Abstract
Context
Single ACTH measurements have limited ability to distinguish patients with Cushing's disease (CD) from those in remission or with other conditions.
Objective
To investigate the changes in ACTH levels before and after transsphenoidal surgery (TSS) to identify trends that could confirm remission from CD and help establish ACTH cutoffs for targeted clinical trials in CD.
Design
Retrospective analysis of CD patients who underwent TSS from 2005 to -2019.
Setting
Referral center.
Patients
CD patients (n = 253) with ACTH measurements before and after TSS.
Interventions
TSS for CD.
Main Outcome Measures
Remission after TSS.
Results
Remission was observed in 223 patients after TSS. Those in remission had higher ACTH variability at AM (P = .02) and PM (P < .001) time points compared to nonremission. The nonremission group had a significantly narrower diurnal range compared to the remission group (P = <.0001). A decrease in plasma ACTH of ≥50% from mean preoperative levels predicted CD remission after TSS, especially when using PM values. The absolute plasma ACTH concentration and ratio of preoperative to postoperative values were significantly associated with nonremission after multivariable logistic regression (adj P < .001 and .001, respectively).
Conclusions
Our findings suggest that ACTH variability is suppressed in CD, and remission from CD is associated with the restoration of this variability. Furthermore, a decrease in plasma ACTH by 50% or more may serve as a predictor of remission post-TSS. These insights could guide clinicians in developing rational outcome measures for interventions targeting CD adenomas.
Keywords: ACTH, variability, diurnal variation, circadian, Cushing's disease
Cushing's disease (CD) is characterized by hypercortisolism caused by excessive ACTH secretion from a pituitary corticotrope tumor. In healthy individuals, pituitary corticotrophs secrete ACTH in pulsatile bursts that exhibit significant diurnal variation, with minimal levels during sleep and peak levels after waking (1).
In contrast, CD patients have elevated plasma ACTH levels during sleep and variable elevations upon waking, leading to both an increased and contracted ACTH diurnal range (1, 2). A reduced ACTH coefficient of variation (CV) below 40% can distinguish CD from pseudo-Cushing's states with 97% sensitivity [confidence interval (CI), 83–100%] and 100% specificity (CI, 71–100%) (1) While the number of ACTH pulses in CD patients may be similar to (3, 4) or higher than (4, 5) those in healthy individuals, the overall ACTH amplitude increases, resulting in a higher 24-hour mean level (3–5).
Single ACTH level measurements have limited ability to predict short- or long-term remission after CD surgery due to the substantial overlap in ACTH levels between normal and diseased states. The sensitivity of timed plasma ACTH for predicting endocrine outcomes is approximately 80% (6, 7). The utility of analyzing dynamical postsurgical patterns to detect endocrine remission CD adenoma surgery has not been previously studied.
We hypothesized that ACTH patterns would normalize shortly after successful resection of CD pituitary adenomas and could serve as an additional confirmatory marker to guide clinical decision-making. In addition, the results of this study could help identify ACTH cutoffs that could be used to test efficacy of therapies targeting CD adenoma ACTH secretion (8). To test this hypothesis, we assessed the intra-patient variability and diurnal range of ACTH in CD patients and analyzed the changes in variability upon remission from CD.
Materials and Methods
Patient Selection
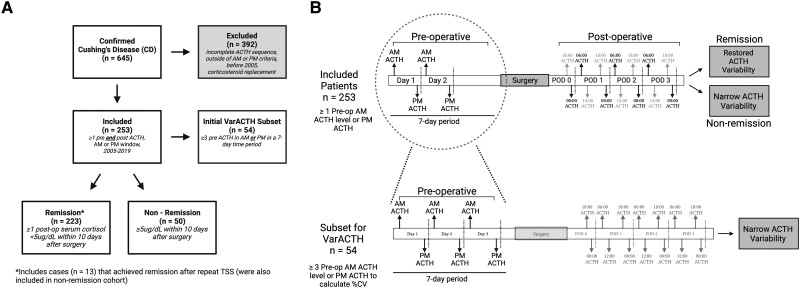
We retrospectively evaluated the National Institutes of Health (NIH) Clinical Center patients with histopathologically confirmed diagnosis of CD after transsphenoidal surgery (TSS) from 2005 to 2019 (n = 645). Of these, 253 patients (pediatric and adult) met the criteria for inclusion: each had 1 or more preoperative and postoperative plasma ACTH values drawn between 4 Am and 8 Am (AM ACTH) and 10 Pm and 2 Am (PM ACTH), as well as postoperative cortisol values to determine remission or lack thereof. This study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health, Bethesda (Clinical Trial identifier NCT00060541). Written informed consent was obtained from each patient for research study participation. The NIH Biomedical Translational Informatics system was used for data extraction.
Diagnosis of Cushing's Disease
Most patients underwent initial hypercortisolism evaluation by their home endocrinologists prior to arriving at the NIH. Cushing's syndrome was diagnosed by at least 2 abnormal test results among 2 late-night salivary cortisol values, 2 24-hour urine free cortisol values, and low-dose dexamethasone suppression testing (DST) (9).
At the NIH, confirmatory diagnostic testing for ACTH-dependent CS included evaluation of serum cortisol and ACTH at 7:30 Am, 8:00 Am and at 11:30 Pm and midnight. These results were used in this study to evaluate the variability in ACTH concentrations in the morning and evening. From 2005 to 2015, ACTH assays were outsourced to another academic center that provided third-party laboratory services (Mayo Medical Laboratories, Rochester, MN). Apart from pre-2015 ACTH, all hormone assays were performed by the Department of Laboratory Medicine at the NIH Clinical Center. ACTH assays were completed by the NIH Clinical Center from 2015 to 2019. Cortisol was measured by immunoassay [AM reference range (RR) 5–25 ug/dL; PM cortisol RR: <7.5 mcg/dL) and ACTH was measured by the Nichols Advantage Immunochemiluminometric Assay or chemiluminescence immunoassay on the Siemens Immulite 2500 analyzer (AM RR 2005 to 2015: 0.0 to 46.0 pg/mL; 2015 to 2019: 5 to 46 pg/mL) (10, 11).
High-resolution (1- to 1.5-mm slice thickness) magnetic resonance imaging of the pituitary was performed to identify adenomas using standard T1 spin echo sequences, as well as spoiled gradient-recalled acquisition sequences (12) A probable pituitary source of ACTH was determined by 8 mg overnight DST and ovine corticotropin-releasing hormone stimulation test as previously described (13–15). Confirmatory inferior petrosal sinus sampling (16) was performed in all patients, except when both DST and ovine corticotropin-releasing hormone tests results were consistent with CD and magnetic resonance imaging showed an adenoma greater than 6 mm.
Postoperatively, ACTH values were obtained at 6-hour intervals for at least 96 hours beginning with a blood draw in the recovery room following surgery.
ACTH Measurements
Patients classified as adults if they were age 18 years or older at the time of TSS. Patients were in remission if found to have a nadir AM serum cortisol <5 mg/dL within 10 days after surgery (17, 18). Repeat surgery was performed on some patients who did not meet criteria for remission after their first surgery and were treated with repeat TSS during the same admission. Patients who entered remission following same-admission repeat surgery were considered as both nonremission and remission patients.
Postoperatively, serum cortisol and ACTH levels were obtained at 6-hour intervals through a patient’s hospital stay starting on postoperative day (POD) 0 until the patient was discharged or required glucocorticoid administration (19–21). Replacement glucocorticoid administration was withheld if the patient developed symptomatic hypocortisolemia. ACTH measurements obtained in the morning (4 Am–8 Am ) were designated as AM, and values obtained at midnight (10 Pm –2 Am) were designated as PM. ACTH variability was defined as the CV of ACTH values, where CV is equal to the standard deviation of the (AM or PM) values divided by the mean of the values.
Coefficient of Variation
The median intra-patient CV for both AM and PM ACTH were initially established in a subset (n = 54) of the total cohort who had 3 or more AM and/or PM ACTH values obtained during a 7-day period prior to undergoing surgery. Intra-patient median CV were assessed for reliability using intraclass correlation coefficient (22).
We then compared the preoperative and postoperative CV at defined time points (AM and PM) of the entire cohort (n = 253). Intra-patient (individual patient) ACTH values at each time point were evaluated and represented as the median CV. Intra-patient and inter-patient values are represented as mean ACTH ± SD. CV was compared across the preoperative dataset and the postoperative dataset for patients in remission and in nonremission using a one-way ANOVA, with the Kruskal-Wallis or the Sidak method for unequal variance.
ACTH diurnal range was defined as the intra-patient CV of ACTH over a single 24-hour period, calculated by dividing the SD of the combined AM and PM values by the mean of the ACTH values. Diurnal range provides a way to capture the intra-patient diurnal range, ie, the amount of variation between the peak (AM) and nadir (PM) ACTH values within an individual.
Statistical Analysis
Baseline clinical characteristics are presented as percentages or means with SDs. They were compared using 2-sample tests of proportions or 2-sample t-tests with unequal variance where appropriate, using Welch approximation for degrees of freedom (23). Lab values that were below the limits of detection were treated as censored data and substituted with the limit of detection divided by 2 (24, 25).
The ability of ACTH variables to predict nonremission were evaluated by univariable and multivariable logistic regression adjusting for age, female sex, and White race, with nonremission as the dependent variable. ACTH variables were evaluated for morning and evening results on postoperative days 0 to 3 and included the postoperative ACTH concentration, the difference between the corresponding timed preoperative and postoperative ACTH value, and the ratio of the average of preoperative ACTH value (AM or PM) divided by the corresponding AM or PM postoperative value. Predictive models were evaluated for goodness-of-fit using area under the receiver operating characteristic curves (AUROCs).
Two-tailed P-values <.05 were considered statistically significant with all confidence intervals as 95%. Statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad Software, La Jolla, CA) and STATA 14/IC software package (Stata- Corp LP, College Station, TX).
Results
Our study included a total of 253 patients with an average age of 28 ± 17 years (ranging from 6 to 77 years). Among them, 68.4% were female (n = 173), and about half were adults (n = 137, 53%). Out of these 253 patients, remission was achieved in 223 patients, with 13 requiring a second (repeat) TSS during the same hospital admission after initial surgery failure (totaling 282 TSS procedures). Nonremission was documented in 50 patients, including those who eventually achieved remission after repeat TSS during the same hospital stay (n = 13). Overall, 88% of patients (n = 223) achieved remission after initial or repeat TSS (Table 1).
Table 1.
Demographics of patients who underwent transsphenoidal surgery for Cushing's disease at the National Institutes of Health Clinical Center from 2005 to 2019 (n = 253) and included in this study
| Demographics | Nonremission n = 50 | Remissiona n = 223 |
P-value |
|---|---|---|---|
| Age (mean ± SD) | 31.2 ± 17.6 | 27.3 ± 17.5 | .1556 |
| Female (%) | 64.7 | 66.8 | .7475 |
| Race | |||
| White (%) | 62.8 | 71.8 | .2009 |
| Black (%) | 17.7 | 11.7 | .2879 |
| Asian (%) | 3.9 | 3.1 | .7820 |
| American Indian/Alaskan (%) | 0.00 | 0.9 | .1578 |
| Hawaiian/Pacific Islander (%) | 2.0 | 0.5 | .4535 |
Remission is defined as ≥ 1 postoperative serum cortisol <5 ug/dL within 10 days after surgery.
P-values derived from two-tailed T-tests with Welch's correction for unequal variance.
Preoperative Intra-patient ACTH Variability in Cushing's Disease
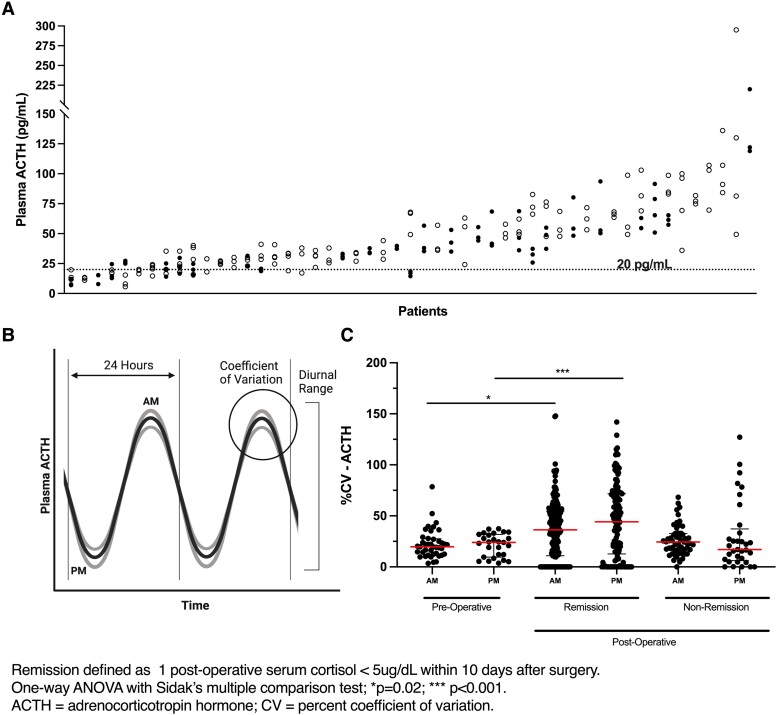
Fifty-four patients had at least 3 preoperative ACTH measurements on different days in the morning (n = 41) and/or evening (n = 27). (Figure 1A and 1B; Table 1). The median CV of ACTH intra-patient variability (Fig. 1B) was 19.8% (95% CI:12.5-27.5) for morning values and 24.0% (95% CI: 9.6-31.8) for evening values (Fig. 1C). CV was not associated with patient age (AM: P = .174; PM: P = .954), the number of ACTH values per patient (AM: P = .433; PM: P = .403), or the duration (number of days) over which ACTH measurements were drawn (AM: P = .217; PM: P = .803) (Supplementary Table S1) (26). The intraclass correlation coefficient of the AM data set was 0.59 and of the PM data set was 0.80, which demonstrates a good and excellent reliability, respectively (27).
Figure 1.
Data collection schematic. (A) Flow chart for patient data collection showing inclusion and exclusion criteria. (B) Schematic showing sequence of perioperative ACTH data collection and subset for VarACTH (n = 54) for determination of ACTH variability in Cushing's disease. Panel B was generated with Biorender.com.
Intra-patient ACTH Variability Rises in Remission
We expanded our analysis to include the entire cohort (n = 253) and compared it to the initial subset (n = 54). We observed that postoperative remission status was associated with increased ACTH variability at each clock time point. Patients in remission demonstrated higher ACTH variability at both morning [median CV: 36.31 (CI: 32.53–42.90) vs 24.38 (CI: 18.85–28.22, P = .02)] and evening time points [median CV: 44.24 (CI: 31.53–55.19) vs 17.02 (CI: 10.26–25.37, P < .001)] compared to patients without remission (Table 2, Fig. 1C).
Table 2.
ACTH variation in Cushing's diseasea
| Postoperative | |||||
|---|---|---|---|---|---|
| Preoperative n = 54 | Nonremissionn = 50 | P-value | Remissiona n = 223 | P-value | |
| Intra-patient ACTH variability | |||||
| median CV | |||||
| AM (4 Am–8 Am) | 19.8 (IQR: 12.5–27.5) |
24.4 (IQR: 16.1–32.8) |
0.962 |
36.3
(IQR: 10.9–54.7) |
.02 |
| PM (10 Pm–2 Am) | 24.0 (IQR: 9.6–31.8) |
17.0 (IQR: 6.2–37.2) |
0.676 |
44.2
(IQR: 12.6–71.8) |
<.001 |
| Inter-patient ACTH variability | |||||
| mean ACTH | |||||
| AM (4 Am–8 Am) | 42.4 ± 27.0 | 42.2 ± 31.4 | 0.7403 | 8.6 ± 8.9 | <.0001 |
| PM (10 Pm–2 Am) | 40.1 ± 29.9 | 36.0 ± 31.1 | 0.7491 | 11.2 ± 10.0 | <.0001 |
Abbreviations: CV, coefficient of variation; IQR, interquartile range.
Remission defined as ≥1 postoperative serum cortisol <5 ug/dL within 10 days after surgery.
P-values are from one-way ANOVA using the Sidak method for unequal variance, showing the significance or lack thereof compared to the preoperative CV for the corresponding time point. Statistically significant findings in boldface font.
Diurnal ACTH Range is Regained in Remission
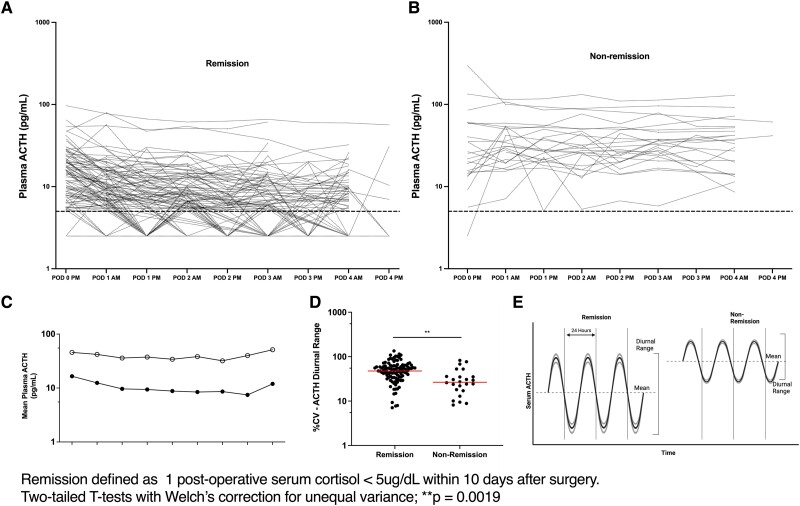
We then analyzed the effect of successful CD treatment on diurnal range of ACTH (Fig. 1C) in 135 evaluable patients. This analysis was carried out using postoperative paired morning and midnight plasma ACTH values within a single 24-hour period for at least 96 hours in patients with and without remission (Fig. 2A and 2B). The nonremission group (n = 24) had a significantly narrower intra-patient diurnal range with a median CV of 28.33 (CI: 25.72–42.89) while the patients who entered remission (n = 114) had a diurnal median CV of 87.77 (CI: 88.38-102.1, P = <.0001) (Fig. 2C). These findings confirm previous observations of reduced diurnal ACTH range in CD (1, 2). As expected, we found resumption of the physiologic nocturnal nadir of plasma ACTH levels following successful remission from CD (Fig. 2D).
Figure 2.
Coefficient of variation for ACTH is restored with remission from Cushing’s disease. (A) Preoperative plasma ACTH measurements obtained on multiple (at least 3) days during the workup for Cushing's disease in 253 patients. The open circles show the Am ACTH values and the closed circles show the midnight values for individual days. Each column represents a patient included in this study. (B) Conceptual representation of the intra-patient variability in plasma ACTH levels and its relationship to the diurnal ACTH changes. (C) The median (bar) coefficient of variation and interquartile range (range bars) of preoperative plasma ACTH and postoperative 6:00 Am and 12:00 Am for patients in remission and nonremission.
Predicting Remission Using ACTH
We further analyzed the remission prediction capability of dynamic ACTH parameters. Initial analysis of the data from the 54 patients, who were used to establish pretreatment ACTH CV, showed that a reduction in plasma ACTH by 50% or more from average preoperative levels effectively predicted remission from CD following TSS, particularly when using evening (PM) values (Supplementary Fig. S1) (26). Moreover, this ACTH reduction by ≥50% preceded the occurrence of undetectable cortisol (≤5 ug/dL) by 6 hours in at least 30% (15/54) of patients (26).
Subsequently, we compared postoperative AM and PM ACTH levels for the entire cohort (n = 253) with their respective preoperative averaged AM or PM values (Table 2). Compared to their average preoperative morning or midnight ACTH levels, patients who achieved remission exhibited significant reductions in the corresponding postoperative ACTH levels (P < .0001, Table 2). Mean ACTH values for each POD were found to be lower in remission patients (n = 114) as compared to nonremission patients (n = 24) (Fig. 3).
Figure 3.
ACTH diurnal range is restored in remission from Cushing’s disease. Intra-patient plasma ACTH diurnal variation for postoperative patients with paired ACTH values (Am and Pm ACTH on the same day). Top panel shows the diurnal variation for each patient, patients that achieved remission (A) and patients in nonremission (B). (C) Mean postoperative plasma ACTH for all paired (Am and Pm) patients in remission (closed dots) and nonremission (open dots). (D) Diurnal variation represented as coefficient of variation, showing the median (bar) for the paired patients in remission and nonremission. (E) Conceptual representation of the differences in diurnal variation of absolute ACTH values in remission patients compared to nonremission patients.
ACTH Variables as Indicators of Nonremission
To evaluate the predictive power of ACTH variables for remission, we performed a formal receiver operating characteristic (ROC) analysis. Changes in absolute plasma ACTH and the ratio of postoperative to preoperative ACTH on POD 0 to 3 at all clock time points were found to be effective in identifying patients who achieved clinical remission (Table 3). Both absolute plasma ACTH concentration and the ratio of preoperative (“Bsl” in Table 3) to postoperative (AM POD 1) values (n = 157) were associated with nonremission after multivariable logistic regression, with adjusted P values of <.001 and .001, respectively (Table 3).
Table 3.
Variables predicting nonremissiona
| OR | 95% CI | P-value | Adj OR | Adj 95% CI | Adj P-value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age | 0.99 | 0.98–1.01 | .1587 | — | — | — |
| Female | 0.96 | 0.58–1.60 | .8687 | — | — | — |
| White | 0.69 | 0.39–1.24 | .2037 | — | — | — |
| ACTH variables | ||||||
| POD 1 Am | ||||||
| ACTH | 1.08 | 1.05–1.11 | <.001 | 1.10 | 1.06–1.14 | <.001 |
| Ratio to Bsl | 1.02 | 1.01–1.02 | <.001 | 1.02 | 1.01–1.02 | .001 |
| Difference from Bsl | 1.02 | 1.00–1.03 | .027 | 1.03 | 1.00–1.03 | .041 |
| POD 0 Pm | ||||||
| ACTH | 1.04 | 1.02–1.06 | .001 | 1.05 | 1.02–1.07 | <.001 |
| Ratio to Bsl | 1.01 | 1.00–1.01 | .004 | 1.01 | 1.00–1.02 | .007 |
| Difference from Bsl | 1.02 | 1.00–1.03 | .013 | 1.02 | 1.00–1.04 | .018 |
| POD 1 Pm | ||||||
| ACTH | 1.12 | 1.07–1.17 | <.001 | 1.16 | 1.09–1.23 | <.001 |
| Ratio to Bsl | 1.03 | 1.02–1.05 | <.001 | 1.03 | 1.02–1.05 | <.001 |
| Difference from Bsl | 1.03 | 1.01–1.05 | .014 | 1.02 | 1.00–1.04 | .044 |
| POD 2 Pm | ||||||
| ACTH | 1.11 | 1.05–1.16 | <.001 | 1.15 | 1.07–1.23 | <.001 |
| Ratio to Bsl | 30.20 | 5.81–157.12 | <.001 | 37.60 | 5.85–241.72 | <.001 |
| Difference from Bsl | 1.02 | 1.00–1.05 | .035 | 1.02 | 1.00–1.05 | .036 |
| POD 3 Pm | ||||||
| ACTH | 1.12 | 1.04–1.21 | .002 | 1.11 | 1.03–1.20 | .005 |
| Ratio to Bsl | 87.20 | 5.04–1507.29 | .002 | 149.66 | 4.03–5560.87 | .007 |
| Difference from Bsl | 1.07 | 1.01–1.13 | .016 | 1.10 | 1.01–1.19 | .031 |
Abbreviations: Bsl, baseline, preoperative ACTH for the corresponding time point; CI, confidence interval; OR, odds ratio; POD, postoperative day.
Remission defined as ≥ 1 post-operative serum cortisol < 5ug/dL within 10 days after surgery.
P-values are from multivariable logistic regression adjusting for age, female, and White race, with nonremission as the dependent variable. Statistically significant findings in boldface font.
To determine the association between the absolute postoperative ACTH and change from preoperative levels, we performed an AUROC analysis (Table 4). There was an excellent correlation for the morning of POD 1 AUROC ACTH: 0.91 (CI: 0.86–0.96) and ACTH ratio: 0.87 (CI: 0.81–0.93). Midnight values on POD 3 provided the highest ROC for ACTH (0.93; CI: 0.85–1.00) and ACTH ratio (0.91; CI: 0.82–1.00); however, the sample size was relatively small (n = 40) (Table 4).
Table 4.
Area under the curve for variables predicting nonremissiona
| ACTH variable | AUROC | 95% CI |
|---|---|---|
| POD 1 Am (n = 174) | ||
| Absolute ACTH | 0.91 | 0.86–0.96 |
| Ratio to Bsl | 0.87 | 0.81–0.93 |
| Difference from Bsl | 0.69 | 0.57–0.81 |
| POD 0 Pm (n = 135) | ||
| Absolute ACTH | 0.73 | 0.62–0.84 |
| Ratio to Bsl | 0.68 | 0.57–0.80 |
| Difference from Bsl | 0.62 | 0.49–0.75 |
| POD 1 Pm (n = 129) | ||
| Absolute ACTH | 0.90 | 0.83–0.97 |
| Ratio to Bsl | 0.81 | 0.71–0.92 |
| Difference from Bsl | 0.69 | 0.55–0.82 |
| POD 2 Pm (n = 96) | ||
| Absolute ACTH | 0.88 | 0.79–0.96 |
| Ratio to Bsl | 0.83 | 0.72–0.93 |
| Difference from Bsl | 0.68 | 0.53–0.83 |
| POD 3 Pm (n = 40) | ||
| Absolute ACTH | 0.93 | 0.85–1.00 |
| Ratio to Bsl | 0.91 | 0.82–1.00 |
| Difference from Bsl | 0.80 | 0.64–0.96 |
Statistically significant findings in boldface font. Abbreviations: AUROC, area under the receiver operator curve; Bsl, baseline; CI, confidence interval; POD, postoperative day.
Remission defined as ≥1 postoperative serum cortisol <5 ug/dL within 10 days after surgery. Ratio baseline is the ratio of ACTH value to the preoperative AM ACTH value. Difference from Bsl is the difference of the POD1 ACTH value from the preoperative Am ACTH value.
Discussion
The hypercortisolism-induced suppression of ACTH secretion from nonadenomatous corticotroph cells in CD patients (28–30) becomes apparent after successful TSS, as ACTH levels rapidly decrease. This offers an opportunity to identify residual tumors since ACTH and cortisol levels remain elevated in about 15% to 35% of patients (18, 31). Early reoperation is effective in most of these cases, emphasizing the significance of postoperative predictors of remission (31–33).
The main postoperative prognostic marker is the well-established cortisol measurement (9). Early remission is identified by a nadir serum cortisol of <5ug/dL or urine-free cortisol <10–20ug/24 hours within the first 10 days postoperatively (18). However, due to the limited number of studies on postoperative ACTH dynamics, including intra-patient variation, there are no well-established criteria for using ACTH levels to predict therapy response.
Diminished ACTH variation is a known feature in CD patients, as demonstrated through inter-patient ACTH analysis (1). Our study aimed to identify altered ACTH variation using intra-patient data from a large cohort with serial data points. We observed minimal variation in morning and midnight plasma ACTH measurements in CD patients prior to TSS. Patients in remission after TSS exhibited larger, more normal variations in ACTH values at each clock time point, as well as a more physiologic diurnal range that started on POD 1 after successful TSS. In contrast, patients who did not achieve remission maintained a narrowed CV at clock time points, a higher overall mean plasma ACTH, and a contracted diurnal range. Compression of ACTH diurnal range has been previously observed in a small cohort of CD patients (2). Our study expands on this finding in a larger cohort (n = 253) by demonstrating an early restoration of diurnal range with CD remission, driven primarily by the return to a physiologic nighttime nadir. However, patients who failed surgery did not regain the nocturnal ACTH nadir, as plasma ACTH remained elevated throughout the day and was similar to preoperative values (34).
Previous studies have shown that ACTH values may decrease within 10 hours postoperatively, before the onset of hypocortisolemia (7). We found that a 50% or greater reduction in postoperative ACTH occurred in 30% of patients before reaching an undetectable cortisol (≤5 ug/dL) level. Temporal pattern of ACTH decrease preceding hypocortisolemia after TSS is consistent with previous studies (35, 36). Srinivasan et al (2011) found that ACTH drops below 20 ng/L in patients with remission 4 to 26 hours before hypocortisolemia (37).
The changes in absolute plasma ACTH values, the ratio of postoperative to preoperative ACTH and, to a lesser extent, the difference in ACTH compared to the patients’ preoperative values, correlated well with remission. The absolute ACTH value on the first POD exhibited the highest predictive value, with a morning and midnight absolute ACTH AUROC of 0.91 and 0.90, respectively. This finding aligns with previous studies that also found that a low ACTH value on POD 1 was most consistent with remission (19, 34, 38). Our study extends these findings by employing AUROC analysis on this large dataset. Previous studies use a set level of ACTH as a cut-off for remission and possible recurrence. Flitsch et al evaluated 147 patients with CD and TSS and reported that ACTH value less than 20 ng/L correlated with a 99% remission rate and 3.5% recurrence rate (34). In our analysis, we investigated the association between the absolute postoperative ACTH value on POD 1 and the change from the preoperative value, finding both to have high predictive value for remission. Using the AUROC values rather than a single ACTH cut-off value allowed us to accurately define remission in a larger subset of patients.
The insights gained from this study could aid in designing clinical trials targeting ACTH secretion from adenomas. The resumption of ACTH variability at clock time points, the restoration of diurnal variation, and changes in absolute ACTH values could all serve as valid outcome measures for trials targeting ACTH secretion. The combined evaluation of postoperative cortisol levels and the absolute ACTH value on the first POD may also enable an earlier and more accurate assessment of remission following TSS.
Limitations
The current study, however, does have limitations. As a retrospective study, there is data that was not recorded consistently across all the patients, causing many data points to be missed within this population. There were patients who did not have the full collection of ACTH values throughout all time points within the 10 PODs, causing fewer data points in the later hospital days. The ACTH assay during 2015 to 2019 was completed within the NIH Clinical Center as compared to ACTH prior to 2015, which was an outsourced assay. Completing the ACTH assay within the Clinical Center allowed improved quality control of patient samples: preventing loss of samples during shipment, minimalizing the risk of denaturation of the sample, and improving turn-around time. An additional limitation of this study is that it was conducted at the NIH Clinical Center, which cares for one of the largest CD patient populations in the world, which may not reflect that of other academic centers. As one of the largest CD patient populations, the high rates of postoperative remission may not reflect those seen at other medical centers. Additionally, the lack of a standardized ACTH assay within the field of endocrinology presents a barrier for external generalizability to all healthcare centers.
Conclusions
Our study first confirmed that ACTH variability was suppressed in CD at each clock time point. Remission of CD was associated with the resumption of intra-patient ACTH variability. We then found that a decrease in plasma ACTH by 50% or more was a predictor of remission from CD. These results may enable clinicians to develop rational outcome measures for interventions targeting CD adenomas. For example, drug trials targeting CD adenoma ACTH secretion could incorporate clock time point variability, diurnal range, and a 50% reduction in absolute plasma ACTH values for a priori power and effect size calculations.
Contributor Information
Reinier Alvarez, Neurosurgery Unit for Pituitary and Inheritable Diseases, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA; Department of Neurosurgery, University of Colorado Anschutz Medical Campus School of Medicine, Aurora, CO, USA; Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Elizabeth Hogan, Neurosurgery Unit for Pituitary and Inheritable Diseases, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA; Department of Neurosurgery, University of Colorado Anschutz Medical Campus School of Medicine, Aurora, CO, USA.
David T Asuzu, Neurosurgery Unit for Pituitary and Inheritable Diseases, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA; Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA; Department of Neurosurgery, University of Virginia, Charlottesville, VA, USA.
Tianxia Wu, Clinical Trials Unit, National Institute of Neurological Diseases and Stroke, Bethesda, MD, USA.
Gloria Oshegbo, Biomedical Translational Research Information System, Clinical Center, National Institutes of Health, Bethesda, MD, USA.
Raven McGlotten, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Michaela Cortes, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Christina Hayes, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Constantine A Stratakis, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Christina Tatsi, Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Lynnette K Nieman, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Prashant Chittiboina, Neurosurgery Unit for Pituitary and Inheritable Diseases, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA; Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Funding
This study was supported by the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke, Eunice Kennedy Shriver National Institute for Child Health and Human Development and the National Institute of Diabetes and Digestive Kidney Diseases. PC was supported by NINDS Intramural Reserch Grant ZIA NS003150-08.
Disclosures
The authors have nothing to declare.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Cunningham JM, Buxton OM, Weiss RE. Circadian variation in Cushing's disease and pseudo-Cushing states by analysis of F and ACTH pulsatility. J Endocrinol Invest. 2002;25(9):791‐799. doi: 10.1007/BF03345514 [DOI] [PubMed] [Google Scholar]
- 2. Sekiya K, Nawata H, Kato K, Motomatsu T, Ibayashi H. Diurnal rhythms of proopiomelanocortin-derived N-terminal peptide, beta-lipotropin, beta-endorphin and adrenocorticotropin in normal subjects and in patients with Addison's disease and Cushing's disease. Endocrinol Jpn. 1986;33(5):713‐719. doi: 10.1507/endocrj1954.33.713 [DOI] [PubMed] [Google Scholar]
- 3. Liu JH, Kazerj RR, Rasmussen DD. Characterization of the twenty-four hour secretion patterns of adrenocorticotropin and cortisol in normal women and patients with Cushing's disease. J Clin Endocrinol Metab. 1987;64(5):1027‐1035. doi: 10.1210/jcem-64-5-1027 [DOI] [PubMed] [Google Scholar]
- 4. Roelfsema F, Keenan DM, Veldhuis JD. Endogenous ACTH concentration-cortisol secretion dose analysis unmasks decreased ACTH potency in Cushing's disease with restoration after successful pituitary adenomectomy. J Clin Endocrinol Metab. 2011;96(12):3768‐3774. doi: 10.1210/jc.2011-1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart PM, Penn R, Gibson R, et al. Hypothalamic abnormalities in patients with pituitary-dependent Cushing's syndrome. Clin Endocrinol (Oxf). 1992;36(5):453‐458. doi: 10.1111/j.1365-2265.1992.tb02245.x [DOI] [PubMed] [Google Scholar]
- 6. Abellán-Galiana P, Fajardo-Montañana C, Riesgo-Suárez P, et al. Prognostic usefulness of ACTH in the postoperative period of Cushing's disease. Endocr Connect. 2019;8(9):1262‐1272. doi: 10.1530/EC-19-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salmon P, Loftus PD, Dodd RL, et al. Utility of adrenocorticotropic hormone in assessing the response to transsphenoidal surgery for Cushing's disease. Endocr Pract. 2014;20(11):1159‐1164. doi: 10.4158/EP14140.OR [DOI] [PubMed] [Google Scholar]
- 8. Lu J, Chatain GP, Bugarini A, et al. Histone deacetylase inhibitor SAHA is a promising treatment of Cushing disease. J Clin Endocrinol Metab. 2017;102(8):2825‐2835. doi: 10.1210/jc.2017-00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526‐1540. doi: 10.1210/jc.2008-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papanicolaou DA, Yanovski JA, Cutler GB, Chrousos GP, Nieman LK. Distinguishes Cushing’s syndrome from pseudo-Cushing. Endocrinol Metab. 2009;83(4):1163‐1167. doi: 10.1210/jcem.83.4.4733 [DOI] [PubMed] [Google Scholar]
- 11. Newell-Price J, Trainer P, Perry L, Wass J, Grossman A, Besser M. A single sleeping midnight cortisol has 100% sensitivity for the diagnosis of Cushing's syndrome. Clin Endocrinol (Oxf). 1995;43(5):545‐550. doi: 10.1111/j.1365-2265.1995.tb02918.x [DOI] [PubMed] [Google Scholar]
- 12. Chowdhury IN, Sinaii N, Oldfield EH, Patronas N, Nieman LK. A change in pituitary magnetic resonance imaging protocol detects ACTH-secreting tumours in patients with previously negative results. Clin Endocrinol (Oxf). 2010;72(4):502‐506. doi: 10.1111/j.1365-2265.2009.03646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Günes M, Celik O, Kadioglu P. Reliability of the diagnostic tests for Cushing's syndrome performed in a tertiary referral center. Pituitary. 2013;16(2):139‐145. doi: 10.1007/s11102-012-0387-7 [DOI] [PubMed] [Google Scholar]
- 14. Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB. A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1994;78(2):418‐422. doi: 10.1210/jcem.78.2.8106630 [DOI] [PubMed] [Google Scholar]
- 15. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB Jr. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1993;77(5):1308‐1312. doi: 10.1210/jcem.77.5.8077325 [DOI] [PubMed] [Google Scholar]
- 16. Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med. 1991;325(13):897‐905. doi: 10.1056/NEJM199109263251301 [DOI] [PubMed] [Google Scholar]
- 17. Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing's disease after transsphenoidal surgery. J Clin Endocrinol Metab. 2011;96(7):2057‐2064. doi: 10.1210/jc.2011-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nieman LK, Biller BMK, Findling JW, et al. Treatment of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807‐2831. doi: 10.1210/jc.2015-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asuzu D, Chatain GP, Hayes C, et al. Normalized early postoperative cortisol and ACTH values predict nonremission after surgery for Cushing disease. J Clin Endocrinol Metab. 2017;102(7):2179‐2187. doi: 10.1210/jc.2016-3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ironside N, Chatain G, Asuzu D, et al. Earlier post-operative hypocortisolemia may predict durable remission from Cushing's disease. Eur J Endocrinol. 2018;178(3):255‐263. doi: 10.1530/EJE-17-0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asuzu DT, Bhatt S, Nwokoye D, et al. Cortisol and ACTH measurements at extubation from pituitary surgery predicts hypothalamic-pituitary-adrenal axis function. J Endocr Soc. 2023;7(4):bvad025. 10.1210/jendso/bvad025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vargha P. A critical discussion of intraclass correlation coefficients. Stat Med. 1997;16(7):821‐823. doi: [DOI] [PubMed] [Google Scholar]
- 23. Welch B. The generalisation of student's problems when several different population variances are involved. Biometrika. 1947;34(1-2):28‐35. doi: 10.1093/biomet/34.1-2.28 [DOI] [PubMed] [Google Scholar]
- 24. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46‐51. doi: 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- 25. Hewett P, Ganser GH. A comparison of several methods for analyzing censored data. Annals of Occupational Hygiene. 2007;51(7):611‐632. doi: 10.1093/annhyg/mem045 [DOI] [PubMed] [Google Scholar]
- 26. Alvarez R, Hogan E, Asuzu DT, et al. Supplemental data for: Diurnal range and intra-patient variability of ACTH is restored with remission in Cushing's disease. Data hosted in NINDS Lab Server. J Clin Endocrinol Metab. 2023;108(11):2812-2820. doi: 10.1210/clinem/dgad309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155‐163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson SM, Jean Kant G, De Souza EB. Effects of chronic stress on anterior pituitary and brain corticotropin-releasing factor receptors. Pharmacol Biochem Behav. 1993;44(4):755‐761. doi: 10.1016/0091-3057(93)90002-b [DOI] [PubMed] [Google Scholar]
- 29. Ochedalski T, Rabadan-Diehl C, Aguilera G. Interaction between glucocorticoids and corticotropin releasing hormone (CRH) in the regulation of the pituitary CRH receptor in vivo in the rat. J Neuroendocrinol. 1998;10(5):363‐369. doi: 10.1046/j.1365-2826.1998.00212.x [DOI] [PubMed] [Google Scholar]
- 30. Monteith SJ, Starke RM, Jane JA, Oldfield EH. Use of the histological pseudocapsule in surgery for Cushing disease: rapid postoperative cortisol decline predicting complete tumor resection—clinical article. J Neurosurg. 2012;116(4):721‐727. doi: 10.3171/2011.12.JNS11886 [DOI] [PubMed] [Google Scholar]
- 31. Dickerman RD, Oldfield EH. Basis of persistent and recurrent Cushing disease: an analysis of findings at repeated pituitary surgery. J Neurosurg. 2002;97(6):1343‐1349. doi: 10.3171/jns.2002.97.6.1343 [DOI] [PubMed] [Google Scholar]
- 32. Dimopoulou C, Schopohl J, Rachinger W, et al. Long-term remission and recurrence rates after first and second transsphenoidal surgery for Cushing's disease: care reality in the Munich metropolitan region. Eur J Endocrinol. 2014;170(2):283‐292. doi: 10.1530/EJE-13-0634 [DOI] [PubMed] [Google Scholar]
- 33. Friedman RB, Oldfield EH, Nieman LK, et al. Repeat transsphenoidal surgery for Cushing's disease. J Neurosurg. 1989;71(4):520‐527. doi: 10.3171/jns.1989.71.4.0520 [DOI] [PubMed] [Google Scholar]
- 34. Flitsch J, Knappe U, Lüdecke D. The use of postoperative ACTH levels as a marker for successful transsphenoidal microsurgery in Cushing's disease. Zentralbl Neurochir. 2003;64(1):6‐11. doi: 10.1055/s-2003-37145 [DOI] [PubMed] [Google Scholar]
- 35. Pimentel-Filho FR, Silva MER, Nogueira KC, Berger K, Cukiert A, Liberman B. Pituitary-adrenal dynamics after ACTH-secreting pituitary tumor resection in patients receiving no steroids post-operatively. J Endocrinol Invest. 2005;28(6):502‐508. doi: 10.1007/BF03347237 [DOI] [PubMed] [Google Scholar]
- 36. Pereira AM, van Aken MO, van Dulken H, et al. Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing's disease. J Clin Endocrinol Metab. 2003;88(12):5858‐5864. doi: 10.1210/jc.2003-030751 [DOI] [PubMed] [Google Scholar]
- 37. Srinivasan L, Laws ER, Dodd RL, et al. The dynamics of post-operative plasma ACTH values following transsphenoidal surgery for Cushing's disease. Pituitary. 2011;14(4):312‐317. doi: 10.1007/s11102-011-0295-2 [DOI] [PubMed] [Google Scholar]
- 38. Czirják S, Bezzegh A, Gál A, Rácz K. Intra- and postoperative plasma ACTH concentrations in patients with Cushing's disease cured by transsphenoidal pituitary surgery. Acta Neurochir (Wien). 2002;144(10):971‐977. discussion 977. doi: 10.1007/s00701-002-0984-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.