Abstract
Objective
Obesity is a growing emergency in type 1 diabetes (T1D). Sex differences in obesity prevalence and its clinical consequences in adult T1D subjects have been poorly investigated. The aim of this study was to investigate the prevalence of obesity and severe obesity, clinical correlates, and potential sex differences in a large cohort of T1D subjects participating to the AMD (Associazione Medici Diabetologi) Annals Initiative in Italy.
Research Design and Methods
The prevalence of obesity [body mass index(BMI) ≥30 kg/m2] and severe obesity (BMI ≥ 35 kg/m2) according to sex and age, as well as obesity-associated clinical variables, long-term diabetes complications, pharmacological treatment, process indicators and outcomes, and overall quality of care (Q-score) were evaluated in 37 436 T1D subjects (45.3% women) attending 282 Italian diabetes clinics during 2019.
Results
Overall, the prevalence of obesity was similar in the 2 sexes (13.0% in men and 13.9% in women; mean age 50 years), and it increased with age, affecting 1 out of 6 subjects ages >65 years. Only severe obesity (BMI >35 kg/m2) was more prevalent among women, who showed a 45% higher risk of severe obesity, compared with men at multivariate analysis. Cardiovascular disease risk factors (lipid profile, glucose, and blood pressure control), and the overall quality of diabetes care were worse in obese subjects, with no major sex-related differences. Also, micro- and macrovascular complications were more frequent among obese than nonobese T1D men and women.
Conclusions
Obesity is a frequent finding in T1D adult subjects, and it is associated with a higher burden of cardiovascular disease risk factors, micro- and macrovascular complications, and a lower quality of care, with no major sex differences. T1D women are at higher risk of severe obesity.
Keywords: obesity, type 1 diabetes, sex, age, AMD Annals Initiative
The prevalence of type 1 diabetes (T1D) is growing worldwide, with an estimate of an incidence increase of 2.7% to 3.4% per year in recent decades (1, 2). In Italy, the prevalence of T1D is 0.5% (3, 4).
Obesity in T1D has progressively increased in recent years. In the DCCT/EDIC study, 30% of youth with T1D had overweight/obesity (5), and a European study reported a prevalence of 15% of obesity in adults with T1D (6).The estimated prevalence of obesity in people with T1D, however, ranges between 2.8% and 37.1%, varying by the definition of obesity, age of the examined patients, and country (7).
Notably, in overweight/obese children, T1D is diagnosed at younger ages, and obesity has been suggested to be an accelerator in the pathogenesis of T1D (8, 9). Obesity is also associated with insulin resistance and other cardiovascular (CV) risk factors in T1D patients (5). In this regard, the FinnDiane study showed that more than 40% of T1D subjects had the diagnostic criteria for the metabolic syndrome (10). Furthermore, the impact of some risk factors, such as hypertension and hyperglyceridemia, seems to increase with the degree of obesity in subjects with T1D (11).
The prevalence of severe obesity, ie, the presence of body mass index (BMI) value ≥ 35 kg/m2, has been reported to be higher in women than in men with type 2 diabetes (T2D) (12), with a higher risk for cardiovascular disease (CVD) mortality (13). Furthermore, obesity phenotypes may differently impact on CVD risk in men and women with acute coronary syndrome, including T2D subjects (14).
Data from the DPV database (Diabetespatienten Verlaufsdokumentation) on 53.108 young T1D patients ages <20 years showed that T1D girls were at higher risk of obesity (15). Conversely, in adult T1D subjects, sex differences in obesity and its correlates are still largely unexplored.
Sex differences in the quality of care relative to the year 2011 were reported in the 28 802 T1D participants to the AMD Annals Initiative, an ongoing study started in 2006 and involving approximately one-third of diabetes clinics in Italy with the aim of exploring quality of care in both T2D and T1D subjects (16). In this report, T1D women showed a 33% higher likelihood of having out-of-target glycated hemoglobin A1C (HbA1c) values, 29% lower risk of high blood pressure values, and 27% lower risk of micro/macroalbuminuria (MAU) than T1D men, while low-density lipoprotein LDL-cholesterol (LDL-c) levels, estimated glomerular filtration rate (eGFR), and a validated indicator of overall quality of care (Q-score) did not significantly differ (17, 18). However, an age- and BMI-stratified analysis was not reported in that study; furthermore, specific correlates of the obese phenotypes have not been investigated separately for T1D men and women.
The aim of the current study was to analyze potential age and sex differences in the prevalence of obesity and its association with major clinical variables, including metabolic characteristics, CVD risk factors, and chronic diabetes complications, as well as with several indicators of the quality of diabetes care, including a validated score (Q-score), separately in T1D men and women participating in the AMD Annals Initiative.
Research Design and Methods
The AMD Annals Initiative
Since 2006, the Italian Association of Clinical Diabetologists [Associazione Medici Diabetologi-(AMD)] promoted a continuous quality improvement initiative called AMD Annals. In this context, AMD identified a set of process and intermediate outcome indicators to be used for benchmarking activities (19, 20). Furthermore, the use of glucose-lowering, antihypertensive, and lipid-lowering drugs is evaluated.
Centers share the same software for data extraction from electronic medical records. Data are collected in a standardized format (AMD Data File). The database is anonymous by design. Information is extracted by the medical records system without any data allowing the identification of patients and centers. Both are identified by numeric codes, and analysis is centralized and based on aggregated data (19, 20). Given the nature of the study, the Italian regulations require an ethics approval but not the signature of the informed consent. The entire project is conducted without allocation of extra resources or financial incentives but simply through a physician-led effort, made possible by the commitment of the specialists involved.
Sample Selection
All patients with diagnosis of T1D were included. Clinical data collected during the year 2019 were extracted from electronic medical records. In case of multiple records collected during the year for the same patient, the last available value was included in the quality-of-care profiling (19-23).
Obesity was defined according to BMI values: BMI < 30 kg/m2 for nonobese and BMI ≥30 kg/m2 for obese subjects. The severity of obesity was categorized according to BMI values: 30-34.9 kg/m2 class I; 35-39.9 kg/m2 class II; ≥40 kg/m2 class III. Class II and III obesity were combined and defined as severe obesity (BMI ≥ 35 kg/m2).
Quality-of-Care Indicators
Process measures are expressed as the proportion of patients with at least 1 evaluation of the following clinical parameters during the previous year: HbA1c, blood pressure (BP), lipid profile [LDL-c or total and high-density lipoprotein cholesterol (HLD-c) and triglycerides], renal function, and eye examination (19-23).
Intermediate outcome measures include the proportion of patients with at-target values for major risk factors. Outcomes are considered satisfactory if HbA1c levels are ≤7.0% (≤53 mmol/mol), BP values are ≤130/80 mmHg, and LDL-c levels are <2.59 mmol/L (100 mg/dL).
Indicators of treatment intensity/appropriateness are also measured, taking into consideration the use of pharmacologic treatments in relation to the achievement of the targets: no lipid-lowering agents despite LDL-c ≥ 3.37 mmol/L (130 mg/dL), no antihypertensive treatments despite BP ≥ 140/90 mmHg, no angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers despite MAU, not treated with antiplatelets in spite of history of CVD events (indicating patients not adequately treated) and LDL-c ≥ 3.37 mmol/L (130 mg/dL) in spite of lipid-lowering treatment, and BP ≥140/90 mmHg in spite of antihypertensive treatment (indicating patients not reaching targets in spite of treatment) (19-23).
The quality-of-care summary score (Q-score) was derived from the combination of process and outcome indicators based on levels and treatment of HbA1c, blood pressure, LDL-c, and MAU (23). The score ranges between 0 and 40; the higher the score, the better the quality of care. Two validation studies (17, 18) documented that the risk to develop a new CV event was 80% higher in patients with a score <15% and 20% higher in those with a score between 15 and 25, as compared to those with a score >25.
If not recorded in electronic medical records, LDL-c was estimated by the Friedwald equation. MAU was defined as albumin excretion rate ≥20 mcg/min, albumin/creatinine ratio >2.5 (men) or >3.5 (women) mg/mmol, or urine albumin >30 mg/L. The glomerular filtration rate was calculated with the Chronic Kidney Disease Epidemiology Collaboration formula (24).
Statistical Analyses
Patients’ characteristics and quality indicators according to sex are described as mean and standard deviation or frequencies, as appropriate. The denominators for the different quality indicators vary according to the availability of the information in the index year. No missing imputation was performed. Patient characteristics and quality indicators by BMI classes or sex (overall and by age classes) were compared using the Mann-Whitney U-test for continuous variables and the χ2 test for categorical ones.
Multivariate analyses were also performed to identify correlates of obesity (BMI ≥30) and severe obesity (≥35 kg/m2) in the total study population and in the 2 sexes separately. Covariates tested in the model were sex, age, diabetes duration, smoking habit, HbA1c levels, lipid profile, BP values, eGFR, MAU, diabetes treatments, treatment with antiplatelets, antihypertensive or lipid-lowering agents, and history of micro- and macrovascular complications. Backward variable selection was adopted. Results are expressed as odds ratios with their 95% confidence intervals. P-values <.05 were considered statistically significant. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
BMI Classes Distribution in T1D Subjects According to Sex
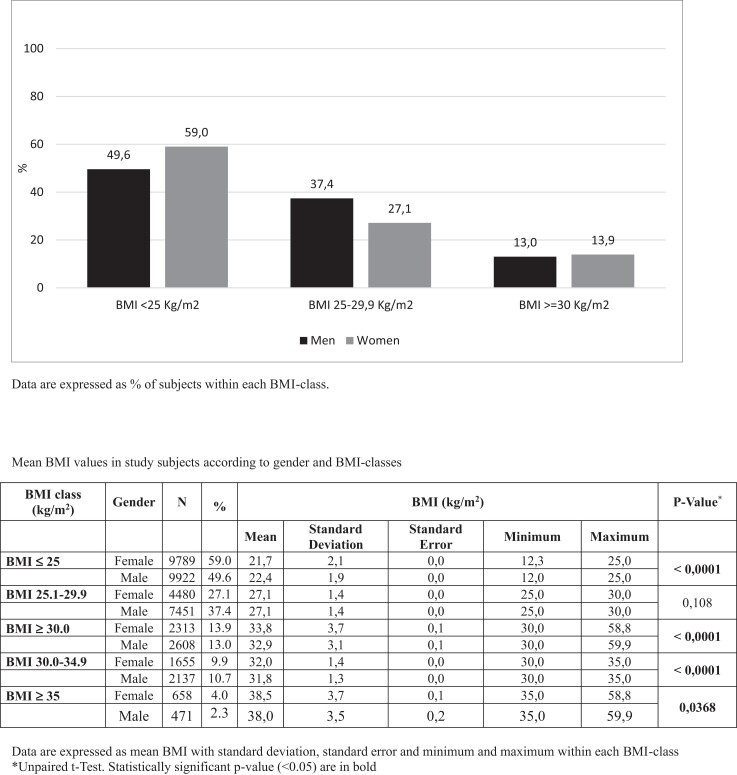
Data from 37 436 T1D subjects, 20 474 men (54.7%) and 16 971 women (45.3%), mean age 49.6 years, mean BMI 28.6 kg/m2, seen in 282 Italian diabetes outpatient clinics during the year 2019 were included in the present analysis. Distribution of BMI classes according to sex is shown in Fig. 1.
Figure 1.
Body mass index classes distribution in men and women with type 1 diabetes from the AMD Annals Initiative according to sex.
Overall, 49.6% of T1D men and 59.0% of T1D women had normal BMI values (<25 kg/m2), while overweight (BMI 25-29.9 kg/m2) was observed in 37.4% of men and 27.1% of women. The prevalence of obesity (BMI >30 kg/m2) was similar in the 2 sexes (13.9% vs 13.0% in women and men, respectively). The distribution of obesity classes is shown in the legend of the same figure (Fig. 1): the prevalence of severe obesity (classes II and III obesity, BMI ≥35 kg/m2) was nearly double among T1D women compared to men (4.0% in women vs 2.3% in men) (P < .0001) (Fig. 1).
Obesity in T1D Subjects by Age and Sex
Distribution of BMI classes by age and sex is shown in Supplementary Table S1 (25). Overall, mean BMI values increased with age in both sexes, ranging from 21.4 ± 4.0 kg/m2 in men and 22.3 ± 4.5 kg/m2 in women ages ≤18 years to 26.1 ± 4.0 kg/m2 in men and 25.6 ± 5.1 kg/m2 in women ages >65 years (P < .0001 for both sexes).
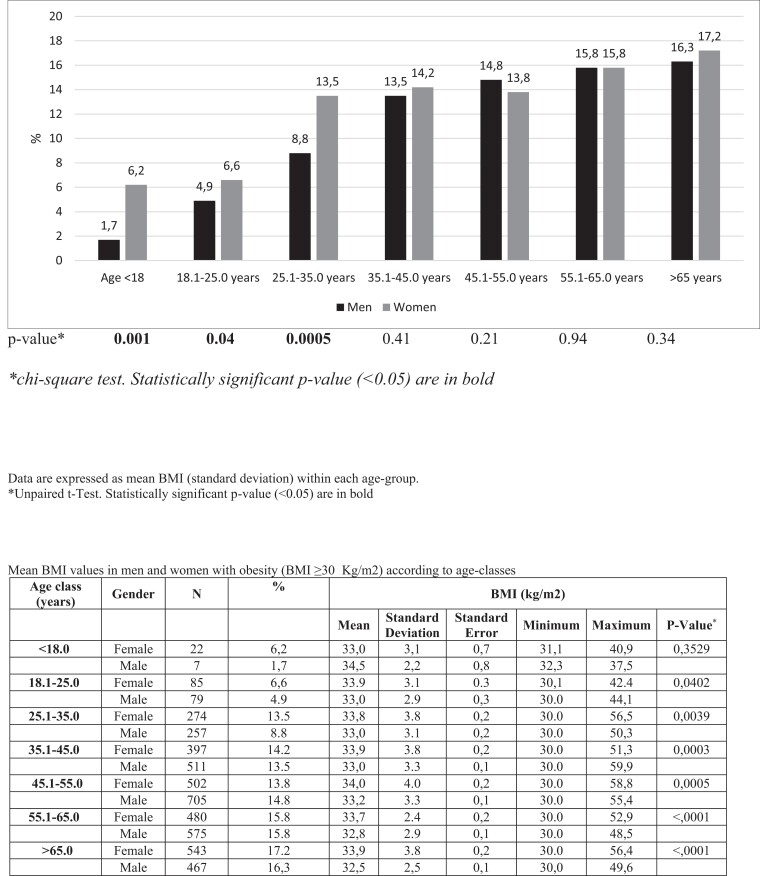
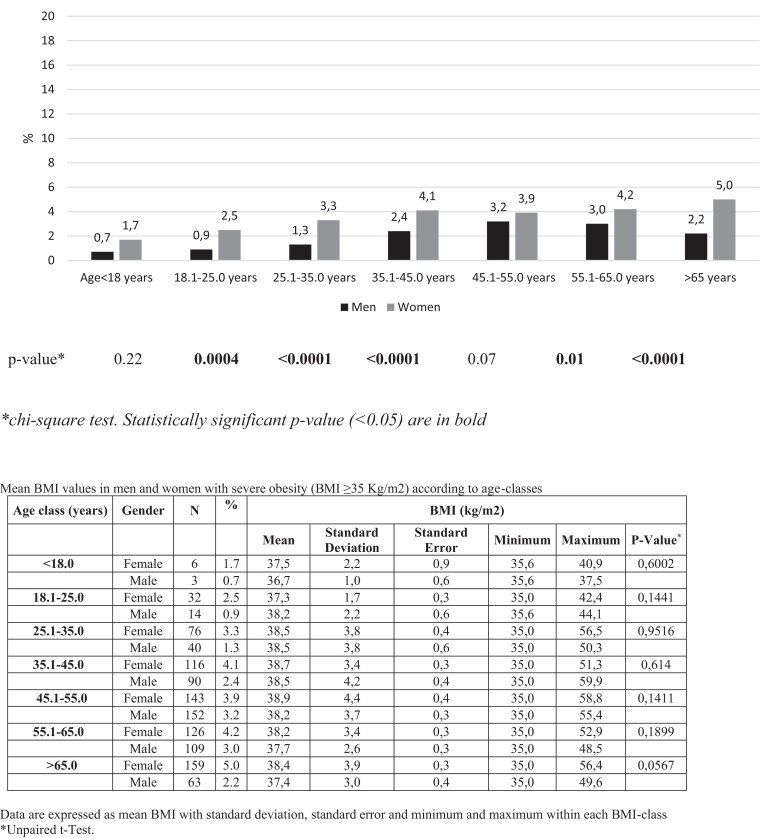
As shown in Fig. 2, the prevalence of obesity progressively increased with age in both sexes. Obesity prevalence ranged from 1.7% before the age of 18 years to 16.3% after 65 years in men and from 6.2% (<18 years) up to 17.2% (>65 years) in women. Consistently, severe obesity also increased with age, ranging from 0.7% to 2.2% in T1D men and from 1.7% to 5.0% in women (Fig. 3).
Figure 2.
Prevalence of obesity in type 1 diabetes subjects participating in the study, according to sex and age classes (years).
Figure 3.
Prevalence of severe obesity in type 1 diabetes subjects participating in the study, according to sex and age classes (years).
Clinical Characteristics and Pharmacological Treatments in T1D Men and Women According to the Presence of Obesity
The distribution of clinical characteristics, major CV risk factors, and diabetes long-term complications in obese and nonobese subjects in T1D men and women separately is shown in Table 1. Overall, obese subjects were older; had a longer diabetes duration; had higher HbA1c, triglycerides, systolic blood pressure (SBP), and diastolic blood pressure (DBP) and lower HDL-c levels; and were less frequently smokers, irrespective of sex, whereas LDL-c levels were higher in obese women only.
Table 1.
Clinical characteristics and pharmacological treatments in T1D men and women participating to the AMD Annals Initiative, according to the presence of obesity (BMI ≥30 kg/m2)
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Nonobese | Obese | Pa | Nonobese | Obese | Pa | |
| n (%) | 17 373 (87.0) | 2608 (13.0) | 14 269 (86.1) | 2313 (13.9) | ||
| BMI (kg/m2) | 24.4 ± 2.9 | 32.9 ± 3.1 | <.0001 | 23.4 ± 3.2 | 33.8 ± 3.7 | <.0001 |
| Age (yrs) median (IQR) | 47.2 (34.2-58.0) | 51.5 (41.7-61.2) | <.0001 | 48.8 (35.0-60.8) | 53.0 (40.6-64.4) | <.0001 |
| Diabetes duration (years) | 18.0 (9.0-29.0) | 22.0 (11.0-33.0) | <.0001 | 18.0 (9.0-30.0) | 22.0 (12.0-33.0) | <.0001 |
| Clinical characteristics | ||||||
| HbA1c (%) (mmol/mol) |
7.7 ± 1.3 61 ± 6.3 |
7.9 ± 1.2 63 ± 5.3 |
<.0001 | 7.8 ± 1.3 62 ± 6.3 |
8.1 ± 1.2 65 ± 5.3 |
<.0001 |
| HbA1c < 7% (≤53 mmol/mol) | 29.1% | 21.4% | <.0001 | 23.6% | 16.2% | <.0001 |
| HbA1c 7-9% (53-75 mmol/mol) | 59.1% | 64.1% | 63.1% | 65.7% | ||
| HbA1c > 9% (≤75 mmol/mol) | 11.8% | 14.5% | 13.3% | 18.1% | ||
| Smokers (%) | 30.6% | 26.4% | .0003 | 22.4% | 15.4% | <.0001 |
| Total cholesterol (mmol/L) | 4.5 ± 0.9 | 4.5 ± 1.0 | .0514 | 4.7 ± 0.9 | 4.6 ± 0.9 | .06 |
| HDL cholesterol (mmol/L) | 1.45 ± 0.36 | 1.27 ± 0.32 | <.0001 | 1.73 ± 0.39 | 1.52 ± 0.38 | <.0001 |
| LDL cholesterol (mmol/L) | 2.56 ± 0.76 | 2.55 ± 0.85 | .0832 | 2.57 ± 0.71 | 2.64 ± 0.97 | .001 |
| LDL cholesterol >1.8 mmol/L | 85.0% | 80.1% | <.0001 | 87.2% | 85.8% | .11 |
| Triglycerides (mmol/L) | 1.04 ± 0.7 | 1.44 ± 1.02 | <.0001 | 0.9 ± 0.53 | 1.21 ± 0.78 | <.0001 |
| SBP (mmHg) | 127.4 ± 17.3 | 136.0 ± 17.9 | <.0001 | 123.3 ± 18.8 | 131.9 ± 18.8 | <.0001 |
| SBP ≥140 mmHg | 27.3% | 46.6% | <.0001 | 21.9% | 37.7% | <.0001 |
| DBP (mmHg) | 75.2 ± 9.4 | 78.8 ± 10.1 | <.0001 | 72.9 ± 9.5 | 76.3 ± 9.8 | <.0001 |
| DBP >90 mmHg | 3.6% | 8.8% | <.0001 | 2.4% | 4.7% | <.0001 |
| eGFR (mL/min) | 94.0 ± 28.5 | 86.8 ± 28.3 | <.0001 | 90.7 ± 28.7 | 85.0 ± 30.3 | <.0001 |
| T1D long-term complications | ||||||
| DKDb | 22.3% | 31.4% | <.0001 | 20.7% | 29.1% | <.0001 |
| GFR ≤60 mL/min | 9.5% | 15.7% | <.0001 | 11% | 17.8% | <.0001 |
| MAU | 20.4% | 27.8% | <.0001 | 16.3% | 21.8% | <.0001 |
| Retinopathy | 32.6% | 47.1% | <.0001 | 30.6% | 43.9% | <.0001 |
| CV eventsc | 5.6% | 9.9% | <.0001 | 3.3% | 6.1% | <.0001 |
| Pharmacological treatments | ||||||
| CSII | 14.6% | 17.1% | 20.7% | 25.4% | ||
| MDI | 83.%1 | 80.5% | 77.3% | 72.7% | ||
| Metformin | 9.1% | 28.3% | <.0001 | 9% | 28% | <.0001 |
| SGLT2i | 1.9% | 7.9% | <.0001 | 1.9% | 7.8% | <.0001 |
| Lipid-lowering agents | 35.2% | 55.4% | <.0001 | 32.3% | 47.4% | <.0001 |
| Antihypertensive treatment | 28.2% | 51.2% | <.0001 | 27% | 48.1% | <.0001 |
| Antiplatelets | 16.4% | 27% | <.0001 | 15% | 25.6% | <.0001 |
Data are N, proportion or mean ± SD; median and IQR where specified.
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; CV, cardiovascular ; DBP, diastolic blood pressure; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; MAU, micro/macroalbuminuria; MDI, multiple daily injection; SBP, systolic blood pressure; SGLT2i, sodium/glucose cotransporter-2 inhibitor; T1D, type 1 diabetes.
Chi-square test or Mann-Whitney U-test, as appropriate.
DKD, MAU, and/or eGFR ≤60 mL/min.
Cardiovascular (myocardial infarction, revascularization/reperfusion procedures), cerebrovascular (stroke), peripheral vascular disease (revascularization/reperfusion procedures).
As for chronic diabetes complications, impaired renal function (eGFR < 60 mL/min) was also overall more frequent among obese than nonobese subjects (9.5% vs 15.7% in nonobese and obese men; 10.9% vs 17.8% in nonobese and obese women, respectively; P < .0001 for both comparisons), especially in women, with only 52% of obese T1D women having an eGFR ≥90 mL/min. The prevalence of MAU was also significantly higher among obese subjects in both sexes, especially in men.
Notably, also retinopathy and CV events were more frequent in obese than in nonobese subjects, both among men (retinopathy: 32.6% vs 47.1%; CV events: 5.6% vs 9.9%) and women (retinopathy: 30.6% vs 43.9%, CV events: 3.3% vs 6.1%) (P < .0001 for all comparisons).
As for treatments, subjects with obesity were more frequently treated with continuous subcutaneous insulin infusion (CSII) than nonobese ones, irrespective of sex. Similarly, also treatment with metformin, SGLT2 inhibitors (SGLT2i), lipid-lowering agents, and antihypertensive and antiplatelets drugs was more frequent among men and women with obesity as compared to nonobese subjects (Table 1).
Diabetes Care Process and Outcome Indicators in T1D Men and Women According to the Presence of Obesity
Diabetes care, process, and outcome indicators in obese and nonobese T1D men and women are shown in Table 2. Process indicators, ie, the proportion of patients who were yearly monitored for major clinical parameters, were also analyzed according to sex and obesity status (Table 2). Obesity did not influence these parameters, with the exceptions of creatinine measurement, which was more frequent among obese than nonobese subjects in both sexes, and BP, which was slightly less monitored in obese T1D men.
Table 2.
Diabetes quality of care, process, and outcome indicators in T1D men and women participating to the AMD Annals Initiative, according to the presence of obesity (BMI ≥30 kg/m2)
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Nonobese | Obese | Pa | Non obese | Obese | Pa | |
| Process indicators (proportion of patients with at least 1 yearly monitoring of | ||||||
| HbA1c (%) | 97.3 | 97.3 | .93 | 97.5 | 97.8 | .35 |
| Lipid profile (%) | 78.8 | 79.9 | .21 | 78.8 | 79.6 | .36 |
| BP (%) | 90.6 | 89.3 | .05 | 90.2 | 89.6 | .35 |
| Albuminuria (%) | 72.9 | 71.3 | .07 | 72 | 72.7 | .53 |
| Creatinine (%) | 85.0 | 86.9 | .01 | 84.7 | 87.2 | .002 |
| Eye examination (%) | 44.7 | 44.9 | .86 | 44.4 | 45.5 | .30 |
| Intermediate favorable outcome indicators (proportion of patients reaching risk factors targets) | ||||||
| HbA1c ≤7.0% (≤53 mmol/mol) (%) | 32.7 | 24.4 | <.0001 | 26.7 | 18.8 | <.0001 |
| LDL-c <2.6 mmol/L (%) | 54.2 | 55.4 | .33 | 54.2 | 50.2 | .001 |
| BP ≤130/80 mmHg (%) | 61.4 | 40.5 | <.0001 | 69.0 | 50.2 | <.0001 |
| Indicators of treatment intensity/appropriateness (proportions of patients not adequately treated) | ||||||
| Not treated with lipid-lowering agents despite LDL-C ≥ 3.36 mmol/L (%) | 62.9 | 50.9 | <.0001 | 64.1 | 55.6 | .005 |
| Not treated with antihypertensive treatments despite BP ≥140/90 mmHg | 53.6 | 40.8 | <.0001 | 50.3 | 37.9 | <.0001 |
| Not treated with ACE-I and/or ARBs despite MAU (%) | 59 | 36.5 | <.0001 | 65.7 | 50.4 | <.0001 |
| Not treated with antiplatelets in spite of the presence of history of CV events (%)b | 22.2 | 17.0 | .07 | 24.8 | 21.8 | .46 |
| Indicators of treatment intensity/appropriateness (proportions of patients not reaching targets in spite of treatment): | ||||||
| With LDL-c ≥ 3.36 mmol/L in spite of lipid-lowering treatment (%) | 13.4 | 13.9 | .63 | 13.4 | 15.6 | .07 |
| With BP ≥140/90 mmHg in spite of antihypertensive treatment (%) | 47.7 | 57.0 | <.0001 | 42.6 | 52.0 | <.0001 |
| Overall quality of care | ||||||
| Q-score (mean ± SD) | 29.0 ± 8.4 | 26.2 ± 8.9 | <.0001 | 29.1 ± 8.3 | 26.1 ± 8.6 | <.0001 |
| Q-score <15 (%) | 3.8 | 8.0 | <.0001 | 3.4 | 7.2 | <.0001 |
Statistically significant comparisons (P < .05) are in bold. Data are proportion or mean ± SD.
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; CV, cardiovascular; HbA1c, glycated hemoglobin A1c; LDL-c, low-density lipoprotein cholesterol; MAU, micro/macroalbuminuria; T1D, type 1 diabetes.
Chi-square test or Mann-Whitney U-test, as appropriate.
Cardiovascular (myocardial infarction, revascularization/reperfusion procedures), cerebrovascular (stroke), peripheral vascular disease (revascularization/reperfusion procedures).
Table 2 also shows that the achievement of HbA1c, LDL-c, and BP recommended targets was less frequent among obese subjects both in T1D men and women. Moreover, indicators of appropriateness of treatment showed that obese T1D subjects were less appropriately treated than nonobese ones in both sexes, with the exception of subjects with previous CV events and those being treated with antiplatelets at the same rate irrespective of obesity status; as for subjects with out-of-target values in spite of treatment, a marker of therapeutic inertia, and/or lack of adherence, no differences were noted for LDL-c, whereas for BP, the lack of target values in spite of treatment was higher among obese subjects, both in men and women. Furthermore, Q-score values, an indicator of the overall quality of diabetes care, were significantly lower in T1D obese that in nonobese patients in both sexes (Table 2).
Factors Associated With Obesity and Severe Obesity in T1D Men and Women
Table 3 shows factors associated with obesity and severe obesity in T1D patients at multivariate analyses. Notably, the impact of sex was larger in severe obesity, as being female conferred a small (10%) increase in obesity risk but a 45% higher risk of severe obesity compared to men.
Table 3.
Factors associated with obesity (BMI > 30 kg/m2) and severe obesity (BMI > 35 kg/m2) in T1D patients participating in the AMD Annals Initiative at the multivariate analysis
| BMI ≥30 kg/m2 | BMI ≥35 kg/m2 | |||
|---|---|---|---|---|
| OR | ORCI | OR | ORCI | |
| Male sex (vs female) | 0.90 | 0.85-0.96 | 0.55 | 0.48-0.62 |
| Age (years) | ||||
| ≤25.0 (RC) | 1.00 (RC) | — | 1.00 (RC) | — |
| 25.1-35.0 | 1.72 | 1.45-2.05 | 1.2 | 0.86-1.67 |
| 35.1-45.0 | 1.79 | 1.51-2.12 | 1.15 | 0.84-1.57 |
| 45.1-55.0 | 1.34 | 1.13-1.59 | 0.88 | 0.64-1.21 |
| >55.0 | 1.04 | 0.87-1.23 | 0.54 | 0.39-0.75 |
| Duration (years) | ||||
| ≤2.0 | 1.00 (RC) | — | 1.00 (RC) | — |
| 2.1-5.0 | 1.19 | 0.98-1.43 | 0.94 | 0.67-1.31 |
| 5.1-10.0 | 1.39 | 1.18-1.64 | 0.78 | 0.57-1.06 |
| >10.0 | 1.66 | 1.44-1.91 | 1.14 | 0.89-1.45 |
| HbA1c | ||||
| <53 mmol/mol (7.0%) | 1.00 (RC) | — | 1.00 (RC) | — |
| 53-64 mmol/mol (7.0-8.0%) | 1.22 | 1.12-1.34 | 1.23 | 1.03-1.47 |
| 65-75 mmol/mol (8.1-9.0%) | 1.53 | 1.39-1.68 | 1.54 | 1.28-1.87 |
| >75 mmol/mol (9.0%) | 1.58 | 1.41-1.76 | 1.64 | 1.33-2.03 |
| Smoking (Yes vs No) | 0.74 | 0.67-0.81 | 0.7 | 0.58-0.84 |
| LDL-cholesterol (mmol/L) | ||||
| <1.8 | 1.00 (RC) | — | 1.00 (RC) | — |
| 1.8-2.6 | 0.94 | 0.84-1.04 | 0.89 | 0.72-1.09 |
| 2.61-3.36 | 1.06 | 0.95-1.19 | 1.15 | 0.93-1.42 |
| 3.36-4.13 | 1.37 | 1.19-1.57 | 1.17 | 0.90-1.53 |
| ≥4.13 | 1.61 | 1.32-1.97 | 1.39 | 0.95-2.03 |
| DBP ≥90 mmHg (Yes vs No) | 1.62 | 1.39-1.88 | 1.9 | 1.48-2.43 |
| SBP ≥140 mmHg (Yes vs No) | 1.64 | 1.52-1.77 | 1.64 | 1.42-1.90 |
| Insulin schemes: | ||||
| CSII | 1.00 (RC) | — | 1.00 (RC) | — |
| Basal-bolus vs CSII | 0.76 | 0.69-0.83 | 0.72 | 0.60-0.87 |
| Premix vs CSII | 0.73 | 0.56-0.95 | 0.49 | 0.26-0.94 |
| Metformin (Yes vs No) | 2.09 | 1.75-2.49 | 1.83 | 1.37-2.44 |
| SGLT2i (Yes vs No) | 1.67 | 1.42-1.97 | 1.61 | 1.26-2.04 |
| Antihypertensive treatment (Yes vs No) | 1.85 | 1.71-2.01 | 2.11 | 1.81-2.47 |
| Lipid-lowering agents (Yes vs No) | 1.35 | 1.25-1.46 | 1.44 | 1.24-1.67 |
| eGFR (>60 vs ≤60 mL/min) | 0.70 | 0.63-0.77 | 0.54 | 0.46-0.64 |
| Retinopathy (Yes vs No) | 1.30 | 1.18-1.43 | 1.27 | 1.04-1.54 |
| MAU (Yes vs No) | — | — | 1.35 | 1.14-1.59 |
| Antiplatelets (Yes vs No) | 1.10 | 1.00-1.20 | — | — |
The following variables were included in the model: sex; age; diabetes duration; smoking habit; HbA1c levels; lipid profile; BP values; eGFR; MAU; diabetes treatment; treatment with antiplatelets, antihypertensive, and lipid-lowering agents; history of micro- and macrovascular complications. Significant P values are in bold.
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitors; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; MAU, micro/macroalbuminuria; OR, odds ratio; RC, reference class; SBP, systolic blood pressure; SGLT2i, sodium/glucose cotransporter-2 inhibitor; T1D, type 1 diabetes.
Furthermore, increasing age, duration of diabetes, LDL-c, and BP levels were associated with a higher likelihood of obesity and severe obesity. Obesity and severe obesity were also associated with a higher likelihood of being treated with CSII, metformin, SGLT2i, and antihypertensive and lipid-lowering drugs. As for diabetes complications, obesity and severe obesity were associated with a higher likelihood of retinopathy and a lower likelihood of eGFR <60 mL/min/1.73m2. Severe obesity was also associated with a higher likelihood of MAU.
Similar associations, although with some differences in the strength of these associations, were noted when multivariate models were applied in T1D men and women separately (Supplementary Tables S4 and S5) (25).
Sex Differences in Clinical Characteristics, Long-term Diabetes Complications, and Process Indicators and Outcomes in the Obese and Nonobese Subgroups
Potential sex differences in the clinical characteristics within the obese and nonobese groups were also analyzed (Supplementary Table S2) (25). In both obese and normal weight groups, when comparing men vs women, women were older than men, despite a similar diabetes duration.
Women also showed higher values of HbA1c, total cholesterol, and HDL-c; lower values of SBP, DBP, and triglycerides; and a lower percentage of smokers when compared to T1D men, both in the group of subjects with obesity and in the group of nonobese subjects.
Mean LDL-c levels were higher in women than in men in the obese group, whereas no differences were observed in the normal-weight group. Cardiovascular events were overall more frequent in T1D men than in women, in both the obese and nonobese groups; conversely, retinopathy was equally frequent in both sexes in the obese group, while it was more frequent among men than in women in the group of nonobese subjects.
The percentage of subjects with impaired eGFR values (eGFR <60 mL/min) was higher in T1D women than in men, whereas MAU was more frequent in men than in women both in the obese and nonobese subgroups.
Sex differences in the pharmacological treatment, process, and outcome indicators in the obese and nonobese subgroups were also evaluated (Supplementary Table S3) (25).
Discussion
The obesity trend is progressively increasing, and, according to the World Health Organization, more than 650 million people (13% of the world population) are affected by obesity (26).
T1D subjects have long been considered to be protected from obesity, but recent data pointed to an increase of this burdensome clinical condition also in T1D, with prevalence estimates ranging from 2.8% to 37.1% (27-29), depending on definition of obesity, age of the examined population, and country. However, most of the evidence comes from pediatric studies (15, 29, 30), while data on adult T1D subjects are sparse.
In our large cohort of T1D subjects, including >37 000 adults followed up in one-third of diabetes centers in Italy, we found a high prevalence of obesity, affecting ∼13% of our population, which is in line with current data in T1D worldwide (7) and 3 recent European studies on adult T1D subjects, in which the prevalence of obesity was 18%, 17%, and 15.3%, respectively (6, 11, 31).
Moreover, severe obesity was detected in a nonnegligible percentage of our study subjects (2.3% men and 4.0% women), comparable with data from a recent study conducted in Catalonia, where 4.3% of subjects with T1D had a BMI value >35 kg/m2 (11).
These figures reflect our country-specific prevalence of obesity in the general population, which has been estimated to be 11% in Italy in 2019 (32), a very different figure when compared with data from the United States (33). Conversely, other authors have found a higher prevalence of obesity in young T1D subjects than in the age-matched nondiabetic population (29).
The progressive ageing of T1D subjects in our cohort has influenced these results. Thus, in our data set, age had an impact on obesity prevalence, which progressively increased with aging in both sexes, reaching 17% of the T1D population ages >65 years old, ie, 1 out 6 T1D subjects. The same increasing trend with aging was reported in Catalonia, with the prevalence of obesity reaching 21% in T1D subjects ages >40 years old (11).
This finding has important clinical implications since the plethora of aging T1D subjects is increasing thanks to a better management of the disease; this fact, coupled with the observed age-related increase in the prevalence of obesity, requires health systems to face a large group of T1D subjects with a long diabetes duration and a high burden of obesity in the next few years.
Also, sex may impact the epidemiology of obesity, and sex differences have been reported both in the general population and in subjects with T2D. Thus, in the general population in Italy, the prevalence of obesity is overall higher in men (11.7% men vs 10.3% women) (32), whereas in T2D subjects, severe obesity is more prevalent among women (12, 21), in whom it is associated with an higher mortality risk than in men (12, 13, 16).
When evaluating the sex-specific distribution of BMI classes in our study, we found a similar prevalence of obesity in the two sexes (13% men vs 13.9% women). T1D women had a higher prevalence of severe obesity only, and this difference was evident in all age groups (Fig. 3). Multivariate analysis also confirmed the effect of sex on severe obesity, as women had a 45% higher risk than men.
Notably, the unfavorable health outcomes associated with obesity have been shown to further increase in case of severe obesity (34, 35), and more severe obesity classes have been shown to predict mortality (36).
Data on sex differences in obesity in T1D are sparse and conflicting (37), and a recent study on a large cohort of T1D subjects did not find any sex difference in the prevalence of obesity (11).
The higher prevalence of severe obesity among T1D women cannot be easily explained. The first hypothesis may be related to insulin treatment in adult patients with long-lasting T1D. Overall, women participating in our study were older, but the duration of diabetes was similar in the 2 sexes. Moreover, women were more frequently treated with CSII than men. Although this data may reflect an indication bias, ie, diabetologists may try a more intensive insulin treatment for the documented difficulties in achieving glucose targets among T1D women, than among T1D men (16, 38, 39), the use of CSII may potentially impact body weight, because of the improvements in blood glucose control, as well as of changes in insulin doses and eating behaviors (40).
However, this explanation is not supported by a recent Australian study in adolescents with T1D, which did not find any increase of BMI-SDS with the implementation of insulin therapy with more intensive schemes (multiple daily injection/CSII) (41). Similarly, a recent Italian study showed comparable body weight gain over a 10-year follow-up in T1D patients on CSII or multiple daily injection, despite improved glycemic control and decreased insulin doses with CSII (42). The higher rate of severe obesity in women than in men is not even related to disparities in the use of adjunctive glucose-lowering therapies, such as metformin and SGLT2i, which was similar in both sexes, potentially reflecting the attempt to reduce weight and/or reach glucose targets in obese T1D subjects.
Conversely, it is likely that the differences in the prevalence of severe obesity observed in both T1D and T2D may involve complex interactions between biological (sex)- and nonbiological (gender)-related variables, many of which have not been fully clarified (43, 44).
Thus, sex-related variables such as hormonal fluctuation through the life span, including menopausal status in women but also sex differences in psychological factors, difficulty in adaptation, and acceptation of the disease and/or depression may be more frequent in T1D women than in T1D men, contributing to their body weight variations. Also, the role of eating disorders and parental control in younger population cannot be ruled out (45, 46).
In spite of all these unsolved questions that might not be adequately addressed by an observational study such as the current one, our data showing the high prevalence of obesity in T1D men and women and of severe obesity among adult women with T1D should be taken into great consideration for the clinical management of these high-risk subjects.
Obesity has an overall similar prevalence in T1D men and women and potentially similar detrimental consequences in terms of CV risk. Thus, our data clearly indicate a worse metabolic and risk factor profile among obese T1D subjects, irrespective of sex. Obese T1D subjects had a worse glucose control and higher values of major CVD risk factors, including SBP, DBP, lipid profile, and micro/macroalbuminuria; moreover, the burden of micro- and macrovascular diabetes complications, including renal disease, retinopathy, and CVD, was also higher among T1D subjects with BMI >30 kg/m2.
The greater burden of CVD risk factors and diabetes-related micro- and macrovascular complications in obese T1D should be considered in the perspective of their high global CVD risk. Thus, it has been estimated that according to European Society of Cardiology guidelines (47), 64.7% of T1D participants to the AMD Annals Initiative were at very high CVD risk and 28.5% were in the high-risk category (48), with important implications in terms of diabetes management.
In this regard, our data also documented significant differences in the quality of several diabetes care indicators, which were systematically less satisfactory in obese T1D subjects, although they did not differ between men and women. Despite a similar rate of annual monitoring of major risk factors and despite similar or even superior treatment of major risk factors in individuals with or without obesity, process and quality-of-care indicators in our data set showed that subjects with obesity more frequently have off-target values of HbA1c, cholesterol, and BP and more frequently do not reach these targets despite therapy, as compared to nonobese subjects. Moreover, the Q-score, a validated score measuring the overall diabetes care, was significantly impaired in obese T1D subjects, in which a larger percentage of subjects showed Q-score values <15, indicating poor diabetes care that has been associated with a higher CVD mortality risk (18). All of these differences between obese and nonobese subjects were evident in both sexes.
We also searched for potential sex differences in study variables between T1D men and women within the obese and nonobese groups, largely confirming those previously reported in this same population irrespective of BMI (16). Overall, our results are in line with the growing body of literature reporting sex and gender differences for diabetes long-term complications, although not consistently (22, 49-53).
Several potential strengths and limitations should be acknowledged when interpreting our results. The large number of subjects included in the current analysis, which is representative of T1D patients in Italy, as well as the collection of data on process indicators and quality of care according to sex and obesity status, should be acknowledged among the strengths of the study. On the other hand, the cross-sectional nature of our analysis prevents exploration of the causality of the detected associations. Moreover, the lack of information on waist circumference, daily insulin dose, dietary habits, and physical activity are among the limitations of the current study.
In conclusion, our data show that obesity is a frequent condition in men and women with T1D, and it is associated with a higher burden of cardiometabolic risk factors and diabetes long-term complications, as well as with an overall poorer quality of care in both sexes. Obesity prevalence increases with age, and T1D women are at higher risk of severe obesity.
The full list of authors in participating the AMD Annals Initiative is provided as supplementary material (25).
Abbreviations
- AMD
Associazione Medici Diabetologi (Italian Association of Clinical Diabetologists)
- BMI
body mass index
- BP
blood pressure
- CSII
continuous subcutaneous insulin infusion
- CV
cardiovascular
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- HbA1c
glycated hemoglobin
- HDL-c
high-density lipoprotein cholesterol
- LDL-c
low-density lipoprotein cholesterol
- MAU
micro/macroalbuminuria
- SBP
systolic blood pressure
- SGLT2i
sodium/glucose cotransporter-2 inhibitor
- T1D
type 1 diabetes
- T2D
type 2 diabetes
Contributor Information
Annalisa Giandalia, Department of Clinical and Experimental Medicine, University of Messina, 98100 Messina, Italy.
Giuseppina Tiziana Russo, Department of Clinical and Experimental Medicine, University of Messina, 98100 Messina, Italy.
Patrizia Ruggeri, UOD Diabetes Center, ASST Cremona, 26100 Cremona, Italy.
Annalisa Giancaterini, UOSD Endocrine, Metabolic and Nutrition Diseases, ASST Brianza, Desio Hospital, 20832 Desio, Italy.
Elisabetta Brun, UOC Endocrine, Metabolic and Nutrition Diseases, Ospedale Civile di Vicenza, 36100 Vicenza, Italy.
Mariarosaria Cristofaro, S.C. Diabetes and Endocrine Diseases, Cardarelli Hospital, 86100 Campobasso, Italy.
Anna Bogazzi, SSVD Diabetes and Endocrine Diseases, ASL TO 3, 10024 Torino, Italy.
Maria Chiara Rossi, Center for Outcomes Research and Clinical Epidemiology, CORESEARCH, 75100 Pescara, Italy.
Giuseppe Lucisano, Center for Outcomes Research and Clinical Epidemiology, CORESEARCH, 75100 Pescara, Italy.
Alberto Rocca, SS Diabetes and Metabolic disease, Bassini Hospital, Cinisello Balsamo, 20019 Milano, Italy.
Valeria Manicardi, Fondazione AMD, 42121 Reggio Emilia, Italy.
Paolo Di Bartolo, AUSL Diabetes Unit Romagna, 48121 Ravenna, Italy.
Graziano Di Cianni, Diabetes and Metabolic Diseases Unit, Health Local Unit North-West Tuscany, 57100 Livorno, Italy.
Chiara Giuliani, Department of Experimental Medicine, Sapienza University of Rome, 00044 Rome, Italy.
Angela Napoli, Israelitico Hospital, 00044 Rome, Italy; Cdc Santa Famiglia, 00044 Rome, Italy; Human Nutrition Sciences, International Medical University Unicamillus, 00044 Rome, Italy.
Funding
This research received external funding from Associazione Medici Diabetologi.
Author Contributions
A.G., G.T.R., A.N., and C.G. developed the study concept and design, interpreted the data, and wrote the paper. M.C.R. and G.L. developed the research methods and performed the statistical analyses. P.G., A.G., E.B., M.C., A.B., A.R., V.M., P.DB. and G.DC. critically revised the paper. G.T.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors declare no conflict of interest.
Data Availability
The datasets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
- 1. Patterson CC, Harjutsalo V, Rosenbauer J, et al. . Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408‐417. doi: 10.1007/s00125-018-4763-3 [DOI] [PubMed] [Google Scholar]
- 2. Lawrence JM, Imperatore G, Dabelea D, et al. . Trends in incidence of type 1 diabetes among non-Hispanic White youth in the U.S., 2002-2009. Diabetes, 2014;63(11):3938‐3945. doi: 10.2337/db13-1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruno G, Pagano E, Rossi E, et al. . Incidence, prevalence, costs and quality of care of type 1 diabetes in Italy, age 0–29 years: the population-based CINECA-SID ARNO Observatory, 2002–2012. Nutr Metab Cardiovasc Dis. 2016;26(12):1104‐1111. doi: 10.1016/j.numecd.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 4. International Diabetes Federation . Diabetes atlas. Accessed2021. https://diabetesatlas.org
- 5. Baskaran C, Volkening LK, Diaz M, Laffel LM. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the Diabetes Control and Complications Trial. Pediatr Diabetes. 2015;16(4):263‐270. doi: 10.1111/pedi.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Mark G, Lanzinger S, Barion R, et al. . Patient and disease characteristics of adult patients with type 1 diabetes in Germany: an analysis of the DPV and DIVE databases. Ther Adv Endocrinol Metab. 2019;10:2042018819830867. doi: 10.1177/2042018819830867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015;22(4):277‐282. doi: 10.1097/MED.0000000000000170 [DOI] [PubMed] [Google Scholar]
- 8. Betts P, Mulligan J, Ward P, Smith B, Wilkin T. Increasing body weight predicts the earlier onset of insulin-dependant diabetes in childhood: testing the “accelerator hypothesis” (2). Diabet Med. 2005;22(2):144‐151. doi: 10.1111/j.1464-5491.2004.01368.x [DOI] [PubMed] [Google Scholar]
- 9. Buzzetti R, Zampetti S, Pozzilli P. Impact of obesity on the increasing incidence of type 1 diabetes. Diabetes Obes Metab. 2020;22(7):1009‐1013. doi: 10.1111/dom.14022 [DOI] [PubMed] [Google Scholar]
- 10. Thorn LM, Forsblom C, Fagerudd J, et al. . Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care. 2005;28(8):2019‐2024. doi: 10.2337/diacare.28.8.2019 [DOI] [PubMed] [Google Scholar]
- 11. Da Genua I, Franch-Nadal J, Navas E, et al. . Obesity and related comorbidities in a large population-based cohort of subjects with type 1 diabetes in Catalonia. Front Endocrinol (Lausanne). 2022;13:1015614. doi: 10.3389/fendo.2022.1015614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kautzky-Willer A, Baggio G, Rossi MC, Lapolla A, Russo GT. Type 2 diabetes and cardiovascular risk in women 2016. Int J Endocrinol. 2017;2017:6905697. doi: 10.1155/2017/6905697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62(10):1761‐1772. doi: 10.1007/s00125-019-4939-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imbalzano E, Russo GT, Giandalia A, et al. . Sex-specific impact of different obesity/metabolic phenotypes on long-term cardiovascular outcomes in acute coronary syndrome patients. Biomedicines. 2022;10(2):424. doi: 10.3390/biomedicines10020424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fröhlich-Reiterer EE, Rosenbauer J, Bechtold-Dalla Pozza S, et al. . Predictors of increasing BMI during the course of diabetes in children and adolescents with type 1 diabetes: data from the German/Austrian DPV multicentre survey. Arch Dis Child. 2014;99(8):738‐743. doi: 10.1136/archdischild-2013-304237 [DOI] [PubMed] [Google Scholar]
- 16. Manicardi V, Russo G, Napoli A, et al. . Gender-disparities in adults with type 1 diabetes: more than a quality of care issue. A cross-sectional observational study from the AMD Annals Initiative. PLoS One. 2016;11(10):e0162960. doi: 10.1371/journal.pone.0162960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Berardis G, Pellegrini F, Franciosi M, et al. . Quality of diabetes care predicts the development of cardiovascular events: results of the QuED study. Nutr Metab Cardiovasc Dis. 2008;18(1):57‐65. doi: 10.1016/j.numecd.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 18. Rossi MC, Lucisano G, Comaschi M, et al. . Quality of diabetes care predicts the development of cardiovascular events: results of the AMD-QUASAR study. Diabetes Care. 2011;34(2):347‐352. doi: 10.2337/dc10-1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossi MC, Nicolucci A, Arcangeli A, et al. . Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31(11):2166‐2168. doi: 10.2337/dc08-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicolucci A, Rossi MC, Arcangeli A, et al. . Four-year impact of a continuous quality improvement effort implemented by a network of diabetes outpatient clinics: the AMD-Annals Initiative. Diabet Med. 2010;27(9):1041‐1048. doi: 10.1111/j.1464-5491.2010.03055.x [DOI] [PubMed] [Google Scholar]
- 21. Rossi MC, Cristofaro MR, Gentile S, et al. . Sex disparities in the quality of diabetes care: biological and cultural factors may play a different role for different outcomes: a cross-sectional observational study from the AMD Annals Initiative. Diabetes Care. 2013;36(10):3162‐3168. doi: 10.2337/dc13-0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo G, Pintaudi B, Giorda C, et al. . Age- and gender-related differences in LDL-cholesterol management in outpatients with type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:957105. doi: 10.1155/2015/957105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossi MC, Candido R, Ceriello A, et al. . Trends over 8 years in quality of diabetes care: results of the AMD Annals continuous quality improvement initiative. Acta Diabetol. 2015;52(3):557‐571. doi: 10.1007/s00592-014-0688-6 [DOI] [PubMed] [Google Scholar]
- 24. Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156(11):785‐795. doi: 10.7326/0003-4819-156-6-201203200-00391 [DOI] [PubMed] [Google Scholar]
- 25.Giandalia A, Russo GT, Ruggeri P, et al. https://osf.io/4bwfj/? view_only=d1144567a29749d4bc45b2e25cc5287e Supplementary data for “Sex-related characteristics of obesity in subjects with type 1 diabetes: the AMD Annals Initiative.” Deposited February 17, 2023.
- 26. World Health Organization . WHO.int. Accessed June 9,2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 27. Melin EO, Thulesius HO, Hillman M, Landin-Olsson M, Thunander M. Abdominal obesity in type 1 diabetes associated with gender, cardiovascular risk factors and complications, and difficulties achieving treatment targets: a cross sectional study at a secondary care diabetes clinic. BMC Obes. 2018;5:15. doi: 10.1186/s40608-018-0193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu LL, Lawrence JM, Davis C, et al. . Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11(1):4‐11. doi: 10.1111/j.1399-5448.2009.00519.x [DOI] [PubMed] [Google Scholar]
- 29. DuBose SN, Hermann JM, Tamborlane WV, et al. . Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr. 2015;167(3):627‐632.e1-4. doi: 10.1016/j.jpeds.2015.05.046 [DOI] [PubMed] [Google Scholar]
- 30. Kurpiewska E, Ciężki S, Jamiołkowska-Sztabkowska M, et al. . Excessive BMI is associated with higher C-peptide level at recognition but also with its greater loss in two years clinical observation in children with new onset type 1 diabetes. Front Immunol. 2023;14:1176403. doi: 10.3389/fimmu.2023.1176403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lavens A, Nobels F, De Block C, et al. . Effect of an integrated, multidisciplinary nationwide approach to type 1 diabetes care on metabolic outcomes: an observational real-world study. Diabetes Technol Ther. 2021;23(8):565‐576. doi: 10.1089/dia.2021.0003 [DOI] [PubMed] [Google Scholar]
- 32. ISTAT . Indagine “aspetti della vita quotidiana.”2019.
- 33. OECD health statistics. Accessed 2020. https://www.oecd.org/els/health-systems/obesityandtheeconomicsofpreventionfitnotfat-italykeyfacts.htm
- 34. Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074‐1081. doi: 10.1093/ajcn/72.5.1074 [DOI] [PubMed] [Google Scholar]
- 35. Sbaraini M, Cureau FV, Sparrenberger K, et al. . Severity of obesity is associated with worse cardiometabolic risk profile in adolescents: findings from a Brazilian national study (ERICA). Nutrition. 2020;75-76:110758. doi: 10.1016/j.nut.2020.110758 [DOI] [PubMed] [Google Scholar]
- 36. Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. . Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211‐2219. doi: 10.1056/NEJMoa1000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valerio G, Iafusco D, Zucchini S, Maffeis C; Study-Group on Diabetes of Italian Society of Pediatric Endocrinology and Diabetology (ISPED) . Abdominal adiposity and cardiovascular risk factors in adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2012;97(1):99‐104. doi: 10.1016/j.diabres.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 38. McKnight JA, Wild SH, Lamb MJ, et al. . Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32(8):1036‐1050. doi: 10.1111/dme.12676 [DOI] [PubMed] [Google Scholar]
- 39. Collier A, Ghosh S, Hair M, Waugh N. Gender differences and patterns of cardiovascular risk factors in type 1 and type 2 diabetes: a population-based analysis from a Scottish region. Diabet Med. 2015;32(1):42‐46. doi: 10.1111/dme.12569 [DOI] [PubMed] [Google Scholar]
- 40. Festa C, Fresa R, Visalli N, et al. . Insulin requirements and carbohydrate to insulin ratio in normal weight, overweight, and obese women with type 1 diabetes under pump treatment during pregnancy: a lesson from old technologies. Front Endocrinol (Lausanne). 2021;12:610877. doi: 10.3389/fendo.2021.610877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marlow AL, King BR, Phelan HT, Smart CE. Adolescents with type 1 diabetes can achieve glycemic targets on intensive insulin therapy without excessive weight gain. Endocrinol Diabetes Metab. 2022;5(4):e352. doi: 10.1002/edm2.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alderisio A, Bozzetto L, Franco L, Riccardi G, Rivellese AA, Annuzzi G. Long-term body weight trajectories and metabolic control in type 1 diabetes patients on insulin pump or multiple daily injections: a 10-year retrospective controlled study. Nutr Metab Cardiovasc Dis. 2019; 29(10):1110‐1117. doi: 10.1016/j.numecd.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 43. Rossi MC, Lucisano G, Pintaudi B, et al. . The complex interplay between clinical and person-centered diabetes outcomes in the two genders. Health Qual Life Outcomes. 2017;15(1):41. doi: 10.1186/s12955-017-0613-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Enzlin P, Mathieu C, Demyttenaere K. Gender differences in the psychological adjustment to type 1 diabetes mellitus: an explorative study. Patient Educ Couns. 2002;48(2):139‐145. doi: 10.1016/s0738-3991(02)00009-5 [DOI] [PubMed] [Google Scholar]
- 45. Marlow AL, Rowe CW, Anderson D, et al. . Young children, adolescent girls and women with type 1 diabetes are more overweight and obese than reference populations, and this is associated with increased cardiovascular risk factors. Diabet Med. 2019;36(11):1487‐1493. doi: 10.1111/dme.14133 [DOI] [PubMed] [Google Scholar]
- 46. Troncone A, Chianese A, Zanfardino A, et al. . Disordered eating behaviors in youths with type 1 diabetes during COVID-19 lockdown: an exploratory study. J Eat Disord. 2020;8(1):76. doi: 10.1186/s40337-020-00353-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cosentino F, Grant PJ, Aboyans V, et al. . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255‐323. doi: 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 48. Pintaudi B, Scatena A, Piscitelli G, et al. . Clinical profiles and quality of care of adults with type 1 diabetes according to their cardiovascular risk: a multicenter, observational, retrospective study. Diabetes Res Clin Pract. 2021;182:109131. doi: 10.1016/j.diabres.2021.109131 [DOI] [PubMed] [Google Scholar]
- 49. Sundquist K, Li X. Type 1 diabetes as a risk factor for stroke in men and women aged 15-49: a nationwide study from Sweden. Diabet Med. 2006;23(11):1261‐1267. doi: 10.1111/j.1464-5491.2006.01959.x [DOI] [PubMed] [Google Scholar]
- 50. Giandalia A, Giuffrida AE, Gembillo G, et al. . Gender differences in diabetic kidney disease: focus on hormonal, genetic and clinical factors. Int J Mol Sci. 2021;22(11):5808. doi: 10.3390/ijms22115808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Russo GT, Giandalia A, Romeo EL, et al. . HDL Subclasses and the common CETP TaqIB variant predict the incidence of microangiopatic complications in type 2 diabetic women: a 9 years follow-up study. Diabetes Res Clin Pract. 2017;132:108‐117. doi: 10.1016/j.diabres.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 52. Russo GT, Manicardi V, Rossi MC, Orsi E, Solini A. Sex- and gender-differences in chronic long-term complications of type 1 and type 2 diabetes mellitus in Italy. Nutr Metab Cardiovasc Dis. 2022;32(10):2297‐2309. doi: 10.1016/j.numecd.2022.08.011 [DOI] [PubMed] [Google Scholar]
- 53. Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019;62(9):1550‐1560. doi: 10.1007/s00125-019-4926-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.