Abstract
We combine the effects of spirocyclization and hyperconjugation to increase the Diels–Alder reactivity of the 4H-pyrazole scaffold. A density functional theory (DFT) investigation predicts that 4H-pyrazoles containing an oxetane functionality at the saturated center are extremely reactive despite having a relatively high-lying lowest unoccupied molecular orbital (LUMO) energy.
Keywords: Click chemistry, cycloaddition, density functional theory (DFT)
Graphical Abstract
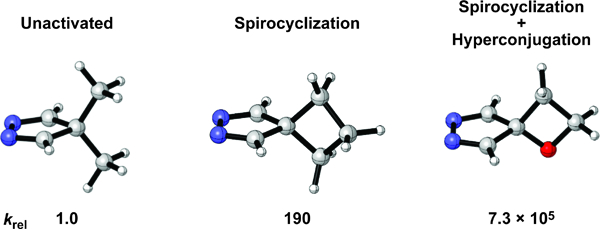
Spirocyclization and hyperconjugation activate 4H-pyrazoles as Diels–Alder dienes. The harmonization of spirocyclization and hyperconjugation was used to design a 4H-pyrazole that is predicted to be 730,000 times more reactive than an unactivated 4H-pyrazole.
Introduction
Sauer and coworkers reported the rapid reactivity of 1,2,4,5-tetrazines as inverse-electron demand Diels–Alder dienes with strained dienophiles such as cyclopropene, trans-cyclooctene, cyclooctyne, and norbornadiene.1,2 The Fox group took advantage of the reactivity of the tetrazine-trans-cyclooctene reaction, using it to modify a protein at low concentration.3 Since then, efforts have been made to enhance the reactivity of the tetrazine and trans-cyclooctene scaffolds without sacrificing stability.4,5
We have focused on developing 4H-pyrazoles as bioorthogonal reagents. Fluorination of the saturated center in a 5-membered diene invokes antiaromatic electron delocalization and increases Diels–Alder reactivity.6–9 We have reported a 4,4-difluoro-4H-pyrazole that reacts twice as fast as an equivalently substituted tetrazine towards the strained alkyne, bicyclononyne (BCN).10 Although fluorination increased the reactivity, it also compromised the stability of the 4H-pyrazole scaffold.11
A purely computational study predicted that spirocyclic 4H-pyrazoles were more reactive than their acyclic analogs.12,13 This study inspired the synthesis and experimental analysis of spirocyclic 4-oxo-4H-pyrazoles.14 Whereas the spirocyclic 4-oxo-4H-pyrazoles were not as reactive as the 4,4-difluoro-4H-pyrazole, they showed robust biological stability, allowing them to be utilized in biological applications. Herein, we further optimize the spirocyclic 4-oxo-4H-pyrazole motif and disclose a new spirocyclic 4-oxo-4H-pyrazole with promising reactivity and suggestive stability.
Results and discussion
The structures of the 4H-pyrazoles included in this study are shown in Scheme 1. The lowest unoccupied molecular orbital (LUMO) energies of these compounds as well as their activation energies towards the strained alkyne BCN are reported in Figure 1. All calculations were carried out at the M06-2X/6-311++G(d,p)-SMD(H2O)//M06-2X/6-31G(d) level of theory.15 The M06-2X functional has been shown to accurately predict the reactivity of bioorthogonal cycloaddditions.16–20
Scheme 1.
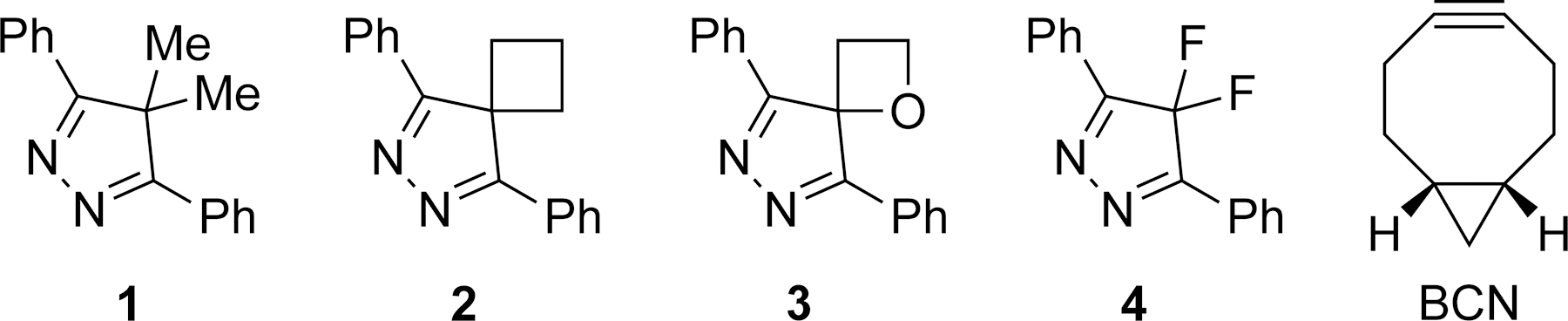
Structures of 4H-pyrazoles 1–4 and BCN.
Figure 1.
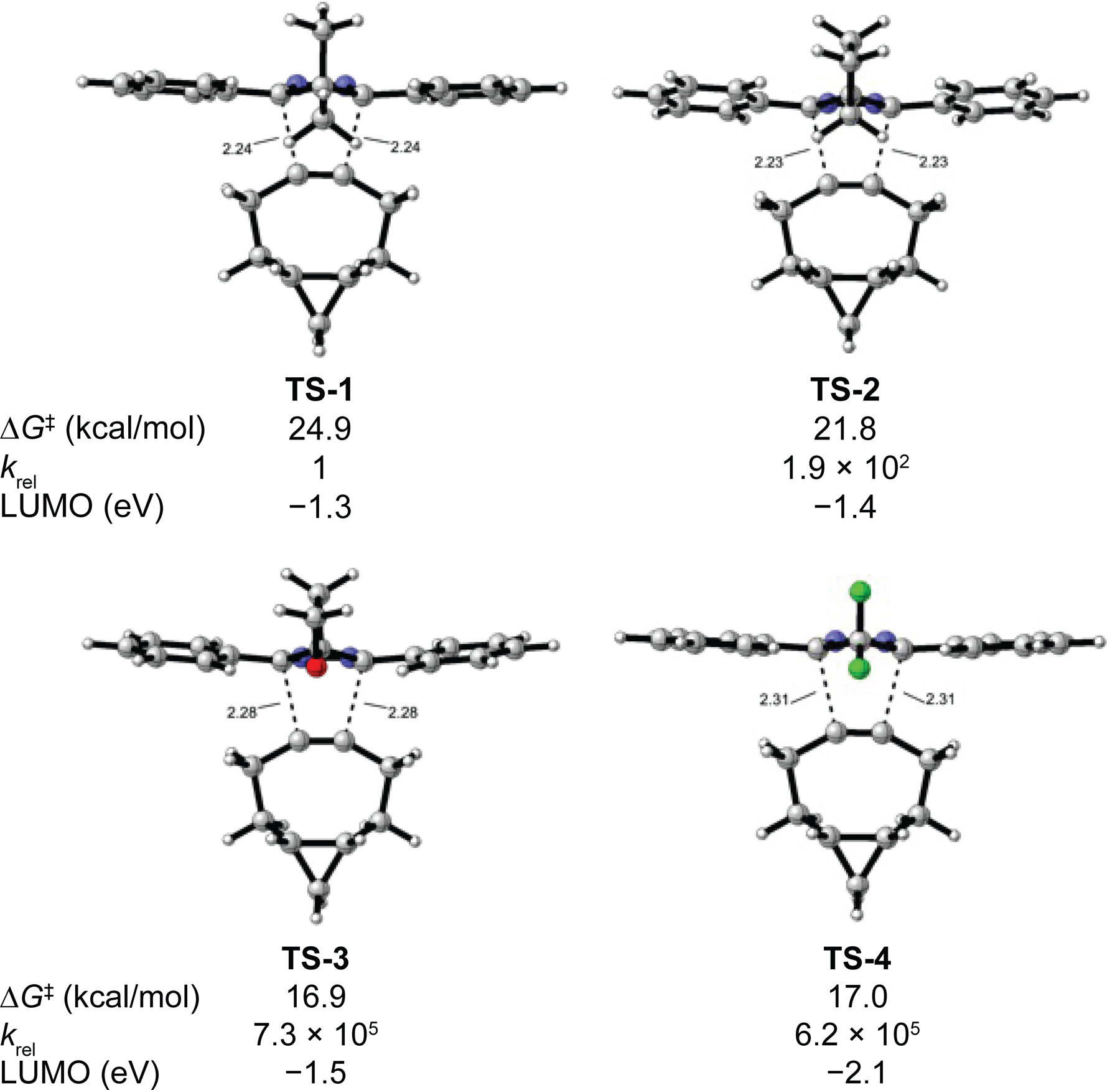
Gibbs free energies of activation in kcal/mol for the Diels–Alder reaction of dienes 1–4 with BCN. Relative rates were obtained from the Arrhenius equation. LUMO energies are reported in electron volts calculated from the diene ground state geometries. Forming bond lengths of the transition state structures are reported in Ångstroms.
The dimethyl 4H-pyrazole, 1, is the least reactive 4H-pyrazole with an activation energy of 24.9 kcal/mol. The difluoro 4H-pyrazole, 4, is predicted to be 6.2 × 105 times more reactive than 1 with an activation energy of 17.0 kcal/mol. The cyclobutane 4H-pyrazole, 2, has a calculated activation energy of 21.8 kcal/mol and is predicted to be 190 times more reactive than dimethyl 4H-pyrazole 1. The oxetane 4H-pyrazole, 3, combines the effects of spirocyclization and hyperconjugation in an optimal fashion to promote reactivity. This diene has a predicted activation energy of 16.9 kcal/mol, which is comparable to that of highly reactive difluoro 4H-pyrazole 4.
The computed LUMO energies are −1.3, −1.4, −1.5, and −2.1 eVs for 4H-pyrazoles 1, 2, 3, and 4, respectively. It has been shown that the relevant unoccupied molecular orbitals of appropriate symmetry in 1,2,4,5-tetrazines correlate with their stability in biological media.5 4H-Pyrazoles 3 and 4 have similar predicted reactivities towards BCN, but the LUMO of 3 is 0.55 eVs higher lying than the LUMO of 4. This increase suggests that oxetane 4H-pyrazole 3 will have greater stability towards biological nucleophiles relative to difluoro 4H-pyrazole 410,11 and could expand the scope of bioorthogonal reactions based on the Diels–Alder reaction.21
Conclusions
We find that oxetane 4H-pyrazole 3 is a promising motif worthy of experimental study. The harmonization of spirocyclization and hyperconjugation in the oxetane 4H-pyrazole induces a synergetic effect that promotes the reactivity of the 4H-pyrazole scaffold without significantly disturbing the electronic properties of the diene. This computational studyx suggests that oxetane 4H-pyrazole 3 will have similar Diels–Alder reactivity and increased biological stability relative to 4H-pyrazoles that are activated by fluorination.
Supplementary Material
Acknowledgments
B.J.L. was supported by postdoctoral fellowship F32 GM137543 from the NIH. N.S.A. was supported by a Graduate Research Fellowship from the NSF. This work was supported by Grants R01 GM044783 and R35 GM148220 from the NIH. Computational resources were provided by the Extreme Science and Engineering Discovery Environment (XSEDE) Bridges at the Pittsburgh Supercomputing Center through allocation TG-CHE190066. XSEDE is supported by Grant ACI-1548562 from the NSF.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tetlet.2023.xxxxxx.
References
- [1].Thalhammer F, Wallfahrer U, Sauer J J, Tetrahedron Lett 31 (1990) 6851–6854. [Google Scholar]
- [2].Sauer J, Heinrichs G, Tetrahedron Lett 7 (1966) 4979–4984. [Google Scholar]
- [3].Blackman ML, Royzen M, Fox JM, J. Am. Chem. Soc 130 (2008) 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Taylor MT, Blackman ML, Dmitrenko O, Fox JM, J. Am. Chem. Soc 133 (2011) 9646–9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Svatunek D, Wilkovitsch M, Hartmann L, Houk KN, Mikula H, J. Am. Chem. Soc 144 (2022) 8171–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fernández I I, Wu JI, von R Schleyer P. , Org. Lett 15 (2013) 2990–2993. [DOI] [PubMed] [Google Scholar]
- [7].Levandowski BJ, Zou L, Houk KN, J. Comput. Chem 37 (2016) 117–123. [DOI] [PubMed] [Google Scholar]
- [8].Levandowski BJ, Zou L, Houk KN, J. Org. Chem 83 (2018) 14658–14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nyulászi L, von R P. Schleyer, J. Am. Chem. Soc 121 (1999) 6872–6875. [Google Scholar]
- [10].Levandowski BJ, Abularrage NS, Houk KN, Raines RT, Org. Lett 21 (2019) 8492–8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Abularrage NS, Levandowski BJ, Raines RT, Int. J. Mol. Sci 21 (2020) 3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Levandowski BJ, Abularrage NS, Raines RT, J. Phys. Org. Chem 36 (2023) e4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Levandowski BJ, Abularrage NS, Raines RT, Tetrahedron 91 (2021) 132160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abularrage NS, Levandowski BJ, Giancola JB, Graham BJ, Raines RT, Chem. Commun 59 (2023) 4451–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao Y, Truhlar DG, Theor. Chem. Acc 120 (2008) 215–241. [Google Scholar]
- [16].Yang J, Liang Y, Šečkutė J, Houk KN, Devaraj NK, Chem. Eur. J 20 (2014) 14893–14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kamber DN, Liang Y, Blizzard RJ, Liu F, Mehl RA, Houk KN, Prescher JA, J. Am. Chem. Soc 137 (2015) 8388–8391. [DOI] [PubMed] [Google Scholar]
- [18].Narayanam MK, Liang Y, Houk KN, Murphy JM, Chem. Sci 7 (2016) 1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Levandowski BJ, Gamache RF, Murphy JM, Houk KN, J. Am. Chem. Soc 140 (2018) 6426–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Levandowski BJ, Svatunek D, Sohr B, Mikula H, Houk KN, J. Am. Chem. Soc 141 (2019) 2224–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levandowski BJ, Raines RT, Chem. Rev 121 (2021) 6777–6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


