Abstract
Context
Secondary hyperparathyroidism (SHPT) is a complication of chronic kidney disease (CKD) affecting mineral and bone metabolism and characterized by excessive parathyroid hormone (PTH) production and parathyroid hyperplasia.
Objective
The objective of this analysis was to compare the efficacy and adverse effects of extended-release calcifediol (ERC) and paricalcitol (PCT) by assessing their effect on the biomarkers PTH, calcium, and phosphate in patients with non-dialysis CKD (ND-CKD).
Methods
A systematic literature research was performed in PubMed to identify randomized control trials (RCTs). Quality assessment was done with the GRADE method. The effects of ERC vs PCT were compared using random effects in a frequentist setting.
Results
Nine RCTs comprising 1426 patients were included in the analyses. The analyses were performed on 2 overlapping networks, due to nonreporting of outcomes in some of the included studies. No head-to-head trials were identified. No statistically significant differences in PTH reduction were found between PCT and ERC. Treatment with PCT showed statistically significant increases in calcium compared with ERC (0.2 mg/dL increase; 95% CI, −0.37 to −0.05 mg/dL). No differences in effects on phosphate were observed.
Conclusion
This network meta-analysis showed that ERC is comparable in lowering PTH levels vs PCT. ERC displayed avoidance of potentially clinically relevant increases in serum calcium, offering an effective and well-tolerated treatment option for the management of SHPT in patients with ND-CKD.
Keywords: bone/mineral metabolism, hypercalcemia, hyperparathyroidism, metabolic bone disease, vitamin D
Secondary hyperparathyroidism (SHPT) is a common and major complication of chronic kidney disease (CKD) among patients on dialysis and in patients with non-dialysis chronic kidney disease (ND-CKD). SHPT in CKD is caused by disturbances in metabolic parameters, including phosphate, calcium, fibroblast growth factor 23 (FGF23), and vitamin D. The decreasing ability of the kidney to activate vitamin D (25[OH]D) to its most active metabolite (1,25(OH)2D) is a key factor that leads to an excessive secretion of parathyroid hormone (PTH) and high blood levels of PTH. If left unaddressed, elevated levels of PTH can cause bone disease and extraskeletal calcification and increase cardiac disease risk through vascular and visceral calcification. Additionally, prolonged hyperparathyroidism leads to parathyroid hyperplasia (enlargement of the parathyroid glands) which can cause therapeutic resistance and the need for parathyroidectomy (a high-risk procedure to remove or partially remove the parathyroid glands) (1). As such, simultaneous control of various biomarkers, including PTH, calcium, and phosphate, is essential for effective treatment of SHPT and PTH-related issues in CKD.
Paricalcitol, other active vitamin D analogues (doxercalciferol and alfacalcidol), and active vitamin D (calcitriol) have been commonly used to treat SHPT in ND-CKD for several years. However, studies indicate that these therapies aversively increase serum calcium, phosphate, and FGF23 levels (2–4). The increased risk of hypercalcemia from these treatments has been demonstrated in randomized controlled trials (RCTs), such as the PRIMO and OPERA trials of paricalcitol vs placebo (5, 6), and in Cozzolino et al (2021) (7). Consequently, the latest Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (8) highlighted this risk as the predominant reason for changing the treatment recommendations for ND-CKD stage 3-5 patients. In the updated guidelines, routine use of paricalcitol, calcitriol and the other vitamin D receptor activators (VDRAs) in CKD stages 3 to 5 is no longer recommended. Instead, these agents should be reserved for severe and progressive SHPT in CKD stages 4-5 (8). Extended-release (ER) calcifediol (extended-release calcifediol is the term used within the United States, while in Europe, the term prolonged-release calcifediol is used) has been developed as an alternative treatment for SHPT in CKD stages 3-4.
The objective of this study was to compare the effectiveness of paricalcitol and ER calcifediol in impacting the biomarkers PTH, calcium, and phosphate in patients with ND-CKD. A systematic literature review was conducted to build a comprehensive collection of RCTs in which paricalcitol and ER calcifediol were evaluated. To study the comparative effectiveness, study-level results identified in the systematic literature review were synthesized using network meta-analysis (NMA).
Methods
Search Strategy and Study Selection
We conducted a systematic literature review, according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (9), to identify studies for inclusion in the NMA. The systematic literature review was not registered with PROSPERO. The PubMed database was searched using a predefined search strategy with database-appropriate terms for CKD and outcomes and treatment alternatives for SHPT. No restrictions were imposed on publication date. Non-English language publications and publications that were reviews, comments, or meta-analyses were excluded in the search. The reference lists of all studies that fulfilled the inclusion criteria were also searched for additional publications which had not been identified in the search strategy. Two reviewers independently performed all stages of the study selection and data extraction.
To be included in the NMA, the publication had to present results from an RCT comprising more than 20 adult patients (18 years+) with documented ND-CKD. At least one patient group in the study must have been administered ER calcifediol or paricalcitol, and the comparator group(s) had to receive placebo treatment or no treatment. In line with the approved posology of paricalcitol and ERC, only data from the trial arms in which patients were administered 1 to 2 µg/day of paricalcitol and 30 to 60 µg/day of ER calcifediol were included in the analyses. If 2 or more publications reported results from the same underlying trial and the reporting of results overlapped, only one of the publications was included (without loss of data). The full set of inclusion criteria are described in Table 1 and the PubMed search facets are included in Table 2.
Table 1.
Eligibility criteria for clinical studies used in the systematic literature review
| Eligibility criteria | Included | Excluded |
|---|---|---|
| Population |
|
|
| Interventions/comparators |
|
|
| Outcomes |
|
|
| Study design |
|
Comparative CCT, comparative observational studies, case studies, case reports, economic evaluations, reviews, meta-analyses |
| Other eligibility criteria |
|
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; BMD, bone mineral density; CKD, chronic kidney disease; FGF23, fibroblast growth factor 23; ND-CKD, non-dialysis chronic kidney disease; PTH, parathyroid hormone; RCT, randomized controlled trial; SHPT, secondary hyperparathyroidism.
Table 2.
PubMed search facets
| Ref | Facet | Search terms |
|---|---|---|
| 1 | Chronic kidney disease | (“chronic kidney disease” OR CKD OR “kidney failure” OR “renal outcome” OR “renal outcomes” OR “renal failure”):[tiab] |
| 2 | Biomarkers or outcomes | (“secondary hyperparathyroidism” OR SHPT OR calcium OR phosphorus OR “vitamin D” OR “25(OH)D” OR “1,25(OH)” OR pth OR “parathyroid hormone” OR ipth OR parathormone OR FGF-23 OR FGF23 OR “fibroblast growth factor 23” OR BSAP OR P1NP OR CTX OR hepcidin OR “TRAP 5b” OR “calcification propensity” OR proteinuria OR hypercalcaemia OR hypercalcemia OR hyperphosphatemia OR progression OR CVD OR “cardiovascular disease” OR “cv event” OR “cardiovascular event” OR “cardiovascular events” OR mortality OR death OR fracture OR fractures OR fx OR hospitalization OR hospitalisation OR anemia OR anaemia OR parathyroidectomy OR ptx OR “bone mineral density” OR “bone turnover” OR “bone metabolic” OR BMD OR “bone disease” OR MBD OR “CKD-MBD”):[tiab] |
| 3 | Treatments | (calcitriol OR paricalcitol OR alfacalcidol OR alphacalcidol OR 1-hydroxycholecalciferol OR doxercalciferol OR “vitamin D receptor agonist” OR “vitamin D receptor activator” OR “vitamin D receptor activation” OR VDRA OR VDRAs OR calcifediol OR calcidiol OR NVD OR “nutritional vitamin D” OR cholecalciferol OR ergocalciferol OR calcimimetic OR cinacalcet):[tiab] |
| 4 | Language | English[lang] |
| 5 | Publication type | NOT (review OR comment OR meta-analysis) |
| 6 | Effect of drug therapy on biomarker levels or outcomes in CKD | #1 AND #2 AND #3 AND #4 NOT #5 |
Data Extraction
A standardized data extraction form was created and tested using a random sample of included publications. The 2 reviewers independently extracted all data to the prespecified data form. Once extracted, disparities in the data were corrected by joint re-examination of the source material by the reviewers. Any unresolved disagreements were adjudicated by an arbiter.
Quality Assessment of Evidence
The quality of the evidence was assessed using the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) methodology. Each outcome was assessed to be of high, moderate, low, or very low quality through joint assessment of the 5 domains included in the GRADE approach: risk of bias (domain 1), inconsistency (domain 2), indirectness (domain 3), imprecision (domain 4), and publication bias (domain 5). Evidence gathered from RCTs are initially regarded as high-quality evidence, but can be downgraded to moderate, low, or very low quality depending on the evaluation of these 5 domains (10).
Outcomes
The treatment outcomes recorded were the mean or median differences in absolute values of the biomarkers PTH, calcium, and phosphate from baseline to the end of the study for all patient groups in the included studies. When not reported directly, the mean or median difference was calculated by subtracting the baseline biomarker values from the values at the end of the study period. In such cases, the SD of the effect measure was calculated using the formula presented in the Cochrane Handbook for Systematic Reviews of Interventions, with the correlation coefficient set to a conservative value of 0.4 (11). If a biomarker value was reported with an accompanying interquartile range, the SD of the value was approximated by dividing the range of the interquartile range by 1.35 (11).
Values for PTH were converted to the common unit of picograms per milliliter (pg/mL) and values for calcium and phosphate were converted to milligrams per deciliter (mg/dL).
Statistical Analysis
The association between treatment and impact on PTH, calcium, and phosphate were estimated using mean or median differences in all analyses.
Random-effects NMA in a frequentist framework was used to synthesize evidence from indirect comparisons within a single analytical framework (12, 13). Transitivity is assumed in conducting an NMA, ie, that the comparisons of treatments A and B can be made using indirect evidence if both have been tested against treatment C. If evidence from direct and indirect comparisons are available, the validity of the transitivity assumption can be tested by assessing the consistency of direct and indirect evidence. Given that no direct comparisons between paricalcitol and ER calcifediol have been identified, the data collected for this study does not allow for such tests of consistency. Rather, regular meta-analytic procedures were used to assess the heterogeneity of study-level results using the common I2-statistic.
To utilize the results from studies that had 2 intervention arms and one placebo arm, the data from the placebo arm was duplicated and compared separately to each intervention arm. The number of patients in these duplicated placebo arms was divided by 2 to avoid double counting of these patients in the analyses (11).
The NMA was performed in Stata16, using the network family of commands (14). Forest plots at the intervention level illustrate the treatment effects on each outcome. These are presented both separately, for paricalcitol and ER calcifediol compared to placebo-treated patients, and for paricalcitol and ER calcifediol compared to each other using the indirect evidence.
Forest plots from a random effects meta-analysis model were produced to assess heterogeneity between studies through the I2-statistic. Funnel plots were used to assess publication bias and small study effects.
Results
Study Selection
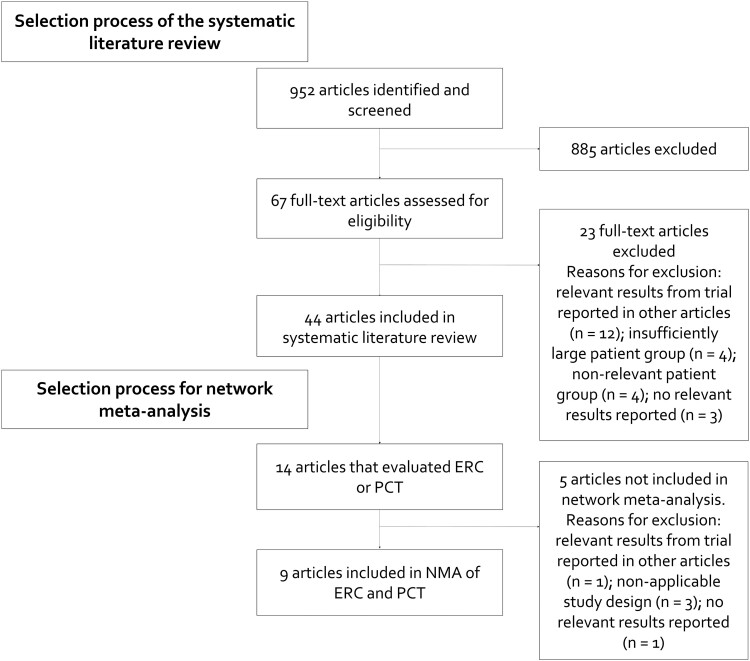
The initial search for articles yielded a total of 1175 hits on May 31, 2022. Of these, 18 publications were eligible for inclusion in the network meta-analysis (NMA) and 9 articles were included in the final NMA. Figure 1 shows the PRISMA diagram for the search process.
Figure 1.
Study selection.
Study and Patient Characteristics
A total of 1443 patients were randomized to study arms in the included studies; 507 in the studies that evaluated ER calcifediol and 936 in studies that evaluated paricalcitol (Table 3). Levels of PTH at baseline were elevated above normal levels in all studies (average over all studies and patient groups: 126.8 pg/mL). Baseline levels of calcium and phosphorus were 9.3 and 3.7 mg/dL, respectively, in the included studies.
Table 3.
Patient characteristics of the included publications
| Author (year) | Study inclusion in networks | Study arms | Dose | Number of patients | Age | % female | Baseline eGFR (mL/min/1.73m2) | Baseline PTH (pg/mL) | Baseline calcium (mg/dL) | Baseline phosphate (mg/dL) | Baseline vitamin D (25(OH)D) (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Panel A: Publications comparing extended-release calcifediol to placebo | |||||||||||
| Sprague (2014) (17) | PTH, Ca, P | ER calcifediol | 30 µg/day | 13 | 58.2 | 53.8 | 36.7 | 156.3 | 9.3 | 3.8 | 21.1 |
| ER calcifediol | 60 µg/day | 17 | 64.7 | 41.2 | 42.6 | 118.5 | 9.3 | 3.6 | 23.6 | ||
| Placebo | NA | 31 | 62.8 | 64.5 | 38.7 | 145.7 | 9.4 | 3.5 | 19.7 | ||
| Sprague (2016) (20) | PTH, Ca, P | ER calcifediol | 30 µg/day for weeks 0-12 with possible up titration to 60 µg/day weeks 13-26, based on levels of PTH, 25(OH)D and calcium | 285 | 66.0 | 49.8 | 30.6 | 147.2 | 9.2 | 3.7 | 19.9 |
| Placebo | NA | 144 | 64.9 | 50.0 | 32.0 | 148.9 | 9.2 | 3.8 | 19.3 | ||
| Summary Panel A: | 507 (sum) | 63.7 (average) | 52.0 (average) | 36.3 (average) | 144.0 (average) | 9.3 (average) | 3.7 (average) | 19.9 | |||
| Panel B: Publications comparing paricalcitol to placebo | |||||||||||
| Alborzi (2008) (16) | PTH | Paricalcitol | 1 µg/day | 8 | 72.6 | 25.0 | 47.5 | 66.8 | 9.5 | 3.2 | N/A |
| Paricalcitol | 2 µg/day | 8 | 67.5 | 25.0 | 47.4 | 76.0 | 9.5 | 3.3 | N/A | ||
| Placebo | NA | 8 | 68.4 | 0.0 | 44.0 | 124.9 | 9.3 | 3.4 | N/A | ||
| Coyne (2006) (15) | Ca, P | Paricalcitol | Starting dose 1 µg/day or 2 µg thrice weekly if PTH <500 pg/mL or 2 µg/day or 4 µg thrice weekly if PTH ≥500 pg/mL, with subsequent dose titration based on levels of PTH, calcium and phosphate (average daily dose of 1.36 µg/day) | 107 | 63.6 | 32.0 | 23.1 | 265.0 | 9.3 | 4.0 | N/A |
| Placebo | NA | 113 | 61.8 | 33.0 | 23.0 | 280.0 | 9.4 | 4.0 | N/A | ||
| Coyne (2013) (18) | PTH, Ca, P | Paricalcitol | 1 µg/day | 93 | 64.0 | 29.0 | 40.0 | 97.0 | 9.3 | 3.9 | N/A |
| Paricalcitol | 2 µg/day | 95 | 65.0 | 27.0 | 42.0 | 91.0 | 9.4 | 3.8 | N/A | ||
| Placebo | NA | 93 | 65.0 | 35.0 | 39.0 | 105.0 | 9.3 | 3.8 | N/A | ||
| Lundwall (2015) (19) | PTH, Ca, P | Paricalcitol | 1 µg/day | 12 | 66.1 | 8.0 | 38.9 | 68.8 | 9.1 | 3.4 | 28.7 |
| Paricalcitol | 2 µg/day | 12 | 70.8 | 33.0 | 42.1 | 66.0 | 9.1 | 3.4 | 27.8 | ||
| Placebo | NA | 12 | 59.1 | 25.0 | 41.6 | 87.7 | 9.1 | 3.1 | 25.9 | ||
| Thadhani (2012) (5) | PTH, Ca, P | Paricalcitol | Starting dose 2 µg/d with possible downtitration to 1 µg/d based on levels of calcium | 115 | 64.0 | 31.3 | 36.01 | 100.01 | 9.61 | 3.71 | N/A |
| Placebo | NA | 112 | 66.0 | 29.5 | 31.01 | 106.01 | 9.61 | 3.51 | N/A | ||
| Wang (2014) (6) | PTH, Ca, P | Paricalcitol | Starting dose 1 µg/d if PTH <500 pg/mL or 2 µg/d if PTH ≥500 pg/mL, with subsequent dose titration based on levels of calcium | 30 | 60.8 | 34.0 | 23.91 | 156.01 | 9.3 | 4.2 | N/A |
| Placebo | NA | 30 | 62.2 | 53.0 | 19.71 | 158.01 | 9.4 | 3.9 | N/A | ||
| Zoccali (2014) (4) | PTH, Ca, P | Paricalcitol | starting dose 2 µg/d with possible downtitration to 1 µg every other day based on levels of PTH and calcium | 44 | 60.8 | 41.0 | 34.0 | 102.01 | 9.0 | 3.7 | 13.2 |
| Placebo | NA | 44 | 62.0 | 30.0 | 29.0 | 102.01 | 8.9 | 3.8 | 15.2 | ||
| Summary Panel B: | 936 (sum) | 64.7 (average) | 28.9 (average) | 35.4 (average) | 120.7 (average) | 9.3 (average) | 3.7 (average) | 18.1 | |||
| Summary all studies: | 1443 (sum) | 64.4 (average) | 34.9 (average) | 35.7 (average) | 126.8 (average) | 9.3 (average) | 3.7 (average) | 19.5 | |||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration rate; ER, extended-release; PTH, parathyroid hormone.
Effect sizes were reported as unadjusted mean changes in nearly all publications and for all outcomes. The exceptions are Wang et al (2014) (6), which reported median changes in PTH and Thadhani et al (2012) (5), which reported adjusted least-squares mean changes from a model including treatment, visit, treatment by visit interaction, country, and baseline value of the biomarker for all outcomes. One publication (15) reported no numerical results for PTH and another publication (16) presented no results for calcium and phosphate. Therefore, it was possible to utilize 8 publications in the analyses of each outcome and different networks are used in the analysis of PTH compared to analyses of Ca and P (see Table 3).
Four included publications (1 evaluating ER calcifediol and 3 evaluating paricalcitol) reported results from 3-armed trials, where patients in 2 of the arms were administered different doses of the active intervention (1 or 2 µg/day of paricalcitol or 30 or 60 µg/day of ER calcifediol) (16–19). The 5 remaining publications (4 evaluating paricalcitol (4–6, 15) and 1 evaluating ER calcifediol (20)) reported results from 2-armed trials, in which dosing of the intervention was allowed to be titrated over the study period.
Quality of Evidence
The assessed risk of study-level bias was generally “very low” or “low,” with the exception of 2 studies; Alborzi et al (2008) (16) and Lundwall et al (2015) (19) were assessed to have a “moderate” and “high” risk of bias, respectively. All studies were reported to be randomized and double-blinded. No trial was stopped early. The randomization process was described in more detail in 4 publications (6, 15, 16, 18). The randomization process for the study by Coyne (2013) (19) is described in de Zeeuw (2010) (15), which presents the study design of the underlying trial more thoroughly, while the method of blinding was described more specifically in 2 publications (4, 15). The number of dropouts were low in the included studies and the dropout rate was higher than 20% in only 2 studies (5, 18).
No publication bias was detected. While some association to the pharmaceutical industry was reported in all publications, no asymmetries or small study effects indicative of publication bias was detected in the funnel plots for any of the outcomes (Supplementary Fig. S1 in the supplementary materials (21)). The sample sizes in the publications were generally of low to moderate size and the number of publications utilized in the analyses were moderate (8 publications, in all analyses). All in all, no limitations in the GRADE domains of risk of bias, imprecision, or publication bias were considered severe enough to warrant a downgrading of the overall quality of evidence.
Forest plots (Supplementary Fig. S2, S3, and S4 in the supplementary materials (21)) indicate a likely presence of substantial heterogeneity in study-level effect sizes (with I2-statistics ranging from 47.1% to 78.4%). Additionally, the lack of any direct comparison between paricalcitol and ER calcifediol prohibits any evaluation of the consistency of the network. This heterogeneity and lack of direct comparison constitute limitations in the GRADE domains of inconsistency and indirectness. Given these limitations, the quality of the evidence was downgraded from high to low quality for all outcomes.
Treatment Outcomes
PTH results
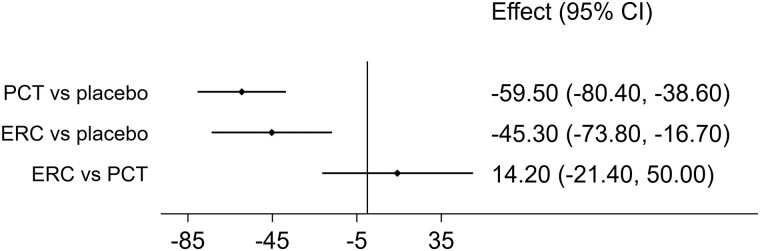
Paricalcitol and ER calcifediol both showed statistically significant PTH-lowering effects compared to placebo (Fig. 2). While the estimated PTH reduction from paricalcitol (−59.5 pg/mL, 95% CI: −80.4 to −38.6 pg/mL) was larger than the PTH reduction from ER calcifediol (−45.3 pg/mL, 95% CI: −73.8 to −16.7 pg/mL), the resulting 14.2 pg/mL difference (95% CI: −21.4 to 50.0 pg/mL) in treatment effects did not show statistical significance.
Figure 2.
Estimated effects on PTH (pg/mL) from treatment with paricalcitol (PCT) and extended-release calcifediol (ERC), compared with placebo (first rows) and directly compared using the indirect evidence (last row).
Calcium results
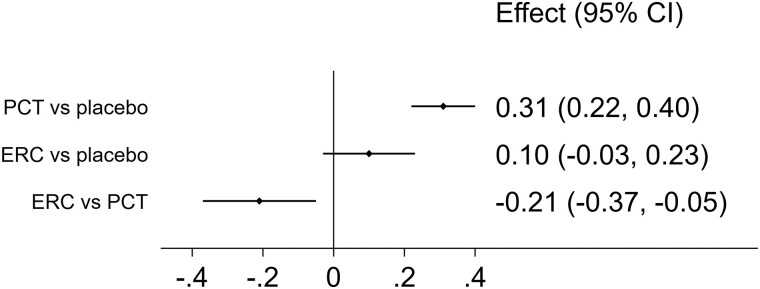
Treatment with paricalcitol caused statistically significant increases in calcium vs placebo (increase: 0.31 mg/dL, 95% CI: 0.22 to 0.40 mg/dL), while the marginal increase in calcium from treatment with ER calcifediol (increase: 0.10 mg/dL, 95% CI: −0.03 to 0.23 mg/dL) did not exhibit statistical significance (Fig. 3). The estimated difference in effects showed that paricalcitol raised the level of calcium by a statistically significant 0.2 mg/dL compared to ER calcifediol (95% CI: −0.37 to −0.05 mg/dL). Hypercalcemia was more common among patients that received PCT (9.25%, 47/508 patients) than among patients who received ER calcifediol (2.1%, 7/332 patients).
Figure 3.
Estimated effects on calcium (mg/dL) from treatment with paricalcitol (PCT) and extended-release calcifediol (ERC), compared with placebo (first rows) and directly compared using the indirect evidence (last row).
Phosphate results
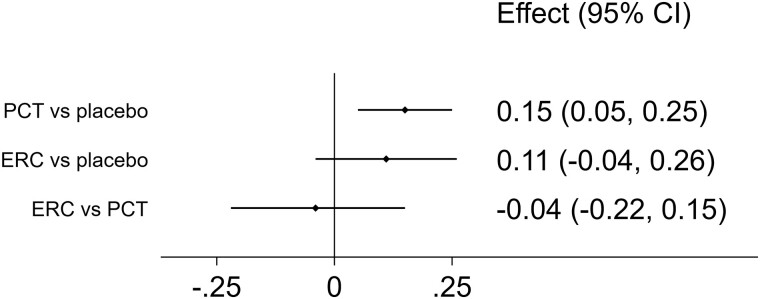
Levels of phosphate increased marginally from treatment with paricalcitol (increase: 0.15 mg/dL, 95% CI: 0.05 to 0.25 mg/dL) and ER calcifediol (increase: 0.11 mg/dL, 95% CI: −0.04 to 0.26 mg/dL) when compared to placebo (Fig. 4). While the increase in phosphate from paricalcitol vs placebo was statistically significant, the marginal 0.04 mg/dL difference (95% CI: −0.22 to 0.15 mg/dL) in effect between paricalcitol and ER calcifediol did not show statistical significance. In most publications, the number of patients with hyperphosphatemia was either not reported or no cases were observed. One publication reports 1 case of hyperphosphatemia (20) and one publication reports that 10% of patients treated with PCT and 12% of patients given placebo experienced hyperphosphatemia (15).
Figure 4.
Estimated effects on phosphate (mg/dL) from treatment with paricalcitol (PCT) and extended-release calcifediol (ERC), compared with placebo (first rows) and directly compared using the indirect evidence (last row).
Discussion
In this network meta-analysis, we found comparable reductions in PTH from treatment with ER calcifediol compared to treatment with paricalcitol, whereas treatment with paricalcitol increased levels of calcium compared to treatment with ER calcifediol. Increases in phosphate were observed upon treatment with both paricalcitol and ER calcifediol, but these increases were small and similar between the 2 drugs. As such, reductions in PTH achieved through treatment with paricalcitol come with a risk of simultaneous increases in levels of calcium.
The beneficial effect of paricalcitol in reducing PTH levels in patients with CKD has been widely established but has been accompanied by an unproven belief of a greater PTH-lowering effect compared with calcitriol (22, 23). Additionally, the 2016 meta-analysis by Cai et al (10 trials, 734 patients), which compared the efficacy and safety of paricalcitol and nonselective vitamin D receptor activators (VDRAs), did not find any difference between the therapeutic options (24). In a recent comparison of ER calcifediol and the immediate-release formulations of calcifediol, it was shown that ER calcifediol produces larger reductions in PTH while consistently attaining threshold levels of 25(OH)D (30 and 50 ng/mL) that immediate-release calcifediol was not able to reliably attain (25). The spikes in 25(OH)D levels from immediate-release calcifediol can also lead to overexpression of CYP24A1, further causing increased catabolism of 25(OH)D and 1,25(OH)2D and increased expression of FGF23 (26, 27).
The findings of the present analyses indicate that there are no statistically significant differences in PTH reduction between paricalcitol and the extended-release formulation of calcifediol. We thereby reach a similar conclusion of nonsuperior PTH reductions from paricalcitol as have been found in the previous comparative studies vs calcitriol and other VDRAs. While clearly defined target levels of PTH are lacking, this study shows that ER calcifediol can be used to effectively combat persistently elevated and progressively rising levels of PTH in patients with SHPT, as recommended by the latest KDIGO guidelines (8).
The decrease in PTH associated with paricalcitol and ER calcifediol partly stems from a reaction to increases in serum calcium. Our analysis shows that treatment with paricalcitol is associated with statistically significant increases in calcium, both when compared to placebo and to ER calcifediol. Given the interlinkage between the biomarkers, it is plausible to believe that measures taken to reduce any calcium-increasing effects from paricalcitol would also contribute to a weakening of the desired PTH-lowering effects. The risk of calcium increases from treatment with paricalcitol has previously been highlighted, eg, in the most recent KDIGO guidelines and in the meta-analyses by Han et al (2013) and by Cozzolino et al (2021). As a result, the use of paricalcitol in ND-CKD has been cautioned against, due to the well-established link between increases in calcium and an increased risk of vascular calcification (7, 8, 28). This NMA hence re-confirms that prescribers need to carefully monitor calcium levels when prescribing paricalcitol. Our results suggest a lower need for such monitoring efforts with ER calcifediol, although further assessments of the safety of ER calcifediol in clinical practice are needed to verify this.
The main limitations in the present study stem from the limited amount of data that was available for inclusion in the NMA. Additional studies comprising comparable populations would allow more precise estimation of true overall effect sizes and provide statistical power to enable analyses of effects among, for example, subsets of patients and different drug dosing regimens. Such subset analyses could be used to better understand the mechanisms underlying the observed treatment effects and would hence be useful in guiding day-to-day treatment with these drugs. Furthermore, the lack of head-to-head trials between paricalcitol and ER calcifediol prevents evaluation of the consistency of the network. While this issue is somewhat diminished by the generally comparable study populations in the included articles, the lack of a direct comparison contributed to the downgrading of the quality of evidence using the GRADE approach. At the individual study level, however, the overall risk of bias was assessed to be low, and the study designs were typically of high quality.
The measured values of the biomarker outcomes also contain uncertainties and variability, depending on, for example, test timing, unobserved patient characteristics, and the test assay used. PTH in particular has been observed to have a wide variability within patients over short time spans. Such inherent measurement uncertainties in the biomarkers can affect the study-level effects and contribute to the heterogeneity of study-level estimates that was observed. This type of variability might also be amplified by features of the designs of the included studies, for instance, if the patient-level effect in a study is determined by a single measurement or repeated measurements at the end of the study period.
Despite these limitations, this NMA presents a comprehensive collection of evidence regarding the comparative effectiveness of paricalcitol and ER calcifediol in controlling the biomarkers PTH, calcium, and phosphate in the treatment of SHPT and PTH-related issues in CKD. The evidence presented suggests that while both paricalcitol and ER calcifediol are equally effective in reducing levels of PTH, calcium levels tended to increase from treatment with paricalcitol. Therefore, ER calcifediol may be a treatment option for SHPT in ND-CKD patients, especially in those for whom paricalcitol is not recommended by KDIGO guidelines, with CKD–mineral and bone disorder (CKD-MBD).
Disclosures
M.F. has no interests to disclose. O.S. and J.G. are employed by Quantify Research. E.G.P. has been supported by grants from Instituto de Salud Carlos III (ISCIII, FIS-FEDER PI21/01430). A.F. received lecture and consultancy fees from Vifor Pharma. G.C. received research support from the European Community (EC), the Italian Agency of Drugs (AIFA) and the Italian Ministry for University and Research (MIUR), G.C. took part in a variety of projects that were funded by pharmaceutical companies (ie, Novartis, GSK, Roche, AMGEN and BMS), G.C. received honoraria as a member of the advisory board to Roche.
The study was funded by Vifor Pharma.
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- CKD
chronic kidney disease
- ER
extended-release
- ERC
extended-release calcifediol
- FGF23
fibroblast growth factor 23
- GRADE
Grades of Recommendation, Assessment, Development and Evaluation
- KDIGO
Kidney Disease: Improving Global Outcomes
- ND-CKD
non-dialysis chronic kidney disease
- PCT
paricalcitol
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PTH
parathyroid hormone
- RCT
randomized controlled trial
- SHPT
secondary hyperparathyroidism
- VDRA
vitamin D receptor activator
Contributor Information
Matteo Franchi, Department of Statistics and Quantitative Methods, Bicocca University Milan, 20126 Milan, Italy.
Joel Gunnarsson, Quantify Research, 112 21 Stockholm, Sweden.
Emilio Gonzales-Parra, Nephrology and Hypertension Clinic, Fundación Jiménez Díaz, 28040 Madrid, Spain.
Anibal Ferreira, NOVA Medical School, Faculty of Medical Sciences, Nova University of Lisbon, 1169-056 Lisbon, Portugal.
Oskar Ström, Quantify Research, 112 21 Stockholm, Sweden; Department of Medicine, Huddinge, Karolinska Institutet, 141 57 Stockholm, Sweden.
Giovanni Corrao, Department of Statistics and Quantitative Methods, Bicocca University Milan, 20126 Milan, Italy.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Cannata-Andia JB, Carrera F. The pathophysiology of secondary hyperparathyroidism and the consequences of uncontrolled mineral metabolism in chronic kidney disease: the role of COSMOS. NDT Plus. 2008;1(Suppl 1):i2‐i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D'Arrigo G, Pizzini P, Cutrupi S, et al. FGF23 And the PTH response to paricalcitol in chronic kidney disease. Eur J Clin Invest. 2020;50(2):e13196. [DOI] [PubMed] [Google Scholar]
- 3. Hansen D, Rasmussen K, Pedersen SM, Rasmussen LM, Brandi L. Changes in fibroblast growth factor 23 during treatment of secondary hyperparathyroidism with alfacalcidol or paricalcitol. Nephrol Dial Transplant. 2012;27(6):2263‐2269. [DOI] [PubMed] [Google Scholar]
- 4. Zoccali C, Curatola G, Panuccio V, et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension. 2014;64(5):1005‐1011. [DOI] [PubMed] [Google Scholar]
- 5. Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674‐684. [DOI] [PubMed] [Google Scholar]
- 6. Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD–the OPERA trial. J Am Soc Nephrol. 2014;25(1):175‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cozzolino M, Bernard L, Csomor PA. Active vitamin D increases the risk of hypercalcaemia in non-dialysis chronic kidney disease patients with secondary hyperparathyroidism: a systematic review and meta-analysis. Clin Kidney J. 2021;14(11):2437‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) . Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7(1):1‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schünemann H, Brożek J, Guyatt G, Oxman A, ed. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (Updated October 2013). GRADE Working Group; 2013. [Google Scholar]
- 11. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2011. [Google Scholar]
- 12. Salanti G, Higgins JPT, Ades AE, Ioannidis JPA. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279‐301. [DOI] [PubMed] [Google Scholar]
- 13. White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White IR. Network meta-analysis. Stata J. 2015;15(4):951‐985. [Google Scholar]
- 15. Coyne D, Acharya M, Qiu P, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47(2):263‐276. [DOI] [PubMed] [Google Scholar]
- 16. Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52(2):249‐255. [DOI] [PubMed] [Google Scholar]
- 17. Sprague SM, Silva AL, Al-Saghir F, et al. Modified-release calcifediol effectively controls secondary hyperparathyroidism associated with vitamin D insufficiency in chronic kidney disease. Am J Nephrol. 2014;40(6):535‐545. [DOI] [PubMed] [Google Scholar]
- 18. Coyne DW, Andress DL, Amdahl MJ, Ritz E, de Zeeuw D. Effects of paricalcitol on calcium and phosphate metabolism and markers of bone health in patients with diabetic nephropathy: results of the VITAL study. Nephrol Dial Transplant. 2013;28(9):2260‐2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lundwall K, Jörneskog G, Jacobson SH, et al. Paricalcitol, microvascular and endothelial function in non-diabetic chronic kidney disease: a randomized trial. Am J Nephrol. 2015;42(4):265‐273. [DOI] [PubMed] [Google Scholar]
- 20. Sprague SM, Crawford PW, Melnick JZ, et al. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol. 2016;44(4):316‐325. [DOI] [PubMed] [Google Scholar]
- 21. Franchi M, Gunnarsson J, Gonzales-Parra E, et al. Supplemental materials repository for paricalcitol and extended release calcifediol for treatment of secondary hyperparathyroidism in non-dialysis chronic kidney disease: results From a network meta-analysis. March 3 2023. [DOI] [PMC free article] [PubMed]
- 22. Coyne DW, Goldberg S, Faber M, Ghossein C, Sprague SM. A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3-4 CKD. Clin J Am Soc Nephrol. 2014;9(9):1620‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riccio E, Sabbatini M, Bruzzese D, et al. Effect of paricalcitol vs calcitriol on hemoglobin levels in chronic kidney disease patients: a randomized trial. PLoS One. 2015;10(3):e0118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai P, Tang X, Qin W, Ji L, Li Z. Comparison between paricalcitol and active non-selective vitamin D receptor activator for secondary hyperparathyroidism in chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Int Urol Nephrol. 2016;48(4):571‐584. [DOI] [PubMed] [Google Scholar]
- 25. Strugnell SA, Csomor P, Ashfaq A, Bishop CW. Evaluation of therapies for secondary hyperparathyroidism associated with vitamin D insufficiency in chronic kidney disease. Kidney Dis (Basel). 2023;9(3):206‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petkovich M, Melnick J, White J, Tabash S, Strugnell S, Bishop CW. Modified-release oral calcifediol corrects vitamin D insufficiency with minimal CYP24A1 upregulation. J Steroid Biochem Mol Biol. 2015;148:283‐289. [DOI] [PubMed] [Google Scholar]
- 27. Strugnell SA, Sprague SM, Ashfaq A, Petkovich M, Bishop CW. Rationale for raising current clinical practice guideline target for serum 25-hydroxyvitamin D in chronic kidney disease. Am J Nephrol. 2019;49(4):284‐293. [DOI] [PubMed] [Google Scholar]
- 28. Han T, Rong G, Quan D, et al. Meta-analysis: the efficacy and safety of paricalcitol for the treatment of secondary hyperparathyroidism and proteinuria in chronic kidney disease. Biomed Res Int. 2013;2013:320560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.