Abstract
Objectives:
The variability in outcomes of cochlear implantation is largely unexplained, and clinical factors are not sufficient for predicting performance. Genetic factors have been suggested to impact outcomes, but the clinical and genetic heterogeneity of hereditary hearing loss makes it difficult to determine and interpret postoperative performance. It is hypothesized that genetic mutations that affect the neuronal components of the cochlea and auditory pathway, targeted by the cochlear implant (CI), may lead to poor performance. A large cohort of CI recipients was studied to verify this hypothesis.
Design:
This study included a large German cohort of CI recipients (n = 123 implanted ears; n = 76 probands) with a definitive genetic etiology of hearing loss according to the American College of Medical Genetics (ACMG)/Association for Molecular Pathology (AMP) guidelines and documented postoperative audiological outcomes. All patients underwent preoperative clinical and audiological examinations. Postoperative CI outcome measures were based on at least 1 year of postoperative audiological follow-up for patients with postlingual hearing loss onset (>6 years) and 5 years for children with congenital or pre/perilingual hearing loss onset (≤6 years). Genetic analysis was performed based on three different methods that included single-gene screening, custom-designed hearing loss gene panel sequencing, targeting known syndromic and nonsyndromic hearing loss genes, and whole-genome sequencing.
Results:
The genetic diagnosis of the 76 probands in the genetic cohort involved 35 genes and 61 different clinically relevant (pathogenic, likely pathogenic) variants. With regard to implanted ears (n = 123), the six most frequently affected genes affecting nearly one-half of implanted ears were GJB2 (21%; n = 26), TMPRSS3 (7%; n = 9), MYO15A (7%; n = 8), SLC26A4 (5%; n = 6), and LOXHD1 and USH2A (each 4%; n = 5). CI recipients with pathogenic variants that influence the sensory nonneural structures performed at or above the median level of speech performance of all ears at 70% [monosyllable word recognition score in quiet at 65 decibels sound pressure level (SPL)]. When gene expression categories were compared to demographic and clinical categories (total number of compared categories: n = 30), mutations in genes expressed in the spiral ganglion emerged as a significant factor more negatively affecting cochlear implantation outcomes than all clinical parameters. An ANOVA of a reduced set of genetic and clinical categories (n = 10) identified five detrimental factors leading to poorer performance with highly significant effects (p < 0.001), accounting for a total of 11.8% of the observed variance. The single strongest category was neural gene expression accounting for 3.1% of the variance.
Conclusions:
The analysis of the relationship between the molecular genetic diagnoses of a hereditary etiology of hearing loss and cochlear implantation outcomes in a large German cohort of CI recipients revealed significant variabilities. Poor performance was observed with genetic mutations that affected the neural components of the cochlea, supporting the “spiral ganglion hypothesis.”
Keywords: Cochlear implant, Hereditary hearing loss, Outcome, Spiral ganglion hypothesis
INTRODUCTION
The variability in cochlear implantation outcomes is largely unexplained, and known clinical factors do not sufficiently predict cochlear implant (CI) performance. More recently, genetic factors have been proposed to impact cochlear implantation outcomes. However, the clinical and genetic heterogeneity of hereditary hearing loss confounds preoperative counseling on cochlear implantation outcome prediction and, conversely, the interpretation of postoperative performance in relation to a confirmed genetic background. The current hypothesis and data suggest that patients affected by genetic factors that primarily compromise the function of sensory cochlear tissue components, such as the stria vascularis, the cochlear lateral wall, and the sensory organ of Corti, including the afferent synapse, have favorable outcomes as they are presumed of limited relevance to CI function. In contrast, it is hypothesized that genetic mutations that functionally deteriorate the relevant neuronal components of the cochlea and the auditory pathway that are targeted by the electric stimulation of the CI may cause poor performance—also termed the “spiral ganglion hypothesis” (Eppsteiner et al. 2012; Shearer et al. 2017). We sought to verify this interpretation of CI outcomes within the background of a broad spectrum of confirmed genetic diagnoses in a large cohort of CI recipients.
Severe, profound, and complete hearing loss grades (≥65 dB HL) affect 60.5 million people (3.9% of all hearing loss) (World Health Organization 2021) and usually necessitate intervention with CIs to achieve functionally useful hearing rehabilitation. Hereditary hearing loss is the most common etiology of hearing loss in children (Morton & Nance 2006; Korver et al. 2017), and genetic causes may be responsible for up to 50% of cases of adult-onset hearing loss, including late-adult-onset hearing loss (Van Eyken et al. 2007a,b). Timely detection of hearing loss and intervention with hearing aids or CIs are key to prevent delays in speech, language, and cognitive skill development in children (Ciorba et al. 2017; Kral 2017). Hearing rehabilitation by hearing aids or CIs in adults may be critical to the prevention of associated comorbidities such as depression, cognitive decline, and dementia (Livingston et al. 2017, 2020; Mitchell et al. 2020; Abidin et al. 2021; Powell et al. 2021; Trpchevska et al. 2022). However, prospective, longitudinal studies that have restored hearing via appropriately fitted hearing aids (Sarant et al. 2020) or CIs (Mosnier et al. 2015; Claes et al. 2018; Sarant et al. 2019; Mertens et al. 2021) have shown either a decline, stability, or improvement in cognitive function in adults and call for a need to verify the effects of cochlear implantation further.
The CI has evolved as one of the most consequential developments in modern medicine (Niparko 2013). Despite this accomplishment, the relatively high interindividual variability in cochlear implantation outcomes remains a therapeutic concern in both children (Niparko et al. 2010; Barnard et al. 2015; Ching et al. 2017) and adults (Blamey et al. 2015; Holden et al. 2013; Krueger et al. 2008; Lenarz et al. 2012). Known clinical factors that influence cochlear implantation outcomes include variability in cochlear anatomy (Rask-Andersen et al. 2011; Avci et al. 2014) and surgical techniques, electrical coupling between electrode contacts and spiral ganglion cells (Senn et al. 2017), hearing impairment history, hearing aid use, individual success of postoperative hearing rehabilitation, and general cognitive ability (Lazard et al. 2012).
However, these currently known clinical factors account for only a small fraction of the variability in cochlear implantation outcomes observed in postlingual CI recipients. In a large multicenter cohort of 2251 adult patients, a model of four clinical factors, including the duration of severe/profound hearing loss (PHL), the age at the onset of severe/PHL, the duration of CI experience, and etiology, accounted for only 10% of the variance in CI auditory performance (Blamey et al. 2013). Using the same data set and extending the analysis to nine additional significant factors increased the proportion of explainable variance to 22% (Lazard et al. 2012). The extended list of relevant clinical factors included the duration of moderate hearing loss, hearing aid use, the pure-tone average of the implanted ear, the pure-tone average of the better ear, the hearing loss at 500 Hz in the implanted ear or the better ear, the ranked preoperative scores, the CI brand, and the percentage of active electrodes (Lazard et al. 2012). The majority of the variance (78%) remained unexplained. This unexplained variance was partly attributed to the test/retest reliability of the speech perception measurements (Lazard et al. 2012). A more recently identified factor that influences the CI outcome variance of speech recognition in adults is CI processor use time (Schvartz-Leyzac et al. 2019; Holder & Gifford 2021).
Similar observations on CI outcome variability have been made in children. Children with CIs demonstrated slower and more variable language development than hearing children. The rate of speech comprehension and expression was dependent on the age at implantation, with children undergoing cochlear implantation before 18 months of age having more favorable results than children undergoing cochlear implantation after 18 months of age (Niparko et al. 2010). Apart from the age at implantation, other clinical factors, such as less functional hearing before implantation, delayed amplification, lower maternal sensitivity to communication needs, minority status, and complicated perinatal history, have been associated with poor performance in children (Barnard et al. 2015). Similar to adults, CI processor use time has been identified as another critical factor in CI outcome variance when measuring communication skills in children (Busch et al. 2020; Wiseman et al. 2021).
Despite the identification of these clinical factors, the high degree of unexplained outcome variability in speech perception has remained the major challenging question for hearing rehabilitation with CIs, also termed the “enigma of poor performance” (Moberly et al. 2016). Individuals with poor CI performance who realized a worsening of, no improvement in, or an improvement of less than 10% in speech perception made up 13% of a large cohort of 445 patients (Bodmer et al. 2007). Depending on the speech test applied and definitions used, the rate of poor performance is highly variable (Krueger et al. 2008; Lenarz et al. 2012). To better understand outcome variability and poor performance, novel factors not yet routinely collected need to be sought to overcome this limitation.
Recently, genetic factors have been addressed as a potential additional element impacting cochlear implantation outcomes. This element may have been previously underestimated, although hereditary hearing loss represents a major etiology of hearing loss among CI recipients (Eshraghi et al. 2020; Usami et al. 2020). In a Japanese cohort of CI recipients, targeted genetic testing successfully identified causal variants in 49% (n = 84 out of 173) of the probands—referred to as the “diagnostic rate.” CI recipients with prelingual (early) hearing loss onset had a diagnostic rate of 60% (n = 55 out of 92). In comparison, CI recipients with postlingual (late) hearing loss onset had a lower diagnostic rate of 36% (n = 29 out of 81) (Miyagawa et al. 2016). In a comprehensive genetic testing study in an American CI cohort, 28% (n = 128 out of 459) of the CI recipients had a positive genetic testing result. Pediatric CI recipients with early hearing loss onset had a diagnostic rate of 48% (n = 48 out of 100), while CI recipients with late hearing loss onset had a lower diagnostic rate of 22% (n = 80 out of 359) (Seligman et al. 2021), similar to the Japanese cohort. These observations compare to general diagnostic rates in pre- and postlingual hearing loss in the literature. In a large American cohort, congenital hearing loss onset had a diagnostic rate of 44%. In comparison, patients with a later onset in childhood (29%) or adult age (28%) hearing loss onset had lower diagnostic rates (Sloan-Heggen et al. 2016). In our own German genetic cohort, the diagnostic rate was 42% for congenital and pre/perilingual hearing loss onset and declined to 12% for postlingual hearing loss onset (Tropitzsch et al. 2022).
These studies demonstrate that the genetic diagnostic yield in CI cohorts has increased considerably with the general diagnostic rates for genetic hearing loss and reached appropriate levels. However, the general evaluation of genetic factors as a contributing component to cochlear implantation outcomes is complicated by the extraordinary complexity owing to both the genetic and clinical heterogeneity of hereditary hearing loss. Hence, assessing the collective contribution of genetic factors to cochlear implantation outcomes is not expedient and will not solve the variability question. Despite this limitation, in a large multicenter cohort, patients with a genetic etiology collectively performed significantly above the average speech performance, while patients with a clinically diagnosed etiology of auditory neuropathy spectrum disorders (ANSDs) performed significantly below the average speech performance of individuals with hearing loss due to any etiology (Blamey et al. 2013). This finding raised the hypothesis that specific genetic factors that underlie certain types of clinically diagnosed ANSDs may negatively affect cochlear implantation outcomes.
Currently, more than 200 genes have been causally associated with hearing loss and are evaluated in current routine clinical testing (Rubinstein et al. 2013; Thorpe & Smith 2020). Furthermore, at the level of genetic variants, the mutational spectrum of a single hearing loss gene encompasses up to several hundred pathogenic variants (Azaiez et al. 2018), further increasing the complexity of genotype–phenotype relationships in hereditary hearing loss (Vona et al. 2019). Therefore, translating this complexity into cochlear implantation outcome interpretation may prove even more intricate.
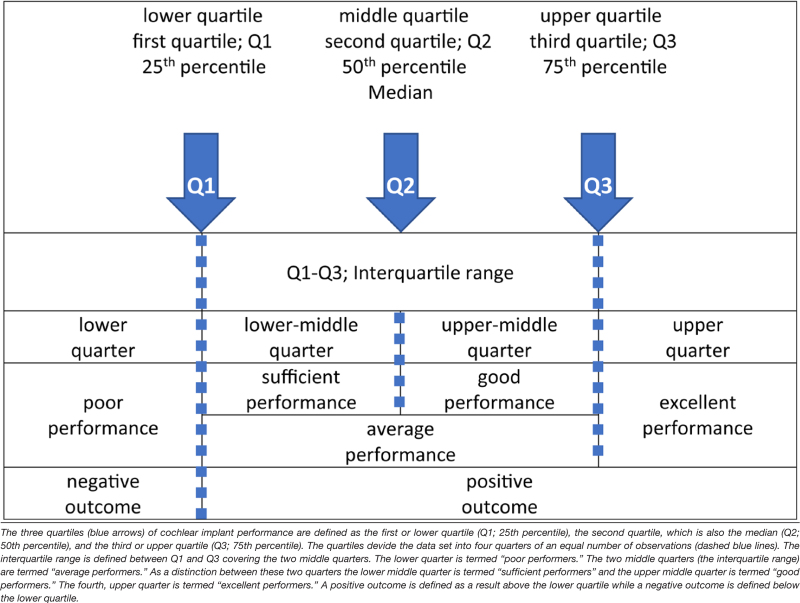
A decade ago, the “spiral ganglion hypothesis” was introduced and presented as a targeted approach addressing genetic factors negatively affecting cochlear implantation outcomes. The “spiral ganglion hypothesis” was based on the initial observations of two patients with poorly performing CIs who were found to have causal mutations in the hearing loss gene TMPRSS3, which were thought to negatively impact the function of spiral ganglion cells (Eppsteiner et al. 2012). Since then, further evidence has accumulated, that genetic deficits related to the spiral ganglion, the auditory nerve (Shearer et al. 2017, 2018; Shearer & Hansen 2019), and potentially the central nervous system may negatively affect cochlear implantation outcomes. Two recent reports aggregated the results of published CI cases of patients with pathogenic TMPRSS3 variants. CI outcomes were variable with the majority of the studies showing “positive outcomes” (Moon et al. 2021) or “favorable outcomes” (Chen et al. 2022) relating to an improvement. While “positive outcomes” or “favorable outcomes” are not well defined in the literature, we have calculated quartiles for word recognition score outcomes for the cohort in this study (for a detailed description, see the section “Materials and Methods” and Table 1). Using this calculation, a “positive outcome” is defined as a result above the lower quartile, while a poor or “negative outcome” is defined below the lower quartile.
TABLE 1.
Terminology for cochlear implant performance outcomes (for the WRS65)
The three quartiles (blue arrows) of cochlear implant performance are defined as the first or lower quartile (Q1; 25th percentile), the second quartile, which is also the median (Q2; 50th percentile), and the third or upper quartile (Q3; 75th percentile). The quartiles devide the data set into four quarters of an equal number of observations (dashed blue lines). The interquartile range is defined between Q1 and Q3 covering the two middle quarters. The lower quarter is termed “poor performers.” The two middle quarters (the interquartile range) are termed “average performers.” As a distinction between these two quarters the lower middle quarter is termed “sufficient performers” and the upper middle quarter is termed “good performers.” The fourth, upper quarter is termed “excellent performers.” A positive outcome is defined as a result above the lower quartile while a negative outcome is defined below the lower quartile.
published online ahead of print July 13, 2023.
In summary, current predictions based on specific genetic diagnoses, such as in the TMPRSS3 gene, have remained controversial. This is related to the limited number of reports and relatively small numbers of patients investigated, the variant-based complexity in single genes, and the considerable variation in reporting cochlear implantation outcomes (Nishio & Usami 2017; Usami et al. 2020; Nisenbaum et al. 2021). To improve the understanding of the relationship of specific genetic diagnoses to functional cochlear implantation outcomes, the present study evaluated a large German cohort (123 implanted ears; n = 76 probands) with a definitive genetic diagnosis according to American College of Medical Genetics (ACMG)/Association for Molecular Pathology guideline criteria (Richards et al. 2015; Oza et al. 2018) and the localization of gene expression to neural and nonneural cochlear tissue components (Ida-Eto et al. 2011; Schoen et al. 2013; Nishio et al. 2015; Usami et al. 2020).
MATERIALS AND METHODS
Patient Cohort
This retrospective study was approved by the Ethics Committee of the University of Tübingen (institutional review board). Written informed consent was obtained from patients or, in the case of minors, from their legal guardians. This retrospective data analysis included 76 probands with a definitive genetic diagnosis and available audiological results for 123 implanted ears. The genetic diagnosis for some probands (n = 48) has been described in a previous report (Tropitzsch et al. 2022).
This retrospective study evaluated patients for CI candidacy and a potential hereditary etiology of hearing loss. Among patients with a possible hereditary etiology of hearing loss in whom other known hearing loss causes were excluded, CI candidates or implanted patients were informed about the option of genetic testing that ensued when desired. According to these criteria, one family may have had more than one proband. In addition, CI patients without a definitive genetic diagnosis, those with hearing loss due to nongenetic etiologies, and a lack of German language proficiency were excluded.
All patients included in this retrospective data analysis had undergone molecular genetic diagnostic testing (2011–2020) and cochlear implantation over the course of 25 years (1997–2021) without phenotype restriction, including patients of all ages and with all implant types.
The detailed genetic results for a subgroup of patients (n = 48) have been described in a separate publication (Tropitzsch et al. 2022) that focused on the results of a gene distribution analysis in a large German hereditary hearing loss cohort (n = 305 families) (see Table in Supplemental Digital Content 1, http://links.lww.com/EANDH/B156, which references all patients, including the previously reported patients, who are marked by “&”). The present study focuses on cochlear implantation outcomes. It has been extended to include additional individuals, including the family members of index patients from the previous study (Tropitzsch et al. 2022), as well as new families with a definitive genetic diagnosis according to ACMG/AMP criteria (Richards et al. 2015; Oza et al. 2018). Patients with hearing loss due to other etiologies, such as chronic otitis media, cholesteatoma, otosclerosis, previous middle ear surgery, noise-induced hearing loss, sudden deafness, meningitis, Meniérè’s disease, measles, mumps, rubella, other viral infections including congenital cytomegalovirus, birth trauma, or other nongenetic etiologies were excluded. Preoperative evaluation included otologic examinations, imaging studies, and audiological testing comprising pure tone and speech audiometry. Audiological follow-up was carried out at yearly fixed time intervals postoperatively as part of routine postoperative care.
Statistical Analysis
Clinical and audiological phenotypic data were recorded for all probands in the cohort.
The word recognition score at 65 dB SPL (WRS65; see audiological assessment below) was employed as the primary parameter to rank CI performance (see Table 1 for a schematic overview of the terminology). Three quartiles were calculated for the ranked data set of the WRS65 speech recognition. The three quartiles were defined as the first or lower quartile (Q1; 25th percentile), the second quartile or median (Q2; 50th percentile), and the third or upper quartile (Q3; 75th percentile). This allowed dividing the ranked data set into four quarters of an equal number of observations (for the WRS65). The interquartile range was defined as between Q1–Q3 covering the two middle quarters. In terms of a qualitative assessment of CI performance for single or grouped observations (i.e., for probands diagnosed for a causal pathogenic variant in a particular gene), the lower quarter was termed “poor performers.” The two middle quarters (the interquartile range) were termed “average performers.” To allow a distinction between these two middle quarters, the lower mid-quarter was termed “sufficient performers,” and the upper mid-quarter was termed “good performers.” The fourth, upper quarter was termed “excellent performers.” “Positive outcomes” were defined as results above the lower quartile, while a “negative outcome” (equivalent to “poor performers”) was defined below the lower quartile (see Table 1 for a schematic overview of the terminology).
The median cochlear implantation outcome based on speech recognition of the total cohort was compared to specific genotypic and phenotypic features (single gene–gene comparisons, the localization of gene expression, the mode of inheritance, the age at implantation, the age at hearing loss onset, the delay of implantation, the grade of hearing loss at implantation, the laterality of hearing loss and the sequence of implantation) using Fisher’s exact test (levels of significance: p* < 0.05; p** < 0.005; p*** < 0.001).
Using a reduced set of 10 factors, we performed an ANOVA with the word recognition score at 65 dB SPL (WRS65; see audiological assessment below) as the dependent variable and two ranks for five groups: (1) gene expression, (2) age at implantation, (3) hearing-loss onset, (4) delay between hearing loss onset and implantation, (5) and sequence of implantation as independent variables. Further details on this statistical analysis, including distributions of WRS65, are described in Supplemental Digital Content 5, http://links.lww.com/EANDH/B161, Supplemental Digital Content 6, http://links.lww.com/EANDH/B162, and Supplemental Digital Content 7, http://links.lww.com/EANDH/B163.
This first statistical analysis, as described above was performed using 123 ears as independent items; we also performed a second analysis using 76 subjects as independent items. When using subjects as independent items, the WRS65 had to be computed as the mean value of both ears in the case of bilateral implantations. In addition, for these bilateral cases, the age at implantation, as well as the delay between hearing loss onset and the date of implantation had to be computed as mean values before entering the analysis as a factor.
Data were compiled using Microsoft Excel and analyzed using IBM SPSS Statistics V24 (as previously described in Tropitzsch et al. 2022).
Genetic Testing Workflow
Genetic testing was offered to and performed for 76 CI candidates or previously implanted probands with a suspected genetic etiology of hearing loss. Inheritance patterns were based on family history. The genetic testing workflow consisted of single-gene screening and custom-designed hearing loss gene panel sequencing targeting known nonsyndromic and syndromic hearing loss genes, as described in detail previously (Tropitzsch et al. 2022). Patients undergoing genetic testing after 2019 were also diagnosed by whole-genome sequencing.
CI Candidacy and Audiological Assessment
Candidacy for cochlear implantation was governed by applicable national guidelines as regulated by the Association of the Scientific Medical Societies in Germany (AWMF) (Dazert et al. 2020; Zahnert et al. 2020). In children with congenital or pre/perilingual hearing loss onset (≤6 years), cochlear implantation was considered at a threshold of up to >70 dB HL as measured by auditory brainstem responses or the pure-tone average threshold for frequencies of 0.5, 1, 2, and 4 kHz (PTA4) for each ear in air conduction (Leigh et al. 2011; Vickers et al. 2016; Dazert et al. 2020). In patients with postlingual hearing loss onset (>6 years), speech intelligibility was assessed via the WRS determined by the Freiburger monosyllable speech test. The Freiburger monosyllable speech test was also employed for the postoperative evaluation of cochlear implantation outcomes.
The Freiburger speech test is the standard reference speech test in quiet for the German language in German-speaking countries and has been widely used over more than five decades (Hahlbrock 1957; DIN 45621-1 1995; Krueger et al. 2008; Lenarz et al. 2012; Hoppe et al. 2019, 2021) and adapted to German language local variations (Kompis et al. 2006). The Freiburger monosyllable speech test consists of 20 phonemically balanced monosyllable word lists with 20 items each. Normal‐hearing subjects achieve a WRS of 50% at a presentation level of 30 dB sound pressure level (SPL) (WRS30) and 100% at 50 dB SPL (WRS50) (DIN 45621-1 1995). For hearing‐impaired subjects, typically the WRS65 is evaluated. Using further test lists, the presentation level may be increased in steps of 5, 10, or 15 dB (i.e., WRS70, WRS80, WRS90, respectively) until a maximum score (WRSmax) up to 100% or the level of discomfort is reached. The Freiburger speech test serves as an essential measure to ascertain audiological indication parameters (DIN 45621-1 1995) for hearing aids (Gemeinsamer Bundesausschuss 2021) and CIs in Germany (Hoppe & Hast 2017; Dazert et al. 2020; Zahnert et al. 2020).
In the present study, test lists of phonemically balanced monosyllabic words were presented via headphones or loudspeakers in quiet at the level of 65 dB SPL, the conversational speech level. The WRS65 was used to establish the audiological indication for cochlear implantation. According to current national guidelines, an audiological indication for cochlear implantation was considered if the measured monaural WRS65 was ≤ 60% based on optimized hearing aid fitting (Dazert et al. 2020; Zahnert et al. 2020). In the previously published national guidelines that were valid throughout most of the study period, the indication criteria were not fixed to a WRS of ≤ 60% (Lenarz et al. 2012); however, the indication criteria were common practice based on the current literature incorporating German language word recognition criteria (Hoppe et al. 2015; Leigh et al. 2016; Hoppe et al. 2017, 2019).
The audiological assessment was performed with a standard audiometer (AT900 or AT1000; AURITEC Medizindiagnostische Systeme GmbH, Hamburg, Germany) equipped with air‐conduction headphones (DT48; Beyerdynamic, Heilbronn, Germany) or a loudspeaker. Both the Freiburger monosyllable speech test and pure-tone audiometry were performed separately for each ear, and grades of hearing impairment were classified as PTA4 in seven mutually exclusive severity categories as recommended by the GBD Expert Group on Hearing Loss (Stevens et al. 2013; Olusanya et al. 2019; GBD Hearing Loss Collaborators 2021). Accordingly, the preoperative bilateral PTA4 results for all probands (n = 76), including all implanted single ears (n = 123) and nonimplanted single ears (n = 29), were classified into seven hearing loss grades designated as normal (0–19.9 dB HL), mild (20–34.9 dB HL), moderate (35–49.9 dB HL), moderately severe (50–64.9 dB HL), severe (65–79.9 dB HL), profound (80–94.9 dB HL), and complete (≥95 dB HL) hearing loss.
Speech recognition outcomes of cochlear implantation were assessed with the Freiburger monosyllabic speech test (Hahlbrock 1957) for the German language at 65 dB and 80 dB SPL in quiet to determine the WRS65 (CI) and WRS80 (CI), respectively, including contralateral masking if applicable such as in SSD or contralateral residual hearing (Kompis et al. 2006; Sukowski et al. 2009; Kollmeier et al. 2014; Hoppe et al. 2015; Hoppe & Hast 2017; Hoppe et al. 2017, 2019; Dazert et al. 2020; Volter et al. 2020; Zahnert et al. 2020; Hoppe et al. 2021). To allow a sufficient postoperative rehabilitation period, cochlear implantation outcome measures were based on at least 1 year of postoperative audiological follow-up for patients with postlingual hearing loss onset (>6 years)/implantation age and 5 years for children with congenital or pre-/perilingual hearing loss onset (≤6 years)/implantation age. This postimplantation time intervals were chosen to ensure, that all probands had reached a postlingual age of > 6 years after implantation (Dazert et al. 2020; Zahnert et al. 2020).
Results for CI performance were categorized in percentiles with a 70% WRS65 defining the median outcome. “Poor” performance was defined as a performance within the lower quarter defined as a 0%–45% WRS65. “Sufficient” performance was defined within the second quarter, ranging from 46% to 69 % WRS65. “Good” performance was defined within the third quarter with a range of 70%–79%, and “Excellent” performance was defined within the fourth, upper quarter with a range from 80% to 100% (for definitions of percentiles, see section “Materials and Methods” and Table 1).
Cochlear Implantation Surgery and Postimplantation Hearing Rehabilitation
All patients were implanted using an atraumatic, structure-preserving approach through the round window or extended round window access as an institutional standard. Intraoperative assessment by direct microscopic visualization and review of postoperative imaging films and radiologic reports for all implants indicated that all electrode contacts of the stimulation electrodes were located in the cochlea, consistent with a full insertion, regardless of the manufacturer and stimulation electrode type. Only probands with documented full insertion of the stimulation electrodes were included. All patients underwent a standardized and certified postoperative hearing rehabilitation program according to national CI care guidelines (Dazert et al. 2020; Zahnert et al. 2020).
Localization of Gene Expression
Gene expression profiles of hearing loss genes were classified according to localization in the neural and nonneural tissue components of the cochlea as described and compiled in the current literature (Ida-Eto et al. 2011; Schoen et al. 2013; Nishio et al. 2015; Usami et al. 2020) and the databases gEAR (Orvis et al. 2021) and SHIELD (Shen et al. 2015). Nonneural tissue components of the cochlea included the following designated categories: (1) hair cells (including synaptopathy), (2) structural loci in the cochlear duct (i.e., in supporting cells and interdental cells), (3) the spiral lamina and the stria vascularis (including genes related to homeostasis at these locations) and (4) mitochondria (mitochondrial genes). Due to the ubiquitous localization of the mitochondria in both neural and nonneural tissue components, it remains difficult to predict their functional significance for either of those categories. Also, some of the single genes (i.e., COL11A1) are expressed in more than one cluster and were assigned to their most probable functional significance according to the current literature. The specific cellular expression patterns of the single solving genes in the respective tissue components, their functional significance, and their allocation to the five designated clusters of the cochlea are described in Table in Supplemental Digital Content 2, http://links.lww.com/EANDH/B157.
RESULTS
Patient Cohort Summary
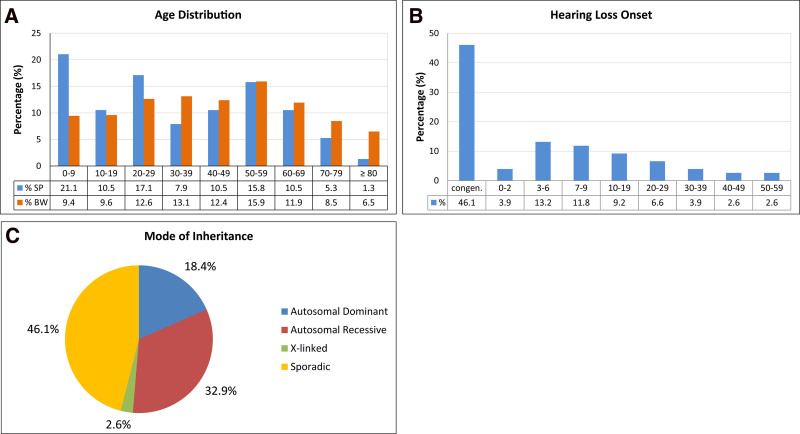
In this genetic CI cohort, the ages of the 76 probands were distributed across all age groups (mean age: 35.7; year 2021) (Fig. 1A; Table 2; Supplemental Digital Content 1 http://links.lww.com/EANDH/B156). The mean age of this genetic CI cohort was almost a decade below the mean age of the population in southwest Germany at a close reference date [mean population age in the State of Baden-Württemberg: 43.8 years; year 2020 (State Statistical Office Baden-Württemberg 2021)]. As expected for a CI cohort, pediatric probands in the first decade of life (0–9 years) made up a relatively high proportion, corresponding to more than one-fifth of the study cohort (21.1%; n = 16). This high proportion of 0- to 9-year-old patients explains the shift (by 8.1 years) toward a younger mean age of the genetic CI cohort than of the general population. Otherwise, the age distribution of the genetic CI cohort showed an even distribution across all age groups, with an average of approximately 12% of participants per decade in the second to seventh decades. Similar to the general population, there was a slight peak in patients in the sixth decade and a decline thereafter. Although lower proportions of individuals in the eighth and ninth decades were seen in the study cohort than in the general population, it is remarkable to have these age groups represented in a CI cohort with a definitive genetic diagnosis (Fig. 1A; Table 2).
Fig. 1.
Demographic and phenotypic characteristics of the genetic cochlear implant cohort. A, Age distribution of the study population (SP) (n = 76) across all age groups in decades (blue bars). The percentage for each decade is shown below the bar. The age distribution of the cohort (2021) was compared to the age distribution of the population in southwest Germany (BW) for similar reference years (2019) and decades (data from State Statistical Office Baden-Württemberg 2021) (orange bars). B, Age-related distribution of hearing loss onset in the genetic cochlear implant cohort. The phases of congenital onset and prelingual (0–2 years) and perilingual (3–6 years) hearing loss onset are shown in the first three bars (≤6 years). Postlingual hearing loss onset (>6 years) starts at the second bar (7–9 years) and continues through subsequent decades. The percentage for each time window is shown below the bar. C, Overview of the modes of inheritance in the total patient cohort. Pie chart showing the respective proportions of patients with autosomal-dominant, autosomal-recessive, and X-linked inheritance patterns, as well as patients with sporadic hearing impairment.
TABLE 2.
Phenotypic, audiological, and implantation characteristics of probands and implanted ears
| n | % | ||
|---|---|---|---|
| Subjects | 76 | ||
| Phenotypic characteristics | |||
| Sex | Male | 35 | 46.1 |
| Female | 41 | 53.9 | |
| Age | 0–9 y | 16 | 21.1 |
| 10–29 y | 21 | 27.6 | |
| 30–59 y | 26 | 34.2 | |
| 60–80+ y | 13 | 17.1 | |
| Onset of hearing loss | Congenital | 35 | 46.0 |
| Pre/perilingual (0–6) | 13 | 17.1 | |
| Postlingual (>6–19) | 16 | 21.1 | |
| Postlingual (≥20–59) | 12 | 15.8 | |
| Laterality of hearing loss | Bilateral symmetric | 70 | 92.1 |
| Bilateral asymmetric | 2 | 2.6 | |
| Single-sided deafness | 4 | 5.3 | |
| Ethnicity | Western European | 52 | 68.4 |
| Western Asian | 10 | 13.2 | |
| Eastern European | 8 | 10.5 | |
| Southern European | 5 | 6.6 | |
| Southern Asian | 1 | 1.3 | |
| Family history | Autosomal-recessive | 25 | 32.9 |
| Autosomal-dominant | 14 | 18.4 | |
| X-linked | 2 | 2.6 | |
| Sporadic | 35 | 46.1 | |
| Ears implanted | 123 | ||
| Characteristics of implanted ears | |||
| Side of cochlear implant | Left | 59 | 48.0 |
| Right | 64 | 52.0 | |
| Preoperative grades of hearing loss for implanted ears | Moderately severe | 1 | 0.8 |
| Severe | 5 | 4.1 | |
| Profound | 24 | 19.5 | |
| Complete | 93 | 75.6 | |
| Age at implantation | 0–2 y | 31 | 25.2 |
| 3–5 y | 14 | 11.4 | |
| 6–59 y | 66 | 53.7 | |
| ≥60 y | 12 | 9.7 | |
| Time delay between age of hearing loss onset vs. age at implantation | ≤1 y | 21 | 17.0 |
| 2–5 y | 26 | 21.2 | |
| 6–29 y | 45 | 36.6 | |
| 30–69 y | 31 | 25.2 | |
| Laterality of cochlear implantation | Unilateral | 29 | 23.6 |
| Bilateral simultaneous | 15 | 12.2 | |
| Bilateral sequential | 32 | 26.0 | |
| Bilateral all | 47 | 38.2 | |
The genetic CI cohort included 35 males (46.1%; state of BW: 49.7%, year 2019) and 41 females (53.9%; state of BW: 50.3%, year 2019), which is representative of the sex distribution in the population of southwest Germany (State Statistical Office Baden-Württemberg 2021) (Table 2).
The ethnic background of the probands was primarily western European (68.4%; n = 52), with the majority coming from the local region of southwest Germany. Other ethnicities representing the general migration background of the regional population (31.6%; n = 24) were western Asian (13.2%; n = 10), eastern European (10.5%; n = 8), southern European (6.6%; n = 5), and southern Asian (1.3%; n = 1) (Table 2). This distribution in the genetic CI cohort is based on a slightly higher migration background in the general population in southwest Germany (33.4% for state of BW; year 2019; State Statistical Office Baden Württemberg 2021). In total, patients of European ethnicity made up 86% (n = 65) of the genetic CI cohort.
Hearing loss onset ranged from congenital onset to late adult onset in the sixth decade, with a distribution that spread over all age groups of the cohort. By far, the largest group of probands had congenital hearing loss onset (46.1%; n = 35), followed by pre/perilingual (age 0–6 years) hearing loss onset (17.1%; n = 13). Altogether, this early-onset group (congenital, pre/perilingual; ≤6 years of age) made up almost two-thirds of the cohort (63.2%; n = 48), while one-third of the probands in the cohort (36.8%; n = 28) had a late onset of hearing loss in the postlingual domain (>6 years of age). In the postlingual domain, the largest group of probands (21.0%; n = 16) had late childhood and juvenile (age 7–19 years) hearing loss onset. In the following decades of hearing loss onset, the proportion of patients slowly declined, but included patients with late adult hearing loss onset that was recognized as late as the sixth decade (Table 2; Fig. 1B).
With regard to hearing loss laterality, the majority of probands presented with bilateral symmetric hearing loss (n = 70; 92.1%). Bilateral asymmetric hearing loss (AHL) (n = 2; 2.6%) (better ear: PTA >30 and ≤55 dB HL; poorer ear PTA ≥70 dB HL; interaural threshold gap ≥15 dB HL), as defined by the consensus framework for single-sided deafness (SSD) and AHL (Van de Heyning et al. 2016), and unilateral hearing loss within the definition of SSD (5.3%; n = 4) (better ear: PTA ≤30 dB HL; poorer ear: PTA ≥70 dB HL; interaural threshold gap: ≥40 dB HL) (Van de Heyning et al. 2016) were relatively rare in this genetic CI cohort (Table 2).
Based on family history, the pattern of inheritance included 25 probands (32.9%) with autosomal-recessive hearing loss, 14 probands (18.4%) with autosomal-dominant hearing loss, two male probands (2.6%) with X-linked hearing loss and 35 probands (46.1%) with a sporadic type of hearing loss (Table 2; Fig. 1C; Supplemental Digital Content 1, http://links.lww.com/EANDH/B156).
Cochlear implantations were evenly distributed between the left (48.0%; n = 59) and right (52.0%; n = 64) ears (Table 2).
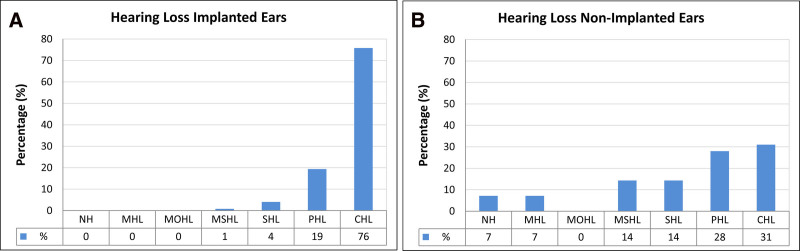
Preoperative grades of hearing impairment were classified as recommended by the GBD Expert Group on Hearing Loss (GBD Hearing Loss Expert Group et al. 2013; Olusanya et al. 2019; GBD Hearing Loss Collaborators 2021) for all ears (n = 152), including implanted and nonimplanted ears. Hearing loss for all implanted ears (n = 123; 80.9% of all ears) was distributed across the four highest hearing loss grades: moderately severe hearing loss (MSHL; 50.0 to 64.9 dB HL) (n = 1; 0.8%), severe hearing loss (SHL; 65.0 to 79.9 dB HL) (n = 5; 4.1%), profound hearing loss (PHL; 80.0 to 94.9 dB HL) (n = 24; 19.5%), and complete or total hearing loss (CHL; ≥95.0 dB HL) (n = 93; 75.6%) (Table 2; Fig. 2A). Hearing loss for single nonimplanted contralateral ears (n = 29; 19.1% of all ears) of patients who underwent unilateral implantation was distributed among normal hearing (−10.0 to 19.9 dB HL) (n = 2; 6.9%), mild hearing loss (20.0 to 34.9 dB HL) (n = 2; 6.9%), moderate hearing loss (MOHL; 35.0 to 49.9 dB HL) (n = 0; 0%), MSHL (50.0 to 64.9 dB HL) (n = 4; 13.8%), SHL (65.0 to 79.9 dB HL) (13.8%; n = 4), PHL (80.0 to 94.9 dB HL) (27.6%; n = 8), and complete or total hearing loss (CHL; ≥ 95.0 dB HL) (31.0%; n = 9) (Fig. 2B).
Fig. 2.
Distribution of hearing loss grades in the genetic cochlear implant cohort. A, Distribution of preoperative hearing loss grades for all implanted ears (n= 123). B, Distribution of preoperative hearing loss grades for all nonimplanted ears (n=29). NH indicates normal hearing (−10.0 to 19.9 dB HL), MHL mild hearing loss (20.0 to 34.9 dB HL), MOHL moderate hearing loss (35.0 to 49.9 dB HL), MSHL moderately severe hearing loss (50.0 to 64.9 dB HL), SHL severe hearing loss (65.0 to 79.9 dB HL), PHL profound hearing loss (80.0 to 94.9 dB HL), and CHL complete or total hearing loss (≥95.0 dB HL). Classification follows the recommendations of the Global Burden of Disease (GBD) Expert Group on Hearing Loss (Global Burden of Disease Hearing Loss Expert Group et al. 2013; Olusanya et al. 2019). Percentage values for different hearing loss grades are shown below bars.
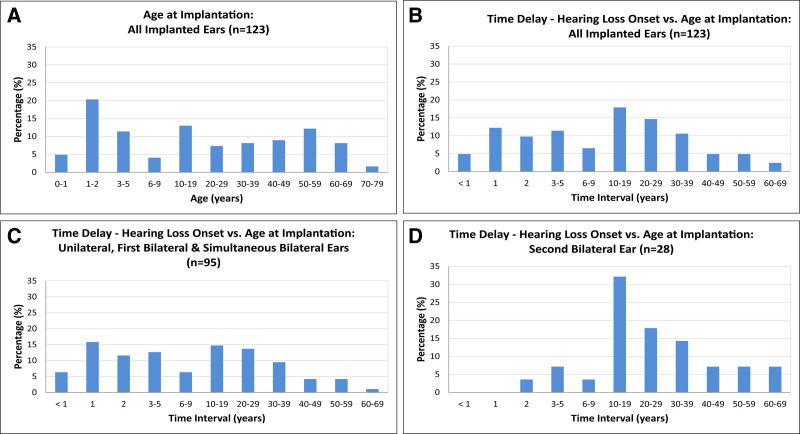
The age at implantation for all implanted ears (n = 123) was distributed across the full age range, from less than 1 year to the eighth decade (Table 2). A quarter of implantations occurred in ears of infants up to 2 years old (0–2) (25.2%; n = 31). For the remainder of implanted ears, implantations occurred in the first decade and the following decades in an even distribution, with an average of ≈10% per decade up to the seventh decade. There were slight peaks in the first, second, and sixth decades and a decline in the eighth decade (Fig. 3A).
Fig. 3.
Distribution of age at implantation and time delay between hearing loss onset and age at cochlear implantation. A, Distribution of age at cochlear implantation of all implanted ears (n = 123). Phases of early implantation at prelingual age are shown in the first two bars (0–1 and 1–2 years). Implantation at perilingual age is shown in the third bar (3–5 years). Implantation in late childhood (6–9 years) is shown in the fourth bar. Further distribution of age at implantation is shown in decades. Impacts of phenotypic characteristics on diagnostic solution rates. B, Time delay between hearing loss onset and age at cochlear implantation for all implanted ears (n = 123). C, Time delay between hearing loss onset and age at cochlear implantation for all unilateral implanted ears, first implanted ears in sequential bilateral implantations and simultaneous bilateral implantations (n = 95). D, Time delay between hearing loss onset and age at cochlear implantation for second implanted ears in sequential bilateral implantations (n = 28).
The time interval or delay between hearing loss onset and cochlear implantation also showed a broad range of latencies, from less than 1 year to seven decades. Short time intervals of up to 2 years (0–2) were found for more than one-quarter of implantations (26.8%; n = 33). The time interval was 3 to 9 years for a further one-fifth (17.9%; n = 22) of implantations. Altogether, slightly less than one-half of the implantations (44.7%; n = 55) were performed within the first decade (0–9 years) after hearing loss onset. For more than one-half of the implantations (55.3%; n = 68), the latency between hearing loss onset and cochlear implantation was very long, ranging from 10 to 69 years. One-fifth of the implantations (17.9%; n = 22) occurred in the second decade after hearing loss onset. The following five decades showed a continuous decline (at ≈ 3% per decade) in the number of implantations (Table 2; Fig. 3B).
Both the first and second implanted ears showed this broad range of time intervals of up to seven decades between hearing loss onset and implantation. The “first implanted ears” group included cases of unilateral implantations, simultaneous bilateral implantations and first ears of bilateral but consecutively implanted cases. This “first implanted ears” group (77.2%; n = 95 implanted ears out of 123) was larger than the “second implanted ears” group (22.8%; n = 28 implanted ears out of 123), with a ratio of three to one. In the “first implanted ears” group, more than one-half of the implantations (52.6%; n = 50) occurred within the first decade after hearing loss onset, and less than one-half (47.4%; n = 45) occurred within much longer time intervals of up to seven decades (Fig. 3C). For the “second implanted ears” group (n = 28), the proportion of implantations that occurred within the first decade (14.3%; n = 4) was much smaller than the proportion of implantations that occurred in later decades (85.7%; n = 24), shifting the peak of implantations for the “second implanted ear” group to the second decade (32.1%; n = 9) after hearing loss onset (Fig. 3D).
Gene Distribution
The genetic diagnosis of the 76 solved cases in the genetic CI cohort involved 35 genes and 61 different causative mutations in clinically relevant (P, LP) variants (see Table in Supplemental Digital Content 1, http://links.lww.com/EANDH/B156, for detailed gene and variant distributions of all solved cases).
For the remainder of this article, the gene distribution of causative mutations for hearing loss is described for implanted ears (n = 123) rather than probands (n = 76), as the relevant readout of CI performance relates to individually implanted ears. The gene distribution analysis showed that the hearing loss in nearly one-half of the implanted ears (48%; n = 59 of 123) was related to medically relevant variants (P, LP) in the six most frequently affected genes: GJB2 (21%; n = 26), TMPRSS3 (7%; n = 9), MYO15A (7%; n = 8), SLC26A4 (5%; n = 6), and LOXHD1 and USH2A (each 4%; n = 5). One-fourth of the genetic hearing loss cases (20%; n = 24) was correlated with causative variants in six additional genes, namely, MITF, WFS1, CDH23, MARVELD2, COL4A3, and MYO7A (all 3%; n = 4). Overall, in two-thirds of implanted ears (67%; n = 83 of 123) genetic hearing loss was attributed to causative variants in only 12 genes. The genetic hearing loss in the remaining one-third (33%; n = 40 of 123) of the implanted ears was attributable to causative variants in 23 additional genes, PAX3, MYO6, SMPX, and MYH14 (all 2.4%; n = 3); LHFPL5, DIAPH1, MYO3A, TMIE, KCNE1, POU3F4, OTOF, SOX10, and ACTG1 (all 1.6%; n = 2); and COL2A1, MT-TL1, POU4F3, EYA4, COCH, TFAP2A, PTPRQ, RRM2B, EDNRB, and COL11A1 (all 0.8%; n = 1) (see Figure in Supplemental Digital Content 3, http://links.lww.com/EANDH/B158, for the gene distribution of causative variants for the genetic hearing loss in all implanted ears).
CI Performance and Ranking According to Single Genes
Cochlear implantation performance was measured for speech recognition (WRS65). The median CI performance for the total of all implanted ears (n = 123) was calculated at 70% WRS65, with upper and lower quartiles at 80% and 45%, respectively. For children and adolescents (n = 42 implanted ears; n = 23 probands; age 6–17 years), the median CI performance reached 80%, while for adults (n = 81 implanted ears; n = 53 probands; age ≥ 18 years), the median CI performance remained at 60%.
In a first attempt to correlate CI performance with a definitive genetic diagnosis, cochlear implantation performance for the WRS65 was ranked on the basis of the medians of all implantations for every single gene.
The genetic diagnosis of the 76 probands and 123 implanted ears in the present genetic CI cohort involved 35 genes and 61 different causative mutations. This implies that an average of 3.5 implanted ears were available for analysis for every single gene. However, as expected for a genetic hearing loss cohort, the diagnostic yield per gene showed a highly asymmetric distribution and ranged from 1 to 26 implanted ears per gene. Accordingly, two-thirds of implanted ears were distributed among only 12 genes affecting at least four implanted ears in the present cohort (see Figure in Supplemental Digital Content 3, http://links.lww.com/EANDH/B158). This asymmetric distribution of genetic diagnoses is also seen in other genetic CI cohorts (Miyagawa et al. 2016; Seligman et al. 2021). Although we attempted to relate cochlear implantation outcomes for all 35 single genes (Fig. 3), this comparison has to be taken with caution, as for two-thirds of the single genes (n = 23), the median performance was based on only three or fewer implanted ears.
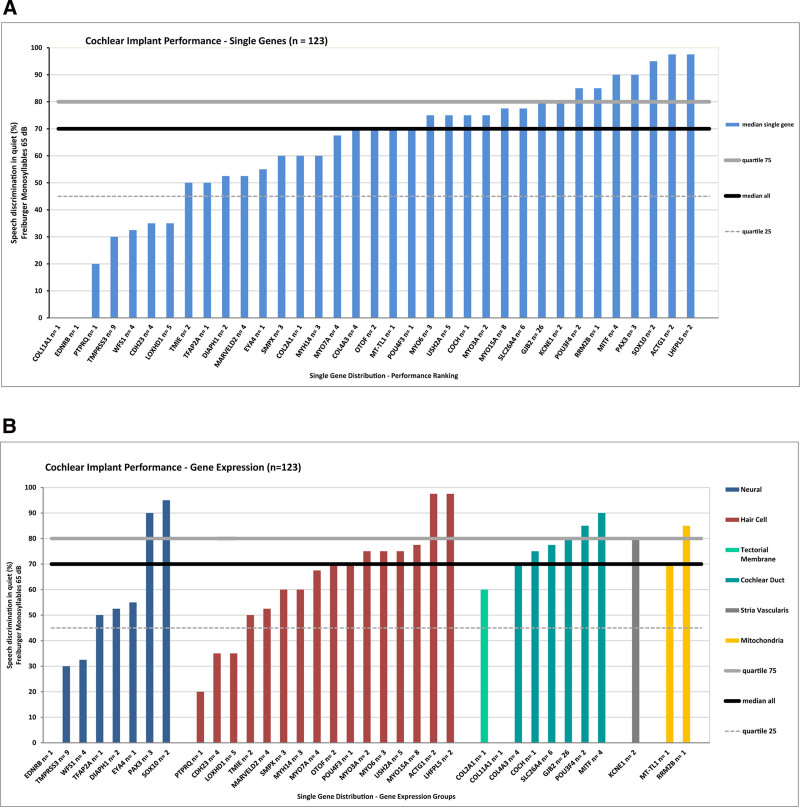
The most frequently affected gene, GJB2 (n = 26), showed a median CI performance of 80%. For all genes, the CI performance ranged from 0% associated with the genes COL11A1 and EDNRB (single implantation each) to 97.5% for the genes ACTG1 and LHFPL5 (two implantations each). The CI performance results for most genes (n = 21 of 35) remained within the 25th–75th quartile or the “average performance” range. Seven genes (COL11A1, EDNRB, PTPRQ, TMPRSS3, WFS1, CDH23, and LOXHD1; n = 25 implanted ears) showed “poor performance” below the 25th quartile while seven other genes (POU3F4, RRM2B, MITF, PAX3, SOX10, ACTG1, and LHFPL5; n = 16 implanted ears) demonstrated “excellent performance” above the 75th quartile (Fig. 4A).
Fig. 4.
Cochlear implant performance for single genes according to the word recognition scores in the Freiburger monosyllabic speech intelligibility test at 65 dB SPL (WRS65). A, Performance ranking for all single genes (n = 35). B, Performance ranking of single genes within five different gene expression groups according to the localization gene expression within the cochlea designated (1) Neural, (2) Hair Cell, (3) Cochlear Duct and Tectorial Membrane, (4) Stria Vascularis, and (5) Mitochondria. The black solid line shows the median of the overall cohort at 70%, the gray solid line shows the upper (80%) quartile, and the gray dashed line shows the lower quartile (45%).
There were no “nonperforming” ears among this genetic CI cohort. The two implanted ears diagnosed with medically significant variants in the genes COL11A1 and EDNRB that had a result of 0% for the WRS65, but showed an increase to 50% and 15% at WRS80, respectively, at this increased speech level. The overall mean increased from 70% for the WRS65 to 85% for the WRS80, indicating a performance reserve in this genetic CI cohort (see Figure in Supplemental Digital Content 4, http://links.lww.com/EANDH/B159, for CI performance of single genes for the WRS80).
CI Performance According to the Localization of Gene Expression in Single Genes
As a next step, CI performance was related to the localization of gene expression in the cochlea for single genes. Each gene was classified with regard to the currently established expression pattern in the cochlea as reviewed in the literature (see Table in Supplemental Digital Content 2, http://links.lww.com/EANDH/B157, for a detailed description of gene expression patterns and the localization of the gene expression of all single genes within the cochlea) (Ida-Eto et al. 2011; Schoen et al. 2013; Kantarci et al. 2015; Nishio et al. 2015; Neuhaus et al. 2017; Usami et al. 2020; Wu et al. 2020; Nisenbaum et al. 2021). According to the primary localization of gene expression and associated functional relevance, five expression clusters were established for cochlear tissue components, designated as (1) Neural, (2) Hair Cell, (3) structural genes in the Tectorial Membrane and the Cochlear Duct, (4) Stria Vascularis, and (5) Mitochondria. The Neural cluster comprised eight genes (EDNRB, TMPRSS3, WFS1, TFAP2A, DIAPH1, EYA4, PAX3, and SOX10; n = 23 implanted ears), the Hair Cell cluster 16 genes (PTPRQ, CDH23, LOXHD1, TMIE, MARVELD2, SMPX, MYH14, MYO7A, OTOF, POU4F3, MYO3A, MYO6, USH2A, MYO15A, ACTG1, and LHFPL5; n = 51 implanted ears), the combined structural genes of the Tectorial Membrane and Cochlear Duct cluster eight genes (COL2A1, COL11A1, COL4A3, COCH, SLC26A4, GJB2, POU3F4, and MITF; n = 45 implanted ears), the Stria Vascularis cluster one gene (KCNE1; n = 2 implanted ears), and the Mitochondria cluster two genes (MT-TL1 and RRM2B; n = 2 implanted ears).
The distribution of the medians of cochlear implantation outcomes of the WRS65 for the eight genes of the Neural cluster ranged from 0% to 95%, for the twelve genes of the Hair Cell cluster from 20–97.5%, for the eight structural genes in the Cochlear Duct and Tectorial Membrane cluster from 0–90%, for the single gene in the Stria Vascularis cluster at 80% and for the two genes in the Mitochondrial cluster from 70% to 85% (Fig. 4B).
Due to the broad range of gene cluster outcomes, no obvious trends were inferred from the comparison of the expression clusters. In fact, the three large clusters Neural (n = 25), Hair Cell (n = 51) and the combined structural cluster for the Tectorial Membrane and the Cochlear Duct cluster (n = 45) all covered a range of outcomes that was very similar to that of the total cohort (0% to 97.5%). Therefore, all single genes within an expression cluster were combined as a further step in the analysis.
CI Performance According to the Localization of Gene Expression in Cochlear Tissue Clusters
Combining the single genes of the cochlear tissue clusters allowed a comprehensive comparison of the five clusters with different expression and functional backgrounds. Surprisingly, this comparison revealed relatively uniform median outcomes of 70% to 80% (WRS65) for all four nonneural clusters. The Hair Cell cluster performed at 70%, the combined structural genes of the Tectorial Membrane and Cochlear Duct cluster at 80%, the Stria Vascularis cluster at 80% and the Mitochondria cluster at 77.5%. These results for all the nonneural clusters are at or near the upper quartile of 80% of the total cohort. However, the Neural cluster showed a significantly (p < 0.05) lower median of only 45% than the overall median of 70% of the total cohort and stayed below the lower quartile of 45% of the total cohort. These results show a strong divergence between the outcomes in the nonneural clusters and the neural cluster (Fig. 5A). As a next step of the analysis, the outcome for the genetic clusters was compared with other clinical parameters.
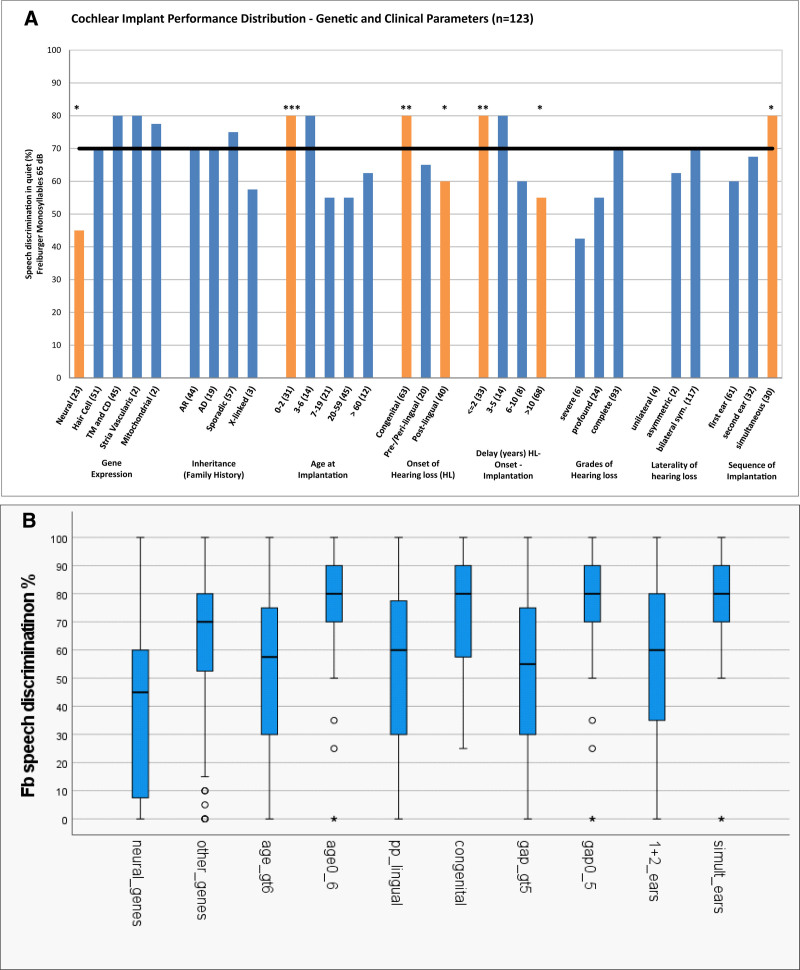
Fig. 5.
Cochlear implant performance including gene expression and demographic and clinical parameters according to the word recognition scores in the Freiburger monosyllabic speech intelligibility test at 65 dB SPL (WRS65) for 123 implanted ears. A, Cochlear implant performance for 30 categories. Demographic and clinical parameters consisted of inheritance based on the family history, onset of hearing loss, delay of implantation between the onset of hearing loss and cochlear implantation, grades of hearing loss, laterality of hearing loss and sequence of hearing loss. Statistical significance was determined with Fisher’s exact test (*p < 0.05, **p < 0.005, ***p < 0.0005). B, Cochlear implant performance for 10 broader genetic and clinical categories. Median value, interquartile range (IQR) and min/max estimates based on 1.5 IQR of word recognition scores. These represent five groups designated as (1) Gene expression: neural versus other nonneural genes; (2) age at implantation: 0–6 years (0-6y) of age versus age >6 years (gt 6y); (3) onset of hearing loss: congenital versus non-congenital (pre/peri/postlingual; pp_lingual); (4) implantation delay (gap): 0–5 years (0-5y) versus >5 years (gt 5y); (5) sequence of implantation: simultaneous bilateral implantation (simul_ears) versus subsequent bilateral or monaural only implantation (1 and 2 ears).
CI Performance According to the Mode of Inheritance Based on Family History and Other Clinical Phenotypic Features
In addition to the direct comparison of the genotypic clusters according to the localization of gene expression, cochlear implantation outcomes were assessed for the mode of inheritance based on family history and other specific demographic and phenotypic features, such as the age at implantation, the onset of hearing loss, the delay between the onset of hearing loss and cochlear implantation, grade of hearing loss at implantation, laterality of hearing loss, and the sequence of implantation. Finally, all these clinical parameters were compared to the median outcome of the total cohort (Fig. 5A).
The median for modes of inheritance according to family history ranged between 57.5% and 75% (WRS65). Autosomal-recessive (n = 44; 36%) and autosomal-dominant (n = 19; 15%) modes of inheritance reached a performance of 70%, sporadic cases (n = 57; 46%) were slightly above that at 75%, and X-linked cases (n = 3; 2%) were below the other three modes of inheritance at 57.5%. All four different modes of inheritance were not significantly different from the median of the total cohort at 70% (Fig. 5A).
In relation to age at implantation, the CI performance was evaluated for five different age groups. In early childhood, the youngest (0–2 years) age at implantation group, comprising infants and toddlers in the prelingual domain (n = 31; 25%), and the 3 to 6 years age at implantation group, comprising preschool children in the perilingual domain (n = 14; 11%), both reached an outcome of 80% WRS65 at the upper quartile. All of these children who underwent implantation between 0 and 6 years of age were tested at least 5 years after implantation at a postlingual age of >6 years to allow sufficient speech development (school age) (Dazert et al. 2020; Zahnert et al. 2020). The 7 to 19 years age at implantation group in middle childhood and adolescence (n = 21; 17%) reached an outcome of 55%. The 20 to 59 years age at implantation group (n = 45; 37%) in young and middle adulthood reached the same outcome of 55%. In older adulthood, the ≥60 years age at implantation group (n = 12; 10%) achieved an outcome of 62.5%. Except for the youngest (0–2 years) age group, the results for the four older age groups were not significantly different from the median of the total cohort at 70%. The youngest (0–2 years) age group was the only age group that reached a significantly (p < 0.001) better result than the total cohort (Fig. 5A).
The onset of hearing loss was evaluated for three different age groups of hearing loss onset, designated congenital (n = 47; 38%), pre/perilingual (n = 20; 16%; ≤6 years of age), and postlingual (n = 56; 46%; >6 years of age). The congenital group showed a significantly (p < 0.01) better performance of 80%, at the upper quartile; the pre/perilingual group showed a performance of 67.5%, which was near the median of 70% for the total cohort; and the postlingual group showed a significantly (p < 0.05) poorer performance, at 57.5% (Fig. 5A).
The time delay between the onset of hearing loss and cochlear implantation was evaluated for four groups: a short delay group (≤2 years; n = 33; 27%), a medium-short delay group (3–5 years; n = 14; 11%), a medium-long delay group (6–10 years; n = 8; 7%), and a relatively large long delay group (>10 years; n = 68; 55%). The short delay group had a significantly (p < 0.01) better performance of 80%, at the upper quartile. The medium-short delay group also had an outcome of 80% but did not reach significance. The medium-long delay group showed a decrease, with an outcome of 60%, which also was not significant. However, the large long delay group showed a significant (p < 0.05) decline, with an outcome of 55% (Fig. 5A).
The grades of hearing loss for implanted ears were analyzed for three different preoperative grades: SHL (n = 5; 4%), PHL (n = 24; 20%), and CHL (n = 93; 76%). The relatively small SHL group showed an outcome of only 35% but did not reach significance due to the small sample size. The PHL group had an outcome of 55%, and the large CHL group performed at 70% (Fig. 5A).
The laterality of hearing loss was assessed for three different groups, consisting of two small groups of unilateral (n = 4; 3%) and asymmetric (n = 2; 2%) hearing loss and a very large group with bilateral symmetric hearing loss (n = 117; 95%). The unilateral group had an outcome of 0%, and the asymmetric group achieved an outcome of 62.5%. The bilateral symmetric group performed at 70%. None of the laterality groups reached significance (Fig. 5A).
The sequence of implantation was evaluated for three different groups consisting of the first ear only (n = 64; 52%), the second ear only (n = 34; 28%), and the simultaneous bilateral implantation (n = 32; 26%) groups. The first ear only group had an outcome of 60%, and the second ear only group had an outcome of 62.5%, while the simultaneous bilateral implantation group demonstrated a significantly (p < 0.05) better result of 80%, at the upper quartile (Fig. 5A).
In summary, among the genetic factors, the outcome for the neural-genetic group fell below the lower quartile into the lower quarter related to “poor performance” or a “negative outcome.” Also, the neural-genetic group was the only genetic group with a significantly poorer result than all four other nonneural genetic groups. In relation to nongenetic clinical factors, all other significant median values for clinical parameters remained above the value of the neural-genetic group.
When using 76 subjects instead of 123 ears as independent items, the median WRS65 dropped from 70% to 65%. As can be expected for a reduction of the number of items, the p values generally increased for most of the investigated comparisons. For the neural-genetic group, the probability for a random result increased from p = 0.014 to p = 0.091 and thus can be regarded as a trend at p ≤ 0.1; for the “age at implantation,” the favorable factor “0 to 2 years” was reduced to p = 0.055, and thus can also be regarded as a trend at p ≤ 0.1; the nonfavorable factor “3 to 6 years” increased to p = 0.047 and thus reached significance; in the “hearing loss onset” group, none of the three categories reached a trend or significance; in the “delay” group, the favorable factor “≤2 years” and the nonfavorable factor “>10 years” did not reach a trend or significance. The favorable factor “3 to 5 years” became significant at p = 0.011. For the sequence of implantation, the simultaneous implantation was reduced to a trend with p = 0.091 (Supplemental Digital Content 5, http://links.lww.com/EANDH/B161).
For further statistical investigation, we condensed the 30 detailed categories shown in Figure 5A into 10 broader categories representing the significant outcome categories of the first analysis, as shown in Figure 5A. We investigated these with 123 ears as items entering the analysis. These 10 outcome categories are represented in five putative factor groups that can assume two values each. These five putative factor groups are designated as: (1) Gene expression: - neural versus other nonneural genes; (2) age at implantation: 0 to 6 years of age versus >6 years; (3) onset of hearing loss: congenital versus noncongenital (pre/peri/postlingual); (4) implantation delay (gap): 0 to 5 versus >5 years; (5) sequence of implantation: simultaneous bilateral implantation versus subsequent bilateral or monaural only implantation. The median WRS65 results for the five groups relate to known or at least expected effects: the first outcome category of each of the five groups (e.g., neural expression) always corresponds to the expected nonfavorable category, the second outcome category to the expected favorable category (Fig. 5B; Supplemental Digital Content 5, http://links.lww.com/EANDH/B161).
Welch-ANOVA treating these 10 factors as independent variables resulted in 5 significant factors influencing variability, namely “neural genetic,” age at implantation: >6 years, hearing loss onset: noncongenital (pre/peri/postlingual), implantation delay: >5 years, sequence of implantation: monaural or subsequent bilateral implantation (but not simultaneous bilateral implantation). These five categories all represented detrimental factors leading to poorer performance and had highly significant effects (p < 0.001), accounting for a total of 11.8% of the observed variance. The single strongest category was neural gene expression accounting for 3.1% of the variance. The other expected nonfavorable categories contributed 2.3% for age at implantation: >6 years, 2.3 % for hearing loss onset: noncongenital (pre/peri/postlingual); 2.4% for delay of implantation: >5 years; 1.7% for the sequence of implantation: nonsimultaneous bilateral or monaural (Supplemental Digital Content 5, http://links.lww.com/EANDH/B161).
When repeating the Welch-ANOVA for the above-mentioned reduced set of 10 categories with n = 76 subjects instead of n = 123 ears, almost the same set of factors contributed significantly to the observed variance with similar numbers: 3.4% for neural genetic; 2.2% for age at implantation: >6 years; 1.9% for hearing loss onset: noncongenital; 2.3% for delay of implantation: >5 years. However, for the sequence of implantation, simultaneous implantation explained 1.7% of the variance as a favorable factor, whereas for n = 123 ears, nonsimultaneous implantation explained 1.7% of the variance as an adverse factor. Altogether, for the reduced number of items with n = 76 subjects instead of n = 123 ears, 11.5% of the variance was explained by a similar set of factors (Supplemental Digital Content 5, http://links.lww.com/EANDH/B161).
DISCUSSION
Phenotypic Characteristics of the Cohort
This study presents findings from a large German genetically defined CI cohort from the regional population of southwest Germany. As may be expected for a CI cohort, there was a shift in the mean age toward almost a decade younger age (mean age: 35.7 years; year 2021) than the mean age of the regional population of southwest Germany (mean age: 43.8 years; year 2020; State Statistical Office Baden-Württemberg 2021). This shift is mainly based on a high proportion (more than one-fifth) of pediatric probands (21.1%; 16 out of 76) in their first decade of life, while the proportions of probands in the other age groups in the following decades appear to be representative of the age distribution of the general population (Fig. 1A). In a recent study on a hereditary hearing loss cohort from our Hearing Center that included 305 unrelated hearing-impaired probands/families with a suspected hereditary hearing loss etiology, the mean age (42.4 years; year 2018) was very close to the mean age of the population in southwest Germany at the same reference date (mean age: 43.5 years; year 2018) (State Statistical Office Baden-Württemberg 2021), and the age distribution was very similar to that of the regional population (Tropitzsch et al. 2022; includes n = 48 patients in the present study). However, this represents the mean age of all 305 probands, including those patients with a suspected hereditary hearing loss etiology who remained without a genetically defined diagnosis.
Interestingly, in this hereditary hearing loss cohort, the calculated mean age for the solved cases only (n = 75) was considerably lower (mean age: 29.8 years; year 2018) than that of the total cohort (mean age: 43.5; year 2018) (Tropitzsch et al. 2021, 2022). This shift toward a younger mean age is based on a relatively high proportion (32%; n = 24 out of 75) of pediatric probands in their first decade among the solved cases (Tropitzsch et al. 2022), which is similar to the proportion in the present study (21.1%; 16 out of 76). The diagnostic rate for hereditary hearing loss is well established to be highly sensitive to phenotypic characteristics (Sloan-Heggen et al. 2016). As recently confirmed, single phenotypic characteristics, such as autosomal-recessive inheritance, early hearing loss onset, and a hearing loss grade of CHL, significantly elevate the diagnostic rate up to 60% (Tropitzsch et al. 2022). These phenotypic characteristics lead to a high diagnostic rate and select for younger patients and CI candidacy. Thus, the relatively young mean age of the present genetic CI cohort is more likely related to the phenotypic characteristics that increase the probability of a confirmed genetic diagnosis rather than the context of CI use.
The genetic component of the cohort is also reflected in the distribution of hearing loss onset, with the majority of patients presenting with an early hearing loss onset in the congenital and pre/perilingual domains (≤6 years) (63.2%; n = 48 out of 76) rather than with late hearing loss onset in the postlingual domain (>6 years) (36.8%; n = 28 out of 76) (Fig. 1B). This two-to-one ratio of early versus late hearing loss onset was also seen in another genetic CI cohort. In a Japanese genetic CI cohort of 84 probands with a confirmed genetic diagnosis, approximately two-thirds had early hearing onset (65.5%; n = 55 out of 84), and one-third had late hearing loss onset (34.5%; n = 29 out of 84) (Miyagawa et al. 2016). However, in other genetic CI studies, the relation between early and late-onset with a confirmed genetic diagnosis is less well defined (Park et al. 2014, 2017) or even shows an inverse ratio (Seligman et al. 2021).
As expected for a CI cohort, the preoperative hearing loss grade for implanted ears (n = 123) was predominantly complete hearing loss, which was present in three-quarters (76%; n = 93) of the implanted ears at the time of implantation, while the moderately severe to profound grades were present in only one-quarter (24%; n = 30) of the implanted ears. This distribution of preoperative hearing loss grades is in accordance with national guidelines (Dazert et al. 2020; Zahnert et al. 2020) and international indication criteria (Papsin & Gordon 2007; Carlson 2020). The age at implantation ranged from < 1 year to octogenarians, which is similar to national and international practice (Carlson et al. 2010, 2018; Dazert et al. 2020; Zahnert et al. 2020).
The time delay between hearing loss onset and cochlear implantation ranged from very short (<1 year) to very long time-intervals (up to 7 decades). While the short delay group (≤2 years of time delay) had a significantly (p < 0.01) better performance of 80% in the WRS65 at the upper quartile, the long delay group (>10 years of time delay) showed a significant (p < 0.05) decline, with an outcome of 55% in the WRS65. This outcome corresponds to the known poor speech perception outcomes that occur due to relatively late timing of cochlear implantation in relation to the onset of SHL in postlingual adults (Dowell 2016). In children with an early (congenital, pre/perilingual) hearing loss onset, this is even more critical since early implantation after the onset of profound to complete hearing loss may take advantage of the neuronal plasticity inherent to critical time periods of auditory pathway development (Sharma et al. 2002). Accordingly, the rate of speech comprehension and expression was shown to depend on the age of implantation, with more favorable outcomes in children undergoing cochlear implantation before 18 months of age (Niparko et al. 2010).
In the present study, the majority of cochlear implantations for the first implanted ear occurred within the first decade after hearing loss onset (52.6%; 50 out of 95), and relatively few implantations for the second implanted ear occurred within that first decade (14.3%; 4 out of 28). Interestingly, first implanted ears performed at 60%, and second implanted ears at 67.5% in the WRS65 (Fig. 5A). Both categories showed no significant difference from the median total performance at 70%. In this context, it is important to note that the success of sequential bilateral implantation performance for the second implanted ear does not necessarily depend on a short interval between the first and second ear implantations (Smulders et al. 2017).
Overall Performance of the Genetic CI Cohort
The overall median performance of all cochlear implantations (n = 123) for this genetic CI cohort was calculated at a median of 70% for the WRS65. For the pediatric and adolescent patients (n = 42 implanted ears; n = 23 probands; age 6–17 years), the median WRS65 was noted at 80%, and for adult patients (n = 81 implanted ears; n = 53 probands; age ≥ 18 years), it was noted at 60%.
For pediatric and adolescent implanted ears, long-term results (80% WRS65; at ≥ 5 years after implantation) were within the range of long-term outcomes in other German pediatric CI cohorts (Lesinski-Schiedat et al. 2004, 2006; Illg et al. 2017; Dazert et al. 2020). For the adult cases, the performance can be related to the largest German adult CI cohort to date (n = 1005) from a single center (Hannover) that reached a mean performance of 47.7% ± 30.7% WRS65 1 year after implantation and then remained stable at a plateau over a period of 22 years (Lenarz et al. 2012). In the present genetic CI study, the WRS65 of adult probands was also acquired at least 1 year after implantation in the plateau phase for this speech test, allowing for a comparison between the two cohorts. Another more recent German adult multicenter CI cohort with clinically representative mixed etiologies using a single implant type reached a mean WRS65 of 54.9 ± 24.8% and a median of 55.0% (n = 147) at 6 months postimplantation (Hey et al. 2020). The median of 60% for the WRS65 found in the present adult genetic CI cohort is slightly (5%–10%) higher than the reported values of these two reference studies. This finding correlates with the result of a large international multicenter cohort showing that postlingual CI probands with a genetic etiology (n = 232 out of 2251) collectively performed significantly above the average of all etiologies (Blamey et al. 2013).
A comprehensive Japanese study compared the cochlear implantation outcomes of patients with hearing loss due to a nonsyndromic genetic etiology with those of patients with hearing loss due to other etiologies. For early hearing loss onset (prelingual), no statistically significant differences were observed between the group with a defined nonsyndromic genetic hearing loss etiology and the group with other hearing loss etiologies. For late hearing loss onset (postlingual), no statistically significant differences were found between the group with defined genetic hearing loss etiology and the group with other hearing loss etiologies, but the group with defined genetic hearing loss etiology tended to show better outcomes than the other etiology group (Miyagawa et al. 2016).
In summary, a defined genetic hearing loss etiology may lead to cochlear implantation outcomes at or above the average level of mixed hearing loss etiologies for both pediatric and adult cohorts. Nevertheless, the general prognostic value based on the evaluation of genetic factors as a contributing component to cochlear implantation outcomes is limited. As demonstrated in this study CI outcomes in a genetically defined background range from very poor to excellent performance (median of 0%–97.5% for the WRS65). This wide range of results is due to the complex and heterogeneous background of hereditary hearing loss at both the molecular-genetic and clinical levels. To address the variability question and to reach a better understanding of the association between more specific genetic etiologies and cochlear implantation outcomes, a single gene performance analysis appears to be appropriate as the next step.
CI Performance Ranking Based on Single Genes Affected
The six most frequently affected genes covered one-half of the diagnoses and involved the genes GJB2 (18%; 14 out of 76 probands), TMPRSS3 (8%; 6 out of 76 probands), MYO15A (7%; 5 out of 76 probands), SLC26A4, LOXHD1, and USH2A (each 4%; 3 out of 76 probands). Much larger and possibly multicenter cohorts will have to be investigated to address a single gene-based discussion in the remaining group of more rarely affected genes.
In an American study involving 128 genetically defined CI probands and 44 causative genes, the six most frequently affected genes were GJB2 (16%), TMPRSS3 (10%), SLC26A4 (8%), MYO7A (7%), MT-RNR1 (6%), and POU4F3 (5%), which also covered one-half of the diagnoses (Seligman et al. 2021). Ethnicity is unlikely to account for the differences, as non-Hispanic white participants represented 98% percent of the American cohort (Seligman et al. 2021). Similarly, 86% percent of the present cohort were of European ethnicity. In a Japanese genetic CI cohort, the most frequently affected genes were GJB2, CDH23, SLC26A4, OTOF, MYO7A, and TMPRSS3 in the pre- and postlingual domains (Miyagawa et al. 2016; Nishio & Usami 2017; Usami et al. 2020). The genes GJB2, TMPRSS3, and MYO15A were identified as the three most frequently affected genes in the present German cohort, and GJB2 and TMPRSS3 overlapped as the most frequently affected genes in the American (Seligman et al. 2021) and Japanese (Miyagawa et al. 2016) cohorts. These three most frequently affected single genes GJB2, TMPRSS3, and MYO15A were also representative of the three large expression clusters of the present study. GJB2 was representative of the combined structural genes of the Tectorial Membrane and Cochlear Duct cluster (eight genes; 37%, n = 45 implanted ears), TMPRSS3 of the Neural cluster (eight genes; 19%, n = 23 implanted ears) and MYO15A of the Hair Cell cluster (12 genes; 42%, n = 51 implanted ears).
In the present study, the most frequently affected gene, GJB2 (18%; 14 out of 76 probands or 21%; n = 26 out of 123 implanted ears), showed a median performance of 80% in the WRS65. The expression pattern for GJB2 was classified as a structural gene in the cluster of the Cochlear Duct. GJB2 is the most frequently affected hereditary hearing loss gene worldwide, with a reported prevalence of 17.3% in the world, 27.1% in Europe, and 14.1% in Germany (Chan & Chang 2014). Two recent meta-analyses on GJB2-related pediatric cochlear implantation outcomes showed a significantly better outcome for a GJB2 etiology than for acquired environmental etiologies. However, GJB2 cochlear implantation outcomes were not significantly better than those for other nonsyndromic hereditary hearing loss (genetic diagnosis unknown and genetically defined) etiologies in the absence of neurological deficits (Abdurehim et al. 2017; Nishio & Usami 2017). The median of 80% for the WRS65 in GJB2-related hearing loss in the present study is the same as the 80% for the WRS65 for all pediatric genetic cases and confirms an equivalent performance of GJB2 compared to other genetic causes with a similar phenotypic background in the present cohort. Correspondingly, a recent review also noted that no report in the literature stated a poorer-than-average CI outcome for GJB2 (Usami et al. 2020).
The second most frequently involved gene in the present genetic CI cohort was the autosomal-recessive gene TMPRSS3 (DFNB8/10) (8%; n = 6 out of 76 probands or 7%; n = 9 out of 123 implanted ears) in the Neural expression cluster. TMPRSS3 showed a median performance of only 30% in the WRS65, which is below the lower quartile of 45% and is designated as “poor performance.” Consistent with other CI genetic studies in a Japanese cohort (Miyagawa et al. 2016) and an American cohort (Seligman et al. 2021) TMPRSS3 is one of the most frequently affected genes. TMPRSS3 is known for its association with a variable hearing loss onset related to two phenotypes that are based on different types of mutations. These two phenotypes comprise a severe early hearing loss onset prelingual type (DFNB10) and a milder late hearing loss onset postlingual progressive type (DFNB8) (Weegerink et al. 2011). There were no pediatric patients with an early hearing loss onset in whom TMPRSS3 was the affected gene in either the American (Seligman et al. 2021) or our own cohort.
TMPRSS3 is a member of the type II transmembrane serine protease family. The function of TMPRSS3 has been associated with the activation of ENaC-mediated currents as a candidate substrate in the inner ear (Guipponi et al. 2002) and the expression of Kcnma1 potassium channel in the inner hair cells of mice (Molina et al. 2013). In the human cochlea, we recently localized the expression of TMPRSS3 in the cytoskeleton of inner and outer pillar cells, Deiters cells and in subcuticular organelles of outer hair cells, suggesting that TMPRSS3 proteolysis is linked to hair and supporting cell function in the organ of Corti (Liu et al. 2018). Experimentally, in a mouse model with the protein-truncating nonsense mutation Tmprss3Y260X, compromised gene function resulted in rapid early developmental hair cell loss starting at postnatal day 12, at hearing onset, followed by late spiral ganglion degeneration at 4 months of age (Fasquelle et al. 2011). This early progressive degeneration pattern of hair cells observed in this mouse model is consistent with the human progressive high-frequency hearing loss phenotype of DFNB8/10 (Weegerink et al. 2011) and its expression in the human organ of Corti (Liu et al. 2018).
In the present study, the TMPRSS3 expression pattern was classified in the Neural gene cluster, assuming its expression and functional relevance in the neural tissue components of the cochlea. This classification is based on the expression of Tmprss3 mRNA and protein in spiral ganglion neurons (Guipponi et al. 2002, 2008; Nishio et al. 2015) and the observation of a late-onset loss of spiral ganglion neurons in the Tmprss3Y260X mouse model (Fasquelle et al. 2011). Therefore, it appears plausible that the observed poor CI performance is related to a late loss of spiral ganglion neurons. However, CI outcomes in TMPRSS3 patients are highly variable and include favorable outcomes (Elbracht et al. 2007; Weegerink et al. 2011; Eppsteiner et al. 2012; Miyagawa et al. 2013, 2015a, 2016; Battelino et al. 2016; Shearer et al. 2017; Holder, Morrel, et al. 2021). Interestingly, an electrocochleography study demonstrated that auditory nerve neurophonic responses were reduced in probands affected by pathogenic variants in TMPRSS3 compared to those in probands with genetic variants affecting the sensory part of the cochlea. These results imply that pathogenic variants in TMPRSS3 impact the function of the spiral ganglion (Shearer et al. 2018). However, a recent study of a pediatric case series showed more favorable cochlear implantation outcomes (Holder et al. 2021) than the reported adult cochlear implantation outcomes (Eppsteiner et al. 2012; Shearer et al. 2017), including the present study. This suggests that cochlear implantation outcomes in TMPRSS3 may be age-sensitive (Holder et al. 2021). Furthermore, two recent reports compiled the results of published CI cases of patients with pathogenic TMPRSS3 variants and described “positive outcomes” (Moon et al. 2021) or “favorable outcomes” (Chen et al. 2022). An analysis of Tmprss3/TMPRSS3 in mouse/human cochlear specimens revealed a limited expression in spiral ganglion neurons. This finding questioned the functional relevance of spiral ganglion expression of TMPRSS3 in the context of cochlear implantation and raised the argument that duration of deafness appeared as a key factor predictive of poor performance in TMPRSS3 cases in the literature (Chen et al. 2022).
Other genes classified in the Neural cluster, such as SOX10 and PAX3, demonstrated excellent performance. SOX10 and PAX3 are associated with Waardenburg syndrome, and a recent review described that in most cases, CI outcomes are similar to those for nonsyndromic hearing loss patients (Nishio & Usami 2017).
The third most frequently affected gene in this genetic CI cohort was MYO15A (DFNB3) (7%; 5 out of 76 probands or 7%; 8 out of 123 implanted ears) in the Hair Cell expression cluster. MYO15A showed a median performance of 77.5% in the WRS65, which is close to the upper quartile of 80% of the total cohort and higher than the median performance for the Hair Cell cluster of 70%. MYO15A was among the second most frequently affected genes (5%) in our previous genetic cohort (Tropitzsch et al. 2022), which is in line with similar proportions in the results of a Dutch genetic hearing loss cohort (5.9%) (Zazo Seco et al. 2017) and a large Iowa genetic hearing loss cohort (4.8%) (Sloan-Heggen et al. 2016). MYO15A was also represented in the pediatric segment of the Japanese genetic CI cohort (3%) (Miyagawa et al. 2016) and the American genetic CI cohort (8%) (Seligman et al. 2021) as expected for an autosomal-recessive gene and early hearing loss onset (Usami & Nishio 2021). Previous reports on cochlear implantation outcomes in probands affected by pathogenic variants in MYO15A showed similar favorable outcomes (Miyagawa et al. 2013, 2015b; Shearer et al. 2017). The “good outcome” (upper middle quarter) for MYO15A is representative of the Hair Cell expression cluster although single genes in the same cluster such as CDH23 and LOXHD1 have shown poor outcomes. Other reports in the literature describe “favorable” outcomes for both CDH23 (Usami et al. 2012) and LOXHD1 (Eppsteiner et al. 2012).
CI Performance Comparing Neural and Nonneural Clusters
Based on the assumptions of the “spiral ganglion hypothesis,” mutations in genes that primarily affect cochlear sensory tissue elements and synapses are associated with good cochlear implantation outcomes since CIs bypass these structures and do not depend on their functional status. However, mutations in genes that primarily or in addition to the sensory partition affect the spiral ganglion and/or the auditory nerve show poor CI performance, since the electrical signal of the CI cannot be received or transformed into neural activity (Eppsteiner et al. 2012; Shearer et al. 2017; Shearer & Hansen 2019). In the present cohort, the combination of single genes in five cochlear expression clusters allowed a comprehensive comparison. This comparison revealed surprisingly uniform median outcomes of 70% to 80% for the WRS65 for all four nonneural clusters (Hair cells, Tectorial Membrane and Cochlear Duct, Stria Vascularis, and Mitochondria) at or near the upper quartile of 80% of the total cohort. In a surprisingly large divergence, the Neural cluster performing at the lower quartile of 45% of the total cohort strongly supported the “spiral ganglion hypothesis.” However, the distribution of outcomes in the Neural cluster is very broad and ranges from 0% to 100% for individual implanted ears. The two genes PAX3 and SOX10 that contributed 22% (n = 5 out of 23) to the Neural cluster demonstrated “excellent performance” of 90% to 95%.
It is important to note that the lesion sites of the “ANSD” are not equivalent to the spiral ganglion and auditory nerve but are defined by objective audiological measures that distinguish sensory hearing loss from four different entities of synaptic and neural hearing loss under the ANSD definition (Rance et al. 2015). In detail, the diagnostic criteria for ANSDs allow to differentiate ANSDs according to the site of the lesion into (1) presynaptic disorders affecting inner hair cells and ribbon synapses; (2) postsynaptic disorders affecting unmyelinated auditory nerve dendrites; (3) postsynaptic disorders affecting auditory spiral ganglion cells and their myelinated axons and dendrites; and (4) central neural pathway disorders affecting the auditory brainstem (Rance & Starr 2015). While the anatomical structures of the sites in categories (1) and (2) are bypassed by the CI, the sites in categories (3) and (4) are relevant to the optimal function of a CI and may have implications for preoperative counseling on treatment expectations (Shearer & Hansen 2019).
Genetic variants of genes expressed in the relevant neural domain of the peripheral auditory pathway in categories (3) and (4) of ANSD have been demonstrated to significantly affect adult cochlear implantation outcomes (Shearer et al. 2017)—in the following termed the “Iowa cohort.” This result was based on the identification of causative mutations leading to a genetic diagnosis in the seven genes OPA1, DIAPH3, DFNB59, AIFM1, TBC1D24, MT-RNR1, or TMPRSS3 associated with a spiral ganglion type auditory neuropathy or spiral ganglion expression (Nishio et al. 2015; Moser & Starr 2016). In addition, variants in the same seven genes predicted to be deleterious by combined annotation-dependent depletion score analysis (Kircher et al. 2014) were classified as a neural-genetic etiology. In the study of the “Iowa cohort” (Shearer et al. 2017), the total genetic diagnostic rate was 17% (27 of 155 patients). When re-analyzing their data by the same statistical approach used in the present study (for details, see Supplemental Digital Content 5, http://links.lww.com/EANDH/B161) (Shearer et al. 2017) we identified three significant factors in their study [bilateral sudden sensorineural hearing loss (favorable), sensory genetic (favorable), and SSD (nonfavorable)] that accounted for 11.6% of the variance. The pathogenic “genetic load” in spiral ganglion cells of the “neural-genetic” in their data accounted for 1.4% (p = 0.146) of the total variance but was not significant.
In the present study, 23 of 123 implanted ears contributed to the “Neural cluster” of the cohort, and all were based on a definitive genetic diagnosis that excludes diagnostic uncertainty based on combined annotation-dependent depletion score analysis. According to the ANOVA analysis of our data (Supplemental Digital Content 5, http://links.lww.com/EANDH/B161), we identified five significant factors including a nonfavorable neural genetic etiology and demographic factors that added to 11.8% of the observed variance. Thus, the analysis explained almost the same percentage of variance as did the significant factors in the data of Shearer et al. (2017) (11.6%).
Importantly, in the present study, the neural-genetic etiology turned out to be a significant factor and also the largest factor, accounting for 3.1% (p < 0.001) of the variance. However, if we combine both genetic categories, that is, take the nonsignificant nonneural genes category (p = 0.10; variance: 0.4%) into account, the explained variance sums up to 3.5%, whereas in the Iowa cohort, both, the nonsignificant neural-genetic (see above) and the significant sensory-genetic category (p = 0.022; variance: 3.5%) summed up to 4.9% for genetic etiologies. One potential reason for the observed difference might be related to the inclusion of a different set of genes assigned to the analyses of both studies (Supplemental Digital Content 5, http://links.lww.com/EANDH/B161). As shown in Figure 4, the neural-genetic category of the present study included five ears related to two genes (PAX3, SOX10) with surprisingly good performance. These two genes were not included in the neural-genetic group of the Iowa cohort (Shearer et al. 2017).
Study Limitations
Although this retrospective study is based on one of the largest CI cohorts with a defined genetic diagnosis, the number of ears and subjects is still rather low in relation to the high number of 30 investigated parameters in the statistical analysis. When using 76 subjects instead of 123 ears as items entering the statistical analysis, the significance of all seven significant parameters vanished, but three of these factors remained at least at a trend of p ≤ 0.01. On the other hand, two new favorable factors gained significance. When inspecting the distributions, it appears to be plausible that the reason for this shift in outcome is partly due to the group of unilateral implantations which comprises four cases of 0% WRS65, which now gain weight when reducing the dataset from 123 items to 76 items. This also contributed to the reduction of the median WRS65 from 70% to 65%. The results for the condensed analysis on a reduced number of 10 broader categories remained more robust, when reducing the number of items from 123 ears to 76 subjects. Almost the same amount of variance is explained by five factors. In only one factor group, namely the sequence of implantation, the significant contributing factor switched from the nonfavorable factor “unilateral and sequential implantation (1 and 2 ears)” to the favorable factor “simultaneous implantation,” putatively due to the reduction in the median WRS65. Noteworthy, the neural-genetic group remained the strongest factor with 3.4% (instead of 3.1% for the 123 ears) of the explained variance.
Whether the analysis should be based on ears or subjects as items is debatable: The genetic groups (i.e., for genetic factors/mode of inheritance) may inflict the same influence on both ears within the same subject. This implies that subjects should be used as items in a CI genetic study. However, even for a defined genetic diagnosis, the hearing status may vary significantly between both ears and exhibit phenotypes of AHL or SSD (Tropitzsch et al. 2022). Furthermore, many other clinical factor groups (i.e., for hearing loss onset, delay of implantation, laterality, implantation sequence) may vary significantly between both ears within the same subject. This implies that individual ears should be treated as separate items.
Another limitation of this study is that the relationship between CI processor use time and CI performance was not included in the CI variance analysis. CI processor use time is an important clinical factor for communication skills in children (Busch et al. 2020; Wiseman et al. 2021) and speech recognition in adults (Schvartz-Leyzac et al. 2019; Holder et al. 2021a). Due to the very long inclusion period for cochlear implantations over the course of 25 years between 1997 and 2021 CI data logging information was not available for a large proportion of the probands.
Nevertheless, compared to previously identified clinical factors (Lazard et al. 2012; Blamey et al. 2013) and clinical parameters included in the present study, neural-genetic factors are confirmed as a significant and substantial predictive factor for cochlear implantation outcome (Shearer et al. 2017; Shearer & Hansen 2019).
CONCLUSION
Despite the complex interaction between genotype and clinical phenotype the relationship of molecular genetic diagnoses and cochlear implantation outcomes in a large German cohort of CI recipients revealed significant variabilities. Poor performance was observed with genetic mutations that affected the neural components of the cochlea, supporting the “spiral ganglion hypothesis.” Neural genetic factors accounted for 3.1% of the total variance and represented a significant and the single largest factor within the total of the observed variance of 11.8% including other significant demographic and clinical factors. Future extension of cohort size may allow addressing CI outcomes at the single gene and possibly variant level.
ACKNOWLEDGMENTS
We thank the patients and their families for participating in our study, as well as the personnel at the Department of OHNS Tübingen, Praxis für Humangenetik Tübingen and CeGaT GmbH, Tübingen, Germany, for technical assistance.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and text of this article on the journal’s Web site (www.ear-hearing.com).
The authors have no conflicts of interest to disclose.
Variants have been submitted to ClinVar under the accession IDs xxx (www.ncbi.nlm.nih.gov/clinvar/).
This publication is dedicated to the memory of our dear colleague and friend Dr. Marcus Müller, who unexpectedly died during the course of this project.
Saskia Dofek is currently at the Department of Otolaryngology-Head & Neck Surgery, Facial Plastic Surgery, St. Vincentius-Kliniken, Steinhäuserstraße 18, 76135 Karlsruhe, Germany. Barbara Vona is currently at the University Medical Center Göttingen Institute of Human Genetics and Institute for Auditory Neuroscience & InnerEarLab, Heinrich-Düker-Weg 12, 37073 Göttingen, Germany.
M.M. is deceased.
M.H., A.T., T.S.-M., and H.L. conceived the study; B.S., S.F., M.S., F.B., and S.B. were involved in the experimental design; P.G., T.S.-M., B.S., M.S., S.F., T.H., P.S., H.L., and B.V. analyzed the sequencing data or were involved in data curation; E.D., J.H., A.L.-S., A.B., T.L., A.W., and T.S.-M. analyzed audiological data; A.T., M.M., T.S.-M., and H.L. prepared the original draft; A.T., H.L., T.S.-M., E.D., J.H., A.L.-S., A.B., T.L., A.W., and T.H. reviewed and edited the manuscript; E.D. and A.H. performed statistical analysis and generated figures; H.L. and A.T. provided supervision. All authors—except M.M.—have read and approved the final revised manuscript.
REFERENCES
- Sprache für Gehörprüfung - Teil 1: Ein- und mehrsilbige Wörter (German). In G. I. f. S. R. n. c. D. G.-H. DIN, Audiometer und Kuppler (Ed.): Beuth Verlag GmbH. [Google Scholar]
- Abdurehim Y., Lehmann A., Zeitouni A. G. (2017). Predictive value of GJB2 mutation status for hearing outcomes of pediatric cochlear implantation. Otolaryngol Head Neck Surg, 157, 16–24. [DOI] [PubMed] [Google Scholar]
- Abidin F. N. Z., Wells H. R. R., Altmann A., Dawson S. J. (2021). Hearing difficulty is linked to Alzheimer’s disease by common genetic vulnerability, not shared genetic architecture. npj Aging Mech Dis, 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci E., Nauwelaers T., Lenarz T., Hamacher V., Kral A. (2014). Variations in microanatomy of the human cochlea. J Comp Neurol, 522, 3245–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaiez H., Booth K. T., Ephraim S. S., Crone B., Black-Ziegelbein E. A., Marini R. J., Shearer A. E., Sloan-Heggen C. M., Kolbe D., Casavant T., Schnieders M. J., Nishimura C., Braun T., Smith R. J. H. (2018). Genomic landscape and mutational signatures of deafness-associated genes. Am J Hum Genet, 103, 484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard J. M., Fisher L. M., Johnson K. C., Eisenberg L. S., Wang N. -Y., Quittner A. L., Carson C. M., Niparko J. K.; CDaCI Investigative Team. (2015). A prospective longitudinal study of U.S. children unable to achieve open-set speech recognition 5 years after cochlear implantation. Otol Neurotol, 36, 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelino S., Klancar G., Kovac J., Battelino T., Trebusak Podkrajsek K. (2016). TMPRSS3 mutations in autosomal recessive nonsyndromic hearing loss. Eur Arch Otorhinolaryngol, 273, 1151–1154. [DOI] [PubMed] [Google Scholar]
- Blamey P., Artieres F., Başkent D., Bergeron F., Beynon A., Burke E., Dillier N., Dowell R., Fraysse B., Gallégo S., Govaerts P. J., Green K., Huber A. M., Kleine-Punte A., Maat B., Marx M., Mawman D., Mosnier I., O’Connor A. F., O’Leary S., et al. (2013). Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol, 18, 36–47. [DOI] [PubMed] [Google Scholar]
- Blamey P. J., Maat B., Başkent D., Mawman D., Burke E., Dillier N., Beynon A., Kleine-Punte A., Govaerts P. J., Skarzynski P. H., Huber A. M., Sterkers-Artières F., Van de Heyning P., O’Leary S., Fraysse B., Green K., Sterkers O., Venail F., Skarzynski H., Vincent C., et al. (2015). A Retrospective Multicenter Study comparing speech perception outcomes for bilateral implantation and bimodal rehabilitation. Ear Hear, 36, 408–416. [DOI] [PubMed] [Google Scholar]
- Bodmer D., Shipp D. B., Ostroff J. M., Ng A. H. C., Stewart S., Chen J. M., Nedzelski J. M. (2007). A comparison of postcochlear implantation speech scores in an adult population. Laryngoscope, 117, 1408–1411. [DOI] [PubMed] [Google Scholar]
- Busch T., Vanpoucke F., van Wieringen A. (2017). Auditory environment across the life span of cochlear implant users: Insights from data logging. J Speech Lang Hear Res, 60, 1362–1377. [DOI] [PubMed] [Google Scholar]
- Carlson L., M. (2020). Cochlear implantation in adults. N Engl J Med, 382, 1531–1542. [DOI] [PubMed] [Google Scholar]
- Carlson L., M., Breen J. T., et al. (2010). Cochlear implantation in the octogenarian and nonagenarian. Otol Neurotol, 31, 1343–1349. [DOI] [PubMed] [Google Scholar]
- Carlson L., M., Sladen D. P., et al. (2018). Survey of the American Neurotology Society on Cochlear Implantation: Part 1, candidacy assessment and expanding indications. Otol Neurotol, 39, e12–e19. [DOI] [PubMed] [Google Scholar]
- Chan D. K., Chang, K W. (2014). GJB2-associated hearing loss: Systematic review of worldwide prevalence, genotype, and auditory phenotype. Laryngoscope, 124, E34–E53. [DOI] [PubMed] [Google Scholar]
- Chen Y. S., Cabrera E., Tucker B. J., Shin T. J., Moawad J. V., Totten D. J., Booth K. T., Nelson R. F. (2022). TMPRSS3 expression is limited in spiral ganglion neurons: Implication for successful cochlear implantation. J Med Genet, 59, 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. Y. C., Dillon H., Button L., Seeto M., Van Buynder P., Marnane V., Cupples L., Leigh G. (2017). Age at intervention for permanent hearing loss and 5-year language Outcomes. Pediatrics, 140, e20164274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba A., Corazzi V., Negossi L., Tazzari R., Bianchini C., Aimoni C. (2017). Moderate-severe hearing loss in children: A diagnostic and rehabilitative challenge. J Int Adv Otol, 13, 407–413. [DOI] [PubMed] [Google Scholar]
- Claes A. J., Van de Heyning P., Gilles A., Van Rompaey V., Mertens G. (2018). Cognitive performance of severely hearing-impaired older adults before and after cochlear implantation: Preliminary results of a prospective, longitudinal cohort study using the RBANS-H. Otol Neurotol, 39, e765–e773. [DOI] [PubMed] [Google Scholar]
- Dazert S., Thomas J. P., Loth A., Zahnert T., Stöver T. (2020). Cochlear implantation. Dtsch Arztebl Int, 117, 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsches Institut für Normung. (1995). DIN 45626-1:1995-07 Tonträger mit Sprache für Gehörprüfung, Teil1: Tonträger mit Wörtern nach DIN45621-1. Beuth. [Google Scholar]
- Dowell R. C. (2016). The case for earlier cochlear implantation in postlingually deaf adults. Int J Audiol, 55, S51–S56. [DOI] [PubMed] [Google Scholar]
- Elbracht M., Senderek J., Eggermann T., Thürmer C., Park J., Westhofen M., Zerres K. (2007). Autosomal recessive postlingual hearing loss (DFNB8): Compound heterozygosity for two novel TMPRSS3 mutations in German siblings. J Med Genet, 44, e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppsteiner R. W., Shearer A. E., Hildebrand M. S., Deluca A. P., Ji H., Dunn C. C., Black-Ziegelbein E. A., Casavant T. L., Braun T. A., Scheetz T. E., Scherer S. E., Hansen M. R., Gantz B. J., Smith R. J. H. (2012). Prediction of cochlear implant performance by genetic mutation: The spiral ganglion hypothesis. Hear Res, 292, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi A. A., Polineni S. P., Davies C., Shahal D., Mittal J., Al-Zaghal Z., Sinha R., Jindal U., Mittal R. (2020). Genotype-phenotype correlation for predicting cochlear implant outcome: Current challenges and opportunities. Front Genet, 11, 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasquelle L., Scott H. S., Lenoir M., Wang J., Rebillard G., Gaboyard S., Venteo S., François F., Mausset-Bonnefont A. -L., Antonarakis S. E., Neidhart E., Chabbert C., Puel J. -L., Guipponi M., Delprat B. (2011). Tmprss3, a transmembrane serine protease deficient in human DFNB8/10 deafness, is critical for cochlear hair cell survival at the onset of hearing. J Biol Chem, 286, 17383–17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Hearing Loss Expert Group, Stevens G., Flaxman S., Brunskill E., Mascarenhas M., Mathers C. D., Finucane M., et al. (2013). Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur J Public Health, 23, 146–152. [DOI] [PubMed] [Google Scholar]
- GBD Hearing Loss Collaborators. (2021). Hearing loss prevalence and years lived with disability, 1990-2019: Findings from the Global Burden of Disease Study 2019. Lancet, 397, 996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemeinsamer Bundesausschuss. (2021). Hilfsmittel-Richtlinie; Richtlinie über die Verordnung von Hilfsmitteln in der vertragsärztlichen Versorgung. In G. B. Federal Joint Committee (Ed.), BAnz AT 15.04.2021 B3. https://www.g-ba.de/downloads/62-492-2467/HilfsM-RL_2021-03-18_iK-2021-04-01.pdf. [Google Scholar]
- Guipponi M., Vuagniaux G., Wattenhofer M., Shibuya K., Vazquez M., Dougherty L., Scamuffa N., Guida E., Okui M., Rossier C., Hancock M., Buchet K., Reymond A., Hummler E., Marzella P. L., Kudoh J., Shimizu N., Scott H. S., Antonarakis S. E., Rossier B. C. (2002). The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet, 11, 2829–2836. [DOI] [PubMed] [Google Scholar]
- Guipponi M., Antonarakis S. E., Scott H. S. (2008). TMPRSS3, a type II transmembrane serine protease mutated in non-syndromic autosomal recessive deafness. Front Biosci, 13, 1557–1567. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K. H. (1957). Sprachaudiometrie: Grundlagen und Praktische Anwendung Einer Sprachaudiometrie für das Deutsche Sprachgebiet. Thieme. [Google Scholar]
- Hey M., Neben N., Stover T., Baumann U., Mewes A., Liebscher T., Schüssler M., Aschendorff A., Wesarg T., Büchner A., Greenham P., Hoppe U. (2020). Outcomes for a clinically representative cohort of hearing-impaired adults using the Nucleus(R) CI532 cochlear implant. Eur Arch Otorhinolaryngol, 277, 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden L. K., Finley C. C., Firszt J. B., Holden T. A., Brenner C., Potts L. G., Gotter B. D., Vanderhoof S. S., Mispagel K., Heydebrand G., Skinner M. W. (2013). Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear, 34, 342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. T., Gifford, R H. (2021a). Effect of increased daily cochlear implant use on auditory perception in adults. J Speech Lang Hear Res, 64, 4044–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. T., Morrel W., Rivas A., Labadie R. F., Gifford R. H. (2021b). Cochlear implantation and electric acoustic stimulation in children with TMPRSS3 genetic mutation. Otol Neurotol, 42, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe U., & Hast A. (2017). Speech audiometry for indication of conventional and implantable hearing aids. HNO, 65, 195–202. [DOI] [PubMed] [Google Scholar]
- Hoppe U., Hast A., Hocke T. (2015). Audiometry-based screening procedure for cochlear implant candidacy. Otol Neurotol, 36, 1001–1005. [DOI] [PubMed] [Google Scholar]
- Hoppe U., Hocke T., Hast A., Hornung J. (2017). Longterm results of a screening procedure for adult cochlear implant candidates. Laryngorhinootologie, 96, 234–238. [DOI] [PubMed] [Google Scholar]
- Hoppe U., Hocke T., Hast A., Iro H. (2019). Maximum preimplantation monosyllabic score as predictor of cochlear implant outcome. HNO, 67, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe U., Hocke T., Hast A., Iro H. (2021). Cochlear implantation in candidates with moderate-to-severe hearing loss and poor speech perception. Laryngoscope, 131, E940–E945. [DOI] [PubMed] [Google Scholar]
- Ida-Eto M., Ohgami N., Iida M., Yajima I., Kumasaka M. Y., Takaiwa K., Kimitsuki T., Sone M., Nakashima T., Tsuzuki T., Komune S., Yanagisawa M., Kato M. (2011). Partial requirement of endothelin receptor B in spiral ganglion neurons for postnatal development of hearing. J Biol Chem, 286, 29621–29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illg A., Haack M., Lesinski-Schiedat A., Büchner A., Lenarz T. (2017). Long-term outcomes, education, and occupational level in cochlear implant recipients who were implanted in childhood. Ear Hear, 38, 577–587. [DOI] [PubMed] [Google Scholar]
- Kantarci H., Edlund R. K., Groves A. K., Riley B. B. (2015). TFAP2A promotes specification and maturation of neurons in the inner ear through modulation of BMP, FGF and notch signaling. PLoS Genet, 11, e1005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M., Witten D. M., Jain P., O’Roak B. J., Cooper G. M., Shendure J. (2014). A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet, 46, 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmeier B., Lenarz T., Kiessling J., et al. (2014). Contribution to the discussion surrounding the Freiburg speech test. HNO, 62, 49–53. [DOI] [PubMed] [Google Scholar]
- Kompis M., Krebs M., Hausler R. (2006). Verification of normative values for the Swiss version of the Freiburg speech intelligibility test. HNO, 54, 445–450. [DOI] [PubMed] [Google Scholar]
- Korver A. M., Smith R. J., Van Camp G., Schleiss M. R., Bitner-Glindzicz M. A. K., Lustig L. R., Usami S.-I., Boudewyns A. N. (2017). Congenital hearing loss. Nat Rev Dis Primers, 3, 16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A. (2017). Pathophysiology of hearing loss: Classification and treatment options. HNO, 65, 290–297. [DOI] [PubMed] [Google Scholar]
- Krueger B., Joseph G., Rost U., Strauss-Schier A., Lenarz T., Buechner A. (2008). Performance groups in adult cochlear implant users: speech perception results from 1984 until today. Otol Neurotol, 29, 509–512. [DOI] [PubMed] [Google Scholar]
- Lazard D. S., Vincent C., Venail F., et al. (2012). Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: A new conceptual model over time. PLoS One, 7, e48739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J., Dettman S., Dowell R., Sarant J. (2011). Evidence-based approach for making cochlear implant recommendations for infants with residual hearing. Ear Hear, 32, 313–322. [DOI] [PubMed] [Google Scholar]
- Leigh J. R., Moran M., Hollow R., Dowell R. C. (2016). Evidence-based guidelines for recommending cochlear implantation for postlingually deafened adults. Int J Audiol, 55(Suppl 2), S3–S8. [DOI] [PubMed] [Google Scholar]
- Lenarz T., & Laszig R. (2012). AWMF-Leitlinie Cochlea-Implantat-Versorgung und zentral-auditorische Implantate 05/2012. Register-Nr. 017–071.
- Lenarz M., Sönmez H., Joseph G., Büchner A., Lenarz T. (2012). Long-term performance of cochlear implants in postlingually deafened adults. Otolaryngol Head Neck Surg, 147, 112–118. [DOI] [PubMed] [Google Scholar]
- Lesinski-Schiedat A., Illg A., Heermann R., Bertram B., Lenarz T. (2004). Paediatric cochlear implantation in the first and in the second year of life: A comparative study. Cochlear Implants Int, 5, 146–159. [DOI] [PubMed] [Google Scholar]
- Lesinski-Schiedat A., Illg A., Warnecke A., Heermann R., Bertram B., Lenarz T. (2006). Paediatric cochlear implantation in the first year of life: Preliminary results. HNO, 54, 565–572. [DOI] [PubMed] [Google Scholar]
- Liu W., Lowenheim H., Santi P. A., Glueckert R., Schrott-Fischer A., Rask-Andersen H. (2018). Expression of trans-membrane serine protease 3 (TMPRSS3) in the human organ of corti. Cell Tissue Res, 372, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S. G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., Cooper C., Fox N., Gitlin L. N., Howard R., Kales H. C., Larson E. B., Ritchie K., Rockwood K., Sampson E. L., Samus Q., et al. (2017). Dementia prevention, intervention, and care. Lancet, 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., Costafreda S. G., Dias A., Fox N., Gitlin L.N., Howard R., Kales H. C., Kivimäki M., Larson E. B., Ogunniyi A., Orgeta V. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens G., Andries E., Claes A. J., Topsakal V., Van de Heyning P., Van Rompaey V., Calvino M., Sanchez Cuadrado I., Muñoz E., Gavilán J., Bieńkowska K., Świerniak W., Skarżyński P. H., Skarżyński H., Tapper L., Killan C., Ridgwell J., McGowan J., Raine C., Tavora-Vieira D., et al. (2021). Cognitive improvement after cochlear implantation in older adults with severe or profound hearing impairment: A Prospective, Longitudinal, Controlled, Multicenter Study. Ear Hear, 42, 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B. L., Thorp J. G., Evans D. M., Nyholt D. R., Martin N. G., Lupton M. K. (2020). Exploring the genetic relationship between hearing impairment and Alzheimer’s disease. Alzheimers Dement (Amst), 12, e12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa M., Nishio S. Y., Ikeda T., Fukushima K., Usami S.-I. (2013). Massively parallel DNA sequencing successfully identifies new causative mutations in deafness genes in patients with cochlear implantation and EAS. PLoS One, 8, e75793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa M., Nishio S. Y., Sakurai Y., Hattori M., Tsukada K., Moteki H., Kojima H., Usami S.-I. (2015a). The patients associated with TMPRSS3 mutations are good candidates for electric acoustic stimulation. Ann Otol Rhinol Laryngol, 124, 193S–204S. [DOI] [PubMed] [Google Scholar]
- Miyagawa M., Nishio S. Y., Hattori M., Moteki H., Kobayashi Y., Sato H., Watanabe T., Naito Y., Oshikawa C., Usami S.-I. (2015b). Mutations in the MYO15A gene are a significant cause of nonsyndromic hearing loss: Massively parallel DNA sequencing-based analysis. Ann Otol Rhinol Laryngol, 124, 158S–168S. [DOI] [PubMed] [Google Scholar]
- Miyagawa M., Nishio S. Y., Usami S.-I. (2016). A comprehensive study on the etiology of patients receiving cochlear implantation with special emphasis on genetic epidemiology. Otol Neurotol, 37, e126–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly A. C., Bates C., Harris M. S., Pisoni D. B. (2016). The enigma of poor performance by adults with cochlear implants. Otol Neurotol, 37, 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina L., Fasquelle L., Nouvian R., Salvetat N., Scott H. S., Guipponi M., Molina F., Puel J.-L., Delprat B. (2013). Tmprss3 loss of function impairs cochlear inner hair cell Kcnma1 channel membrane expression. Hum Mol Genet, 22, 1289–1299. [DOI] [PubMed] [Google Scholar]
- Moon I. S., Grant A. R., Sagi V., Rehm H. L., Stankovic K. M. (2021). TMPRSS3 gene variants with implications for auditory treatment and counseling. Front Genet, 12, 780874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton C. C., Nance, W E. (2006). Newborn hearing screening—a silent revolution. N Engl J Med, 354, 2151–2164. [DOI] [PubMed] [Google Scholar]
- Moser T., & Starr A. (2016). Auditory neuropathy—neural and synaptic mechanisms. Nat Rev Neurol, 12, 135–149. [DOI] [PubMed] [Google Scholar]
- Mosnier I., Bebear J. P., Marx M., Fraysse B., Truy E., Lina-Granade G., Mondain M., Sterkers-Artières F., Bordure P., Robier A., Godey B., Meyer B., Frachet B., Poncet-Wallet C., Bouccara D., Sterkers O. (2015). Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg, 141, 442–450. [DOI] [PubMed] [Google Scholar]
- Neuhaus C., Lang-Roth R., Zimmermann U., Heller R., Eisenberger T., Weikert M., Markus S., Knipper M., Bolz H. J. (2017). Extension of the clinical and molecular phenotype of DIAPH1-associated autosomal dominant hearing loss (DFNA1). Clin Genet, 91, 892–901. [DOI] [PubMed] [Google Scholar]
- Niparko J. K. (2013). The significance of cochlear implant history. JAMA Otolaryngol Head Neck Surg, 139, 454. [DOI] [PubMed] [Google Scholar]
- Niparko J. K., Tobey E. A., Thal D. J., Eisenberg L. S., Wang N.-Y., Quittner A. L., Fink N. E.; CDaCI Investigative Team. (2010). Spoken language development in children following cochlear implantation. JAMA, 303, 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum E., Prentiss S., Yan D., Nourbakhsh A., Smeal M., Holcomb M., Cejas I., Telischi F., Liu X. Z. (2021). Screening strategies for deafness genes and functional outcomes in cochlear implant patients. Otol Neurotol, 42, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio S. Y., Hattori M., Moteki H., Tsukada K., Miyagawa M., Naito T., Yoshimura H., Iwasa Y. -I., Mori K., Shima Y., Sakuma N., Usami S. -I. (2015). Gene expression profiles of the cochlea and vestibular endorgans: Localization and function of genes causing deafness. Ann Otol Rhinol Laryngol, 124, 6S–48S. [DOI] [PubMed] [Google Scholar]
- Nishio S. Y., Usami, S I. (2017). Outcomes of cochlear implantation for the patients with specific genetic etiologies: A systematic literature review. Acta Otolaryngol, 137, 730–742. [DOI] [PubMed] [Google Scholar]
- Olusanya B. O., Davis A. C., Hoffman H. J. (2019). Hearing loss grades and the International classification of functioning, disability and health. Bull World Health Organ, 97, 725–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvis J., Gottfried B., Kancherla J., Adkins R. S., Song Y., Dror A. A., Olley D., Rose K., Chrysostomou E., Kelly M. C., Milon B., Matern M. S., Azaiez H., Herb B., Colantuoni C., Carter R. L., Ament S. A., Kelley M. W., White O., Bravo H. C., et al. (2021). gEAR: Gene Expression Analysis Resource portal for community-driven, multi-omic data exploration. Nat Methods, 18, 843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza A. M., DiStefano M. T., Hemphill S. E., Cushman B. J., Grant A. R., Siegert R. K., Shen J., Chapin A., Boczek N. J., Schimmenti L. A., Murry J. B., Hasadsri L., Nara K., Kenna M., Booth K. T., Azaiez H., Griffith A., Avraham K. B., Kremer H., Rehm H. L., et al. (2018). ClinGen Hearing Loss Clinical Domain Working Group. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum Mutat, 39, 1593–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papsin B. C., Gordon, K A. (2007). Cochlear implants for children with severe-to-profound hearing loss. N Engl J Med, 357, 2380–2387. [DOI] [PubMed] [Google Scholar]
- Park J. H., Kim N. K., Kim A. R., Rhee J., Oh S. H., Koo J. -W., Nam J. -Y., Park W. -Y., Choi B. Y. (2014). Exploration of molecular genetic etiology for Korean cochlear implantees with severe to profound hearing loss and its implication. Orphanet J Rare Dis, 9, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Kim A. R., Han J. H., Kim S. D., Kim S. H., Koo J. -W., Oh S. H., Choi B. Y. (2017). Outcome of cochlear implantation in prelingually deafened children according to molecular genetic etiology. Ear Hear, 38, e316–e324. [DOI] [PubMed] [Google Scholar]
- Powell D. S., Oh E. S., Reed N. S., Lin F. R., Deal J. A. (2021). Hearing loss and cognition: what we know and where we need to go. Front Aging Neurosci, 13, 769405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G., & Starr A. (2015). Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. Brain, 138, 3141–3158. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen H., Erixon E., Kinnefors A., Löwenheim H., Schrott-Fischer A., Liu W. (2011). Anatomy of the human cochlea—implications for cochlear implantation. Cochlear Implants Int, 12, S8–13. [DOI] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W. W., Hegde M., Lyon E., Spector E., Voelkerding K., Rehm H. L.; ACMG Laboratory Quality Assurance Committee. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 17, 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein W. S., Maglott D. R., Lee J. M., Kattman B. L., Malheiro A. J., Ovetsky M., Hem V., Gorelenkov V., Song G., Wallin C., Husain N., Chitipiralla S., Katz K. S., Hoffman D., Jang W., Johnson M., Karmanov F., Ukrainchik A., Denisenko M., Fomous C., et al. (2013). The NIH genetic testing registry: a new, centralized database of genetic tests to enable access to comprehensive information and improve transparency. Nucleic Acids Res, 41(Database issue), D925–D935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarant J., Harris D., Busby P., Maruff P., Schembri A., Dowell R., Briggs R. (2019). The effect of cochlear implants on cognitive function in older adults: Initial baseline and 18-month follow up results for a Prospective International Longitudinal Study. Front Neurosci, 13, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarant J., Harris D., Busby P., Maruff P., Schembri A., Lemke U., Launer S. (2020). The effect of hearing aid use on cognition in older adults: Can we delay decline or even improve cognitive function? J Clin Med, 9, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen C. J., Burmeister M., Lesperance M. M. (2013). Diaphanous homolog 3 (Diap3) overexpression causes progressive hearing loss and inner hair cell defects in a transgenic mouse model of human deafness. PLoS One, 8, e56520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac K. C., Conrad C. A., Zwolan T. A. (2019). Datalogging statistics and speech recognition during the first year of use in adult cochlear implant recipients. Otol Neurotol, 40, e686–e693. [DOI] [PubMed] [Google Scholar]
- Seligman K. L., Shearer A. E., Frees K., Nishimura C., Kolbe D., Dunn C., Hansen M. R., Gantz B. J., Smith R. J. H. (2022). Genetic causes of hearing loss in a large cohort of cochlear implant recipients. Otolaryngol Head Neck Surg, 166, 734–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn P., Roccio M., Hahnewald S., Frick C., Kwiatkowska M., Ishikawa M., Bako P., Li H., Edin F., Liu W., Rask-Andersen H., Pyykkö I., Zou J., Mannerström M., Keppner H., Homsy A., Laux E., Llera M., Lellouche J. P., Ostrovsky S., et al. (2017). NANOCI-Nanotechnology Based Cochlear Implant With Gapless Interface to Auditory Neurons. Otol Neurotol, 38, e224–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Dorman M. F., Spahr A. J. (2002). A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear, 23, 532–539. [DOI] [PubMed] [Google Scholar]
- Shearer A. E., Eppsteiner R. W., Frees K., Tejani V., Sloan-Heggen C. M., Brown C., Abbas P., Dunn C., Hansen M. R., Gantz B. J., Smith R. J. H. (2017). Genetic variants in the peripheral auditory system significantly affect adult cochlear implant performance. Hear Res, 348, 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer A. E., Tejani V. D., Brown C. J., Abbas P. J., Hansen M. R., Gantz B. J., Smith R. J. H. (2018). In vivo electrocochleography in hybrid cochlear implant users implicates TMPRSS3 in spiral ganglion function. Sci Rep, 8, 14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer A. E., Hansen M. R. (2019). Auditory synaptopathy, auditory neuropathy, and cochlear implantation. Laryngoscope Investig Otolaryngol, 4, 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Scheffer D. I., Kwan K. Y., Corey D. P. (2015). SHIELD: an integrative gene expression database for inner ear research. Database (Oxford), 2015, bav071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Heggen C. M., Bierer A. O., Shearer A. E., Kolbe D. L., Nishimura C. J., Frees K. L., Ephraim S. S., Shibata S. B., Booth K. T., Campbell C. A., Ranum P. T., Weaver A. E., Black-Ziegelbein E. A., Wang D., Azaiez H., Smith R. J. H. (2016). Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum Genet, 135, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders Y. E., Hendriks T., Eikelboom R. H., Stegeman I., Santa Maria P. L., Atlas M. D., Friedland P. L. (2017). Predicting sequential cochlear implantation performance: A Systematic Review. Audiol Neurootol, 22, 356–363. [DOI] [PubMed] [Google Scholar]
- State Statistical Office Baden-Württemberg. (2021). Bevölkerung Baden-Württembergs nach Alters- und Geburtsjahren (bev_altersjahre.csv). Bevölkerung in Baden-Württemberg nach Migrationshintergrund im engeren Sinn (MZ-LA-Migr-LR-WE.csv). Durchschnittsalter der Bevölkerung nach Geschlecht (01035100.csv). https://www.statistik-bw.de/BevoelkGebiet.
- Stevens G., Flaxman S., Brunskill E., Mascarenhas M., Mathers C. D., Finucane M.; Global Burden of Disease Hearing Loss Expert Group. (2013). Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur J Public Health, 23, 146–152. [DOI] [PubMed] [Google Scholar]
- Sukowski H., Brand T., Wagener K. C., Kollmeier B. (2009). Comparison of different speech intelligibility tests in German language (Freiburg speech test vs. Gottingen sentence test and monosyllabic rhyme test). HNO, 57, 239–250. [DOI] [PubMed] [Google Scholar]
- Thorpe R. K., Smith R. J. H. (2020). Future directions for screening and treatment in congenital hearing loss. Precis Clin Med, 3, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropitzsch A., Schade-Mann T., Gamerdinger P., Dofek S., Schulte B., Schulze M., Battke F., Fehr S., Biskup S., Heyd A., Müller M., Löwenheim H., Vona B., Holderried M. (2022). Diagnostic yield of targeted hearing loss gene panel sequencing in a large German cohort with a balanced age distribution from a single diagnostic center: An eight-year study. Ear Hear, 43, 1049–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trpchevska N., Freidin M. B., Broer L., Oosterloo B. C., Yao S., Zhou Y., Vona B., Bishop C., Bizaki-Vallaskangas A., Canlon B., Castellana F., Chasman D. I., Cherny S., Christensen K., Concas M. P., Correa A., Elkon R., Estonian Biobank Research Team., Mengel-From J., Gao Y., et al. (2022). Genome-wide association meta-analysis identifies 48 risk variants and highlights the role of the stria vascularis in hearing loss. Am J Hum Genet, 109, 1077–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S., Miyagawa M., Nishio S. Y., Moteki H., Takumi Y., Suzuki M., Kitano Y., Iwasaki S. (2012). Patients with CDH23 mutations and the 1555A>G mitochondrial mutation are good candidates for electric acoustic stimulation (EAS). Acta Otolaryngol, 132, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S. I., Nishio S. Y., Moteki H., Miyagawa M., Yoshimura H. (2020). Cochlear implantation from the perspective of genetic background. Anat Rec (Hoboken), 303, 563–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S. I., Nishio S. Y. (2021). The genetic etiology of hearing loss in Japan revealed by the social health insurance-based genetic testing of 10K patients. Hum Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyken E., Van Camp G., Van Laer L. (2007a). The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol, 12, 345–358. [DOI] [PubMed] [Google Scholar]
- Van Eyken E., Van Laer L., Fransen E., Topsakal V., Hendrickx J. J., Demeester K., Van de Heyning P., Mäki-Torkko E., Hannula S., Sorri M., Jensen M., Parving A., Bille M., Baur M., Pfister M., Bonaconsa A., Mazzoli M., Orzan E., Espeso A., Stephens D., et al. (2007). The contribution of GJB2 (Connexin 26) 35delG to age-related hearing impairment and noise-induced hearing loss. Otol Neurotol, 28, 970–975. [DOI] [PubMed] [Google Scholar]
- Van de Heyning P., Távora-Vieira D., Mertens G., Van Rompaey V., Rajan G. P., Müller J., Hempel J. M., Leander D., Polterauer D., Marx M., Usami S. I., Kitoh R., Miyagawa M., Moteki H., Smilsky K., Baumgartner W. D., Keintzel T. G., Sprinzl G. M., Wolf-Magele A., Arndt S., et al. (2016). Towards a Unified Testing Framework for Single-Sided Deafness Studies: A Consensus Paper. Audiol Neurootol, 21, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers D., De Raeve L., Graham J. (2016). International survey of cochlear implant candidacy. Cochlear Implants Int, 17, 36–41. [DOI] [PubMed] [Google Scholar]
- Volter C., Gotze L., Haubitz I., Dazert S., Thomas J. P. (2020). Benefits of cochlear implantation in middle-aged and older adults. Clin Interv Aging, 15, 1555–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vona B., Muller M., Dofek S., Holderried M., Löwenheim H., Tropitzsch A. (2019). A big data perspective on the genomics of hearing loss. Laryngorhinootologie, 98, S32–S81. [DOI] [PubMed] [Google Scholar]
- Weegerink N. J., Schraders M., Oostrik J., Huygen P. L. M., Strom T. M., Granneman S., Pennings R. J. E., Venselaar H., Hoefsloot L. H., Elting M., Cremers C. W. R. J., Admiraal R. J. C., Kremer H., Kunst H. P. M. (2011). Genotype-phenotype correlation in DFNB8/10 families with TMPRSS3 mutations. J Assoc Res Otolaryngol, 12, 753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman K. B., Warner-Czyz A. D., Kwon S., Fiorentino K., Sweeney M. (2021). Relationships between daily device use and early communication outcomes in young children with cochlear implants. Ear Hear, 42, 1042–1053. [DOI] [PubMed] [Google Scholar]
- Zahnert et al. (2020). Cochlea Implantat Versorgung AWMF-Register-Nr. 017/071 [Register-Nr. 017–071]. https://www.awmf.org/uploads/tx_szleitlinien/017-071l_S2k_Cochlea-Implantat-Versorgung-zentral-auditorische-Implantate_2020-12.pdf.
- zazo Seco C., Wesdorp M., Feenstra I., Pfundt R., Hehir-Kwa J. Y., Lelieveld S. H., Castelein S., Gilissen C., de Wijs I. J., Admiraal R. J., Pennings R. J., Kunst H. P., van de Kamp J. M., Tamminga S., Houweling A. C., Plomp A. S., Maas S. M., de Koning Gans P. A., Kant S. G., de Geus C. M., et al. (2017). The diagnostic yield of whole- exome sequencing targeting a gene panel for hearing impairment in The Netherlands. Eur J Hum Genet, 25, 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.