Abstract
The activity of the pradimicin derivative BMS 181184 was evaluated in a model of invasive pulmonary aspergillosis in persistently neutropenic rabbits and compared with that of amphotericin B deoxycholate. BMS 181184 at total daily doses of 50 and 150 mg/kg of body weight was at least as effective as amphotericin B at 1 mg/kg once a day in conferring survival and had comparable activity in reducing organism-mediated tissue injury and excess lung weight. Although treatment at all dosing regimens of BMS 181184 resulted in significant reductions in fungal tissue burden compared to untreated controls, equivalence to amphotericin B occurred only at the higher dosage level. Similar observations were made in bronchoalveolar lavage fluid cultures obtained postmortem. Monitoring of the animals through ultrafast computerized tomography scan revealed a marked resolution of pulmonary lesions during treatment with BMS 181184. The compound was well tolerated at all dosing regimens, and no toxicity was noted. Pharmacokinetic studies revealed nonlinear drug disposition with increased clearance at higher dosages and some evidence for extravascular drug accumulation. BMS 181184 had excellent activity in the treatment of experimental invasive pulmonary aspergillosis in persistently neutropenic rabbits, thus underscoring the potential of pradimicin derivatives in therapy of invasive aspergillosis in the neutropenic host.
Invasive pulmonary aspergillosis is an important cause of morbidity and mortality in immunosuppressed patients, particularly those with hematological malignancies or aplastic anemia or those undergoing allogeneic bone marrow transplantation (1, 11, 15, 20, 30). Current treatment options for neutropenic patients are limited and include amphotericin B deoxycholate (AmB) and, more recently, lipid formulations of AmB (28). The usefulness of AmB in this population, however, is hampered by the drug’s considerable propensity for nephrotoxicity (24) and overall poor response rates (6). The available lipid formulations of AmB, although better tolerated, are still associated with failures of antifungal efficacy (12). As a consequence, there is a continuing and urgent need for novel, more efficacious, and less toxic therapeutic approaches.
The pradimicin family of antibiotics is a new and unique class of compounds with broad-spectrum fungicidal activity in vitro (17). The pradimicins appear to act by calcium-dependent complexing with the saccharide portion of cell surface mannoproteins, leading to a perturbation of the cell membrane, leakage of intracellular contents, and, ultimately, cell death (23). Earlier studies indicate that the pradimicins possess very promising, non-cross-resistant activity against Aspergillus spp. in vitro and in animal models of systemic infection without major limiting toxicities (8, 9, 14, 18, 19).
In order to better understand the potential use of this novel class of compounds, we investigated the activity of a new pradimicin derivative, BMS 181184, in a model of invasive pulmonary aspergillosis in persistently neutropenic rabbits.
MATERIALS AND METHODS
Animals.
Female New Zealand White rabbits (Hazleton, Denver, Pa.) weighing 2 to 3 kg at the time of inoculation were used in all experiments. They were individually housed and maintained with water and standard rabbit feed ad libitum, according to National Institutes of Health guidelines for laboratory animal care (4) and in fulfillment of American Association for Accreditation of Laboratory Animal Care criteria. Vascular access was established in each rabbit by the surgical placement of a subcutaneous silastic central venous catheter, as previously described (26).
Immunosuppressive regimen and supportive care.
Cytosine arabinoside (provided by Upjohn, Kalamazoo, Mich.) was administered intravenously at 525 mg/m2 on days 1 through 5 and at 484 mg/m2 on days 8 and 9 to produce profound and persistent neutropenia (≤100 neutrophils per μl). Rabbits were closely monitored by daily inspection and complete blood counts and were treated with broad-spectrum antibiotics throughout neutropenia. Granulocyte counts were maintained at below 500/μl and were below 100/μl from day 5 onward. Concomitant platelet counts ranged from 10,000 to 25,000/μl in most rabbits. Ceftazidime (Glaxo, Research Triangle Park, N.C.) at 75 mg/kg of body weight intravenously twice daily, gentamicin (Baxter Health Care Corp., Deerfield, Ill.) at 5 mg/kg intravenously daily and vancomycin (Eli Lilly, Indianapolis, Ind.) at 15 mg/kg intravenously daily were administered from day 4 onward to prevent the emergence of invasive bacterial infections during neutropenia.
Organism and preparation of inoculum.
Rabbits received an endotracheal inoculum of 5 × 107 conidia of Aspergillus fumigatus on day 2 of the experiment after the second dose of cytosine arabinoside. A well-characterized strain of A. fumigatus (isolate 4215) obtained from a fatal case of pulmonary aspergillosis was used in all experiments.
The inoculum of A. fumigatus was prepared from a frozen isolate that was subcultured onto potato dextrose slants, which were incubated for 24 h at 37°C and then kept at room temperature for 5 days. Conidia were harvested under a laminar air flow hood with a solution of 0.025% Tween 20 (Fisher Scientific, Fair Lawn, N.J.) in normal saline, transferred to a 50-ml conical tube, and counted in a hemacytometer. The concentration was adjusted to give each rabbit a predetermined inoculum of 5 × 107 conidia of A. fumigatus in a volume of 200 to 350 μl. The concentrations of inocula were confirmed by serial dilution and culture on Sabouraud dextrose agar check plates.
Inoculation of rabbits was performed on day 2 of the experiments under general intravenous anesthesia with 0.5 to 1.0 ml of a 2:1 (vol/vol) mixture of 100 mg of ketamine (Fort Dodge Laboratories, Fort Dodge, Iowa) per ml and 20 mg of xylazine (Mobay Corp., Shawnee, Kans.) per ml for analgesia, amnesia, and muscle relaxation. Once a satisfactory level of anesthesia was reached, a Flagg 0 straight-blade laryngoscope (Welch-Allyn, Skaneateles Falls, N.Y.) was inserted until the vocal cords were clearly visualized, and the A. fumigatus inoculum was given intratracheally with a tuberculin syringe attached to a 5.25-in. Teflon catheter (Becton Dickinson, Sandy, Utah).
In vitro antifungal susceptibility.
Testing of susceptibility of the experimental isolate to both BMS 181184 and AmB was performed by broth macro- and microdilution with an inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml in RPMI 1640 medium, as previously described (7). Tubes were read after 24 and 48 h of incubation at 35°C. The MIC was defined as the lowest concentration of an antifungal compound which rendered no growth (0) to slight growth (1+) on a 0 to 4+ scale. The minimum fungicidal concentration (MFC) was determined by dispensing and streaking 100 μl of broth from tubes exhibiting no growth onto Sabouraud dextrose agar (Media Department, National Institutes of Health, Bethesda, Md.) and incubating it at 35°C. The MFC was defined as the lowest concentration of an antifungal compound with growth of ≤3 colonies. By this method, the MIC and MFC of BMS 181184 were 8 and 8 μg/ml, respectively, and those of AmB were 2 and 2 μg/ml.
Antifungal therapy.
Four treatment groups were studied: BMS 181184 at 50 mg/kg daily in one single dose (QD) (six rabbits), BMS 181184 at 17 mg/kg three times daily (TID) (five rabbits), BMS 181184 at 150 mg/kg QD (six rabbits), and BMS 181184 at 50 mg/kg TID (six rabbits). Animals treated with AmB at 1 mg/kg QD (n = 8) and untreated but infected animals (n = 9) served as controls.
BMS 181184 (250-mg vials) (Bristol-Myers Squibb, Princeton, N.J.) was provided as a lyophilized powder, maintained at 4°C, reconstituted in 1:1 (vol/vol) sterile normal saline–sterile 5% dextrose in water to a 50-mg/ml solution and given as a slow intravenous push (2 mg/s). AmB (50-mg vials) (Fungizone; Bristol-Myers Squibb) was reconstituted with 10 cm3 of distilled water, maintained at 4°C, and diluted 1:4 (vol/vol) with sterile 5% dextrose in water immediately before use. AmB preparations were sheltered from light and administered intravenously at a rate of 0.2 mg/min after normal saline loading (10 ml/kg) to decrease nephrotoxicity.
Antifungal treatment was begun 24 h after endotracheal inoculation and was administered daily throughout the experiment. Surviving rabbits were sacrificed on the 11th day postinoculation.
Outcome variables.
All experiments were evaluated according to the following outcome variables.
(i) Survival analysis.
Duration of survival in days postinoculation was recorded for each rabbit. Surviving rabbits were euthanized by pentobarbital anesthesia on day 11 postinoculation.
(ii) Morphological and microbiological postmortem studies.
The entire heart-lung block was carefully dissected and removed at autopsy. The heart was then dissected away from the lungs, leaving an intact tracheobronchial tree and lung preparation. The lungs were weighed (Mettler Instrument, Hightstown, N.J.) and inspected by two observers blinded to the treatment group who recorded the number and type of lesions, if any, in each separate lobe.
Bronchoalveolar lavage (BAL) was performed on each lung preparation by the instillation and subsequent withdrawal of 10 ml of sterile normal saline three times into the clamped trachea. A 0.1-ml sample of this fluid was cultured on Sabouraud-glucose agar.
Thereafter, a representative region of each lobe was excised for cultures and histopathologic examination. Each fragment reserved for culture was weighed individually, minced with sterile scissors, and homogenized with 2 ml of sterile saline for 1 min per tissue sample in reinforced sterile polyethylene bags (Stomacher 80; Tekmar, Cincinnati, Ohio) (25). Lung homogenates in 10−2 and 10−4 dilutions were prepared in sterile saline, and aliquots of 100 μl were plated onto Sabouraud-glucose agar and incubated at 37°C for the first 24 h and then at room temperature for another 24 h. After this, CFU of A. fumigatus were counted and recorded for each lobe and CFU per gram were calculated. The lower limit of reproducible quantitation for this method is 10 CFU/g. A finding of one colony of A. fumigatus was considered positive. A finding of no growth was entered as zero in the database. In addition, the percentage of culture-positive lobes was calculated for each rabbit.
Specimens reserved for histopathological examination were sectioned and preserved in 10% neutral buffered formalin. The fixed specimens were then embedded in paraffin, sectioned, stained with hematoxylin-eosin, periodic acid-Schiff, and Gomori methenamine silver, and microscopically examined. Hemorrhagic infarcts (dark red, consolidated lesions) corresponded microscopically to coagulative necrosis and intra-alveolar hemorrhage.
(iii) Radiographic studies.
Serial ultrafast computerized tomography (UFCT) was performed in BMS 181184-treated rabbits in order to monitor the effects of antifungal treatment on infection-mediated tissue injury during life. Findings in untreated and AmB-treated rabbits with experimental pulmonary aspergillosis monitored by UFCT have been reported in previous studies (27).
Computerized tomography (CT) was performed with a C-100XL ultrafast electron beam CT scanner (Imatron, Oyster Point, Calif.). Rabbits were transported between the laboratory animal facility and the UFCT scanner under general anesthesia. An induction dose of a 2:1 mixture (vol/vol) of 20 mg of ketamine (Fort Dodge Laboratories) and 2 mg of xylazine (Animal Health Care Division, Mobay Corp.) was administered intravenously. General anesthesia was maintained during the procedure by an additional dose of 13 mg of ketamine and 1.3 mg of xylazine (2:1, vol/vol). Rabbits were placed prone and head first on the scanning couch. Scans were made in the high-resolution, table-increment, volume-acquisition mode. Three-millimeter-thick slices were made every 4 mm. A small scan circle and a 9-cm reconstruction circle with a 512 by 512 matrix were used, which resulted in a pixel size of less than 1 mm. Scan parameters were 130 kV at 630 mA, and scan duration was 100 ms. In virtually all cases, 30 slices were sufficient to scan the entire thorax of the rabbit. Images were photographed with lung windows with a level of −600 Hounsfield units (HU) and a width of 1,800 HU.
The radiographic course of experimental infection was followed by CT scan on the day after inoculation and at days 5, 7, and 9 after inoculation, if feasible. At each session, a mean pulmonary lesion score was established by evaluating each individual rabbit’s six lung lobes. Each lobe was evaluated independently by using a score ranging from 0 to 3 with increments of 0.5, where 0 was the absence of an infiltrate and 3 was opacification of the entire lobe. Ultimately, the mean pulmonary lesion score for a given day and dosage level represents the mean of all lobes of all rabbits treated at that dosage level. All CT scans were scored by the same observer (E.F.), who was blinded to the identity of the study group of each rabbit.
(iv) Toxicity studies.
Blood samples were collected from each rabbit at days 5, 7, and 9 postinoculation. Plasma samples were stored in Sarsted tubes (Sarsted Inc., Newton, N.C.) at −70°C until all samples were processed simultaneously. Blood urea nitrogen (BUN), serum creatinine, potassium, and hepatic transaminase tests were performed on the last sample drawn from each rabbit (Analytics, Gaithersburg, Md.).
(v) Pharmacokinetic studies.
Serial plasma samples were drawn from groups of three healthy rabbits each after single BMS 181184 administration of 50 mg/kg, 17 mg/kg TID, 150 mg/kg, and 50 mg/kg TID as intravenous bolus in order to determine basic pharmacokinetic parameters. An accurate, sensitive, reproducible, and specific high-performance liquid chromatography (HPLC) assay was used for the quantitative determination of BMS 181184. The method involved precipitation of plasma protein by addition of 1.2 ml of methanol to 100 μL of standard, quality controls, or unknown rabbit plasma, followed by centrifugation and removal of the methanolic layer. The methanolic supernatant was then evaporated to dryness at 40°C under a stream of nitrogen, and the sample was reconstituted in mobile phase (50 mM sterile potassium phosphate buffer–acetonitrile [80:20, vol/vol]; Fisher Scientific) for injection onto the HPLC column. BMS 181184 eluted at approximately 3.5 to 4.5 min using a C18 analytical column maintained at 35°C (Beckman Ultrasphere; Beckman Instruments, Fullerton, Calif.) and was detected by UV absorbance at 510 nm. The lower limit of quantitation of the assay was 0.2 μg/ml. Standard curves were linear from 0.2 to 200 μg/ml, and R2 values were greater than 0.97. Accuracies were within 15%, and intra- and interday variability (precision) was <10%.
Peak plasma concentrations (Cmax) represented values measured at 0.16 h after dosing. Standard techniques were used to calculate the area under the plasma concentration-time curve (AUC). Elimination half-life (t1/2), plasma clearance (CL), and volume of distribution (V) were obtained by nonlinear least-squared regression analysis after modeling the data into a two-compartment open model with the ADAPT II software program (5). Akaike’s information criterion (31) and visual inspection of observed versus fitted concentrations were used for model discrimination. Weighting by the inverse of the square root of the observation variance provided the best fit of the data.
Statistical analysis.
Survival curves were estimated by the Kaplan-Meier product limit method, and differences between groups were analyzed by the Mantel-Haenszel chi-square test. Comparisons between proportions were done by chi-square or Fisher’s exact test, and group-to-group comparisons of continuous variables were analyzed by the Kruskal-Wallis analysis of variance (ANOVA) with Dunn’s correction for multiple comparisons, the Mann-Whitney U test, or t test, as appropriate. Values are stated as means ± standard deviations (SD) for pharmacokinetic parameters and as means ± standard errors of the means (SEM) for all other variables. All P values were two sided, and a P value of <0.05 was considered to be significant.
RESULTS
Survival.
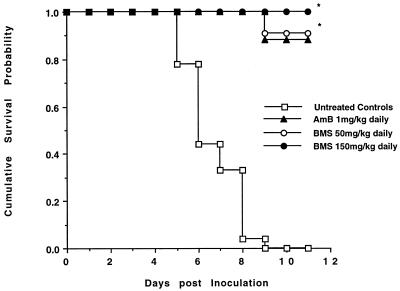
Survival among the six study groups was compared over 11 days by Kaplan-Meier analysis (Fig. 1). None of the nine untreated control rabbits survived beyond day 9 postinoculation. In contrast, 7 of 8 rabbits (88%) treated with AmB at 1 mg/kg/day survived for 11 days (P of <0.001 versus controls). Survival at day 11 postinoculation in rabbits treated with either 50 or 150 mg/kg/day was greater than that of untreated controls (P of <0.001 for both dose levels). Among the 23 rabbits treated with BMS 181184, only one did not survive until the end of the experiment (dosing regimen, 50 mg/kg QD). There was no statistically significant difference in survival between rabbits treated with BMS 181184 or AmB or among the four dosing regimens of BMS 181184.
FIG. 1.
Cumulative survival probability of rabbits treated with BMS 181184 (BMS) at a total daily dosage of 50 or 150 mg/kg or with AmB and of untreated, infected control animals. Asterisks indicate P of <0.001 compared to untreated controls.
Postmortem morphological and microbiological studies.
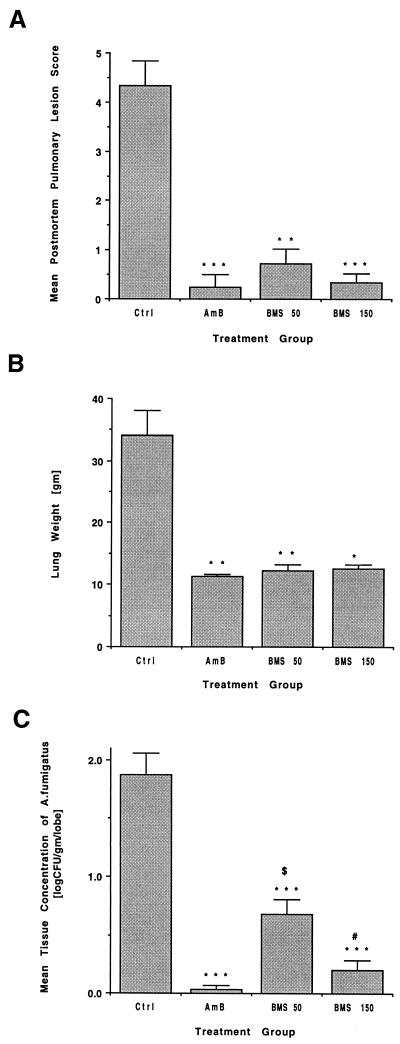
As shown in Fig. 2A, rabbits treated with either AmB (P < 0.001) or BMS 181184 at a total daily dosage of 50 mg/kg (P < 0.01) or 150 mg/kg (P < 0.001) had significantly fewer hemorrhagic infarcts than untreated controls. No statistically significant differences in the mean numbers of hemorrhagic infarcts were observed between BMS-treated and AmB-treated animals.
FIG. 2.
Responses to antifungal therapy in rabbits treated with BMS 181184 at 50 and 150 mg/kg total daily dosage versus AmB-treated rabbits and untreated control animals. (A) Mean postmortem pulmonary infarction score. (B) Mean lung weight postmortem. (C) Mean postmortem pulmonary tissue burden of A. fumigatus. ∗, P of <0.05 versus untreated controls; ∗∗, P of <0.01 versus untreated controls; ∗∗∗, P of <0.001 versus untreated controls; $, P of <0.01 versus AmB-treated rabbits; #, P of <0.05 versus rabbits treated with BMS at 50 mg/kg total daily dosage.
Similarly, the mean lung weights in rabbits treated with AmB (P < 0.01), BMS at 50 mg/kg total daily dose (P < 0.01), and BMS at 150 mg/kg total daily dose (P < 0.05) were consistently lower than in untreated controls, reflecting a reduction in excess postmortem lung weight due to hemorrhage and edema (Fig. 2B). Again, there was no difference between BMS-treated and AmB-treated rabbits.
Compared with untreated controls, rabbits treated with either daily AmB or BMS at a total daily dosage of 50 or 150 mg/kg had highly significant reductions in mean log CFU per gram per lobe (P < 0.001) (Fig. 2C). Similarly, the percentage of culture-positive lobes was 57.1% in untreated controls, 2.1% in AmB-treated rabbits (P < 0.001), 34.8% (P < 0.05) in the 50-mg/kg group and 9.7% (P < 0.001) in the 150-mg/kg group. Treatment with BMS at a total daily dosage of 150 mg/kg was equivalent to AmB in reducing fungal burden and percentage of culture-positive lobes at autopsy. In contrast, BMS administered at a total daily dosage of 50 mg/kg was significantly less effective than AmB in reducing fungal tissue burden (P < 0.01) and was associated with a significantly higher percentage of infected lobes (P < 0.001). Finally, BMS at 150 mg/kg per day was superior to BMS at 50 mg/kg/day in reducing tissue burden (P < 0.05) and in percentage of infected lung lobes at autopsy (P < 0.001).
The analysis of the antifungal effects of daily versus three times daily dosing demonstrated no appreciable differences between QD and TID dosing at the 150 mg/kg/day dose level, but a trend towards reduced tissue injury and fungal burden of the TID regimen at the 50 mg/kg dose level was noticed (Table 1). The effects of antifungal therapy on microbiologic clearance of BAL fluid cultures also are depicted in Table 1. BAL fluid cultures yielded A. fumigatus in 78% of untreated control animals versus 0% of those treated with AmB (P < 0.005). In comparison with untreated controls, BMS-treated rabbits showed statistically significant, dose-dependent reductions in positive BAL cultures (P of < 0.05 at 50 mg/kg and P of < 0.001 at 150 mg/kg total daily dose). No differences were found between BMS-treated groups and AmB-treated rabbits or between the different BMS regimens.
TABLE 1.
Effects of different dosing regimens of BMS 181184 on pulmonary tissue injury, lung weight, pulmonary tissue burden, and detection of A. fumigatus in cultures of BAL fluida
| Dosing regimen (no. of rabbits)b | Lesion score (lesions/lobe) | Lung wt (g) | Fungal burden [log (CFU/g)] | No. (%) of rabbits with culture-positive BAL fluid |
|---|---|---|---|---|
| Untreated controls (9) | 4.33 ± 0.50 | 34.12 ± 4.00 | 1.87 ± 0.19 | 7 (78) |
| AmB, 1 mg/kg (8) | 0.25 ± 0.25 c | 11.27 ± 0.42 c | 0.03 ± 0.03 c | 0 (0) e |
| BMS, 50 mg/kg QD (6) | 0.83 ± 0.47 | 13.17 ± 1.70 | 0.83 ± 0.18 a,d,f | 1 (17) a |
| BMS, 17 mg/kg TID (5) | 0.60 ± 0.40 | 11.32 ± 0.58 b | 0.51 ± 0.16 c | 2 (40) |
| BMS, 150 mg/kg QD (6) | 0.33 ± 0.21 b | 13.02 ± 0.64 | 0.20 ± 0.11 c | 0 (0) b |
| BMS, 50 mg/kg TID (6) | 0.33 ± 0.33 b | 12.00 ± 1.32 b | 0.25 ± 0.12 c | 0 (0) b |
All values are means ± SEM. Statistical significance: a, P < 0.05 versus untreated controls; b, P < 0.01 versus untreated controls; c, P < 0.001 versus untreated controls; d, P < 0.01 versus AmB-treated rabbits; e, P < 0.005 versus AmB-treated rabbits; f, P < 0.05 versus rabbits treated at 50 mg/kg TID and 150 mg/kg QD.
BMS, BMS 181184.
Radiographic studies.
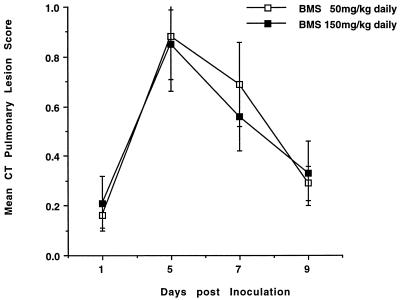
Serial monitoring of invasive aspergillosis by UFCT in rabbits treated at total daily dosages of BMS 181184 of 50 and 150 mg/kg, respectively, revealed marked resolution of pulmonary infiltrates during the course of the experiment (Fig. 3). No dose-dependent difference was seen when the two dosage levels were compared.
FIG. 3.
Mean pulmonary lesion score measured by UFCT scan in rabbits treated with BMS 181184 at total daily doses of 50 and 150 mg/kg.
Toxicity.
In comparison to untreated controls, there were no differences in the mean plasma BUN, creatinine, potassium, and hepatic transaminase levels in rabbits treated with BMS 181184 in any of the dosage groups or when dosage groups were combined for total daily dosages of 50 or 150 mg/kg/day (Table 2). In contrast, animals treated with AmB had significant increases in plasma BUN and creatinine values and significantly decreased plasma potassium compared to untreated controls and rabbits treated with BMS 181184 (data on file). Of note, rabbits receiving BMS 181184 developed variable red discoloration of body fluids and tissues.
TABLE 2.
Effects of treatment with BMS 181184 on levels of BUN, serum creatinine, potassium, aspartate aminotransferase, and alanine aminotransferase compared to untreated controls and AmB-treated rabbitsa
| Dosing regimen (no. of rabbits)b | Creatinine (mg/dl) | BUN (mg/dl) | Potassium (meq/liter) | AST (U/liter) | ALT (U/liter) |
|---|---|---|---|---|---|
| Controls (9) | 1.08 ± 0.04 | 18.6 ± 1.11 | 3.28 ± 0.12 | 8.00 ± 1.22 | 30.7 ± 4.25 |
| AmB, 1 mg/kg/day (8) | 2.62 ± 0.50c | 66.7 ± 13.88d | 2.38 ± 0.13e | 4.50 ± 0.97 | 18.8 ± 1.74 |
| BMS, 50 mg/kg/day (10) | 1.05 ± 0.03 | 16.4 ± 1.10 | 3.12 ± 0.17 | 5.80 ± 0.97 | 27.3 ± 2.68 |
| BMS, 150 mg/kg/day (12) | 1.30 ± 0.18 | 20.0 ± 3.19 | 3.50 ± 0.18 | 5.42 ± 0.64 | 30.5 ± 4.98 |
Values are means ± SEM. AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Doses of BMS 181184 (BMS) are total daily dosages.
P < 0.01 versus controls, P < 0.05 versus BMS 181184 at 150 mg/kg/day, and P < 0.001 versus BMS 181184 at 50 mg/kg/day.
P < 0.05 versus controls, P < 0.01 versus BMS 181184 at 150 mg/kg/day, and P < 0.001 versus BMS 181184 at 50 mg/kg/day.
P < 0.05 versus controls and P < 0.01 versus BMS 181184 at 150 mg/kg/day.
Pharmacokinetics.
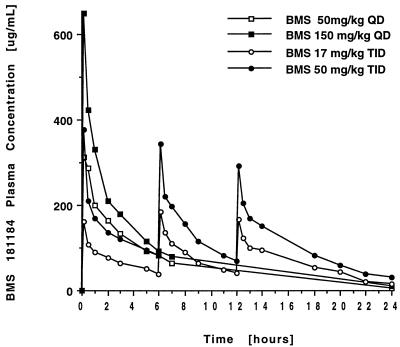
The plasma concentration-versus-time curves after administration of BMS to normal rabbits are shown in Fig. 4, and calculated values are provided in Table 3. Administration of BMS at 50 and 150 mg/kg total daily dose resulted in peak levels in plasma which were at least 20 times above both the MIC and MFC determined for the isolate of A. fumigatus used in the efficacy experiments. Increase of the dosage from 50 to 150 mg/kg QD resulted in nonproportional changes in Cmax and AUC, with increased plasma clearance (P < 0.005) and increased V (P < 0.05) at the higher dosage. Compared to QD dosing, TID administration of a total daily dose of 150 mg/kg of BMS 181184 led to an increased AUC from 0 to 24 h (AUC0–24) (P < 0.05) and apparently reduced CL.
FIG. 4.
Concentration-versus-time curves after administration of four different dosing regimens of BMS 181184.
TABLE 3.
Pharmacokinetic parameters after administration of BMS 181184 in QD and TID doses to normal rabbitsa
| BMS 181184 dosing regimen | Cmax (μg/ml)b | Cmin (μg/ml)c | AUC0–24 (μg · h/ml)d | V (liters/kg)e | CL (liters/h)f | t1/2 (h)g | Cmax/MIC | T above MIC (h) | AUC above MIC (μg · h/ml) |
|---|---|---|---|---|---|---|---|---|---|
| 50 mg/kg QD | 312 ± 23 | 5.0 ± 1.5 | 1,551 ± 253 | 0.54 ± 0.06 | 0.097 ± 0.015 | 4.08 ± 0.15 | 38.9 ± 5.1 | 21.7 ± 1.25 | 1,359 ± 248 |
| 17 mg/kg TID | 162 ± 30 | 16 ± 8.7 | 1,617 ± 134 | 0.44 ± 0.11 | 0.090 ± 0.011 | 3.69 ± 1.16 | 20.20 ± 3.8 | 24 | 1,424 ± 131 |
| 150 mg/kg QD | 648 ± 16 | 9 ± 2.0 | 2,130 ± 289 | 0.79 ± 0.09 | 0.259 ± 0.04 | 2.31 ± 0.38 | 81.0 ± 2.0 | 24 | 1,938 ± 284 |
| 50 mg/kg TID | 375 ± 41 | 29 ± 3.0 | 2,746 ± 162 | 0.42 ± 0.19 | 0.196 ± 0.026 | 1.91 ± 0.82 | 46.4 ± 11.0 | 24 | 2,554 ± 158 |
Values are expressed as means ± SD. Group-to-group comparisons were performed by Kruskal-Wallis ANOVA, as follows: Cmax, Cmin, Cmax/MIC, and T above MIC, P < 0.001; AUC0–24, CL, and AUC above MIC, P < 0.005; V and t1/2, P < 0.05.
P < 0.01 for 150 mg/kg QD (150 QD) versus 50 TID and 50 QD versus 17 TID; P < 0.001 for 150 QD versus 50 QD (t test).
P < 0.001 for 50 TID versus 150 QD (t test).
P < 0.05 for 50 TID versus 150 QD (t test).
P < 0.05 for 150 QD versus 50 TID, 50 QD, and 17 TID (t test).
P < 0.005 for 150 QD or 50 TID versus 50 QD or 17 TID; not significant for 150 QD versus 50 TID (t test).
P < 0.05 for 150 QD and 50 TID versus 50 QD; not significant for 150 QD versus 50 TID (t test).
DISCUSSION
The results of this study demonstrate potent antifungal activity of BMS 181184 in an experimental model of invasive pulmonary aspergillosis in persistently neutropenic rabbits. In comparison to untreated controls, BMS 181184 was at least as effective as standard AmB in conferring survival and had activity comparable to AmB in reducing organism-mediated tissue injury and excess lung weight postmortem. Although treatment with all regimens of BMS 181184 led to a significant reduction in fungal tissue burden, equivalence to AmB was observed only at the higher dosage level. Similar findings were noted in the microbiological analysis of BAL fluid obtained postmortem. Significant resolution of pulmonary lesions during treatment with BMS 181184 was documented by UFCT scan. In comparison to AmB-treated rabbits in this study, BMS-treated animals had no nephrotoxicity. Elevations of hepatic transaminases were not observed in any group.
Preliminary pharmacokinetic studies in uninfected animals revealed dose-dependent disposition of the drug with enhanced CL with increasing dosage. Peak plasma levels were more than 20-fold above both the MIC and MFC of the isolate of A. fumigatus used in the infection model. The V value approximated that of total body water. However, there was a marked increase in V at the 150-mg/kg QD dosage level, suggesting some form of increased extravascular drug disposition; separately submitted studies performed in our laboratories revealed that the kidney may be the site of that accumulation. Dividing the total daily dose of 150 mg/kg into a TID regimen resulted in a trend toward diminished clearance and increased AUC0–24 compared to QD administration; a possible explanation for this observation would be the existence of a saturable tubular reabsorption process and/or saturable protein binding, leading to an increased renal CL of the compound after bolus administration of higher dosages.
The requirement of relatively high doses of BMS 181184 for effective treatment of invasive aspergillosis correlates with the relatively high MIC and MFC values compared to those of AmB. By the broth macrodilution reference methods recommended by the National Committee for Clinical Laboratory Standards, MICs for all six A. fumigatus strains and one strain of Aspergillus nidulans tested were 8 μg/ml. Aspergillus flavus (n = 3) and A. niger (n = 4) were slightly less susceptible (MICs, ≥16 μg/ml). Whereas AmB was fungicidal against all of the nine Aspergillus strains tested, BMS 181184 reduced cell counts for only six of the nine strains on the basis of MFCs at which 95% of the isolates were killed (MFC95) and MFC90, which were within twofold of the MICs (8). By comparison, MICs of BMS 181184 for >95% of all yeasts tested were ≤8 μg/ml (8, 13), and in all strains tested, the compounds’ MFC99 were no more than twofold greater than the MICs (8). With a related, RPMI-based macrodilution method, geometric mean MICs for 54 isolates of Aspergillus were 9.08 μg/ml (range, 4 to 16 μg/ml). BMS 181184 was fungicidal (killing of ≥98%) in only 37% of isolates tested. Susceptibilities varied between species, with A. fumigatus (n = 35) being the most susceptible to the drug (geometric mean MIC, 7.99; range, 4 to 16 μg/ml) (16). The observed differences in susceptibility may be plausibly correlated with inter- and intraspecies differences in cell wall mannoprotein content. Whereas the cell walls of yeasts in general contain abundant amounts of these molecules, they are expressed only in traces in some of the filamentous fungi (2, 8).
The requirement of higher doses of BMS 181184 for effective treatment of invasive aspergillosis is also obvious from the available preclinical data. After a single intravenous bolus dose immediately after intravenous inoculation into normal mice, the calculated protective dose of BMS 181184 necessary to ensure 50% survival at 20 days (PD50) was 8.8 and 31 mg/kg for Candida albicans and A. fumigatus, respectively. In cyclophosphamide-treated animals, the PD50 was substantially higher, increasing to 31 mg/kg in C. albicans and to >50 mg/kg in A. fumigatus. However, when the drug was given for two consecutive days after inoculation, the PD50 for A. fumigatus dropped to 23 mg/kg, resulting in survival of 80% of animals with a dose of 50 mg/kg (19).
In a model of systemic aspergillosis in transiently neutropenic rabbits, BMS 181184 was given at 50 mg/kg QD, 75 mg/kg QD, and 50 mg/kg TID starting 24 h after intravenous challenge for five consecutive days. Whereas the infection was uniformly lethal in untreated controls, survival in BMS-treated rabbits increased in a dose-dependent manner from 27 to 57 and 100%, respectively. Significant reduction of fungal burden in liver and kidney but not lung was observed with 50 mg/kg TID. However, although mortality was high (67%), microbiological efficacy was highest and most consistent in rabbits treated with AmB (1 mg/kg) (21). BMS 181184 has also been compared to itraconazole as the reference azole in mice rendered immunocompromised by triamcinolone and challenged systemically with A. fumigatus. Whereas BMS 181184 (25 mg/kg) and AmB (0.8 mg/kg) increased the survival time in comparison to untreated controls, itraconazole (100 mg/kg) was not effective. BMS 181184 was superior to itraconazole but less active than AmB in clearing tissue (3).
A high therapeutic index permits the administration of relatively large doses of BMS 181184 to animals with minimal toxicity. In comparison with AmB, the compound was 40- to 50-fold less active but at least 130-fold less toxic on a milligrams-per-kilogram basis (19). Although reversible granulomatous inflammation in various tissues in rats and dogs, renal tubular degeneration in dogs at high doses not associated with any renal function test abnormalities, and apparently species-specific hemolysis in Cynomolgus monkeys at high doses have been found in unpublished toxicity studies (29), we and other investigators (10, 21, 22) did not observe significant toxicities at the doses used in the experiments, except for red discoloration of body fluids and some tissues.
In conclusion, BMS 181184 was well tolerated and demonstrated efficacy comparable to that of AmB in a model of invasive pulmonary aspergillosis in persistently neutropenic rabbits closely resembling conditions of human infection. Although early phase I studies in human volunteers revealed hepatic toxicity associated with BMS 181184, perhaps less-toxic congeners of this promising family of antifungal antibiotics with identical or even improved therapeutic efficacy against invasive pulmonary aspergillosis will be developed.
ACKNOWLEDGMENTS
We express our gratitude to Kristina Kligys and William Love for excellent technical assistance.
REFERENCES
- 1.Anaissie, E. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14(Suppl. 1):43–53. [DOI] [PubMed]
- 2.Bartnicki-Gracia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- 3.Clark J M, Ferraro C, Stolfi T, Wewiorski S, Tsai Y H. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Efficacy of BMS-181184 in experimental fungal infections in mice, abstr. 389; p. 190. [Google Scholar]
- 4.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. National Institutes of Health publication no. 85–23. National Institutes of Health; 1985. , Public Health Service, U.S. Department of Health and Human Services, Washington, D.C. [Google Scholar]
- 5.D’Argenio D Z, Schumitzky A. Biomedical simulations resource. University of Southern California, Los Angeles, Calif. 1990. Adapt II user’s guide. [Google Scholar]
- 6.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin E, Dixon D, Fothergil A, Paetznik V, Peter J, Rinaldi M, Walsh T J. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung-Tomc J C, Minassian B, Huczko E, Kolek B, Bonner D P, Kessler R E. In vitro antifungal and fungicidal spectra of a new pradimicin derivative, BMS-181184. Antimicrob Agents Chemother. 1995;39:295–300. doi: 10.1128/aac.39.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furumai T, Saitoh K, Kakushima M, Yamamoto S, Suzuki K, Ikeda C, Kobaru S, Hatori M, Oki T. BMS 181184, a new pradimicin derivative. Screening, taxonomy, directed biosynthesis, isolation and characterization. J Antibiot. 1993;46:265–274. doi: 10.7164/antibiotics.46.265. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez C E, Shetty D, Giri N, Love W, Kigys K, Lyman C, Bacher J, Walsh T J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of pradimicin against disseminated candidiasis, abstr. F181; p. 131. [Google Scholar]
- 11.Groll A H, Shah P M, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33:23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 12.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22(Suppl. 2):S133–S144. [DOI] [PubMed]
- 13.Hoban D, Kabani A, Karlowsky J, Friesen M, Harding G, Zhanel G. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro antifungal activity of BMS181184 against systemic isolates of Candida, Cryptococcus and Blastomyces spp., abstr. F179; p. 131. [Google Scholar]
- 14.Kakushima M, Masuyoshi S, Hirano M, Shinoda M, Ohta A, Kamei H, Oki T. In vitro and in vivo antifungal activities of BMY-28864 a water-soluble pradimicin derivative. Antimicrob Agents Chemother. 1991;35:2185–2190. doi: 10.1128/aac.35.11.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McWhinney P H, Kibbler C C, Hamon M D, Smith O P, Ghandi L, Berger L A, Walesby R K, Hoffbrand A V, Prentice H G. Progress in the diagnosis and management of aspergillosis in bone marrow transplantation: 13 years experience. Clin Infect Dis. 1993;17:397–404. doi: 10.1093/clinids/17.3.397. [DOI] [PubMed] [Google Scholar]
- 16.Oakley K L, Moore C B, Denning D W. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Activity of pradimicin BMS-181184 against Aspergillus spp., abstr. F180; p. 131. [Google Scholar]
- 17.Oki T, Konishi M, Tomatsu K, Tomita K, Saitoh K, Tsunakawa M, Nishio M, Myaki T, Kawaguchi H. Pradimicin, a novel class of potent antifungal antibiotics. J Antibiot. 1988;11:1701–1704. doi: 10.7164/antibiotics.41.1701. [DOI] [PubMed] [Google Scholar]
- 18.Oki T, Tenmyo O, Hirano M, Tomatsu K, Kamei H. Pradimicins A, B and C: new antifungal antibiotics. II. In vitro and in vivo biological activities. J Antibiot. 1990;7:763–770. doi: 10.7164/antibiotics.43.763. [DOI] [PubMed] [Google Scholar]
- 19.Oki T, Kakushima M, Hirano M, Takahashi A, Ohta A, Masuyoshi S, Hatori M, Kamei H. In vitro and in vivo antifungal activities of BMS-181184. J Antibiot. 1992;45:1512–1517. doi: 10.7164/antibiotics.45.1512. [DOI] [PubMed] [Google Scholar]
- 20.Pannuti C S, Gingrich R D, Pfaller M A, Wenzel R P. Nosocomial pneumonia in adult patients undergoing bone marrow transplantation: a 9-year study. J Clin Oncol. 1991;9:77–84. doi: 10.1200/JCO.1991.9.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Patterson T F, Kirkpatrick W R, McAtee R K. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. The activity of pradimicin (BMS-181184) in experimental invasive aspergillosis, abstr. B51; p. 31. [Google Scholar]
- 22.Restrepo M, Najvar L, Bocanegra R, Luther M, Graybill J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Comparison of the efficacy of BMS-181184 (BMS) and amphotericin B (AMB) against hematogenous Candida tropicalis (CT) infection in immunosuppressed mice, abstr. F183; p. 131. [Google Scholar]
- 23.Sawada Y, Numata K, Murakami T, Tanimichi H, Yamamoto S, Oki T. Calcium-dependent anticandidal action of pradimicin A. J Antibiot. 1990;43:715–721. doi: 10.7164/antibiotics.43.715. [DOI] [PubMed] [Google Scholar]
- 24.Sawaya P D, Briggs J P, Schnermann J. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J Am Soc Nephrol. 1995;6:154–164. doi: 10.1681/ASN.V62154. [DOI] [PubMed] [Google Scholar]
- 25.Walsh T J, McEntee C, Dixon D M. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J Clin Microbiol. 1987;25:931–932. doi: 10.1128/jcm.25.5.931-932.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Med. 1988;38:467–470. [PubMed] [Google Scholar]
- 27.Walsh T J, Garrett K, Feuerstein E, Girton M, Allende M, Bacher J, Francesconi A, Schaufele R, Pizzo P A. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob Agents Chemother. 1995;39:1065–1069. doi: 10.1128/aac.39.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh T J, Hiemenz J W, Anaissie E. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin N Am. 1996;10:365–400. doi: 10.1016/s0891-5520(05)70303-2. [DOI] [PubMed] [Google Scholar]
- 29.Walsh T J, Giri N. Pradimicins: a novel class of broad-spectrum antifungal compounds. Eur J Clin Microbiol Infect Dis. 1997;16:93–97. doi: 10.1007/BF01575126. [DOI] [PubMed] [Google Scholar]
- 30.Weinberger M, Elattar I, Marshall D, Steinberg S M, Redner R L, Young N S, Pizzo P A. Patterns of infection in patients with aplastic anemia and the emergence of Aspergillus as major cause of death. Medicine (Baltimore) 1992;71:24–43. doi: 10.1097/00005792-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]