALS AND METHODS
Study design
We designed a prospective study on patients with lumbar spinal stenosis and LDH who underwent simple discectomy or decompressive surgery between November 2021 and May 2022. Patients were from Ege University Hospital, Gazi Hospital Izmir, and Memorial Hospital, Istanbul, Turkey. The local ethics committee approved the study protocol (15.10.2021/E-99166796-050.06.04-359159).
The patients were divided into two groups: LDH (Group 1 – 23 patients) and lumbar spinal stenosis (Group 2 – 22 patients). Five patients were lost to follow-up and excluded from the study. The remaining 40 patients (Group 1 – 20 patient and Group 2 – 20 patient) were analyzed.
Thirty-two of 40 patients were operated on in Ege University Neurosurgery Clinic, and eight of them were operated in other clinics. The operations were performed by a total of 11 different surgeons.
Inclusion criteria
Patients older than 18 years who require only decompressive surgery due to LDH or stenosis.
Exclusion criteria
(1) Patients under the age of 18, (2) patients who had previous low back surgery, and (3) patients with significant instability and requiring fusion surgery.
One of the patients with LDH was reoperated 2.5 months later due to the development of a far lateral disc herniation at the same level.
Clinical evaluation
Apart from routine neurological evaluation, the following tests were performed in the preoperative period and 3 months after surgery: (1) Visual Analog Scale (VAS): Back and leg pain, (2) the Oswestry Disability Index, and (3) Japanese Orthopedic Association (JOA) score. Data on age, gender, comorbidity, gait disturbance (kyphotic/scoliotic gait), duration of surgery, amount of bleeding during surgery, intraoperative complications (dura laceration/bleeding over 500 cc etc.), and length of hospital stay were collected.
Radiologic evaluation
Whole spine radiograms and spinopelvic parameters
Preoperative standing full spine radiographs (including C2 and femoral head) were taken and the radiographs were repeated at the postoperative 3rd month. Patients were asked to stand in a standardized erect posture with the hands placed on his/her chest.
TK, LL, sagittal vertical axis (SVA), T1-pelvic angle (TPA), sacral slope (SS), pelvic tilt (PT), and pelvic incidence (PI) were measured using “Surgimap software” (Nemaris Inc., USA) [Figure 1].
Figure 1.
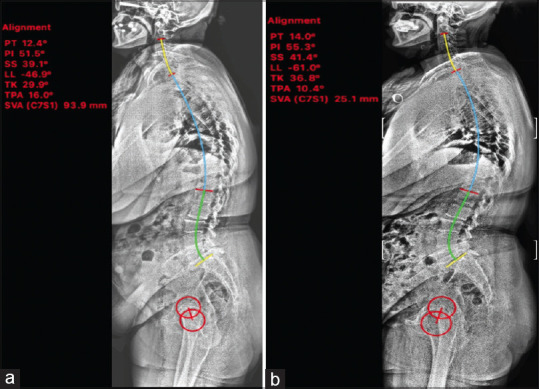
Preoperative and postoperative spinopelvic parameters of one patient measured via Surgimap program. whole spine radiograph before surgery (a) and after surgery (b), red circles represent femoral heads. There was an increase in lumbar lordosis, increase in thoracic kyphosis, and decrease in sagittal vertical axis values. PT - Pelvic tilt, PI - Pelvic incidence, SS - Sacral slope, LL - Lumbar lordosis, TK - Thoracic kyphosis, TPA - T1-pelvic angle, SVA - Sagittal vertical axis
Lumbar magnetic resonance imaging muscle/fat measurements
On axial T2-weighted lumbar magnetic resonance (MR) images, total cross-sectional area (TCSA) was measured in the LM and ES muscles using RadiAnt DICOM viewer (Medixant, PL-Poznan). Measurements were done at L1-2, L2-3, L3-4, L4-5, and L5-S1 level from the right and left sides. LM and ES were measured as total value without muscle/fat distinction [Figure 2].
Figure 2.
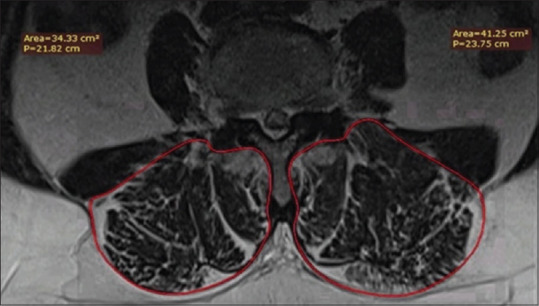
Total cross-sectional area measurements on axial magnetic resonance images
Muscle and fat segmentation were performed using a 3D slicer program.[7] Muscle and fat ratios were measured at L1-S1 levels in total and L2-3; L3-4; and L4-5 separately [Figure 3].
Figure 3.
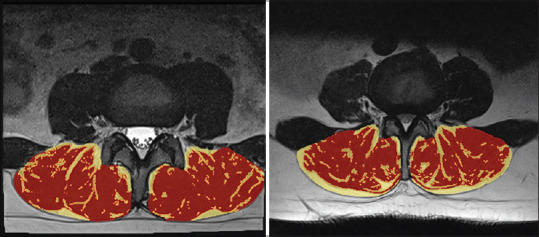
Slicer three-dimensional muscle and fat measurement on axial magnetic resonance image of two different cases. Red areas represent muscle tissue, while yellow areas represent fat tissue.
Statistical analysis
Statistical analyzes were performed using the SPSS software (version 25.0; SPSS IBM; Armonk, NY, USA).[8] Chi-squared test, independent sample t-test, paired sample t-test, and correlation analysis were the tests used.
In Group 1, correlation tests were performed between total lumbar paraspinal muscle ratio, muscle ratio at L4-5-disc level, postoperative LL, and postoperative SVA.
In Group 2, correlation tests were performed between total lumbar paraspinal muscle ratio, postoperative SVA, and VAS leg change.
In Group 1 and 2, correlation tests were performed between lumbar paraspinal muscle ratio, muscle ratio at L4-5-disc level, preoperative and postoperative LL, preoperative and postoperative SVA, preoperative TK, TCSA, preoperative VAS leg scale, preoperative Oswestry scale, and change in the Oswestry scale. P ≤ 0.05 was considered statistically significant.
RESULTS
Table 1 shows demographic data of both the groups. There were more males in the LDH group and more females in the lumbar spinal stenosis group. The mean age of the LDH group was lower than the lumbar stenosis group.
Table 1.
Demographic data and clinical features of patients in Group 1 (lumbar disc herniation) and Group 2 (lumbar spinal stenosis)
| Group 1 | Group 2 | P | |
|---|---|---|---|
| Male/female | 12/8 | 4/16 | 0.024 |
| Age | 48.4±13.36 | 62.5±10.16 | 0.001 |
| Duration of surgery | 88.5±48.39 | 143.5±62.3 | 0.003 |
| Level of surgery, n (%) | |||
| Single | 17 | 9 | 0.02 |
| 2 levels | 3 | 10 | 0.043 |
| 3 levels | 0 | 1 | 0.3 |
| Bleeding volume | 87.75±80.71 | 186.5±155.64 | 0.01 |
| Hospitalization | 1.2±0.61 | 1.5±1.05 | 0.278 |
| Complication, n (%) | |||
| Dura laceration | 1 (5) | 6 (30) | 0.037 |
| Excessive bleeding | 0 | 2 (10) | 0.487 |
| Other | 0 | 1 (5) | 1 |
| Preoperative VAS leg | 7.1±2.59 | 6.7±2.63 | 0.631 |
| Preoperative VAS back | 5.4±2.39 | 5.7±2.55 | 0.704 |
| Oswestry score | 63.10±21.9 | 56.9±20.67 | 0.363 |
| JOA score | 10.8±5.26 | 13.15±4.43 | 0.135 |
| Kyphotic/scoliotic gate, n (%) | 11 (55) | 4 (20) | 0.05 |
VAS - Visual Analog Scale; JOA - Japanese Orthopedic Association
The mean duration of surgery was longer in patients with lumbar spinal stenosis. Besides, decompressive laminectomy was associated with more bleeding, while there was no case with bleeding 500 cc or more in LDH group, there were two patients with 500 cc or more bleeding in lumbar spinal stenosis group.
Dural laceration was more common in patients with lumbar stenosis, depending on age, dural adhesions, and fragility of the dura.
We observed higher VAS leg scores in LDH group. Although VAS back scores were higher in lumbar spinal stenosis group, Oswestry scores were lower and JOA scores were higher.
While gait disturbances such as kyphosis and scoliosis observed in more than half of the patients with LDH, this rate was limited to 20% in patients with lumbar spinal stenosis.
Table 2 gives muscle/fat and TCSA ratios of both the groups. There was no significant difference in the muscle/fat ratio and in TCSA measurements. There was a decrease in muscle ratio caudal to the L2-3-disc level. The average muscle ratio at L3-4 level (75.56) was like the average of total muscle ratio (74.12).
Table 2.
Muscle/fat and total cross-sectional area ratios in Group 1 (lumbar disc herniation) and Group 2 (lumbar spinal stenosis) patients
| Group 1 | Group 2 | P | |
|---|---|---|---|
| L2-3 muscle ratio | 79.41±10.3 | 78.98±5.51 | 0.869 |
| L2-3 fat ratio | 20.58±10.3 | 21.01±5.51 | 0.870 |
| L3-4 muscle ratio | 75.56±9.39 | 74.01±7.5 | 0.567 |
| L3-4 fat ratio | 24.43±9.39 | 25.98±7.53 | 0.566 |
| L4-5 muscle ratio | 70.94±8.71 | 69.17±7.92 | 0.506 |
| L4-5 fat ratio | 29.05±8.76 | 30.82±7.92 | 0.507 |
| Total muscle ratio | 74.12±8.56 | 72.62±5.99 | 0.525 |
| Total fat ratio | 25.87±8.56 | 27.36±5.99 | 0.528 |
| TCSA L1-2 right | 22.22±5.02 | 22.32±5.59 | 0.957 |
| TCSA L1-2 left | 23.31±5.21 | 21.5±4.84 | 0.277 |
| TCSA L2-3 right | 24.44±5.32 | 23.54±4.65 | 0.572 |
| TCSA L2-3 left | 25.62±5.21 | 23.77±4.85 | 0.252 |
| TCSA L3-4 right | 25.96±5.36 | 24.94±4.46 | 0.519 |
| TCSA L3-4 left | 27.02±5.41 | 25.33±4.6 | 0.293 |
| TCSA L4-5 right | 26.17±6.17 | 25.26±5.01 | 0.614 |
| TCSA L4-5 left | 26.44±6.12 | 25.47±4.99 | 0.587 |
| TCSA L5-S1 right | 20.27±4.52 | 20.27±5.81 | 1 |
| TCSA L5-S1 left | 20.22±5.34 | 20.36±5.74 | 0.937 |
| TCSA L1-S1 right | 117.98±24.32 | 115.24±22.25 | 0.713 |
| TCSA L1-S1 left | 121.47±24.56 | 115.38±22.58 | 0.420 |
| TCSA L1-S1 total | 239.45±48.71 | 230.63±44.35 | 0.553 |
| Number of MRI slice | 22.65±4.55 | 24.5±4.66 | 0.212 |
MRI - Magnetic resonance imaging; TCSA - Total cross sectional area
Table 3 shows preoperative and postoperative VAS, Oswestry, and JOA scores of patients in Group 1 and 2. There was a significant improvement in all scores after surgery. We observed that improvement in VAS, Oswestry, and JOA scores was greater in LDH group as compared to lumbar spinal stenosis group.
Table 3.
Pre- and postoperative Visual Analog Scale, Oswestry Disability Index, and Japanese Orthopedic Association scores in Group 1 (lumbar disc herniation) and Group 2 (lumbar spinal stenosis) patients
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Preoperative | Postoperative | P | Preoperative | Postoperative | P | |
| VAS leg | 7.1±2.59 | 0.45±1.27 | <0.001 | 6.7±2.63 | 2.15±2.13 | 0.001 |
| VAS back | 5.44±2.39 | 1.60±1.72 | 0.001 | 5.7±2.55 | 3.15±2.6 | <0.001 |
| Oswestry score | 63.1±21.9 | 8.75±8.13 | <0.001 | 56.9±20.67 | 19.15±18.23 | <0.001 |
| JOA score | 10.80±5.26 | 24.4±3.05 | <0.001 | 13.15±4.43 | 22±3.89 | 0.002 |
VAS - Visual Analog Scale; JOA - Japanese Orthopedic Association
Sagittal parameters of patients in both the groups are in Table 4. TK, TPA, LL, PT, and PI increased, while SS and SVA decreased in patients with LDH after surgery. However, these changes are not statistically significant. TK, LL, and SS increased and PT, PI, SVA, and TPA decreased in patients with lumbar spinal stenosis after surgery. Only the increase in the TK was statistically significant [Figures 4 and 5].
Table 4.
Pre- and postoperative sagittal parameters in Group 1 (lumbar disc herniation) and Group 2 (lumbar spinal stenosis) patients
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Preoperative | Postoperative | P | Preoperative | Postoperative | P | |
| TK | 28.72±7.04 | 29.19±8.008 | 0.76 | 26.88±10.31 | 31.46±9.31 | <0.001 |
| TPA | 11.04±13.84 | 11.53±12.4 | 0.72 | 13.98±8.19 | 13.25±7.69 | 0.349 |
| LL | (−) 46.03±14.91 | (−) 46.86±16.2 | 0.642 | (−) 47.55±12.88 | (−) 50.57±11.52 | 0.058 |
| SS | 31.71±11.04 | 31.14±12.82 | 0.599 | 33.55±10.79 | 33.89±9.48 | 0.372 |
| PV | 15.32±13.29 | 16.87±12.6 | 0.225 | 18.78±7.9 | 18.09±7.38 | 0.371 |
| PI | 47.02±10.93 | 48.01±10.75 | 0.464 | 52.32±14.46 | 51.92±12.79 | 0.689 |
| SVA | 10.06±44.22 | 4.84±34.6 | 0.458 | 10.34±35.55 | 9.75±33.98 | 0.941 |
SVA - Sagittal vertical axis; TPA - T1-pelvic angle; LL - Lumbar lordosis; TK - Thoracic kyphosis; SS - Sacral slope; PV - Pelvic tilt; PI - Pelvic incidence
Figure 4.
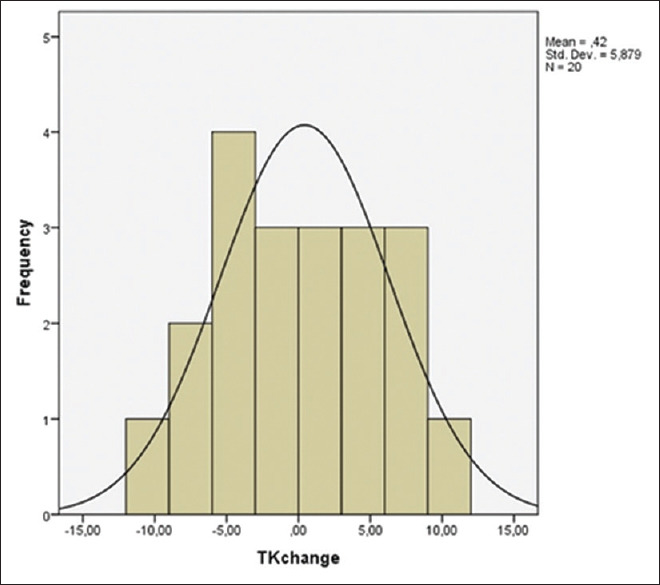
Group 1 (lumbar disc herniation) thoracic kyphosis change histogram. Frequency indicates number of patients, mean change is 0.42, and the amount of change varies between − 10 and 10. TK - Thoracic kyphosis
Figure 5.
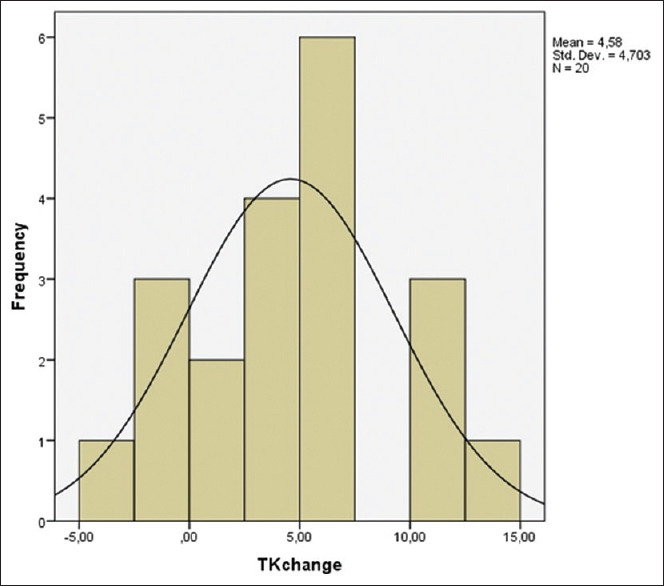
Thoracic kyphosis change histogram in Group 2 (lumbar spinal stenosis). Frequency indicates number of patients, mean change is 4.58, and the amount of change varies between − 5 and 15. TK - Thoracic kyphosis
Change rates in sagittal parameters are shown in Table 5. There was further improvement in TK-LL in patients with lumbar stenosis; on the other hand, SVA improved more in patients with LDH.
Table 5.
Changes of sagittal parameters with surgery in Group 1 (lumbar disc herniation) and Group 2 (lumbar spinal stenosis)
| Group 1 | Group 2 | P | |
|---|---|---|---|
| TK change (%) | 0.42±5.87 | 4.58±4.7 | 0.018 |
| LL change (%) | 2.04±7.56 | 3.01±6.67 | 0.670 |
| SVA change (%) | 6.55±30.48 | 4.07±34.86 | 0.812 |
SVA - Sagittal vertical axis; LL - Lumbar lordosis; TK - Thoracic kyphosis
We also looked Pearson’s correlations of some parameters. Table 6 shows correlation test between lumbar paraspinal muscle ratio, postoperative sagittal parameters, and change in LL in Group 1. We observed that in patients with LDH, as the muscle ratio increased, postoperative LL increased (P = 0.007) and SVA decreased (P = 0.002). It was determined that when the muscle ratio at L4-5 level increased, the LL improved more after surgery (P = 0.017). These correlations were statistically significant.
Table 6.
Correlation between lumbar paraspinal muscle ratio, postoperative sagittal parameters, and change in lumbar lordosis in Group 1 (lumbar disc herniation) patients
| Correlations | |||||
|---|---|---|---|---|---|
|
| |||||
| LL change | L4.5 muscle | Muscle percentile | Postoperative LL | Postoperative SVA | |
| LL change | |||||
| Pearson’s correlation | 1 | −0.528* | −0.341 | 0.225 | 0.125 |
| Significant (two-tailed) | 0.017 | 0.141 | 0.341 | 0.600 | |
| n | 20 | 20 | 20 | 20 | 20 |
| L4.5 muscle | |||||
| Pearson’s correlation | −0.528* | 1 | 0.911** | −0.489* | −0.475* |
| Significant (two-tailed) | 0.017 | 0.000 | 0.029 | 0.034 | |
| n | 20 | 20 | 20 | 20 | 20 |
| Muscle percentile | |||||
| Pearson’s correlation | −0.341 | 0.911** | 1 | −0.584** | −0.642** |
| Significant (two-tailed) | 0.141 | 0.000 | 0.007 | 0.002 | |
| n | 20 | 20 | 20 | 20 | 20 |
| Postoperative LL | |||||
| Pearson’s correlation | 0.225 | −0.489* | −0.584** | 1 | 0.555* |
| Significant (two-tailed) | 0.341 | 0.029 | 0.007 | 0.011 | |
| n | 20 | 20 | 20 | 20 | 20 |
| Postoperative SVA | |||||
| Pearson’s correlation | 0.125 | −0.475* | −0.642** | 0.555* | 1 |
| Significant (two-tailed) | 0.600 | 0.034 | 0.002 | 0.011 | |
| n | 20 | 20 | 20 | 20 | 20 |
*Correlation is significant at the 0.05 level (two-tailed); **Correlation is significant at the 0.01 level (two-tailed). SVA - Sagittal vertical axis; LL - Lumbar lordosis
Similarly, we looked Pearson’s correlations between total paraspinal muscle ratio, postoperative SVA, and change in VAS leg in Group 2 [Table 7]. We found that in patients with lumbar spinal stenosis, as the muscle ratio increased, postoperative SVA decreased (P = 0.041) and VAS leg scores improved more (P = 0.027). These correlations were also statistically significant.
Table 7.
Correlation between total paraspinal muscle ratio, postoperative sagittal vertical axis, and Visual Analog Scale for leg in Group 2 (lumbar spinal stenosis) patients
| Correlations | |||
|---|---|---|---|
|
| |||
| Muscle percentile | Postoperative SVA | VAS leg change | |
| Muscle percentile | |||
| Pearson’s correlation | 1 | −0.460* | −0.494* |
| Significant (two-tailed) | 0.041 | 0.027 | |
| n | 20 | 20 | 20 |
| Postoperative SVA | |||
| Pearson’s correlation | −0.460* | 1 | 0.127 |
| Significant (two-tailed) | 0.041 | 0.592 | |
| n | 20 | 20 | 20 |
| VAS leg change | |||
| Pearson’s correlation | −0.494* | 0.127 | 1 |
| Significant (two-tailed) | 0.027 | 0.592 | |
| n | 20 | 20 | 20 |
*Correlation is significant at the 0.05 level (two-tailed). SVA - Sagittal vertical axis; VAS - Visual Analog Scale
Table 8 examines the Pearson’s correlations between paraspinal muscle ratio, preoperative and postoperative sagittal parameters, TCSA, preoperative and postoperative pain scales, and change in Oswestry Disability Index of patients in Group 1 and 2. When the groups were examined together, we observed that as the muscle ratio increased, preoperative and postoperative LL increased (P = 0.002; P = 0.001), preoperative and postoperative SVA decreased (P < 0.001; P < 0.001); As the muscle ratio increased, preoperative VAS leg scores decreased (P = 0.04), as L4-5 muscle ratio increased, preoperative Oswestry scores decreased (P = 0.044), preoperative VAS leg scores decreased (P = 0.011); As preoperative LL increased, preoperative Oswestry scores decreased (P = 0.041) and the change in Oswestry score increased (P = 0.016); As the SVA value increased, the preoperative VAS leg scores also increased (P = 0.045). It was determined that as the TCSA increased, the preoperative TK also increased (P = 0.025). These correlations were statistically significant.
Table 8.
Correlation between paraspinal muscle ratio, preoperative and postoperative sagittal parameters, total cross-sectional area, pre- and post-operative pain scales, Oswestry Disability Index in Group 1 (lumbar disc herniation) and Group 2 (lumbar spinal stenosis) patients
| Muscle percentile | L4.5 Muscle | Postop LL | Postop SVA | Preop Oswestry | Preop LL | Preop SVA | TCSA Total | Preop TK | Preop VAS Leg | Oswestry Change | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle percentile | |||||||||||
| Pearson Correlation | 1 | 0.902** | -0.487** | -0.563** | -0.197 | -0.478** | -0.543** | 0.028 | -0.170 | -0.326* | -0.098 |
| Sig. (2-tailed) | 40 | 0.000 | 0.001 | 0.000 | 0.223 | 0.002 | 0.000 | 0.865 | 0.294 | 0.40 | 0.548 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| L4.5 Muscle | |||||||||||
| Pearson Correlation | 0.902** | 1 | -0.392* | -0.374* | -0.320* | 0.483** | -0.506** | -0.30 | -0.149 | -0.397* | -0.139 |
| Sig. (2-tailed) | 0.000 | 40 | 0.12 | 0.18 | 0.44 | 0.002 | 0.001 | 0.856 | 0.359 | 0.11 | 0.393 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Postop LL | |||||||||||
| Pearson Correlation | -0.487** | -0.392* | 1 | 0.367* | 0.297 | 0.864** | 0.261 | -0.291 | 0.033 | 0.250 | 0.323* |
| Sig. (2-tailed) | 0.001 | 0.12 | 40 | 0.020 | 0.063 | 0.000 | 0.104 | 0.069 | 0.838 | 0.119 | 0.042 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Postop SVA | |||||||||||
| Pearson Correlation | -0.563** | -0.374* | 0.367* | 1 | 0.080 | 0.206 | 0.615** | -0.095 | 0.174 | 0.163 | 0.000 |
| Sig. (2-tailed) | 0.000 | 0.18 | 0.020 | 40 | 0.623 | 0.201 | 0.000 | 0.560 | 0.284 | 0.315 | 1.000 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Preop Oswestry | |||||||||||
| Pearson Correlation | -0.197 | -0.320* | 0.297 | 0.080 | 1 | 0.324* | 0.056 | -0.039 | 0.090 | 0.727** | 0.754** |
| Sig. (2-tailed) | 0.223 | 0.44 | 0.063 | 0.623 | 40 | 0.041 | 0.733 | 0.812 | 0.579 | 0.000 | 0.000 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Preop LL | |||||||||||
| Pearson Correlation | -0.478** | -0.483** | 0.864** | 0.206 | 0.324* | 1 | 0.361* | -0.187 | -0.196 | 0.288 | 0.378* |
| Sig. (2-tailed) | 0.002 | 0.002 | 0.000 | 0.201 | 0.041 | 40 | 0.022 | 0.249 | 0.226 | 0.071 | 0.016 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Preop SVA | |||||||||||
| Pearson Correlation | -0.543** | -0.506** | 0.261 | 0.615** | 0.056 | 0.361* | 1 | 0.097 | -0.089 | 0.319* | 0.020 |
| Sig. (2-tailed) | 0.000 | 0.001 | 0.104 | 0.000 | 0.733 | 0.022 | 40 | 0.550 | 0.587 | 0.045 | 0.904 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| TCSA Total | |||||||||||
| Pearson Correlation | 0.028 | -0.030 | -0.291 | -0.95 | -0.039 | -0.187 | 0.097 | 1 | 0.353* | -0.100 | -0.046 |
| Sig. (2-tailed) | 0.865 | 0.856 | 0.069 | 0.560 | 0.812 | 0.249 | 0.550 | 40 | 0.025 | 0.539 | 0.776 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Preop TK | |||||||||||
| Pearson Correlation | -0.170 | -0.149 | 0.033 | 0.174 | 0.090 | -0.196 | -0.089 | 0.353* | 1 | -0.036 | -0.057 |
| Sig. (2-tailed) | 0.294 | 0.359 | 0.838 | 0.284 | 0.579 | 0.226 | 0.587 | 0.025 | 40 | 0.827 | 0.726 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Preop VAS Leg | |||||||||||
| Pearson Correlation | -0.326* | -0.397* | 0.250 | 0.163 | 0.727** | 0.288 | 0.319* | -0.100 | -0.036 | 1 | 0.510** |
| Sig. (2-tailed) | 0.40 | 0.11 | 0.119 | 0.315 | 0.000 | 0.071 | 0.045 | 0.539 | 0.827 | 40 | 0.001 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Oswestry Change | |||||||||||
| Pearson Correlation | -0.098 | -0.139 | 0.323* | 0.000 | 0.754** | 0.378* | 0.020 | -0.046 | -0.057 | 0.510** | 1 |
| Sig. (2-tailed) | 0.548 | 0.393 | 0.042 | 1.000 | 0.000 | 0.016 | 0.904 | 0.776 | 0.726 | 0.001 | 40 |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
DISCUSSION
With advancing age, several processes occur in the spine due to degeneration such as decrease in disc height, hypertrophy in facet joints and ligamentum flavum, reconstruction of bone structures, and atrophy in muscles.[9,10] Paraspinal muscles play an important role in maintaining spinal alignment. Forward displacement of sagittal balance can happen due to degeneration of the spine, atrophy of the paraspinal muscles, decreased LL, and increased PT.[11] Compensatory mechanisms come into play to correct the deteriorated sagittal balance due to degeneration such as pelvic retroversion, knee flexion, ankle extension, spine hyperextension, and retrolisthesis. While these mechanisms support the body to stand upright, they can also cause changes in spinopelvic parameters.[11,12] Various studies demonstrate that spinal sagittal imbalance and lumbar paraspinal intramuscular fat infiltration are associated with LBP in adults.[4,13] In our study, we aimed to analyze whether there is a correlation between spinal sagittal imbalance, fatty infiltration of lumbar paraspinal muscles, and LBP.
In the last few decades, our knowledge on the importance of the sagittal balance and associated pathologies has increased. Schwab et al. demonstrated that realignment of SVA values <50 mm is associated with better quality of life in patients with adult spinal deformity.[14] Dohzono et al. examined patients with lumbar spinal stenosis, which they performed laminotomy and found that patients who h a d preoperative forward bending posture had less LL and lower preoperative JOA score as compared to patients without forward bending posture; however, they also found that improvement in JOA scores and postoperative VAS leg scores did not differ between the groups.[15] Another study involving LSS patients also demonstrated correlation between SVA and JOA scores.[3] Endo et al. also showed correlation between SVA and JOA scores in patients with LDH.[16] In our study, we did not demonstrate a statistically significant correlation between SVA and JOA scores; however, we found that patients who had preoperative higher SVA values also had greater VAS leg scores (P = 0.045). In addition, as preoperative LL increased, preoperative Oswestry scores decreased (P = 0.041) and the change in Oswestry scores increased (P = 0.016).
Considering the relationship of lumbar paraspinal muscles with sagittal alignment, measuring the muscle/fat ratio has paramount importance. To evaluate the fatty infiltration in the muscles, qualitative and quantitative methods have been described. Goutallier introduced the first qualitative classification in 1994, and in this computed tomography (CT)-based study, the fat infiltration rates of the rotator cuff muscle were evaluated and classified between 0 and 4: Grade 0, normal muscle; Grade 1, linear adiposity; Grade 2, muscle > fat; Grade 3, muscle = fat; and Grade 4, fat > muscle.[17] In 2000, Kader et al. evaluated fat infiltration in an MRI-based study, in which they evaluated the LM muscle, divided into three groups as <10, <50, and >50 fat infiltration.[18] In 2012, Slabaugh et al.[19] assessed the intraobserver and interobserver reliability of the Goutallier classification as moderate in a study, in which the rotator cuff muscles were evaluated in MRI. Slabaugh et al. proposed a simpler classification which is reduced into 3 grades: Grade 0, normal to mild fatty infiltration; Grade 1: moderate fatty infiltration; and Grade 2: fat > muscle. They suggested that this simpler version has significantly higher intraobserver and interobserver reliability.[19] On the contrary, Battaglia et al. stated that the reliability of the Goutallier classification was high in their study which they evaluated the LM muscle on MRI.[20] In our study, the 3D slicer program was used to evaluate muscle and fat ratio of ES and LM collectively. Although it is time-consuming, this method was preferred to measure the muscle/fat ratio numerically and to obtain more objective measurements.
In addition, there are various quantitative methods such as: measurement of TCSA, functional cross-sectional area (FCSA)using signal intensity in MR and Hounsfield unit in CT;[21] measurement of thickness and echo intensity of paraspinal muscles on ultrasonography.[22] Measuring TCSA is a simple method which may indicate muscle atrophy. However, a review article by Hu et al. has shown that even if there is an increase in the proportion of fat or fibrous tissue in atrophied muscle, TCSA of muscles does not change significantly. They also denote that TCSA is more reliable in assessing fat infiltration, whereas FCSA is in assessing muscle atrophy.[21] In our study, we did not find correlation between TCSA and muscle or fat ratios.
Mengiardi et al. detected considerable amount of fat in LM of patients with chronic LBP.[13] Getzman et al. suggested that fatty infiltration of paraspinal muscles is associated with higher disability and poor health-related quality of life.[23] Zotti et al. demonstrated that less muscle mass of LM was correlated with worse outcome in patients who had decompression surgery for LSS.[24] In line with those studies and our hypothesis, we also found a correlation between increased muscle ratio and patient’s pain. We demonstrated as muscle ratio increased, preoperative VAS leg scores decreased (P = 0.04); as L4-5 muscle ratio increased, preoperative Oswestry scores decreased (P = 0.044), scores and preoperative VAS leg scores decreased (P = 0.011). In addition, we showed an association between increased muscle volume and increased preoperative/postoperative LL (P = 0.002; P = 0.001), decreased preoperative/postoperative SVA value (P < 0.001; P < 0.001).
Our study has several limitations. Follow-up period of 3 months is short for observing the spinopelvic measurements and surgical outcomes of LSS and LDH patients. Small number of patients in each group is one of the limitations. Besides, we did not measure LM and ES muscle separately. We also did not examine interobserver and intraobserver reliability of our radiological measurements.
CONCLUSION
Although this prospective study was conducted with a limited number of patients, we demonstrated a correlation among the lumbar paraspinal muscle/fat ratio, preoperative/postoperative spinopelvic parameters, and surgical outcomes. Our results showed that an increased paraspinal muscle ratio was correlated with lower SVA values and increased LL; lower VAS leg scores; higher Oswestry scores which indicate better surgical outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Berry DB, Padwal J, Johnson S, Parra CL, Ward SR, Shahidi B. Methodological considerations in region of interest definitions for paraspinal muscles in axial MRIs of the lumbar spine. BMC Musculoskelet Disord. 2018;19:135. doi: 10.1186/s12891-018-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter RW. Management of back pain. Crit Q. 1987;29:115–19. [Google Scholar]
- 3.Suzuki H, Endo K, Kobayashi H, Tanaka H, Yamamoto K. Total sagittal spinal alignment in patients with lumbar canal stenosis accompanied by intermittent claudication. Spine (Phila Pa 1976) 2010;35:E344–6. doi: 10.1097/BRS.0b013e3181c91121. [DOI] [PubMed] [Google Scholar]
- 4.Mehta VA, Amin A, Omeis I, Gokaslan ZL, Gottfried ON. Implications of spinopelvic alignment for the spine surgeon. Neurosurgery. 2015;76(Suppl 1):S42–56. doi: 10.1227/01.neu.0000462077.50830.1a. [DOI] [PubMed] [Google Scholar]
- 5.Paalanne N, Niinimäki J, Karppinen J, Taimela S, Mutanen P, Takatalo J, et al. Assessment of association between low back pain and paraspinal muscle atrophy using opposed-phase magnetic resonance imaging:A population-based study among young adults. Spine (Phila Pa 1976) 2011;36:1961–8. doi: 10.1097/BRS.0b013e3181fef890. [DOI] [PubMed] [Google Scholar]
- 6.Standring S. 42nd ed. London: ANZ Elsevier Health Bookshop; 2020. Gray's Anatomy;Hardcover; pp. 955–85. [Google Scholar]
- 7.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30:1323–41. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IBM SPSS Statistics 25. [[Last accessed on 09 Aug 2023]]. [https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-25 .
- 9.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1459–67. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BattiéMC, Videman T, Parent E. Lumbar disc degeneration:Epidemiology and genetic influences. Spine (Phila Pa 1976) 2004;29:2679–90. doi: 10.1097/01.brs.0000146457.83240.eb. [DOI] [PubMed] [Google Scholar]
- 11.Barrey C, Roussouly P, Perrin G, Le Huec JC. Sagittal balance disorders in severe degenerative spine. Can we identify the compensatory mechanisms? Eur Spine J. 2011;20(Suppl 5):626–33. doi: 10.1007/s00586-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Huec JC, Thompson W, Mohsinaly Y, Barrey C, Faundez A. Sagittal balance of the spine. Eur Spine J. 2019;28:1889–905. doi: 10.1007/s00586-019-06083-1. [DOI] [PubMed] [Google Scholar]
- 13.Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers:Quantification with MR spectroscopy. Radiology. 2006;240:786–92. doi: 10.1148/radiol.2403050820. [DOI] [PubMed] [Google Scholar]
- 14.Schwab F, Patel A, Ungar B, Farcy JP, Lafage V. Adult spinal deformity-postoperative standing imbalance:How much can you tolerate?An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010;35:2224–31. doi: 10.1097/BRS.0b013e3181ee6bd4. [DOI] [PubMed] [Google Scholar]
- 15.Dohzono S;HT. The influence of preoperative mental health on clinical outcomes after laminectomy in patients with lumbar spinal stenosis. Clin Neurol Neurosurg. 2019;185:49–54. doi: 10.1016/j.clineuro.2019.105481. [DOI] [PubMed] [Google Scholar]
- 16.Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J. 2010;19:435–8. doi: 10.1007/s00586-009-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 18.Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145–9. doi: 10.1053/crad.1999.0340. [DOI] [PubMed] [Google Scholar]
- 19.Slabaugh MA, Friel NA, Karas V, Romeo AA, Verma NN, Cole BJ. Interobserver and intraobserver reliability of the Goutallier classification using magnetic resonance imaging:Proposal of a simplified classification system to increase reliability. Am J Sports Med. 2012;40:1728–34. doi: 10.1177/0363546512452714. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia PJ, Maeda Y, Welk A, Hough B, Kettner N. Reliability of the Goutallier classification in quantifying muscle fatty degeneration in the lumbar multifidus using magnetic resonance imaging. J Manipulative Physiol Ther. 2014;37:190–7. doi: 10.1016/j.jmpt.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Hu ZJ, He J, Zhao FD, Fang XQ, Zhou LN, Fan SW. An assessment of the intra- and inter-reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine (Phila Pa 1976) 2011;36:E868–74. doi: 10.1097/BRS.0b013e3181ef6b51. [DOI] [PubMed] [Google Scholar]
- 22.Masaki M, Ikezoe T, Fukumoto Y, Minami S, Tsukagoshi R, Sakuma K, et al. Association of sagittal spinal alignment with thickness and echo intensity of lumbar back muscles in middle-aged and elderly women. Arch Gerontol Geriatr. 2015;61:197–201. doi: 10.1016/j.archger.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Getzmann JM, Ashouri H, Burgstaller JM, Valeri F, Winklhofer S, Ulrich NH, et al. The effect of paraspinal fatty muscle infiltration and cumulative lumbar spine degeneration on the outcome of patients with lumbar spinal canal stenosis:Analysis of the lumbar stenosis outcome study (LSOS) data. Spine (Phila Pa 1976) 2023;48:97–106. doi: 10.1097/BRS.0000000000004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zotti MG, Boas FV, Clifton T, Piche M, Yoon WW, Freeman BJ. Does pre-operative magnetic resonance imaging of the lumbar multifidus muscle predict clinical outcomes following lumbar spinal decompression for symptomatic spinal stenosis? Eur Spine J. 2017;26:2589–97. doi: 10.1007/s00586-017-4986-x. [DOI] [PubMed] [Google Scholar]


