Abstract
Background
Peak tricuspid regurgitant velocity (TRV) on transthoracic echocardiography (TTE) is a commonly obtained parameter and robust predictor of subsequent adverse clinical outcomes.
Objectives
The purpose of this study was to determine the predictors and clinical significance of TRV progression.
Methods
We retrospectively linked consecutive outpatient TTE reports from our institution to 2005 to 2017 Medicare claims. Individuals with prior tricuspid surgery, endocarditis, tricuspid stenosis, missing TRV values, TTEs performed during inpatient hospitalization, or <2 TTEs were excluded.
Results
A total of 4,572 patients (mean age 67.8 ± 11.9 years, 50.4% female) received 13,273 TTEs over a median follow-up of 7.4 (IQR: 4.5-6.9) years. TRV increased by a mean of 0.23 (95% CI: 0.22 to 0.23 m/s/y, P < 0.001) (range, 0.01-0.80 m/s/y). Older age, depressed left ventricular ejection fraction, diabetes, hypertension, hyperlipidemia, atrial fibrillation, heart failure, and chronic kidney disease were associated with faster progression (all P < 0.05). Accounting for 23 demographic, clinical, and TTE variables, faster TRV progression was associated with a stepwise increased risk of all-cause mortality (TRV progression quartile 4 vs 1; adjusted HR: 2.17; 95% CI: 1.74-2.71; P < 0.001). Those with regression of TRV (n = 384 [8.4%]) had a lower mortality risk (adjusted HR: 0.40; 95% CI: 0.28-0.57; P < 0.001).
Conclusions
In this large, multidecade study of Medicare beneficiaries with serial TTEs performed in the outpatient setting, the mean rate of TRV progression was 0.23 m/s/y. Older age, left heart disease, and adverse metabolic features were associated with faster progression. Faster progression was associated with a graded risk for all-cause mortality.
Key words: transthoracic echocardiography, tricuspid regurgitant velocity progression
Central Illustration
Pulmonary hypertension (PH), whether primary or secondary in nature, is an independent predictor of mortality.1, 2, 3 The presence of a resting mean pulmonary artery pressure even marginally above the upper limit of normal is a driver of hospitalizations and mortality across a wide variety of medical comorbidities.4 Consequently, screening for PH is essential for early diagnosis and management, and transthoracic echocardiography (TTE) is the modality of choice for screening.5 Echocardiographic assessment of the peak tricuspid regurgitant velocity (TRV) allows for calculation of a tricuspid regurgitant gradient utilizing the modified Bernoulli equation with a consequent estimation of the pulmonary artery systolic pressure (PASP).
While abnormal resting values of TRV are associated with increased all-cause mortality and higher rates of adverse cardiovascular outcomes, limited information is available on expected changes in TRV with serial echocardiography, the association between changes in TRV and baseline demographic and clinical factors, and the independent association of changes in TRV demonstrated via serial echocardiography on survival. Such information may be valuable for identifying individuals with rapid progression in PH and to elucidate potentially modifiable factors that contribute to rapid progression.
Consequently, we performed a retrospective, multidecade analysis of a large cohort of patients referred for TTE to: 1) describe rates of progression in TRV with serial echocardiography; 2) assess for any associations of demographic and clinical factors with TRV progression; and 3) evaluate for any independent association between TRV change and all-cause mortality.
Methods
Study population
We previously linked TTE reports from the Beth Israel Deaconess Medical Center and 4 community-based affiliated satellite sites to Medicare fee-for-service claims as part of the Echocardiography and Health Services Optimization study. Details of this study have previously been published.6 Briefly, as part of routine care at Beth Israel Deaconess Medical Center and affiliated sites, from July 6, 2000, to September 4, 2018, TTE findings and measurements were stored in an electronic data set which was previously directly linked to 100% Medicare claims from January 1, 2003, to December 31, 2017. Among linked individuals (N = 64,063), we selected 45,725 individuals who received their initial TTE from January 1, 2005, to December 31, 2017, to allow for 2 years of historical claims to derive clinical covariate information for all included individuals and verify consistent Medicare enrollment.
In this study, we related a patient’s demographic, clinical, and echocardiographic characteristics at the time of an individual’s initial TTE to subsequent progression of the TRV. Individuals with a prior tricuspid valve repair or replacement, tricuspid valve endocarditis (present or historical), tricuspid stenosis (defined as a mean transvalvular tricuspid gradient of ≥5 mm Hg) at the time of initial TTE were excluded. Additionally, patients were excluded if they had <2 TTEs over the study period, missing values for TRV, or had TTEs performed as an inpatient (to avoid capturing transient changes in TRV that could occur with altered hemodynamics from acute illness). The study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board with a waiver of informed consent. The data supporting this study are not available for review due to prior data use agreements with the Centers for Medicare and Medicaid Services.
Covariates and outcomes
All TTEs were performed according to American Society of Echocardiography guidelines.7 Demographic, anthropometric, and echocardiographic variables are described in Supplemental Table 1 and were recorded at the time of initial TTE. Peak TRV was determined using standard protocols, as the peak velocity on continuous-wave spectral Doppler interrogation of the tricuspid valve (Central Illustration).8
Central Illustration.
Progression of Tricuspid Regurgitant Velocity on Echocardiogram
Peak tricuspid regurgitant velocity (TRV) on transthoracic echocardiogram is a robust predictor of adverse outcomes but the influence of serial changes in TRV is uncertain. In this large, multidecade cohort, TRV progression occurred at a rate of 0.23 m/s/y on average but ranged from an increase of 0.01 to 0.80 m/s/y. Faster TRV progression was associated with older age, depressed left ventricular systolic function, and baseline cardiometabolic risk factors. Accounting for 23 demographic and comorbidity variables, those in the top quartile of TRV progression (>0.28 m/s/y) had a 2.2-fold increased risk of mortality compared to those in the bottom quartile. Regression of TRV was associated with decreased mortality.
Comorbidities were assigned using Medicare Chronic Conditions Warehouse indicator variables,9 which use validated algorithms based on a combination of inpatient and outpatient codes in the 2 years prior to the index TTE to ascertain the presence or absence of comorbid diseases, including tobacco use, diabetes mellitus, history of acute myocardial infarction, hypertension, hyperlipidemia, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), history of ischemic stroke, atrial fibrillation, and heart failure. All-cause mortality was obtained via linkage to the Medicare Beneficiary Summary File and information was complete for all individuals. Information on subsequent tricuspid valve replacement was obtained from review of institutional records.
Statistical analysis
Follow-up time was determined as the time from the index TTE date to the end of the study period (ie, December 31, 2017) or date of death. Categorical variables were displayed using counts and percentages and continuous variables as mean ± SD and compared across categories of PH (TRV ≤2.8 m/s and >2.8 m/s) using Fisher exact tests and t-tests, respectively. To model progression of the TRV, we constructed a linear mixed effects model with TRV as the response variable and both a fixed effect and a random intercept and slope for time since the index TTE. This mixed effects modeling approach both accounts for differences in baseline TRV between individuals and for differences in the time intervals between TTEs in estimating rates of TRV change over time.
We subsequently estimated individual random effects for time and added these values to the marginal slope for the time variable to obtain the conditional predicted slope for time, which represents an individual’s annual rate of TRV progression. A histogram was created to display the distribution of the conditional predicted slope for the time variable. We evaluated the conditional predicted slope for time stratified by baseline characteristic. In the linear mixed effects model, we evaluated a multiplicative time x baseline covariate interaction term to assess the impact of these baseline characteristics on TRV progression rate. Using this approach, we are able to evaluate the average rate of TRV progression within clinical subgroups (eg, hypertension, hyperlipidemia, etc) as well as test for whether rates of TRV progression are significantly different across clinical subgroups. Linear regression was used to model TRV progression rate as a function of number of baseline comorbidities.
Subsequently, among those with TRV progression (ie, conditional predicted TRV progression slope >0), the conditional predicted slope for time was divided into quartiles, representing quartiles of TRV progression rates. Kaplan-Meier curves were constructed for time to all-cause mortality by TRV progression rate quartile and compared using the log-rank test. Cox proportional hazards models were used to estimate the hazard ratio for quartile membership and time to death, both unadjusted and adjusting for all available baseline characteristics (age, sex, presence of suboptimal image quality, body mass index, systolic and diastolic blood pressure, left ventricular ejection fraction (LVEF), mitral regurgitation severity, tricuspid regurgitation severity, peak TRV, presence of right ventricular dilation, presence of right ventricular dysfunction, presence of a pacemaker or implantable cardioverter-defibrillator, diabetes mellitus, hypertension, hyperlipidemia, CKD, smoking, acute myocardial infarction, COPD, ischemic stroke, atrial fibrillation, and heart failure). As a sensitivity analysis, we evaluated differences in time to all-cause mortality between those with a decrease or increase in TRV over the study period using similar survival methods. Schoenfeld residuals were used to confirm the proportionality assumption. Alive individuals were censored on the last study date. Missing covariate data were categorized in analyses. All analyses were conducted in JMP v15.0 (SAS Institute) with a 2-tailed P value <0.05 to define statistical significance.
Results
Overall results
Of 45,725 individuals with linked Medicare claims initially considered for inclusion, 64 (0.03%) were excluded due to prior tricuspid valve replacement or repair, 15 (0.03%) were excluded due to the presence of infective endocarditis on the index TTE, 13,004 (28.4%) had <2 TTEs, 23,665 (51.8%) had TTEs performed as an inpatient, 4,405 (9.6%) had missing TRV values, and no individuals were excluded due to the presence of tricuspid stenosis. A total of 45,725 individuals (99.8%) and 13,273 index and follow-up TTEs were ultimately included (mean age 67.8 ± 11.9 years, 50.4% female; Figure 1). Among these subjects, 19 (0.04%) had a tricuspid valve replacement or repair performed during the follow-up period at a median of 7 (IQR: 3-11) days after baseline TTE. The median time between TTEs was 490 (IQR: 287-960) days.
Figure 1.
Flowchart of Included and Nonincluded Patients
Displayed is a flowchart demonstrating the number of individuals included and excluded from the study sample and reasons for exclusion. Of 45,725 individuals with transthoracic echocardiograms (TTEs) linked to Medicare Fee-for-service claims, 2005 to 2017, 64 (0.1%) were excluded due to prior tricuspid valve replacement or repair, 15 (0.03%) were excluded due to the presence of endocarditis on the individual’s baseline TTE, 13,004 (28.4%) were excluded due to receiving fewer than 2 TTEs, 23,665 (51.8%) were excluded due to having TTEs performed as an inpatient, 4,405 (9.6%) were excluded due to missing TRV values, and no individuals were excluded due to presence of tricuspid stenosis. This resulted in a final sample size of 4,572 individuals.
Individuals with existing PH, as defined by baseline TRV of >2.8 m/s, were older, more frequently female and had a greater prevalence of right atrial (RA) dilation, right ventricle (RV) dilation, and RV dysfunction (all P < 0.001) (Table 1). Individuals with PH also had a slightly lower LVEF, greater degrees of moderate or greater valvular regurgitation, higher E/e’ ratios and a greater prevalence of comorbidities, including the presence of a pacemaker or an implantable defibrillator, diabetes mellitus, hypertension, hyperlipidemia, CKD, heart failure, and atrial fibrillation (all P < 0.001) (Table 1).
Table 1.
Baseline Characteristics of Included Patients by Pulmonary Hypertension Status
| Number of Observations (N = 4,572) | Tricuspid Regurgitant Velocity ≤2.8 m/s (n = 3,508) | Tricuspid Regurgitant Velocity >2.8 m/s (n = 1,064) | P Value | |
|---|---|---|---|---|
| Age (y) | 4,572 | 66.6 ± 11.8 | 71.8 ± 11.5 | <0.001 |
| Female | 4,571 | 577 (54.2) | 1,727 (49.2) | 0.005 |
| Suboptimal image quality | 4,572 | 126 (11.8) | 384 (11.0) | 0.44 |
| Height (cm) | 4,572 | 168.3 ± 10.8 | 167.0 ± 10.4 | <0.001 |
| Weight (kg) | 4,572 | 80.2 ± 19.1 | 80.9 ± 20.9 | 0.33 |
| Body surface area (cm2) | 4,572 | 1.93 ± 0.26 | 1.93 ± 0.28 | 0.93 |
| Body mass index (kg/m2) | 4,572 | 28.3 ± 7.7 | 28.9 ± 6.7 | 0.02 |
| Systolic blood pressure (mm Hg) | 4,518 | 130.2 ± 19.7 | 134.2 ± 21.6 | <0.001 |
| Diastolic blood pressure (mm Hg) | 4,509 | 73.8 ± 10.3 | 72.6 ± 11.6 | 0.0011 |
| Pulse pressure (mm Hg) | 4,507 | 56.3 ± 16.8 | 61.6 ± 19.6 | <0.001 |
| Heart rate (beats/min) | 4,572 | 69.1 ± 13.4 | 69.7 ± 13.6 | 0.22 |
| Left ventricular ejection fraction (%) | 4,570 | 63.9 ± 14.3 | 61.2 ± 16.7 | <0.001 |
| Moderate or greater mitral regurgitation | 4,572 | 214 (6.1) | 158 (14.9) | <0.001 |
| Moderate or greater tricuspid regurgitation | 4,572 | 97 (2.8) | 160 (15.0) | <0.001 |
| Peak tricuspid regurgitant gradient (mm Hg) | 4,572 | 23.5 ± 4.5 | 37.5 ± 4.8 | <0.001 |
| Right atrial length (cm) | 4,463 | 5.0 ± 0.7 | 5.4 ± 0.9 | <0.001 |
| RV dilation | 4,572 | 215 (6.1) | 192 (18.1) | <0.001 |
| RV basal diameter (cm) | 392 | 3.6 ± 0.9 | 4.0 ± 0.9 | <0.001 |
| RV dysfunction | 4,572 | 120 (3.4) | 105 (9.9) | <0.001 |
| Right atrial major dimension (cm) | 4,463 | 5.0 ± 0.7 | 5.4 ± 0.9 | <0.001 |
| Peak transmitral E/e’ ratio | 3,545 | 10.0 ± 3.8 | 12.8 ± 6.0 | <0.001 |
| Peak transmitral E/A ratio | 4,109 | 1.0 ± 0.5 | 1.3 ± 0.8 | <0.001 |
| Peak transmitral E-wave deceleration time (ms) | 4,322 | 231 ± 67 | 224 ± 77 | 0.008 |
| Presence of pacemaker/ICD | 4,572 | 158 (4.5) | 91 (8.6) | <0.001 |
| Smoking | 4,572 | 50 (1.4) | 18 (1.7) | 0.56 |
| Diabetes | 4,572 | 205 (5.8) | 97 (9.1) | <0.001 |
| History of acute myocardial infarction | 4,572 | 56 (1.6) | 13 (1.2) | 0.47 |
| Hypertension | 4,572 | 392 (11.2) | 215 (20.2) | <0.001 |
| Hyperlipidemia | 4,572 | 269 (7.7) | 128 (12.0) | <0.001 |
| Chronic obstructive pulmonary disease | 4,572 | 59 (1.7) | 26 (2.4) | 0.12 |
| Chronic kidney disease | 4,572 | 237 (6.8) | 110 (10.3) | <0.001 |
| History of ischemic stroke | 4,572 | 11 (1.0) | 19 (0.5) | 0.09 |
| Atrial fibrillation | 4,572 | 47 (1.3) | 35 (3.3) | <0.001 |
| Heart failure | 4,572 | 189 (5.4) | 142 (13.4) | <0.001 |
Values are mean ± SD or n (%) unless otherwise indicated. Shown is a comparison of baseline characteristics of patients according to baseline tricuspid regurgitant velocity.
ICD = implantable cardioverter-defibrillator; RV = right ventricular.
Overall progression rates
A total of 384 individuals (8.4%) had a decrease in TRV over time. Among those with TRV progression (N = 4,188), the mean rate of TRV progression was 0.23 m/s/y, 95% CI 0.22 to 0.23 m/s/y, P < 0.001. The estimated individual rates of TRV progression ranged from 0.01 m/s/y to an increase of 0.80 m/s/y (Figure 2).
Figure 2.
Distribution of Estimated Peak Tricuspid Regurgitant Velocity Progression Rate
Displayed is a histogram demonstrating the distribution of the number of individuals (y-axis) with each peak tricuspid regurgitant velocity progression rate (x-axis). Only individuals with progression of the tricuspid regurgitant velocity are included. The estimated rate of progression of the peak tricuspid regurgitant velocity is the conditional predicted slope for time as estimated by the linear mixed effects model.
Progression rates according to baseline comorbidities
Rates of TRV progression were greater in individuals >75 years of age, with baseline RA length >5.1 cm, those with a TRV >2.8 m/s at baseline and those with an LVEF <50% (all interaction P < 0.05) (Table 2). Apart from smoking, COPD, and history of acute myocardial infarction, TRV progression was greater in those with comorbidities (Table 2). There was an unadjusted 0.01 (95% CI: 0.008-0.012) m/s/y increase in TRV associated with each additional comorbidity (P < 0.001) that persisted despite adjustment for age and sex.
Table 2.
Rates of TRV Progression According to Baseline Characteristics
| Levels | N | Rate of Progression of Tricuspid Regurgitant Velocity (m/s/y) | P Valuea | |
|---|---|---|---|---|
| Age (y) | ≤75 | 3,023 | 0.22 ± 0.09 | <0.001 |
| >75 | 1,165 | 0.26 ± 0.09 | ||
| Sex | Female | 2,128 | 0.24 ± 0.09 | 0.02 |
| Male | 2,059 | 0.23 ± 0.09 | ||
| Body surface area (cm2) | >2 | 2,684 | 0.23 ± 0.09 | 0.30 |
| ≤2 | 1,504 | 0.23 ± 0.09 | ||
| Body mass index (kg/m2) | >30 | 2,810 | 0.23 ± 0.09 | 0.04 |
| ≤30 | 1,378 | 0.24 ± 0.09 | ||
| Left ventricular ejection fraction (%) | <50 | 576 | 0.25 ± 0.09 | <0.001 |
| ≥50 | 3,610 | 0.23 ± 0.09 | ||
| Baseline TRV (m/s) | >2.8 | 1,021 | 0.29 ± 0.09 | <0.001 |
| ≤2.8 | 3,167 | 0.21 ± 0.08 | ||
| Right atrial length >5.1 cm | Yes | 1,949 | 0.25 ± 0.09 | <0.001 |
| No | 2,623 | 0.22 ± 0.09 | ||
| Smoking | Yes | 64 | 0.22 ± 0.09 | <0.001 |
| No | 4,124 | 0.23 ± 0.09 | ||
| Diabetes | Yes | 285 | 0.26 ± 0.10 | 0.08 |
| No | 3,903 | 0.23 ± 0.09 | ||
| History of acute myocardial infarction | Yes | 61 | 0.28 ± 0.07 | <0.001 |
| No | 4,127 | 0.23 ± 0.09 | ||
| Hypertension | Yes | 570 | 0.26 ± 0.09 | <0.001 |
| No | 3,618 | 0.23 ± 0.09 | ||
| Hyperlipidemia | Yes | 369 | 0.25 ± 0.09 | 0.21 |
| No | 3,819 | 0.23 ± 0.09 | ||
| Chronic obstructive pulmonary disease | Yes | 82 | 0.24 ± 0.09 | <0.001 |
| No | 4,106 | 0.23 ± 0.09 | ||
| Chronic kidney disease | Yes | 331 | 0.26 ± 0.09 | 0.04 |
| No | 3,857 | 0.23 ± 0.09 | ||
| History of ischemic stroke | Yes | 26 | 0.26 ± 0.07 | <0.001 |
| No | 4,162 | 0.23 ± 0.09 | ||
| Atrial fibrillation | Yes | 78 | 0.27 ± 0.09 | <0.001 |
| No | 4,110 | 0.23 ± 0.09 | ||
| Heart failure | Yes | 312 | 0.28 ± 0.09 | <0.001 |
| No | 3,876 | 0.23 ± 0.09 |
Values are mean ± SD progression rates of the peak tricuspid regurgitant gradient within each clinical and echocardiographic subgroup. Only individuals with TRV progression (nonnegative conditional predicted slopes for time) are included.
Represents the P value for the time × variable interaction term.
Relationship of progression and survival
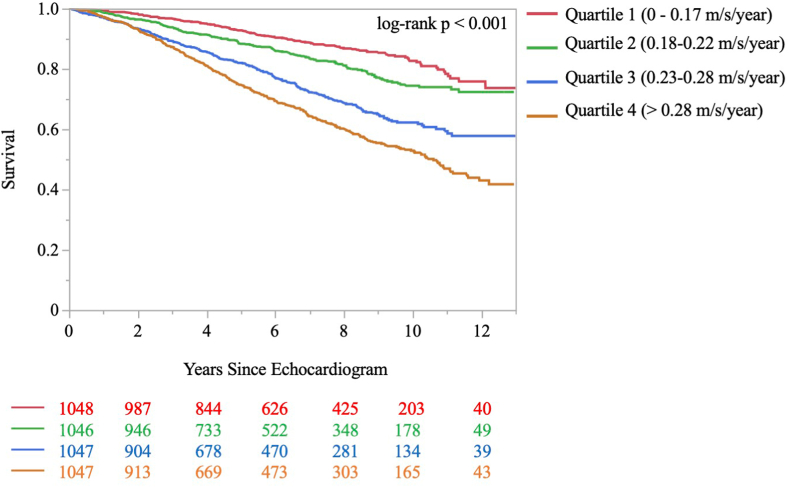
Those with baseline PH had an overall greater risk of mortality (32.6% vs 18.2% in those with a TRV of ≤2.8 m/s; unadjusted HR: 2.11; 95% CI: 1.86-2.41; P < 0.001) (Supplemental Figure 1). Over a median 7.4 (IQR: 4.5-6.9) years of follow-up, a total of 947 individuals with TRV progression (22.6%) died at a median 4.2 (IQR: 2.4-6.6) years after the index TTE. The individual rates of TRV progression were divided into quartiles. A total of 12.7% individuals died in quartile 1 (0-0.17 m/s/y), 16.3% in quartile 2 (0.18-0.22 m/s/y), 25.6% in quartile 3 (0.23-0.28 m/s/y), and 35.8% in quartile 4 (>0.28 m/s/y) (Table 3 and Figure 3, log rank P < 0.001).
Table 3.
Mortality Rates and Mortality Risk by Tricuspid Peak Velocity Progression Quartile
| Quartile | N | Number of Deaths (N = 947), n (%) | Time to Death (y), Median (IQR) | Unadjusted HR (95% CI) for All-Cause Mortality | P Valuea | Adjusted HR (95% CI) for All-Cause Mortality | P Valueb |
|---|---|---|---|---|---|---|---|
| 1 (0-0.17 m/s/y) | 1,048 | 133 (12.7) | 4.9 (2.8-7.3) | Ref | Ref | Ref | Ref |
| 2 (0.18-0.22 m/s/y) | 1,046 | 171 (16.3) | 4.1 (2.3-6.6) | 1.45 (1.16-1.82) | 0.0013 | 1.41 (1.12-1.78) | 0.003 |
| 3 (0.23-0.28 m/s/y) | 1,047 | 268 (25.6) | 3.9 (1.9-6.2) | 2.50 (2.03-3.08) | <0.001 | 1.95 (1.57-2.43) | <0.001 |
| 4 (>0.28 m/s/y) | 1,047 | 375 (35.8) | 4.1 (2.4-6.6) | 3.44 (2.82-4.19) | <0.001 | 2.17 (1.74-2.71) | <0.001 |
Shown are the number of individuals (N) in each quartile of progression rates of the peak tricuspid regurgitant velocity as well as the number and percentage of all-cause deaths in each quartile, the median (IQR) of time to death in years, the unadjusted HR 95% CI, and P value for the risk of all-cause mortality by quartile (with quartile 1 as reference), and the adjusted HR, 95% CI, and P value for the risk of all-cause mortality by quartile (with quartile 1 as reference). Estimates are adjusted for baseline parameters including age, sex, presence of suboptimal image quality, body mass index, systolic and diastolic blood pressure, left ventricular ejection fraction, mitral regurgitation severity, tricuspid regurgitation severity, peak tricuspid regurgitant velocity, presence of right ventricular dilation, presence of right ventricular dysfunction, presence of a pacemaker or implantable cardioverter-defibrillator, and all clinical variables (diabetes mellitus, hypertension, hyperlipidemia, chronic kidney disease, smoking, acute myocardial infarction, chronic obstructive pulmonary disease, ischemic stroke, atrial fibrillation, and heart failure).
Represents the P value for the unadjusted comparison across quartiles.
Represents the P value for the adjusted comparison across quartiles.
Figure 3.
Time to All-Cause Mortality by Peak Tricuspid Regurgitant Velocity Progression Rate Quartile
Displayed is a Kaplan-Meier curve demonstrating the time from the baseline echocardiogram to the occurrence of all-cause mortality according to quartile of peak tricuspid regurgitant velocity progression rates. The proportion surviving at each time point is shown on the y-axis and the number of years since the baseline echocardiogram is shown on the x-axis. The red line represents those in the slowest progression rate quartile (0-0.17 m/s/y), the green line represents those in quartile 2 (0.18-0.22 m/s/y), the blue line represents those in quartile 3 (0.23-0.28 m/s/y), and the orange line represents those in the highest quartile of progression rate (>0.28 m/s/y). The number of individuals in the risk set at 2-year intervals is provided below the x-axis. The log-rank P value for comparison of time to all-cause mortality across quartiles was <0.001.
On an unadjusted basis (Table 3), compared to quartile 1, mortality risk increased stepwise with increasing TRV progression quartiles, (quartile 2; HR: 1.45; 95% CI: 1.16-1.82: P = 0.0013; quartile 3; HR: 2.50; 95% CI: 2.03-3.08; P < 0.001; quartile 4; HR: 3.44; 95% CI: 2.82-4.19; P < 0.001). Using a dichotomous cutoff, a TRV progression >0.23 m/s/y was associated with increased mortality risk (HR: 2.45; 95% CI: 2.15-2.81; P < 0.001).
On an adjusted basis (Table 3) and accounting for 23 demographic, clinical, and TTE variables (Supplemental Table 2), when compared to quartile 1, a similar pattern of increased mortality was observed (quartile 2; adjusted HR [aHR]: 1.41; 95% CI: 1.12-1.78; P = 0.003; quartile 3; aHR: 1.95; 95% CI: 1.57-2.43; P < 0.001; quartile 4; aHR: 2.17; 95% CI: 1.74-2.71; P < 0.001).
Among the 3,508 individuals with a baseline TRV of <2.8 m/s, a TRV progression rate of >0.23 m/s/y was associated with an increased risk of mortality (unadjusted HR: 2.13; 95% CI: 1.81-2.50; P < 0.001) (Supplemental Figure 2).
Sensitivity analysis
Among those with a decrease in TRV over the study period (N = 384), 36 (9.4%) died at a median of 5.8 (IQR: 4.1-8.7) years after TTE compared to 945 (22.6%) of those with TRV progression (Figure 4) (unadjusted HR: 0.31; 95% CI: 0.22-0.43; P < 0.001). This effect persisted despite adjustment for 23 demographic, clinical, and TTE variables (Supplemental Table 3) (aHR: 0.40; 95% CI: 0.28-0.57; P < 0.001).
Figure 4.
Time to All-Cause Mortality Among Those With Progression vs Regression of Tricuspid Regurgitant Peak Velocity
Displayed is a Kaplan-Meier curve demonstrating the time from the baseline echocardiogram to the occurrence of all-cause mortality comparing groups with progression (ie, an increase) vs regression (ie, a decrease) in the tricuspid regurgitant peak velocity (TRV). The proportion surviving at each time point is shown on the y-axis and the number of years since the baseline echocardiogram is shown on the x-axis. The red line represents those with TRV progression and the blue line represents those with TRV regression. The number of individuals in the risk set at 2-year intervals is provided below the x-axis. Shaded areas represent the 95% confidence interval for Kaplan-Meier estimates. The log-rank P-value for comparison of time to all-cause mortality between groups was <0.001.
Discussion
In this large, multidecade study of Medicare beneficiaries with serial TTEs performed in the outpatient setting, the average rate of TRV progression was 0.23 m/s/y but ranged from 0.01 to 0.80 m/s/y. Rates of TRV progression were higher in older individuals and those with cardiometabolic diseases (eg, diabetes, hypertension, hyperlipidemia, CKD) or left-sided heart disease (eg, depressed LVEF, atrial fibrillation, heart failure). Despite multivariable adjustment for 23 demographic, clinical, and TTE factors including baseline TRV, there was a stepwise graded increase in mortality risk with increasing TRV progression rate. Compared to those with TRV progression, those with TRV regression (eg, a decrease in TRV) had a substantially lower risk of all-cause mortality. In total, these findings suggest that TRV progression is independently associated with significantly worsened survival, underlining the value of serial TRV measurements in monitoring PH.
To our knowledge, only one prior study, by Santosh et al,10 has evaluated the echocardiographic progression of estimated PASP. This study retrospectively evaluated 67 patients with end-stage renal disease undergoing TTEs 1 to 4 years apart. The mean increase in PASP was 2.41 mmHg per year, but higher rates of change were predicted by E/e’, a marker of worsened diastolic dysfunction, diabetes, and left-sided valvular heart disease. Santosh et al10 found that those with higher PASP had a higher rate of incident RV dysfunction. In the current study, including individuals across the spectrum of cardiometabolic diseases, the overall rate of TRV progression was substantially lower (0.23 m/s/y, roughly equating to 0.21 mm Hg/y assuming a stable RA pressure). This observed rate of progression reflects the average rate of change, only among those with progression, and thus the average change in TRV in the overall cohort is likely lower. Moreover, we noted significant heterogeneity in TRV progression rates from 0.01 to 0.8 m/s/y. Overall, this rate of change is lower but consistent with observations in the study by Santosh et al.10
While those individuals with baseline renal dysfunction had greater overall progression than those without (mean increase of 0.26 m/s/y), the substantially lower change over time observed in the current study suggests that TRV progression may be more muted in a larger, more diverse population. Nevertheless, in the current study, we confirm the association between baseline cardiometabolic disease—specifically hypertension, hyperlipidemia, diabetes, and CKD, which are all risk factors for diastolic dysfunction—and faster TRV progression. Moreover, in our cohort subjects with baseline increased RA length and RV basal diameters, reflective of adverse right heart hemodynamics, were more likely to have PH based on their baseline TRV values. Furthermore, increased RA length was associated with more rapid TRV progression as well.
In addition to cardiometabolic risk factors, the current study identifies baseline age as associated with TRV progression. It has been shown previously in cross-sectional invasive hemodynamic studies11 that the aging process is associated with increases in PASP, including among those without echocardiographic or clinical diseases and may be associated with decreased survival.12 These changes in PASP with age are felt to be related to loss of distensibility over time, noted on histologic studies.13, 14, 15 We also found that older individuals more frequently had higher PASP values at baseline as well as a faster rate of change in TRV than younger individuals. We additionally identify evidence of left-sided heart disease (ie, atrial fibrillation, depressed LVEF, and heart failure) as associated with faster TRV progression which may be explained by the higher postcapillary pressures (eg, pulmonary capillary wedge pressure or left atrial pressure) in these patients.
Despite the inherent limitations as a proxy for PASP, the current study underlines the value of serial echocardiographic surveillance of TRV. Specifically, there was a graded relationship between TRV progression and risk of all-cause mortality which persisted despite adjustment for 23 clinical and echocardiographic variables, including baseline TRV and right ventricular dilation or dysfunction. Those in the highest quartile or progression (>0.28 m/s/y) had a 2.2-fold increased risk of mortality compared to those in the lowest quartile (0-0.17 m/s/y). Moreover, those who had a decrease in TRV over the study period had a 60% lower risk of all-cause mortality than those with an increase in TRV which persisted despite identical multivariable adjustment. While the exact causal mechanisms remain uncertain currently, these findings suggest that TRV progression may independently and meaningfully impact survival. As such, serial TTEs may have a role in identifying rapid progressors for prognostication and intensification of resources. Moreover, many (but not all) risk factors for TRV progression are potentially modifiable (eg, hypertension, hyperlipidemia, diabetes, etc) through targeted risk factor control. Thus, while this remains to be tested and thus remains speculative, it is plausible that risk factor control may mitigate the increased mortality risk observed in this population. Lastly, it is unclear whether progression of TRV could be used to identify those at higher risk of developing PH prior to observing a TRV >2.8 m/s, though this remains an intriguing topic for future study. In the current study, TRV progression >0.23 m/s/y was associated with a 2.1-fold increased risk of mortality among those with a baseline TRV ≤2.8 m/s, thus reinforcing that TRV progression may be prognostic regardless of the absolute value of TRV.
Study Limitations
Despite multiple strengths of the current study, including the large number of included individuals and duration of follow-up, spanning several decades, there are several limitations to note. As a single-center study, these results should be confirmed in an external cohort. Participants were all Medicare enrolled at the time of TTE. Though Medicare beneficiaries represent the largest share of those receiving TTEs nationally, results nonetheless cannot be readily extrapolated to a non-Medicare or younger population. Though those referred for TTE may differ from those not referred, the shortage of serial longitudinal TTE data in existing population-based cohorts where referral bias is absent, makes it otherwise challenging to evaluate longitudinal changes in TRV. While the modeling technique employed accounts for more frequent imaging among sicker patients, it cannot account for changes in TRV that are unobserved (ie, those without follow-up intervals) and thus may bias slightly toward a faster rate of progression. Notably, those receiving TTEs during an inpatient hospitalization were excluded to reduce the effect of short-term changes in hemodynamics with acute illness and only more stable ambulatory patients were included. Barring exclusions, all-comers were included in the cohort to maximize the generalizability of results while simultaneously evaluating differences in TRV progression within a variety of clinical subgroups including commonly encountered conditions such as heart failure, CKD, and COPD. We did not specifically exclude individuals with primary PH, though these are likely to represent a small minority of included individuals given the mean age of the sample. Though interval tricuspid valve replacement or repair may impact estimates, the rate of tricuspid valve surgery was so low (0.04%) that this is unlikely to influence the observed results. However, it was not possible to ascertain if medical treatments (eg, diuretic use, goal directed medical therapy for heart failure, pulmonary vasodilators, etc) were initiated which could have influenced the observed TRV. Furthermore, it was not possible to distinguish those with end-stage renal disease requiring renal replacement therapy from those with CKD.
While there may have been technical differences in echocardiographs used over the study period, the fundamental techniques and precision of TRV measurement have remained substantially unchanged since inception. Invasive hemodynamic assessment of PASP was not feasible and thus conclusions must be considered within the context of the limitations of TRV as a measure of pulmonary vascular function. Due to inherent inaccuracies in estimation of RA pressure, the analysis focused on TRV rather than estimated PASP. We utilized a TRV >2.8 m/s to define baseline PH, but it is possible that a different cutoff may better identify mortality risk and propose this as a topic for future investigation. As TRV progression was defined as conditional predicted slope >0, it is possible that the confidence limits for the conditional predicted slope cross zero, though this likely represents a small portion of the included population. Furthermore, it is possible that some individuals may experience TRV progression prior to death that was unobserved as they did not receive a second TTE. Thus, these results should be considered exploratory and are conditional on survival to receive a second TTE. Moreover, the current analysis assumes TRV progression is linear, though this assumption should be tested in future investigations. Lastly, while relevant in a minority of cases, the use of TRV to estimate PASP is not accurate in the setting of pulmonic stenosis.
Conclusions
In this large, multidecade study of Medicare beneficiaries with serial TTEs performed in the outpatient setting, the average rate of progression in TRV was 0.23 m/s/y but ranged from a decrease of 0.01 m/s/y to an increase of 0.8 m/s/y. TRV progression was more rapid in older individuals and those with cardiometabolic diseases (eg, diabetes, hypertension, hyperlipidemia, CKD) and those with left-sided heart disease (eg, depressed LVEF, atrial fibrillation, heart failure). Progression of TRV was associated with a graded stepwise independent increased risk of all-cause mortality and those with TRV regression had a lower mortality risk than those with progression. These findings indicate a possible role for serial TTEs to monitor TRV progression to identify high-risk individuals.
Funding support and author disclosures
Dr Strom is supported by grants from the National, Heart, Lung, and Blood Institute (1R01HL169517, 1K23HL144907); has received research grants from Edwards Lifesciences, Anumana, HeartSciences, Ultromics Ltd, EchoIQ, and Lantheus Medical Iamging; has done consulting for Bracco Diagnostics, General Electric Healthcare, and Philips Healthcare; and is a member of the scientific advisory boards for Edwards Lifesciences, Ultromics Ltd, and EchoIQ. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE 1: While TRV on TTE is a powerful clinical risk factor, the importance of serial changes in TRV over time remains unclear.
COMPETENCY IN MEDICAL KNOWLEDGE 2: In this large, multidecade study of outpatient receiving TTEs, baseline cardiometabolic risk factors were associated with faster TRV progression which was independently linked to mortality risk.
TRANSLATIONAL OUTLOOK: These findings indicate a possible role for serial TTEs for TRV monitoring and further investigation into whether risk factor control can mitigate TRV progression.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Benza R.L., Gomberg-Maitland M., Elliott C.G., et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Lam C.S., Roger V.L., Rodeheffer R.J., Borlaug B.A., Enders F.T., Redfield M.M. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan S.D., Barbera J.A., Gaine S.P., et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53 doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron B.A., Hess E., Maddox T.M., et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 6.Hyland P.M., Xu J., Shen C., Markson L.J., Manning W.J., Strom J.B. Race, sex and age disparities in echocardiography among Medicare beneficiaries in an integrated healthcare system. Heart. 2021;108:956–963. doi: 10.1136/heartjnl-2021-319951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell C., Rahko P.S., Blauwet L.A., et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Rudski L.G., Lai W.W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86-8. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services Chronic conditions data warehouse [internet] https://www2.ccwdata.org/web/guest/condition-categories
- 10.Santosh S., Chu C., Mwangi J., et al. Changes in pulmonary artery systolic pressure and right ventricular function in patients with end-stage renal disease on maintenance dialysis. Nephrology. 2019;24:74–80. doi: 10.1111/nep.13183. [DOI] [PubMed] [Google Scholar]
- 11.Davidson W.R., Jr., Fee E.C. Influence of aging on pulmonary hemodynamics in a population free of coronary artery disease. Am J Cardiol. 1990;65:1454–1458. doi: 10.1016/0002-9149(90)91354-9. [DOI] [PubMed] [Google Scholar]
- 12.Lam C.S., Borlaug B.A., Kane G.C., Enders F.T., Rodeheffer R.J., Redfield M.M. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lansing A.I., Rosenthal T.B., Alex M. Significance of medial age changes in the human pulmonary artery. J Gerontol. 1950;5:211–215. doi: 10.1093/geronj/5.3.211. [DOI] [PubMed] [Google Scholar]
- 14.Harris P., Heath D., Apostolopoulos A. Extensibility of the human pulmonary trunk. Br Heart J. 1965;27:651–659. doi: 10.1136/hrt.27.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay E.H., Banks J., Sykes B., Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax. 1978;33:335–344. doi: 10.1136/thx.33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.