Abstract
This article mainly reviews the biomedicine applications of two metal–organic frameworks (MOFs), MIL-100(Fe) and MIL-101(Fe). These MOFs have advantages such as high specific surface area, adjustable pore size, and chemical stability, which make them widely used in drug delivery systems. The article first introduces the properties of these two materials and then discusses their applications in drug transport, antibacterial therapy, and cancer treatment. In cancer treatment, drug delivery systems based on MIL-100(Fe) and MIL-101(Fe) have made significant progress in chemotherapy (CT), chemodynamic therapy (CDT), photothermal therapy (PTT), photodynamic therapy (PDT), immunotherapy (IT), nano-enzyme therapy, and related combined therapy. Overall, these MIL-100(Fe) and MIL-101(Fe) materials have tremendous potential and diverse applications in the field of biomedicine.
This review focuses on drug delivery systems based on MIL-100(Fe) and MIL-101(Fe) that have made significant progress in chemodynamic therapy, photothermal therapy, photodynamic therapy, and combined therapy.
1. Introduction
Traditional cancer treatment methods mainly include surgery, radiotherapy, and chemotherapy, but these methods have significant drawbacks. Surgical treatment has a high risk and large trauma area, and is prone to recurrence. Radiotherapy and chemotherapy have significant toxic side effects, damaging normal cells and tissues and leading to a decline in physiological functions. In recent years, the rapid development of nanomaterial technology has brought new possibilities for cancer diagnosis and treatment. Nanomaterials possess excellent optical, magnetic, and electrical properties,1 making them ideal for cancer diagnosis and targeted drug delivery. The application of nanomaterials in cancer diagnosis and treatment has many advantages, including strong detection signals, high specificity and selectivity, and the ability to reduce drug dosage and alleviate side effects.2
An ideal drug carrier should have the ability to load a large amount of drug and control its release, maximizing efficacy while minimizing harm to the body. Currently, there are many materials available as carriers. For instance, activated carbon possesses a high specific surface area and exhibits excellent adsorption properties. However, its pore size distribution is uneven, making precise regulation challenging. Zeolites demonstrate good adsorption capacity and thermal stability, allowing them to carry certain metals or drugs. Yet, they have a lower specific surface area and show reduced selectivity for adsorbing specific molecules. Organic carrier materials primarily encompass liposomes,3 nanoparticles,4 and micelles.5 They are biocompatible, can target drug delivery, and control drug release. However, their drug-loading capacity is generally quite low (less than 5 wt%). Metal–organic frameworks (MOFs) are crystalline materials assembled from metal nodes and organic ligands. Compared to activated carbon and zeolites, MOFs offer the advantages of adjustable pore sizes and high selectivity. Relative to organic carriers, MOFs have a higher drug-loading capacity. As a new material, MOFs are characterized by a large specific surface area, abundant porosity, high drug-loading capacity, tunable pore size, good biocompatibility, and modifiable surfaces. They have been widely utilized in areas like catalysis, gas adsorption, energy storage, and drug carriers.6,7 Furthermore, MOFs serve as an excellent platform for designing drug delivery systems, holding significant potential in the field of cancer treatment.6
MOFs can be coordinated with various metals, including Fe, Zn, Ca, Mg, and others.7 Among these metals, Fe is abundant in the Earth's crust and has better biocompatibility and safety compared to other transition metal-based compositions. Additionally, iron-based MOFs (Fe-MOFs) have magnetic targeting and magnetic resonance imaging (MRI) potential applications.8 Through adjusting their structure, Fe-MOFs can also achieve active targeting and integrated diagnosis and treatment functions. Therefore, Fe-MOFs are novel drug carriers with high safety and added value, possessing unique advantages in the delivery of anti-tumor drugs.
Among Fe-MOFs, the MIL series (Materials of Institute Lavoisier) is widely applied. It is one of the earliest Fe-MOF series and exhibits highly controllable pore structures, thermal stability, and chemical stability, such as MIL-53, MIL-88, MIL-100, and MIL-101.9 This article mainly focuses on MIL-100(Fe) and MIL-101(Fe), which have advantages such as high specific surface area, adjustable pore size, and chemical stability. Drug delivery systems based on MIL-100(Fe) and MIL-101(Fe) are commonly used for drug transport and release, antibacterial therapy, and cancer treatment. In cancer treatment, these drug delivery systems have shown potential in modalities such as chemotherapy (CT), chemodynamic therapy (CDT), photothermal therapy (PTT), photodynamic therapy (PDT), immunotherapy (IT), nano-enzyme therapy, and combined therapy. In the Web of Science Database, we conducted searches using “MIL-100(Fe) and therapy” and “MIL-101(Fe) and therapy” as the main topics, respectively. The publication records obtained as of August 17, 2023 are shown in Fig. 1A and B. The development of cancer therapy based on MIL-100(Fe) and MIL-101(Fe) in recent years is illustrated in Fig. 1C. Fig. 1D shows the application ratio of MIL-100(Fe), MIL-101(Fe), and other Fe-MOFs in therapy.
Fig. 1. (A) Data obtained with the topic “MIL-100(Fe) and therapy” in the Web of Science. (B) Data obtained with the topic “MIL-101(Fe) and therapy” in the Web of Science. (C) Timeline of MIL-100(Fe) and MIL-101(Fe) used in cancer treatment in recent years. (D) The application ratio of MIL-100(Fe), MIL-101(Fe), and other Fe-MOFs in therapy.
2. Properties of MIL-100(Fe) and MIL-101(Fe)
2.1. Crystal structure of MIL-100(Fe) and MIL-101(Fe)
MIL-100(Fe) and MIL-101(Fe) are two MOF materials with unique crystal structures and pore structures, widely used in adsorption, catalysis, and drug transport. Their crystal structures are composed of metal ions and organic ligands connected by coordination bonds. By adjusting reaction conditions, features like the size, shape, and pore structure of these structural units can be controlled to meet different application requirements.
The crystal structures of MIL-100(Fe) and MIL-101(Fe) are orthorhombic, with numerous straight-through pores and spherical pores of varying sizes and shapes, suitable for adsorbing and catalyzing molecules of different sizes and shapes.10,11 For example, small pores in MIL-100(Fe) can capture small gas molecules like hydrogen, carbon dioxide, and nitrogen, while its large pores can load larger compounds, such as drugs and proteins. Additionally, by altering the metal ion coordination, organic ligand size, shape, and functional groups, the pore size and shape can be adjusted.12 Controlling reaction conditions like reaction time, temperature, and pH can regulate the crystal size and shape. The catalytic activity and selectivity can also be adjusted by changing the metal ion coordination mode and substituting or replacing ligands. The unique crystal structures of MIL-100(Fe) and MIL-101(Fe) enable them to efficiently load drugs, control the drug release rate, and release them at designated sites, thus achieving precise treatment of cancer.
2.2. Physical properties of MIL-100(Fe) and MIL-101(Fe)
The high specific surface area of MIL-100(Fe) and MIL-101(Fe) is due to their highly ordered pore structures, providing favorable conditions for the full adsorption and reaction of various molecules. Their surfaces also have active sites, such as carbonyl (–C O) and hydroxyl groups (–OH), which can undergo chemical reactions with other molecules. Therefore, their surfaces can be chemically modified to achieve targeted delivery, thereby enhancing the effectiveness of cancer treatment. The pore size distribution of MIL-100(Fe) and MIL-101(Fe) depends on the combination of their ligands and metal ions, which can be controlled by adjusting synthesis conditions and the ligand structure. Furthermore, their pore structures not only have multi-peak distributions but also have pores of different shapes, which is one of the reasons why they can simultaneously adsorb molecules of different sizes and shapes. These characteristics allow MIL-100(Fe) and MIL-101(Fe) to adsorb various types of drug molecules, making them suitable for treating multiple kinds of tumors. In the future, they hold the promise of enabling combination drug therapies to improve treatment outcomes.
The thermal stability of MIL-100(Fe) and MIL-101(Fe) is attributed to the stability of their metal–organic framework structures. Simon and his team studied the thermal stability of MIL-100(Fe) using thermogravimetric analysis, and the results showed that the weight loss was only 5% in the temperature range of 60–340 °C.13 This thermal stability ensures that MIL-100(Fe) and MIL-101(Fe) remain relatively stable at certain temperatures, facilitating effective drug delivery and release, and thus enhancing the therapeutic effect of cancer. In addition to the above physical properties, MIL-100(Fe) and MIL-101(Fe) have other features, such as controllable pore structures, adjustable surface chemical properties, and reproducible synthesis, which make them powerful tools in adsorption, separation, catalysis, and drug delivery.
2.3. Adsorption performance of MIL-100(Fe) and MIL-101(Fe)
MIL-100(Fe) and MIL-101(Fe) have many similarities in terms of their adsorption properties. Their gas adsorption performance is influenced by factors such as pore size, molecular size, temperature, pressure, and polarity. Their excellent adsorption performance makes MIL-100(Fe) and MIL-101(Fe) promising candidates for a wide range of applications in catalysis, separation, storage and cancer treatment. For example, in the catalysis field, they exhibit excellent catalytic performance14 and can be used for the separation and recovery of organic substances and catalysts; in the energy storage field, they can be used as storage materials for gases such as hydrogen;15 in the environmental protection field, they can remove various heavy metal ions, volatile organic compounds, antibiotics, dyes, and other pollutants from water or air.16 In terms of cancer treatment, they can adsorb drug molecules, control the drug release rate, and enhance the drug delivery system. Therefore, the research and application of MIL-100(Fe) and MIL-101(Fe) have very broad prospects. Barjasteh et al. used MIL-100(Fe) as an adsorbent to remove dacarbazine (DTIC) from contaminated water, and the results showed that the maximum adsorption capacity of MIL-100(Fe) for DTIC was up to 292.87 mg g−1. Additionally, the MIL-100(Fe) particles were recoverable through ethanol washing, and could be reused for multiple cycles.17
3. Applications of MIL-100(Fe) and MIL-101(Fe) in drug delivery
In long-term drug carrier research, various strategies based on nanoparticles have been developed to improve the delivery efficiency of traditional drugs. Porous and adjustable nanomaterials, such as MOFs, have become potential drug carriers due to their large specific surface area and high porosity. The tunable functional groups and pore sizes give them an advantage in biomedical applications compared to rigid nanoparticle carriers. Although several types of nano-scale organic carriers, such as micelles and liposomes, have been used for drug delivery, the lack of adjustable pore channels makes drug release difficult to control. In contrast, MOF nanoparticles have high drug loading capacity and controlled release performance. In terms of safety, Fe-MOFs have low toxicity, as shown by toxicological studies on MIL-88(Fe) and MIL-101(Fe), which demonstrated their low toxicity.18,19
In 2006, Horcajada and his team successfully synthesized two MOFs, MIL-100 and MIL-101, and applied them in the field of drug loading. The results showed that these materials exhibited higher drug loading capacity compared to traditional carriers, reaching up to 60% drug loading,20 which was the first demonstration of the potential of porous MOFs in drug loading. Since then, the research on MOFs as drug carriers has rapidly developed. Ding et al. prepared RT-MIL100 at room temperature (RT) using acetic acid as a modifier, and ensured storage stability through a non-toxic cyclodextrin coating. They encapsulated the model drug adenosine monophosphate (AMP) in RT-MIL100. The coated RT-MIL100 exhibited degradability, good colloidal stability, low cytotoxicity, and high drug loading capacity.21
3.1. Application of MIL-100(Fe) and MIL-101(Fe) in the delivery of nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat inflammation, but their low solubility and poor stability limit their clinical efficacy. To overcome these limitations, researchers have turned to nanotechnology, where MOFs such as MIL-100(Fe) and MIL-101(Fe) offer promising solutions. Flurbiprofen (FBP), an NSAID, is virtually insoluble in water and requires an appropriate delivery system. In this regard, Haydar et al. used various analytical techniques, including X-ray diffraction, Fourier-transform infrared spectroscopy, scanning electron microscopy, nuclear magnetic resonance, and FBP release spectroscopy, to analyze the drug loading capacity of the solid material obtained from centrifugation and drying of FBP and acetonitrile mixtures. The results showed that the drug loading capacities of FBP on Fe-MIL-101 and Fe-MIL-100 were 37% and 46%, respectively. When examining the drug release pattern of FBP in phosphate-buffered saline with a pH of 7.4, the total release rates for MIL-100(Fe) and MIL-101(Fe) were 75.2% and 90.3%, respectively, after two days. These results demonstrate that using MOFs as drug delivery systems for FBP provides higher drug loading capacities and longer drug release times, making them a very promising therapeutic option.22 Haydar et al. prepared multi-metal organic frameworks (M-MOFs) and investigated their drug loading capacity for aceclofenac (ACF), with MnII-MIL-100(Fe) showing a loading capacity of 57%, significantly higher than traditional carriers.23
Asadi et al. successfully synthesized MOF derivatives with dipeptide functionalization by using amine-containing MIL-101(Fe) as a raw material and applying isocyanate multi-component reactions. An evaluation of the slow-release performance of ibuprofen over six days using these different functional MIL-101(Fe) materials showed good drug adsorption capacity and slow-release properties.24 It is evident that MIL-100(Fe) and MIL-101(Fe) have significant advantages in the delivery of NSAIDs. First, the large specific surface area of these materials allows for high drug loading, reducing the need for frequent dosing. Second, the adjustable pore size of the materials allows selective adsorption based on the specific drug's molecular size, improving drug efficacy and reducing side effects. Third, the stability of the materials under physiological conditions ensures that the drug remains intact during transport, improving bioavailability and reducing toxicity risks. Fourth, the controlled release of the drug from the materials ensures sustained drug delivery, increasing drug efficacy and reducing the need for frequent dosing. MIL-100(Fe) and MIL-101(Fe) have broad application prospects in the delivery of anti-inflammatory drugs. Future research should focus on optimizing the synthesis and functionalization of MIL-100(Fe) and MIL-101(Fe) for specific anti-inflammatory drugs and developing new routes of administration to achieve maximum therapeutic effects.
3.2. Application of MIL-100(Fe) and MIL-101(Fe) in ophthalmic drugs
Ophthalmic drugs are widely used to treat various eye diseases, such as glaucoma, keratitis, and cataracts. However, due to the unique anatomy and physiology of the eye, drug bioavailability is limited, with the main challenges being the protective barrier of the cornea against drugs and the rapid clearance by tears and excretion systems.25 To overcome these challenges, a drug delivery system that can improve drug bioavailability and provide sustained drug release is needed. MOF-based drug delivery systems offer higher drug loading, which is especially important in ophthalmic drug delivery, as the volume of the eye is small, and the amount of deliverable drug is limited. MOFs have high drug loading capacity for a variety of drugs, making them a powerful tool for developing efficient ophthalmic drug delivery systems. Gandara-Loe et al. loaded MIL-100(Fe) with brimonidine tartrate for the treatment of chronic glaucoma and demonstrated that MIL-100(Fe) has a high loading capacity, reaching up to 50–60 wt%.26 As more research and applications progress, we can expect MOF-based drug delivery systems to play an increasingly important role in the field of ophthalmic drugs.
4. Application of MIL-100(Fe) and MIL-101(Fe) in antibacterial therapy
The advent of penicillin marked the beginning of the development of antibacterial drugs. Since then, antibacterial drugs have experienced rapid growth. Scientists have developed various new antibacterial drugs with different mechanisms of action, greatly benefiting clinical applications. However, with the widespread use of antibacterial drugs, a series of problems have emerged, including irrational use, development of resistance, and significant side effects for some drugs.27–29 The traditional drawbacks of antibacterial drugs include antibiotic resistance, side effects, a small antibacterial spectrum, and difficulty in penetrating biofilms.30 Some metal complexes and metal oxides have been used for antibacterial therapy,31–33 and although they have high antibacterial activity, they exhibit high cytotoxicity.34 Fe-MOF materials offer a new option for antibacterial therapy with many advantages,35 including:
a. Broad-spectrum activity: Fe-MOF materials have been proven to have antibacterial activity against a wide range of bacteria, including Gram-positive and Gram-negative bacteria. This broad-spectrum activity is due to the MOFs' ability to act on multiple bacterial pathways, making it more difficult for bacteria to develop resistance.
b. Low toxicity: Fe-MOF materials have lower toxicity to mammalian cells, which is very important for their potential use as antibacterial drugs. Traditional antibacterial drugs may produce side effects, limiting their use, but Fe-MOFs have shown the ability to target bacteria without damaging healthy cells.
c. Stability: MOFs have high stability, which is an advantage over traditional antibacterial drugs that may decompose or lose effectiveness over time. This stability makes MOFs a good candidate for long-term antibacterial therapy.
d. Ease of modification: MOFs can be easily modified by changing the metal ions or ligands used in their synthesis. This flexibility allows researchers to adjust the properties of MOFs to target specific bacterial strains or pathways.
Han et al. synthesized MIL-100(Fe) nanoparticles and tested their antibacterial activity against Escherichia coli and Staphylococcus aureus. The results showed that MIL-100(Fe) nanoparticles had strong bactericidal effects on both bacteria, with a minimum inhibitory concentration of 5 μg mL−1.36 As shown in Fig. 2A, Song et al. employed a layer-by-layer self-assembly method to fabricate a near-infrared responsive β-NaYF4:Yb,Tm,Gd@MIL-100(Fe) (UCNR@MIL-100(Fe)) nanostructure. This nanostructure exhibited impressive photocatalytic performance, effectively killing bacteria. Under near-infrared light excitation (980 nm), this nanostructure can be activated, triggering a photo-Fenton reaction to produce reactive oxygen species (ROS) for antibacterial applications. As depicted in Fig. 2B, under 980 nm laser excitation, this nanostructure undergoes transitions, with electrons migrating to the material's surface. These electrons react with hydrogen peroxide and surrounding oxygen, ultimately generating hydroxyl radicals. Antibacterial tests revealed that after exposure to 980 nm light and treatment with hydrogen peroxide, this nanostructure demonstrated good antibacterial activity against both Gram-negative and Gram-positive bacteria.37
Fig. 2. Schematic of the synthesis process (A) and the antibacterial mechanism (B) of UCNR@MIL-100(Fe). In the figure, H3BTC represents trimesic acid. Reproduced from ref. 37 with permission from The Royal Society of Chemistry, copyright 2022.
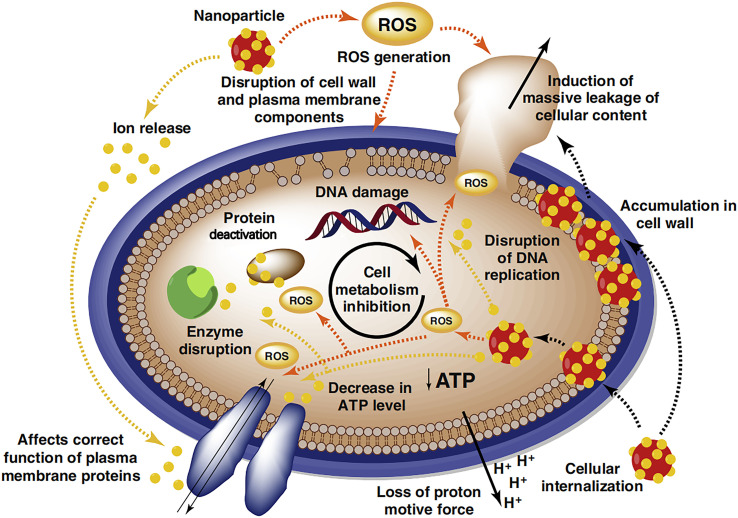
The antibacterial mechanism of Fe-MOFs is mainly through the photocatalytic activity of iron ions, generating ROS and damaging bacterial cell membranes and DNA.38,39 As shown in Fig. 3, Wyszogrodzka and colleagues discovered that the antibacterial mechanism of MOFs is similar to that of metal oxide nanoparticles. The primary actions involve the production of ROS and the release of metal ions. The ROS generated by metal oxide nanoparticles disrupt cell structures, lead to DNA damage, and inhibit cell metabolism. Furthermore, metal oxide nanoparticles can release metal ions, resulting in increased membrane permeability, loss of proton motive force, leakage of cell contents, and disruption of DNA replication, thereby achieving antibacterial effects.40 Liu et al. investigated the antibacterial mechanism of MOFs, finding that their superior antibacterial properties relied mainly on physical contact, metal ions and ligands, oxidative stress, photothermal effects, and synergistic actions.41
Fig. 3. Schematic diagram of the antibacterial mechanism of the metal nanostructured system. Reproduced from ref. 40 with permission from Elsevier, copyright 2016.
In addition, Fe-MOFs can be combined with other materials to form dual or multiple drug resistance mechanisms. Li et al. used MIL-101(Fe)@Ag composite nanoparticles for antibacterial experiments, showing high antibacterial activity and lower cytotoxicity.38 Liu et al. successfully prepared NH2-MIL-101(Fe)@MoS2/ZnO ternary nanocomposites by a wet chemical method, as shown in Fig. 4, which exhibited significant bactericidal performance against both Gram-negative and Gram-positive bacteria under visible light irradiation, with a killing rate of 98.6% for E. coli and 90% for S. aureus after 30 minutes of visible light irradiation.42
Fig. 4. Schematic illustration of the preparation of NH2-MIL-101(Fe)@MoS2/ZnO nanocomposites and the corresponding antibacterial activity. NH2-MIL-101(Fe)@MoS2 microspheres (denoted as F101@MoS2). NH2-MIL-101(Fe)@MoS2/ZnO composites (denoted as F101@MoS2/ZnO). Reproduced from ref. 42 with permission from American Chemical Society, copyright 2022.
The antibacterial properties of Fe-MOFs make them promising candidates for antibacterial therapy. With further research into the properties and applications of these materials, it is expected that Fe-MOFs will have more opportunities for use in antibacterial therapy.
5. Applications of MIL-100(Fe) and MIL-101(Fe) in cancer therapy
Cancer is a disease that poses a serious threat to human health, and its incidence is increasing globally.43 Cancer not only threatens individual health but also imposes a significant burden on the global economy and social development. Therefore, research on cancer prevention and treatment is of great importance. Traditional cancer treatments mainly include surgery, radiotherapy, and chemotherapy, which have certain effects but also some shortcomings. Firstly, these treatments may affect the patient's normal cells, leading to side effects. Secondly, these treatments may not be very effective for some advanced cancers, prone to recurrence or metastasis. Moreover, traditional treatments have limited effectiveness for certain types of cancer, such as pancreatic cancer. Therefore, finding a more effective and safe cancer treatment has become a hot and challenging topic in the field of cancer research. Nanomaterials, as an emerging treatment, offer precision targeting, efficient treatment, and reduced side effects and have been widely used in cancer therapy. MOFs, as excellent drug delivery systems, have great potential in the targeted killing of cancer cells with low toxicity to normal cells. Wang et al. found that MIL-101(Fe) exhibited selective cytotoxicity and angiogenesis inhibition in ovarian cancer cells by downregulating matrix metalloproteinases, with low cytotoxicity.44
The applications of MIL-100(Fe) and MIL-101(Fe) in cancer therapy can be mainly divided into CT, CDT, PTT, PDT, IT, nano-enzyme therapy, and combination therapy. The use of MIL-100(Fe) and MIL-101(Fe) in cancer therapy offers promising prospects for more effective, targeted, and safer treatment options. Further research in this area will help to develop novel MOF-based therapeutics for various types of cancer.
5.1. Chemotherapy
Although traditional chemotherapy for cancer has certain therapeutic effects, it has significant drawbacks, such as poor discrimination between cancer cells and normal cells, lack of targeting, and potential damage to normal cells, leading to side effects. Fe-MOFs are a new type of metal–organic framework material with many excellent properties, which can serve as an effective carrier for chemotherapeutic drugs. Compared to traditional chemotherapy methods, Fe-MOF-based chemotherapy has many advantages. Firstly, Fe-MOFs have stronger targeting ability and can be chemically modified to precisely release drugs into cancer cells, achieving targeted delivery and reducing side effects. Secondly, Fe-MOFs have good controlled-release properties, allowing for the control of the drug release rate and dosage, thereby enhancing the efficacy of the drug. Thirdly, Fe-MOFs have better biocompatibility and can be surface-modified to enhance their biocompatibility, reducing immune reactions and toxicity in the body. Therefore, Fe-MOF-based chemotherapy has broad prospects for application.
In recent years, there has been an increasing amount of research on Fe-MOF-based chemotherapy. Rezaei et al. prepared nanoscale MIL-100(Fe) using ultrasound, encapsulated the anti-cancer drug docetaxel in the nanoscale MIL-100(Fe) for the treatment of breast cancer, and then evaluated its potential as a cancer-targeting nanocarrier. The results showed that the drug-loaded MIL-100(Fe) had a toxic effect on breast cancer cells, with an effective drug payload of 57.2%, far higher than that of traditional carriers.45
Adding some other materials to MIL-100(Fe) can synthesize composite carriers with superparamagnetism, which can improve the efficacy of chemotherapy. Dhawan et al. prepared FeAu@MIL-100(Fe) nanomaterials by wrapping FeAu alloy nanoparticles with MIL-100(Fe). Under the action of an alternating magnetic field (AMF), FeAu@MIL-100(Fe) encapsulating doxorubicin showed superparamagnetism and magnetothermal behavior, with an encapsulation rate and a release rate of 69.95% and 97.19%, respectively. In vitro experiments showed that AMF-induced hyperthermia could cause 90% death of HSC-3 oral squamous cell carcinoma.46 As shown in Fig. 5, Zhao et al. synthesized a graded porous nanocarrier (denoted as USPIO@MIL) by synthesizing MIL-100(Fe) and magnetite through a one-pot method. This nanocarrier has characteristics such as low toxicity, high drug loading capacity, stimulus-responsive drug release, and superparamagnetism. The USPIO@MIL nanocarrier loaded with anti-tumor and anti-inflammatory drugs (doxorubicin and methotrexate) showed highly potent anti-inflammatory and anti-tumor activity.47
Fig. 5. Schematic illustration of (a) the synthesis of USPIO@MIL-100(Fe) nano-objects and (b) their use as theranostic platforms for the treatment of cancer and inflammatory diseases. Reproduced from ref. 47 with permission from The Royal Society of Chemistry, copyright 2023.
Chemotherapy based on Fe-MOFs can also be combined with other treatment methods such as photothermal therapy and photodynamic therapy to achieve multimodal therapy and improve treatment efficacy. In the future, researchers will continue to develop more Fe-MOF materials for chemotherapy, with better control release performance, higher biocompatibility, stronger targeting, etc. The excellent results achieved in the laboratory still require further research to determine their safety, efficacy, and reliability for clinical application, in order to achieve clinical translation.
5.2. Chemodynamic therapy
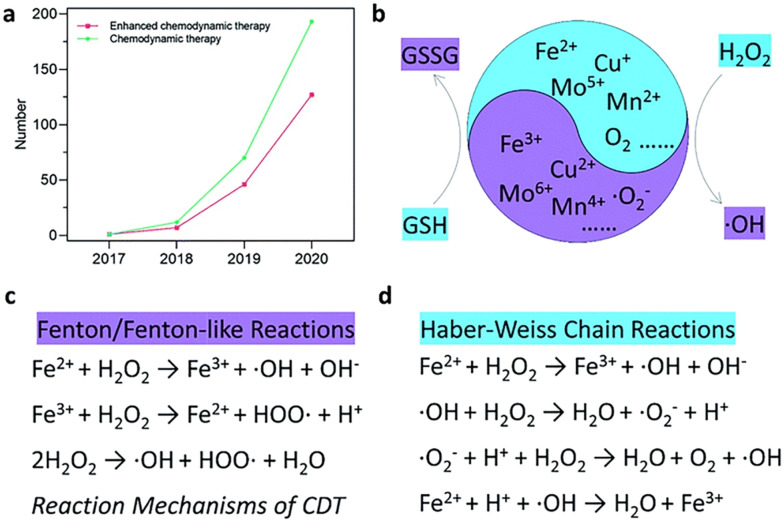
As shown in Fig. 6, CDT is a new cancer treatment strategy that uses Fenton reactions to convert intracellular hydrogen peroxide into highly toxic hydroxyl radicals (˙OH) to kill cancer cells.48,49 CDT demonstrates minimal invasiveness and high tumor specificity by reacting with the acidic and high-concentration hydrogen peroxide microenvironment in tumors, reducing systemic toxicity and providing better therapeutic effects and biocompatibility.50 CDT is less dependent on external stimuli, simplifying the treatment process, and can regulate the hypoxic and immunosuppressive states in the tumor microenvironment, showing great application prospects.
Fig. 6. (a) Number of published research articles for “chemodynamic therapy” and “enhanced chemodynamic therapy” from the Web of Science. (b) Schematic illustration of the chemodynamic therapy mechanism. In the figure, GSH represents glutathione and GSSG represents oxidized glutathione. (c and d) Specific reaction mechanism of Fenton/Fenton-like reactions and Haber–Weiss chain reactions. Reproduced from ref. 48 with permission from The Royal Society of Chemistry, copyright 2022.
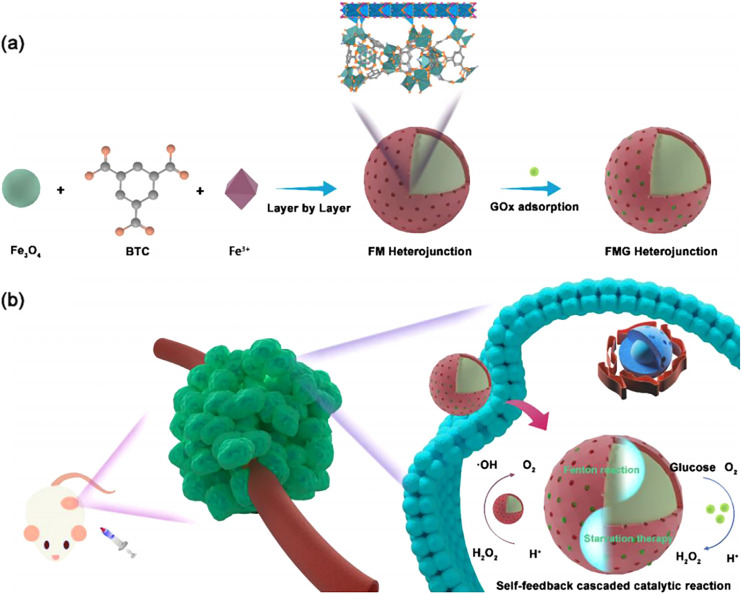
MIL-100(Fe) and MIL-101(Fe) as carriers for Fenton reaction catalysts have many advantages.51 Firstly, MIL-100(Fe) and MIL-101(Fe) have large specific surface areas and pore sizes, enabling high loading of Fenton catalysts. Secondly, by altering the surface functional groups of MOFs, they can achieve precise control of catalyst release, thus reducing systemic toxicity. Thirdly, MIL-100(Fe) and MIL-101(Fe) have good biocompatibility and are less likely to trigger severe immune reactions. Finally, their toxicity in cancer cells is significantly higher than in normal cells, due to the lower pH and higher hydrogen peroxide levels in the tumor microenvironment, which favor Fenton reactions. As shown in Fig. 7, Ni et al. have successfully constructed an Fe3O4@MIL-100(Fe) heterostructure. The heterostructure (recorded as FMG) formed after encapsulation of glucose oxidase (GOx) is an intelligent pH-responsive Fenton nano-system with a starvation therapy synergistic effect.52 GOx can catalyze the conversion of β-d-glucose into gluconic acid and hydrogen peroxide, thus providing an abundant supply of hydrogen peroxide. Moreover, the absence of glucose might cut off the energy supply to cancer cells, leading to starvation therapy for the cancer. The local acidity of the tumor microenvironment accelerates the delivery of GOx, resulting in a sustained production of hydrogen peroxide and gluconic acid, thereby offering a more conducive catalytic microenvironment for the FMG heterostructure. Simultaneously, the oxygen produced during the CDT process can enhance the enzymatic reaction of GOx. This intelligent Fenton nano-reactor can improve the performance of the Fenton reaction, increase the efficiency of CDT, exhibit potent anticancer effects, and has high safety.
Fig. 7. (a) Synthetic procedure of the FMG heterojunction; (b) illustration of enhanced CDT/accelerated ST synergistic cancer therapy. Reproduced from ref. 52 with permission from Elsevier, copyright 2023.
CDT still has some limitations, such as its dependence on hydrogen peroxide levels in the tumor microenvironment, and the unstable and uneven distribution of hydrogen peroxide in the body. The hydroxyl radicals produced by CDT have strong reactivity, but their limited diffusion distance may not effectively kill deep cancer cells. These drawbacks affect the therapeutic effects of CDT. Currently, a common approach to improve the therapeutic effect of CDT is to increase the level of hydrogen peroxide in the tumor microenvironment through alternative methods. Ji et al. used NH2-MIL-101(Fe) as a carrier for the copper chelator d-penicillamine (d-PEN). NH2-MIL-101(Fe)/d-PEN effectively entered cancer cells in the form of nanoparticles and continuously released d-PEN. The released d-PEN chelates with copper, which is highly expressed in the cancer environment, generating additional hydrogen peroxide, which is then decomposed by iron in NH2-MIL-101(Fe) to produce ˙OH. NH2-MIL-101(Fe)/d-PEN has a toxic effect on cancer cells but not on normal cells.53
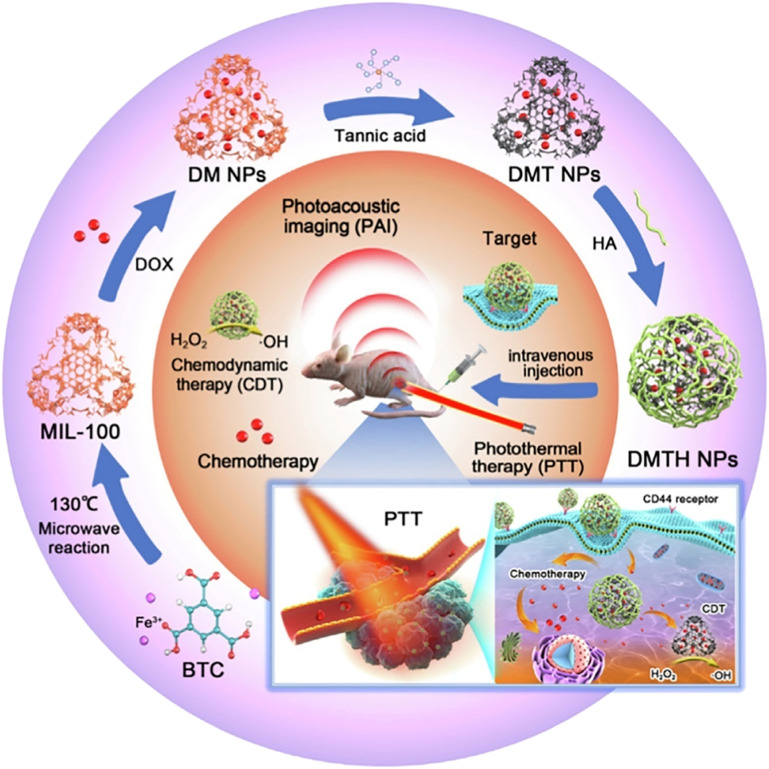
In recent years, the development of CDT strategies based on multifunctional MOFs has become a research hotspot. These systems can combine CDT with other cancer treatment strategies, such as chemotherapy, photothermal, and photodynamic therapy, to overcome the limitations of single treatments. Chen et al. synthesized MIL-101(Fe) as a catalyst and simultaneously delivered photosensitizers, combining chemodynamic and photodynamic effects to generate more ROS in tumors and enhance therapeutic effects.54 As shown in Fig. 8, Hu et al. loaded doxorubicin (DOX) into MIL-100(Fe), and then modified it with tannic acid (TA) coordination and hyaluronic acid (HA) encapsulation to prepare multifunctional nanoparticles (DMTH NPs) for photoacoustic imaging (PAI)-guided chemodynamic/chemo/photothermal synergistic therapy. DMTH NPs target tumors with low in vivo toxicity and effectively treat breast cancer.55
Fig. 8. The preparation of multifunctional nanoparticles (DMTH NPs) by loading doxorubicin (DOX) into MIL-100 modified with tannic acid (TA), encapsulated in hyaluronic acid (HA), and used for chemodynamic/chemo/photothermal synergistic therapy guided by photoacoustic imaging (PAI), which resulted in antitumor therapy. Reproduced from ref. 55 with permission from Elsevier, copyright 2022.
Further improvements and optimizations of these systems are needed to enhance their therapeutic effects and application prospects. By addressing these limitations, MOF-based CDT may become a more effective and promising cancer treatment method.
5.3. Photothermal therapy
PTT is an alternative, minimally invasive, and targeted cancer treatment method that offers a promising approach for improving cancer therapy. As shown in Fig. 9, under the irradiation of external light sources like near-infrared light (NIR), photothermal agents (PTA) can absorb photon energy, transitioning from the ground state to the excited state. They then collide with surrounding molecules, producing kinetic energy, which eventually converts into heat. This localized heat induces therapeutic effects, leading to cancer cell death through various mechanisms, such as protein denaturation, membrane damage, and cell apoptosis.56 Compared to traditional cancer treatments, PTT has fewer side effects, higher specificity, the possibility of repeated treatments, and relatively less damage to surrounding healthy tissues.
Fig. 9. Schematic diagram of tumor imaging and PTT in the second near-infrared window. Reproduced from ref. 56 with permission from Frontiers, copyright 2022.
The core of PTT lies in PTAs. Light-absorbing materials such as gold nanoparticles, carbon nanotubes, and organic dyes have been extensively studied, but their limitations in toxicity, stability, and photothermal conversion efficiency are evident. MOFs have attracted widespread attention in PTT applications due to their unique structural and functional characteristics. Firstly, the porosity of MOFs enables the loading of various materials, including PTAs, drugs, or imaging agents, promoting the use of MOFs as multifunctional therapeutic platforms. Secondly, the porous structure of MOFs allows for controlled release of therapeutic agents, enabling the design of stimulus-responsive systems that can be activated by light, pH, etc. Su et al. combined NH2-MIL-101(Fe) with graphitic carbon nitride (g-C3N4) nanosheets, improving its photothermal performance and stability.57 Chien et al. synthesized an MCP-1/GNR@MIL-100(Fe) composite material, utilizing the tumor-targeting ability of macrophages to achieve PTT, effectively inhibiting tumors.58 As shown in Fig. 10, a triple-negative breast cancer (TNBC) targeted peptide (CTVRTSADC, ZD2)-engineered and a single gold nanostar (AuNS) encapsulated within MIL-101-NH2(Fe) through a process of coating the MOF with four cycles, results in well-defined core–shell AuNS@MOF-ZD2 nanocomposites. This composite material exhibits excellent biocompatibility and stable photothermal conversion capability with a photothermal conversion efficiency of 40.5%. The fabricated AuNS@MOF-ZD2 nanoprobes can specifically target TNBC cells (MDA-MB-231) but not other breast cancer subtypes (MDA-MB-435, MDA-MB-468, and MCF-7). This composite material demonstrates effective and targeted therapeutic outcomes in TNBC treatment.59
Fig. 10. Schematic illustration of the synthesis of AuNS@MOF-ZD2 nanoprobes and application for T1-weighted MR imaging and photothermal therapy specifically toward MDA-MB-231 tumor (TNBC). Reproduced from ref. 59 with permission from Wiley, copyright 2018.
MIL-100(Fe) and MIL-101(Fe) have been identified as promising candidates for PTT treatment of tumors due to their unique structural properties, high specific surface areas, and excellent thermal stability. However, challenges still remain regarding their biocompatibility, toxicity, and in vivo performance. Future research should focus on optimizing their combination with other materials, developing novel MOFs with enhanced photothermal properties, functionalizing them for targeted therapy and evaluating their safety and effectiveness in clinical settings.
5.4. Photodynamic therapy
PDT is a novel cancer treatment method60,61 that primarily relies on photosensitizers to catalyze oxygen production of ROS under light irradiation, killing cancer cells.62,63 Compared to traditional cancer treatments, PDT has several advantages, including non-invasiveness, selective targeting, low toxicity, and potential effectiveness for various cancers. It can be applied to a wide range of cancers, including skin cancer,64 lung cancer,65 and esophageal cancer,66 among others.
Nanomaterials used as photosensitizers in PDT offer several advantages over traditional photosensitizers. Firstly, they have a much higher surface area-to-volume ratio, which can improve the solubility and bioavailability of the photosensitizer. Secondly, they can be engineered to have specific size, shape, and surface properties, which can improve the targeting and internalization by cancer cells. Thirdly, their optical properties can be tuned to absorb light at specific wavelengths, allowing for deeper tissue penetration and higher selectivity for tumor tissues. Finally, their small size allows for rapid clearance from the body, reducing the risk of long-term toxicity.67
Nanomaterials such as gold nanoparticles, carbon-based nanomaterials, and semiconductor nanocrystals have been explored as potential photosensitizers in PDT. However, these materials have limitations in terms of stability, biocompatibility, and specificity. MOFs, on the other hand, have emerged as a promising class of nanomaterials for PDT due to their high surface area, tunable porosity, and excellent biocompatibility.
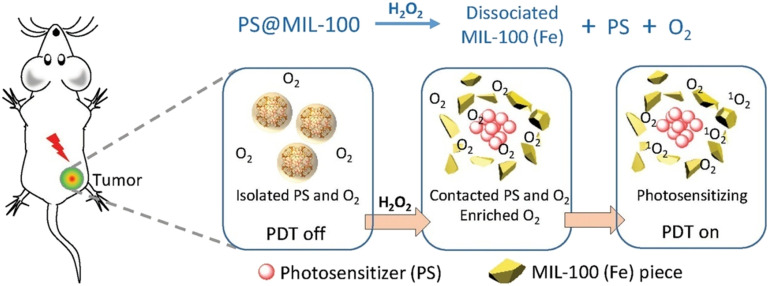
MOFs can be designed to have high loading capacities for photosensitizers, enabling efficient delivery of the photosensitizer to cancer cells. Additionally, the porous structure of MOFs can be engineered to allow for controlled release of the photosensitizer in response to specific stimuli, such as light or changes in pH. As shown in Fig. 11, Hu et al. found that when a photoactivatable photosensitizer (PS) is encapsulated in MIL-100(Fe), the photosensitizing ability is suppressed due to isolation from oxygen. Upon reaction of iron ions with hydrogen peroxide, the MIL-100(Fe) framework collapses, and the encapsulated PS is re-exposed to oxygen, activating the photosensitizer. The decomposition of hydrogen peroxide generates oxygen, which alleviates tumor hypoxia and enhances the PDT effect.68 Ma et al. performed an engineered design using MIL-101(Fe) based on iron(iii) carboxylate to graft DNA onto PSs after loading. After reacting with hydrogen peroxide in the tumor, MIL-101 selectively degrades, releasing the loaded PDT reagent and restoring its photosensitivity, allowing for the controlled killing of cancer cells.69
Fig. 11. Illustration of the inhibition and activation of the photosensitization of the PS in tumor after its encapsulation into MIL-100 (Fe). Reproduced from ref. 68 with permission from Wiley, copyright 2018.
Furthermore, MOFs can enhance the therapeutic effect of PDT by loading chemotherapy drugs. Wang et al. prepared small-sized metal–organic framework MIL-101(Fe) as a smart delivery system to load the chemotherapeutic drug dihydroartemisinin (DHA) and photosensitizer methylene blue (MB). In the tumor environment, the iron ions released from MOFs not only enhance the efficacy of DHA but also catalyze the release of oxygen from hydrogen peroxide, further improving the photodynamic therapy effect of the nanocomposite.70
Overall, the unique properties of MOFs make them an attractive option for developing new photodynamic therapies for cancer treatment. With further research and development, MOF-based PDT may become a more effective and targeted treatment option for various types of cancer.
5.5. Immunotherapy
Immunotherapy (IT) is an effective cancer treatment method that primarily enhances the immune system's recognition and attack capabilities against cancer cells.71–73 Compared to traditional therapies such as chemotherapy and radiation therapy, IT has higher survival rates and fewer adverse reactions. Cancer immunotherapy has various types, including monoclonal antibodies, cancer vaccines, and cell therapies.74 One of the key challenges of IT is developing effective drug delivery systems that can modulate immune responses and target immune cells.
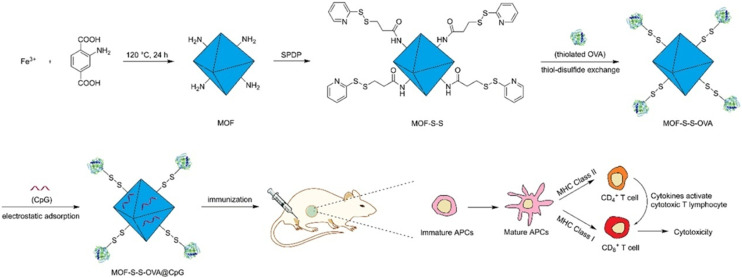
MOFs can serve as drug delivery carriers in IT, where controlled release of drugs from MOFs can enhance their efficacy and reduce systemic toxicity.75 MIL-100(Fe) and MIL-101(Fe) have demonstrated excellent drug loading capacity and controlled release performance for a variety of therapeutic drugs, including chemotherapy drugs, small molecule inhibitors, and immunomodulatory drugs. As shown in Fig. 12, Yang and colleagues utilized MIL-101-Fe-NH2 as a carrier to construct an innovative redox-responsive antigen delivery system for the transport of ovalbumin (OVA) and cytosine–phosphate–guanine (CpG) oligonucleotide. In vitro cell experiments demonstrated that the MOF nanoparticles not only significantly enhance the uptake of OVA by antigen-presenting cells but also deliver both OVA and CpG to the same cell. Through the controlled release of OVA upon reduction and the enhancing effects of CpG, this delivery system can induce a robust cellular immunity and cytotoxic T lymphocyte response in mice.76 Hidalgo and others conducted immune induction experiments using MIL-100(Al), MIL-100(Fe), and ZIF-8(Zn) (ZIF stands for zeolitic imidazolate framework). The results showed that MIL-100(Fe) induced a higher immune response.77
Fig. 12. Schematic representation of the preparation of the MOF-based antigen delivery system and its role in eliciting strong cellular immunity response. Reproduced from ref. 76 with permission from American Chemical Society, copyright 2018.
Additionally, MOFs can function as scaffolds for the development of tumor vaccines, representing another promising application area. These MOFs can be loaded with tumor-associated antigens (TAAs) or neoantigens, activating the immune system and enhancing its ability to recognize and attack cancer cells. Duan et al. developed a dual delivery platform based on MOFs for antigens and immunostimulatory molecules, and this nanocarrier enhanced the antitumor effect on melanoma by recruiting tumor-killing immune cells.78
Despite significant achievements in cancer IT with MOFs, several challenges remain to be addressed. The first challenge is to improve the targeting of these MOFs to tumor tissues. Some studies have functionalized them with antibody or peptide targeting moieties to enhance their specificity towards tumor cells. The second challenge is to optimize drug loading and release performance for specific therapeutic agents. MOFs' physicochemical properties, such as pore size, specific surface area, and surface chemistry, must be optimized to achieve the best drug loading and release kinetics. This may require the development of new synthesis strategies or the exploration of other MOFs with suitable properties for IT applications. Lastly, understanding the mechanisms of action of these MOFs in modulating the immune system is necessary. Although some studies have shown that they have immunostimulatory effects, their exact mechanisms of action remain unclear. Further research is needed to elucidate the potential mechanisms of these MOFs in immune system regulation. Additionally, the safety and toxicity of these MOFs in vivo need to be assessed. While MIL-100(Fe) and MIL-101(Fe) have demonstrated good biocompatibility in vitro, further investigation is required to evaluate their in vivo toxicity and long-term effects on the immune system.
5.6. Nanoscale enzyme therapy for tumors
Enzymes are highly specific and efficient protein or RNA catalysts that catalyze various reactions effectively within living organisms. Currently, enzymes are utilized in various fields, such as the food industry,79 detergent industry,80 textile industry,81 and pharmaceutical industry.82 However, the high cost, low stability, and challenges in transportation and storage of enzymes have limited their widespread applications. With the continuous development of nanotechnology and biotechnology, nanozymes, a new type of biomaterial with broad application prospects, have received widespread attention. Due to their excellent biocompatibility, high catalytic activity, and strong stability, nanozymes have enormous potential in the fields of biosensing,83 antibacterial treatment,84 and tumor therapy.85 MOF nanozymes have good degradability, and in comparison to traditional inorganic nanozymes, degradable MOF nanozymes have significantly lower toxicity in the body.86 Fe-MOFs possess catalytic activity and selectivity similar to various natural enzymes, making them ideal for the development of new nanozymes. Fe-MOF nanozymes can catalyze the hydrolysis of hydrogen peroxide to produce a significant amount of hydroxyl radicals, which generate a large amount of ROS. In the tumor microenvironment, the concentration of hydrogen peroxide is relatively high, so these nanozymes can generate a considerable amount of ROS at the tumor site. ROS can damage the DNA, proteins, and lipids of tumor cells, leading to apoptosis. Fe-MOF nanozymes can also modulate the tumor microenvironment by reducing the acidity at the tumor site, helping inhibit the growth and spread of tumor cells.
Ma et al. designed a degradable MIL-101(Fe) nanozyme for tumor therapy, which can catalyze the generation of ˙OH from hydrogen peroxide in the tumor microenvironment. Under microwave radiation, a substantial amount of ˙OH can enter the tumor in a highly efficient and non-invasive manner, resulting in microwave-enhanced dynamic therapy (MEDT).87 As shown in Fig. 13, Wu et al. developed a Pt-MIL-101(Fe)-based nano-catalytic drug that catalyzes the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) and the subsequent nanozyme-catalyzed cascade reaction to produce hydroxyl radicals and block glutathione (GSH) regeneration, promoting cancer cell ferroptosis for iron-based tumor therapy.88
Fig. 13. Schematic illustration of Pt-MIL-101 nanocatalytic medicine-initiated cascade catalytic reactions for ferroptosis sensitivity. The NOX/SOD (NOX: NADPH oxidase, SOD: superoxide dismutase)-mimic activities of the Pt-MIL-101 nanomedicine produce superoxide anions (O2˙−), H2O2, and ˙OH in a cascade manner. Based on the cascade enzyme-mimic reaction activities, the Fenton reaction activity and NADPH depletion capacity of Pt-MIL-101 not only trigger the ROS production for lipid peroxidation, but also facilitate the GSH regeneration prevention for glutathione peroxidase 4 (GPX4) deactivation and drive the consequent cancer cell and tumor ferroptotic suppression. Reproduced from ref. 88 with permission from Elsevier, copyright 2022.
Although significant progress has been made at the experimental level, the application of Fe-MOF nanoscale enzymes in tumor treatment still faces several challenges. Firstly, the studies on biodistribution and pharmacokinetics are insufficient, requiring further exploration of the behavior of nanoscale enzymes within living organisms. Secondly, achieving precise targeting and controlled release of nanoscale enzymes still requires breakthroughs. Lastly, in-depth research is needed to evaluate the long-term safety and potential toxicity of nanoscale enzymes.
In the future, the development of more novel nanoscale enzymes will continue, such as those based on other metal–organic framework materials, further expanding the application scope of nanoscale enzymes in tumor treatment. Additionally, the combination of nanoscale enzymes with other treatment strategies, such as photodynamic therapy and immunotherapy, is expected to achieve more effective and safer tumor treatment.
5.7. Combined therapy
Although progress has been made in MOF-based nanoscale drug delivery systems,89 the therapeutic effect of single treatments is still insufficient during the treatment process. For example, CDT is ineffective in the weakly acidic tumor microenvironment, and PDT's extreme dependence on oxygen leads to poor results in the treatment of hypoxic solid tumors. In comparison to single therapies, combined therapies have several advantages in treating tumors, including synergistic effects, reduced resistance, enhanced tumor response, enhanced immune response, and low toxicity. Various combined cancer treatments have been developed, such as PTT with CT, CDT with PTT, and PDT with IT.
5.7.1. PTT + CT
The combination of PTT and CT has always been a research hotspot in the field of cancer treatment. The use of PTT or chemotherapy alone has limitations, and the combination of PTT and CT improves cancer treatment. MOFs have large pore sizes, allowing for high drug loading and delivery of chemotherapy drugs to tumor sites. Mileo et al. investigated the drug-loading capacity of MIL-100(Fe) for the anticancer drug 5-fluorouracil (5-FU), and the results demonstrated that MIL-100(Fe) had a high drug loading capacity.90
Combining PTT and CT based on MOFs can improve the therapeutic effect of tumor treatment and reduce the toxic side effects of traditional chemotherapy. As shown in Fig. 14, Wang et al. prepared a new core–shell structure of Pb@MIL100(Fe) bimetallic MOF (d-MOF) nanoparticles and studied the in vitro and in vivo combined effects. The d-MOFs have a high load of artemisinin (ART), and the inner layer of PB MOFs can be used for PTT due to their strong absorption in the near-infrared region. The combination of chemical and photothermal therapy produced excellent synergistic therapeutic effects.91 Gao et al. designed an AuNRs@MIL-101(Fe)-NH2@CP[5]A nanosystem by wrapping gold nanorods in MIL-101(Fe) and then installing carboxylatopillar[5]arene (CP[5]A)-based supramolecular gates. This system can load the anticancer drug 5-fluorouracil and has effective chemo-photothermal synergistic treatment capabilities.92 Liu et al. loaded oxaliplatin (OXA) and indocyanine green (ICG) into hyaluronic acid (HA)-modified MIL-100(Fe) nanoparticles to obtain multifunctional nanoparticles (OIMH NPs). OIMH NPs demonstrated good synergistic effects in killing tumor cells by combining chemotherapy and photothermal therapy.93
Fig. 14. Schematic illustrations of d-MOFs targeting tumors for combined therapy. (a) The loading and delivery process of d-MOFs in tumors through the EPR effect. (b) pH responsive degradation of outer MOFs for drug release and dual-modal fluorescence optical imaging (FOI) and magnetic resonance imaging (MRI)-guided cancer therapy in vitro and in vivo. Reproduced from ref. 91 with permission from Elsevier, copyright 2016.
Despite the positive results obtained in this study, there are still many questions worth further exploration. Firstly, it is necessary to investigate deeply the interaction mechanism between MOFs and drugs to optimize the drug loading and release process. Secondly, research should focus on how to regulate synthesis conditions and drug loading processes to further enhance the drug loading capacity and therapeutic effects of MOFs. Moreover, improving the PTT equipment and treatment parameters to enhance the effectiveness and precision of PTT is also an important direction of research. In the future, MOF-based PTT combined with chemotherapy may become one of the significant means of cancer treatment. We believe that with in-depth research and technological development, more breakthroughs will be achieved in this field, bringing hope to cancer patients worldwide.
5.7.2. CDT + PTT
The combined application of MOF-based CDT and PTT in cancer treatment can produce a synergistic effect. In this combination therapy, photothermal therapy can increase the local temperature within the tumor, improving the penetration rate and bioavailability of drugs used in CDT. At the same time, CDT can generate ROS that effectively damage tumor cells, increasing tumor destruction. The combined therapy helps improve treatment outcomes, reduce side effects, and minimize damage to normal tissues, and personalized treatment can be achieved by adjusting the composition and structure of MOFs based on tumor types and individual differences.
As shown in Fig. 15, Lu et al. designed and synthesized a multifunctional therapeutic system where a CuFeSe2-based heterojunction was controllably constructed by coating a MIL-100(Fe) shell layer by layer. The large mesoporous cavities were subsequently filled with a polymerization initiator (AIPH) and phase change material (tetradecanol) to achieve higher drug loading and controlled heat release of radicals. Under 808 nm laser irradiation, the basic PTT was achieved, and the Fenton reaction of the MIL-100(Fe) shell layer was also greatly promoted. The nanoplatform showed superior therapeutic effects in the hypoxic tumor environment.94
Fig. 15. (a) Schematic illustration for the synthesis procedures of the CuFeSe2@MIL-100(Fe)-AIPH nanoplatform. (b) Schematic illustration showing the process of how the multifunctional nanoplatform integrating PTT, heat promoted CDT and AIPH-based therapy takes effect under 808 nm irradiation. Reproduced from ref. 94 with permission from The Royal Society of Chemistry, copyright 2021.
Bai et al. constructed a smart near-infrared carbon dot MIL-100(Fe) assembly. They developed near-infrared emission carbon dots (RCDs) using glutathione (GSH) as the precursor. Then, RCDs@MIL-100 self-assemblies were obtained using RCDs, FeCl3, and trimesic acid solutions as raw materials. Under the guidance of fluorescence imaging responsive to the tumor microenvironment, an efficient synergistic chemodynamic-photothermal dual-mode therapy was achieved.95
In the future, research will focus on enhancing the biosafety evaluation of MOF-based CDT and PTT combined therapies to ensure their safety in clinical applications. Exploring more MOF materials with photothermal conversion and chemical reaction carrier functions will help achieve more efficient and personalized treatment strategies.
5.7.3. PDT + IT
PDT is a minimally invasive anti-tumor therapy with fewer side effects, and IT has important clinical applications in cancer treatment. However, the individual use of these two methods has certain limitations and cannot fully meet the current needs of cancer treatment. The combination of PDT and IT can improve the therapeutic effects on tumor metastasis and recurrence.96 PDT can induce immunogenic cell death (ICD), promote the release of tumor-associated antigens (TAAs) in tumor cell residues, and increase the proliferation, activation, and infiltration of antigen-presenting cells and antigen-specific T cells.97,98 The combination of PDT and IT not only improves the anti-tumor immune response induced by PDT but also promotes the proliferation and activation of immune memory cells, which inhibit tumor metastasis and prevent tumor recurrence.99,100
MOFs can serve as efficient carriers by co-encapsulating photosensitizers and immune stimulants in nanoparticles, which allows for precise tumor targeting and controlled drug release. Additionally, MOFs can induce tumor cells to release antigens through PDT and activate the body's immune system through immune stimulants, enabling combined treatment inside and outside the tumor. Shao et al. designed an MOF-based nanoplatform that can achieve better cancer treatment in vitro and in vivo through the combination of near-infrared light-induced photodynamic therapy and hypoxia-activated chemotherapy. By integrating anti-programmed death ligand 1 (α-PD-L1), the platform combines photodynamic therapy and hypoxia-activated chemotherapy with immunotherapy to overcome the current limitations of tumor treatment.101
Currently, researchers are exploring more effective methods for combined treatment and making constant improvements. One promising approach is the use of MOF multifunctional nanoplatforms, which can enhance therapeutic effects, particularly for refractory cancers. By applying MOFs, significant improvements in tumor treatment outcomes can be achieved, making this approach highly promising for future clinical applications.
6. Conclusion
This article reviews the biomedical properties of MIL-100(Fe) and MIL-101(Fe) in drug delivery, antimicrobial therapy, and tumor treatment. The two classical materials MIL-100(Fe) and MIL-101(Fe) possess unique crystal structures and large pore size, providing several advantages such as high specific surface area, adjustable pore size, excellent adsorption performance, and chemical stability. They are widely used in fields such as adsorption, catalysis, and drug delivery. As drug delivery carriers, MIL-100(Fe) and MIL-101(Fe) have high drug loading capacity, controlled drug release, and good biocompatibility, demonstrating unique advantages in the transport and release of nonsteroidal anti-inflammatory drugs and ophthalmic drugs.
MIL-100(Fe) and MIL-101(Fe) have many advantages in antimicrobial therapy, including broad-spectrum activity, low toxicity, stability, and ease of modification. These properties allow them to achieve therapeutic effects through the photocatalytic activity of iron ions, generating ROS and damaging bacterial cell membranes and DNA. As excellent drug delivery systems, MIL-100(Fe) and MIL-101(Fe) also have enormous potential in the field of tumor treatment, enabling targeted killing of tumor cells with low toxicity to normal cells. CT based on MIL-100(Fe) and MIL-101(Fe) exhibits stronger targeting performance and better biocompatibility. They can act as carriers for Fenton reaction catalysts in CDT, improving therapeutic effects, and can load photothermal conversion agents in PTT to achieve good therapeutic effects through PTT. Furthermore, they can act as photosensitizers in PDT, improving selectivity and stability, enhancing light absorption, and reducing toxicity. In IT, the controlled release of immunomodulatory drugs through MIL-100(Fe) and MIL-101(Fe) can improve their efficacy and reduce systemic toxicity. Fe-MOF nanocatalysts generate ROS through the catalysis of hydrogen peroxide, destroying tumor cells. Combined treatments based on MIL-100(Fe) and MIL-101(Fe) have synergistic effects, reduced drug resistance, improved tumor response, enhanced immune response, and low toxicity, offering broad application prospects in the field of tumor treatment.
In summary, we can observe that compared to traditional carriers, MOFs based on MIL offer unique advantages as drug delivery systems. MIL-100(Fe) and MIL-101(Fe) possess a high surface area, allowing them to efficiently adsorb a large amount of drug molecules, thus achieving a high drug loading capacity. The pore sizes of these MOFs can be finely tuned under certain conditions, making them more compatible with the size of the desired drug molecules, thereby ensuring efficient loading and release. Due to their porous structure, MIL-100(Fe) and MIL-101(Fe) can provide steady and sustained drug release, preventing abrupt fluctuations in drug concentration in the body, which in turn increases therapeutic efficacy and reduces side effects. In terms of safety, iron-based MOFs exhibit low toxicity to cells, ensuring a higher degree of safety in biomedical applications. Regarding targeted treatment, the surfaces of MIL-100(Fe) and MIL-101(Fe) can undergo chemical modifications, such as the addition of targeting ligands, to enhance their specificity towards certain cells, improving therapeutic outcomes and minimizing damage to healthy tissues. In cancer therapy, MIL-100(Fe) and MIL-101(Fe) can enhance therapeutic effects against tumors in CT, CDT, PTT, PDT, IT, nanoenzyme treatment, and combination therapies while reducing toxicity. They can also simultaneously carry multiple drugs or functional molecules, allowing them to serve diagnostic purposes while treating.
While MOFs based on MIL have achieved significant progress in drug delivery, antimicrobial therapy, and tumor treatment, they still face several technical and application challenges. Firstly, there are concerns about the stability of MOFs. In complex biological environments, especially in vivo, MOFs may be vulnerable to proteins, ions, and other biomolecules that could compromise their structure, potentially affecting their therapeutic effectiveness. Secondly, even though these MOFs can be prepared at laboratory scales, ensuring high yields and consistency in industrial-scale production remains a challenge. Lastly, there's limited clinical research on MOFs, necessitating further validation regarding their in vivo performance and long-term toxicity.
With the progression of scientific research, the performance and applications of MOFs based on MIL will be further refined and expanded in the future. Efforts will be directed towards optimizing the structure, functional groups, ligands, composite effects with other materials, and the fabrication processes of MOFs. This will lead to improvements in their stability, biocompatibility, and controllability of drug release, a reduction in potential toxicity, and a decrease in production costs. More combined therapies based on MOFs will be developed to enhance the treatment efficacy against cancer. As the relationship between the structure and properties of MOFs is further studied, it is foreseeable that more tailor-made, functional MOF materials will be designed and synthesized to meet diverse application needs. Additionally, more clinical research will be conducted to further validate their in vivo performance and long-term toxicity, which is paramount for addressing challenges such as cancer therapy.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research was partially funded by the Guangdong Basic and Applied Basic Research Foundation (2021A1515011616 and 2023A1515011536), the Key Scientific Research Project of Colleges and Universities of Education Department of Guangdong Province (20202ZDZX2046 and 2021ZDZX2052, 2022ZDZX2022), the Featured Innovation Project of Guangdong Province (2022KTSCX045) and the Dongguan Social Development Science and Technology Project (No. 20211800904802), the Guangdong Medical University Research Project (1019k2022003 and 4SG23285G), the open research fund of Songshan Lake Materials Laboratory (2022SLABFN12) and the National Natural Science Foundation of China (82102782).
References
- Mishra B. Munisha B. Nanda J. Sankaran K. J. Suman S. Mater. Chem. Phys. 2022;292:126791. [Google Scholar]
- Siddique S. Chow J. C. L. Nanomaterials. 2020;10:1700. doi: 10.3390/nano10091700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J. Zhao R. Wang B. Sun X. R. Guo X. Tan S. W. Liu W. J. Front. Oncol. 2021;11:758143. doi: 10.3389/fonc.2021.758143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. J. Billingsley M. M. Haley R. M. Wechsler M. E. Peppas N. A. Langer R. Nat. Rev. Drug Discovery. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambe P. Kumar P. Paknikar K. M. Gajbhiye V. J. Controlled Release. 2019;299:64–89. doi: 10.1016/j.jconrel.2019.02.026. [DOI] [PubMed] [Google Scholar]
- (a) Mallakpour S. Nikkhoo E. Hussain C. M. Coord. Chem. Rev. 2022;451:214262. [Google Scholar]; (b) Ma D. Y. Li Z. Zhu J. X. Zhou Y. P. Chen L. L. Mai X. F. Liufu M. L. Wu Y. B. Li Y. W. J. Mater. Chem. A. 2020;8:11933–11937. [Google Scholar]; (c) Qin T. R. Zhang X. Y. Li D. C. Dong X. Y. Qi N. Shang Y. J. Sakiyamad H. Afzal M. Alarifi A. J. Mol. Struct. 2023;1291:136074. [Google Scholar]; (d) Qin T. R. Shi Z. Zhang W. J. Dong X. Y. An N. Sakiyama H. Muddassir M. Srivastava D. Kumar A. J. Mol. Struct. 2023;1282:135220. [Google Scholar]
- (a) Singh N. Qutub S. Khashab N. M. J. Mater. Chem. B. 2021;9:5925–5934. doi: 10.1039/d1tb01044a. [DOI] [PubMed] [Google Scholar]; (b) Hu J. T. Xu Z. J. Liao D. H. Jiang Y. H. Pu H. J. Wu Z. Y. Xu X. T. Zhao Z. Liu J. Q. Lu X. W. Liu X. B. Li B. Adv. Healthcare Mater. 2023:2301316. doi: 10.1002/adhm.202301316. [DOI] [PubMed] [Google Scholar]; (c) Hu W. B. Wang S. Y. Jiang C. J. Zheng M. B. Bai Z. Srivastava D. Kumar A. Liu J. Q. Dyes Pigm. 2023;219:111596. [Google Scholar]; (d) Tan G. J. Wang S. Y. Yu J. L. Chen J. H. Liao D. H. Liu M. Nezamzadeh-Ejhieh A. Pan Y. Liu J. Q. Food Chem. 2024;430:136934. doi: 10.1016/j.foodchem.2023.136934. [DOI] [PubMed] [Google Scholar]; (e) Luo D. W. Huang J. F. Jian Y. H. Singh A. Kumar A. Liu J. Q. Pan Y. Ouyang Q. J. Mater. Chem. B. 2023;11:6802–6822. doi: 10.1039/d3tb00505d. [DOI] [PubMed] [Google Scholar]; (f) Zhong Y. Y. Peng Z. X. Peng Y. Q. Li B. Pan Y. Ouyang Q. Sakiyama H. Muddassir M. Liu J. Q. J. Mater. Chem. B. 2023;11:6335–6345. doi: 10.1039/d3tb00749a. [DOI] [PubMed] [Google Scholar]; (g) Qin L. Liang F. L. Li Y. Wu J. N. Guan S. Y. Wu M. Y. Xie S. L. Luo M. S. Ma D. Y. Inorganics. 2022;10:202. [Google Scholar]; (h) Zhang X. Y. Zhang W. J. Xiang R. F. Lan L. Dong X. Y. Sakiyma H. Muddassir M. Polyhedron. 2023;244:116625. [Google Scholar]
- (a) Gao X. Cui R. Song L. Liu Z. Dalton Trans. 2019;48:17291–17297. doi: 10.1039/c9dt03287h. [DOI] [PubMed] [Google Scholar]; (b) Zhong Y. Y. Liu W. C. Rao C. Y. Li B. H. Wang X. X. Liu D. Pan Y. Liu J. Q. Curr. Med. Chem. 2021;28:6179–6198. doi: 10.2174/0929867328666210511014129. [DOI] [PubMed] [Google Scholar]
- Zhang H. Hu X. Li T. Zhang Y. Xu H. Sun Y. Gu X. Gu C. Luo J. Gao B. J. Hazard. Mater. 2022;429:128271. doi: 10.1016/j.jhazmat.2022.128271. [DOI] [PubMed] [Google Scholar]
- Du M. L. Xu G. L. Zhang J. B. Guan Y. P. Guo C. Chen Y. J. J. Solid State Chem. 2023;322:123950. [Google Scholar]
- Zorainy M. Y. Alalm M. G. Kaliaguine S. Boffito D. C. J. Mater. Chem. A. 2021;9:22159–22217. [Google Scholar]
- Ahmadi M. Ebrahimnia M. Shahbazi M. A. Kecili R. Ghorbani-Bidkorbeh F. J. Ind. Eng. Chem. 2022;115:1–11. [Google Scholar]
- Simon M. A. Anggraeni E. Soetaredjo F. E. Santoso S. P. Irawaty W. Thanh T. C. Hartono S. B. Yuliana M. Ismadji S. Sci. Rep. 2019;9:16907. doi: 10.1038/s41598-019-53436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. W. Kim H. G. Cho D. H. Catal. Commun. 2016;73:69–73. [Google Scholar]
- Otun K. O. Inorg. Chim. Acta. 2020;507:119563. [Google Scholar]
- Fang Y. Yang Z. Li H. Liu X. Environ. Sci. Pollut. Res. 2020;27:4703–4724. doi: 10.1007/s11356-019-07318-w. [DOI] [PubMed] [Google Scholar]
- Barjasteh M. Vossoughi M. Bagherzadeh M. Bagheri K. P. Chem. Eng. J. 2023;452:138987. [Google Scholar]
- Hinks N. J. McKinlay A. C. Xiao B. Wheatley P. S. Morris R. E. Microporous Mesoporous Mater. 2010;129:330–334. [Google Scholar]
- Keskin S. Kizilel S. Ind. Eng. Chem. Res. 2011;50:1799–1812. [Google Scholar]
- Horcajada P. Serre C. Vallet-Regí M. Sebban M. Taulelle F. Férey G. Angew. Chem., Int. Ed. 2006;45:5974–5978. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]
- Ding M. Qiu J. Rouzière S. Rihouey C. Picton L. Gref R. Int. J. Mol. Sci. 2023;24:1757. doi: 10.3390/ijms24021757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Haydar M. Abid H. R. Sunderland B. Wang S. Drug Des., Dev. Ther. 2017;11:2685–2695. doi: 10.2147/DDDT.S145716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Haydar M. Abid H. R. Sunderland B. Wang S. Drug Des., Dev. Ther. 2019;13:23–35. doi: 10.2147/DDDT.S182983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi F. R. Hamzavi S. F. Shahverdizadeh G. H. Ilkhchi M. G. Hosseinzadeh-Khanmiri R. Appl. Organomet. Chem. 2018;32:e4552. [Google Scholar]
- Weng Y. Liu J. Jin S. Guo W. Liang X. Hu Z. Acta Pharm. Sin. B. 2017;7:281–291. doi: 10.1016/j.apsb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandara-Loe J. Ortuño-Lizarán I. Fernández-Sanchez L. Alió J. L. Cuenca N. Vega-Estrada A. Silvestre-Albero J. ACS Appl. Mater. Interfaces. 2019;11:1924–1931. doi: 10.1021/acsami.8b20222. [DOI] [PubMed] [Google Scholar]
- D'Costa V. M. King C. E. Kalan L. Morar M. Sung W. W. Schwarz C. Froese D. Zazula G. Calmels F. Debruyne R. Golding G. B. Poinar H. N. Wright G. D. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- Kim W. Zhu W. Hendricks G. L. Van Tyne D. Steele A. D. Keohane C. E. Fricke N. Conery A. L. Shen S. Pan W. Lee K. Rajamuthiah R. Fuchs B. B. Vlahovska P. M. Wuest W. M. Gilmore M. S. Gao H. Ausubel F. M. Mylonakis E. Nature. 2018;556:103–107. doi: 10.1038/nature26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassahun H. Ayfokru T. Infect. Drug Resist. 2020;13:2717–2722. doi: 10.2147/IDR.S257086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh A. J. Kwon Y. J. J. Controlled Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Firinci R. Firinci E. Coban E. P. Sevincek R. Biyik H. Aygun M. Inorg. Chem. Commun. 2022;141:109509. [Google Scholar]
- Sang Y. L. Zou L. F. Jin R. F. Liu Y. H. Lin X. S. Zhang X. H. J. Coord. Chem. 2021;74:1929–1946. [Google Scholar]
- Abu-Dief A. M. El-Metwaly N. M. Alzahrani S. O. Alkhatib F. Abualnaja M. M. El-Dabea T. El-Remaily M. A. E. A. A. J. Mol. Liq. 2021;326:115277. [Google Scholar]
- Chinthamreddy A. Karreddula R. Pitchika G. K. Surendrababu M. S. J. Inorg. Organomet. Polym. 2021;31:1381–1394. [Google Scholar]
- Nong W. Q. Wu J. Ghiladi R. A. Guan Y. G. Coord. Chem. Rev. 2021;442:214007. [Google Scholar]
- Han L. Qi H. Zhang D. Ye G. Zhou W. Hou C. M. Xu W. Sun Y. Y. New J. Chem. 2017;41:13504–13509. [Google Scholar]
- Song J. Sun L. Geng H. Tan W. Zhen D. Cai Q. New J. Chem. 2022;46:4806–4813. [Google Scholar]
- Li X. Zheng H. Y. Chen J. H. Xu M. Y. Bai Y. Liu T. T. Molecules. 2022;27:3497. doi: 10.3390/molecules27113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. N. Mi X. C. Qian J. L. Ma N. Dai W. ACS Appl. Mater. Interfaces. 2022;14:48285–48295. doi: 10.1021/acsami.2c14489. [DOI] [PubMed] [Google Scholar]
- Wyszogrodzka G. Marszalek B. Gil B. Dorozynski P. Drug Discovery Today. 2016;21:1009–1018. doi: 10.1016/j.drudis.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Liu J. H. Wu D. Zhu N. Wu Y. N. Li G. L. Trends Food Sci. Technol. 2021;109:413–434. [Google Scholar]
- Liu J. L. Cheng W. X. Zhang K. T. Liu H. Li J. Q. Tressel J. Chen S. W. ACS Appl. Bio Mater. 2022;5:3912–3922. doi: 10.1021/acsabm.2c00439. [DOI] [PubMed] [Google Scholar]
- Sung H. Ferlay J. Siegel R. L. Laversanne M. Soerjomataram I. Jemal A. Bray F. Ca-Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Wang J. Q. Chen D. M. Li B. He J. Duan D. L. Shao D. D. Nie M. F. Sci. Rep. 2016;6:26126. doi: 10.1038/srep26126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei M. Abbasi A. Varshochian R. Dinarvand R. Jeddi-Tehrani M. Artif. Cells, Nanomed., Biotechnol. 2018;46:1390–1401. doi: 10.1080/21691401.2017.1369425. [DOI] [PubMed] [Google Scholar]
- Dhawan U. Tseng C. L. Wu P. H. Liao M. Y. Wang H. Y. Wu K. C. W. Chung R. J. Nanomedicine: Nanotechnology, Biology and Medicine. 2023;48:102652. doi: 10.1016/j.nano.2023.102652. [DOI] [PubMed] [Google Scholar]
- Zhao H. Sene S. Mielcarek A. M. Miraux S. Menguy N. Ihiawakrim D. Ersen O. Pechoux C. Guillou N. Scola J. Greneche J. M. Nouar F. Mura S. Carn F. Gazeau F. Dumas E. Serre C. Steunou N. J. Mater. Chem. B. 2023;11:3195–3211. doi: 10.1039/d2tb02094g. [DOI] [PubMed] [Google Scholar]
- Cao C. Wang X. Yang N. Song X. Dong X. Chem. Sci. 2022;13:863–889. doi: 10.1039/d1sc05482a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X. J. Pei Z. C. Pei Y. X. James T. D. Coord. Chem. Rev. 2023;484:215098. [Google Scholar]
- Wang W. Q. Jin Y. L. Xu Z. A. Liu X. Bajwa S. Z. Khan W. S. Yu H. J. Wires. Nanomed. Nanobi. 2020;12:e1614. doi: 10.1002/wnan.1614. [DOI] [PubMed] [Google Scholar]
- Taha A. A. Huang L. B. Ramakrishna S. Liu Y. J. Water Process Eng. 2020;33:101004. [Google Scholar]
- Ni W. Jiang K. Ke Q. Su J. Cao X. Zhang L. Li C. J. Mater. Sci. Technol. 2023;141:11–20. [Google Scholar]
- Ji H. B. Kim C. R. Min C. H. Han J. H. Kim S. N. Lee C. Choy Y. B. Bioeng. Transl. Med. 2023;8:e10477. doi: 10.1002/btm2.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J. Wang Y. T. Niu H. C. Wang Y. W. Wu A. J. Shu C. Q. Zhu Y. F. Bian Y. H. Lin K. L. ACS Appl. Mater. Interfaces. 2021;13:45201–45213. doi: 10.1021/acsami.1c11032. [DOI] [PubMed] [Google Scholar]
- Hu Z. M. Xu C. N. Liang Y. H. Liu T. Y. Tian H. Y. Zhang Y. C. Mater. Des. 2022;223:111132. [Google Scholar]
- Fan F. Hou Y. Zhang Y. Zeng Y. Zhang Y. Zhang S. Meng X. Wang X. Front. Oncol. 2022;12:987491. doi: 10.3389/fonc.2022.987491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. Y. Xing Z. P. Zhang S. Y. Du M. Wang Y. Li Z. Z. Chen P. Zhu Q. Zhou W. Appl. Surf. Sci. 2021;537:147890. [Google Scholar]
- Chien W. C. Cheng P. H. Cheng X. J. Chuang C. C. Huang Y. T. Anilkumar T. S. Liu C. H. Lu Y. J. Wu K. C. W. ACS Appl. Mater. Interfaces. 2021;13:52092–52105. doi: 10.1021/acsami.1c09518. [DOI] [PubMed] [Google Scholar]
- Zhang L. Y. Liu C. Gao Y. Li Z. H. Xing J. Ren W. Z. Zhang L. L. Li A. G. Lu G. M. Wu A. G. Zeng L. Y. Adv. Healthcare Mater. 2018;7:1801144. doi: 10.1002/adhm.201801144. [DOI] [PubMed] [Google Scholar]
- Chen Z. X. Liu M. D. Zhang M. K. Wang S. B. Xu L. Li C. X. Gao F. Xie B. R. Zhong Z. L. Zhang X. Z. Adv. Funct. Mater. 2018;28:1803498. [Google Scholar]
- Gao S. T. Zheng P. L. Li Z. H. Feng X. C. Yan W. X. Chen S. Z. Guo W. S. Liu D. D. Yang X. J. Wang S. X. Liang X. J. Zhang J. C. Biomaterials. 2018;178:83–94. doi: 10.1016/j.biomaterials.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Fan W. P. Huang P. Chen X. Y. Chem. Soc. Rev. 2016;45:6488–6519. doi: 10.1039/c6cs00616g. [DOI] [PubMed] [Google Scholar]
- Shen Z. J. Ma Q. M. Zhou X. Y. Zhang G. M. Hao G. Z. Sun Y. Cao J. NPG Asia Mater. 2021;13:39. [Google Scholar]
- Braathen L. R. Szeimies R. M. Basset-Seguin N. Bissonnette R. Foley P. Pariser D. Roelandts R. Wennberg A. M. Morton C. A. J. Am. Acad. Dermatol. 2007;56:125–143. doi: 10.1016/j.jaad.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Allison R. Moghissi K. Downie G. Dixon K. Photodiagn. Photodyn. Ther. 2011;8:231–239. doi: 10.1016/j.pdpdt.2011.03.342. [DOI] [PubMed] [Google Scholar]
- Wu H. Minamide T. Yano T. Dig. Endosc. 2019;31:508–516. doi: 10.1111/den.13353. [DOI] [PubMed] [Google Scholar]
- Escudero A. Carrillo-Carrion C. Castillejos M. C. Romero-Ben E. Rosales-Barrios C. Khiar N. Mater. Chem. Front. 2021;5:3788–3812. [Google Scholar]
- Hu F. Mao D. Kenry A. Wang Y. X. Wu W. B. Zhao D. Kong D. L. Liu B. Adv. Funct. Mater. 2018;28:1707519. [Google Scholar]
- Ma Y. H. Chen R. Z. Chen X. H. Sun Y. Q. Wang Y. B. Wang B. Nanoscale Adv. 2023;5:361–367. doi: 10.1039/d2na00509c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Jia X. D. Zhen W. Y. Zhang M. C. Jiang X. E. ACS Biomater. Sci. Eng. 2019;5:4435–4441. doi: 10.1021/acsbiomaterials.9b00813. [DOI] [PubMed] [Google Scholar]
- Mellman I. Coukos G. Dranoff G. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. R. Wu X. L. Sun Y. L. Signal Transduction Targeted Ther. 2022;7:331. doi: 10.1038/s41392-022-01136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra A. Sathiyamoorthy P. Park I. K. Pharmaceutics. 2021;13:1867. doi: 10.3390/pharmaceutics13111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Mooney D. J. Nat. Mater. 2018;17:761–772. doi: 10.1038/s41563-018-0147-9. [DOI] [PubMed] [Google Scholar]
- Ni K. Y. Luo T. K. Nash G. T. Lin W. B. Acc. Chem. Res. 2020;53:1739–1748. doi: 10.1021/acs.accounts.0c00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Chen Q. Q. Wu J. P. Kirk T. B. Xu J. K. Liu Z. H. Xue W. ACS Appl. Mater. Interfaces. 2018;10:12463–12473. doi: 10.1021/acsami.8b01680. [DOI] [PubMed] [Google Scholar]
- Hidalgo T. Simon-Vazquez R. Gonzalez-Fernandez A. Horcajada P. Chem. Sci. 2022;13:934–944. doi: 10.1039/d1sc04112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan F. Feng X. C. Yang X. J. Sun W. T. Jin Y. Liu H. F. Ge K. Li Z. H. Zhang J. C. Biomaterials. 2017;122:23–33. doi: 10.1016/j.biomaterials.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Ozatay S. Eng. Sci. Technol. 2020;6:17–30. [Google Scholar]
- Hasan F. Shah A. A. Javed S. Hameed A. Afr. J. Biotechnol. 2010;9:4836–4844. [Google Scholar]
- Madhu A. Chakraborty J. N. J. Cleaner Prod. 2017;145:114–133. [Google Scholar]
- Basso A. Serban S. Methods Mol. Biol. 2020;2100:27–63. doi: 10.1007/978-1-0716-0215-7_2. [DOI] [PubMed] [Google Scholar]
- Navyatha B. Singh S. Nara S. Biosens. Bioelectron. 2021;175:112882. doi: 10.1016/j.bios.2020.112882. [DOI] [PubMed] [Google Scholar]
- Mei L. Q. Zhu S. Liu Y. P. Yin W. Y. Gu Z. J. Zhao Y. L. Chem. Eng. J. 2021;418:129431. [Google Scholar]
- Chakraborty N. Gandhi S. Verma R. Roy I. Biomedicines. 2022;10:1378. doi: 10.3390/biomedicines10061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. M. Xia F. Yang Y. Gong B. Y. Xie A. J. Shen Y. H. Zhu M. Z. J. Mater. Chem. B. 2017;5:8600–8606. doi: 10.1039/c7tb01680h. [DOI] [PubMed] [Google Scholar]
- Ma X. Y. Ren X. L. Guo X. D. Fu C. H. Wu Q. Tan L. F. Li H. B. Zhang W. Chen X. D. Zhong H. S. Meng X. W. Biomaterials. 2019;214:119223. doi: 10.1016/j.biomaterials.2019.119223. [DOI] [PubMed] [Google Scholar]
- Wu C. Y. Xu D. L. Ge M. Luo J. J. Chen L. S. Chen Z. X. You Y. L. Zhu Y. X. Lin H. Shi J. L. Nano Today. 2022;46:101574. [Google Scholar]
- Rojas S. Arenas-Vivo A. Horcajada P. Coord. Chem. Rev. 2019;388:202–226. [Google Scholar]
- Mileo P. G. M. Gomes D. N. Goncalves D. V. Lucena S. M. P. Adsorption. 2021;27:1123–1135. [Google Scholar]
- Wang D. D. Zhou J. J. Chen R. H. Shi R. H. Zhao G. Z. Xia G. L. Li R. Liu Z. B. Tian J. Wang H. J. Guo Z. Wang H. B. Chen Q. W. Biomaterials. 2016;100:27–40. doi: 10.1016/j.biomaterials.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Gao J. Yu H. M. Wu M. Chen Q. Yang Y. Qu Y. Sun M. Qin J. C. Ma L. Yang Y. W. Mater. Today Chem. 2022;23:100716. [Google Scholar]
- Liu H. S. Xu C. N. Meng M. Li S. Sheng S. Zhang S. J. Ni W. D. Tian H. Y. Wang Q. Acta Biomater. 2022;144:132–141. doi: 10.1016/j.actbio.2022.03.023. [DOI] [PubMed] [Google Scholar]
- Lu J. Yang J. Yang D. Hu S. S. Sun Q. Q. Yang G. X. Gai S. L. Wang Z. Yang P. P. J. Mater. Chem. B. 2021;9:336–348. doi: 10.1039/d0tb01599g. [DOI] [PubMed] [Google Scholar]
- Bai Y. L. Zhao J. J. Zhang L. L. Wang S. L. Hua J. Zhao S. L. Liang H. Adv. Healthcare Mater. 2022;11:2102759. doi: 10.1002/adhm.202102759. [DOI] [PubMed] [Google Scholar]
- Hua J. F. Wu P. Gan L. Zhang Z. K. He J. Zhong L. P. Zhao Y. X. Huang Y. Front. Oncol. 2021;11:738323. doi: 10.3389/fonc.2021.738323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. Limaye A. Oyer J. L. Igarashi R. Kittipatarin C. Copik A. J. Khaled A. R. Cytokine. 2017;97:123–132. doi: 10.1016/j.cyto.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turubanova V. D. Balalaeva I. V. Mishchenko T. A. Catanzaro E. Alzeibak R. Peskova N. N. Efimova I. Bachert C. Mitroshina E. V. Krysko O. Vedunova M. V. Krysko D. V. J. Immunother. Cancer. 2019;7:350. doi: 10.1186/s40425-019-0826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti G. Calastretti A. Bevilacqua A. Reddi E. Palumbo G. Nicolin A. Neoplasma. 2010;57:184–188. doi: 10.4149/neo_2010_02_184. [DOI] [PubMed] [Google Scholar]
- Rajendrakumar S. K. Uthaman S. Cho C. S. Park I. K. Biomacromolecules. 2018;19:1869–1887. doi: 10.1021/acs.biomac.8b00460. [DOI] [PubMed] [Google Scholar]
- Shao Y. L. Liu B. Di Z. H. Zhang G. Sun L. D. Li L. L. Yan C. H. J. Am. Chem. Soc. 2020;142:3939–3946. doi: 10.1021/jacs.9b12788. [DOI] [PubMed] [Google Scholar]