Abstract
Background:
Venous thromboembolism (VTE) is a common and serious complication after colorectal cancer (CRC) surgery. Few large-sample studies have reported VTE incidence and management status after CRC surgery in China. This study aimed to investigate the incidence and prevention of VTE in Chinese patients after CRC surgery, identify risk factors for developing VTE, and construct a new scoring system for clinical decision-making and care planning.
Methods:
Participants were recruited from 46 centers in 17 provinces in China. Patients were followed up for 1 month postoperatively. The study period was from May 2021 to May 2022. The Caprini score risk stratification and VTE prevention and incidence were recorded. The predictors of the occurrence of VTE after surgery were identified by multivariate logistic regression analysis, and a prediction model (CRC-VTE score) was developed.
Results:
A total of 1836 patients were analyzed. The postoperative Caprini scores ranged from 1 to 16 points, with a median of 6 points. Of these, 10.1% were classified as low risk (0–2 points), 7.4% as moderate risk (3–4 points), and 82.5% as high risk (≥5 points). Among these patients, 1210 (65.9%) received pharmacological prophylaxis, and 1061 (57.8%) received mechanical prophylaxis. The incidence of short-term VTE events after CRC surgery was 11.2% (95% CI 9.8–12.7), including deep venous thrombosis (DVT) (11.0%, 95% CI 9.6–12.5) and pulmonary embolism (PE) (0.2%, 95% CI 0–0.5). Multifactorial analysis showed that age (≥70 years), history of varicose veins in the lower extremities, cardiac insufficiency, female sex, preoperative bowel obstruction, preoperative bloody/tarry stool, and anesthesia time at least 180 min were independent risk factors for postoperative VTE. The CRC-VTE model was developed from these seven factors and had good VTE predictive performance (C-statistic 0.72, 95% CI 0.68–0.76).
Conclusions:
This study provided a national perspective on the incidence and prevention of VTE after CRC surgery in China. The study offers guidance for VTE prevention in patients after CRC surgery. A practical CRC-VTE risk predictive model was proposed.
Keywords: colorectal cancer, prediction model, prophylaxis, risk factors, venous thromboembolism
Background
Highlights
After colorectal cancer (CRC) surgery, 82.5% of the patients were at high risk for venous thromboembolism (VTE) and only 24.3% received appropriate prophylaxis.
VTE incidence was 11.2% within 1 month after CRC surgery. Most VTE events occurred within 5–9 days, and 78% were asymptomatic deep venous thrombosis events.
The CRC-VTE risk score was developed involving seven variables, which showed better predictive performance than the Caprini score.
Active cancer patients are at increased risk for venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), with a prevalence of 1–20%1. VTE is also a frequent and serious complication after colorectal surgery due to specific procedural risk factors, including the unique location of the lithotomy, the prolonged operation time, and the pelvic dissection2. Radiologically and clinically diagnosed VTE rates were up to 40% for DVT and 5% for PE after colorectal cancer (CRC) surgery. VTE has serious consequences for patients, including prolonged hospitalization, delays in cancer treatment, post-thrombotic syndrome, and even death, resulting in significantly increased healthcare costs3.
Appropriate thromboprophylaxis reduces the risk of VTE in settings such as hospitalization and major surgery and has attracted significant public health attention. However, even after receiving the thromboprophylaxis recommended by the guideline, VTE events occurred in 9.4% of patients who underwent colorectal procedures4. The CERTAIN and the ACS NSQIP studies showed relatively high rates of short-term thromboprophylaxis (86.4–91.4%) and low incidences of VTE events (2.0–2.2%) in patients after CRC surgery5,6. However, these studies were conducted in the United States, making the results impossible to extrapolate to Chinese patients.
In 2018, the DissolVE-2 study (13 609 patients), a multicenter cross-sectional study in China, revealed that only 14.2% of patients received thromboprophylaxis and 3.1% of those received appropriate prophylaxis7. Although VTE events only occurred in 0.3% of patients, the DissolVE-2 study was a retrospective analysis of patients admitted due to acute medical conditions or surgery. The design can inevitably underestimate the risk of VTE as some VTE events can be asymptomatic, and patients generally suffer a higher risk of VTE after CRC surgery. Therefore, a large prospective cohort study is needed to further investigate both the VTE’s prevalence and thromboprophylaxis practice in patients after CRC surgery in China.
In recent times, considerable attention has been paid to the analysis of thrombotic risk assessment models (RAMs) with the aim of reducing the incidence of VTE by implementing early preventive measures in high-risk patients. The Caprini score is currently the most commonly used assessment tool for predicting postoperative VTE; however, its application in clinical practice is complicated by a multitude of factors and additional risk factors. Furthermore, as the Caprini score is predominantly employed in retrospective studies to evaluate VTE risk in general surgery, the present standard precautions for high-risk patients in most hospitals often lead to inaccurate assessments8,9. Therefore, it is imperative to undertake a prospective cohort study to validate the accuracy of the Caprini score and to devise a model that specifically predicts the risk of VTE following CRC surgery.
Methods
Study design and population
The CRC-VTE trial was a prospective multicenter cohort study. The study aimed to investigate the incidence of VTE events and the practice of VTE prophylaxis in patients after CRC surgery in China; identify clinically significant predictors of postoperative VTE; develop a RAM to predict the occurrence of VTE after CRC surgery; and assess the predictive value of the established CRC-VTE model and do an external validation.
Participants were continuously recruited from 46 centers in 17 provinces in China from May 2021 to May 2022. All participating centers were tertiary hospitals in major cities and all received uniform training before the study is conducted. The inclusion criteria were patients (1) at least 18 years old, (2) diagnosed with CRC, and (3) who received CRC surgery, including radical surgery, palliative surgery, and other limited surgeries. Patients were excluded if they (1) underwent emergency surgery, (2) were diagnosed with benign disease, and (3) suffered VTE before surgery. The sample size was determined based on the assumption that the perioperative incidence rate of VTE in patients with CRC was 24% (P), with an allowable error of 0.1 P and α=0.0510–12. A total of 1217 cases were needed based on the sample size estimation13.
This study was conducted according to the principles of the Declaration of Helsinki (as revised in 2013). Ethics Committee of Capital Medical University Affiliated Beijing Friendship Hospital approved the study protocol. All participants signed written informed consent. The work has been reported in line with the STROCSS criteria14 (Supplemental Digital Content 1, http://links.lww.com/JS9/A723).
Study procedure
Since this was an observational study, investigators did not interfere with clinical decision-making during treatment. Perioperative clinical data were collected using pre-designed collection forms, and routine follow-up (21–28 days after surgery) was provided. Preoperative data included patient demographics, VTE risk within 24 h of admission, medical history, and baseline laboratory tests. Intraoperative information included surgical method, anesthesia time, and bleeding. After the operation, laboratory tests and examination results, tumor information, VTE risk, prophylaxis methods, and VTE events were recorded during the 28-day follow-up. Data were entered into the network filling system (https://vte.dpclouds.com:8081/login).
Outcomes
The primary outcomes were the status of the risk assessment and prevention of VTE and the VTE events 28 days after surgery. Secondary outcomes were VTE events within 5–9 days (early stage) and 21–28 days (late stage) after surgery; predictors of postoperative VTE; and the development of a prediction model for 28-day VTE events after CRC surgery.
The risk of VTE was evaluated using the Caprini RAM15. Patients were classified into low-risk, medium-risk, or high-risk groups based on Caprini RAM and assessed within 24 h of admission and after CRC surgery. Prophylactic treatment for VTE included drugs [low-molecular weight heparin (LMWH), heparin, and oral anticoagulants] and non-pharmacological techniques [graduated compression stockings (GCS), intermittent pneumatic compression devices (IPCD), and other devices]. In our cohort, we adhere to the ACCP-9 guidelines (Table S1, Supplemental Digital Content 2, http://links.lww.com/JS9/A724) for perioperative VTE prophylaxis in CRC patients.
The VTE events were DVT or PE. DVT was diagnosed based on clinical symptoms (swelling and pain in the lower extremities, tenderness behind the lower leg and/or medial thigh) and/or routine screening of the lower extremities by color ultrasound or venography. PE was diagnosed based on clinical manifestations (dyspnea and shortness of breath), laboratory values (plasma D-dimer), and computed tomography pulmonary arteriography (CTPA). The VTE events were evaluated 5–9 days and 21–28 days after surgery. If the patient suffered a VTE event, they will be seen further in vascular surgery and be evaluated for thrombotic progression by ultrasound of both lower extremity veins after 2 weeks.
Statistical analysis
Continuous variables are presented as means±standard deviations, and categorical variables are reported as numbers and percentages. The incidence of VTE events after CRC and adverse events during anticoagulation therapies was summarized as percentages and 95% confidence intervals (95% CI). Data were separated into training and validation cohorts in the prediction model. The training group consisted of eight provinces with a sample size greater than or equal to 100 cases, while the validation group consisted of nine provinces with a sample size of less than 100 cases. In the training cohort, univariate and multivariate logistic regression analyses were performed to test the associations of candidate variables with VTE after CRC surgery. A variable was included in the final prediction model if the P-value was ≤0.05 in the univariate analysis (indicative of VTE) and with a plausible association with VTE based on the Caprini RAM scores. Weighted points, proportional to the β regression coefficient multiplied by 2 to the nearest integer, were assigned for the factors determined in the multivariate analysis. The overall performance of the model was assessed by the C-statistic, as well as by the Brier score and Nagelkerke R 2. The Brier score is an evaluation function used to measure probability calibration, with lower Brier scores indicating better calibration of predictions. The Nagelkerke R 2 is a generalized coefficient of determination, similar to the R 2 in linear regression analysis. It ranges between 0 and 1, with larger values indicating a higher proportion of variance the model explains and greater accuracy of its predictions. External validation was conducted in the validation cohort to analyze the same performance metrics. Four risk categories were created using this scoring system: low risk, intermediate risk, high risk, and very high risk. The trend in the risk for VTE among risk categories was evaluated by the Cochran–Armitage trend test. Decision curve analysis was performed to evaluate the net clinical benefit of the models, with zero indicating no benefit and values greater than 0 indicating increased benefit16.
Data were analyzed using SPSS V25.0 (IBM Corp, Armonk, New York, USA) and the R software V4.2.1 (the R Foundation for Statistical Computing, Vienna, Austria). A P-value <0.05 was considered statistically significant. This study was registered with ClinicalTrials.gov, number NCT04588805.
Results
Characteristics of participants
A total of 1920 patients were enrolled (Figure S1, Supplemental Digital Content 2, http://links.lww.com/JS9/A724 and Table S2, Supplemental Digital Content 2, http://links.lww.com/JS9/A724), and 1836 were included in the analyses (36 were excluded for preoperative VTE events, and 48 had missing data). Among these patients, 205 were diagnosed with VTE during follow-ups (Figure S2, Supplemental Digital Content 2, http://links.lww.com/JS9/A724). The baseline demographic and clinical characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics of the cohort.
| Variables | Overall (n=1836) |
|---|---|
| Demographics | |
| Age, years, mean (SD) | 62.6 (11.8) |
| Gender, male, n (%) | 1098 (59.8) |
| BMI, kg/m2, mean (SD) | 23.7 (3.3) |
| Smoking, n (%) | 366 (19.9) |
| ECOG score, n (%) | |
| 0 | 803 (43.7) |
| 1 | 888 (48.3) |
| 2 | 127 (6.9) |
| 3 | 18 (0.9) |
| Disease history, n (%) | |
| Hypertension | 582 (31.6) |
| Diabetes | 264 (14.3) |
| Hepatic insufficiency | 16 (0.8) |
| Renal insufficiency | 15 (0.8) |
| Cardiac insufficiency | 41 (2.2) |
| CHD or cerebrovascular disease | 172 (9.3) |
| Varicose veins | 37 (2.0) |
| Lung disease | 77 (4.1) |
| History of tumor in other sites | 45 (2.4) |
| Previous surgical history (excluding ambulatory surgery) | 578 (31.4) |
| Procedure | |
| Operation approach, n (%) | |
| Laparoscopic | 1757 (95.6) |
| Open | 79 (4.3) |
| Operation time, min, mean (SD) | 212.3 (88.3) |
| Anesthesia time, min, mean (SD) | 233.92 (101.0) |
| Intraoperative bleeding, ml, median (P25–P75) | 50 (30–100) |
| Intraoperative blood transfusion, n (%) | 119 (6.4) |
| Histological type of tumor, n (%) | |
| Adenocarcinoma | 1712 (93.2) |
| Mucous adenocarcinoma | 88 (4.7) |
| Othersa | 36 (1.9) |
| Differentiation grade, n (%) | |
| Undifferentiated | 33 (1.7) |
| Poorly differentiated | 210 (11.4) |
| Moderately differentiated | 1502 (81.8) |
| High differentiation | 91 (4.9) |
| TNM staging, n (%) | |
| Phase 0 | 103 (5.6) |
| Phase I | 365 (19.9) |
| Phase II | 625 (34.0) |
| Phase III | 653 (35.6) |
| Phase IV | 90 (4.9) |
| Preoperative length of hospital stays, n (%) | |
| 0–4 days | 180 (9.8) |
| 5–10 days | 866 (47.2) |
| >10 days | 790 (43.0) |
Others including squamous cell carcinoma and undifferentiated carcinoma.
BMI, body mass index; CHD, coronary heart disease; ECOG, Eastern Cooperative Oncology Group.
The mean age of the patients was 62.6 years, and 59.8% were men. The mean body mass index (BMI) was 23.7 kg/m2. The most common comorbidities were hypertension (31.6%) and diabetes (14.3%). 31.4% of the patients had a history of major surgeries. 95.6% of the patients received laparoscopic surgeries, and 4.3% had open surgeries. Regarding tumor staging, 25.5% of patients were at stages 0–I, 69.6% were at stages II–III, and only 4.9% were at stage IV. In particular, 2.4% of the patients had a history of malignancies other than CRC.
Current status of VTE risk assessment and prophylaxis
Within 24 h of admission for CRC surgery, 23.6% of the patients were considered high risk for VTE. However, only 13.6% of those patients received prophylactic treatments (Fig. 1A). Reassessment of VTE risk within 24 h after CRC surgery revealed that 185 (10.1%), 136 (7.4%), and 1515 patients (82.5%) were at low, medium, and high risk of VTE, respectively. A total of 82.6% of the patients received prophylactic VTE treatments, and 24.3% received them appropriately. Appropriate prevention refers to prevention in accordance with the ACCP-9 guidelines (Table S1, Supplemental Digital Content 2, http://links.lww.com/JS9/A724). Among high-risk patients, 83.9% (1271/1515) received preventive treatments: 7.0% continued for more than 7 days, and 0.5% continued for 4 weeks (Fig. 1B, Table S3, Supplemental Digital Content 2, http://links.lww.com/JS9/A724).
Figure 1.

Caprini score risk stratification and prevention status of colorectal cancer (CRC) patients. (A) Risk stratification based on Caprini score and prophylaxis for high-risk patients at admission. (B) Risk stratification based on Caprini score and prophylaxis for high-risk patients within 24 h after CRC surgery. (C) Postoperative pharmacological and physical prophylaxis for CRC patients. (D) Start time and duration of drug prophylaxis after CRC surgery. GCS, graduated compression stockings; IPCD, intermittent pneumatic compression devices; LMWH, low-molecular weight heparin; VTE, venous thromboembolism.
A total of 65.9% (1210/1836) patients received prophylactic anticoagulation therapies: LMWH (94.5%, 1143/1210) and other anticoagulants (5.5%, 67/1210). A total of 57.8% (1061/1836) patients received non-pharmacologic prophylactic treatment: GCS (44.8%, 475/1061), IPCD (26.2%, 278/1061), a combination of GCS and IPCD (24.2%, 257/1061), or other devices (4.8%, 51/1061) (Fig. 1C). Anticoagulants were started mainly within 1–2 days after surgery (76.5%, 926/1210) and continued for 1–5 days (51.2%, 619/1210) or 6–10 days (38.1%, 461/1210). Only 52 patients (4.3%) continued anticoagulant therapy after discharge (Fig. 1D).
Efficacy and safety during VTE prophylaxis after CRC surgery
VTE events occurred in 205 patients (11.2%, 95% CI 9.8–12.7) within 1 month after CRC surgery: 202 (11.0%, 95% CI 9.6–12.5) had DVT and 3 (0.2%; 95% CI, 0–0.5) had PE (Table 2). During follow-up, 1743 patients (94.9%) completed an ultrasound examination in the first postoperative review (5–9 days after surgery), and 183 (10.5%, 95% CI 9.1–12.0) developed VTE. A total of 1204 patients (65.6%) completed the ultrasound examination in the second postoperative review (21–28 days after surgery), and 22 (1.8%, 95% CI 1.5–2.8) developed VTE. In patients with VTE, 78% of patients (160/205) were asymptomatic. Of the 1188 patients who underwent anticoagulation prophylaxis, 147 patients had VTE (12.4%), while only 58 of the 648 patients who did not undergo anticoagulation prophylaxis had VTE (9.0%).
Table 2.
The incidence of VTE events.
| Endpoints | Overall (n=1836) | First stage (5−9 days) (n=1743) | Second stage (21−28 days) (n=1204) |
|---|---|---|---|
| VTE, n (%; 95% CI) | 205 (11.2; 9.8–12.7) | 183 (10.5; 9.1–12.0) | 22 (1.8; 1.5–2.8) |
| PE | 3 (0.2; 0−0.5) | 3 (0.2; 0−0.5) | 0 |
| DVT without PE | 202 (11.0; 9.6–12.5) | 180 (10.3; 8.9–11.9) | 22 (1.8; 1.5–2.8) |
| Symptom of VTE, n (%; 95% CI) | |||
| Symptomatic events | 45 (2.5; 1.8–3.3) | 41 (2.4; 1.7–3.2) | 4 (0.3; 0–0.8) |
| Asymptomatic events | 160 (8.7; 7.5–10.1) | 142 (8.1; 6.9–9.5) | 18 (1.5; 0.8–2.3) |
Data are shown as number (%; 95% CI).
DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Treatments for VTE after CRC surgery are summarized in Table 3. Of the patients who had confirmed VTE events, 76.1% (156/205) received medical treatment, including anticoagulants (150 patients, 73.2%) and interventional surgical treatment (6 patients, 2.9%). Subcutaneous LMWH was the most commonly prescribed anticoagulant (78 patients, 38.0%), followed by oral anticoagulants (53 patients, 25.9%). Regarding the clinical outcome of VTE events, 49.3% of the patients had thrombus resolution, and 50.7% developed unresolved thrombus.
Table 3.
Treatments and outcome of VTE events.
| Treatments and outcomes | Overall (n=205) | First stage (5−9 days) (n=183) | Second stage (21−28 days) (n=22) |
|---|---|---|---|
| Medical treatment, n (%) | 156 (76.1) | 140 (76.5) | 16 (72.7) |
| Anticoagulants | 150 (73.2) | 134 (73.2) | 16 (72.7) |
| LMWH | 78 (38.0) | 73 (39.9) | 5 (22.7) |
| Oral anticoagulants | 53 (25.9) | 42 (3.0) | 11 (50.0) |
| LMWH plus oral anticoagulants | 11 (5.4) | 11 (6.0) | 0 |
| Heparin | 8 (3.9) | 8 (4.4) | 0 |
| Interventional surgical treatment | 6 (2.9) | 6 (3.3) | 0 |
| Outcomes, n (%) | |||
| Thrombus resolution | 101 (49.3) | 93 (50.8) | 8 (36.4) |
| Unresolved thrombus | 104 (50.7) | 90 (49.2) | 14 (63.6) |
Unresolved thrombus, thrombus does not dissolve and even develops mechanization.
LMWH, low-molecular weight heparin; n, number; VTE, venous thromboembolism.
The adverse events caused by prophylactic anticoagulants after CRC surgery are shown in Table 4. The most common adverse event was abnormal liver function due to heparin (7.2%, 85/1188, 95% CI 5.8–8.8), followed by bleeding (2%, 24/1188, 95% CI 1.3–3.0) and heparin-induced thrombocytopenia (0.4%, 5/1188, 95% CI 0.1–1.0). Twenty-four bleeding episodes occurred: seven were major, and 17 were minor.
Table 4.
Adverse events of prophylactic anticoagulants.
| Adverse events | Number of events (total number=1188) | Incidence (95% CI) |
|---|---|---|
| Bleeding | 24 | 2.0 (1.3–3.0) |
| Minor bleeding | 17 | 1.4 (0.8–2.3) |
| Major bleeding | 7 | 0.6 (0.2–1.2) |
| Abnormal liver function due to heparin | 85 | 7.2 (5.8–8.8) |
| HIT | 5 | 0.4 (0.1–1.0) |
HIT, heparin-induced thrombocytopenia.
Risk factors and prediction models for short-term VTE after CRC surgery
Figure 2 presents the development of the predictive risk model. Patients were divided by sample size. Patients from provinces with more than 100 patients were assigned to the training cohort; otherwise, they were assigned to the validation cohort. Finally, 1515 patients (179 VTE events, 11.8%) and 321 patients (26 VTE events, 8.1%) were included in the training and validation sets (Fig. 2A), respectively. The demographics and characteristics of the patients are summarized in Table S4 (Supplemental Digital Content 2, http://links.lww.com/JS9/A724).
Figure 2.
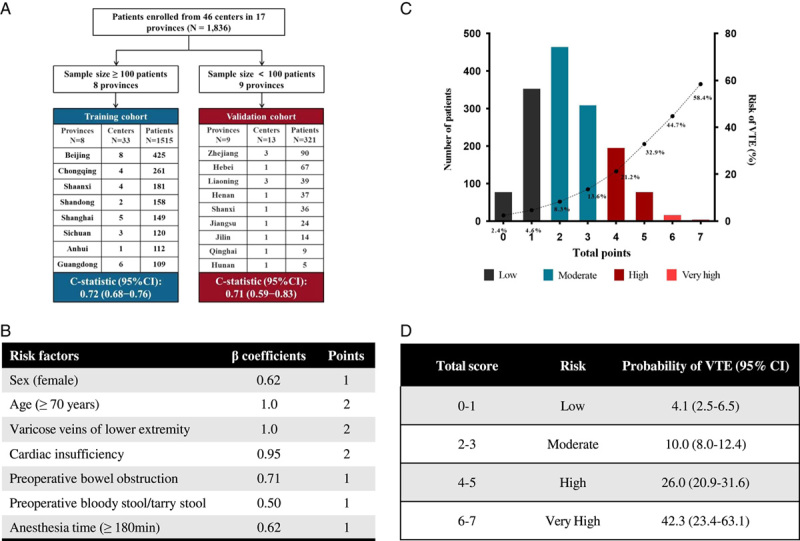
The model development overview. (A) Division of the training and validation sets. (B) Independent risk factors and corresponding scores associated with VTE occurrence in patients after colorectal cancer (CRC) surgery. (C) VTE incidence was distinguished by score quartiles (low risk, 0–1; moderate risk, 2–3; high risk, 4–5; very high risk, ≥6), with an exponential increase in VTE incidence among top-quartile patients. (D) Risk stratification based on the Caprini-CRC scoring system. VTE, venous thromboembolism.
In the univariate analysis (Table S5, Supplemental Digital Content 2, http://links.lww.com/JS9/A724), the following 12 variables were identified as associated with the risk of VTE after CRC: age, sex, length of hospital stay, history of varicose veins of the lower extremity, history of cardiovascular and cerebrovascular diseases, hypertension, cardiac insufficiency, preoperative intestinal obstruction, preoperative bloody or tarry stool, preoperative D-dimer abnormality, anesthesia time, and intraoperative blood transfusion (P<0.05 for each predictor). Two variables (smoking, P=0.064; preoperative hemoglobin ≥100 g/l, P=0.073) associated with VTE were also included in multiple regression analyses based on the Khorana score and other VTE assessment scales. Finally, 15 candidate predictors were retained to develop the prediction model, and seven were identified as predictors of VTE after CRC surgery (Table S6, Supplemental Digital Content 2, http://links.lww.com/JS9/A724).
Based on the adjusted β regression coefficient, the scores of the selected predictors were assigned to integer scores: 2 points each for age (≥70 years), history of varicose veins of the lower extremity, and cardiac insufficiency, and 1 point each for female sex, preoperative intestinal obstruction, preoperative bloody or tarry stool, and anesthesia time ≥180 min (Fig. 2B and Table S5, Supplemental Digital Content 2, http://links.lww.com/JS9/A724). The developed RAM model (CRC-VTE score) had a good overall performance in the training set (C-statistic 0.72, 95% CI 0.68–0.76) and the validation set (C-statistic 0.71, 95% CI 0.59–0.83), higher than that of the Caprini RAM score (C-statistic 0.59, 95% CI 0.55–0.64) (Table S7, Supplemental Digital Content 2, http://links.lww.com/JS9/A724). The decision curve analysis also showed that the CRC-VTE score was superior to the Caprini RAM score (Fig. S3, Supplemental Digital Content 2, http://links.lww.com/JS9/A724).
The CRC-VTE score classified the risks of short-term VTE after CRC surgery into low risk (0–1 points), medium risk (2–3 points), high risk (4–5 points), or very high risk (≥6 points) (Fig. 2C). The incidence of VTE in each risk category was 4.1%, 10.0%, 26.0%, and 42.3%, respectively (Fig. 2D and Table S8, Supplemental Digital Content 2, http://links.lww.com/JS9/A724). A significant trend of increasing risk from the low-risk to the very high-risk group was observed (P<0.001, Cochran–Armitage trend test). The distribution of CRC-VTE scores and VTE rates in the external validation cohort is shown in Table S9 (Supplemental Digital Content 2, http://links.lww.com/JS9/A724).
Here is an example of VTE risk prediction in a 72-year-old woman who underwent CRC surgery based on the CRC-VTE model. The patient had preoperative symptoms of intestinal obstruction and was anesthetized for 200 min during the operation. The CRC-VTE score would be 5: 2 points for the female sex, 1 point for age ≥70 years, 1 point for preoperative bowel obstruction, and 1 point for anesthesia time ≥180 min. Therefore, this patient would be in the very high-risk group, with a short-term VTE risk of 42.3% after CRC surgery.
Discussion
Major findings
To our knowledge, this is the first large-scale, prospective, multicenter study to systematically investigate the incidence and prevention practice of short-term VTE after CRC surgery in China. The three main findings were (1) after CRC surgery, 82.5% of the patients were at high risk for VTE, and 82.6% of them received pharmacological or non-pharmacological prophylaxis while only 24.3% of them received appropriate prophylaxis, (2) the incidence of VTE was 11.2% within 1 month after CRC surgery. Most VTE events occurred within 5–9 days, and 78% were asymptomatic DVT events, and (3) a CRC-VTE score involving seven variables was developed, which showed predictive performance superior to that of the Caprini RAM score.
Overall status of VTE risk and prophylaxis
The Caprini RAM score is a widely used VTE risk assessment tool15. The percentage of patients at high risk increased from 23.4 to 82.5% after CRC surgery in this study based on the Caprini RAM score. The risks of VTE increased in patients who underwent laparoscopic surgery, major surgery (more than 45 min), and deep vein catheterization. Reasonable preventive measures are needed to prevent VTE events after CRC surgery. Although 82.6% of the patients received prophylactic treatments for postoperative VTE in this study, less than 25% received them appropriately. However, this rate is higher than in the DissolVE-2 study, which was 16.3%7.
The ENDORSE study (n=33 797), a multinational cross-sectional survey designed to assess the prevalence of VTE risk and the proportion of patients at risk who received effective prophylaxis in the acute hospital care setting, reported that almost 60% of surgical patients received ACCP-recommended prophylaxis17. The percentages of patients who received guideline-recommended prophylactic treatment for VTE in our study and the DissolVE-2 study were much lower than in other international studies. Several factors may contribute to lower prophylaxis rates in Chinese patients, including surgeons’ awareness of VTE prophylactic guidelines and adherence to consensus treatment18.
Analysis of postoperative VTE prophylaxis
The ACCP guidelines recommend mechanical prophylaxis for all postoperative patients, except for very low-risk patients. Pharmacological prophylaxis should be added for those at intermediate or high risk19. The effectiveness of mechanical methods has been demonstrated to prevent DVT or PE. Mechanical methods combined with drugs were superior to using anticoagulants alone20–22. In our study, 57.8% and 65.9% of the patients received mechanical and pharmacological prophylaxis, respectively. The rate of pharmacological prophylaxis was much lower than that of the CERTAIN research conducted in the United States (91.4%)5. Since approximately one-third of postoperative VTE can occur after colorectal surgery23,24, the duration of pharmacological prophylaxis is actively discussed. The ENOXACAN II study demonstrated that 4-week prevention with enoxaparin after abdominal or pelvic cancer surgeries was more effective than 1-week prophylaxis in reducing the risk of venography-confirmed DVT without increasing bleeding or other complications25.
Similarly, the ACCP guidelines recommend 4 weeks of LMWH for cancer patients undergoing abdominal or pelvic surgery after excluding the risk of bleeding19. Our data showed that only 4.6% of patients received anticoagulants for more than 15 days, and fewer (2.8%) continued treatment after discharge, much lower than the 11.6% reported in the CERTAIN study. Possible barriers to prolonging anticoagulant use include patient-specific social factors, drug costs, prescribing patterns, and the inconvenience of subcutaneous administration of LMWH26.
Regarding the timing of prophylaxis initiation, the ACCP guidelines recommend that medications be administered as soon as the risk of definite bleeding is ruled out19. More than 75% of the patients in our study received pharmacological prophylaxis for VTE within 2 days after the operation. However, 20.3% of the patients did not receive VTE prophylaxis until the third day postoperatively or later, increasing the risk of VTE. More studies are needed to evaluate the outcomes based on the timing and duration of VTE prophylaxis in Chinese patients.
Incidence of postoperative VTE
Several large international retrospective databases have reported an event rate of 1.1–2.5% of VTE after CRC surgery5,6,23,24,27–38. However, these studies have some limitations, such as a lack of information on patient characteristics, details of previous medical history, and the implementation of preventive measures. The rate of symptomatic VTE in our prospective study was 2.5%, consistent with the rates in these database studies, indicating that most of the VTE events in the database studies were symptomatic. The incidence of asymptomatic VTE events reported in the literature is unexpectedly high, reaching 38%39, much higher than the 8.6% rate in our study.
The RISTOS project reported that 40% of the VTE events occurred after 21 days of operation40. Another study found that almost 33% of 30-day postoperative VTE were diagnosed after discharge23. In contrast, only one-tenth of the VTE events occurred after 21 days postoperatively in our study. Anticoagulation is the cornerstone for preventing postoperative VTE, but its clinical use is challenging in these fragile patients due to concerns about bleeding. A meta-analysis found that 4.3% of CRC patients receiving drug prophylaxis experienced postoperative bleeding41, higher than the 2.0% in our cohort. Furthermore, the treatment of postoperative VTE events was suboptimal in our study. Although most patients have asymptomatic interstitial vein thrombosis, treating VTE events after CRC surgery in Chinese medical centers deserves attention.
Risk factors for postoperative VTE
Thirty-four candidate predictors were evaluated, and seven independent factors associated with VTE were identified. We proposed the CRC-VTE score based on these independent variables. Advanced age, varicose veins in the lower extremities, and cardiac insufficiency were critical predictors of VTE development after CRC surgery. These are also significant risk factors based on the Caprini RAM score42. Female sex is a substantial risk factor43. In addition, the symptoms of CRC patients at admission, including preoperative bowel obstruction and bloody or tarry stools, could predict the risk of postoperative VTE. Preoperative bowel obstruction increases preoperative bed rest and causes stagnant blood flow44. Persistent bloody or tarry stools cause disturbances in the coagulation system and activate exogenous and endogenous coagulation systems, all of which promote thrombosis44.
The effect of anesthetic drugs, peripheral venous vasodilatation, and intravenous blood pooling also favored the formation of DVT. Additionally, our study found that the duration of anesthesia greater than 180 min was an independent risk factor for the development of VTE. The longer the anesthesia time, the higher the risk of VTE45.
Prediction model for postoperative VTE
Currently, various scales, such as Caprini score15 (for surgical patients), Khorana RAM46 (for outpatient cancer patients), and Padua RAM47 (for medical patients), are widely used for VTE risk assessment. However, these scales are too general to accurately assess the risk of VTE occurrence in specific oncology patients. Therefore, personalized assessment of VTE has garnered attention due to its simplicity, speed, and validity. For instance, Sultan et al.48 developed a postpartum VTE risk assessment scale based on clinical information from nearly 1 million women in the United Kingdom and Sweden, which quantified the absolute risk of postpartum VTE and was externally validated. Similarly, Jianchao Yao et al.49 developed the Sir-Run-Run-Shaw VTE RAM based on 541 patients after CRC surgery in China, which included four scoring items and risk stratification, but lacked external validation from multiple centers. Patients undergoing CRC surgery have a higher incidence of VTE than those undergoing general abdominal surgery. However, a simple, rapid, and efficient VTE risk assessment tool for CRC surgery patients for widespread use in China is still lacking. Hence, we conducted this prospective cohort study.
Risk stratification helps implement prevention strategies to avoid over-prophylactic or under-prophylactic treatment. The CRC-VTE score classified patients into low, moderate, high, and very high-risk categories to predict postoperative VTE. Mechanical prophylaxis (early ambulation or pneumatic pump for the lower extremities) is recommended for low-risk patients. LMWH or oral anticoagulants are recommended for intermediate-risk patients. Non-pharmacological combined with pharmacological prophylaxis is recommended for high-risk and very high-risk patients.
External validation was performed with a cohort of patients from different regions, yielding a good result with an area under the curve (AUC) of 0.71. The CRC-VTE score was compared with the Caprini RAM score, resulting in an AUC of 0.72 and 0.59, respectively, suggesting that the CRC-VTE score is better than the Caprini score for the postoperative evaluation of VTE. Furthermore, the CRC-VTE score, which consists of only seven scoring items, is easier and faster to use than the Caprini score. Indeed, the Caprini score was initially developed to predict symptomatic VTE in hospitalized surgical patients, and it may not be sensitive enough to detect asymptomatic VTE. In contrast, our study established a model based on a prospective cohort, which could better reflect the real-world scenario and identify high-risk individuals for VTE. Therefore, the CRC-VTE model may have higher accuracy and clinical relevance in predicting the risk of VTE after CRC surgery compared to the Caprini score.
Limitations
Our study has several limitations. First, there was an even regional enrollment of patients due to the distribution of population density in China. More patients from eastern and southern provinces were enrolled. Second, our follow-up period was limited to only 1 month, although VTE events occurred most frequently within 1 month after surgery, a small portion might have happened after the follow-up period and was omitted. This has also led to the majority of observed VTE treatment outcomes being no remission. Finally, external validation was based on the study cohort. The predictive model may not be generalizable to other patient populations or countries. More studies with extended follow-up are needed to validate the CRC-VTE score.
Conclusions
This study provides a national perspective on the clinical practice of VTE prophylaxis and VTE incidence after CRC surgery in China. Many patients were at high risk for VTE, and VTE prophylaxis was underutilized. Most postoperative VTE events occurred within 10 days and were asymptomatic DVT events. A practical CRC-VTE score was derived involving seven predictors to identify at-risk patients. The findings highlight areas of improvement in VTE management.
Ethical approval
Ethical approval has been obtained for this research study. The relevant ethics committee is the Capital Medical University Affiliated Beijing Friendship Hospital Life Ethics Committee and the reference number for their judgement is 2020-P2-183-02. This information has already been described in the manuscript.
Consent
Ethical approval from a life ethics committee has been obtained for this study and is described in the methodology section. A statement has been added to the end of the manuscript confirming that written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal upon request.
Sources of funding
This study was supported by the National Key Technologies R&D Program (No. 2015BAI13B09); National Key Technologies R&D Program of China (No. 2017YFC0110904); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (No. ZYLX201504); Clinical University (No. YYQDKT2016-5); Beijing Key Clinical Discipline Fund (No. 2018-118).
Author contribution
Q.W. and Z.-C.G. are the guarantors of the entire manuscript; H.-W.Y. and Z.-T.Z. contributed to the study conception and design and critical revision of the manuscript for important intellectual content; X.-D.P., Z.-Q.W., Y.-X.L., D.-B.Z., M.-B.L., X.-L.H., F.L., Q.L., J.-Y.Z., G.-Y.W., S.-L.T., Z.-J.W., A.L., G.X., J.Z., L.B., H.H., Y.L., W.S., Z.-L.L., Z.L.S., F.-L.L., Y.D., X.-J.Z., M.D., H.W., J.Q., L.Z., Z.-Q.W., H.Z., Q.W., M.-H.P., H.-B.W., and Z.-Q.H. contributed to the data acquisition; Y.C. and Y.-D.Y. contributed to the analysis and interpretation; H.-W.Y. and Z.-T.Z. contributed to the important guidance for this study. All the authors have read and approved the final version of this manuscript.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: ClinicalTrials.gov.
Unique identifying number or registration ID: NCT04588805.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Home - ClinicalTrials.gov.
Guarantor
The guarantors for this study are Professor Zhong-Tao Zhang, Hong-Wei Yao, and Zhi-Chun Gu.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Provenance and peer review
Our paper was not invited. Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
We would like to thank all patients who participated in the present study. We also express our gratitude to the remaining researchers who participated in the contribution: Hong-Yu Zhang, Xu-Dong Peng, Feng Tian, Liang Shang, Shang-Xin Zhao, Bo Yang, Long Qin, Qing Teng, Hai-Long Liu, Ai-Li Wang, Nan Wang, Bo Zhang, Yue Tian, Fan Zhang, Fu-Qiang Zhao, Zhi-Jie Wang, Gang Ji, Kun-Li Du, Bin Yu, Wen-Bo Niu, Hang Yuan, Xin-Ye Hu, Jia-Gang Han, Hao-Yu Zhang, Zhi-Gang Xue, Wei Zhang, Guo-Ju Wu, Qi An, Zhan-Dong Zhang, Jin-Bang Wang, Ze-Lin Wen, Pei Wu, Ai-Gang Ren, Yao-Liang Huo, De-Qing Wu, Ze-Jian Lv, Zhi-Hui Chen, Yu-Jie Yuan, Wen-Jun Ding, Peng Du, Shi-Dong Zhao, Song Wang, Dong Chen, Yan-Lei Wang, Wei Guo, Yu-Di Zhou, Qi-Yang Zhou, Jian-Ping Zhou, Jing-Tong Tang, Teng-Hui Ma, Qi-Yuan Qin, Rui-Yan Liu, Guo-Rong Wang, Hua Yang, Chao-Feng Li, Wen-Jian Meng, Zi-Xuan Zhuang, Ding-Sheng Liu, Shuo Xu, Liang He, Yin-Quan Zhao, Wei Xie, Zhen-Jia Dan, Tu-Feng Chen, Zong-Hang Zheng, Xin-Xing Li, Kai Xu, Xue-Jun Sun, Jian-Bao Zheng, Jun-Hui Yu, Bo Tang, Ao Mo, Lin-Feng Gao, Ke Zhang, Wei-Dong Liu, Jie Chen, Wei Wang, Wen-Jun Xiong, Li-Jie Lou, Hai-Jun Deng, Zhi-Yong Shen, Shen-Yuan Guan, Wei Zhang, Zheng Lou, Chen Wang, Ke-Feng Ding, Jun Li and Feng Yu.
Footnotes
Qi Wei, Zheng-Qiang Wei, Chang-Qing Jing, Yong-Xiang Li, Dong-Bing Zhou, Mou-Bin Lin, Xian-Li He, Fan Li, and Qian Liu contributed equally to this work and should be considered as co-first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 19 June 2023
Contributor Information
Qi Wei, Email: 15810563578@163.com.
Zheng-Qiang Wei, Email: 384535713@qq.com.
Chang-Qing Jing, Email: jingchangqing@sdfmu.edu.cn.
Yong-Xiang Li, Email: liyongxiang@ahmu.edu.cn.
Dong-Bing Zhou, Email: zhoudb2005@126.com.
Mou-Bin Lin, Email: 1500142@tongji.edu.cn.
Xian-Li He, Email: wanghe@fmmu.edu.cn.
Fan Li, Email: levinecq@163.com.
Qian Liu, Email: fcwpumch@163.com.
Jian-Yong Zheng, Email: zhjy68@163.com.
Gui-Ying Wang, Email: wangguiyingtgzy@163.com.
Shi-Liang Tu, Email: tushiliang@126.com.
Zhen-Jun Wang, Email: drzhenjun@163.com.
Ang Li, Email: anglixwabc@163.com.
Gang Xiao, Email: xgbj@sina.com.
Jing Zhuang, Email: 13383817858@189.cn.
Lian Bai, Email: 2840996478@qq.com.
He Huang, Email: hh93003@163.com.
Yong Li, Email: liyong@gdph.org.cn.
Wu Song, Email: songwu@mail.sysu.edu.cn.
Zhong-Lin Liang, Email: xhgclzl@126.com.
Zhan-Long Shen, Email: shenzhanlong@pkuph.edu.cn.
Fan-Long Liu, Email: fanlong_liu@163.com.
Yong Dai, Email: yong_dai@163.com.
Xiao-Jun Zhou, Email: chowxj@126.com.
Ming Dong, Email: cmudongming@sohu.com.
Hui Wang, Email: wang89@mail.sysu.edu.cn.
Jian Qiu, Email: qiujian263@126.com.
Lei Zhou, Email: zhouleizryy@163.com.
Xin-Xiang Li, Email: lxx1149@163.com.
Zi-Qiang Wang, Email: wangziqiang@scu.edu.cn.
Hong Zhang, Email: haojiubujian1203@sina.cn.
Quan Wang, Email: wangquanjdyy@163.com.
Ming-Hui Pang, Email: mhpang@uestc.edu.cn.
Hong-Bo Wei, Email: drweihb@126.com.
Zhi-Qian Hu, Email: huzq62@163.com.
Yi-Dan Yan, Email: yanyidan_cpu@163.com.
Yan Che, Email: cheyan2004@163.com.
Zhi-Chun Gu, Email: guzhichun213@163.com.
Hong-Wei Yao, Email: yaohongwei@ccmu.edu.cn.
Zhong-Tao Zhang, Email: Zhongtao.z@139.com.
References
- 1.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost 2017;117:219–30. [DOI] [PubMed] [Google Scholar]
- 2.Wille-Jørgensen P, Kjaergaard J, Jørgensen T, et al. Failure in prophylactic management of thromboembolic disease in colorectal surgery. Dis Colon Rectum 1988;31:384–386. [DOI] [PubMed] [Google Scholar]
- 3.Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum 2006;49:1620–1628. [DOI] [PubMed] [Google Scholar]
- 4.McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DVT prophylaxis trial: a randomized, double-blind trial. Ann Surg 2001;233:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson DW, Simianu VV, Bastawrous AL, et al. Thromboembolic complications and prophylaxis patterns in colorectal surgery. JAMA Surg 2015;150:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res 2012;129:568–572. [DOI] [PubMed] [Google Scholar]
- 7.Zhai Z, Kan Q, Li W, et al. VTE risk profiles and prophylaxis in medical and surgical inpatients: the identification of Chinese hospitalized patients’ risk profile for venous thromboembolism (DissolVE-2) – a cross-sectional study. Chest 2019;155:114–22. [DOI] [PubMed] [Google Scholar]
- 8.Barber EL, Clarke-Pearson DL. The limited utility of currently available venous thromboembolism risk assessment tools in gynecological oncology patients. Am J Obstet Gynecol 2016;215:445.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAlpine K, Breau RH, Mallick R, et al. Current guidelines do not sufficiently discriminate venous thromboembolism risk in urology. Urol Oncol 2017;35:457.e1–457.e8. [DOI] [PubMed] [Google Scholar]
- 10.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and Prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 suppl):e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang SM, Jang MJ, Kim KH, et al. Prevention of venous thromboembolism, 2nd edition: Korean society of thrombosis and hemostasis evidence-based clinical practice guidelines. J Korean Med Sci 2014;29:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahl V, Hu HM, Henke PK, et al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010;251:344–350. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Wang L, Wu X, et al. Validation of a venous thromboembolism risk assessment model in hospitalized Chinese patients: a case–control study. J Atheroscler Thromb 2014;21:261–272. [DOI] [PubMed] [Google Scholar]
- 14.Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case–control studies in surgery. Int J Surg (London, England) 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 15.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon 2005;51:70–78. [DOI] [PubMed] [Google Scholar]
- 16.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet (London, England) 2008;371:387–394. [DOI] [PubMed] [Google Scholar]
- 18.Yao HW, Han JG, Zhang ZT. Questioniare analysis of the status of venous thromboembolism after colorectal surgery in China. Chin J Pract Surg 2020;40:551–556. [Google Scholar]
- 19.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 suppl):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agu O, Hamilton G, Baker D. Graduated compression stockings in the prevention of venous thromboembolism. Br J Surg 1999;86:992–1004. [DOI] [PubMed] [Google Scholar]
- 21.Urbankova J, Quiroz R, Kucher N, et al. Intermittent pneumatic compression and deep vein thrombosis prevention. A meta-analysis in postoperative patients. Thromb Haemost 2005;94:1181–1185. [DOI] [PubMed] [Google Scholar]
- 22.Wille-Jørgensen P, Rasmussen MS, Andersen BR, et al. Heparins and mechanical methods for thromboprophylaxis in colorectal surgery. Cochrane Database Syst Rev 2003;4:CD001217. [DOI] [PubMed] [Google Scholar]
- 23.Davenport DL, Vargas HD, Kasten MW, et al. Timing and perioperative risk factors for in-hospital and post-discharge venous thromboembolism after colorectal cancer resection. Clin Appl Thromb Hemost 2012;18:569–575. [DOI] [PubMed] [Google Scholar]
- 24.Moghadamyeghaneh Z, Alizadeh RF, Hanna MH, et al. Post-hospital discharge venous thromboembolism in colorectal surgery. World J Surg 2016;40:1255–1263. [DOI] [PubMed] [Google Scholar]
- 25.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975–980. [DOI] [PubMed] [Google Scholar]
- 26.Merkow RP, Bilimoria KY, Sohn MW, et al. Adherence with postdischarge venous thromboembolism chemoprophylaxis recommendations after colorectal cancer surgery among elderly Medicare beneficiaries. Ann Surg 2014;260:103–108. [DOI] [PubMed] [Google Scholar]
- 27.Alizadeh RF, Sujatha-Bhaskar S, Li S, et al. Venous thromboembolism in common laparoscopic abdominal surgical operations. Am J Surg 2017;214:1127–32. [DOI] [PubMed] [Google Scholar]
- 28.Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? a study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum 2010;53:1355–1360. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum 2011;54:1496–1502. [DOI] [PubMed] [Google Scholar]
- 30.Buchberg B, Masoomi H, Lusby K, et al. Incidence and risk factors of venous thromboembolism in colorectal surgery: does laparoscopy impart an advantage? Arch Surg 2011;146:739–43. [DOI] [PubMed] [Google Scholar]
- 31.Kwon S, Meissner M, Symons R, et al. Perioperative pharmacologic prophylaxis for venous thromboembolism in colorectal surgery. J Am Coll Surg 2011;213:596–603; e603.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henke PK, Arya S, Pannucci C, et al. Procedure-specific venous thromboembolism prophylaxis: a paradigm from colectomy surgery. Surgery 2012;152:528–534; discussion 34-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallaert JB, De Martino RR, Marsicovetere PS, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum 2012;55:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moghadamyeghaneh Z, Hanna MH, Carmichael JC, et al. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg 2014;18:2169–2177. [DOI] [PubMed] [Google Scholar]
- 35.Walker AJ, West J, Card TR, et al. Variation in the risk of venous thromboembolism in people with colorectal cancer: a population-based cohort study from England. J Thromb Haemost 2014;12:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Dhuwaib Y, Selvasekar C, Corless DJ, et al. Venous thromboembolism following colorectal resection. Colorectal Dis 2017;19:385–94. [DOI] [PubMed] [Google Scholar]
- 37.Beal EW, Tumin D, Chakedis J, et al. Which patients require extended thromboprophylaxis after colectomy? modeling risk and assessing indications for post-discharge harmacoprophylaxis. World J Surg 2018;42:2242–51. [DOI] [PubMed] [Google Scholar]
- 38.Emoto S, Nozawa H, Kawai K, et al. Venous thromboembolism in colorectal surgery: Incidence, risk factors, and prophylaxis. Asian J Surg 2019;42:863–73. [DOI] [PubMed] [Google Scholar]
- 39.Cheung HY, Chung CC, Yau KK, et al. Risk of deep vein thrombosis following laparoscopic rectosigmoid cancer resection in Chinese patients. Asian J Surg 2008;31:63–68. [DOI] [PubMed] [Google Scholar]
- 40.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006;243:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li YD, Li HD, Zhang SX. Effect of thromboprophylaxis on the incidence of venous thromboembolism in surgical patients with colorectal cancer: a meta-analysis. Int Angiol 2020;39:353–60. [DOI] [PubMed] [Google Scholar]
- 42.Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost 1991;17(Suppl 3):304–312. [PubMed] [Google Scholar]
- 43.Lopez-Gomez M, Gomez-Raposo C, Lobo Samper F, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2008;113:223–224; author reply 4. [DOI] [PubMed] [Google Scholar]
- 44.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet (London, England) 2016;388:3060–73. [DOI] [PubMed] [Google Scholar]
- 45.Yang SS, Yu CS, Yoon YS, et al. Symptomatic venous thromboembolism in Asian colorectal cancer surgery patients. World J Surg 2011;35:881–887. [DOI] [PubMed] [Google Scholar]
- 46.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost 2010;8:2450–2457. [DOI] [PubMed] [Google Scholar]
- 48.Sultan AA, West J, Grainge MJ, et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: multinational cohort study. BMJ (Clinical research ed) 2016;355:i6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao J, Lang Y, Su H, et al. Construction of risk assessment model for venous thromboembolism after colorectal cancer surgery: a Chinese single-center study. Clin Appl Thromb Hemost 2022;28:10760296211073748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.


