Abstract
Background:
Oral antibiotics (OA) combined with mechanical bowel preparation (MBP) significantly decrease the rate of surgical site infections (SSIs). However, the prophylactic effects in region-specific colorectal surgery have not been assessed.
Materials and methods:
A single-centre, single-blind, randomized controlled trial was conducted from 2019 to 2022. Patients were eligible if they were diagnosed with nonmetastatic colorectal malignancy, and laparoscopic colorectal surgery was indicated. Participants were randomly assigned (1:1) to the experimental (OA+MBP preparation) or control group (MBP preparation). The randomization was further stratified by resected region. The primary outcome was the incidence of SSIs. Patients were followed up for 1 month postoperatively, and all complications were recorded.
Result:
Between 2019 and 2022, 157 and 152 patients were assigned to the experimental and control groups, respectively, after 51 patients were excluded. The incidence of SSIs in the control group (27/152) was significantly higher than that in the experimental group (13/157; P=0.013), as was the incidence of superficial SSIs (5/157 vs. 14/152, P=0.027) and deep SSIs (7/157 vs. 16/152, P=0.042). After redistribution according to the resected region, the incidence of SSIs was significantly higher in the control group with left-sided colorectal resection (descending, sigmoid colon, and rectum) (9/115 vs. 20/111, P=0.022) but was similar between the groups with right-sided colon resection (ascending colon) (3/37 vs. 7/36, P=0.286). No differences were noted between the groups in terms of other perioperative complications.
Conclusion:
OA+MBP before colorectal surgery significantly reduced the incidence of SSIs. Such a prophylactic effect was particularly significant for left-sided resection. This preparation mode should be routinely adopted before elective left-region colorectal surgeries.
Keywords: colorectal surgery, laparoscopy, mechanical bowel preparation, oral antibiotics, surgical site infection
Introduction
Highlights
Oral antibiotics (OA) combined with mechanical bowel preparation (MBP) reduced the incidence of surgical site infections after laparoscopic colorectal surgery.
OA combined with MBP was effective only for left-sided colorectal surgery.
OA combined with MBP was not superior for right-sided colon surgery.
Surgical site infections (SSIs) are the most common surgical complication and significantly affect hospital stays, readmission rates, total expenses, and mortality1,2. Given the abundance of bacteria in the bowel, patients undergoing colorectal surgery are particularly at risk of SSIs3,4. Bowel preparation strategies have been proven to be associated with postoperative morbidity and have been controversial for several decades5–7.
Simple mechanical bowel preparation (MBP) was initially applied because it can theoretically remove the human faecal content and bacterial load5,8. However, subsequent studies have indicated that simple MBP has no benefit on the incidence of SSIs or other complications9–11. MBP combined with oral antibiotics (OA) was then applied for patients before colorectal surgery, with the hypothesis that MBP-mediated reduction in bacterial burden promotes better delivery of OA to the entire length of the colon, improving prophylactic activity12,13. As a general implementation of the enhanced recovery after surgery (ERAS) principle, surgeons have recently sought to minimize perioperative physiologic perturbations, leading to increasing concern regarding and abandonment of the use of MBP or OA14,15. An online survey conducted by the European Society of Coloproctology indicated that MBP was routinely conducted by 126 of 426 surgeons before colonic surgery and 328 surgeons before rectal surgery in Europe and that only 47 surgeons gave preoperative OA16. Another survey conducted by the Association of Coloproctology of Great Britain and Ireland showed that prophylactic OA was only prescribed by 12–20% of surgeons17.
Recently, the merits of OA and MBP have been rediscovered in several related studies demonstrating a significant decrease in the rate of SSIs18–21. WHO guidelines22 and American Society for Enhanced Recovery guidelines23 suggest that MBP should not be given alone and recommend that MBP be combined with OA before elective colorectal surgery.
However, none of these studies assessed the relative prophylactic effects of the bowel preparation mode in region-specific colorectal surgery. Combinational preparation for all patients may prolong preoperative hospital stays and expenses, in addition to causing increased patient discomfort and reduced compliance. Our previous retrospective study reported the prophylactic efficacy of MBP+OA in patients undergoing left-sided colon or rectum resection24. Herein, we report a randomized controlled trial (RCT) of a single-centre comparison of MBP+OA with MBP alone, assessing the rates of prophylactic combinations between groups with specific surgical locations.
Materials and methods
Study design and participants
Patients were recruited sequentially from a single centre from 2019 to 2022 in the current single-blind, pragmatic, RCT. The flowchart for patient selection is shown in Figure 1. The inclusion criteria were as follows: patients over 18 years old; patients who were diagnosed with neoplasia disease of colorectal malignancy and a laparoscopic partial colon resection or total colectomy; patients who agreed to participate voluntarily in the study and signed an informed consent form. The exclusion criteria were as follows: patients who underwent emergency surgery, natural orifice specimen extraction surgery or abdominal perineal resection; patients who underwent other surgeries due to infections, such as appendectomy and cholecystectomy; patients who suffered from severe comorbidities, such as uncontrolled hypertension and diabetes mellitus; patients with a combined histological diagnosis of inflammatory bowel disease (IBD), active acute diverticulitis, ischaemic colitis, or infectious colitis; patients who were diagnosed with peritoneal implantation or detection of distant metastasis during surgery; patients who underwent neoadjuvant chemo/radiotherapy; patients who were allergic to OA or polyethylene glycol (PEG); patients who underwent preoperative dermatosis; patients with long-term use of corticosteroids or with autoimmune disease; patients who did not comply with the assigned prophylaxis regimen or who refused in writing to participate in the study.
Figure 1.
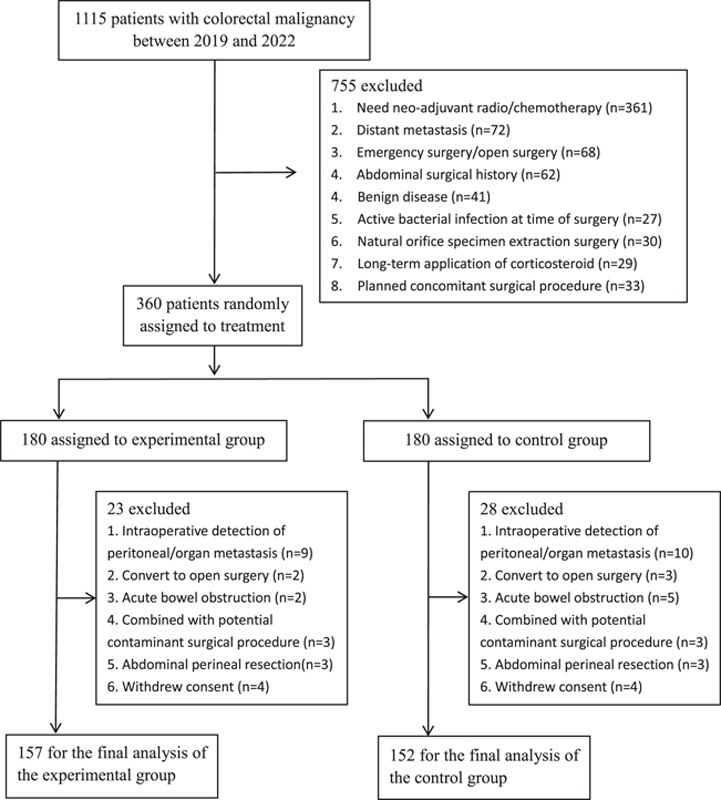
Flowchart for patient selection.
Written informed consent was obtained from all eligible patients before inclusion in the study. This study followed the consolidated standards of reporting trials (CONSORT) guidelines25. This RCT was approved by the Ethics Committee of the single centre and was performed in accordance with the Declaration of Helsinki guidelines.
Randomization and procedure
Eligible patients were randomly assigned (1:1) to the experimental group (OA combined with MBP) or the control group (only MBP). The randomization was conducted using online randomization software and stratified by resection site (left side included descending colon, sigmoid colon, and rectum resection; right side included ascending colon resection only). The investigators, surgeons and patients were unmasked to the group to which the patient was randomly assigned. However, the research assistant who assessed the presence or absence of outcomes and the statistician were masked to treatment assignment.
The study intervention for patients assigned to the experimental group was as follows: A liquid diet and PEG with 2 l water were administered orally 1 day before surgery. A combination of streptomycin 1 g and metronidazole 0.2 g every 6 h three times was also administered. For patients assigned to the control group, only a liquid diet and PEG with 2 l water were administered orally 1 day before surgery. Enteroclysis was prescribed for all patients on the morning of surgery.
All patients received the same intravenous antimicrobial prophylaxis, namely, a second-generation cephalosporin with anaerobic activity (ceftriaxone 2 g) as recommended, which was administered to all patients 30 min before skin incision and readministered intraoperatively if the procedure lasted 3 h or more. Continuous administration was continued once a day until 48 h after surgery. All patients underwent laparoscopic partial or total/subtotal colorectal resection by the same surgical team with a similar surgical procedure and the same suture technique for the peritoneum, fascial layer, subcutaneous layer and skin. Skin preparation with an alcohol-based antiseptic agent was performed before the surgical incision. The ERAS principle was conducted during the postoperative period as early mobilization on the first day after surgery, pain control, early food intake, chewing gum application, etc26.
Outcomes
The primary endpoint of the current study was the incidence of SSIs within 30 days after surgery, which included superficial, deep and organ–space infections according to the US Centers for Disease Control and Prevention criteria. The secondary endpoints included antibiotic-associated adverse effects, length of hospital stay after surgery, time interval from surgery to defecation, postoperative urinary complications within 30 days, postoperative cardiovascular complications within 30 days, postoperative respiratory complications within 30 days, the need for unplanned hospital readmissions, unexpected admission to the intensive care unit and all-cause mortality within 30 days after surgery. Research assistants who were unaware of the trial group assignments obtained the data for outcome measures.
Calculations
The primary endpoint of the current study was the incidence of SSIs within 30 days after surgery. According to our previous retrospective analysis, the incidence of SSIs was 16.4 and 6.2% for the routine MBP preparation mode and OA+MBP mode in laparoscopic colorectal resection, respectively. And a total of 300 patients were needed to achieve a power of 80% and a significance level of 95%. Because we have set a strict criterion for the patients’ inclusion and exclusion, we have set a 20% for patient loss and, finally 360 patients were required.
Data were collected prospectively by the investigators and research assistants. Patients were followed up per protocol with individual case report forms (CRFs), and CRFs were stored in a specialized clinical trial office. CRFs were also converted manually to electronic CRFs and stored in the database of our centre. Frequencies are presented for categorical variables, and means±standard deviations are given for continuous variables. Pearson’s χ 2 or Fisher’s exact tests were used to analyse categorical variables. Student’s t-tests were used to analyse normally distributed data. Otherwise, Mann–Whitney U tests were used for continuous variables. We conducted two prespecified subgroup analyses of the primary outcome according to the resection region. Interaction terms in the random effect regression model were used to test for heterogeneity of the effect between subgroups. A post hoc analysis was conducted to test for a difference in specified patients. All statistical analyses were conducted using R (version 3.4.2), and P<0.05 was considered significant.
Results
Between 1 February 2019, and 18 October 2022, 1115 patients were assessed for eligibility, and 360 patients were randomly assigned to either the OA+MBP group (experimental group; n=180) or the MBP-only group (control group; n=180). After excluding another 51 patients, 157 patients received OA+MBP and 152 patients received MBP the day before surgery (Fig. 1).
The baseline characteristics, including age, sex, smoking or drinking status, comorbidities, preoperative haemoglobin level, serum CEA (carcinoembryonic antigen) level, albumin level and ASA (American Society of Anesthesiologists) status, were comparable between the groups. Overall, 188 patients underwent laparoscopic colon resection, 117 patients underwent rectal resection, and 4 patients underwent multiple region colorectal resection. The surgical region was stratified before patients were enroled and was similar between both groups. The AJCC (American Joint Committee on Cancer) 8th staging system was applied in the current study. Carcinoma in situ was diagnosed in 23 patients without lymph node involvement as stage 0, while 66 patients were diagnosed as stage I, and 110 patients were diagnosed as stages II and III. The majority of enroled patients had moderately differentiated adenocarcinoma, while 2 patients suffered from neuroendocrine carcinoma, and 1 patient was diagnosed with lymphoma. All characteristics are shown in Table 1.
Table 1.
Baseline characteristics of all participants who were assigned to both groups.
| Characteristics | Overall N=309 (100%) |
Experimental group N=157 (100%) |
Control group N=152 (100%) |
P |
|---|---|---|---|---|
| Sex (male) | 197 | 102 | 95 | 0.652 |
| Age (median [IQR]) | 61 [54–69] | 62 [54–70] | 61 [54–68] | 0.361 |
| Smoker | 69 | 38 | 31 | 0.260 |
| Ex-smoker | 2 | 2 | 0 | 0.492 |
| Alcohol addicted | 13 | 6 | 7 | 0.732 |
| Comorbidities | ||||
| Hypertension | 90 | 46 | 44 | 0.946 |
| Diabetes mellitus | 74 | 38 | 36 | 0.915 |
| Dyslipidaemia | 60 | 33 | 27 | 0.469 |
| Coronary disease | 15 | 9 | 6 | 0.465 |
| COPD | 16 | 9 | 7 | 0.655 |
| Arrhythmia | 69 | 35 | 34 | 0.987 |
| ASA grade (1/2/3) | 107/153/49 | 53/81/23 | 54/72/26 | 0.726 |
| BMI (median [IQR]) (kg/m2) | 22.86 [20.96–24.91] | 22.89 [20.89–25] | 22.77 [21.08–24.85] | 0.948 |
| Preoperative albumin [mean (SD)] (g/l) | 39.549 (4.385) | 39.641 (4.282) | 39.454 (4.501) | 0.709 |
| CEA (median [IQR]) (ng/ml) | 2.210 [1.400–4.960] | 2.490 [1.300–4.930] | 2.000 [1.400–5.210] | 0.397 |
| HGB (median [IQR]) (g/l) | 124 [110–138] | 126 [109–138] | 122.500 [110.750–138] | 0.602 |
| Primary site | 0.805 | |||
| Right colon | 73 | 37 | 36 | |
| Transverse colon | 6 | 2 | 4 | |
| Descending colon | 31 | 14 | 17 | |
| Sigmoid colon | 78 | 39 | 39 | |
| Rectum | 117 | 62 | 55 | |
| Multiple lesions | 4 | 3 | 1 | |
ASA, American Society of Anesthesiologists; BMI, body mass index; CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease; HGB, haemoglobin; IQR, interquartile range.
The incidence of SSIs, including superficial SSIs, deep SSIs, and organ–space SSIs, was the primary outcome of the study. The occurrence of overall SSIs was significantly lower in the experimental group (13/157, 8.28%) than in the control group (27/152, 17.76%, P=0.013), as was the incidence of superficial SSIs (5/157, 3.18% vs. 14/152, 9.21%, P=0.027) and deep SSIs (7/157, 4.46% vs. 16/152, 10.53%, P=0.042).
The incidence of other overall complications was defined as the secondary outcome in the current RCT, which was also similar in both groups (55/157 vs. 71/152). Furthermore, each morbidity was analysed, and the incidence of anastomotic fistula, anastomotic stenosis, postoperative diarrhoea, urinary/respiratory infection, haemorrhage, and ileus was similar. The other 12 complications included 1 incisional hernia, 1 acute appendicitis, 2 acute pancreatitis, 4 intra-abdominal chylous fistula and 4 acute coronary syndromes within 30 days. Most reoperations were attributed to anastomotic fistula (6/7), and only 1 patient underwent reoperation due to SSIs. Two patients from the control group were transferred to the intensive care unit, and one patient died within 30 days due to a severe infection caused by an anastomotic fistula. All data are shown in Table 2.
Table 2.
Surgical and other perioperative characteristics of participants who were assigned to both groups.
| Characteristics | Overall N=309 (100%) |
Experimental group N=157 (100%) |
Control group N=152 (100%) |
P |
|---|---|---|---|---|
| Operation time (median [IQR]) (min) | 200 [163–240] | 194 [160–235] | 203 [165–241.25] | 0.289 |
| Intraoperative bleeding volume (median [IQR]) (ml) | 50 [20–50] | 50 [20–50] | 50 [20–50] | 0.632 |
| Incision length (median [IQR]) (cm) | 6 [6–8] | 6 [6–8] | 7 [6–8] | 0.697 |
| Tumour size (median [IQR]) (cm) | 3.500 [2.500–5] | 3.600 [2.500–5] | 3.500 [2–5] | 0.423 |
| Defecation time (median [IQR]) (day) | 3 [2–4] | 3 [2–4] | 3 [2.875–4] | 0.823 |
| Postoperative hospitalization days (median [IQR]) (day) | 7 [6–9] | 7 [6–9] | 7 [6–9] | 0.717 |
| Stoma | 26 | 11 | 15 | 0.365 |
| Intraoperative complications | 5 | 1 | 4 | 0.345 |
| Haemorrhage | 4 | 1 | 3 | |
| Shock | 1 | 0 | 1 | |
| 8th AJCC stage (0/I/II/III) | 23/66/110/110 | 10/32/65/50 | 13/34/45/60 | 0.178 |
| Grade | 0.072 | |||
| Intraepithelial neoplasia and atypical hyperplasia | 32 | 12 | 20 | |
| Well-differentiated adenocarcinoma | 2 | 0 | 2 | |
| Moderately differentiated adenocarcinoma | 254 | 134 | 120 | |
| Poorly differentiated adenocarcinoma | 18 | 11 | 7 | |
| Other | 3 | 0 | 3 | |
| Transfer to ICU | 2 | 0 | 2 | 0.241 |
| Reoperation | 7 | 5 | 2 | 0.471 |
| Readmission within 30 days | 4 | 4 | 0 | 0.123 |
| Mortality within 30 days | 1 | 1 | 0 | 1.000 |
| Postoperative complications | ||||
| Anastomotic fistula | 21 | 7 | 14 | 0.097 |
| Overall SSI | 40 | 13 | 27 | 0.013 |
| Superficial SSI | 19 | 5 | 14 | 0.027 |
| Deep SSI | 23 | 7 | 16 | 0.042 |
| Organ–space SSI | 5 | 2 | 3 | 0.971 |
| Anastomotic stenosis | 2 | 1 | 1 | 1.000 |
| Postoperative haemorrhage | 21 | 12 | 9 | 0.548 |
| Postoperative ileus | 9 | 3 | 6 | 0.468 |
| Postoperative urinary infection | 3 | 2 | 1 | 1.000 |
| Postoperative diarrhoea | 50 | 20 | 30 | 0.095 |
| Postoperative pulmonary infection | 8 | 5 | 3 | 0.755 |
| Other complications | 12 | 5 | 7 | 0.518 |
AJCC, American Joint Committee on Cancer; ICU, intensive care unit; SSI, surgical site infection.
To further explore the protective function of OA in region-specific surgeries in accordance with our previous retrospective study, patients were stratified into right-sided and left-sided colorectal resection subgroups during the recruiting phase. Right-sided surgery included ascending colon resection only, while left-sided colorectal resection included descending colon resection, sigmoid colon resection and rectum resection.
A total of 73 patients underwent right-sided colon resection, with 37 patients in the experimental group and 36 in the control group. Additionally, 115 patients in the experimental group and 111 patients in the control group who underwent left-sided colorectal resection were analysed. Patient baseline characteristics, surgical variables and pathological features were comparable between the experimental and control groups in both subgroup analyses. For the primary outcome, the incidence of overall SSIs and subtypes of SSIs was not significant in right-sided surgeries. However, the incidence of overall SSIs and deep SSIs was significantly higher in the control group (9/115 vs. 20/111, P=0.022; 3/115 vs. 13/111, P=0.008) with left-sided surgeries. Other postoperative morbidities showed no difference in any subgroup analysis. The detailed data are shown in Tables 3 and 4.
Table 3.
Surgical and other perioperative characteristics of participants who underwent right-sided colon resection.
| Characteristics (right) | Overall N=73 (100%) |
Experimental group N=37 (100%) |
Control group N=36 (100%) |
P |
|---|---|---|---|---|
| Sex (male) | 40 | 21 | 19 | 0.733 |
| Age [mean (SD)] | 60.781 (13.426) | 61.378 (15.119) | 60.167 (11.616) | 0.703 |
| Smoker | 16 | 8 | 8 | 0.951 |
| Alcohol addicted | 3 | 1 | 2 | 0.981 |
| Comorbidities | ||||
| Hypertension | 22 | 8 | 14 | 0.108 |
| Diabetes mellitus | 20 | 10 | 10 | 0.943 |
| Dyslipidaemia | 14 | 7 | 7 | 0.955 |
| Coronary disease | 6 | 5 | 1 | 0.214 |
| COPD | 4 | 2 | 2 | 1.000 |
| Arrhythmia | 18 | 10 | 8 | 0.634 |
| ASA grade (1/2/3) | 22/32/19 | 12/17/8 | 10/15/11 | 0.682 |
| BMI (median [IQR]) (kg/m2) | 22.474 (3.068) | 22.04 (3.096) | 22.92 (3.016) | 0.223 |
| Preoperative albumin [mean (SD)] (g/l) | 38.478 (4.709) | 37.678 (4.334) | 39.3 (4.993) | 0.143 |
| CEA (median [IQR]) (ng/ml) | 2 [1.22–6.2] | 2.4 [1.3–6.7] | 1.8 [1.2–5.15] | 0.231 |
| HGB (median [IQR]) (g/l) | 111.808 (25.413) | 107.811 (25.763) | 115.917 (24.730) | 0.175 |
| Operation time (median [IQR]) (min) | 203.397 (53.224) | 200.108 (55.978) | 206.778 (50.806) | 0.596 |
| Intraoperative bleeding volume (median [IQR]) (ml) | 50 [20–50] | 50 [20–50] | 50 [45–62.5] | 0.081 |
| Incision length (median [IQR]) (cm) | 7 [6–8] | 6 [6–8] | 7 [6–8] | 0.321 |
| Tumour size (median [IQR]) (cm) | 5 [3–6] | 4.5 [2.5–6.5] | 5 [3–6] | 0.982 |
| Defecation time (median [IQR]) (day) | 3 [3–4] | 3 [3–4] | 3 [2.875–5] | 0.596 |
| Postoperative hospitalization days (median [IQR]) (day) | 7 [6–9] | 7 [6–8] | 7 [6–9.5] | 0.733 |
| Intraoperative complications | ||||
| Haemorrhage | 1 | 0 | 1 | 0.493 |
| 8th AJCC stage (0/I/II/III) | 5/6/34/28 | 2/1/21/13 | 3/5/13/15 | 0.181 |
| Grade | 0.052 | |||
| Intraepithelial neoplasia and atypical hyperplasia | 9 | 2 | 7 | |
| Moderately differentiated adenocarcinoma | 56 | 30 | 26 | |
| Poorly differentiated adenocarcinoma | 6 | 5 | 1 | |
| Other | 2 | 0 | 2 | |
| Reoperation | 2 | 1 | 1 | 1.000 |
| Postoperative complications | ||||
| Anastomotic fistula | 5 | 1 | 4 | 0.338 |
| Overall SSI | 10 | 3 | 7 | 0.286 |
| Superficial SSI | 4 | 0 | 4 | 0.116 |
| Deep SSI | 6 | 3 | 3 | 1.000 |
| Organ–space SSI | 1 | 0 | 1 | 0.493 |
| Postoperative haemorrhage | 3 | 1 | 2 | 0.981 |
| Postoperative ileus | 5 | 1 | 4 | 0.338 |
| Postoperative urinary infection | 1 | 0 | 1 | 0.493 |
| Postoperative diarrhoea | 11 | 3 | 8 | 0.092 |
| Postoperative pulmonary infection | 4 | 2 | 2 | 1.000 |
| Other complications | 3 | 2 | 1 | 1.000 |
AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease; HGB, haemoglobin; IQR, interquartile range; SSI, surgical site infection.
Table 4.
Surgical and other perioperative characteristics of participants who underwent left-sided colon resection.
| Characteristics (left) | Overall N=226 (100%) |
Experimental group N=115 (100%) |
Control group N=111 (100%) |
P |
|---|---|---|---|---|
| Sex (male) | 153 | 79 | 74 | 0.744 |
| Age [mean (SD)] | 61.270 (10.970) | 61.635 (11.452) | 60.892 (10.485) | 0.612 |
| Smoker | 52 | 30 | 22 | 0.185 |
| Alcohol addicted | 10 | 5 | 5 | 1.000 |
| Comorbidities | ||||
| Hypertension | 67 | 38 | 29 | 0.255 |
| Diabetes mellitus | 50 | 25 | 25 | 0.887 |
| Dyslipidaemia | 44 | 25 | 19 | 0.380 |
| Coronary disease | 9 | 4 | 5 | 0.957 |
| COPD | 12 | 7 | 5 | 0.596 |
| Arrhythmia | 49 | 25 | 24 | 0.983 |
| ASA grade (1/2/3) | 84/113/29 | 41/60/14 | 43/53/15 | 0.801 |
| BMI (median [IQR]) (kg/m2) | 22.935 [21.093–24.893] | 23.110 [21.215–25.015] | 22.720 [21.075–24.740] | 0.497 |
| Preoperative albumin [mean (SD)] (g/l) | 39.928 (4.230) | 40.157 (4.133) | 39.691 (4.334) | 0.409 |
| CEA (median [IQR]) (ng/ml) | 2.3 [1.4–4.952] | 2.58 [1.4–4.850] | 2.060 [1.4–5.34] | 0.527 |
| HGB (median [IQR]) (g/l) | 127 [113–139] | 128 [113.5–139] | 126 [112–139] | 0.386 |
| Primary site | 0.727 | |||
| Descending colon | 31 | 14 | 17 | |
| Sigmoid colon | 78 | 39 | 39 | |
| Rectum | 117 | 62 | 55 | |
| Operation time (median [IQR]) (min) | 194.5 [162–236.5] | 185 [158.5–230] | 202 [165–238.5] | 0.221 |
| Intraoperative bleeding volume (median [IQR]) (ml) | 50 [20–50] | 50 [20–50] | 50 [20–50] | 0.740 |
| Incision length (median [IQR]) (cm) | 6 [6–8] | 6 [6–8] | 6 [5–8] | 0.845 |
| Tumour size (median [IQR]) (cm) | 3.5 [2–4.475] | 3.5 [2.5–4.5] | 3 [2–4] | 0.112 |
| Defecation time (median [IQR]) (day) | 3 [2–4] | 3 [2–4] | 3 [2.5–4] | 0.947 |
| Postoperative hospitalization days (median [IQR]) (day) | 7 [6–9] | 7 [6–9] | 7 [6–9] | 0.769 |
| Stoma | 26 | 11 | 15 | 0.352 |
| Intraoperative complications | 4 | 1 | 3 | 0.490 |
| Haemorrhage | 3 | 1 | 2 | |
| Shock | 1 | 0 | 1 | |
| 8th AJCC stage (0/I/II/III) | 15/60/74/77 | 5/31/44/35 | 10/29/30/42 | 0.176 |
| Grade | 0.178 | |||
| Intraepithelial neoplasia and atypical hyperplasia | 20 | 7 | 13 | |
| Well-differentiated adenocarcinoma | 2 | 0 | 2 | |
| Moderately differentiated adenocarcinoma | 193 | 104 | 89 | |
| Poorly differentiated adenocarcinoma | 10 | 4 | 6 | |
| Other | 1 | 0 | 1 | |
| Transfer to ICU | 2 | 0 | 2 | 0.462 |
| Reoperation | 5 | 4 | 1 | 0.387 |
| Readmission within 30 days | 4 | 4 | 0 | 0.139 |
| Mortality within 30 days | 1 | 1 | 0 | 1.000 |
| Postoperative complications | ||||
| Anastomotic fistula | 16 | 6 | 10 | 0.267 |
| Overall SSI | 29 | 9 | 20 | 0.022 |
| Superficial SSI | 15 | 5 | 10 | 0.159 |
| Deep SSI | 16 | 3 | 13 | 0.008 |
| Organ–space SSI | 4 | 2 | 2 | 1.000 |
| Anastomotic stenosis | 2 | 1 | 1 | 1.000 |
| Postoperative haemorrhage | 16 | 9 | 7 | 0.656 |
| Postoperative ileus | 4 | 2 | 2 | 1.000 |
| Postoperative urinary infection | 1 | 1 | 0 | 1.000 |
| Postoperative diarrhoea | 38 | 16 | 22 | 0.235 |
| Postoperative pulmonary infection | 3 | 2 | 1 | 1.000 |
| Other complications | 9 | 3 | 6 | 0.463 |
AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; BMI, body mass index; CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease; HGB, haemoglobin; ICU, intensive care unit; IQR, interquartile range; SSI, surgical site infection.
To clarify the advantage of the bowel preparation mode in specific categories for both groups, a post hoc analysis was conducted including all related factors. The results suggested that patients with male sex, non-smoking status, nonalcohol addiction status, no comorbidities [hypertension, dyslipidaemia, coronary disease, chronic obstructive pulmonary disease (COPD)], left-sided surgery and stage II colorectal malignancy may benefit from preoperative OA combined with MBP. All results are shown in Figure 2.
Figure 2.
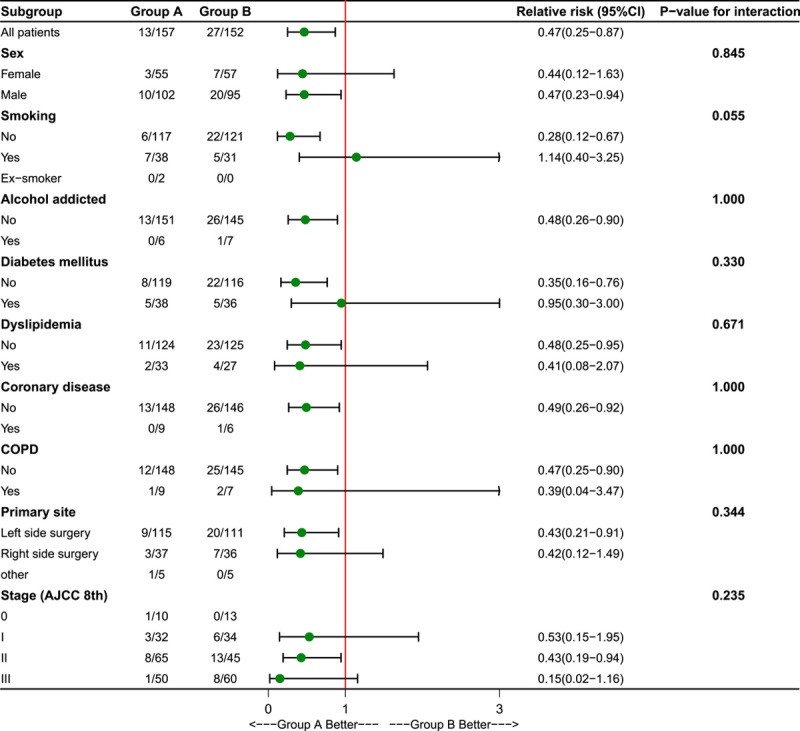
Relative risks with 95% confidence intervals for the primary outcome of surgical site infections within 30 days after surgery in the experimental and control groups.
Discussion
The current RCT revealed that patients receiving prophylactic treatment with OA combined with MBP suffered fewer SSIs within 30 days after elective laparoscopic colorectal resection due to malignancy. The protection effect was particularly significant in those with left-sided surgeries (descending colon, sigmoid colon, and rectum), while those with right hemi-colon resection did not benefit from this effect.
SSIs are some of the most common complications after colorectal surgery and substantially increase patient morbidity and healthcare expenses1,2. Colonic bacterial flora is the major cause of SSIs, and bowel preparation is performed preoperatively to reduce the bacterial burden and human faecal content27. Recently, the combination of OA and MBP has been evaluated in many RCTs, large retrospective studies, and several meta-analyses, demonstrating a significant decrease in the rate of SSIs18–21. However, these studies had some limitations. The majority of the studies included the open surgery approach, which is associated with a greater risk of SSIs than laparoscopic surgery and thus biased the results28. Surgical indications include both malignant and benign factors, such as IBD and diverticulitis. Patients with IBD/diverticulitis undergoing elective colectomy have significantly increased rates of SSIs29,30, specifically deep and organ–space infections30. Furthermore, postoperative prophylactic intravenous antibiotic choice and duration were not reported and restricted in previous studies. The combination of OA and intravenous antibiotics was also proven to be significantly associated with a lower incidence of SSIs, thus interfering with the results31. Finally, in some long-term retrospective studies, the choice of OA, dosages, timings, and administration of antiseptic agents varied in different decades, making the results difficult to translate into clinical practice.
In our study, only patients with malignancy who underwent laparoscopic surgery were enroled. Strict inclusion criteria were applied to minimize bias. Patients with an abdominal surgical history who underwent neoadjuvant chemotherapy–radiotherapy combined with a potential contaminant procedure, administration of corticosteroids or immune suppression drugs, natural orifice specimen extraction surgery, abdominal perineal resection or emergency surgery were excluded. Furthermore, the incision length was recorded to maintain comparability, which was not carried out in previous studies. The same surgical team performed the resection procedure, along with the same suture technique, in different layers. Lap-Protector was abandoned in the current study to maintain reliability. Preoperative oral antibiotic was adopted according to the local principles, as non-gastrointestinal tract absorbed antibiotics, with the ability to resist Gram-negative bacteria and anaerobic bacteria32. A preoperative oral dose of streptomycin 1 g and metronidazole 0.2 g, which has a spectrum of activity extended to most anaerobes encountered in the colon and the rectum, was administered every 6 h three times for all patients. Postoperative application of ceftriaxone 2 g was strictly restricted until 48 h after surgery unless a definitive infection occurred and was recorded in CRFs. Furthermore, to clarify the conclusion we reported in a previous retrospective analysis, patients were stratified into right-sided and left-sided colorectal resection subgroups. All perioperative treatment was applied according to ERAS principles. However, patient enrolment was delayed, as our hospital was selected as a designated hospital for the treatment of COVID-19 during the epidemic period; thus, one additional year for patient recruitment was needed.
The current study revealed that the application of MBP+OA can significantly decrease the overall incidence of SSIs (13/157, 8.28% vs. 27/152, 17.76%, P=0.013), superficial SSIs (5/157, 3.18% vs. 14/152, 9.21%, P=0.027) and deep SSIs (7/157, 4.46% vs. 16/152, 10.53%, P=0.042) relative to MBP alone in patients undergoing laparoscopic elective colorectal resection. The incidence of anastomotic fistula, postoperative ileus, urinary infection, pulmonary infection, haemorrhage, and 30-day readmission was comparable in both groups. These findings were in accordance with our previous retrospective study and other trials. However, the postoperative length of stay (LOS) was similar between both groups (P=0.717), which was different from previous results. LOS was calculated separately for patients with SSIs in both groups and indicated a comparable result (17.17±11.05 vs. 18.14±12.0, P=0.803). Such a phenomenon was attributed to the development of wound outpatient services in modern nursing. Patients could be discharged earlier than expected instead of waiting for the wound to heal totally. However, the accurate recovery interval was not recorded during the follow-up period.
The incidence of SSIs in the experimental group was slightly higher in our analysis than in other studies (8.28 vs. 6.2%). There are some explanations for this finding. First, we abandoned the application of wound protectors, which can separate surgical sites from the potential contaminant environment caused by colorectal cavity exposure, thus reducing SSIs33. Wound protectors were applied in recent decades in large-scale retrospective studies and RCTs, and they may influence the results and were prohibited in the current study. Second, as recently reported, intravenous application of antibiotics combined with OA also helps decrease the local bacterial load of the surgical site31. Former retrospective studies failed to report the precise administration duration of intravenous antibiotics. Prolonged application of intravenous antibiotics also affects the incidence of SSIs. Thus, antibiotics were strictly restricted within 48 h postoperatively and affected the occurrence of SSIs. Third, the majority of patients had left-sided colorectal malignancy, which is intrinsically associated with a higher incidence of SSIs.
The most prominent finding of the current study was the region-specific protective function of preoperative OA combined with MBP, which has never been studied before. There are a few reasons for such subgroup definition: first, different embryonic origins and anatomical structures. The right colon is supplied by the superior mesenteric artery, and venous blood flows back mainly to the right liver through the superior mesenteric vein. The inferior mesenteric artery supplies the left-side colorectal tract, which drains into the splenic vein through the inferior mesenteric vein and then into the left liver via the left branch of the portal vein. Consequently, microbial communities in the colonic lumen’s distribution differ significantly, as evidenced in previous literature34. Second, patients with right-colon cancer are more likely to suffer from systemic symptoms, whereas those with left-sided colorectal cancer may develop digestive obstructions or changes in defecation preference. Additionally, there are notable disparities in the prognosis and sensitivity to chemotherapy drugs among right-sided and left-sided colorectal cancer patients. Furthermore, there is actual difficulty in estimating sample size according to detailed anatomical classifications such as ascending colon/transverse colon/descending colon/sigmoid colon/rectum in SSIs. As a result, this subgroup analysed was conducted solely into the left/right sides to meet the statistical test power and in accordance with the hypothesis in our previous retrospective study24.
Left-sided colorectal resection included descending, sigmoid colon, and rectal resection, while right-sided resection included ascending colon resection only. Our previous retrospective analysis indicated that the OA+MBP mode provided a protective function in left-sided colorectal resection. In the current study, patients were stratified during the recruiting phase to further prove our hypothesis. Strict inclusion and exclusion criteria helped diminish intrinsic interference, indicating similar baseline characteristics overall and in subgroups among enroled patients. The comparison results proved that the OA+MBP mode showed no superiority in right-sided hemi-colon resection. However, this bowel preparation mode manifested a significant advantage in left-side resection. It can significantly reduce the incidence of overall SSIs and deep SSIs. Physiological function, tumorigenesis and clinical features of malignancy were quite different according to side. The concentration of bacteria ranges from 106 to 107 bacteria/g of stool content in the right hemi-colon, whereas these numbers rise to 1011–1012 bacteria/g in the rectosigmoid region34. Such a contaminant environment in the left-side colon and rectum increases the risk of postoperative SSIs35. Several studies have also demonstrated a lower risk of SSIs for right-colon resection36. Furthermore, patients who underwent left-sided colorectal resection also suffered from a higher incidence of anastomotic fistula and other complications36,37. Theoretically, the OA+MBP mode could remove the hard stool content and associated higher bacterial load within the left-side bowel, thus reducing the risk of SSIs. The application of OA is questionable due to its potential side effects, such as postoperative Clostridium difficile infection, drug allergy, dysbacteriosis and patient refusal. This study identified the appropriate population for OA combined with MBP, preventing complications associated with the overuse of this procedure.
To identify those who might benefit from OA combined with MBP, a post hoc analysis was performed on all patients, while only chi-square (χ 2) test was performed to distinguish which patient feature may benefit from the OA+MBP mode. Male patients with no tobacco or alcohol addiction, no coronary heart disease, no COPD, no dyslipidaemia, no diabetes, and stage II colorectal malignancy may benefit from this bowel preparation mode. However, some confounding factors may exist and fluctuate the results, post hoc analysis only provided a statistically significant correlation and did not establish causality. Specific causality needs to be further confirmed by prospective trials.
Our study had several limitations. We did not use any placebo for the control group, which might be a source of bias. Furthermore, surgeons and patients were not masked in treatment group assignments because of logistical issues. Lack of blindness may also confer some bias in the current trial. In addition, according to ERAS principles, MBP should be given up for all patients undergoing colorectal resection. However, MBP is still routinely conducted according to surgeon suggestions, especially for rectal cancer patients. Furthermore, detailed anatomical distribution subgroup analysis was not analysed in the current study. Future investigation of the benefits of administering OA in the absence of MBP or multicentre-specific anatomical subgroup analysis should help solve these problems.
To our knowledge, this is the first RCT investigating the benefit of OA according to region-specific function. Adequate patient recruitment and an experienced surgical team provided a sufficient sample size to analyse the association between bowel preparation mode and postoperative outcomes. With the application of ERAS, future assessments of patients who receive simple OA or placebo without MBP will be conducted, thereby helping to overcome the limitations of the present study.
Conclusion
Administration of OA combined with MBP before colorectal surgery significantly reduced the incidence of SSIs. Such a prophylactic effect was particularly significant for left-region surgeries. The preparation mode should be routinely adopted before elective left-region colorectal surgeries.
Ethical approval
The study was approved by the Ethics Committee of Third Affiliated Hospital of Sun Yat-sen University and the reference No. is [2019]02-008-01.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
This study is supported by the Medical Scientific Research Foundation of Guangdong Province, China (No. A2021163), the Guangzhou Science and Technology Program Project (No. 2023A03J0210) and the Clinical Medical Research 5010 Program of Sun Yat-sen University (2018023).
Author contribution
P.L. and T.C.: were responsible for the study design and conduction; G.J.: performed the statistical analysis and made all tables and figures; X.Y. and Y.R.: performed the CRF data collection and conversion into electrical case report forms; P.L. and B.W.: wrote the manuscript; T.C.: made the language correction.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Clinicaltrials.gov
Unique identifying number or registration ID: NCT03856671.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://clinicaltrials.gov/ct2/show/NCT03856671?term=NCT03856671&draw=1&rank=1.
Guarantor
Purun Lei and Tufeng Chen.
Data availability statement
The data that support the finding of the current study are available on request from the corresponding author, Tufeng Chen (tufengchen1@126.com), upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 11 August 2023
Contributor Information
Purun Lei, Email: leipurun@163.com.
Guiru Jia, Email: jiagr@mail.sysu.edu.cn.
Xiaofeng Yang, Email: young5100@126.com.
Ying Ruan, Email: ruany5@mail.sysu.edu.cn.
Bo Wei, Email: sanpi2013@163.com.
Tufeng Chen, Email: tufengchen1@126.com.
References
- 1.Fry DE. Colon preparation and surgical site infection. Am J Surg 2011;202:225–232. [DOI] [PubMed] [Google Scholar]
- 2.Fields AC, Pradarelli JC, Itani KMF. Preventing surgical site infections: looking beyond the current guidelines. JAMA 2020;323:1087–1088. [DOI] [PubMed] [Google Scholar]
- 3.Ramsay G, Watson A. Reducing surgical site infection rates in colorectal surgery – a quality improvement approach to implementing a comprehensive bundle. Colorectal Dis 2022;24:239. [DOI] [PubMed] [Google Scholar]
- 4.Lei PR, Liao JW, Ruan Y, et al. Risk factors analysis for surgical site infection following elective colorectal resection: a retrospective regression analysis. Chin Med J (Engl) 2020;133:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols RL, Choe EU, Weldon CB. Mechanical and antibacterial bowel preparation in colon and rectal surgery. Chemotherapy 2005;51(Suppl 1):115–121. [DOI] [PubMed] [Google Scholar]
- 6.Nichols RL, Smith JW, Garcia RY, et al. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin Infect Dis 1997;24:609–619. [DOI] [PubMed] [Google Scholar]
- 7.Beck DE, Fazio VW. Current preoperative bowel cleansing methods – results of a survey. Dis Colon Rectum 1990;33:12–15. [DOI] [PubMed] [Google Scholar]
- 8.Lindsey JT, Smith JW, McClugage SG, et al. Effects of commonly used bowel preparations on the large bowel mucosal-associated and luminal microflora in the rat model. Dis Colon Rectum 1990;33:554–560. [DOI] [PubMed] [Google Scholar]
- 9.Dahabreh IJ, Steele DW, Shah N, et al. Oral mechanical bowel preparation for colorectal surgery: systematic review and meta-analysis. Dis Colon Rectum 2015;58:698–707. [DOI] [PubMed] [Google Scholar]
- 10.Slim K, Vicaut E, Launay-Savary MV, et al. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg 2009;249:203–209. [DOI] [PubMed] [Google Scholar]
- 11.Wille-Jörgensen P, Guenaga KF, Matos D, et al. Pre-operative mechanical bowel cleansing or not? An updated meta-analysis. Colorectal Dis 2005;7:304–310. [DOI] [PubMed] [Google Scholar]
- 12.Clarke JS, Condon RE, Bartlett JG, et al. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg 1977;186:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols RL, Condon RE, Gorbach SL, et al. Efficacy of preoperative antimicrobial preparation of the bowel. Ann Surg 1972;176:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zmora O, Mahajna A, Bar-Zakai B, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg 2003;237:363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miettinen RPJ, Laitinen ST, Mäkelä JT, et al. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery: prospective, randomized study. Dis Colon Rectum 2000;43:669–675. [DOI] [PubMed] [Google Scholar]
- 16.Devane LA, Proud D, O’Connell PR, et al. A European survey of bowel preparation in colorectal surgery. Color Dis 2017;19:O402–O406. [DOI] [PubMed] [Google Scholar]
- 17.Battersby CLF, Battersby NJ, Slade DAJ, et al. Preoperative mechanical and oral antibiotic bowel preparation to reduce infectious complications of colorectal surgery – the need for updated guidelines. J Hosp Infect 2019;101:295–299. [DOI] [PubMed] [Google Scholar]
- 18.Scarborough JE, Mantyh CR, Sun Z, et al. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg 2015;262:331–337. [DOI] [PubMed] [Google Scholar]
- 19.Futier E, Jaber S, Garot M, et al. Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: multicentre, randomised, double blind, placebo controlled trial. BMJ 2022;379:e071476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:729–738. [DOI] [PubMed] [Google Scholar]
- 21.Willis MA, Toews I, Soltau SL, et al. Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database Syst Rev 2023;2:CD014909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allegranzi B, Bischoff P, de Jonge S, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e276–e287. [DOI] [PubMed] [Google Scholar]
- 23.Holubar SD, Hedrick T, Gupta R, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond) 2017;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei P, Ruan Y, Yang X, et al. Preoperative mechanical bowel preparation with oral antibiotics reduces surgical site infection after elective colorectal surgery for malignancies: results of a propensity matching analysis. World J Surg Oncol 2020;18:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019;43:659–695. [DOI] [PubMed] [Google Scholar]
- 27.Krezalek MA, Alverdy JC. The role of the gut microbiome on the development of surgical site infections. Clin Colon Rectal Surg 2023;36:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni N, Arulampalam T. Laparoscopic surgery reduces the incidence of surgical site infections compared to the open approach for colorectal procedures: a meta-analysis. Tech Coloproctol 2020;24:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordeianou L, Cauley CE, Patel R, et al. Prospective creation and validation of the PREVENTT (Prediction and Enaction of Prevention Treatments Trigger) scale for surgical site infections (SSIs) in patients with diverticulitis. Ann Surg 2019;270:1124–1130. [DOI] [PubMed] [Google Scholar]
- 30.Bhakta A, Tafen M, Glotzer O, et al. Increased incidence of surgical site infection in IBD patients. Dis Colon Rectum 2016;59:316–322. [DOI] [PubMed] [Google Scholar]
- 31.Bellows CF, Mills KT, Kelly TN, et al. Combination of oral non-absorbable and intravenous antibiotics versus intravenous antibiotics alone in the prevention of surgical site infections after colorectal surgery: a meta-analysis of randomized controlled trials. Tech Coloproctol 2011;15:385–395. [DOI] [PubMed] [Google Scholar]
- 32.Dou RX, Zhou ZL, Wang JP. Bowel preparation before elective surgery for colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2022;25:645–647. [DOI] [PubMed] [Google Scholar]
- 33.Edwards JP, Ho AL, Tee MC, et al. Wound protectors reduce surgical site infection: a meta-analysis of randomized controlled trials. Ann Surg 2012;256:53–59. [DOI] [PubMed] [Google Scholar]
- 34.Nichols RL, Broido P, Condon RE, et al. Effect of preoperative neomycin erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degrate L, Garancini M, Misani M, et al. Right colon, left colon, and rectal surgeries are not similar for surgical site infection development. Analysis of 277 elective and urgent colorectal resections. Int J Colorectal Dis 2011;26:61–69. [DOI] [PubMed] [Google Scholar]
- 36.Turrado-Rodriguez V, Targarona Soler E, Bollo Rodriguez JM, et al. Are there differences between right and left colectomies when performed by laparoscopy? Surg Endosc 2016;30:1413–1418. [DOI] [PubMed] [Google Scholar]
- 37.Campana JP, Pellegrini PA, Rossi GL, et al. Right versus left laparoscopic colectomy for colon cancer: does side make any difference? Int J Colorectal Dis 2017;32:907–912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finding of the current study are available on request from the corresponding author, Tufeng Chen (tufengchen1@126.com), upon reasonable request.


