Abstract
Environmental temperature rises are powerful stimuli that can alter both the sympathetic nervous system and the hypothalamic-pituitary-adrenocortical axis (HPA). Heat stress has been shown to harm pregnancy outcomes such as causing spontaneous abortion, low birth weight, growth retardation and stillbirth. Supplementation of bee bread in pregnant rats under heat stress exposure has been shown to improve the pregnancy outcomes. However, whether supplementation of bee bread during heat stress exposure may also reduce the level of the stress hormone, corticosterone has yet been reported. Therefore, this study aims to determine the effect of bee bread on corticosterone level, progesterone level, oestradiol level and zonation of the adrenal cortex of pregnant rats under heat stress exposure. Pregnant rats were randomly categorised into four groups (n = 6): Control (C: standard feeding), Treatment 1 (T1: 0.5 g bee bread/kg body weight/day), Treatment 2 (T2: standard feeding with heat exposure), and Treatment 3 (T3: 0.5 g bee bread/kg body weight/day with heat exposure). Bee bread (0.5 g/kg body weight/day) was force-fed to pregnant rats through oral gavage beginning on day 0 of pregnancy and continuing until delivery. Heat stress was generated experimentally by putting both T2 and T3 rats in an egg incubator for 45 min daily at a temperature of 43°C till delivery. On a postnatal Day 21, dams were euthanised to assess serum corticosterone, progesterone, oestradiol levels and adrenal gland histology. Rats in the T2 group had a significantly (P < 0.05) increase in the zona fasciculata thickness (94.95 ± 1.55 μm) and higher corticosterone levels (49.57 ± 1.57 ng/mL) compared with control. However, supplementation of bee bread during heat stress was able to show an improvement in adrenal zona fasciculata thickness by decreasing to 79.89 ± 3.08 μm and corticosterone level reduced to 35.31 ± 1.73 ng/mL significantly (P < 0.05). Therefore, these findings may imply that bee bread is effective as a neutralizer in lowering the production of stress hormone.
Keywords: Heat Stress, Corticosterone, Adrenal Gland, Female Reproduction, Bee Bread
Abstrak
Peningkatan suhu persekitaran adalah rangsangan kuat yang boleh mengubah kedua-dua sistem saraf simpatetik dan paksi hipotalamus-pituitari-adrenokortikal (HPA). Tekanan haba telah terbukti membahayakan hasil kehamilan seperti pengguguran spontan, berat lahir rendah, terencat pertumbuhan dan kelahiran mati. Suplemen roti lebah pada tikus hamil di bawah pendedahan tekanan haba telah menunjukkan pemulihan dalam hasil kehamilan. Walau bagaimanapun, sama ada suplemen roti lebah semasa pendedahan tekanan haba juga boleh mengurangkan tahap hormon tekanan, kortikosteron masih belum dilaporkan. Oleh itu kajian ini bertujuan untuk menentukan kesan roti lebah terhadap paras kortikosteron, paras progesteron, paras estradiol dan pengezonan korteks adrenal tikus hamil di bawah pendedahan tekanan haba. Tikus hamil secara rawak dikategorikan kepada empat kumpulan (n = 6): Kawalan (C: pemakanan standard), Rawatan 1 (T1: 0.5 g roti lebah/kg berat badan/hari), Rawatan 2 (T2: pemakanan standard dengan pendedahan haba), dan Rawatan 3 (T3: 0.5 g roti lebah/kg berat badan/hari dengan pendedahan haba). Roti lebah (0.5 g/kg berat badan/hari) telah diberi makan secara paksa kepada tikus hamil melalui gavaj oral bermula pada hari 0 kehamilan dan berterusan sehingga bersalin. Tekanan haba dijana secara eksperimen dengan memasukkan kedua-dua tikus T2 dan T3 ke dalam inkubator telur selama 45 minit setiap hari pada suhu 43°C sehingga kelahiran. Pada hari ke-21 selepas kelahiran, ibu tikus telah dikorbankan untuk menilai paras kortikosteron, progesteron, estradiol dan histologi kelenjar adrenal. Tikus dalam kumpulan T2 mempunyai peningkatan ketara (P < 0.05) dalam ketebalan zona fasciculata (94.95 ± 1.55 μm) dan tahap kortikosteron yang lebih tinggi (49.57 ± 1.57 ng/mL) berbanding dengan kawalan. Walau bagaimanapun, suplemen roti lebah semasa tekanan haba telah menunjukan kesan positif dengan pengurangan ketebalan zona fasciculata (79.89 ± 3.08 μm) dan hormon kortikosteron (35.31 ± 1.73 ng/mL) secara ketara (P < 0.05). Oleh itu, penemuan ini mungkin membayangkan bahawa roti lebah berkesan sebagai peneutral dalam menurunkan pengeluaran hormon tekanan.
Kata kunci: Tekanan Haba, Kortikosteron, Kalenjar Adrenal, Reproduksi Betina, Roti Lebah
Highlights.
Bee bread is a nutritious, health-promoting food, rich in antioxidants, antibacterial, anticancer and hepatoprotective properties that is also packed with essential nutrients, such as carbohydrates, lipids, proteins, fatty acids and minerals.
Rats in the T2 (heat stress) group had a significantly (P < 0.05) increase in the zona fasciculata thickness and higher corticosterone levels compared with control.
Supplementation of 0.5 g bee bread during heat stress was able to reduce the zona fasciculate thickness and corticosterone level significantly compared with rats under heat stress exposure.
INTRODUCTION
In both clinical and experimental studies, heat stress has been shown to have a negative impact on pregnancy outcomes such as spontaneous abortion, low birth weight, growth retardation and stillbirth (Schifano et al. 2016; Arroyo et al. 2016; Auger et al. 2017; Ha, Liu, Zhu, Kim, Sherman & Mendola 2017; Ha, Liu, Zhu, Kim, Sherman, Grantz & Mendola 2017). The precise mechanism of heat stress causing problems with pregnancy outcomes is still unclear. According to Sadek et al. (2021), both the hypothalamic-pituitary-adrenocortical (HPA) and sympatho-adrenomedullary axes are associated with maintaining homeostasis during stress. Elevated environmental rise are powerful stimuli that can alter both the sympathetic nervous system and the hypothalamic-pituitary-adrenocortical axis (HPA) (Huh et al. 2021). Elevated plasma corticosterone levels have previously been reported in heat-stressed broilers due to hypothalamic-pituitary-adrenal axis activation (Quinteiro-Filho et al. 2012; Xu et al. 2018). There is scientific proof that exposure to 8 h of heat stress at 32°C for 7 days increased adrenal weight in male rats while also increasing adrenocorticotrophic hormone (ACTH) and corticosterone levels (Wang et al. 2015).
Moreover, the activation of HPA axis has a strong inhibitory effect on hypothalamic-pituitary-gonadal axis (HPG) as reported by Valsamakis et al. (2019). According to El-Ratel et al. (2020), the high ambient temperature was found to have an impact on female reproductive hormones, resulting in lower levels of LH, FSH, progesterone and estradiol. Furthermore, a study by Fotsing et al. (2016) found that chronic immobilisation stress caused a decrease in uterus weight in rats due to a lack of oestrogen and progesterone, which are required for uterus development suggesting that activation of HPA axis may disturb the female reproduction process.
Bee bread is one of the bee products with antioxidants, antibacterial, anticancer, and hepatoprotective characteristics (Kieliszek et al. 2018). It is also considered a well-balanced diet since it contains carbs, lipids, proteins fatty acids and trace minerals (Urcan et al. 2017; Tomás et al. 2017; Kieliszek et al. 2018; Belina-Aldemita et al. 2019; Bakour et al. 2022). Bee bread also contains necessary amino acids that humans are unable to produce (da Silva et al. 2014; Donkersley et al. 2017). Recently, supplementation of bee bread in pregnant rats under heat stress exposure has shown a recovery in pregnancy outcomes such as an increase in litter size, increase in foetal birth weight and reduction in the percentage of embryo resorption when compared with heat-stressed dams (Nor et al. 2021). Furthermore, a previous study by Haron et al. (2014) reported that supplementation of honey during stress was able to counteract the effect of stress and reduced the corticosterone level that leading to an improvement in pregnancy outcomes. However, whether supplementation of bee bread during heat stress exposure may also reduce the level of the stress hormone, corticosterone has yet been reported.
Therefore, this study aims to determine the effect of bee bread on corticosterone level, progesterone level, oestradiol level and zonation of the adrenal cortex of pregnant rats under heat stress exposure.
MATERIALS AND METHODS
Experimental Design
Twenty-four (24) eight-week-old sexually developed female Sprague Dawley rats were used for this study. The rats were housed separately in a cage with clean and absorbent bedding, commercial pellet food, water ad libitum, and were kept at a temperature of 20°C–24°C in a 12-hour light/dark cycle. This study protocol was approved by the UniSZA Animal and Plant Research Ethics Committee (UAPREC/04/043).
The oestrous regularity was first checked by taking the vagina fluid smear. This procedure was repeated for 10 days consecutively. Using a blunt-ended pipette, the vagina was flushed with 0.9% normal saline. Following that, a little quantity of the cell solution was evacuated onto a labelled glass slide before being viewed under a light microscope at 100× and 400× magnifications. The proestrous or oestrous phase of the cycle was determined once the regularity of the cycle is obtained. The female rats were then mated overnight with proven fertile male rats (males who have achieved mature sexual age and have successfully mated before) to induce conception. Each female rat was rechecked the next morning to detect the presence of sperm, and a positive sperm smear was designated as day 0 of pregnancy (Organisation for Economic Co-operation and Development [OECD] 2008).
Pregnant rats were randomly categorised into four groups (n = 6): Control (C: standard feeding), Treatment 1 (T1: 0.5 g bee bread/kg body weight/day), Treatment 2 (T2: standard feeding with heat exposure), and Treatment 3 (T3: 0.5 g bee bread/kg body weight/day with heat exposure). All treatment group rats received the treatment once daily in the morning until delivery. Bee bread (0.5 g/kg body weight/day) was force-fed to pregnant rats through oral gavage beginning on day 0 of pregnancy and continuing until delivery. The bee bread samples utilised in this investigation were the same as one of the bee bread samples tested for nutritional value by Othman et al. (2019). To make the administration of the bee bread simpler, it was diluted with 1.0 mL distilled water. To guarantee the exact intestinal administration of bee bread, the dilution was administered through oral gavage. The bee bread dosages were calculated based on human intake, which was one tablespoon daily or roughly 30 g/60 kg body weight (Kieliszek et al. 2018). Rats in C and T2 groups were given 1.0 mL of distilled water daily to experience the same force-feeding procedure as the T1 and T3 groups. Heat stress was generated experimentally by putting both T2 and T3 rats in an egg incubator (M&M Ternak) for 45 min daily at a temperature of 43°C till delivery. This procedure was modified from Mohd Nor and Haron (2018) study’s temperature and duration. After delivery, each dam was sacrificed via cervical dislocation (American Veterinary Medical Association [AVMA] 2013). Blood was drawn directly from the inferior vena cava to obtain serum, which was later used to determine the level of corticosterone, progesterone and estradiol hormone. The dam’s adrenal glands were also removed for histopathological examination.
Assay of Hormone Level
Serum corticosterone, progesterone, and oestradiol levels were measured by using an enzyme-linked immunosorbent assay kit from Elabscience. All samples and reagents for ELISA were brought to room temperature prior to performing the assay. An aliqout of 50 μL standard and samples were added to each well. Another 50 μL of Biotinylated Detection antibory (Ab) were immediately added to each well. The wells were incubated for 45 min at 37°C. After being incubated, the contents of the wells were aspirated and rinsed with 350 μL of wash buffer per well for three times. The residual droplets were removed. Next, 100 μL horseradish peroxidase (HRP) conjugate working solution were added to each well. Then, the mixture was mixed thoroughly for 10 s before incubating for 30 min at room temperature. Following incubation, the well contents were vigorously shaken, and the washing procedure was carried out five more times. Each well received 90 μL of the substrate reaction, which was then incubated at room temperature for 15 min. The enzymatic reaction was stopped by adding 50 μL of stop solution to each well. Within 10 min of adding the stop solution, the plate was read at 450 ± 10 mm using an Ultra Microplate Reader.
Histological Assessment of Adrenal Gland
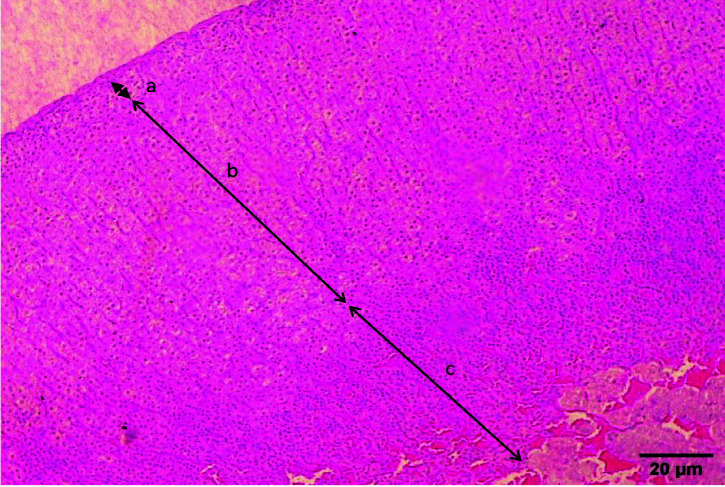
The adrenal glands of the rats were taken immediately after they were slaughtered and submerged in 10% formalin for 24 h. After 24 h, the adrenal gland was cut with a sharp surgical blade and put inside a histology cassette before being dried and embedded in paraffin. On a microtome, solidified paraffin-embedded tissues were sliced into 4 μm thick slices. The section on the slides was stained using standard haematoxylin and eosin (H&E) staining methods ((National Society for Histotechnology [NSH] 2001). The thickness of the zona glomerulosa, zona fasciculata and zona reticularis was evaluated using the LAZ X image analyzer. The thickness was measured in two distinct locations of the adrenal gland, as illustrated in the histological section (Fig. 1) and was represented as the average mean of the two measurements (Haron et al. 2014).
Figure 1.
Light microscope feature of a histological section of rat adrenal gland, (a) Zona Glomerulosa, (b) Zona Fasciculata, (c) Zona Reticularis. (H&E staining, magnification of 400x).
Statistical Analysis
The statistical analysis was performed using Minitab software. All normal distribution data were analysed using one way ANOVA, followed by Tukey’s post hoc test, and the results were expressed as mean ± SEM. Statistical significance was accepted at P < 0.05.
RESULTS AND DISCUSSION
Hormonal Level
Fig. 2 depicts the results of all treatment groups’ serum corticosterone levels. When compared to the control and T3 groups, the T2 group had substantially (P < 0.05) greater corticosterone levels (49.57 ± 1.57 ng/mL). The corticosterone level in the T3 group, on the other hand, was significantly (P < 0.05) lower (35.31 ± 1.73 ng/mL) than in the T1 and T2 groups.
Figure 2.
The corticosterone level (mean ± SEM) in different treatment groups which are Control (C: standard feeding treatment), Treatment 1 (T1: Bee bread), Treatment 2 (T2: Heat stress) and Treatment 3 (T3: Bee bread + Heat stress). Significant differences were determined by one-way ANOVA followed by Tukey’s post hoc test with P < 0.05. Different superscript (a, b, c) indicates a significant difference between the treatment groups. a indicates significant difference between C and T2; b indicates significant differences between T1 and T3 and c indicates significant difference between T2 and T3.
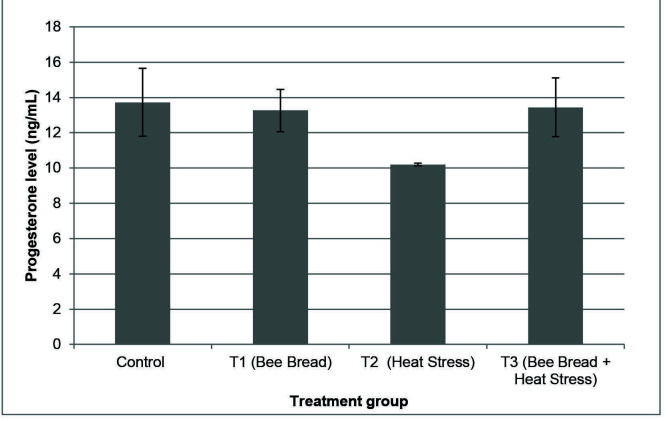
Figs. 3 and 4 show the level of progesterone and estradiol levels in all experimental groups. However, no significant differences were observed in the progesterone and estradiol levels between all experimental groups.
Figure 3.
The progesterone level (mean ± SEM) in different treatment groups which are Control (C: standard feeding treatment), Treatment 1 (T1: Bee bread), Treatment 2 (T2: Heat stress) and Treatment 3 (T3: Bee bread + Heat stress).
Figure 4.
The oestradiol level (mean ± SEM) of in different treatment groups which are Control (C: standard feeding treatment), Treatment 1 (T1: Bee bread), Treatment 2 (T2: Heat stress) and Treatment 3 (T3: Bee bread + Heat stress).
The higher corticosterone hormone level in T2 group proved that the HPA axis was activated during exposure to heat stress. The HPA axis is one of the main endocrine components involved in the stress system response (Sheng et al. 2021). The activation of the HPA axis has resulted in the production of corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP). Both CRH and AVP stimulate the anterior pituitary to generate and release ACTH synergistically. Subsequently, the ACTH stimulates the adrenal cortex’s synthesis of glucocorticoids such as cortisol and corticosterone (Lucy 2019). In a rat’s adrenal cortex, corticosterone is one of the most abundant glucocorticoids produced (Ieko et al. 2019).
Rats in T2 group had a higher level of corticosterone than in the control and T3 groups which suggests that exposure to heat stress during pregnancy has resulted in increased corticosterone levels in dams of the present study. Similar findings were also reported by Wang et al. (2015), Zhang et al. (2019) and Sadek et al. (2021) showing that stressed dams had greater levels of plasma corticosterone than control dams. Previous research has demonstrated that high cortisol levels have a negative feedback effect on the production of corticotropin-releasing hormone (CRH) from the hypothalamus, causing the decrease of corticosterone release from the adrenal cortex (Allen & Sharma 2021). However, with prolonged stress, that negative feedback fails to limit cortisol release, and its levels rise to dangerously high levels, eventually resulting in neuroendocrine, cardiovascular and immunological diseases (Sadek et al. 2021).
According to Valsamakis et al. (2019), the HPA axis hormones have powerful, mostly inhibitory effects on female reproductive axis, the hypothalamic pituitary ovarian (HPO) axis. HPO axis is suppressed by restriction of GnRH production leading to inhibition of the release of LH by the pituitary (Wagenmaker & Moenter 2017). This was evident in the reduction of LH, FSH and estradiol secretion upon stressor exposure (El-Ratel et al. 2020). Therefore, these findings might suggest that higher corticosterone level due to stressor exposure including heat stress could potentially harm the reproductive activity in female which required further investigations.
Meanwhile, supplementation of bee bread during heat stress exposure (T3) has demonstrated a significant reduction in the corticosterone level suggesting that consumption of bee bread could minimise the stress effect on the body. The same findings were reported by Haron et al. (2014), showing that the level of corticosterone was reduced with the consumption of honey during stress in pregnant rats. According to Morsy et al. (2021), the presence of flavonoids in red propolis extract (RPE) may have contributed in lowering cortisol concentrations found in propolis-treated sheep. This can be rationalised by the function of antioxidants in honey or bee bread which are responsible in removing reactive oxygen and reactive nitrogen species, thereby protecting neurons in the brain from harm (Abdulmajeed et al. 2016). Bee bread is also rich in vitamins including vitamin C that ranged from 0.1087 to 0.1152 mg/g (Mohammad et al. 2020). A study found that supplementation of vitamin C in heat-stressed broiler has also resulted in lower corticosterone levels (del Barrio et al. 2020). This implies that bee bread supplementation was able to lower the corticosterone level possibly by the action of the antioxidant activity and its nutritional content that leads to better pregnancy outcomes
Histological Analysis of Adrenal Gland
Table 1 shows the findings on the thickness of three zonas of the adrenal cortex. There is no significant difference was observed in the thickness of zona glomerulosa and zona reticularis among all experimental groups.
Table 1.
The thickness of zona glomerulosa, zona fasciculata and zona reticularis of rats’ adrenal gland.
| Zona | Control | T1 (Bee bread) | T2 (Heat stress) | T3 (Bee bread + Heat stress) |
|---|---|---|---|---|
| Zona Glomerulosa (μm) | 7.05 ± 0.21 | 6.85 ± 0.21 | 7.98 ± 0.40 | 7.51 ± 0.17 |
| Zona Fasciculata (μm) | 76.93 ± 3.36a | 74.27 ± 4.31a | 94.95 ± 1.55b | 79.88 ± 3.08a |
| Zona Reticularis (μm) | 45.83 ± 3.26 | 32.80 ± 2.46 | 30.42 ± 2.93 | 43.18 ± 6.34 |
Notes: Data are presented as mean ± SEM. Significant differences were determined by one-way ANOVA followed by Tukey’s post hoc test with P < 0.05. Different superscript (a, b) indicates a significant difference between the treatment groups.
However, the thickness of zona fasciculata in T2 group was significantly higher when compared to control, T1, and T3 groups as shown in Table 1. This finding conforms with previous studies that found significant increase in the thickness of zona fasciculata layer of rat’s subjected to chronic unpredictable mild stress paradigm (CUMS) (Sadek et al. 2021). Zona fasciculata is the main source of glucocorticoids production such as corticosterone that being stimulated by adrenocorticotrophic hormone (ACTH) during stressful conditions (Levin et al. 2019; Sheng et al. 2021). Increase in the thickness of the zona fasciculata during stress may reflect its cell’s secretory activity in terms of generating and secreting corticosterone upon activation of the HPA axis. Another study by Zaki et al. (2018) also observed that stress induced changes in adrenal cortex by forming deformed irregulars capsule observed in both zona glomerulosa and zona fasciculata. Heat stress in T2 has altered normal functions of adrenal gland by increasing glucocorticoids to some extent thus resulting in a higher corticosterone level in T2 group of the present study. Although there is no significant difference was evident in the thickness of zona glomerulosa, however, the increased thickness of zona glomerulosa in T2 group might suggest its response in synthesising aldosterone for water and sodium maintenance during dehydration that may be caused by heat stress (Rakova et al. 2017) although no further analysis was undertaken in this study. This suggests that more research needs to be undertaken to determine the exact effect of heat stress on zona glomerulosa. The results also suggest that consumption of bee bread during heat stress by rats in T3 group has significantly reduced the thickness of zona fasciculata compared to the T2 group, reflecting the drop in corticosterone hormone in rats.
CONCLUSION
In conclusion, exposure to heat stress significantly increased corticosterone levels. Heat stress also significantly increases the thickness of zona fasciculata which imply the cell’s secretory activity in terms of generating and secreting corticosterone upon activation of the HPA axis. Furthermore, supplementation of bee bread during heat stress significantly reduced the thickness of zona fasciculata and corticosterone levels. These findings may suggest that bee bread is effective as a neutralizer in lowering the production of stress hormone.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Universiti Sultan Zainal Abidin for funding this study (RAGS/1/2015/SKK0/UNISZA/03/1 and UniSZA/LABMAT/2018/01).
Footnotes
AUTHORS’ CONTRIBUTIONS
Mohd Nizam Haron: Devised the project and main conceptual ideal.
Nur Akmar Nadhirah Mohd Nor: Conceived and designed the analysis, collected the data performed the analysis and wrote the manuscript.
Asmad Kari: Review and improved the content of manuscript.
Connie Fay Komilus: Review and improved the content of manuscript.
REFERENCES
- Abdulmajeed WI, Sulieman HB, Zubayr MO, Imam A, Amin A, Biliaminu SA, Oyewole LA, Owoyele BV. Honey prevents neurobehavioural deficit and oxidative stress induced by lead acetate exposure in male Wistar rats: A preliminary study. Metabolic Brain Disease. 2016;31(1):37–44. doi: 10.1007/s11011-015-9733-6. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Sharma S. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2021. Physiology, Adrenocorticotropic Hormone (ACTH) [Updated 17 August 2021] https://www.ncbi.nlm.nih.gov/books/NBK500031/ [PubMed] [Google Scholar]
- American Veterinary Medical Association (AVMA) AVMA guidelines for the euthanasia of animals: 2013 edition. 2013. https://works.bepress.com/cgi/viewcontent.cgi?article=1014&context=cheryl_greenacre .
- Arroyo V, Díaz J, Carmon R, Ortiz C, Linares C. Impact of air pollution and temperature on adverse birth outcomes: Madrid, 2001–2009. Environmental Pollution. 2016;218:1154–1161. doi: 10.1016/j.envpol.2016.08.069. [DOI] [PubMed] [Google Scholar]
- Auger N, Fraser WD, Smargiassi A, Bilodeau-Bertrand M, Kosatsky T. Elevated outdoor temperatures and risk of stillbirth. International Journal of Epidemiology. 2017;46(1):200–208. doi: 10.1093/ije/dyw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakour M, Laaroussi H, Ousaaid D, El Ghouizi A, Es-Safi I, Mechchate H, Lyoussi B. Bee bread as a promising source of bioactive molecules and functional properties: An up-to-date review. Antibiotics (Basel, Switzerland) 2022;11(2):203. doi: 10.3390/antibiotics11020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belina-Aldemita MD, Opper C, Schreiner M, D’Amico S. Nutritional composition of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines. Journal of Food Composition and Analysis. 2019;82:103215. doi: 10.1016/j.jfca.2019.04.003. [DOI] [PubMed] [Google Scholar]
- da Silva GR, da Natividade TB, Camara CA, da Silva EMS, dos Santos FDAR, Silva TMS. Identification of sugar, amino acids and minerals from the pollen of jandara stingless bees (Melipona subnitida) Food and Nutrition Sciences. 2014;05(11):1015–1021. doi: 10.4236/fns.2014.511112. [DOI] [Google Scholar]
- del Barrio AS, Mansilla W, Navarro-Villa A, Mica J, Smeets J, den Hartog L, García-Ruiz A. Effect of mineral and vitamin C mix on growth performance and blood corticosterone concentrations in heat-stressed broilers. Journal of Applied Poultry Research. 2020;29(1):23–33. doi: 10.1016/j.japr.2019.11.001. [DOI] [Google Scholar]
- Donkersley P, Rhodes G, Pickup RW, Jones KC, Power EF, Wright GA, Wilson K. Nutritional composition of honey bee food stores vary with floral composition. Oecologia. 2017;185(4):749–761. doi: 10.1007/s00442-017-3968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ratel IT, Abdel-Khalek AE, Fouda SF. Effect of ovarian stimulation by different gonadotrophin treatments on in vivo and in vitro reproductive efficiency of rabbit does under high ambient temperature. Tropical Animal Health and Production. 2020;53(1):22. doi: 10.1007/s11250-020-02429-w. [DOI] [PubMed] [Google Scholar]
- Fotsing D, Njapdounke KJ, Kenneth YA, Ngo Bum E. Effect of Nelsonia canescens (Acanthaceae) on the stress induced behavioral and reproductive changes in female rats. World Journal Pharmacy and Pharmaceutical Science. 2016;12:31–49. [Google Scholar]
- Ha S, Liu D, Zhu Y, Kim SS, Sherman S, Mendola P. Ambient temperature and early delivery of singleton pregnancies. Environmental Health Perspectives. 2017;125(3):453–459. doi: 10.1289/EHP97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Liu D, Zhu Y, Kim SS, Sherman S, Grantz KL, Mendola P. Ambient temperature and stillbirth: A multi-center retrospective cohort study. Environmental Health Perspectives. 2017;125(6):067011. doi: 10.1289/EHP945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haron MN, Wan F, Siti AS, Mahaneem M. Tualang honey ameliorates restraint stress-induced impaired pregnancy outcomes in rats. European Journal of Integrative Medicine. 2014;6(6):657–663. doi: 10.1016/j.eujim.2014.07.001. [DOI] [Google Scholar]
- Huh E, Lee W, Choi Y, Lee TH, Oh MS. Geongangbuja-tang decoction and its active ingredient, aconiti lateralis radix preparata, exerts inhibitory effects on heat stress-induced inflammation in mice. Applied Sciences. 2021;11(15):6902. doi: 10.3390/app11156902. [DOI] [Google Scholar]
- Ieko T, Sasaki H, Maeda N, Fujiki J, Iwano H, Yokota H. Analysis of corticosterone and testosterone synthesis in rat salivary gland homogenates. Frontiers in Endocrinology. 2019;10:479. doi: 10.3389/fendo.2019.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Piwowarek K, Kot AM, Błażejak S, Chlebowska-Śmigiel A, Wolska I. Pollen and bee bread as new health-oriented products: A review. Trends in Food Science and Technology. 2018;71:170–180. doi: 10.1016/j.tifs.2017.10.021. [DOI] [Google Scholar]
- Levin G, Elchalal U, Rottenstreich A. The adrenal cortex: Physiology and diseases in human pregnancy. European Journal of Obstetrics, Gynecology and Reproductive Biology. 2019;240:139–143. doi: 10.1016/j.ejogrb.2019.06.036. [DOI] [PubMed] [Google Scholar]
- Lucy MC. Stress, strain, and pregnancy outcome in postpartum cows. Animal Reproduction. 2019;16(3):455–464. doi: 10.21451/1984-3143-AR2019-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad SM, Mahmud-Ab-Rashid N, Zawawi N. Botanical origin and nutritional values of bee bread of stingless bee (Heterotrigona itama) from Malaysia. Journal of Food Quality. 2020;2020:2845757. doi: 10.1155/2020/2845757. [DOI] [Google Scholar]
- Mohd Nor NAN, Haron MN. Effect of heat stress on pregnancy outcomes in Sprague Dawley rats. International Journal of Engineering Technology. 2018;7(4.43):6–9. [Google Scholar]
- Morsy A, Soltan Y, El-Zaiat H, Alencar S, Abdalla A. Bee propolis extract as a phytogenic feed additive to enhance diet digestibility, rumen microbial biosynthesis, mitigating methane formation and health status of late pregnant ewes. Animal Feed Science and Technology. 2021;273:114834. doi: 10.1016/j.anifeedsci.2021.114834. [DOI] [Google Scholar]
- National Society for Histotechnology (NSH) Guidelines for hematoxylin and eosin staining. Maryland: NSH; 2001. [Google Scholar]
- Nor NANM, Komilus CF, Haron MN, Lananan F, Chew HH, Yaakub N, Kari A. Effect of bee bread on pregnancy outcomes and reproductive system of rats under heat stress exposure. Malaysian Journal of Fundamental and Applied Sciences. 2021;17(6):781–793. doi: 10.11113/mjfas.v17n6.2308. [DOI] [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD) OECD Guidelines for the Testing of Chemicals. OECD; 2008. Part 5: Preparation, reading and reporting of vaginal smears; pp. 116–125. https://www.oecd.org/chemicalsafety/testing/40581357.pdf . [Google Scholar]
- Othman ZA, Wan Ghazali WS, Nordin L, Omar N, Mohamed M. Nutritional, phytochemical and antioxidant analysis of bee bread from different regions of Malaysia. Indian Journal Pharmacology Science. 2019;81:955–960. doi: 10.36468/pharmaceutical-sciences.590. [DOI] [Google Scholar]
- Quinteiro-Filho WM, Gomes AVS, Pinheiro ML, Ribeiro A, Ferraz-de-Paula V, Astolfi-Ferreira CS, Palermo-Neto J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella enteritidis. Avian Pathology. 2012;41(5):421–427. doi: 10.1080/03079457.2012.709315. [DOI] [PubMed] [Google Scholar]
- Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, Kopp C, Pedchenko T, Zhang Y, Beck L, Johannes B, Marton A, Müller DN, Rauh M, Luft FC, Titze J. Increased salt consumption induces body water conservation and decreases fluid intake. The Journal of Clinical Investigation. 2017;127(5):1932–1943. doi: 10.1172/JCI88530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek MT, El-Abd SS, Ibrahim MAA. Effect of chronic unpredictable mild stress on adrenal cortex of adult rat and the possible protective role of licorice extract: A histological and immunohistochemical study. Egyptian Journal of Histology. 2021;44(4):887–901. [Google Scholar]
- Schifano P, Asta F, Dadvand P, Davoli M, Basagana X, Michelozzi P. Heat and air pollution exposure as triggers of delivery: A survival analysis of population-based pregnancy cohorts in Rome and Barcelona. Environment International. 2016;88:153–159. doi: 10.1016/j.envint.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, Hale TM, Handa RJ. The hypothalamic-pituitary-adrenal axis: Development, programming actions of hormones, and maternal-fetal interactions. Frontiers in Behavioral Neuroscience. 2021;14(January):1–21. doi: 10.3389/fnbeh.2020.601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás A, Falcão SI, Russo-Almeida P, Vilas-Boas M. Potentialities of beebread as a food supplement and source of nutraceuticals: Botanical origin, nutritional composition and antioxidant activity. Journal of Apicultural Research. 2017;56(3):219–230. doi: 10.1080/00218839.2017.1294526. [DOI] [Google Scholar]
- Urcan AC, Marghitas LA, Dezmirean DS, Bobis O, Bonta V, Muresan CI, Margaoan R. Chemical composition and biological activities of beebread: Review. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Animal Science and Biotechnologies. 2017;74(1):6–14. [Google Scholar]
- Valsamakis G, Chrousos G, Mastorak-os G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology. 2019;100:48–57. doi: 10.1016/j.psyneuen.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Wagenmaker ER, Moenter SM. Exposure to acute psychosocial stress disrupts the luteinizing hormone surge independent of estrous cycle alterations in female mice. Endocrinology. 2017;158(8):2593–2602. doi: 10.1210/en.2017-00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu F, Luo Y, Zhu L, Li G. Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats. Biomedical Reports. 2015;3(3):425–429. doi: 10.3892/br.2015.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lai X, Li Z, Zhang X, Luo Q. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poultry Science. 2018;97(11):4073–4082. doi: 10.3382/ps/pey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki SM, Abdelgawad FA, El-Shaarawy EAA, Radwan RAK, Aboul-Hoda BE. Stress-induced changes in the aged-rat adrenal cortex. Histological and histomorphometric study. Folia Morphologica (Warsz) 2018;77(4):629–641. doi: 10.5603/FM.a2018.0035. [DOI] [PubMed] [Google Scholar]
- Zhang S, Mesalam A, Lee KL, Song SH, Khan I, Yuan Y, Wenfa LV, Kong IK. Effect of predator stress on the reproductive performance of female mice after nonsurgical embryo transfer. Journal of the American Association for Laboratory Animal Science. 2019;58(3):304–310. doi: 10.30802/AALAS-JAALAS-18-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]