Abstract
Context
Crinecerfont, a corticotropin-releasing factor type 1 receptor antagonist, has been shown to reduce elevated adrenal androgens and precursors in adults with congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21OHD), a rare autosomal recessive disorder characterized by cortisol deficiency and androgen excess due to elevated adrenocorticotropin.
Objective
To evaluate the safety, tolerability, and efficacy of crinecerfont in adolescents with 21OHD CAH.
Methods
This was an open-label, phase 2 study (NCT04045145) at 4 centers in the United States. Participants were males and females, 14 to 17 years of age, with classic 21OHD CAH. Crinecerfont was administered orally (50 mg twice daily) for 14 consecutive days with morning and evening meals. The main outcomes were change from baseline to day 14 in circulating concentrations of ACTH, 17-hydroxyprogesterone (17OHP), androstenedione, and testosterone.
Results
8 participants (3 males, 5 females) were enrolled; median age was 15 years and 88% were Caucasian/White. After 14 days of crinecerfont, median percent reductions from baseline to day 14 were as follows: ACTH, −57%; 17OHP, −69%; and androstenedione, −58%. In female participants, 60% (3/5) had ≥50% reduction from baseline in testosterone.
Conclusion
Adolescents with classic 21OHD CAH had substantial reductions in adrenal androgens and androgen precursors after 14 days of oral crinecerfont administration. These results are consistent with a study of crinecerfont in adults with classic 21OHD CAH.
Keywords: congenital adrenal hyperplasia, 21-hydroxylase deficiency, crinecerfont, CRF type 1 receptor antagonist, adolescents, pediatric
Classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21OHD) is a rare autosomal recessive disorder that results in impaired synthesis of cortisol and oftentimes aldosterone (1, 2). The lack of cortisol feedback on the hypothalamus and pituitary leads to the increased secretion of adrenocorticotropic hormone (ACTH) and the shunting of accumulated cortisol precursors toward adrenal androgen production. The resulting excess in adrenal androgens contributes to advanced bone age in children and adolescents, precocious puberty, and ultimately to decreased final adult height (2–4). Classic 21OHD CAH requires an individualized therapeutic approach based on each patient's clinical symptoms and their response to exogeneous glucocorticoids (GCs) and other treatments. GC therapies are used to replace cortisol deficiency and control excess androgen production (1, 5); however, supraphysiologic doses of GCs are typically needed for disease control, which can lead to long-term complications (5, 6).
In patients with classic 21OHD CAH, production of cortisol is disrupted, resulting in a buildup of androgen precursors such as 17-hydroxyprogesterone (17OHP) and androstenedione. This is driven by the loss of normal negative feedback by cortisol on the hypothalamus and pituitary, which results in excessive ACTH synthesis, release, and levels in the systemic circulation. Since corticotropin releasing factor (CRF) stimulates ACTH production, medications that antagonize the type 1 CRF receptor may be effective in treating 21OHD CAH (7). Crinecerfont, an orally administered, CRF type 1 (CRF1) receptor antagonist, has been shown in adults with 21OHD CAH to reduce ACTH, 17OHP, androstenedione in males and females, testosterone in females, and androstenedione/testosterone ratio (A4/T) in males (8). This reduction was particularly apparent during the early-morning hours when the normal rise in ACTH is exaggerated and leads to markedly increased production of androgen precursors.
Since supraphysiologic GC dosing is primarily needed to treat the androgen excess in 21OHD CAH, CRF1 receptor antagonism could potentially decrease adrenal androgens and allow patients to reduce their GC dose to more physiologic levels, thereby reducing the short- and long-term consequences of supraphysiologic GC exposure (9, 10). Building on the prior study in adults with classic 21OHD CAH, this phase 2 study (NCT04045145) was conducted to explore the safety, tolerability, and efficacy of crinecerfont in adolescents with classic 21OHD CAH.
Materials and Methods
Study Design and Participants
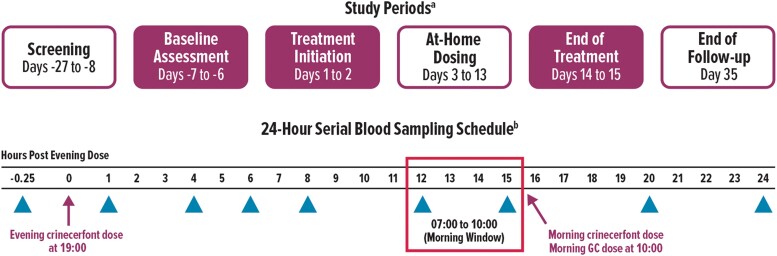
This phase 2, open-label study evaluated oral crinecerfont 50 mg, administered twice daily for 14 days in adolescents with classic 21OHD CAH (Fig. 1). The study drug dose was selected based on pediatric estimations incorporating allometric scaling that indicated the exposure of crinecerfont in adolescents would be similar to 100 mg in adult patients with CAH that was safe and well tolerated in a 2-week, open-label study (8). The study was conducted in accordance with Good Clinical Practice guidelines at 4 study centers in the United States with approval by the Institutional Review Board at each site. Participating patients provided assent and their parents or legal guardians provided written informed consent before any study-related procedures were initiated.
Figure 1.
Study design. aShaded boxes indicate overnight stay at study center for 24-hour serial blood sampling. bNo crinecerfont dose was administered on days −7/−6 (baseline visit). However, sample collection timepoints during this overnight stay were the same as days 1/2 and 14/15 (postbaseline visits). Triangles indicate timepoints when blood samples were collected. GC, glucocorticoid.
Key inclusion criteria were as follows: male or female, 14-17 years of age; diagnosis of classic 21OHD CAH, confirmed by hormonal and/or molecular testing; 17OHP ≥800 ng/dL, ACTH ≥20 pg/mL, and cortisol <5 μg/dL prior to the morning GC dose at screening; body weight ≥10th percentile for age and sex; and stable GC regimen for ≥30 days prior to study entry. Participants remained on their current GC regimen throughout the study.
Key exclusion criteria were as follows: known or suspected diagnosis of other forms of CAH (eg, 11β-hydroxylase deficiency, 17α-hydroxylase deficiency, 3β-hydroxysteroid dehydrogenase deficiency, P450 side-chain cleavage deficiency, and P450 oxidoreductase deficiency); prior or current medical condition requiring daily GC therapy other than classic 21OHD CAH; concomitant use of strong cytochrome P450 3A4 inhibitors or inducers, strong cytochrome P450 2B6 inhibitors or inducers, anxiolytics, anticoagulants, or antiplatelet therapies during the study; QT interval adjusted with Fridericia's correction ≥450 ms or other significant cardiac abnormality; clinically significant unstable medical condition, chronic disease, or malignancy; clinically significant illness within 30 days before screening; currently pregnant or lactating; history of substance abuse or dependence within 3 months before study entry; or significant risk of suicidal or violent behavior.
Procedures and Assessments
During a 21-day screening period to determine study eligibility (Fig. 1), blood samples were collected between 07:00 and 10:00. On the evening of day −7, eligible participants were admitted overnight at their study site for a 24-hour serial sampling procedure to obtain baseline ACTH, 17OHP, androstenedione, and testosterone concentration profiles.
Participants were admitted on day 1 for a second overnight stay, with the first oral dose of crinecerfont 50 mg administered at 19:00 with an evening meal. On day 2, participants received crinecerfont doses with morning and evening meals (at 10:00 and 19:00, respectively) prior to being discharged from the study site. On days 3 through 13 with parent/guardian supervision, participants continued to receive crinecerfont 50 mg twice daily with meals in the morning and evening at approximately 07:00 and 19:00, respectively. Participants were again admitted overnight to their study site on day 14 to receive their last dose of crinecerfont, which was administered at 19:00 with an evening meal, and to undergo another 24-hour serial sampling procedure. For all 3 overnight inpatient admissions (ie, days −7/–6 [“baseline”], days 1/2 [“day 1”], and days 14/15 [“day 14”]), blood samples were collected at 18:45, 20:00, 23:00, 01:00, 03:00, 07:00, 10:00, 15:00, and 19:00, corresponding to 15 minutes prior to the evening crinecerfont dose and 1, 4, 6, 8, 12, 15, 20, and 24 hours after the evening dose. Participants' usual morning GC dose (on days −6, 2, 15) and morning dose of study drug (on day 2) were delayed until after 10:00 for all serial blood sampling procedures. Samples were analyzed for the following: plasma ACTH; serum 17OHP, androstenedione, testosterone, and cortisol; and crinecerfont concentration to assess pharmacokinetics (for details see 11).
Treatment-emergent adverse events (TEAEs) were assessed throughout the study. Additional safety assessments, including clinical laboratory tests, vital signs, physical examination, electrocardiogram, the Brief Psychiatric Rating Scale for Children, and the Columbia-Suicide Severity Rating Scale were performed at the study center on days 1, 7, 14, and 35 (end of follow-up).
Statistical Analyses
The sample size was based on practical clinical considerations for a rare disease, without statistical power calculations. Data were analyzed using descriptive statistics for all participants who received at least 1 dose of study drug.
For height, weight, and body mass index (BMI), Z-scores were calculated using a program from the Centers for Disease Control and Prevention (CDC) to compare each participant with an age- and sex-specific population of adolescents in the United States (12, 13). Efficacy analyses were based on participants' ACTH, 17OHP, androstenedione, and testosterone values, at baseline and day 14, over the 24-hour sampling period, and during the “morning window,” calculated by averaging the 2 samples collected at 07:00 and 10:00. This morning window was selected to isolate and evaluate the effect of crinecerfont on the early-morning surge of ACTH and the resulting rise in adrenal androgens. Analyses included median change from baseline to day 14 (24-hour period and morning window); median percent change from baseline to day 14 (24-hour period and morning window); proportion of participants with ≥50% reduction from baseline (morning window); and proportion of male participants with elevated A4/T (≥0.5) at baseline who achieved a target ratio of <0.5 at day 14 (morning window) (8, 9).
Post hoc responder analyses (based on morning window values) included the proportion of participants with elevated androstenedione or testosterone (for females), defined as exceeding 1.2 times the upper limit of normal at baseline, who returned to normal values at day 14. Crinecerfont exposure was assessed over a 12-hour dosing interval following the last dose to estimate the total daily exposure as area under the concentration–time curve from 0 to 24 hours (AUC0-24h).
Results
Participants
Eight participants were enrolled in this study, all of whom completed the study and received 14 days of 50 mg twice daily crinecerfont treatment. Median crinecerfont exposure (AUC0-24h) was 25.5 μg·hour/mL.
Of the 8 participants, 5 (63%) were female and 7 (88%) were Caucasian/White; ages ranged from 14 to 16 years (Table 1). Based on age- and sex-specific 2-20 years growth charts from the CDC, the median Z-scores for height and weight prior to starting crinecerfont were 0.2 and 0.7, respectively. The median Z-score for BMI (based on height at screening and weight on day 1) was 1.2. Three (38%) participants were between the 85th and 95th percentiles for BMI, corresponding to the CDC threshold for “overweight” in children and adolescents (14). Two (25%) additional participants were above the 95th percentile, corresponding to the CDC threshold for “obese.” Of the 5 female participants, 4 (80%) were postmenarchal, with a median age of 14 years at menarche and a median menstrual cycle length of 28 days.
Table 1.
Participant demographics and clinical characteristicsa
| All participants (n = 8) | |
|---|---|
| Age, years | 15 (14, 16) |
| Female, n (%) | 5 (63) |
| Race, n (%) | |
| Whiteb | 7 (88) |
| Asian | 1 (13) |
| Height at screening, cm | 165 (155, 175) |
| Z-scorec | 0.2 (−2.1, 0.8) |
| Weight on day 1, kg | 62 (52, 115) |
| Z-scorec | 0.7 (−0.4, 2.8) |
| Body mass index, kg/m2 | 25 (19, 38) |
| Z-scorec | 1.2 (−0.2, 2.6) |
| Number of adrenal crises within past 2 years | 0 (0, 1) |
| Age at menarche—females, yearsd | 14 (13, 14) |
| Menstrual cycle interval—females, daysd | 28 (21, 56) |
| Glucocorticoid treatment at screening, n (%) | |
| Hydrocortisone alone | 6 (75) |
| Prednisone alone | 2 (25) |
| Glucocorticoid dose (hydrocortisone equivalente), mg/m2/day | 16.2 (11.9, 18.5) |
Values are presented as median (minimum, maximum) unless indicated otherwise.
Includes 1 participant who also self-identified as Hispanic or Latino.
Centers for Disease Control Growth Chart used as reference, with Z-scores based on chronological age.
Based on available data for postmenarchal females (n = 4).
Hydrocortisone equivalents were calculated as 1 mg prednisone = 4 mg hydrocortisone.
At baseline, 6 (75%) participants were receiving hydrocortisone alone and 2 (25%) were receiving prednisone alone (Table 1). No participants were taking dexamethasone. The median GC dose in hydrocortisone equivalents was 16.2 mg/m2/day, based on a hydrocortisone equivalency factor of 4 for prednisone. Seven (88%) participants were also receiving fludrocortisone.
One participant was considered to have an important protocol deviation for having taken their morning GC dose prior to the blood sample collection at 10:00 on day 2. This participant was included in all baseline and day 14 analyses. No important COVID-related protocol deviations were reported.
Effects of Crinecerfont on ACTH, Adrenal Androgens, and Androgen Precursors
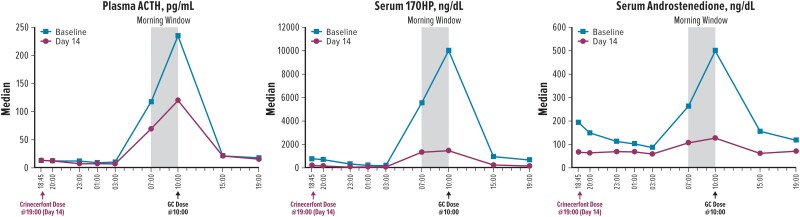
The 24-hour profiles of ACTH, 17OHP, and androstenedione at baseline showed expectedly high circulating concentrations, peaking around 10:00, after which the morning GC dose was administered (Fig. 2). After 14 days of treatment with crinecerfont 50 mg twice daily, participants had substantially lower concentrations of ACTH, androstenedione, and 17OHP throughout the day, particularly during the morning window period before the morning GC dose (Fig. 2).
Figure 2.
24-hour concentration profiles. 17OHP, 17-hydroxyprogesterone; ACTH, adrenocorticotropic hormone; GC, glucocorticoid.
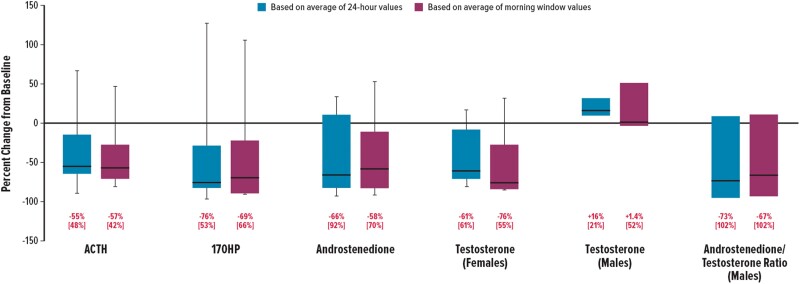
Median ACTH, 17OHP, androstenedione, testosterone (in females), and A4/T (in males) decreased from baseline to day 14, regardless of whether the analysis was based on the 24-hour period or the morning window (Table 2). For ACTH, 17OHP, and androstenedione, median percent decreases ranged from −55% to −76% for the 24–hour period and from −57% to −69% for the morning window (Fig. 3). Testosterone (in females) also decreased substantially, with median percent reductions of −61% and −76% in the 24-hour period and the morning window, respectively. In males, a high A4/T can indicate a greater proportion of testosterone originating from the adrenal glands vs the testes. After 14 days of crinecerfont treatment, testosterone did not decrease in male participants; however, androstenedione decreased in both female and male participants (Fig. 3). As a result, a median percent A4/T reduction of –67% was found among male participants during the morning window. There were no clinically meaningful changes in cortisol exposure for the participants treated with hydrocortisone.
Table 2.
Changes in adrenal androgen and precursor concentrationsa
| Median (IQR)b | 24-Hour periodc | Morning windowd | ||||
|---|---|---|---|---|---|---|
| At baseline (BL) | At day 14 (D14) | Change from BL to D14 | At baseline (BL) | At day 14 (D14) | Change from BL to D14 | |
| ACTH, pg/mL | 59 (113) | 34 (18) | −28 (79) | 226 (377) | 74 (100) | −127 (224) |
| 17OHP, ng/dL | 2740 (3850) | 668 (1277) | −1965 (3543) | 7704 (7123) | 1789 (3847) | −6265 (7425) |
| Androstenedione, ng/dL | 216 (277) | 80 (98) | −105 (273) | 368 (393) | 134 (184) | −236 (391) |
| Testosterone (females), ng/dL | 26 (116) | 25 (18) | −16 (109) | 64 (270) | 40 (40) | −53 (247) |
| Testosterone (males), ng/dL | 187 (67) | 245 (63) | 28 (33) | 222 (140) | 332 (128) | 3 (120) |
| Androstenedione/testosterone ratio (males) | 1.6 (1.9) | 0.2 (1.6) | −0.4 (2.5) | 1.9 (1.3) | 0.3 (1.9) | −0.6 (2.3) |
Abbreviations: 17OHP, 17-hydroxyprogesterone; ACTH, adrenocorticotropic hormone; IQR, interquartile range (Q3-Q1); SI, International System of Units.
Upper limits of normal for adolescents aged 13-17 are as follows: ACTH, 55 pg/mL; 17OHP (females), 208 ng/dL; 17OHP (males), 140-192 ng/dL; androstenedione (females), 200-212 ng/dL; androstenedione (males), 94-113 ng/dL; total testosterone (females), 76 ng/dL; total testosterone (males), 800-827 ng/dL. For SI calculation, conversion factors are as follows: ACTH (pg/mL to pmol/L), 0.22; 17OHP (ng/dL to nmol/L), 0.0303; androstenedione (ng/dL to nmol/L), 0.0349; testosterone (ng/dL to nmol/L), 0.0347.
The number of participants included for analyses were as follows: ACTH, 17OHP, and androstenedione, n = 8; testosterone (females), n = 5; testosterone (males) and androstenedione/testosterone ratio (males), n = 3.
Based on each participant's average values from all timepoints in the serial blood sampling period (from 18:45 [pre-dose] to 19:00 the following day).
Based on each participant's average values from the morning window timepoints (07:00, 10:00).
Figure 3.
Percent reductions from baseline to day 14. Boxes represent the IQR: lower edge (25th percentile), upper edge (75th percentile), horizontal bar (median). Whiskers extend beyond the box to the minimum and maximum values. Data below represent median [IQR] values, except for the testosterone and androstenedione/testosterone ratio in males, where data represent median [minimum and maximum] values (n = 3). 17OHP, 17-hydroxyprogesterone; ACTH, adrenocorticotropic hormone; IQR, interquartile range.
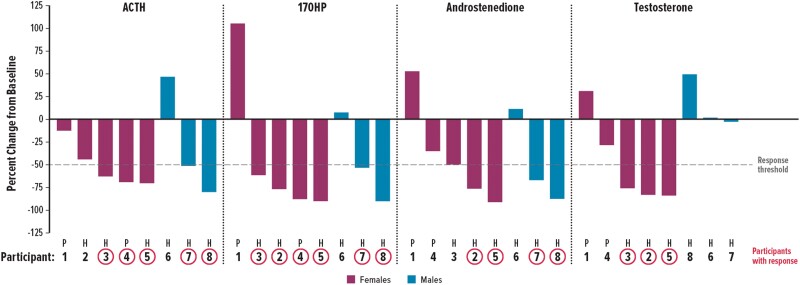
One-half to three-quarters of participants achieved ≥50% reduction from baseline to day 14 in morning window values for ACTH, 17OHP, and androstenedione (Table 3). Of the 5 female participants, 3 (60%) had ≥50% reduction in testosterone. Of the 3 male participants with A4/T ≥ 0.5 at baseline, 2 (67%) achieved a target ratio of <0.5 by day 14. Three participants achieved ≥50% reductions in all 3 biomarkers (ie, ACTH, 17OHP, androstenedione) (Fig. 4). Only 1 (13%) participant had 17OHP concentrations <500 ng/dL during the morning window, before the morning GC dose.
Table 3.
Proportion of participants who achieved ≥50% reduction in morning window hormone values or a return to normal values
| Parameter, n/N (%) | All participants |
|---|---|
| ≥50% reduction from baseline | |
| ACTH | 5/8 (63) |
| 17-Hydroxyprogesterone | 6/8 (75) |
| Androstenedione | 4/8 (50) |
| Testosterone (females) | 3/5 (60) |
| Androstenedione/testosterone ratio (males) | 2/3 (67) |
| Return to normal valuesa | |
| Androstenedione | 3/6 (50) |
| Testosterone (females) | 1/2 (50) |
| Androstenedione/testosterone ratio (males) | 2/3 (67) |
Abbreviations: ACTH, adrenocorticotropic hormone; ULN, upper limit of normal.
In the subset of participants who had baseline androstenedione or (female) testosterone values >1.2 × ULN or (male) androstenedione/testosterone ratio ≥0.5 at baseline. ULN was defined as follows: androstenedione (females aged 14-15), 200 ng/dL; androstenedione (females aged 16-17), 212 ng/dL; androstenedione (males aged 14-15), 94 ng/dL; androstenedione (males aged 16-17), 113 ng/dL; total testosterone (females), 76 ng/dL; total testosterone (males aged 14-15), 800 ng/dL; total testosterone (males aged 16-17), 827 ng/dL; and androstenedione/testosterone ratio (males), 0.5.
Figure 4.
Percent changes from baseline to day 14 in individual participants. Based on morning window (ie, average of values taken from 07:00 and 10:00). For glucocorticoid therapy, 6 participants received hydrocortisone (H) and 2 received prednisone (P). 17OHP, 17-hydroxyprogesterone; ACTH, adrenocorticotropic hormone.
Safety
A total of 12 TEAEs were reported in 6 participants (Table 4). All of these were judged by the investigator to be mild in intensity, with no serious TEAEs, discontinuations due to TEAEs, or deaths. Headache (n = 2) was the only TEAE reported in more than 1 participant. Two TEAEs, headache and dizziness (in 1 participant each), were judged by the investigator as “possibly” related to study drug. The headache was described as intermittent, and the dizziness resolved in 1 day; neither TEAE resulted in a change in crinecerfont dosing. No clinically significant findings were observed in routine laboratory tests, vital signs, electrocardiograms, or neuropsychiatric assessments.
Table 4.
Treatment-emergent adverse events
| All participants (n = 8) | |
|---|---|
| TEAE summary, n (%) | |
| Any TEAE | 6 (75) |
| Any serious TEAE | 0 |
| Any TEAE leading to discontinuation | 0 |
| Any TEAE resulting in death | 0 |
| All TEAEs by MedDRA preferred term, n (%) | |
| Headachea | 2 (25) |
| Arthropod sting | 1 (13) |
| Blepharospasm | 1 (13) |
| Dermatitis contact | 1 (13) |
| Dizzinessa | 1 (13) |
| Frequent bowel movements | 1 (13) |
| Gastritis | 1 (13) |
| Myalgia | 1 (13) |
| Nasopharyngitis | 1 (13) |
| Pyrexia | 1 (13) |
| Vomiting | 1 (13) |
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event.
Mild headache and mild dizziness (each in 1 participant) were judged by the investigator as “possibly” related to study drug.
Discussion
This is the first clinical trial of crinecerfont, a CRF1 receptor antagonist, in adolescents with classic CAH due to 21OHD. The results from this phase 2 study indicated that study participants experienced substantial and clinically meaningful reductions in adrenal androgens and androgen precursors after 14 days of crinecerfont administration.
This trial included 8 adolescents with elevated adrenal androgens and precursors, despite treatment with GC doses that would generally be considered supraphysiologic and likely to impact growth. The study was designed to provide a comprehensive assessment of disease control through frequent blood sampling over a 24-hour period. In addition, the participants' morning GC doses were withheld until 10:00 for all serial blood sampling procedures to assess the effects of crinecerfont alone during the morning window. It should be noted, however, that patients usually take their first GC dose earlier in the morning, and thus the androgen values observed during the study might be higher than what is observed during typical clinical practice, especially in patients who are only taking hydrocortisone.
After 14 days of twice-daily treatment with crinecerfont, circulating concentrations of ACTH, 17OHP, and androstenedione were substantially reduced during the morning window (prior to the morning GC dose) and throughout the 24-hour monitoring period. Testosterone was also lowered in most female participants, and 2 of the 3 male participants achieved an A4/T ratio <0.5, reflecting improved control of adrenal androgens. Furthermore, all TEAEs experienced by study participants were assessed as mild and no clinically significant findings were reported based on routine safety assessments.
Crinecerfont was initially evaluated in adults with classic 21OHD CAH (8). In the adult population, crinecerfont dosing was associated with reductions in ACTH, 17OHP, androstenedione, and testosterone (in women). The largest reductions in androstenedione were observed in those who received 100 mg twice daily. Evaluation in adolescents was equally important, because early treatment of this genetic disorder is needed to promote normal growth and development. The supraphysiologic GC doses typically needed for disease control during childhood and adolescence can impair skeletal growth and attainment of predicted adult height. Over the long-term, this treatment regimen can lead to well-known metabolic complications of excess steroids such as weight gain, obesity, insulin resistance, dyslipidemia, hypertension, increased risk of cardiovascular disease, myopathy, poor wound healing, negative effects on accrual of bone mineral density, and suppressive effects on growth (15–24). Therefore, most patients are prone to suffering the consequences of excess androgens and/or excess GCs, while the clinician has the challenging task of making treatment adjustments that will minimize the risks associated with both androgen and GC excess.
This balance can be particularly difficult to achieve in children and adolescents, as GC dose adjustments become more complicated with age-related physiological changes, including rapid growth, changing body surface area, gonadal contributions to circulating androgens, pubertal changes in other hormones, increased cortisol clearance rates, and wide interindividual and intra-individual variability of pharmacokinetic and pharmacodynamic response to GC dosing due to variations in GC receptor and tissue sensitivity (5, 25–27). In addition, treatment goals often shift during adolescence from the optimization of growth and development to the preservation of fertility and mitigation of long-term treatment-related complications. The increased responsibility for self-care, combined with greater autonomy and complex daily activity schedules, can contribute to suboptimal treatment adherence in adolescents, particularly with regimens that require multiple daily doses of hydrocortisone (5, 25, 28).
Given the dilemmas facing physicians who treat patients with 21OHD CAH, there is an unmet need for an agent that can help reduce pituitary ACTH secretion and subsequent adrenal androgen production through a GC-independent mechanism. Ideally, such a medication would allow patients to achieve adequate disease control using lower or even physiologic doses of GCs. This study, which confirms the findings from the earlier study of adults with 21OHD CAH, indicates that crinecerfont, an investigational CRF1 receptor antagonist, can improve disease control by reducing ACTH secretion and adrenal androgen concentrations.
Several other investigational therapies have been shown to decrease adrenal androgens in adults with classic 21OHD CAH. The CRF1 antagonist tildacerfont was investigated in a phase 2 study of 11 adults with poorly controlled classic 21OHD, with biomarker reductions observed in ACTH (−59 to −28%), 17OHP (−38 to +0.3%), and androstenedione (−24 to −18%) after 14 days of treatment at all doses (200-1000 mg every day or 100/200 mg twice daily), with no clear dose–response relationship (29). In a 12-week study with tildacerfont (400 mg every day), 5 adults with poorly controlled classic 21OHD had ∼80% maximum mean reductions in ACTH, 17OHP and androstenedione concentrations, with ACTH and androstenedione normalized for 3 and 2 participants, respectively (29). In a study of the 17-hydroxylase inhibitor abiraterone acetate, 6 adult women with elevated androstenedione were dosed with 250 mg/day for 6 days, and all 6 women achieved normal androstenedione concentrations on day 7 (30). A study of nevanimibe reported that 70% of the 10 adult participants achieved ≥50% decrease in 17OHP during at least 1 of the treatment periods (125-1000 mg twice daily for 14 days) with less consistent changes observed for androstenedione (31); however, development of this compound was discontinued. To our knowledge, however, the impact of these therapies on adrenal androgen secretion in adolescents with 21OHD CAH has not been reported.
In the current study of adolescents with 21OHD CAH, the crinecerfont dose was 50 mg twice daily, which was one-half of the maximum dose used in the adult study (100 mg twice daily) (8). This is despite the fact that many of the study subjects were already at an adult size. Crinecerfont is expected to reach steady state plasma exposure after approximately 7 days of treatment. With this lower dose, crinecerfont steady-state exposure (AUC0-24h) was also approximately one-half the exposure observed at steady-state in adults. However, the effects of crinecerfont 50 mg twice daily on circulating androgens and androgen precursors in adolescents were remarkably similar to those observed with crinecerfont 100 mg twice daily in adults. If suppression of androgen production persists with long-term crinecerfont treatment, this drug has the potential to allow clinicians to reduce GC doses to more physiologic levels, thus striking a balance between controlling androgen excess and at the same time avoiding long-term treatment-related complications.
Several lines of evidence suggest that CRF can contribute to the pathophysiology of various stress-related disorders, including inflammatory, neuropsychiatric, and reproductive disorders. Consequently, CRF receptor antagonism might be a therapeutic intervention for such disorders and not just CAH (32, 33). Furthermore, adults and children with CAH have been reported to have a higher rate of psychiatric disorders (34, 35), and even carriers of 21OHD are predisposed to an increased risk of mood disorders (36). Beyond the contribution of CRF to the central stress reaction, both CRF itself and pharmacological doses of GCs can precipitate the development of psychiatric disorders in predisposed individuals. Centrally acting CRF receptor antagonists have been shown to reduce anxiety and fear reactions in primates (37). Participants in this study of adolescent CAH had no baseline history of psychiatric diagnoses, and there were no reported suicidal ideation or behavior or neuropsychiatric-related adverse events during the study. Based on preclinical data, crinecerfont is expected to have limited brain penetration and is therefore unlikely to have central effects in the brain, but rather is expected to act primarily on the pituitary gland, which sits outside the blood–brain barrier. However, larger and longer ongoing trials involving crinecerfont administration in patients with CAH might provide additional insight to these questions.
The limitations of this study include the small number of participants and the short-term, open-label treatment without a placebo arm. However, the magnitude of reduction in participants' hormone concentrations is beyond what would be expected with stable GC dosing, including the variations that normally occur even when patients consistently adhere to their treatment regimens. Strengths of the study include the detailed pharmacodynamic characterization with multiple samples drawn serially over a 24-hour time period at baseline and after 14 days of treatment.
In conclusion, this open-label study in adolescents with classic 21OHD CAH demonstrated that crinecerfont led to substantial and clinically meaningful decreases in ACTH, 17OHP, androstenedione, and testosterone (in females) after 14 days of administration, consistent with what was previously observed in adult patients with 21OHD CAH. Long-term studies of crinecerfont in pediatric and adult patients (NCT04806451 and NCT04490915, respectively) with this disorder are underway to assess whether the improvements in androgen control can translate to more physiologic GC dosing and improvement in clinical endpoints such as weight, growth, and development.
Acknowledgments
The authors thank the clinical staff and patients who participated in the study, along with the following coinvestigators and study coordinators: Sue Kearns, RN (Seattle Children's); Shanlee Davis, MD (University of Colorado Anschutz Medical Campus); Marcelle Terry (Children's Hospital Colorado); Marcela Vargas, MD, and Michael Gottschalk, MD, PhD (University of California San Diego and Rady Children's Hospital San Diego). Medical writing assistance was provided by Christina Chan and Mildred Bahn (Prescott Medical Communications Group, Chicago, Illinois) with support from the study sponsor.
Abbreviations
- 17OHP
17-hydroxyprogesterone
- 21OHD
21-hydroxylase deficiency
- A4/T
androstenedione/testosterone ratio
- ACTH
adrenocorticotropic hormone
- AUC0-24h
area under the concentration–time curve from 0 to 24 hours
- BMI
body mass index
- CAH
congenital adrenal hyperplasia
- CDC
Centers for Disease Control and Prevention
- CRF
corticotropin-releasing factor
- CRF1
CRF type 1
- GC
glucocorticoid
- TEAE
treatment-emergent adverse event
Contributor Information
Ron S Newfield, Pediatric Endocrinology, University of California San Diego and Rady Children’s Hospital, San Diego, CA 92123, USA.
Kyriakie Sarafoglou, Department of Pediatrics, Division of Endocrinology, University of Minnesota Medical School, Minneapolis, MN 55454, USA.
Patricia Y Fechner, Departments of Pediatrics, Division of Pediatric Endocrinology, University of Washington School of Medicine, Seattle Children’s, Seattle, WA 98105, USA.
Natalie J Nokoff, Department of Pediatric Endocrinology, University of Colorado Anschutz Medical Campus, Children’s Hospital Colorado, Aurora, CO 80045, USA.
Richard J Auchus, Departments of Pharmacology and Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
Maria G Vogiatzi, Division of Endocrinology and Diabetes, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
George S Jeha, Neurocrine Biosciences, Inc., San Diego, CA 92130, USA.
Nagdeep Giri, Neurocrine Biosciences, Inc., San Diego, CA 92130, USA.
Eiry Roberts, Neurocrine Biosciences, Inc., San Diego, CA 92130, USA.
Julia Sturgeon, Neurocrine Biosciences, Inc., San Diego, CA 92130, USA.
Jean L Chan, Neurocrine Biosciences, Inc., San Diego, CA 92130, USA.
Robert H Farber, Neurocrine Biosciences, Inc., San Diego, CA 92130, USA.
Disclosures
R.S.N.: clinical trial investigator for Neurocrine Biosciences and Spruce Biosciences; consultant for Spruce Biosciences on behalf of UCSD but does not receive personal income for this consultancy. K.S.: research funding from Neurocrine Biosciences, Spruce Biosciences, and Adrenas Therapeutics. P.Y.F.: research funding from and previously served as a consultant to Neurocrine Biosciences; research funding from Spruce Biosciences and Diurnal, Ltd; consulting fees from Eton Pharmaceuticals. N.J.N.: consulting fees for Neurocrine Biosciences and Ionis Pharmaceuticals. R.J.A.: research funding and consulting fees from Neurocrine Biosciences; research funding from Spruce Biosciences and Diurnal, Ltd; consulting fees from OMass Therapeutics, Crinetics Pharmaceuticals, Novo Nordisk, and Adrenas Therapeutics. M.G.V.: research funding from Neurocrine Biosciences and Spruce Biosciences; consulting for Adrenas Therapeutics and Eton Pharmaceuticals. G.S.J., N.G., E.R., J.S., J.L.C., and R.H.F.: employees of Neurocrine Biosciences, Inc.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
NCT04045145 (registered August 1, 2019).
References
- 1. El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. 2017;390(10108):2194‐2210. [DOI] [PubMed] [Google Scholar]
- 2. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043‐4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonfig W. Growth and development in children with classic congenital adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):39‐42. [DOI] [PubMed] [Google Scholar]
- 4. Trapp CM, Speiser PW, Oberfield SE. Congenital adrenal hyperplasia: an update in children. Curr Opin Endocrinol Diabetes Obes. 2011;18(3):166‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mallappa A, Merke DP. Management challenges and therapeutic advances in congenital adrenal hyperplasia. Nat Rev Endocrinol. 2022;18(6):337‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turcu AF, Auchus RJ. The next 150 years of congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. 2015;153:63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prete A, Auchus RJ, Ross RJ. Clinical advances in the pharmacotherapy of congenital adrenal hyperplasia. Eur J Endocrinol. 2021;186(1):R1‐R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Auchus RJ, Sarafoglou K, Fechner PY, et al. Crinecerfont lowers elevated hormone markers in adults with 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2022;107(3):801‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turcu AF, Spencer-Segal JL, Farber RH, et al. Single-dose study of a corticotropin-releasing factor receptor-1 antagonist in women with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2016;101(3):1174‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turcu AF, Auchus RJ. Novel treatment strategies in congenital adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newfield RS, Sarafoglou K, Fechner PY, et al. Data from: supplementary material for “Crinecerfont (NBI-74788), a CRF type 1 receptor antagonist, lowers adrenal androgens and precursors in adolescents with classic congenital adrenal hyperplasia”. Mendeley Data. 2023. Deposited May 26, 2023. [Google Scholar]
- 12. Centers for Disease Control and Prevention . A SAS program for the 2000 CDC growth charts (ages 0 to < 20 y). Accessed June 15, 2022.http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 13. Sarafoglou K, Forlenza GP, Yaw Addo O, et al. Obesity in children with congenital adrenal hyperplasia in the Minnesota cohort: importance of adjusting body mass index for height-age. Clin Endocrinol (Oxf). 2017;86(5):708‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . Defining childhood weight status: BMI for children and teens. Accessed June 15, 2022.https://www.cdc.gov/obesity/basics/childhood-defining.html
- 15. Bonfig W, Pozza SB, Schmidt H, Pagel P, Knorr D, Schwarz HP. Hydrocortisone dosing during puberty in patients with classical congenital adrenal hyperplasia: an evidence-based recommendation. J Clin Endocrinol Metab. 2009;94(10):3882‐3888. [DOI] [PubMed] [Google Scholar]
- 16. Volkl TM, Simm D, Beier C, Dorr HG. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 2006;117(1):e98‐e105. [DOI] [PubMed] [Google Scholar]
- 17. Torky A, Sinaii N, Jha S, et al. Cardiovascular disease risk factors and metabolic morbidity in a longitudinal study of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2021;106(12):e5247‐e5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodrigues TM, Barra CB, Santos JL, Goulart EM, Ferreira AV, Silva IN. Cardiovascular risk factors and increased carotid intima-media thickness in young patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Arch Endocrinol Metab. 2015;59(6):541‐547. [DOI] [PubMed] [Google Scholar]
- 19. Espinosa Reyes TM, Leyva Gonzalez G, Dominguez Alonso E, Falhammar H. Bone mass in young patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2021;94(1-2):1‐8. [DOI] [PubMed] [Google Scholar]
- 20. Auchus RJ, Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98(7):2645‐2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol. 2014;10(2):115‐124. [DOI] [PubMed] [Google Scholar]
- 22. Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects. StatPearls; 2022. [PubMed] [Google Scholar]
- 23. Bomberg EM, Addo OY, Kyllo J, et al. The relation of peripubertal and pubertal growth to final adult height in children with classic congenital adrenal hyperplasia. J Pediatr. 2015;166(3):743‐750. [DOI] [PubMed] [Google Scholar]
- 24. Sarafoglou K, Addo OY, Turcotte L, et al. Impact of hydrocortisone on adult height in congenital adrenal hyperplasia-the Minnesota cohort. J Pediatr. 2014;164(5):1141‐1146.e1. [DOI] [PubMed] [Google Scholar]
- 25. Merke DP, Poppas DP. Management of adolescents with congenital adrenal hyperplasia. Lancet Diabetes Endocrinol. 2013;1(4):341‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al-Kofahi M, Ahmed MA, Jaber MM, et al. An integrated PK-PD model for cortisol and the 17-hydroxyprogesterone and androstenedione biomarkers in children with congenital adrenal hyperplasia. Br J Clin Pharmacol. 2021;87(3):1098‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarafoglou K, Zimmerman CL, Gonzalez-Bolanos MT, Willis BA, Brundage R. Interrelationships among cortisol, 17-hydroxyprogesterone, and androstenendione exposures in the management of children with congenital adrenal hyperplasia. J Investig Med. 2015;63(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 28. Charmandari E, Brook CG, Hindmarsh PC. Classic congenital adrenal hyperplasia and puberty. Eur J Endocrinol. 2004;151(Suppl 3):U77-U82. [DOI] [PubMed] [Google Scholar]
- 29. Sarafoglou K, Barnes CN, Huang M, et al. Tildacerfont in adults with classic congenital adrenal hyperplasia: results from two phase 2 studies. J Clin Endocrinol Metab. 2021;106(11):e4666‐e4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auchus RJ, Buschur EO, Chang AY, et al. Abiraterone Acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(8):2763‐2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Maouche D, Merke DP, Vogiatzi MG, et al. A phase 2, multicenter study of nevanimibe for the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(8):2771‐2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13(10):436‐444. [DOI] [PubMed] [Google Scholar]
- 33. Zoumakis E, Chrousos GP. Corticotropin-releasing hormone receptor antagonists: an update. Endocr Dev. 2010;17:36‐43. [DOI] [PubMed] [Google Scholar]
- 34. Engberg H, Butwicka A, Nordenstrom A, et al. Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: a total population study. Psychoneuroendocrinology. 2015;60:195‐205. [DOI] [PubMed] [Google Scholar]
- 35. Mueller SC, Ng P, Sinaii N, et al. Psychiatric characterization of children with genetic causes of hyperandrogenism. Eur J Endocrinol. 2010;163(5):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Charmandari E, Merke DP, Negro PJ, et al. Endocrinologic and psychologic evaluation of 21-hydroxylase deficiency carriers and matched normal subjects: evidence for physical and/or psychologic vulnerability to stress. J Clin Endocrinol Metab. 2004;89(5):2228‐2236. [DOI] [PubMed] [Google Scholar]
- 37. Habib KE, Weld KP, Rice KC, et al. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci U S A. 2000;97(11):6079‐6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.