The mathematical relationship between the corrected refractive power and the reduction in corneal nerve parameters in SMILE and LASIK is reported.
Abstract
Purpose:
To evaluate the impact of corrected refractive power on the corneal denervation and ocular surface in small-incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK).
Setting:
Singapore National Eye Center, Singapore.
Design:
Prospective study.
Methods:
88 eyes undergoing SMILE or LASIK were divided into low-moderate (manifest refractive spherical equivalent [MRSE] <−6.0 diopters [D]) and high myopic (MRSE ≥−6.0 D) groups. In vivo confocal microscopy and clinical assessments were performed preoperatively and at 1 month, 3 months, 6 months, and 12 months postoperatively.
Results:
In SMILE, high myopic treatment presented with significantly greater reduction in the corneal nerve fiber area (CNFA) and nerve fiber fractal dimension (CFracDim) compared with low-moderate myopic treatment (both P < .05). There was a significant and negative correlation between the corrected MRSE and the reduction in corneal nerve fiber density (CNFD), corneal nerve branch density (CNBD), corneal nerve fiber length, CNFA, and CFracDim after SMILE (r = −0.38 to −0.66, all P < .05). In LASIK, a significant correlation between the MRSE and the changes in CNBD, corneal nerve fiber total branch density, CNFA (r = −0.37 to −0.41), and corneal nerve fiber width (r = 0.43) was observed (all P < .05). Compared with SMILE, LASIK had greater reduction in CNBD and CNFA for every diopter increase in the corrected MRSE. High myopic SMILE, compared with low-moderate myopic SMILE, resulted in significantly lower tear break-up time at 1 and 6 months (both P < .05). The changes in CNFA and CFracDim were significantly associated with Schirmer test values (both P < .001).
Conclusions:
Postoperative corneal denervation was related to corrected refractive power in both SMILE and LASIK. With the same refractive correction, LASIK led to more prominent corneal denervation.
Corneal nerves are responsible for the perceptions of touch, pain, and temperature and regulate the blink reflex and tear production and secretion.1,2 They release various trophic factors, including neurotrophins, neuropeptides, and neurotransmitters, which play a significant role in physiological homeostatic processes, as well as modulation of epithelial integrity, proliferation, and wound healing on the ocular surface.3 Cornea laser refractive surgery is one of the most performed surgical procedures; it alters the shape and refractive power of the cornea by surgically removing corneal tissue.4,5 However, the corneal nerve plexus is damaged to varying degrees because of the corneal incision and laser ablation or photodisruption.6 Corneal denervation leads to a postoperative neuropathic ocular surface, with the clinical manifestations ranging from decreased tear secretion and volume, decreased tear film stability, increased corneal and conjunctival staining, and decrease or loss of corneal sensitivity to corneal epithelial erosions with neurotrophic epitheliopathy or keratopathy in severe cases.7
Factors that may influence the denervation and regeneration of corneal nerves after refractive surgeries include surgical procedure, age, the flap/ablation parameters, the location of hinge, flap or cap depth, and the refractive power of correction.8 Based on the Munnerlyn formula, the ablation depth in laser in situ keratomileusis (LASIK) or the extracted lenticule thickness is directly proportional to the refractive power to be corrected, and therefore, it is conceivable that the stromal volume removal and resultant-affected corneal nerves are greater in high myopic correction given the same diameter of the optical zone.9 Moreover, the stromal tissue devoid of keratocytes is greater in high myopic correction, which may lead to a greater extent of impairment of biological and nutritional cues from keratocytes for nerve regeneration.10
Zhao et al. demonstrated that nerve recovery in patients with high myopia was slower than that in patients with low-moderate myopia after LASIK.11 In 29.6% and 44.4% of the eyes with high myopia, 1 or 2 subbasal nerve fibers were detectable in an in vivo confocal microscopy (IVCM) micrograph 7 days and 6 months after the surgery, respectively, whereas the figures were 38.5% and 64.8%, respectively, in the eyes with low-moderate myopia. The results showed that the recovery of nerve fibers required an extended period in patients with higher myopia after LASIK. The authors concluded that higher myopic treatment, which is associated with deeper ablation depth and more removal of tissue, caused increased nerve depletion after LASIK. This might further delay the nerve recovery of patients with high myopia after LASIK. Similarly, our group previously showed that compared with low-moderate treatment, high myopic small-incision lenticule extraction (SMILE) (corrected spherical equivalent [SE] >−6.0 diopters [D]) resulted in significantly greater reduction in corneal nerve fiber density (CNFD) at 1 week, 1 month, and 6 months.12 In a separate randomized controlled, paired-eye, cross-sectional study from our group, we demonstrated that most eyes that had undergone LASIK, with low-moderate myopia, had equivalent or better CNFD, corneal nerve branch density (CNBD), and corneal nerve fiber total branch density (CTBD) recovery compared with SMILE at 5.5 years postoperatively.10 These data suggest that the corrected refractive power plays a role in postoperative corneal nerve depletion and restoration. However, the detailed relationship between the extent of corneal denervation, corneal nerve regeneration, and the corrected refractive power has not been elucidated.
In this study, we aimed to investigate the corneal denervation and subsequent regeneration in relation to the corrected refractive power after SMILE and LASIK. We also evaluated the impact of the different refractive power corrected on the postoperative ocular surface status.
METHODS
Study Population
This prospective study recruited a total of 44 patients (88 eyes) who had SMILE and LASIK at Singapore National Eye Center between July 2020 and January 2022. The study was conducted in accordance with the tenets of the Declaration of Helsinki, and approval for the study was granted by the institutional review board of SingHealth, Singapore (2020/2050/A). We collected patients' characteristics, including preoperative spherical power, cylindrical power, and preoperative manifest refractive spherical equivalent (MRSE). Patients were divided into low-moderate (MRSE <−6.00 D) and high myopia (MRSE >−6.00 D) groups. The intraoperative parameters, including the cap/flap thickness, and optical zone/ablation zone were also recorded. Due to the fact that the study was not in a randomized controlled trial design, propensity score matching was performed to take into account the factors that may affect baseline corneal nerve status included patient's age, the cap/flap thickness, and optical zone/ablation zone. The propensity score is a scalar summary of all measured pretreatment characteristics (potential confounders).13 The propensity score e(X) is the conditional probability of receiving a certain treatment, vs a comparator, given the measured pretreatment characteristics, X, denoted as e(X) = pr(Z = 1∣∣X), where Z = 1 in the SMILE group and Z = 0 in the LASIK group.14
LASIK and SMILE Procedures
For the LASIK procedure, a superiorly hinged, 100 to 130 μm thick flap was created using the VisuMax femtosecond laser (VisuMax, Carl Zeiss Meditec AG), and the flap diameter was 8.0 ± 0.2 mm (range 7.5 to 8.1 mm). The mean optical zone was 6.5 ± 0.1 mm (range 6.5 to 7.0 mm), and the mean treatment zone was 8.0 ± 0.8 mm (range 6.7 to 9.0 mm). Excimer ablation was performed with the WaveLight EX500 excimer laser (Alcon Laboratories, Inc.) after lifting the flap with a spatula. The flap was carefully repositioned after ablation, and a bandage contact lens was placed.
For the SMILE procedure, suction fixation was applied after the eye was centered and docked with an S-sized curved interface cone. The anterior cap with the anterior surface of the lenticule exceeding the posterior lenticule diameter by 0.5 mm was formed, and a 2.1 mm vertical circumferential incision was placed at 120 degrees with the femtosecond laser.15 The cap thickness was 100 to 130 μm, the mean optical zone was 6.4 ± 0.1 mm (range 6.0 to 6.5 mm), and the mean treatment zone was 6.6 ± 0.1 mm (range 6.2 to 6.7 mm). The dissection of the anterior plane and the posterior border was performed by inserting a SMILE dissector (ASICO LLC) through the incision after suction release. The lenticule was then grasped and removed through the incision using an EndoGlide microforceps (AngioTech/Network Medical Products).
All the procedures were performed under topical anesthesia by the same refractive surgeon (J.S.M.). All the patients received the same postoperative regimen, consisting of topical preservative-free dexamethasone (Maxidex, Alcon Laboratories, Inc.) and moxifloxacin (Vigamox, Alcon Laboratories, Inc.), 3 hourly for a week and then 4 times a day for 2 weeks. Artificial tears (Tears Naturale Free, Alcon Laboratories, Inc.) were also prescribed, with the frequency of 4 times daily for the first 2 months, and were applied if needed afterward.
IVCM and Image Analysis
IVCM (Heidelberg Retina Tomography III, Rostock Cornea Module, Heidelberg Engineering GmbH) was used for the evaluation of the subbasal nerve plexus preoperatively and at 1 month, 3 months, 6 months, and 12 months postoperatively. Patients were asked to fixate on a light source, and the subbasal nerve plexus of the central cornea and 4 quadrants of peripheral areas, which were 3 mm away from the corneal apex, were recorded with a field of view of 400 × 400 mm2. For the image analysis, 5 most representative and best-focused images of subbasal nerves were selected for each area, and each nerve (main truck or branched nerve) was selected only once. These 25 micrographs selected for each eye were analyzed using ACCMetrics software (University of Manchester) for the following nerve metrics: CNFD (the number of fibers/mm2, each frame area = 0.16033 mm2), CNBD (the number of branch points on the main fibers/mm2), corneal nerve fiber length (CNFL; the total length of fibers in mm/mm2), CTBD (the total number of branch points/mm2), corneal nerve fiber area (CNFA; the total nerve fiber area in mm2/mm2), corneal nerve fiber width (CNFW; mean nerve fiber width in mm/mm2), and nerve fiber fractal dimension (CFracDim). CFracDim represents the spatial loss of nerves, and the higher CFracDim value corresponds to the more evenly distribution of the corneal nerve fiber.16,17 We used 1-month nerve data to assess the relationship between the postoperative corneal denervation status and refractive power because 1-month data were less interfered by subsequent nerve regeneration activity. We expressed the percentage of .
Clinical Assessments
Ocular surface assessments contained the Schirmer test (without anesthesia, mm/5 minutes), ocular surface fluorescein staining (Oxford score; 0: absent, 5: severe), corneal fluorescein staining (National Eye Institute scale; 0: minimal, 15: maximal), tear break-up time (TBUT), and corneal sensitivity (Cochet-Bonnet aesthesiometer; Luneau Ophthalmologia; 0 to 6 cm for each quadrant and central cornea, 0 to 30 cm for the entire cornea), as described previously.18 Three measurements in the same visit were taken for all the assessments, and the mean was used for analysis. All patients received assessment preoperatively and at 1 month, 3 months, 6 months, and 12 months postoperatively.
Statistical Analysis
The sample size was calculated using the results of the first 5 patients and CNFL as the primary outcome because CNFL has been shown the most reliable parameter.16 The CNFL reduction was 43.2% ± 13.2% and 26.8% ± 8.5% in high myopic SMILE and low-moderate myopic SMILE, respectively, and was 47.9% ± 6.2% and 34.1% ± 6.0% in high myopic LASIK and low-moderate myopic LASIK, respectively. Considering a statistical power of 80% and a significance level of 5%, a sample size of 9 and 5 eyes was required to confirm the differences between the high myopic and low-moderate myopic groups for SMILE and LASIK, respectively. The comparisons between the low-moderate and high myopia groups or between SMILE and LASIK eyes were performed using an independent t test. The data of both eyes were used for statistical analysis. The Pearson correlation was used to screen and visualize the parameters. A linear mixed model was further applied to analyze the relationship between the nerve parameters and corrected MRSE, as well as the association between the nerve variables and clinical assessments, to take into account the correlation between both eyes and repeated measures. A P value less than 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
In the propensity score matching analysis, there was no significant difference in the probability between the SMILE and LASIK groups (P = .159). The mean age of patients was 29.2 ± 5.0 years and 31.4 ± 5.7 years for the SMILE and LASIK groups, respectively (P = .190). Among them, 68.1% of patients were female. Among the 88 eyes in our study, 24 eyes had SMILE treatment for their low-moderate myopia (−4.14 ± 1.14 D), 36 eyes had LASIK treatment for their low-moderate myopia (−4.26 ± 0.88 D), 21 eyes had SMILE treatment for their high myopia (−8.80 ± 1.96 D), and 7 eyes had LASIK for their high myopia (−6.48 ± 0.37 D).
Impact of Corrected Refractive Power on Corneal Denervation in SMILE
The high myopic SMILE, compared with the low-moderate myopic SMILE, presented with a significantly greater extent of corneal denervation postoperatively in the aspect of CNFA reduction (48.6% ± 6.2% vs 20.9% ± 4.9%, P = .003, respectively) and CFracDim reduction (68.1% ± 1.6% vs 32.7% ± 0.4%, P = .049, respectively; Table 1). There was a significant and negative correlation between the corrected MRSE and the reduction in CNFD (r = −0.66, P < .001), CNBD (r = −0.47, P = .007), CNFL (r = −0.61, P = .002), CNFA (r = −0.55, P = .001), and CFracDim (r = −0.38, P = .032), suggesting that greater corrected MRSE resulted in more significant reduction in corneal nerve metrics. Figure 1 illustrates the impacts of the corrected MRSE on corneal nerve metrics in SMILE with quadratic regression plots.
Table 1.
Reduction in each nerve parameter sorted by surgery and refractive power
| Parameter | Low-moderate myopic SMILE (%) | High myopic SMILE (%) | P valuea | Low-moderate myopic LASIK (%) | High myopic LASIK (%) | P valueb | P valuec | P valued |
| 1 mo | ||||||||
| CNFD | 48.1 ± 10.0 | 55.4 ± 11.5 | .640 | 85.4 ± 5.4 | 81.6 ± 9.6 | .744 | .001* | .177 |
| CNBD | 48.1 ± 12.2 | 72.6 ± 9.6 | .128 | 94.4 ± 2.4 | 91.4 ± 8.6 | .635 | <.001* | .246 |
| CNFL | 34.7 ± 5.2 | 46.9 ± 8.4 | .241 | 61.9 ± 4.0 | 64.7 ± 4.4 | .732 | <.001* | .194 |
| CTBD | 39.1 ± 11.5 | 56.4 ± 7.2 | .208 | 57.8 ± 5.2 | 74.6 ± 8.4 | .132 | .100 | .156 |
| CNFA | 20.9 ± 4.9 | 48.6 ± 6.2 | .003* | 39.6 ± 5.1 | 48.4 ± 8.6 | .422 | .023* | .989 |
| CNFW | −3.5 ± 2.7 | −8.5 ± 4.2 | .346 | −16.2 ± 2.3 | −31.4 ± 7.9 | .018* | .002* | .014* |
| CFracDim | 3.3 ± 0.4 | 6.8 ± 1.6 | .049* | 7.3 ± 1.0 | 7.5 ± 1.0 | .906 | .010* | .777 |
| 3 mo | ||||||||
| CNFD | 36.3 ± 17.2 | 22.4 ± 13.0 | .540 | 75.5 ± 5.7 | 86.8 ± 6.6 | .453 | .010* | .021* |
| CNBD | 14.7 ± 49.9 | 12.4 ± 23.3 | .970 | 84.0 ± 5.4 | 100 | .259 | .043* | .144 |
| CNFL | 35.3 ± 10.6 | 30.7 ± 8.1 | .741 | 62.7 ± 4.7 | 75.0 ± 5.5 | .325 | .004* | .015* |
| CTBD | 25.3 ± 23.9 | 36.8 ± 14.8 | .697 | 70.1 ± 5.3 | 67.1 ± 17.1 | .840 | .012* | .057 |
| CNFA | 20.0 ± 13.8 | 33.1 ± 9.2 | .455 | 47.9 ± 4.7 | 64.6 ± 9.5 | .193 | .011* | .078 |
| CNFW | −2.7 ± 5.0 | 1.5 ± 1.9 | .471 | −7.5 ± 1.7 | −27.8 ± 9.3 | .001* | .259 | .024* |
| CFracDim | 5.2 ± 1.6 | 32.0 ± 17.6 | .127 | 9.4 ± 1.2 | 12.9 ± 1.8 | .263 | .037* | .261 |
| 6 mo | ||||||||
| CNFD | 48.9 ± 7.4 | 46.9 ± 7.4 | .887 | 71.5 ± 5.3 | 90.8 ± 9.2 | .192 | .001* | .015* |
| CNBD | 51.3 ± 11.0 | 57.3 ± 17.0 | .760 | 78.8 ± 5.7 | 88.1 ± 11.9 | .557 | .043* | .045* |
| CNFL | 41.2 ± 5.2 | 41.3 ± 8.3 | .995 | 54.3 ± 3.1 | 66.7 ± 8.9 | .166 | .002* | .010* |
| CTBD | 38.2 ± 16.1 | 49.5 ± 10.5 | .614 | 64.8 ± 5.4 | 42.7 ± 21.9 | .177 | .015* | .270 |
| CNFA | 32.7 ± 6.6 | 36.2 ± 6.1 | .723 | 38.0 ± 4.0 | 48.8 ± 14.8 | .354 | .021* | .078 |
| CNFW | −1.1 ± 2.2 | −2.8 ± 2.5 | .620 | −28.5 ± 6.8 | −9.3 ± 2.3 | .007* | .022* | .022* |
| CFracDim | 5.6 ± 0.9 | 5.1 ± 1.6 | .774 | 7.1 ± 0.7 | 10.8 ± 1.9 | .064 | .013* | .512 |
| 12 mo | ||||||||
| CNFD | 29.4 ± 7.6 | 32.3 ± 6.4 | .781 | 62.2 ± 4.6 | 80.7 ± 19.3 | .189 | <.001* | .007* |
| CNBD | 41.8 ± 17.0 | 49.6 ± 8.9 | .658 | 72.4 ± 4.1 | 88.5 ± 11.5 | .170 | <.001* | .261 |
| CNFL | 29.4 ± 4.5 | 34.5 ± 5.7 | .503 | 46.8 ± 3.3 | 54.3 ± 7.1 | .421 | .001* | .026* |
| CTBD | 41.5 ± 18.9 | 39.6 ± 8.3 | .912 | 49.4 ± 5.2 | 71.6 ± 14.2 | .139 | .585 | .126 |
| CNFA | 36.0 ± 9.9 | 27.3 ± 5.3 | .403 | 29.7 ± 4.1 | 45.4 ± 13.0 | .190 | .488 | .026* |
| CNFW | 1.7 ± 2.8 | −1.5 ± 1.2 | .233 | −5.9 ± 1.6 | −30.2 ± 14.3 | .001* | .017* | .004* |
| CFracDim | 4.3 ± 1.0 | 2.9 ± 0.6 | .248 | 6.2 ± 0.8 | 7.3 ± 0.7 | .620 | .006* | .007* |
CFracDim = corneal nerve fiber fractal dimension; CNBD = corneal nerve branch density; CNFA = corneal nerve fiber area; CNFD = corneal nerve fiber density; CNFL = corneal nerve fiber length; CNFW = corneal nerve fiber width; CTBD = corneal nerve fiber total branch density
Statistically significant
P values comparing low-moderate myopic SMILE vs high myopic SMILE
P values comparing low-moderate myopic LASIK vs high myopic LASIK
P values comparing low-moderate myopic SMILE vs low-moderate myopic LASIK
P values comparing high myopic SMILE vs high myopic LASIK
Figure 1.
Regression plots showing the correlation between the corrected MRSE and the reduction in CNFD (A), CNBD (B), CNFL (C), CNFA (D), and CFracDim (E) in SMILE. Red line indicates the quadratic fitted line. CFracDim = corneal nerve fiber fractal dimension; CNBD = corneal nerve branch density; CNFA = corneal nerve fiber area; CNFD = corneal nerve fiber density; CNFL = corneal nerve fiber length; MRSE = manifest refraction spherical equivalent
Impact of the Corrected Refractive Power on Corneal Denervation in LASIK
Compared with low-moderate myopic LASIK, high myopic LASIK led to a significant increase in CNFW at all the timepoints (P = .018, P = .001, P = .007, and P = .001; Table 1). The corrected MRSE presented a significant and negative correlation with the reduction in CNBD, CTBD, and CNFA (r = −0.37, P = .015; r = −0.42, P = .005; and r = −0.41, P = .006, respectively), suggesting that greater corrected MRSE resulted in more significant reduction in corneal nerve metrics. There was also a significant correlation between the corrected MRSE and CNFW (r = 0.43, P = .004) in a positive direction. Figure 2 demonstrates the relationship between the corrected MRSE and corneal nerve parameters after LASIK.
Figure 2.
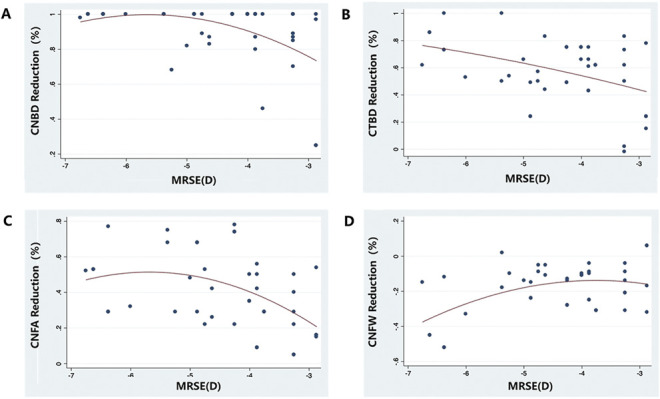
Regression plots showing the correlation between the corrected MRSE and the reduction in CNBD (A), CTBD (B), CNFA (C), and CNFW (D) in LASIK. Red line indicates the quadratic fitted line. CNBD = corneal nerve branch density; CNFA = corneal nerve fiber area; CNFW = corneal nerve fiber width; CTBD = corneal nerve fiber total branch density; MRSE = manifest refraction spherical equivalent
Comparison of Corneal Denervation in SMILE and LASIK
CNBD and CNFA were included for the regression analysis to compare the corneal denervation in SMILE vs LASIK because these 2 parameters are the common significant parameters associated with the corrected MRSE for both surgical types. In the mixed linear model analysis with the adjustment of the cap/flap thickness and optical zone/ablation zone, we found that when the corrected MRSE increased 1 D, the CNBD reduced 4.9% after SMILE (R2 = 0.220, β0 = 30.1%, P = .007), whereas it was 6.1% after LASIK (R2 = 0.140, β0 = 63.4%, P = .015). Similarly, with the increase in every diopter in the corrected MRSE, the CNFA reduced 4.0% after SMILE (R2 = 0.304, β0 = 10.5%, P = .001) but 9.1% after LASIK (R2 = 0.172, β0 = 1.7%, P = .006) (Figure 3).
Figure 3.

Linear mixed model analysis demonstrating the relationship between the preoperative MRSE and the reduction in CNBD (A) or CNFA (B) in SMILE and LASIK. Blue line represents the quadratic fitted line in SMILE, and red line represents the quadratic fitted line in LASIK. CNBD = corneal nerve branch density; CNFA = corneal nerve fiber area; MRSE = manifest refraction spherical equivalent
For low-moderate myopic correction, post-SMILE patients had significantly less reduction in CNFD, CNBD, CNFL, and CFracDim than post-LASIK patients at all timepoints (all P values < 0.05). LASIK led to significantly greater reduction in CTBD at 3 and 6 months (P = .012, P = .015), as well as in CNFA at 1, 3, and 6 months (P = .023, P = .011, and P = .021, respectively). There was also a significant difference in the postoperative changes in CNFW between SMILE and LASIK at postoperative 1, 6, and 12 months (P = .002, P = .022, and P = .017, respectively; Table 1).
For high myopic correction, LASIK resulted in significantly greater changes in CNFW at 1 month (P = .014). Post-SMILE patients had significantly less reduction in CNFD, CNFL, and CNFW than post-LASIK patients at 3 months (P = .021, P = .015, and P = .024, respectively). There was also a significant difference in the changes of CNFD, CNBD, CNFL, and CNFW between 2 groups at 6 months, as well as in the CNFD, CNFL, CNFA, CNFW, and CFracDim at 12 months (all P values < 0.05; Table 1).
Impact of Refractive Power and Corneal Denervation on Ocular Surface Parameters
There was a significant association between postoperative Schirmer test values and the reduction in CNFA (β0 = −8.93; P < .001) and CFracDim (β0 = −59.70; P < .001) after the adjustment of the cap/flap thickness, optical/ablation zone, and MRSE (Figure 4).
Figure 4.

Relationship between Schirmer test values and the reduction in CNFA (A) and CFracDim (B). Red line indicates the quadratic fitted line. CFracDim = corneal nerve fiber fractal dimension; CNFA = corneal nerve fiber area
In the SMILE group, high myopic treatment led to significantly worse TBUT than low-moderate myopic treatment (P = .049 at 1 month; P < .001 at 6 months). We did not observe this difference in the post-LASIK eyes (Table 2).
Table 2.
Clinical parameters sorted by surgery and refractive power
| Parameter | Low-moderate myopic SMILE | High myopic SMILE | P valuea | Low-moderate myopic LASIK | High myopic LASIK | P valueb | P valuec | P valued |
| 1 mo | ||||||||
| Schirmer's values | 14.6 ± 1.6 | 12.4 ± 1.9 | .839 | 8.5 ± 1.0 | 10.2 ± 1.9 | .832 | .002* | .580 |
| Oxford score | 0.7 ± 0.1 | 0.5 ± 0.2 | .761 | 0.7 ± 0.2 | 0.8 ± 0.5 | .431 | .003* | .240 |
| NEI score | 1.3 ± 0.4 | 1.2 ± 0.3 | .237 | 1.3 ± 0.4 | 0.8 ± 0.5 | .527 | .874 | .550 |
| TBUT | 8.2 ± 0.4 | 6.5 ± 0.8 | <.001* | 6.2 ± 0.5 | 3.8 ± 0.9 | .349 | .002* | .110 |
| Corneal sensitivity | 27.7 ± 0.4 | 28.3 ± 0.4 | .406 | 25.8 ± 0.8 | 30.0 ± 0.0 | .098 | .037* | .040* |
| 3 mo | ||||||||
| Schirmer's values | 12.1 ± 2.1 | 10.0 ± 2.5 | .530 | 8.9 ± 0.9 | 8.9 ± 2.5 | .321 | .118 | .791 |
| Oxford score | 0.2 ± 0.1 | 1.4 ± 0.7 | .061 | 0.9 ± 0.1 | 0.3 ± 0.3 | .713 | .005* | .493 |
| NEI score | 0.3 ± 0.1 | 0.9 ± 0.3 | .051 | 1.2 ± 0.2 | 0.3 ± 0.3 | .091 | .016* | .404 |
| TBUT | 8.8 ± 1.0 | 11.3 ± 0.7 | .057 | 6.7 ± 0.5 | 6.7 ± 1.1 | .748 | .057 | .031* |
| Corneal sensitivity | 29.5 ± 0.2 | 29.1 ± 0.5 | .537 | 26.8 ± 0.6 | 27.3 ± 0.2 | .075 | .002* | .039* |
| 6 mo | ||||||||
| Schirmer's values | 13.4 ± 2.3 | 10.0 ± 2.2 | .071 | 6.1 ± 0.7 | 4.5 ± 0.8 | .811 | <.001* | .193 |
| Oxford score | 0.3 ± 0.1 | 0.4 ± 0.1 | .091 | 0.9 ± 0.2 | 0.7 ± 0.3 | .112 | .053 | .520 |
| NEI score | 0.4 ± 0.2 | 0.6 ± 0.2 | .089 | 1.4 ± 0.3 | 0.7 ± 0.3 | .097 | .050 | .840 |
| TBUT | 9.5 ± 0.6 | 6.3 ± 0.7 | <.001* | 6.1 ± 0.5 | 5.7 ± 0.8 | .341 | <.001* | .712 |
| Corneal sensitivity | 29.2 ± 0.4 | 28.7 ± 0.4 | .084 | 27.6 ± 0.4 | 25.7 ± 1.8 | .072 | .012* | .018* |
| 12 mo | ||||||||
| Schirmer's values | 9.9 ± 1.7 | 10.9 ± 1.9 | .078 | 9.3 ± 1.3 | 5.0 ± 2.0 | .131 | .811 | .206 |
| Oxford score | 0.1 ± 0.1 | 0.2 ± 0.1 | .062 | 0.6 ± 0.2 | 1.0 ± 0.0 | .098 | .091 | .021* |
| NEI score | 0.1 ± 0.1 | 0.3 ± 0.2 | .067 | 0.8 ± 0.3 | 1.7 ± 0.3 | .116 | .111 | .005* |
| TBUT | 10.4 ± 1.3 | 9.0 ± 0.6 | .071 | 7.0 ± 0.8 | 6.7 ± 1.2 | .341 | .025* | .079 |
| Corneal sensitivity | 29.1 ± 0.3 | 28.8 ± 0.4 | .570 | 28.2 ± 0.4 | 28.2 ± 0.4 | .237 | .151 | .481 |
NEI = National Eye Institute; TBUT = tear break-up time
Statistically significant
P values comparing low-moderate myopic SMILE vs high myopic SMILE
P values comparing low-moderate myopic LASIK vs high myopic LASIK
P values comparing low-moderate myopic SMILE vs low-moderate myopic LASIK
P values comparing high myopic SMILE vs high myopic LASIK
Comparison of Ocular Surface Assessment in SMILE and LASIK With Respect to Refractive Power
For low-moderate myopic treatment, there was a significant difference in corneal sensitivity between SMILE and LASIK at 1, 3, and 6 months postoperatively (P = .037, P = .002, and P = .012, respectively). At 1 month, TBUT, Schirmer test, and the Oxford score after SMILE were significantly better than those after LASIK at 1 month (P = .002, P = .002, and P = .003, respectively). At 3 months, the SMILE group still presented with significantly better Oxford and National Eye Institute (NEI) scores (P = .005 and P = .016). At 6 months, post-SMILE patients had better TBUT and Schirmer test results than post-LASIK patients (P < .001 and P = .001). At 12 months, the SMILE group had significantly better TBUT (P = .025) (Table 2).
When patients underwent high myopic treatment, SMILE had significantly better TBUT at 3 months (P = .031) and significantly better corneal sensitivity at 1, 3, and 6 months postoperatively (P = .040, P = .039, and P = .018, respectively) than LASIK. The Oxford and NEI scores were significantly worse in the LASIK at 12 months (P = .021 and P = .005; Table 2).
DISCUSSION
In this study, we reported a significant correlation between the corrected MRSE and the reduction in various corneal nerve parameters in both SMILE and LASIK treatment, suggesting the impact of the refractive power correction on corneal denervation. We observed that high myopic SMILE, compared with low-moderate SMILE, resulted in significantly greater CNFA and CFracDim reduction and significantly lower TBUT. Compared with the SMILE group, LASIK had a greater extent of CNBD and CNFA reduction for every diopter increase in the corrected MRSE. To the authors' knowledge, this is the first study describing the mathematical relationship between the postoperative corneal nerve metrics and refractive power to be corrected. We also presented the association between postoperative corneal denervation and clinical ocular surface parameters.
We used an automatic software, ACCMetrics, to quantify the nerve metrics. ACCMetrics has been shown to have good reproducibility and repeatability in the evaluation of corneal nerve status after refractive surgery.16 We demonstrated that corneal denervation was correlated with the refractive power to be corrected in both SMILE and LASIK. This finding is supported by our previous report, in which we showed that the total nerve length in the extracted stromal lenticule increased with the refractive power to be corrected.19 Moreover, we have also previously presented that patients who underwent high myopic SMILE had a significantly greater decrease in CNFD than those who underwent low-moderate myopic SMILE at 1 month postoperatively.12 These findings can be explained by the Munnerlyn formula. Hence, it is expected that the more stromal volume ablation leads to greater impairment of corneal nerves in high myopic treatment.20 Of note, we found that the relationship between the corrected MRSE and corneal nerve metrics was mathematically more apparent in SMILE, with greater correlation coefficients being observed in SMILE. This might be because postoperative corneal nerves were markedly decreased in LASIK at 1 month, and the relationship could not be established (Table 1).
The correlation between the MRSE and changes of CNFW was in a positive manner, and CNFW increased in both types of the surgery. This increase in the fiber width might be due to the following reasons: (1) Nerves became swollen because of postoperative inflammation; an increased nerve fiber width was reported in patients with dry eye and diabetic neuropathy.21,22 (2) Chronic inflammation that occurred postoperatively stimulates the release of neurotrophic factors, which in turn causes compensatory peripheral nerve hypertrophy.23,24 (3) The smaller nerve fibers in the central ablation zone or optical zone were ablated or removed, whereas the thicker nerve bundles in the corneal periphery were preserved.
Compared with SMILE, LASIK led to more prominent corneal denervation with the same power of refractive correction throughout observation postoperative 1-year follow-up, which is in agreement with previous studies on the comparison of the impact on corneal nerves between LASIK and SMILE.25,26 The flapless nature and small incision in SMILE caused less nerve truncation compared with in LASIK. Schwann cells, which have been shown to provide a favorable environment for nerve regeneration, are also preserved to a greater extent in SMILE.10 In this study, it might be also because the treatment zone in LASIK was larger than the optical zone in SMILE, leading to more denervation in LASIK. The results of our regression analysis showed that there were 4.86% and 6.09% of CNBD reduction (β1) for every diopter of increase in MRSE in SMILE and LASIK, with the β0 constant of 30.1% and 63.4% (β0: the constant of regression analysis, which is the predicted value when all other variables are 0), respectively. Given the β0 and β1 value, the postoperative denervation of CNBD could be predicted. For example, for a patient who plans to have −4.0 D MRSE correction, the anticipated postoperative CNBD loss at 1 month would be 49.7% (30.1% + 4 × 4.86%) in SMILE but 87.8% (63.4% + 4 × 6.09%) in LASIK. Similar calculation can be performed for the CNFA loss postoperatively. In view of the preservation of corneal nerves, SMILE would be a more favorable option for the treatment of patients with high myopia and at risk of postoperative dry eye.
High myopic treatment results in not only greater corneal denervation but also a greater negative impact on the ocular surface. We found that high myopic SMILE resulted in significantly lower TBUT compared with low-moderate myopic SMILE. The subbasal nerve plexus is fundamental for the maintenance of tear film stability and ocular surface health, and decreased nerve density and length will contribute to the impairment of tear and ocular surface function.27,28 The difference in the ocular surface parameters between the high and low-moderate myopia groups was not observed in LASIK treatment because all the parameters were remarkedly altered in both groups at all timepoints postoperatively. Moreover, the postoperative reduction in CNFA and CFracDim was significantly associated with the Schirmer test values in our study. As the main aqueous volume of tear film is produced by the parasympathetic and sympathetic innervated lacrimal glands, any damage to the branch of the corneal trigeminal nerve or the reflex arc of the lacrimal gland would reduce the tear production and secretion, reducing the values of the Schirmer test.28 All the ocular surface parameters were better after SMILE than LASIK at all the timepoints in both low-moderate and high myopic groups. This further confirms that SMILE has less damage to the corneal sensory neural loop.29
In this study, there were only 7 eyes that underwent high myopic LASIK because SMILE is a preferable surgical option for high myopic patients in our clinic. The data of earlier timepoints, such as 1 week postoperatively, can be considered in future studies to minimize the effect of the possible nerve regeneration activity within postoperative 1 month.
In conclusion, we present, for the first time, the mathematic relationship between the corneal denervation and refractive power after LASIK and SMILE. The results showed that the extent of corneal denervation and impairment of TBUT increases with the increase of refractive power to be corrected. LASIK was associated with a more prominent negative impact on corneal nerves than SMILE, given the same corrected refractive power. The estimation of the postoperative status of corneal denervation also provides surgeons additional information for preoperative consultation and surgical option.
WHAT WAS KNOWN
SMILE and LASIK surgery result in corneal denervation.
Corrected refractive power may influence the denervation and regeneration of corneal nerves after refractive surgeries based on the Munnerlyn formula.
WHAT THIS PAPER ADDS
We present, for the first time, the mathematic relationship between the corneal denervation and refractive power after LASIK and SMILE.
High myopic SMILE, compared with low-moderate SMILE, resulted in significantly greater reduction of the corneal nerve fiber area and nerve fiber fractal dimension. Compared with low-moderate SMILE, high myopic SMILE resulted in significantly lower tear break-up time.
LASIK led to a more prominent corneal denervation than SMILE in response to every diopter increase in the corrected refractive power.
Footnotes
Supported by Singapore National Medical Research Council (NMRC) Clinician Scientist Award (CSA) grant (MOH-CSAINV21jun-0001).
Disclosures: None of the authors has any financial or proprietary interest in any material or method mentioned.

First author:
Chang Liu, MD
Tissue Engineering and Cell Therapy Group, Singapore Eye Research Institute, Singapore
Contributor Information
Chang Liu, Email: chang1226@qq.com.
Molly Tzu-Yu Lin, Email: lgmolly24@gmail.com.
Isabelle Xin Yu Lee, Email: isabelleleexy.96@gmail.com.
Jodhbir S. Mehta, Email: jodmehta@gmail.com.
REFERENCES
- 1.Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf 2017;15:15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol 2014;59:263–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang LWY, Mehta JS, Liu YC. Corneal neuromediator profiles following laser refractive surgery. Neural Regen Res 2021;16:2177–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou L, Yao C, Jin Y, Perez V, Ye J. Global patterns in health burden of uncorrected refractive error. Invest Ophthalmol Vis Sci 2016;57:6271–6277 [DOI] [PubMed] [Google Scholar]
- 5.Kim TI, Alio Del Barrio JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet 2019;393:2085–2098 [DOI] [PubMed] [Google Scholar]
- 6.Liu YC, Rosman M, Mehta JS. Enhancement after small-incision lenticule extraction: incidence, risk factors, and outcomes. Ophthalmology 2017;124:813–821 [DOI] [PubMed] [Google Scholar]
- 7.Kobashi H, Kamiya K, Shimizu K. Dry eye after small incision lenticule extraction and femtosecond laser-assisted LASIK: meta-analysis. Cornea 2017;36:85–91 [DOI] [PubMed] [Google Scholar]
- 8.Donnenfeld ED, Ehrenhaus M, Solomon R, Mazurek J, Rozell JC, Perry HD. Effect of hinge width on corneal sensation and dry eye after laser in situ keratomileusis. J Cataract Refract Surg 2004;30:790–797 [DOI] [PubMed] [Google Scholar]
- 9.Gatinel D, Weyhausen A, Bischoff M. The percent volume altered in correction of myopia and myopic astigmatism with PRK, LASIK, and SMILE. J Refract Surg 2020;36:844–850 [DOI] [PubMed] [Google Scholar]
- 10.Liu YC, Jung ASJ, Chin JY, Yang LWY, Mehta JS. Cross-sectional study on corneal denervation in contralateral eyes following SMILE versus LASIK. J Refract Surg 2020;36:653–660 [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Yu J, Upsilonang L, Liu Y, Zhao S. Changes in the anterior cornea during the early stages of severe myopia prior to and following LASIK, as detected by confocal microscopy. Exp Ther Med 2017;14:2869–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin JY, Lin MT, Lee IXY, Mehta JS, Liu YC. Tear neuromediator and corneal denervation following SMILE. J Refract Surg 2021;37:516–523 [DOI] [PubMed] [Google Scholar]
- 13.Ali MS, Prieto-Alhambra D, Lopes LC, Ramos D, Bispo N, Ichihara MY, Pescarini JM, Williamson E, Fiaccone RL, Barreto ML, Smeeth L. Propensity score methods in health technology assessment: principles, extended applications, and recent advances. Front Pharmacol 2019;10:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281 [DOI] [PubMed] [Google Scholar]
- 15.Liu YCRA, Mehta JS. Small incision lenticule extraction (SMILE). In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. 4th ed. Philadelphia, PA: Elsevier Mosby; 2016 [Google Scholar]
- 16.Chin JY, Yang LWY, Ji AJS, Nubile M, Mastropasqua L, Allen JC, Mehta JS, Liu YC. Validation of the use of automated and manual quantitative analysis of corneal nerve plexus following refractive surgery. Diagnostics (Basel) 2020;10:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YC, Lin MT, Mehta JS. Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural Regen Res 2021;16:690–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu YC, Yam GH, Lin MT, Teo E, Koh SK, Deng L, Zhou L, Tong L, Mehta JS. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK). J Adv Res 2021;29:67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandeira F, Yam GH, Liu YC, Devarajan K, Mehta JS. Three-dimensional neurite characterization of small incision lenticule extraction derived lenticules. Invest Ophthalmol Vis Sci 2019;60:4408–4415 [DOI] [PubMed] [Google Scholar]
- 20.Chang AW, Tsang AC, Contreras JE, Huynh PD, Calvano CJ, Crnic-Rein TC, Thall EH. Corneal tissue ablation depth and the Munnerlyn formula. J Cataract Refract Surg 2003;29:1204–1210 [DOI] [PubMed] [Google Scholar]
- 21.Mansoor H, Tan HC, Lin MT, Mehta JS, Liu YC. Diabetic corneal neuropathy. J Clin Med 2020;9:3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brines M, Culver DA, Ferdousi M, Tannemaat MR, van Velzen M, Dahan A, Malik RA. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci Rep 2018;8:4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannaccare G, Pellegrini M, Sebastiani S, Moscardelli F, Versura P, Campos EC. In vivo confocal microscopy morphometric analysis of corneal subbasal nerve plexus in dry eye disease using newly developed fully automated system. Graefes Arch Clin Exp Ophthalmol 2019;257:583–589 [DOI] [PubMed] [Google Scholar]
- 24.Giannaccare G, Pellegrini M, Taroni L, Bernabei F, Bolognesi F, Biglioli F, Sebastiani S, Moscardelli F, Cazzola FE, Campos EC. Longitudinal morphometric analysis of sub-basal nerve plexus in contralateral eyes of patients with unilateral neurotrophic keratitis. Curr Eye Res 2019;44:1047–1053 [DOI] [PubMed] [Google Scholar]
- 25.Jiang X, Wang Y, Yuan H, Li Y, Wang H, An Z, Li X. Influences of SMILE and FS-LASIK on corneal sub-basal nerves: a systematic review and network meta-analysis. J Refract Surg 2022;38:277–284 [DOI] [PubMed] [Google Scholar]
- 26.Recchioni A, Siso-Fuertes I, Hartwig A, Hamid A, Shortt AJ, Morris R, Vaswani S, Dermott J, Cervino A, Wolffsohn JS, O'Donnell C. Short-term impact of FS-LASIK and SMILE on dry eye metrics and corneal nerve morphology. Cornea 2020;39:851–857 [DOI] [PubMed] [Google Scholar]
- 27.Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res 2019;73:100762. [DOI] [PubMed] [Google Scholar]
- 28.Labetoulle M, Baudouin C, Calonge M, Merayo-Lloves J, Boboridis KG, Akova YA, Aragona P, Geerling G, Messmer EM, Benitez-Del-Castillo J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol 2019;97:137–145 [DOI] [PubMed] [Google Scholar]
- 29.Moshirfar M, Desautels JD, Walker BD, Murri MS, Birdsong OC, Hoopes PCS. Mechanisms of optical regression following corneal laser refractive surgery: epithelial and stromal responses. Med Hypothesis Discov Innov Ophthalmol 2018;7:1–9 [PMC free article] [PubMed] [Google Scholar]



