Abstract
Steroidal pyridines are a class of compounds that have been the subject of extensive research in recent years due to their potential biological activities. The introduction of a pyridine ring into the steroid skeleton can significantly alter the chemical and biological properties of the compound, making it more potent and/or selective for a particular target. Different synthetic methods have been developed for the preparation of steroidal pyridines. This review provides an overview of the synthesis, biological activities, and future perspectives of steroidal monocyclic dihydropyridines, tetrahydropyridines, and pyridines from 2005 to the present. The different synthetic methods that have been developed for the preparation of these steroids are discussed, as well as the proposed mechanisms and the biological activities that have been reported. Finally, the potential of steroidal monocyclic pyridines for the development of new drugs is discussed. This review is intended to provide a comprehensive overview of the field of steroidal monocyclic pyridines for researchers and scientists who are interested in this area of research. It is also hoped that this review will stimulate further research into the synthesis and biological activities of steroidal pyridines to develop new and improved drugs for the treatment of diseases.
The current review highlights the synthesis and biological activities of steroidal monocyclic pyridines. The synthetic approaches, proposed mechanisms, biological activities are described.
1. Introduction
Steroids are a class of organic compounds that have a four-ringed structure. They are found in all living organisms, including humans, animals, plants, and fungi. All types of steroids, including androgens, oestrogens, progestogens and glucocorticoids, have variable effects on the brain.1 Androgens and oestrogens are involved in the sexual differentiation of the brain, and correspondingly influence cognition.2 The incorporation of heterocycles or heteroatoms in the basic skeleton of the parent steroids frequently results in modifications of their biological character, e.g., improving the cytotoxic activity against diverse tumor cell lines.3–5 In particular, pyridosteroids are a promising new class of compounds with a wide range of potential applications.6,7 More research is needed to fully understand their biological activities and potential therapeutic uses. However, the early research is very promising, and pyridosteroids could potentially be used to treat a variety of diseases.8–10
Abiraterone (I)11,12 has been applied in the clinical field for the treatment of prostate cancers, in which its performance is related to the incorporation of a pyridine ring. The lone pair of electrons on the nitrogen atom enables the formation of a coordination bond with the heme-iron atom at the active site of the enzyme.13 A series of 5α-reductase inhibitors (II) demonstrated respectable in vitro anti-proliferative activity against PC-3 cell lines.14 In recent research, steroidal pyridines (III) exhibited potential cytotoxic activity on prostate cancer cells utilizing the abiraterone “androgen inhibitor” as a control drug.15 In addition, androst[17,16-b] pyridines (IV) revealed a moderate cytotoxic effect on PC-3 cells, in which most of the cells treated with this steroid died at the higher dose (165 μM).6 Furthermore, heterosteroids (structures V and VI) have displayed potent anti-inflammatory activities (Fig. 1).16
Fig. 1. Privileged steroidal bioactive structures incorporating binary and fused pyridine, and dihydropyridine, cores.
Miller17 reported the synthesis of pyridosteroids for the first time through multi-step reactions under harsh conditions and high temperatures. Chelucci et al.18 reported the synthesis of pyridosteroids through a three-step procedure involving the reactions of N,N-dimethylhydrazones with bromoethyl-1,3-dioxolane. Additionally, Abbiati et al.19 reported the synthesis of pyridosteroids starting from carbonyl compounds under catalytic conditions using Au(iii) salts. Recently, Yan et al.20 reported the synthesis of pyridosteroids under copper(ii)-catalyzed reactions of ketosteroids with propargylamine, but this method failed to produce promising product yields (6–51%). The synthesis of fused pyridosteroids was extended to include the reactions of steroidal enamides by treatment with the Vilsmeier reagent21 and other approaches dealing with the reactions of dicarbonyl compounds under microwave irradiation conditions.22 To date, the search for modified heterocycles fused to ring A of the steroid skeleton has increased. The effect on biological features produced by the fusion of the six-membered ring with nitrogen atoms such as pyridines, pyrimidines,23etc., to the 2,3-positions of androstanes and 19-norandrostanes was originally reported by Clinton et al.24,25 and De Ruggieri et al.26 for the synthesis of androstano[3,2-b]pyridines.27
Our recent work was directed toward the exploration of the chemistry and biological diversity of heterocyclic steroids incorporating pyridine moieties of biological importance28,29 in addition to the chemistry of heterocycle-integrated pyridine scaffolds.30–36 In this work, we focus on discussion of the various reported procedures for the synthesis of fused and binary steroidal pyridines and their structural importance, in addition to highlighting their biological significance.
2. General routes for synthetic strategies
The choice of synthetic strategy will depend on a number of factors, including the complexity of the target molecule, the availability of starting materials, and the desired yield and purity of the product. Fig. 2 presents various examples of the reactions that have been adopted to synthesize steroidal fused and binary pyridine and dihydropyridine systems that will be discussed and highlighted in this review. There are many different general routes for the synthetic strategies, among which the most commonly applied strategies are the divergent syntheses. Divergent syntheses involve building a target molecule from two or more different starting materials, which can be a more efficient way to synthesize complex molecules.
Fig. 2. Examples of different synthetic routes reported to prepare steroidal monocyclic pyridines.
3. Synthesis of pyridines fused to steroidal skeleton
3.1. Multicomponent synthesis
MCRs are a versatile and efficient way to synthesize pyridines and their derivatives, and these reactions offer several benefits over traditional methods, including high yields, one-pot procedures, and green protocols under mild conditions.37,38 Several reactions can be used to synthesize pyridines, including common methods39–42 such as Hantzsch pyridine synthesis, which involves the condensation of an aldehyde, an amine, and malonic acid or malonitrile. The Skraup quinoline synthesis involves heating aniline with sulfuric acid, glycerol and an oxidizing agent such as nitrobenzene. The Chichibabin pyridine synthesis is a procedure for the synthesis of pyridine rings. The reaction involves the condensation reaction of aldehydes, ketones, α,β-unsaturated carbonyl compounds, or any combination of the above, with ammonia. Shekarrao et al.43 reported an efficient protocol for the multicomponent synthesis of pyridosteroids under catalytic and microwave irradiation conditions (Scheme 1). Copper-catalyzed multicomponent reactions of steroidal β-bromovinyl aldehyde 1 with substituted alkynes and tert-butylamine under the optimized conditions gave the corresponding steroid-annulated pyridine ring at the D-ring (2a–f) in relatively good yields (70–82%). The reactions proceeded under ligand-free conditions using catalytic copper iodide in DMF for improved product yields. To optimize the conditions, the authors performed the reaction using 1,2-dichloroethane as a solvent and catalytic copper(i) iodide under thermal conditions. This reaction proceeded through a smooth annulation of the regioselective product with a very low yield. DMF was a more efficient solvent than toluene or 1,4-dioxane for improved yield under microwave irradiation conditions (720 W) using 10 mol% of the catalyst (CuI). The microwave irradiation conditions enable a high reaction rate with reduced reaction time compared to the use of thermal conditions. The reaction mechanism was postulated to proceed through the initial formation of the π-complex produced from the interaction of the alkyne with the Cu(i) ion through the deprotonation of the alkyne in the presence of basic tert-butylamine. The generated intermediate A-1 tended to undergo oxidative addition with β-bromovinyl aldehyde in the presence of the amine to generate the possible copper(iii) imine intermediate B-1. The reductive elimination of intermediate B-1 generated the copper-coordinated intermediate C-1. Next, 6-endo-dig cyclization of the intermediate C-1 delivered the regioselective iminium intermediate D-1. The tert-butyl group of the intermediate D-1 was cleaved to neutralize the charge on the nitrogen atom to give the pyridosteroids 2 after the release and recycle the Cu(i) catalyst (Scheme 1).
Scheme 1. Synthesis and the proposed mechanism for the annulations of pyridosteroids.
Ansari et al.9 reported the multicomponent synthesis of steroids fused to annulated pyridines at the B-ring of the steroid skeleton. One-pot multicomponent synthesis of ketosteroids 3–5 with aryl aldehydes, active methylene components, and ammonium acetate under catalyzed and microwave-assisted conditions produced the target products 6–11 in brilliant yields (80–89%) (Scheme 2). MgO NPs were used as a heterogeneous catalyst to improve the product yield, and the reaction exhibited increased reaction rate, insignificant chemical waste, cost-effective sustainability, green conditions, and facile catalyst preparation, product isolation and purification. The low solubility of the nanocatalyst allowed for its simple separation from the reaction mixture by filtration, and correspondingly, the reuse of the nanocatalyst for further reactions without a deficit in its activity. This nanocatalyst could be used for five runs with no notable effect on the product yield. The reactions were achieved in ethanol by heating at 70 °C under microwave-assisted conditions. The ecological alternative protocol delivered high rates of product formation after a short time. The mechanistic progression of this reaction might be managed through the formation of imine intermediate A-2 through the reactions of steroidal ketones with ammonium acetate via the activation of the metal oxide nanocatalyst of the carbonyl group of the steroids and ammonium acetate. The reactions of active methylenes with aryl aldehydes produced the arylidene intermediates B-2 through the Knoevenagel condensation step. Michael-type addition of imine intermediate A-2 to the arylidene intermediates generated the intermediates C-2. Intramolecular nucleophilic addition of the exocyclic imino group at the terminal nitrile group and subsequent oxidation and aromatization gave the steroidal pyridines 6–11 (Scheme 2). In these reactions, the Mg2+ and O2− of the nanocatalyst act as a Lewis acid and Lewis base, respectively; these sites have beneficially activated the substrates for Michael addition and Knoevenagel condensation reactions. The role of the nanocatalyst is to activate the reactions by stabilization of the charge sites of the intermediates, enable condensation, and facilitate the nucleophilic attack during the reaction pathway.9
Scheme 2. Heterogeneous MgO NP catalyzed multicomponent synthesis of pyridosteroids.
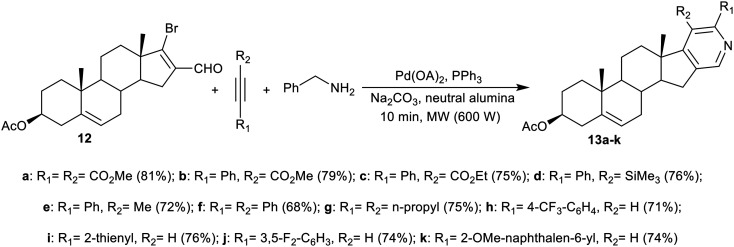
Palladium-catalyzed ring cyclization has been applied on a large scale in organic synthesis to synthesize various nitrogen heterocycles comprising substituted pyridines.44 The process always faces some disadvantages, such as in the multistep synthesis of tert-butylimines. In this route, Shekarrao et al.45 reported the synthesis of 5,6-disubstituted-pyridosteroids through annulations of pyridine rings under palladium-catalyzed multicomponent reactions (Schemes 3 and 4). The steroidal β-bromovinyl aldehyde 12 and β-bromovinyl aldehyde 14 were efficiently synthesized by the treatment of 3-acetoxy-androst-5-en-17-one and 5α-cholestan-3-one, correspondingly, with the Vilsmeier reagent under heating conditions in (PBr3/DMF) or chloroform, respectively.46 Multicomponent reactions of steroids 12 and 14 with benzylamine and alkyne derivatives under Pd-catalyzed (5 mol%), and microwave irradiation (600 Watt, 140 °C, and 12 bar) conditions gave the desired pyridosteroids 13a–k (68–81%) (Scheme 3), and 15a–e (66–76%) (Scheme 4), respectively. The palladium catalyst was recycled in these reactions for two runs with no great effect on the product yield. The yield of the synthesized pyridosteroids is affected by the nature of the substituents on the alkyne substrate.
Scheme 3. The Pd-catalyzed multicomponent synthesis of pyridines fused to ring D of the steroid skeleton.
Scheme 4. The Pd-catalyzed multicomponent synthesis of pyridines fused to ring A of the steroid skeleton.
The mechanism for the synthesis of pyridosteroids through imino-annulation under Pd-catalyzed conditions was projected based on the previous literature reports.47,48 As shown in Scheme 5, the vinyl halide group of steroid 12 or 14 underwent oxidative addition with the transformation of Pd(ii) to Pd(0), and then further reacted with benzylamine to generate the imine intermediate A-3. The alkyne annulations of A-3 occurred at the more hindered position of the alkyne to generate vinyl palladium intermediate B-3, which regioselectively produced the pyridine derivative.48 The ammonium salt intermediate C-3 with a seven-membered ring was generated by the intramolecular interaction of B-3 with vinylic palladium. The reductive elimination of C-3 generated benzylpyridinium salt D-3 with the release of Pd(0). The benzyl group was cleaved in the next step; this was supported by the neutralization of the charge on the nitrogen atom of the pyridine ring, and reduced the steric hindrance with the substituents on the ortho-position of the pyridine ring to yield the respective pyridosteroids 13 or 15 along with the release of benzyl halide.45
Scheme 5. Conceivable mechanism of the Pd-mediated formation of pyridosteroids.
Mohamed et al.49 reported a one-pot three-component procedure for the synthesis of aminoandrostano-1,4-dihydropyridine 17 in an excellent yield (85%). Specifically, the reaction of epi-androsterone 16 with benzaldehyde and malononitrile in refluxing ethanol containing ammonium acetate gave the respective pyridosteroid 17. The product formation was illustrated through the formation of a benzylidene intermediate, which reacted with malononitrile, followed by cyclocondensation after an intramolecular nucleophilic attack of the amino group at the nitrile group. Previous reports demonstrated the possible aromatization of the pyridine ring through an oxidation or dehydrogenation step,50 but in the case reported by Mohamed et al.,49 this step did not occur. The reactivity of the enaminonitrile moiety of 17 was examined in the reaction with DMF-DMA. Gentle heating of 17 in acetonitrile with DMF-DMA efficiently yielded the formamidine 18 after a short time. Consequently, the reaction of pyridosteroid 17 with triethyl orthoformate by heating in acetic anhydride afforded the desired ethoxymethanamine 19 in a magnificent yield (Scheme 6).
Scheme 6. Multicomponent one-pot synthesis and reactivity of pyridosteroids.
Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules. There are two main types of stereoisomers: enantiomers and diastereomers. Enantiomers are molecules that are mirror images of each other but are not superimposable. This means that they cannot be superimposed on each other, no matter how they are rotated. Diastereomers are molecules that are not mirror images of each other, but are not identical either. There are a number of different methods for representing the three-dimensional structure of molecules. Some common methods include ball-and-stick models, space-filling models, and wedge-and-dash models. On the other hand, a diastereoselective product is a product of a chemical reaction that is one of two or more diastereomers. Diastereomers are molecules that have the same connectivity but different arrangements of their atoms in space. Unlike enantiomers, they are not mirror images of each other. Diastereoselective products can be formed by a variety of chemical reactions. Some common methods for achieving diastereoselectivity include the use of chiral catalysts, chiral reagents, kinetic control, and thermodynamic control.
The Diels–Alder reaction of steroidal N-sulfonyl-1-azadienes with active carbonyl analogs in the presence of pyrrolidine was attempted for the synthesis of pyridosteroids fused at ring D (Scheme 7). Lopes et al.51 reported this reaction sequence using various steroidal N-sulfonyl-1-azadienes in the reactions with aldehydes and pyrrolidine. The steroidal N-sulfonyl-1-azadiene 20 was synthesized as a versatile precursor from 16-dehydropregnenolone acetate (16-DPA).52 The steroidal N-sulfonyl-1-azadiene 27 was prepared from 16-dehydroprogesterone through multi-step reactions involving basic hydrolysis of the acetate group, reaction with hydroxylamine hydrochloride, Oppenauer oxidation of the hydroxyl group at C3, protection of the produced carbonyl group with ethylene glycol, and finally, treatment with p-toluene-sulfinyl chloride. The reactions of both substrates, i.e., steroidal N-sulfonyl-1-azadienes 20 and 27, with various aldehydes and pyrrolidine afforded diastereoselective steroids 21–26 and 28–33. The pyridosteroids produced from this reaction incorporated three chiral carbons in the pyridine ring, which enabled the stereoselectivity of these products. The reaction of 20 with pyrrolidine (20 mol%) failed to give the desired product. Thus, the authors attempted the reaction of 20 with enamine “in situ prepared from the reaction of phenylacetaldehyde with pyrrolidine”. The steroid 21 was isolated in 66% yield. The steroid 21 was also obtained in 75% yield by carrying out the reaction under microwave irradiation at 50 °C for 10 min in dichloromethane. Efforts to explore the reactivity of steroid 20 with enamines prepared from iso-butyraldehyde, 2,2-diphenylacetaldehyde, or cyclohexane-carbaldehyde and pyrrolidine failed to give the desired product. The reaction of 1-azadiene 27 with phenylacetaldehyde and pyrrolidine (1.5 equiv.) under MW irradiation at 60 °C for 30 min in dichloromethane afforded pyridosteroid 28 in 69% yield. The trans-enamines were specially formed starting from alkyl aldehydes, including phenyl-acetaldehyde. Accordingly, the projected stereochemistry is in accordance with an approach of the dienophile from the less-hindered α-face of the steroid53 with the endo pyrrolidine ring and retention of the enamine trans geometry. The pyrrolidine acts as a reactive substrate in these reactions, as well as being considered a catalyst. Thus, the enamine catalysis prompted the formation of pyridosteroids through an interesting diastereoselective annulation of the pyridine ring.
Scheme 7. Stereoselective synthesis of substituted pyridosteroids.
Patel et al.54 reported a regioselective route to synthesize a series of pyridosteroids with high selectivity under solvent-free conditions. The reaction of ketosteroid 34 with 3-chloro-1-arylpropan-1-ones and ammonium acetate gave steroidal substituted pyridines 35 as the major product, and 36 as the minor product (Scheme 8). The formation of the products involved the formation of new C–C and C–N–C bonds through the interaction between ketosteroid, which “provided two carbons”, with 3-chloro-1-arylpropan-1-ones, which “provided three carbons”, and ammonium acetate, which was “a source of nitrogen atom”. The green procedure reported in this case provided various benefits, such as exceptional stereoselectivity, exceptional yields, accessible substrates, and solvent- and catalyst-free conditions, but it did not provide high reaction rates.
Scheme 8. Regioselective synthesis of substituted pyridosteroids.
The mechanism of the formation of the stereoselective steroidal pyridines was proposed to involve two basic routes for the formation of the major and minor products (Scheme 9).54 In the first case, 3-chloro-1-arylpropan-1-ones were transferred into the unsaturated ketones by HCl molecule elimination in the presence of ammonium acetate. The formed intermediate reacted with ammonium acetate through the aza-Michael step to form intermediate A-5 (“β-aminoketone intermediate,55 C–N bond formation”). The ketosteroid 34 subsequently underwent imino-condensation in the reaction with intermediate A-5 to form intermediate B-5, which was transformed into the enamine form “C-5” with the aid of acetate anions in the solution. Intramolecular cyclization of the intermediate C-5 generated intermediate D-5 with “C N and C–C bonds”, which upon dehydration produced the desired intermediate E-5. The oxidation of E-5 in air with “C C bond formation” led to the aromatization of the pyridine ring with the formation of the major products 35. Subsequently, the ketosteroid 34 in the second case tended to interact with ammonium acetate to form the enamine intermediate A-6. The 1-arylprop-2-en-1-one intermediate was formed and reacted with intermediate A-6 to form the intermediate B-6 through 1,4-addition. The enamine intermediate C-6 was formed by the influence of acetate anions in the solution and subsequent intramolecular cyclization to form intermediate D-6. The dehydration of intermediate D-6 generated intermediate E-6, which upon oxidation in air produced the minor product 36.
Scheme 9. The reaction mechanism proposed for the formation of regioselective pyridosteroid isomers.
3.2. Synthesis from enaminones
Enaminones are a versatile and efficient way to synthesize pyridines, as they offer several advantages over traditional methods. These advantages include high yields, one-pot reactions, and green procedures. A primary amine is reacted with a carbonyl compound to form an enaminone, which subsequently reacted with an aldehyde or ketone to form a pyridine ring.56–58
Zhang et al.59 reported the synthesis of enaminonitrile pyridosteroid 42 through a multi-step synthetic approach via the Friedländer reaction. Acetylation of dehydroepiandrosterone 37 produced steroid 38 through treatment with acetic anhydride under catalytic conditions at room temperature. Vilsmeier formylation of steroid 38 with the Vilsmeier reagent (DMF/POCl3) in chloroform gave the desired β-halo aldehyde 39; the product in this step was isolated using column chromatography. Treatment of 39 with sodium azide in a mixture of DMF/H2O (“water was utilized to dissolve sodium azide”) afforded azido-formyl derivative 40. The Staudinger reaction of 40 with triphenyl phosphine in a mixture of THF/H2O (9 : 1) afforded enamine steroid 41. During the synthesis of pyridosteroid 42, the use of sodium hydroxide in the reactions with active methylene components led to the formation of a deacetylation product. The reaction of enamine 41 with malononitrile in ethanol containing piperidine yielded pyridosteroid 42 in a moderate yield (55.2%). Product 42 was isolated via silica gel chromatography using ethyl acetate/petroleum (1/3) as an eluent. The same reaction with other active methylene components failed to provide the desired product; this might be due to the reactivity of malononitrile toward the other active methylene derivatives. The authors stated that this might also be attributed to the decreased reactivity of the amino group of steroid 41, which is adjacent to an electron-withdrawing double bond (Scheme 10).
Scheme 10. Synthesis of pyridosteroid-integrated enaminonitrile moiety.
Zhang et al.60 extended their work for the synthesis of pyridosteroid derivatives 44 to find suitable conditions for the pyridine ring annulations that failed to give the target fused pyridines in their previous work.59 One-pot reactions of β-amino aldehydes 43 with active methylene components gave the corresponding annulated products 44 under the optimized conditions in reasonably good yields (61.4–73.4%) (Scheme 11). Therefore, the use of iodine (1% mol) as a catalyst in this reaction under thermal conditions in an ethanol/THF mixture delivered the pyridosteroids 44a–d. The products were purified by silica gel chromatography using ethyl acetate/petroleum (1 : 3) as an eluent.
Scheme 11. The pyridine ring annulations fused to the D-ring of the steroid skeleton.
3.3. Synthesis from enamides
Enamides are a versatile class of organic compounds that can be used for the synthesis of a wide variety of pyridine derivatives.61 One of the most common methods for using enamides to synthesize pyridines is the [4 + 2] cycloaddition reaction. In this reaction, an enamide reacts with a maleimide to form a pyridine ring. This reaction is typically catalyzed by a metal, such as Mn(OAc)2.62
Nongthombam et al.6 reported a multi-step synthetic sequence for the annulations of pyridosteroids 46 and 50 from steroidal enamide 45 (Scheme 12). A series of substituted alkynes were applied in this reaction to discover the scope and applicability of the reactants for proven yields. The reaction of steroid 16-DPA 47 with hydroxylamine hydrochloride in methanol afforded the respective oxime 48. The Vilsmeier–Haack reaction of the oxime derivative with Vilsmeier reagent produced the desired steroidal β-formyl enamide 45, which upon reaction with alkyne derivative under Cu(ii)-mediated conditions (10 mol%) and 1,10-phen (20 mol%) afforded the pyridosteroid analogs 49. These conditions were explored based on their trials for the reaction of steroidal β-formyl enamide 45 with alkynes. To explore the best reaction conditions, the reaction was performed using copper iodide as a catalyst, in addition to the use of other catalysts, such as copper chloride, copper bromide, palladium acetate, zinc chloride, ferric chloride, etc. Ligands including 1,10-phen, DABCO, and 2,2′-bipyridine were tested, as well as ligand-free conditions. In addition, solvents such as DMF, DMSO, DEC, THF, etc., containing basic carbonate salts such as potassium, sodium, cesium, etc., were applied. The best conditions using catalytic copper iodide and the ligand 1,10-phen in DMF containing potassium carbonate provided an improved yield. The base hydrolysis of the acetyl group of pyridosteroids 49 by treatment with potassium carbonate in methanol gave 3-deacetylated analogs 50 in respectable yields (76–80%).
Scheme 12. Multi-step synthesis of pyridosteroid analogs.
Under the optimized conditions, the pyridine ring annulations were proposed to occur as outlined in Scheme 13.6 Initially, the base enables abstraction of the NH proton from the amide group of steroids 45 by the action of potassium carbonate. A nucleophilic attack of the generated anion on the alkynes, which was activated by interaction with the Cu(ii) catalyst, generated intermediate A-7. The iodonium ion attacked the generated intermediate A-7; this step enabled the intramolecular cyclization to form the desired dihydropyridine intermediate B-7. The catalyst was released in this step. The cleavage of the acetyl group was achieved by the action of the base to neutralize the charge on the nitrogen atom with pyridine ring aromatization and release of the acetic acid molecule to give the steroidal pyridines 50.
Scheme 13. The schematic mechanism for the synthesis of pyridosteroids from β-formyl enamide.
Barthakur et al.21 reported the synthesis of pyridine analogs fused to ring A of the steroid skeleton through the transformation of ketosteroids, e.g., 5α-cholestan-3-one into enamides 51 (Scheme 14). Therefore, the three-step reactions involving the formation of enamides (“N-acetyl-5α-cholest-2-en-3-amine”) occurred by treatment of 5α-cholestan-3-one with hydroxylamine hydrochloride followed by acetylation with acetic anhydride under catalyzed conditions using aluminum oxide and pyridine. The Vilsmeier–Haack reaction of the enamides in DMF/POCl3 under gentle heating conditions gave the annulated pyridine derivatives fused to ring A of the steroid nucleus. The consequent products, “6′-chloro-5α-cholest[3,2-b]pyridines” 52 and 53, were obtained in excellent yields. Various ketosteroids were applied in this reaction sequence to prepare linear and angular pyridines fused to ring A of the steroid skeleton. The reaction was also applied for the synthesis of bis-pyridines fused to rings A and D of the steroid core “pyridosteroid 57”, in which a pyridine ring fused to ring A is at an angular configuration to the basic skeleton. All of the reactions proceeded with excellent yields of the products depending on the nature of the substituents and the reacted substrates. The use of various ketosteroids to prepare the respective enamides and subsequently to accomplish the pyridine ring annulation proved the versatility of this reaction. The reaction mechanism is shown in Scheme 14, in which the proposed sequence was initiated by the interaction of the enamides with the Vilsmeier reagent to generate the enamine intermediate A-8. The intermediate A-8 underwent chlorination of the amidic carbonyl group through the reaction with the Vilsmeier reagent after enolization of the amide group to generate intermediate B-8. Intramolecular cyclization of the intermediate B-8 gave the final pyridosteroids 52. Under the cold conditions of the reaction, the corresponding 2-formyl analog was obtained as a sole product that failed to cyclize in the final cyclization step.
Scheme 14. The synthesis and planned reaction mechanism for the preparation of pyridosteroids.
3.4. Synthesis from cyclic unsaturated ketones
Yan et al.20 reported a one-pot synthesis of pyridines fused to ring A of the steroid core under Cu(ii)-catalyzed conditions. The reactions of 17β-hydroxyandrost-4-en-3-ones 58, 59 and 17β-hydroxyestr-4-en-3-one 60 with propargylamine in ethanol containing a catalytic amount of copper nitrate trihydrate under reflux conditions gave a mixture of two pyridosteroids 61–63a,b in each case. Pyridine ring annulation by this procedure avoids the multi-step sequence for the preparation of pyridosteroids and excludes high reaction temperature. The pyridosteroid structure 61a was established via adequate X-ray analysis. The formed product mixture was difficult to isolate. The projected mechanism shown in Scheme 15 involved a condensation reaction between propargylamine and ketosteroids 58–60 in the first step. The imine intermediate A-9 was generated by the activation of the substrates by the Cu(ii) catalyst. Next, isomerization of the imine A-9 generated the enamine intermediate B-9. A regioselective intramolecular “6-endo-dig” nucleophilic attack of the terminal enamine carbon at the carbon–carbon triple bond that was activated by copper catalyst generated intermediate C-9 next occurred. The catalyst release along with protonolysis of the Csp2–C bond generated the intermediate D-9. Oxidation of the D-9 intermediate in air catalyzed by Cu2+ generated the intermediate E-9. The process involved the transfer of an electron to Cu2+ to produce Cu+ and a cation radical and the subsequent loss of a proton to generate intermediate E-9. Cu+ was transformed into Cu2+ with the aid of air. Protonation of the intermediate F-9 generated the desired intermediate G-9. The pyridine products were formed in the last step through the loss of a proton in a proton chain transfer.
Scheme 15. Synthesis and the plausible mechanism of pyridosteroid derivatives.
Savić et al.63 reported the synthesis of pyridine analogs fused to the A-ring of androstane under copper-catalyzed conditions. This approach is an extension of the work reported by Yan et al.20 The reactive precursor, 3,17-dihydroxy-17-(pyridin-2-ylmethyl)androst-5-ene (64) was utilized in the multi-step synthetic sequence to acquire the target pyridines fused to ring A of the steroid skeleton. Oppenauer or Jones oxidation64,65 of 64 by treatment with Al-(O-i-Pr)3 in toluene gave the corresponding ketosteroid 65. In another sequence of the reactions, steroid 64 underwent acetylation by treatment with acetic anhydride under reflux temperature to yield steroid 67. The reaction led to condensation of the hydroxyl group at C17 at the same time. Basic hydrolysis of 67 with potassium hydroxide in methanol gave the hydroxy-steroid 68. The respective ketosteroid 69 was also obtained by Oppenauer or Jones oxidation of 68. The reactions of androstan-4-en-3-one derivatives 65 and 69 with propargylamine in ethanol containing copper nitrate as a catalyst under reflux conditions yielded the respective pyridyl-pyridosteroids 66 and 70, respectively, in moderate-to-good yields (Scheme 16).
Scheme 16. Synthesis of pyridine analogs fused to the A-ring of androstane.
3.5. Synthesis from acyclic α,β-unsaturated ketones
One procedure for the synthesis of pyridine derivatives is from the reactions of unsaturated ketones with active methylene compounds.66 The reactions might proceed through a Michael addition reaction, followed by a cyclization reaction. Amr et al.67 reported a versatile and efficient procedure for the synthesis of pyridosteroids utilizing the reactivity of steroids with an unsaturated ketone core in their reactions with various reagents. The reactions of steroidal α,β-unsaturated ketones 71a,b with ethyl cyanoacetate afforded the desired pyridines 72a,b. The catalytic p-toluenesulfonyl chloride supported the selective condensations of 72a,b to afford the respective cyclohexenes 73a,b. The analogous pyridine-thiones 75a,b were obtained from the reactions of steroids 71a,b with 2-cyanoethanethioamide. Moffat oxidation is a chemical reaction for the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively. The oxidant is a combination of DMSO and dicyclohexyl-carbodiimide. The Moffat oxidation is often compared to the Swern oxidation, which is another mild and selective oxidation reaction for alcohols. The mechanism of the Moffat oxidation is as follows:1 DMSO reacts with DCC to form a sulfonium ylide.2 The sulfonium ylide attacks the alcohol, forming an alkoxysulfonium ion.3 The alkoxysulfonium ion eliminates dimethyl sulfide to form a carbonyl compound. In this route, the Moffat oxidation of pyridosteroids 72a,b and 75a,b produced the respective 3-oxo-derivatives 74a,b and 76a,b (Scheme 17).
Scheme 17. Synthesis of steroidal pyridines from acyclic unsaturated ketosteroids.
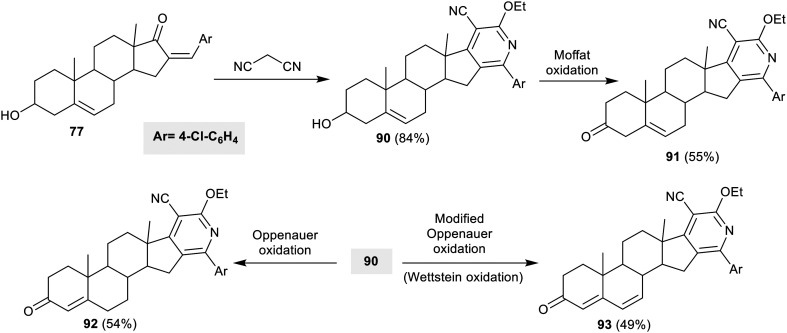
Scheme 18 exemplifies the typical reactivity of steroidal α,β-unsaturated ketone 77 with ethyl cyanoacetate and 2-cyanoethanethioamide. The reactions gave the corresponding pyridine and pyridine-thione derivatives 78 and 82. The reaction of the steroid with ethyl cyanoacetate produced an amended yield based on the reactivity of the substrate with the steroidal α,β-unsaturated ketone. Similarly, p-toluenesulfonic acid reinforced the formation of diene 79. The Moffat oxidation reactions of steroids 78 and 82 gave the anticipated ketosteroids 80 and 83, respectively. On the other hand, Oppenauer oxidation reactions of steroids 78 and 82 gave the corresponding steroidal α,β-unsaturated ketones 81 and 84 (Scheme 18).67
Scheme 18. Synthesis of steroidal pyridines fused to ring D.
The Oppenauer oxidation is a chemical reaction for the selective oxidation of secondary alcohols to ketones. It is typically carried out at room temperature, and it does not require the use of strong acids or bases. The reaction is also relatively tolerant of functional groups, making it a versatile tool for organic synthesis. The mechanism of the Oppenauer oxidation is as follows:1 The alcohol is reacted with aluminum isopropoxide in the presence of acetone.2 The aluminum isopropoxide abstracts a hydrogen atom from the alcohol, forming an alkoxide ion.3 The alkoxide ion then attacks the carbonyl carbon of the acetone, forming a ketone.4 The acetone is regenerated by the oxidation of the alkoxide ion by the aluminum isopropoxide. In another sequence of the reactions, malononitrile has been employed as another example of the activated nitriles in their reactions with steroidal α,β-unsaturated ketones. The reactions of steroidal α,β-unsaturated ketones 71a,b with malononitrile gave pyridosteroids 85a,b, respectively. Treatment of 85a,b with p-toluenesulfonyl chloride gave pyridosteroids 86a,b, while Moffat oxidation yielded pyridosteroids 87a,b, respectively. The reactivity of pyridosteroids 87a,b was studied in reactions with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and ethylidenetriphenyl-λ5-phosphane. DDQ, as a prevailing oxidizing agent, was utilized also for the dehydrogenation of steroids. The reactions of 87a,b with DDQ gave the corresponding quinoids 88a,b, while the reactions with ethylidenetriphenyl-λ5-phosphane produced the corresponding ethylidenes 89a,b, respectively (Scheme 19).67
Scheme 19. Synthesis of steroidal pyridines fused to ring D.
Similarly, the reaction of steroidal α,β-unsaturated ketone 77 with malononitrile gave pyridosteroid 90 in an excellent yield. Moffat oxidation of 90 yielded the corresponding ketone 91, while Oppenauer oxidation gave the α,β-unsaturated ketone 92. Wettstein oxidation is an organic chemical process used to oxidize 3-β-hydroxysterol to 3-ketosterol. The reaction medium is an alkaline environment, and the 3-β-hydroxysterol is heated with DDQ. Wettstein oxidation of pyridosteroid 90 yielded desired dienone 93 in a moderate yield (Scheme 20).67
Scheme 20. Synthesis of steroidal pyridines fused to ring D.
Abdalla et al.68 utilized the reactivity of 3β-hydroxyandrostan-17-one 94 “as an α,β-unsaturated ketone analog” towards reactions with various active methylene components to synthesize a series of pyridines fused to ring D of the steroid skeleton (Scheme 21). The reactions of 3β-hydroxyandrostan-17-one 94 with 2-cyanoacetamide or 2-cyanoethanethioamide in sodium ethoxide solution under heating conditions afforded steroidal cyanopyridones 95 and steroidal cyanopyridine-thiol derivatives 96, respectively. It was noted that the reaction of 3β-hydroxyandrostan-17-one 94 with malononitrile in sodium ethoxide solution afforded pyridosteroids 97. Product 97 was obtained after treatment of the reaction mixture with 10% HCl, while treatment with sodium carbonate solution after completing the reaction produced steroidal dihydropyridines 98. The product from the reaction of steroidal unsaturated ketone with malononitrile changed based on the pH of the medium.
Scheme 21. Synthesis of pyridine derivatives fused to ring D of the steroid core.
3.6. Synthesis from ketosteroids
One way to achieve pyridine synthesis from ketones is through the Hantzsch pyridine synthesis.69,70 The reaction involves the condensation of an aldehyde, an amine, and malonic acid or malonitrile. The aldehyde and amine react to form an imine, which then reacts with malonic acid or malonitrile to form a cyanohydrin. The cyanohydrin is then cyclized in the presence of a base to form a pyridine ring. In the case of ketosteroids, the aldehyde and amine can be derived from the ketosteroid itself.
Recently, Potter et al.71 reported the synthesis of E-ring-improved steroids, which were evaluated as inhibitors of 17β-hydroxysteroid dehydrogenase (17β-HSD) and remarked on the ineffectiveness of routes to pyridine annulated derivatives. Catozzi et al.72 reported an efficient protocol for the synthesis of disubstituted pyridosteroid 101 in a good yield. The procedure is a one-pot reaction of estrone (99) with triazine 100 and pyrrolidine in a sealed tube for 10 hours. The reaction mixture was subsequently treated with silica to furnish disubstituted pyridosteroid 101 (Scheme 22). This reaction was also run under reflux conditions, but the product yield was found to be very low (37%). Fernández Sainz et al.73 reported the reaction mechanism for the annulations of pyridine ring from the reactions of triazine derivatives with ketones in the presence of pyrrolidine under various thermal and microwave irradiation conditions. Predominantly, the reactions under thermal conditions possess the sequence of an inverse electron-demand Diels–Alder reaction followed by a retro-Diels–Alder reaction with the generation of a triazabicyclo[2.2.2]octa-2,5-diene intermediate that was transformed into a dihydropyridine intermediate through the elimination of a pyrrolidine molecule. The aromatization was achieved in the final step to produce the pyridosteroid 101. It was noted that the addition of silica gel enabled the aromatization and facilitated the elimination steps.
Scheme 22. Synthesis of bispyridyl steroids.
Dutta et al.22 developed a new procedure for the synthesis of fused pyridosteroids in excellent yields. Three-component one-pot reactions of ketosteroids 102 with aldehydes, and ketones gave Michael adducts 103a–j. The reactions were accomplished in toluene containing basic potassium hydroxide. Subsequently, the 1,5-diketones 103a–j were utilized as reactive precursors for the synthesis of pyridosteroids fused to either ring A or ring D of the steroid skeleton. Consequently, the one-pot reaction of diketones 103a–j with urea under catalyzed and MW conditions gave the corresponding pyridosteroids 104a–j, respectively (Scheme 23). The preparation of various pyridosteroids fused to rings A and D of the steroid nucleus reflect the significance and derivatization of the reaction. The catalyst enabled high reaction rates and product yields. The product yield depends on the chemical nature of the substituents linked to the steroid compounds or aldehydes or ketones. The authors optimized the reaction conditions in the second step by applying thermal and MW conditions and using iso-propanol, acetonitrile, DMF, and solvent-free conditions under various metal-catalyzed conditions, i.e., aluminum chloride, titanium chloride, zinc chloride, BF3·OEt2 or catalyst-free conditions, to prepare steroid 104a with the highest yield. The best reaction conditions were documented using MW and solvent-free conditions with catalytic BF3·OEt2, in which the product was obtained with 90% yield and reduced reaction time (8 min).
Scheme 23. Synthesis of various pyridines fused to rings A and D of the steroid skeleton.
Scheme 24 presents the planned synthetic route for the annulation of pyridosteroids fused to either ring A or ring D of the steroid nucleus.22 The first step of this reaction involved a multicomponent reaction, in which the respective aldehyde reacted with a ketone to generate α,β-unsaturated ketone intermediate A-12. The generated intermediate subsequently reacted via a Michael-type reaction with the ketosteroids to give the Michael adduct “1,5-diketone” 103 in a basic medium. The reaction of Michael adducts 103 with urea followed in a one-pot manner. Under microwave irradiation, the urea was transformed into ammonia, which condensed with the exocyclic carbonyl group of the Michael adduct to generate intermediate B-12. The boron trifluoride diethyl etherate, as a Lewis acid, activated the cyclocondensation for pyridine ring closure to form intermediate C-12 and was released in the next step to form intermediate D-12. Aromatization of 1,4-dihydropyridine intermediate D-12 gave the pyridosteroid products 104.
Scheme 24. The assumed mechanism for the synthesis of fused pyridosteroids from diketones.
4. Synthesis of binary systems
4.1. Multicomponent synthesis of binary systems
Successively, a series of binary steroidal aminonicotinonitrile analogs 106a–p were perfectly synthesized through the procedure reported by Song et al.74 (Scheme 25). Thus, multicomponent one-pot reactions of 3-hydroxy-17-acetyl-androst-5-ene 105 with aryl aldehydes, malononitrile, and ammonium acetate in ethanol afforded binary aminonicotinonitrile analogs 106a–p. Many trials to optimize the conditions of the reactions were accomplished. Thus, the synthesis of heterosteroid 106a was achieved using different solvents, i.e., ethanol, toluene, water, trifluoroethanol, and a mixture of water and polyethylene glycol (1.5 : 0.5 equiv.), among which ethanol is the best solvent in terms of the product yield. The reaction was also carried out under catalytic and catalyst-free conditions using cesium carbonate, VB1, and TBAB. The use of the catalyst reduces the reaction time and consequently improves the reaction rate. No reaction proceeded using water as a solvent under either catalytic or catalyst-free conditions. In an alternative route, the synthesis of the target steroidal pyridines was achieved through two-step synthesis. Condensation of 3-hydroxy-17-acetyl-androst-5-ene 105 with aryl aldehydes in ethanol containing a basic medium of sodium hydroxide (30%) produced the desired acyclic unsaturated ketones 107, which upon reactions with malononitrile and ammonium acetate under reflux conditions gave products 106. The cyclization of the pyridine ring was proposed to occur through condensation of the carbonyl group with ammonium acetate and subsequent intramolecular cyclization.
Scheme 25. Multicomponent synthesis of binary steroidal aminonicotinonitrile analogs.
In a continuation of the work in the synthesis of pyridosteroids reported by Ansari et al.,9 a series of steroidal binary pyridines 109 and 110a–d was efficiently prepared under the optimized conditions. Multicomponent reactions of ketosteroid 108 with aryl aldehydes, active methylene components, and ammonium acetate under catalyzed (MgO NPs) and microwave irradiation conditions afforded the respective steroidal binary pyridines 109 and 110a–d in remarkable yields (84–89%) (Scheme 26). Microwave irradiation is preferred to the conventional heating method for improved product yield and shortened reaction time through increased reaction rate, e.g., steroidal pyridine 109a was prepared in 74% yield after heating the reaction mixture for 7.5 h, while the same steroid was prepared in 84% yield after 20 min. The mechanism of the acquired products was proposed based on the initial generation of imine intermediate A-13 through the reaction of ketosteroid with ammonium acetate. On the other hand, the arylidene intermediate B-13 was generated by the Knoevenagel condensation of aryl aldehydes with active methylene components. This step of the reaction is supported by the action of the nanocatalyst, MgO NPs, which include Mg2+, which acts as a Lewis acid, and O2−, which act as a Lewis base. Subsequent Michael-type addition of imine intermediates A-13 with the arylidene intermediate B-13 generated the intermediate C-13, which underwent intramolecular cyclization, oxidation, and aromatization to afford steroidal binary pyridines 109 and 110a–d. The reactive ionized sites on the nanocatalyst surface promoted facile interaction between substrates and stabilized the charged atoms, which thus reduced the reaction time and improved the reaction rate and product yield. The surface defects of the MgO NPs as an irreducible oxide influence the catalytic performance. The nanocatalyst binds the reacted substrates to the active sites; this behavior improved the synthetic efficiency of the products.
Scheme 26. Synthesis and the proposed mechanism of steroidal binary pyridines.
A series of 17-cyanopyridinyl steroids were synthesized through one-pot multicomponent reactions as reported by Sun et al.75 Pregnenolone with an acetyl group at the C17 position was involved as a reactive precursor for the synthesis of 17-cyanopyridinyl steroids through a simple procedure. Under heating conditions, pregnenolone reacted with aldehyde derivative, ammonium acetate, and malononitrile in a one-pot multicomponent reaction to give the corresponding pyridinyl steroids 111a–l in reasonable yields. Ammonium acetate was transformed into ammonia under heating conditions and contributed to the condensation reaction with the acetyl group of pregnenolone. The mechanism shown in Scheme 27 proposes that the imine–amine tautomerization of the intermediates A-14 generated the intermediate B-14. Knoevenagel condensation took place between malononitrile and aryl aldehydes on the other side to form the arylidene intermediates C-14. Michael addition subsequently occurred between intermediates B-14 and C-14 to generate the desired intermediate D-14. The intramolecular cyclization of intermediate D-14 through nucleophilic attack of the active imino group at the terminal nitrile group led to pyridine ring closure with the formation of intermediate E-14. The final step is suggested to be [1,3]H-migration followed by aromatization through oxidation by removal of a hydrogen molecule to improve the product stability; this step afforded the target pyridinyl steroids 111a–l. The authors verified the proposed structures of the synthesized pyridinyl steroids based on precise spectral data, i.e.,1H-NMR, 13C-NMR, and HRMS data.
Scheme 27. Multicomponent synthesis and the proposed reaction mechanism of pyridinyl steroids.
Wang et al.76 reported the synthesis of 17-pregnenolone cyanopyridines 113a–l through a simple procedure in moderate-to-good yields. First, pregnenolone reacted with acetic anhydride under catalyzed conditions to give the acetylated product 112. Multicomponent one-pot reactions of steroid 112 with malononitrile, substituted aldehydes, and ammonium acetate under heating conditions gave the anticipated pyridinyl steroids 113a–l (Scheme 28). In this approach to the reactions, ammonium acetate acts as a substrate “nitrogen source”, and also acts as a catalyst for the activation of cyclization through acetate anions in the reaction mixture. The reaction rate, as well as the product yield, are affected by the influence of the substituents on the phenyl ring of the reacted aldehydes or the hetero-aldehyde type.
Scheme 28. Multicomponent synthesis of pyridinyl steroids linked at the C17 position of the steroid scaffold.
4.2. Synthesis from ketosteroids
Koch et al.77 reported the synthesis of binary steroidal pyridines from ketosteroids through two-step sequences (Scheme 29). The transformation of epi-androsterone (114) into the corresponding steroid substituted with bromine (115a) or iodine (115b) at C17 was achieved by treatment with hydrazine to generate the hydrazine intermediate, which was then treated with NBS/pyridine or iodine in TEA.78 The Stille reaction is a palladium-catalyzed cross-coupling reaction between an organotin compound (also known as an organostannane) and an organic halide or pseudohalide. Koch et al.77 developed the synthesis of steroidal pyridines 116a–d using the Stille reaction using steroidal halides as substrates. The Stille reaction was achieved in DMF containing 20 mol% Pd(PPh3)4 as a catalyst. The mechanism of the Stille reaction proceeded through four steps:1 Oxidative addition: The palladium catalyst was oxidized by the halide in the organic electrophile, forming a palladium(ii) complex.2 Transmetalation: The organotin compound transferred its tin atom to the palladium complex, forming a new palladium(ii) complex with the R group.3Trans–cis isomerization: the palladium complex underwent an isomerization reaction, forming a new palladium(ii) complex with the R group in the cis position.4 Reductive elimination: the palladium complex underwent a reductive elimination reaction, forming the desired product and regenerating the palladium catalyst. The adaptable applicability of this procedure was indicated through the incorporation of various heterocycles.
Scheme 29. Synthesis of binary steroidal pyridines from ketosteroids.
4.3. Synthesis from coupling and substitution reactions
Xu et al.79 reported an efficient procedure for the synthesis of steroidal pyridine-3-sulfonate 118 (Scheme 30). In this route, a dried extract of standard estrogen 117 in a vial was treated with sodium bicarbonate buffer and a solution of pyridine-3-sulfonyl chloride in acetone (1.0 mg mL−1) under vortex and heating conditions to give the corresponding steroidal pyridine-3-sulfonate 118. The product was formed through the elimination of hydrogen chloride molecules.
Scheme 30. Synthesis of steroidal pyridine-3-sulfonate.
Dutour et al.80 developed the synthesis of pyridinyl steroids linked to C2, C3, and C4 of estra-1,3,5(10)-triene. In this route, different pyridinyl steroids were synthesized, and the final step of the applied procedure involved a catalytic reduction of the carbonyl group at the C17 position of the steroid skeleton using sodium boron hydride. The pyridine-2-yl steroids 122a,b were synthesized through multi-step reactions. The steroid 1 was prepared in two steps through iodination at the C2 position using mercuric acetate and iodine followed by protection of the hydroxyl group at C3 of the steroid core by reaction with methoxymethyl (MOM) chloride. The Suzuki coupling reaction of steroid 119 with 1 and 3- or 4-pyridine boronic acid yielded the desired steroids 120a,b. Acid hydrolysis of 120a,b using HCl solution (10%) yielded 121a,b, which underwent reduction with sodium boron hydride in a methanol/dichloromethane mixture to give pyridinyl steroids 122a,b, respectively (Scheme 31).
Scheme 31. Synthesis of pyridine-2-yl steroids.
In addition, the estrone E1 was treated with triflic anhydride under catalytic conditions to produce steroid 123, which upon reaction with 3- or 4-pyridine boronic acid through Suzuki coupling reactions afforded the desired pyridinyl steroids 124a,b. The final step involved the reduction of 124a,b with sodium boron hydride to yield pyridinyl steroids 125a,b, respectively (Scheme 32).80
Scheme 32. Synthesis of pyridine-3-yl steroids.
The introduction of pyridine-based steroids through the C4 position of the steroid nucleus reflects the versatility and derivatization of this method. Scheme 33 exemplifies the synthetic route to prepare these hetryl steroids through a multi-step sequence. Estrone E1 was selectively brominated at the C4 position via treatment with bromine in acetic acid to yield steroid 126. The protection of the hydroxyl group at the C3 position of steroid 126 was achieved by reaction with methoxymethyl (MOM) chloride to give steroid 127. The Suzuki coupling reactions of 127 with 3- or 4-pyridine boronic acid under palladium-catalyzed conditions yielded the respective pyridinyl steroids 128a,b. The acidic hydrolysis of 128a,b gave the desired 129a,b, respectively. Finally, the selective reduction of 129a,b with sodium boron hydride gave the corresponding pyridine-4-yl steroids 130a,b, respectively (Scheme 33).80
Scheme 33. Synthesis of pyridine-4-yl steroids.
Improving the procedures for the synthesis of alkylated pyridines has attracted increasing attention as chemical modifications of the pyridine ring, mostly at advanced stages, will offer prompt access to analogs of drug candidates, which is highly significant in the progression of drug-discovery.81 Zhou et al.82 intended that a radical generated from photochemical reaction of a particular alkene would serve as an alternative substrate in the reaction with pyridine N-oxides. The photo-irradiation of the steroidal alkene supported by a photocatalyst produced an electrophilic substrate in the reaction with pyridine N-oxide. Intramolecular ortho-addition of the generated radical intermediate followed by subsequent elimination of a carbonyl compound furnished the target product. Zhou et al.82 reported an efficient procedure for the synthesis of steroidal pyridine 133 through photocatalytic alkene cleavage. In this method, the reaction of steroidal ethyl propionate 131 with pyridine N-oxide proceeded in dichloromethane containing catalysts (HBF4/9-mesityl-10-phenylacridinium tetrafluoroborate) under irradiation conditions using blue LEDs to give the desired steroidal pyridine product 133 (Scheme 34). The authors confirmed the formation of the steroidal pyridine product 133 based on the suggested mechanism shown in Scheme 34. Electron transfer occurred between the steroidal ethyl propionate 131 to the activated PC* with the generation of a cation radical intermediate A-15. The interaction of the generated intermediate A-15 and pyridine N-oxide generated the cation radical intermediate C-15 involving the cyclization of the isoxazolidine ring. The deprotonation and intramolecular electron 1,2-shift generated the radical intermediate D-15. The β-N–O bond cleavage was controlled by aromatization, and protonation produced the alkoxy radical intermediate E-15. Further β-C–C bond cleavage with the release of the aldehyde fragment generated intermediate F-15. The previously formed intermediate accepted an electron to give the final product 133 with the release of the photocatalyst.
Scheme 34. Synthesis and the proposed mechanistic route to steroidal pyridine-ring A connection.
4.4. Synthesis from acyclic unsaturated ketones
Shi et al.15 developed the synthesis of binary pyridinyl-based steroids through multi-step reactions. Aluminum oxide/KF prompted the Aldol condensation reaction of pregnenolone with aryl aldehydes to give the respective arylidenes 134a–p. A similar procedure has been reported by the same authors.83,84 The reactions of α,β-unsaturated ketones 134a–p with malononitrile were accomplished in the sodium ethoxide solution to give the corresponding pyridinyl steroids 135a–n and 136a–l. The ethoxide solution acts as a base for the activation of the reacted substrates, as well as the source of an ethoxy group that is linked to the pyridine ring of the formed products. The steroid 137j was obtained in an excellent yield as a sole product from the acetylation reaction of steroid 135j (R2 = 3-pyridyl) with acetyl chloride through treatment under catalyzed conditions (Scheme 35).
Scheme 35. Synthesis of binary pyridinyl-based steroids.
The mechanism of these reactions is suggested to be through the initial Aldol condensation of pregnenolone with aryl aldehydes to form the anticipated intermediates, α,β-unsaturated ketones 134a–p (Scheme 36).16 Subsequent 1,4-Michael addition of the generated intermediates 134 with malononitrile in sodium ethoxide solution generated intermediates A-16. The sodium ethoxide, as a base, prompted the reaction with malononitrile via the elimination of the acidic proton from the active methylene group. Also, the nitrile group of intermediates A-16 was attacked by sodium ethoxide to generate the intermediates B-16. The imine–amine tautomerization is supported by sodium ethoxide to form the enamine intermediate C-16. The intramolecular condensation of the enamine intermediate C-16 occurred between the amino group and the carbonyl group to form 1,4-dihydropyridine intermediates D-16. The air-catalyzed oxidation of intermediates D-16 produced pyridinyl steroids 136 as the major products. The formation of the minor steroids 135 was exemplified through a reasonable decyanation reaction from 1,4-dihydropyridine intermediates D-16. The study suggested the possible use of other alkoxy salts for the preparation of other derivatives of this steroid-based binary pyridine. Also, the plans for this approach recommended the utility of other active methylene substrates such as α-substituted acetonitriles as a new route for future approaches.
Scheme 36. The plausible mechanism for the synthesis of pyridinyl-based steroids from unsaturated ketones.
4.5. Synthesis from arylidenes
Zhang et al.85 reported a different protocol for the synthesis of 3-cyano-2-aminopyridine-based steroids in moderate-to-respectable yields through two reaction steps. The synthesis of steroid 138 was previously reported via the reaction of 3β-acetyl pregnenolone with malononitrile through an aldol-condensation type reaction catalyzed by ammonium acetate in ethanol.86 Accordingly, the reaction of steroidal α,α-dicyanoalkene (138) with DMF-DMA in equimolar amounts under solvent-free conditions gave the respective steroidal enamine 139. The reactions of various aryl amines with steroid 139 under heating and solvent-free conditions gave the steroidal pyridines 140a–k. The products were isolated based on the complete consumption of the reacted substrates, which were monitored by TLC, and the products were purified by column chromatography over silica gel. These reactions were done in a green procedure to obtain the steroidal pyridines with reasonable yields.
The mechanism of this reaction, which is shown in Scheme 37, supposes the formation of enamine derivatives 139 in the first step by reaction of steroidal α,α-dicyanoalkene (138) with DMF-DMA. The enamine derivatives 139 interacted with primary amines, with a nucleophilic attack of the amino group taking place at the nitrile group of 139 to generate intermediate A-17. The tautomerization of A-17 produced imine anion intermediate B-17. The imine group of intermediate B-17 followed an intramolecular nucleophilic attack at the enamine carbon to form the dihydropyridine intermediate C-17. All the derivatives prepared by this method followed the same sequence of intramolecular nucleophilic attack for pyridine ring closure. Interestingly, the products possessed adjacent amine and nitrile groups, which permit for late-stage structural modifications; this is supported by direct modifications on the amino or nitrile groups or the heterocyclic ring closure retaining the adjacent amino and nitrile groups. In the last step, the dimethyl amino group acts as a very good leaving group, leading to the release of the dimethylamine molecule. The aromatization of the pyridine ring was achieved by [1,3]H migration to give the steroidal pyridines 140a–k.85
Scheme 37. Synthetic route and the proposed mechanism for the pyridine ring annulation.
5. Synthesis of complex materials
Pyridine metal complexes87–89 are coordination complexes that contain pyridine as a ligand. Pyridine is a very versatile ligand and can form complexes with a wide variety of metal ions.
The coordination number of pyridine in metal complexes can vary from 2 to 6, depending on the metal ion and the other ligands present. Pyridine metal complexes are known for their varied colors, their ability to catalyze reactions, and their potential biological applications. They are also used in a variety of industrial applications, such as the production of polymers and pharmaceuticals. Koch et al.90 reported the synthesis of a series of ruthenium and iridium complexes 141–146 utilizing the accessibility of steroidal pyridines 116a–c for coordination bonds with different metals. In particular, treatment of 1/2 [RuCl2(p-cymene)]2 with ligands 116a and 116c in methanol containing potassium acetate afforded pyridinyl Ru(ii) complexes 142 and 141, respectively, after stirring at room temperature for 24 h. The complex products were purified by flash column chromatography on silica gel. In addition, treatment of ligands 116c and 116a with 1/2 [IrCp*Cl2]2 under the same conditions yielded pyridinyl iridium(iii) complexes 143 and 144, respectively. The treatment of ligand 116b with 1/2 [RuCl2(p-cymene)]2 in dichloromethane at room temperature afforded the pyridinyl Ru(ii) complex 145 (70%). The ligand 116b also reacted with 1/2 [IrCp*Cl2]2 under the same conditions to give the desired pyridinyl iridium(iii) complex 146 (76%) (Scheme 38).
Scheme 38. Synthesis of iridium(iii) and ruthenium(ii) metal complexes from steroidal pyridine ligands.
6. Biological features
6.1. Anti-neuroinflammatory activity
The anti-neuroinflammatory and anti-oxidative stress activities of pyridosteroid 19 were assessed in vivo in the brain (Fig. 3). A neuro-inflammation model in rats was induced by the cerebral injection of lipopolysaccharide. The cerebral inflammatory state caused by the intracerebral administration of bacterial endotoxin is an indicated by increased lipid peroxidation marker MDA, reduced GSH level, increased NO level, and increased AChE activity in the brain. The steroid 19 was administered subcutaneously to rats treated with LPS. Steroid 19 increased GSH, which reached the normal level. MDA was reduced to the normal level after treatment with steroid 19. The results possibly indicate dihydropyridosteroid 19 as a beneficial candidate for cerebral inflammation treatment.49
Fig. 3. The structure of the pyridosteroid identified as an efficient anti-neuroinflammatory agent.
6.2. Antiviral activity
Wang et al.76 reported the evaluation of pyridinyl steroids 113a–l (Fig. 4) as antiviral agents against respiratory syncytial virus (RSV). The results indicated potent activity for steroid 113l, which was better than ribavirin, “the positive control”. The steroid 113l displayed a remarkable selective therapeutic index value at 172.09, while steroids 113b and 113i displayed comparable results to ribavirin. The in vitro assessment of steroid 113l as an anti-RSV agent against Hep-2 cells indicated that this steroid reduced the infectivity of RSV depending on the sample dose. The steroid 113i was also assessed for anti-RSV activity at diverse treatment times, and the addition of this steroid was revealed to have an improved inhibitory influence on the virus 24 h before virus infection. Additionally, after 24 h of viral infection, the upregulation of TLR-3, interleukin (IL)-6, RIG-I, and its downstream cytokine interferon (IFN)-β was inhibited by either steroid 113l or ribavirin. Steroid 113l inhibited the expression of cytokines through its antiviral impact. Reduced viral replication was documented for this steroid with an increase in inflammatory factors, as well as inhibition of the growth in lung index affected by RSV infection.
Fig. 4. The results and SARs of the antiviral activity of pyridinyl steroids.
6.3. Anti-proliferative activity
The cytotoxic activity was assessed in vitro for steroids annulated with a pyridine ring at the D-ring (2a–f, Fig. 4) against the cervical HeLa and prostate DU 205 tumor cell lines utilizing the MTT assay. Unfortunately, the results indicated very weak cytotoxic effects against both cell lines (IC50 values ≥100 mM) compared to the results of doxorubicin (IC50 = 9.76 ± 0.1141 and 9.00 ± 0.721 mM) respectively.43 On the other hand, the cytotoxic activity of pyridosteroid 42 (Fig. 5) was assessed against the EC-109, EC-9706, and MGC-803 human tumor cell lines using MTT assay as reported by Zhang et al.59 The results verified that this pyridosteroid exhibited more potent cytotoxicity for inhibition of the growth of the MGC803 cell lines than against the EC109 and EC9706 cell lines. The pyridosteroid skeleton provided improved cytotoxicity against the tested cell lines, along with the introduction of an enaminonitrile core and acetate group, which are electron-rich.
Fig. 5. The structure and antiproliferative results of pyridosteroid 42.
The anti-proliferation activity of the investigated pyridosteroids 44a–d (Fig. 6)60 against the growth of the EC109, EC9706, and MGC-803 tumor cell lines was assessed in vitro using MTT assay. In general, the results indicated good-to-moderate cytotoxicity against the growth of the tumor cell lines. In particular, heterosteroid 44b is the most potent cytotoxic agent against the EC109 cell line (IC50 = 14.32 μM), with greater potency than the standard 5-fluorouracil (IC50 = 21.12 μM). The rest of the pyridosteroids also revealed very strong cytotoxicity compared to the standard 5-FU. All pyridosteroids revealed moderate potency against the growth of the EC9706 tumor cell line with slightly improved cytotoxicity for 44c (IC50 = 26.52 μM). The steroids 44b and 44d revealed promising cytotoxic effects with IC50 values of 10.34 and 9.84 μM, respectively. The rest of the pyridosteroids also revealed strong cytotoxicity compared to the result for 5-FU (IC50 = 26.35 μM). The cytotoxic activities of these pyridosteroids against the growth inhibition of the MGC-803 cell line are affected by the types of substituents on the pyridine ring; it is preferable to incorporate an ethyl ester group at the C3 position and a chloro-methane at the C2 position of the pyridine ring. The most common factor in the impacts of these pyridosteroids against the MGC-803 cell lines is that the fused pyridine ring is beneficial for potent cytotoxicity.
Fig. 6. SARs and the anti-proliferative activity results of pyridosteroids 44a–d.
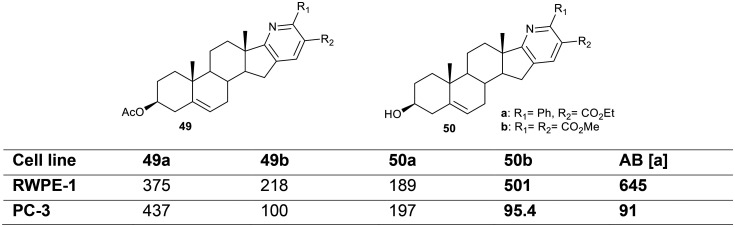
The pyridosteroids of series 49 and 50 (Fig. 7)6 were assessed as cytotoxic agents against the PC-3 cell line using sulforhodamine B (SRB) assay. The results verified that all the steroids exhibited moderate cytotoxicity. In particular, aza-steroids 49b and 50b displayed remarkable cytotoxicity against the PC-3 cell line in a dose-dependent manner in comparison to the results for the standard drug AB (91 μM). On the other hand, steroid 49b demonstrated potent cytotoxicity (218 μM) against human non-tumorigenic prostate epithelial cell RWPE-1 compared to the results for 50b and AB, which showed reduced cytotoxicity (501 μM and 645 μM, respectively). It is worth mentioning that steroid 50b exhibited the most potent cytotoxicity with a broader range of IC50 values against PC-3 and RWPE-1 cells as well as in comparison to abiraterone (AB).
Fig. 7. The cytotoxicity results of pyridosteroids expressed as IC50 values (μM). [a] Anti-prostate cancer drug abiraterone (AB).
The antiproliferative activity of pyridinyl steroids and pyridosteroids 65, 66, 69, and 70 (Fig. 8) was assessed in vitro against various tumor cell lines by MTT assay. The results verified the reduced cytotoxic potency of all the steroids against the growth of the MCF-7, MDA-MB-231, HeLa, and HT-29 cell lines; additionally, they showed no notable cytotoxic effects against the normal MRC-5 cell line. In particular, pyridosteroid 70 is the most potent cytotoxic agent against MCF-7, with higher potency than formestane. The pyridosteroid 66 revealed better cytotoxicity against MDA-MB-231 and HT-29 cells in comparison to the other steroids. The steroid 65 was the most potent against the HeLa cell line, but it still has very weak potency in comparison to the standard anticancer compounds. It was noted that pyridosteroids 66, 69, and 70 demonstrated very strong cytotoxic effects on the growth of PC-3 with IC50 values of 7.93, 12.9, and 11.47 μM, respectively. Pyridosteroid 70 also exhibited remarkable cytotoxicity against the growth of the A549 cell line with an IC50 of 11.89 μM. Thus, the mechanism of action can be illustrated to be based on several factors; the cytotoxic potency of the tested steroids is affected by the sample concentration, structure configuration of the sample, and the type of cell line. Based on the chemical structures, it is worth mentioning that the incorporation of a pyridine ring fused to ring A of the steroid skeleton is crucial for improved cytotoxic results. The binary pyridine ring linked to the steroid skeleton has also a good impact on the cytotoxicity results. It was more likely for a given steroid to inhibit the growth of the tumor cell lines based on the aforementioned factors.63
Fig. 8. The antiproliferative activity results of pyridosteroids against various tumor cell lines.
The anti-proliferative activity of the binary steroidal aminonicotinonitrile analogs 106a–p (Fig. 9) was assessed in vitro via MTT assay against various cancer cell lines.74 Most of the steroids exhibited potent cytotoxicity against all the tumor cell lines. In general, pyridinyl steroids 106f (IC50 = 5.08 ± 0.37–12.39 ± 0.04 μM) and 106p (IC50 = 6.28 ± 0.06–13.84 ± 0.24 μM) were the most active against the SKOV3, HeLa, MCF-7, and HepG2 cell lines. Furthermore, steroid 106a revealed more potent cytotoxicity against the growth of SKOV3 (IC50 = 7.66 ± 0.32 μM) than 5-fluorouracil (IC50 = 25.0 ± 1.03 μM). Less-potent activity was documented for 106j; this might be due to the introduction of a 3-pyridyl substituent on the pyridine ring. Also, noticeable variations in the morphology of the treated cells could be detected. The SARs suggested that the incorporation of groups with electron-donating character, such as hydroxyl groups, linked to the phenyl group at the C4 position of the pyridine ring is essential for potent cytotoxicity. This hypothesis could not be the key factor for potent cytotoxicity, as the introduction of a methoxy group (steroid 106e) diminished the activity, while the bromine substituents improved the cytotoxicity. The key influence might be attributed to the possible formation of a stable free radical on the hydroxyl group through proton dissociation, while the bromine atom is an electron-withdrawing group.
Fig. 9. SARs and cytotoxic activity results of steroidal aminonicotinonitrile analogs.
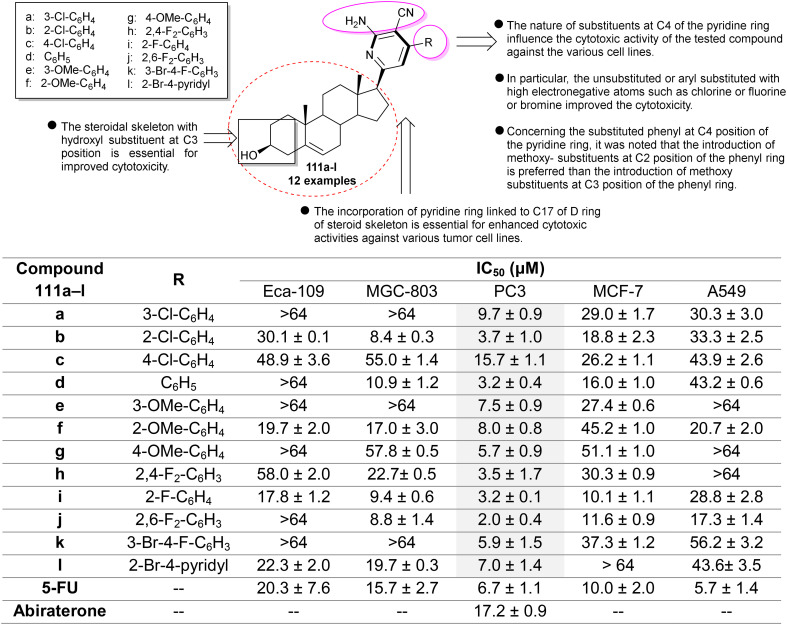
A series of pyridinyl steroids 111a–l (Fig. 10) were evaluated as potential anti-prostate cancer agents using an MTT assay.75 The authors assessed the anticancer activity to investigate the influence of the incorporation of an amino-aryl-cyanopyridine moiety into the steroid skeleton. The results indicated the potential cytotoxicity of steroid 111j against various cell lines, especially the PC3 cell line (IC50 = 2.0 ± 0.4 μM). Steroids 111f (IC50 = 19.7 ± 2.0 μM) and 111i (IC50 = 17.8 ± 1.2 μM) displayed potent activities against the Eca-109 cell line. Additionally, steroids 111b (IC50 = 8.4 ± 0.3 μM), 111d (IC50 = 10.9 ± 1.2 μM), 111f (IC50= 17.0 ± 3.0 μM), 111i (IC50= 9.4 ± 0.6 μM), 111j (IC50 = 8.8 ± 1.4 μM), and 111l (IC50 = 19.7 ± 0.3 μM) demonstrated strong cytotoxicity for the growth inhibition of MGC-803 cells. It is worth mentioning that these steroids effectively inhibited the growth of PC3 cells, with the strongest potency being observed for steroid 111j. These steroids also produced good cytotoxic effects for the growth inhibition of MCF-7 cells (steroid 111i: IC50 = 10.1 ± 1.1 μM; 111j: IC50 = 11.6 ± 0.9 μM). The most reduced cytotoxic activities documented for these steroids were found against A549 cells. In the case of steroid 111j, the injection of the steroid led to the inhibition of cell colonies and migration with an increase in the level of reactive oxygen species in the PC3 cell line depending on the sample concentration, and induced necroptosis. The mechanism of cytotoxicity through the cell proliferation cycle was found to be the phosphorylation of receptor-interacting protein 1/3 (P-RIP1/3) and mixed lineage kinase domain-like protein (P-MLKL) pathway. The pyridinyl steroids exhibit auspicious prospects as anti-proliferative agents, with numerous steroids being applicable in the synthesis for preparing new heterosteroids to develop potent and selective anticancer agents.
Fig. 10. The cytotoxicity results of pyridinyl steroids against miscellaneous tumor cell lines.
Shi et al.15 assessed the potential antiproliferative activity of steroid-based binary pyridines 135a–n, 136a–l, and 137j (Fig. 11) against various cancer cell lines using MTT assay. The results verified the potent activity of steroids 135j (R2 = 3-pyridyl) (IC50 = 1.55 ± 0.19 μM), 136b (IC50 = 4.39 ± 0.64 μM), and 136j (IC50 = 5.84 ± 0.76 μM) against PC-3 cells. Cytotoxic potency was observed for steroids 135j (IC50 = 5.32 ± 0.72 μM) and 136j (IC50 = 2.84 ± 0.45 μM) for the growth inhibition of EC-109 cells. Furthermore, the steroids 135j (IC50 = 2.75 ± 0.44 μM) and 135n (IC50 = 4.48 ± 0.65 μM) revealed very strong cytotoxicity for the growth inhibition of MGC-803 cells. The steroids 135j (IC50 = 7.76 ± 0.89 μM) and 136b (IC50 = 7.80 ± 0.89 μM) are the most potent cytotoxic agents against GES-1 cells. Specifically, the most privileged cytotoxicity was found for steroid 135j in the growth inhibition of the human prostate cancer cell line (IC50 = 1.55 ± 0.19 μM). The SARs recommended the introduction of binary pyridine rings at C17 of the steroid skeleton for improved cytotoxicity. These steroids' mechanism of cytotoxicity was appreciated for the inhibition of colony formation, migration, and evasion of PC-3 cancer cell lines based on the sample dose. The induced apoptosis of these cancer cells (PC-3 cells) is probably achieved through mitochondria-related apoptotic pathways.
Fig. 11. The cytotoxicity results of pyridine-based steroids against different cancer cells.
6.4. Cytochrome P450 (CYP) 1B1 inhibitors
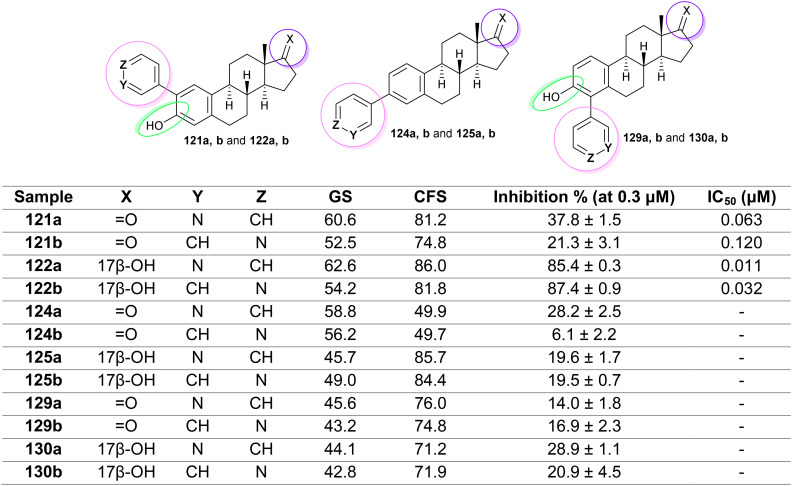
Cytochrome P450 (CYP) 1B1 is an enzyme that is involved in the metabolism of a wide variety of compounds, including drugs, environmental toxins, and endogenous substances. CYP1B1 is highly expressed in the liver, lungs, and skin. Dutour et al.80 assessed the inhibitory activity of steroidal pyridines (Fig. 12) against recombinant human CYP1B1 enzyme by EROD assay, in which NADPH was used as a cofactor. The results indicated very close activities of steroids 122a and 122b against CYP1B1 compared to the results for ANF (94.0 ± 3.2%). Thus, the incorporation of pyridin-3-/4-yl moieties at the C2 position of the steroidal core is preferred for potent activities. In particular, the reduced inhibitory activity of steroids 121a,b compared to steroids 122a,b indicated the impact of reduced forms, exemplified as hydroxyl groups in steroids 122a,b, compared to the corresponding carbonyl groups at C17 in steroids 121a,b. The steroids with pyridine rings linked to the C3 position of the steroid core (124a,b, and 125a,b) exhibited weaker activities than the respective pyridine isomers linked to the steroids at the C2 position. An exception was noticed concerning the lower activity of steroid 124b than the other steroidal pyridines, as these steroids have comparable inhibitory potency. Regarding the inhibitory activity of pyridines linked to the C4 position of the steroid skeleton (“steroids 129a,b and 130a,b”), these steroids displayed similar inhibitory activities to steroids 124a and 125a,b. Slightly improved activities were noted for steroids 130a,b compared to the corresponding isomers in oxidized forms (129a,b).
Fig. 12. The results and structures of steroidal pyridines as cytochrome P450 (CYP) 1B1 inhibitors.
6.5. Androgenic-anabolic activity
Abdalla et al.68 assessed the androgenic-anabolic activity of pyridosteroids 95–98 (Fig. 13) on male albino rats. The results of the mass ratio acquired by the levator ani muscle to the mass acquired by the prostate gland verified the noteworthy androgenic and anabolic effects. For steroidal pyridines, the steroids displayed more anabolic activity than the pyridone derivatives. This behavior is related to the possible formation of hydrogen bonds with the human androgen receptor in the case of pyridine derivatives. Pyridone derivatives revealed fewer effects, which was attributed to the possible tautomerization of these steroids. It was noted that substituted steroidal pyridines with thiol and ethoxy groups had reduced activity, which was attributed to the difficulty of hydrogen bond formation with the less bulky group.
Fig. 13. The results of the androgenic-anabolic activity of steroidal pyridines.
Conclusion
Steroidal monocyclic pyridines have shown a wide range of biological activities, including anti-neuroinflammatory, antiviral, antiproliferative, and androgenic-anabolic activity, cytochrome P450 (CYP) 1B1 inhibition, and acute toxicity. The synthesis of these heterosteroids has been well studied, and several different methods have been developed. Multicomponent synthesis provides a suitable procedure for the preparation of these heterosteroids with good yields, high purity, and reduced reaction time. The reactions of steroidal β-bromovinyl aldehyde with substituted alkynes and alkyl amines yielded the desired fused pyridosteroids. It was preferable to run the reactions using nanocatalysis, e.g., multicomponent reactions of ketosteroids with active methylene components and substituted aldehydes. Additionally, palladium catalyzed the multicomponent reactions of steroidal β-bromovinyl aldehyde with alkynes and benzylamine to give the fused pyridosteroids. Steroidal pyridine fused systems could be also prepared from enaminones, enamides, cyclic unsaturated ketones, acyclic unsaturated ketones, and ketosteroids; in this case, the reactions proceeded through two-component reactions or multi-step syntheses. Binary systems of steroidal pyridines were prepared through multicomponent reactions of aldehydes, active methylene components, and steroids linked to the acetyl group, in which the pyridine ring annulation was constructed on the active acetyl group. These binary systems could also be prepared from ketosteroids through a two-step synthetic route by coupling and substitution reactions from acyclic unsaturated ketones and arylidenes. The pyridine ring is beneficial for the synthesis of metal complexes through the formation of coordination bonds with metal ions. The research into steroidal monocyclic pyridines is still in its early stages, but the results to date are very promising. These heterosteroids have the potential to revolutionize the treatment of a wide range of diseases.
Future perspectives
Recent reviews on steroids have highlighted their potential benefits for drug discovery, pharmacological activity, and steroid diversification, as well as the need for further research.91–93 There are several areas in which future research on steroidal monocyclic pyridines is likely to focus. One area is the development of more potent and selective compounds. The introduction of different functional groups into the pyridine ring or the steroid skeleton can significantly alter the biological properties of the compound. By carefully designing the structure of steroidal monocyclic pyridines, it is possible to create compounds that are more effective and less toxic than existing drugs. Another area of focus for future research is the development of steroidal monocyclic pyridines that can target specific molecular pathways. Many diseases are caused by the activation of specific genes or proteins. By targeting these pathways with steroidal monocyclic pyridines, it may be possible to develop more effective and targeted treatments for diseases. Future research is also likely to focus on the development of steroidal monocyclic pyridines that can be delivered to specific tissues or organs. This is important for the treatment of diseases such as cancer, in which it is necessary to deliver the drug to the tumor cells. By developing steroidal monocyclic pyridines that can be targeted to specific tissues, it becomes possible to improve the efficacy of treatment and reduce side effects. Overall, the future perspectives of research into steroidal monocyclic pyridines are very promising. These steroids have the potential to revolutionize the treatment of a wide range of diseases. With further research, steroidal monocyclic pyridines will likely become a leading class of drugs for the treatment of cancer, viral infections, and other diseases. Future research directions could be based on the development of more potent and selective steroidal monocyclic pyridines with improved pharmacokinetic properties, as well as development for targeting specific molecular pathways involved in disease. There should be increased interest in preparing more pyridine steroids and studying their biological activity extensively in the fields of pharmacology and clinical studies. In addition, future research could be also directed toward the development of steroidal monocyclic pyridines that can be delivered to specific tissues or organs, the study of the mechanisms of action at the molecular level, the development of less-toxic compounds with fewer side effects, and clinical evaluations for the treatment of diseases.
Ethical approval
The research does not comprise dealing with any of the human and/or animal findings and accordingly does not necessitate the agreement of ethical committees, internal review boards, and confirmed guidelines.
The convenience of data and materials
The datasets utilized are the available literature regarding the article topic.
Abbreviations
- MgO NPs
Magnesium oxide nanoparticles
- MW
Microwave
- PPh3
Triphenylphosphine
- WB
Water bath
- DMF-DMA
N,N-Dimethylformamide dimethyl acetal
- DMAP
4-Dimethylaminopyridine
- K2HPO4
Dipotassium phosphate
- Al-(O-i-Pr)3
Aluminum isopropoxide
- p-TsCl
p-Toluenesulfonyl chloride
- DDQ
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
- BF3-OEt2
Boron trifluoride diethyl etherate
- TEA
Triethylamine
- NBS
N-Bromosuccinimide
- DCM
Dichloromethane
- LEDs
Light-emitting diodes
- GSH
Glutathione
- AChE
Acetylcholinesterase
- LPS
Lipopolysaccharide
Cell lines
- MGC-803
Human gastric
- PC-3
Human prostate
- EC-109
Human esophageal
- GES-1
Normal human gastric epithelial
- RWPE1
Epithelial cell
- MCF-7
Breast cancer
- MDA-MB-231
Human breast cancer
- HeLa
Cervical cancer cells
- HT-29
Human colorectal adenocarcinoma
- A549
Adenocarcinomic human alveolar basal epithelial cells
- MRC-5
Human fetal lung fibroblast cells
- SK-OV-3
Human ovarian cancer cell line with epithelial-like morphology
- HepG2
Hepatoblastoma
- Eca 109
Epithelial cell line of human esophageal carcinoma
- GES-1
Normal human gastric epithelium cell line
- EROD
The ethoxyresorufin-O-deethylase
- CFS
Chronic fatigue syndrome
- LD50
Lethal dose, 50%
- CYP
Cytochrome P450 is a hemeprotein that plays a significant role in the metabolism of drugs and other xenobiotics
Conflicts of interest
The authors assert no conflict of interest.
Supplementary Material
Acknowledgments
This study is reinforced via funding from Prince Sattam Bin Abdulaziz University, project number (PSAU/2023/R/1444).
Biographies
Biography
Mohamed M. Hammouda.

Dr. Mohamed M. Hammouda was born in Mansoura, Egypt, in 1983. He received his B.Sc. in 2004 from the Faculty of Science, Mansoura University, Egypt, and his M.Sc. in 2008 from the Faculty of Science, Mansoura University, Mansoura, Egypt. He obtained his Ph.D. in Organic Chemistry in 2013 from the Faculty of Science, Mansoura University, Egypt (Ph.D. thesis: Synthesis and Reactions of some New Isatin Mannich Bases and Related Compounds of Expected Biological Activity). In 2013 he joined the Erasmus Mundus postdoctoral fellowship, Laboratory of Organic and Bio-Organic Synthesis, Gent University, Belgium. His post-doctoral research was in the development of a new type of chiral catalyst for a wide variety of enantioselective reactions. In 2017 he joined the Chemistry Department, Faculty of Science, Mansoura University as a Lecturer of Organic Chemistry, “Synthesis of nitrogen-containing compounds for antioxidant activity”. In 2021 he joined the Department of Chemistry, College of Science and Humanities in Al-Kharj, Prince Sattam Bin Abdulaziz University, Saudi Arabia as an Assistant Professor of Organic Chemistry.
Biography
Khaled M. Elattar.

Dr. Khaled M. Elattar was born in Menyet Sammanoud, Aga, El-dakahlia, Egypt (1979). He received his B.Sc. in 2002 from the Faculty of Science, Mansoura University, Egypt, and his M.Sc. in 2006 from the Faculty of Science, Benha University, Benha, Egypt. He obtained his Ph.D. in Organic Chemistry in 2011 from the Faculty of Science, Mansoura University, Egypt (Ph.D. supervisors: Prof. A. A. Fadda and Prof. A. S. Fouda). He was a Lecturer in Organic Chemistry at the Faculty of Education of the Sert University, Sert, Libya from 2012–2015. He is a member of the Egyptian Chemical Society. He is a reviewer for some international scientific journals. His main research interests are in the fields of organic synthesis, the synthesis of heterocyclic compounds of pharmaceutical interest and medicinal chemistry. He joined the editorial board of OA Journal - Pharmaceutics (2018). https://oa.enpress-publisher.com/index.php/Pha/about/editorialTeam.
References
- Adhya D. Annuario E. Lancaster M. A. Price J. Baron-Cohen S. Srivastava D. P. J. Neuroendocrinol. 2018;30(2):e12547. doi: 10.1111/jne.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab D. F. Gynecol. Endocrinol. 2004;19(6):301–312. doi: 10.1080/09513590400018231. [DOI] [PubMed] [Google Scholar]
- Duh C.-Y. Lo I. W. Wang S.-K. Dai C.-F. Steroids. 2007;72:573–579. doi: 10.1016/j.steroids.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Hanson J. R. Nat. Prod. Rep. 2006;23:100–107. doi: 10.1039/B512848J. [DOI] [PubMed] [Google Scholar]
- Ibrahim-Ouali M. Santelli M. Steroids. 2006;71:1025–1044. doi: 10.1016/j.steroids.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Nongthombam G. S. Borah K. Muinao T. Silla Y. Pal M. Deka Boruah H. P. Boruah R. C. ACS Comb. Sci. 2018;21(1):11–27. doi: 10.1021/acscombsci.8b00140. [DOI] [PubMed] [Google Scholar]
- Kiss M. A. Peřina M. Bereczki L. Baji Á. Bělíček J. Jorda R. Frank É. J. Steroid Biochem. Mol. Biol. 2023;231:106315. doi: 10.1016/j.jsbmb.2023.106315. [DOI] [PubMed] [Google Scholar]
- Rassokhina I. V. Volkova Y. A. Kozlov A. S. Scherbakov A. M. Andreeva O. E. Shirinian V. Z. Zavarzin I. V. Steroids. 2016;113:29–37. doi: 10.1016/j.steroids.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Ansari A. Ali A. Asif M. New J. Chem. 2018;42(1):184–197. doi: 10.1039/C7NJ03742B. [DOI] [Google Scholar]
- Dar A. M. Gatoo M. A. Steroids. 2015;104:163–175. doi: 10.1016/j.steroids.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Attard G. Reid A. H. M. Yap T. A. Raynaud F. Dowsett M. Settatree S. et al. . J. Clin. Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- Ryan C. J. Smith M. R. de Bono J. S. Molina A. Logothetis C. J. de Souza P. et al. . N. Engl. J. Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurášek M. Dzubák P. Sedlák D. Dvoráková H. Hajdúch M. Bartunek P. et al. . Steroids. 2013;78:356–361. doi: 10.1016/j.steroids.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Amr A. E. Abdalla M. M. Hussein M. M. M. Safwat H. M. Elgamal M. H. Russ. J. Gen. Chem. 2017;87(2):305–310. doi: 10.1134/S1070363217020256. doi: 10.1134/S1070363217020256. [DOI] [Google Scholar]
- Shi Y.-K. Wang B. Shi X.-L. Zhao Y.-D. Yu B. Liu H.-M. Eur. J. Med. Chem. 2018;145:11–22. doi: 10.1016/j.ejmech.2017.12.094. [DOI] [PubMed] [Google Scholar]
- (a) Fischer D. S. Allan G. M. Bubert C. Vicker N. Smith A. Tutill H. J. et al. . J. Med. Chem. 2005;48:5749–5770. doi: 10.1021/jm050348a. [DOI] [PubMed] [Google Scholar]; (b) Amr A. E. Abdalla M. M. Bioorg. Med. Chem. 2006;14:4341–4352. doi: 10.1016/j.bmc.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Miller T. C. J. Heterocycl. Chem. 1966;3:338–342. doi: 10.1002/jhet.5570030321. [DOI] [Google Scholar]
- Chelucci G. Gladiali S. Marchetti M. A. J. Heterocycl. Chem. 1988;25:1761–1765. doi: 10.1002/jhet.5570250630. [DOI] [Google Scholar]
- Abbiati G. Arcadi A. Bianchi G. Di Giuseppe S. Marinelli F. Rossi E. J. Org. Chem. 2003;68:6959–6966. doi: 10.1021/jo0347260. [DOI] [PubMed] [Google Scholar]
- Yan J. Z. Li J. Rao G. W. Steroids. 2007;72(11–12):736–739. doi: 10.1016/j.steroids.2007.06.002. doi: 10.1016/j.steroids.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Barthakur M. G. Borthakur M. Boruah R. C. Steroids. 2008;73(11):1137–1142. doi: 10.1016/j.steroids.2008.04.016. doi: 10.1016/j.steroids.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Dutta M. Saikia P. Gogoi S. Boruah R. C. Steroids. 2013;78(4):387–395. doi: 10.1016/j.steroids.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Ackerman J. H. Potts G. O. Beyler A. L. Clinton R. O. J. Med. Chem. 1964;7(2):238–240. doi: 10.1021/jm00332a027. [DOI] [PubMed] [Google Scholar]
- Clinton R. O. Manson A. J. Stonner F. W. Clarke R. L. Jennings K. F. Shaw P. E. J. Org. Chem. 1962;27(4):1148–1154. doi: 10.1021/jo01051a007. [DOI] [Google Scholar]
- Clinton R. O. Manson A. J. Stonner F. W. Neumann H. C. Christiansen R. G. Clarke R. L. Ackerman J. H. Page D. F. Dean J. W. Dickinson W. B. Carabateas C. J. Am. Chem. Soc. 1961;83(6):1478–1491. doi: 10.1021/ja01467a047. [DOI] [Google Scholar]
- De Ruggieri P. Gandolfi C. Chiaramonti D. Gazz. Chim. Ital. 1962;92:768–798. [Google Scholar]
- Shimizu M. Ohta G. Ueno K. Takegoshi T. Chem. Pharm. Bull. 1964;12(1):77–86. doi: 10.1248/cpb.12.77. [DOI] [PubMed] [Google Scholar]
- Elattar K. M. El-Mekabaty A. J. Heterocycl. Chem. 2021;58(2):389–414. doi: 10.1002/jhet.4174. [DOI] [Google Scholar]
- (a) Hammouda M. M. Elattar K. M. Rashed M. M. Osman A. M. Steroids. 2023:109287. doi: 10.1016/j.steroids.2023.109287. [DOI] [PubMed] [Google Scholar]; (b) Monier M. El-Mekabaty A. Abdel-Latif D. Mert B. D. Elattar K. M. Steroids. 2020;154:108548. doi: 10.1016/j.steroids.2019.108548. [DOI] [PubMed] [Google Scholar]
- Monier M. El-Mekabaty A. Abdel-Latif D. Elattar K. M. Synth. Commun. 2019;49(20):2591–2629. doi: 10.1080/00397911.2019.1643889. [DOI] [Google Scholar]
- Monier M. Abdel-Latif D. El-Mekabaty A. Elattar K. M. Synth. Commun. 2020;50(1):1–32. doi: 10.1080/00397911.2019.1686644. [DOI] [Google Scholar]
- Fadda A. A. Badria F. A. Elattar K. M. Med. Chem. Res. 2010;19:413–430. doi: 10.1007/s00044-009-9199-3. doi: 10.1007/s00044-009-9199-3. [DOI] [Google Scholar]
- Fadda A. A. Etman H. A. El-Seidy M. Y. Elattar K. M. J. Heterocycl. Chem. 2012;49(4):774–781. doi: 10.1002/jhet.855. [DOI] [Google Scholar]
- Monier M. Abdel-Latif D. El-Mekabaty A. Elattar K. M. J. Heterocycl. Chem. 2019;56(12):3172–3196. doi: 10.1002/jhet.3727. [DOI] [Google Scholar]
- Fadda A. A. Abdel-Galil E. Elattar K. M. Synth. Commun. 2015;45(18):2053–2082. doi: 10.1080/00397911.2015.1048369. [DOI] [Google Scholar]
- Elattar K. M. Youssef I. Fadda A. A. Synth. Commun. 2016;46(9):719–744. doi: 10.1080/00397911.2016.1166252. [DOI] [Google Scholar]
- Jiang B. Rajale T. Wever W. Tu S. J. Li G. Chem. – Asian J. 2010;5(11):2318–2335. doi: 10.1002/asia.201000310. [DOI] [PubMed] [Google Scholar]
- Ramón D. J. Yus M. Angew. Chem., Int. Ed. 2005;44(11):1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]
- Wang D. Désaubry L. Li G. Huang M. Zheng S. Adv. Synth. Catal. 2021;363(1):2–39. doi: 10.1002/adsc.202000910. [DOI] [Google Scholar]
- Bull J. A. Mousseau J. J. Pelletier G. Charette A. B. Chem. Rev. 2012;112(5):2642–2713. doi: 10.1021/cr200251d. [DOI] [PubMed] [Google Scholar]
- Heusler A. Fliege J. Wagener T. Glorius F. Angew. Chem., Int. Ed. 2021;60(25):13793–13797. doi: 10.1002/anie.202104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D. Yang H. Zhang L. Shao X. Xu X. Li Z. Tetrahedron Lett. 2023;116:154071. doi: 10.1016/j.tetlet.2022.154071. [DOI] [Google Scholar]
- Shekarrao K. Kaishap P. P. Gogoi S. Boruah R. C. RSC Adv. 2014;4(27):14013–14023. doi: 10.1039/C3RA46722H. [DOI] [Google Scholar]
- Larock R. C. J. Organomet. Chem. 1999;576:111–124. doi: 10.1016/S0022-328X(98)01053-5. [DOI] [Google Scholar]
- Shekarrao K. Nath D. Kaishap P. P. Gogoi S. Boruah R. C. Steroids. 2013;78(11):1126–1133. doi: 10.1016/j.steroids.2013.08.002. doi: 10.1016/j.steroids.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Gogoi S. Shekarrao K. Duarah A. Bora T. C. Gogoi S. Boruah R. C. Steroids. 2012;77:1438–1445. doi: 10.1016/j.steroids.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang H. Larock R. C. J. Org. Chem. 2003;68:5132–5138. doi: 10.1021/jo0343228. [DOI] [PubMed] [Google Scholar]
- Roesch K. R. Zhang H. Larock R. C. J. Org. Chem. 2001;66:8042–8051. doi: 10.1021/jo0105540. [DOI] [PubMed] [Google Scholar]
- Mohamed N. R. Abdelhalim M. M. Khadrawy Y. A. Elmegeed G. A. Abdel-Salam O. M. Steroids. 2012;77(13):1469–1476. doi: 10.1016/j.steroids.2012.09.001. doi: 10.1016/j.steroids.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Hassan K. M. El-Dean A. M. K. Youssef M. S. K. Atta F. M. Abbady M. S. Phosphorus, Sulfur Silicon Relat. Elem. 1990;47:283–289. doi: 10.1080/10426509008037980. [DOI] [Google Scholar]
- Lopes S. M. Santos J. R. e Melo T. M. P. Org. Biomol. Chem. 2021;19(5):1122–1132. doi: 10.1039/D0OB02344B. [DOI] [PubMed] [Google Scholar]
- Lopes S. M. M. Gomes C. S. B. Pinho e Melo T. M. V. D. Org. Lett. 2018;20:4332–4336. doi: 10.1021/acs.orglett.8b01783. [DOI] [PubMed] [Google Scholar]
- Lopes S. M. M. Correia C. F. O. Nunes S. C. C. Pereira N. A. M. Ferreira A. R. F. Sousa E. P. Gomes C. S. B. Salvador J. A. R. Pais A. A. C. C. Pinho e Melo T. M. V. D. Org. Biomol. Chem. 2015;13:9127–9139. doi: 10.1039/C5OB01110H. [DOI] [PubMed] [Google Scholar]
- Patel A. K. Rathor S. S. Samanta S. Org. Biomol. Chem. 2022;20(34):6759–6765. doi: 10.1039/D2OB01193J. [DOI] [PubMed] [Google Scholar]
- Hammouda M. M. Elattar K. M. RSC Adv. 2022;12(38):24681–24712. doi: 10.1039/D2RA03864A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vchislo N. V. Asian J. Org. Chem. 2019;8(8):1207–1226. doi: 10.1002/ajoc.201900275. [DOI] [Google Scholar]
- Agamy S. M. Abdel-Khalik M. M. Mohamed M. H. Elnagdi M. H. Z. Naturforsch., B: J. Chem. Sci. 2001;56(10):1074–1078. doi: 10.1515/znb-2001-1016. [DOI] [Google Scholar]
- Stanovnik B. Eur. J. Org. Chem. 2019;2019(31–32):5120–5132. doi: 10.1002/ejoc.201900797. [DOI] [Google Scholar]
- Zhang B. L. Zhang E. Pang L. P. Song L. X. Li Y. F. Yu B. Liu H. M. Steroids. 2013;78(12–13):1200–1208. doi: 10.1016/j.steroids.2013.07.006. doi: 10.1016/j.steroids.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Zhang B. L. Song L. X. Li Y. F. Li Y. L. Guo Y. Z. Zhang E. Liu H. M. Steroids. 2014;80:92–101. doi: 10.1016/j.steroids.2013.12.003. doi: 10.1016/j.steroids.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Duan J. Zhang L. Xu G. Chen H. Ding X. Mao Y. Rong B. Zhu N. Guo K. J. Org. Chem. 2020;85(12):8157–8165. doi: 10.1021/acs.joc.0c01081. [DOI] [PubMed] [Google Scholar]
- Li X. Zhang X. Zhang F. Luo X. Luo H. Adv. Synth. Catal. 2022;364(10):1683–1688. doi: 10.1002/adsc.202200251. [DOI] [Google Scholar]
- Savić M. P. Ajduković J. J. Plavša J. J. Bekić S. S. Ćelić A. S. Klisurić O. R. Jakimov D. S. Petri E. T. Djurendić E. A. MedChemComm. 2018;9(6):969–981. doi: 10.1039/C8MD00077H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraldeschi R. Sharifi N. Auchus R. J. Attard G. Clin. Cancer Res. 2013;19:3353–3359. doi: 10.1158/1078-0432.CCR-12-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurendić E. A. Zaviš M. P. Sakač M. N. Čanadi J. J. Kojić V. V. Bogdanović G. M. Penov Gaši K. M. Steroids. 2009;74:983–988. doi: 10.1016/j.steroids.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Zhou J. F. Tu S. J. Feng J. C. J. Chem. Res. 2001;2001(7):268–269. doi: 10.3184/030823401103169874. [DOI] [Google Scholar]
- Amr A. G. E. Abdulla M. M. Bioorg. Med. Chem. 2006;14(13):4341–4352. doi: 10.1016/j.bmc.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Abdalla M. M. Amr A. E. G. E. Al-Omar M. A. Hussain A. A. Amer M. S. Russ. J. Bioorg. Chem. 2014;40(5):568–578. doi: 10.1134/S106816201405001X. [DOI] [Google Scholar]
- Phillips A. P. J. Am. Chem. Soc. 1949;71(12):4003–4007. doi: 10.1021/ja01180a037. [DOI] [PubMed] [Google Scholar]
- Vanden Eynde J. J. Mayence A. Molecules. 2003;8(4):381–391. doi: 10.3390/80400381. [DOI] [Google Scholar]
- and references therein; Fischer D. S. Allan G. M. Bubert C. Vicker N. Smith A. Tutill H. J. Wood A. P. L. Packham G. Mahon M. F. Reed M. J. Potter B. V. L. J. Med. Chem. 2005;48:5749. doi: 10.1021/jm050348a. [DOI] [PubMed] [Google Scholar]
- Catozzi N. Bromley W. J. Wasnaire P. Gibson M. Taylor R. J. Synlett. 2007;2007(14):2217–2221. doi: 10.1055/s-2007-984918. [DOI] [Google Scholar]
- Fernández Sainz Y. Raw S. A. Taylor R. J. J. Org. Chem. 2005;70(24):10086–10095. doi: 10.1021/jo0518304. doi: 10.1021/jo0518304. [DOI] [PubMed] [Google Scholar]
- Song Y. L. Tian C. P. Wu Y. Jiang L. H. Shen L. Q. Steroids. 2019;143:53–61. doi: 10.1016/j.steroids.2018.12.007. doi: 10.1016/j.steroids.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Sun Y. Gao P. Zhu L. Li Z. Zhao R. Li C. Shan L. Steroids. 2021;171:108841. doi: 10.1016/j.steroids.2021.108841. [DOI] [PubMed] [Google Scholar]
- Wang Z. Hou D. Fang J. Zhu L. Sun Y. Tan Y. Gu Z. Shan L. J. Med. Virol. 2021;93(6):3428–3438. doi: 10.1002/jmv.26604. [DOI] [PubMed] [Google Scholar]
- Koch V. Nieger M. Bräse S. Adv. Synth. Catal. 2017;359(5):832–840. doi: 10.1002/adsc.201601289. [DOI] [Google Scholar]
- (a) Barton D. H. R. O'brien R. E. Sternhell S. J. Chem. Soc. 1962:470–476. doi: 10.1039/JR9620000470. [DOI] [Google Scholar]; (b) Mori H. Tsuneda K. Chem. Pharm. Bull. 1963;11(11):1413–1417. doi: 10.1248/cpb.11.1413. [DOI] [PubMed] [Google Scholar]
- Xu L. Spink D. C. Anal. Biochem. 2008;375(1):105–114. doi: 10.1016/j.ab.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutour R. Cortés-Benítez F. Roy J. Poirier D. ACS Med. Chem. Lett. 2017;8(11):1159–1164. doi: 10.1021/acsmedchemlett.7b00265. doi: 10.1021/acsmedchemlett.7b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Wencel-Delord J. Glorius F. Nat. Chem. 2013;5:369–375. doi: 10.1038/nchem.1607. [DOI] [PubMed] [Google Scholar]; (b) Cernak T. Dykstra K. D. Tyagarajan S. Vachal P. Krska S. W. Chem. Soc. Rev. 2016;45:546–576. doi: 10.1039/C5CS00628G. [DOI] [PubMed] [Google Scholar]
- Zhou W. Miura T. Murakami M. Angew. Chem., Int. Ed. 2018;57(18):5139–5142. doi: 10.1002/anie.201801305. [DOI] [PubMed] [Google Scholar]
- Yu B. Sun X.-N. Shi X.-J. Qi P.-P. Zheng Y.-C. Yu D.-Q. Liu H.-M. Steroids. 2015;102:92–100. doi: 10.1016/j.steroids.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Yu B. Shi X.-J. Zheng Y.-F. Fang Y. Zhang E. Yu D.-Q. Liu H.-M. Eur. J. Med. Chem. 2013;69:323–330. doi: 10.1016/j.ejmech.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Zhang Y. L. Li Y. F. Shi X. L. Yu B. Shi Y. K. Liu H. M. Steroids. 2016;115:147–153. doi: 10.1016/j.steroids.2016.09.005. doi: 10.1016/j.steroids.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Yu B. Shi X.-J. Ren J.-L. Sun X.-N. Qi P.-P. Fang Y. et al. . Eur. J. Med. Chem. 2013;66:171–179. doi: 10.1016/j.ejmech.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Happ B. Winter A. Hager M. D. Schubert U. S. Chem. Soc. Rev. 2012;41(6):2222–2255. doi: 10.1039/C1CS15154A. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Orvilelle-Thomas W. J. J. Mol. Struct. 1977;37(2):321–327. doi: 10.1016/0022-2860(77)80096-3. [DOI] [Google Scholar]
- Chelucci G. Coord. Chem. Rev. 2013;257(11–12):1887–1932. doi: 10.1016/j.ccr.2012.12.002. [DOI] [Google Scholar]
- Koch V. Meschkov A. Feuerstein W. Pfeifer J. Fuhr O. Nieger M. Schepers U. Bräse S. Inorg. Chem. 2019;58(23):15917–15926. doi: 10.1021/acs.inorgchem.9b02402. [DOI] [PubMed] [Google Scholar]
- Bansal R. Suryan A. ACS Bio Med Chem Au. 2022;2(4):340–369. doi: 10.1021/acsbiomedchemau.1c00071. doi: 10.1021/acsbiomedchemau.1c00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito V. Alves G. Almeida P. Silvestre S. Molecules. 2021;26(7):2032. doi: 10.3390/molecules26072032. doi: 10.3390/molecules26072032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera L. Attorresi C. I. Ramírez J. A. Rivera D. G. Beilstein J. Org. Chem. 2019;15(1):1236–1256. doi: 10.3762/bjoc.15.121. doi: 10.3762/bjoc.15.121. [DOI] [PMC free article] [PubMed] [Google Scholar]