Abstract
Mitochondria are organelles involved in the regulation of various important cellular processes, ranging from ATP generation to immune activation. A healthy mitochondrial network is essential for cardiovascular function and adaptation to pathological stressors. Mitochondria undergo fission or fusion in response to various environmental cues and these dynamic changes are vital for mitochondrial function and health. In particular, mitochondrial fission is closely coordinated with the cell cycle, and is linked to changes in mitochondrial respiration and membrane permeability. Another key function of fission is the segregation of damaged mitochondrial components for degradation by mitochondrial autophagy (mitophagy). Mitochondrial fission is induced by the large GTPase dynamin-1-like protein (DNM1L; also known as dynamin-related protein 1 (DRP1)) and is subject to sophisticated regulation. Activation requires various post-translational modifications of DNML1, actin polymerization and the involvement of other organelles such as the endoplasmic reticulum, Golgi and lysosomes. A decrease in mitochondrial fusion can also shift the balance towards fission. Although mitochondrial fission is necessary for cellular homeostasis, this process is often aberrantly activated in cardiovascular disease. In fact, strong evidence exists that aberrant mitochondrial fission directly contributes to disease development. In this Review, we compare the physiological and pathophysiological roles of mitochondrial fission and discuss the therapeutic potential of preventing excessive mitochondrial fission in the heart and vasculature.
Introduction
Mitochondria are multifaceted organelles that regulate various important cellular processes including metabolism, ATP generation and activation of inflammation. These organelles are also involved in determining cell fate during differentiation and activating cell death1. Regulation of these processes is often associated with changes in mitochondrial morphology, where mitochondria undergo fission or fusion in response to changes in the cellular environment. Studies initially indicated a connection between increased mitochondrial fission and activation of apoptosis2, but it is now clear that mitochondrial morphology is also closely linked to bioenergetics and is affected by changes in metabolic demand3,4. In addition, mitochondrial fission contributes to quality control by separating damaged organelles from the healthy network for degradation by mitochondrial autophagy (mitophagy)5. Mitochondrial fission is also closely coordinated with the cell cycle6 and facilitates the equal segregation of mitochondrial DNA (mtDNA) in daughter organelles7. Considering the vital role of mitochondria in cellular homeostasis, it is not surprising that dysregulation of mitochondrial dynamics is associated with disease development.
Because of its high energy demand, the heart is enriched with mitochondria and well-balanced mitochondrial fission and fusion are essential for cardiac homeostasis. Although fission is important for functional mitophagy in the heart,8, excessive mitochondrial fission contributes to various cardiovascular pathologies, including ischaemia–reperfusion injury (IRI)9,10, pathological hypertrophy11 and atherosclerosis12. In this Review, we detail the molecular mechanisms underlying mitochondrial fission mediated by dynamin-1-like protein (DNM1L; also known as dynamin-related protein 1 (DRP1)) and its function in various cellular processes. In particular, we highlight novel mechanisms for DNML1 activation and interorganellar regulation of mitochondrial morphology. We also discuss the increasing evidence for the role of mitochondrial fission in cardiovascular disease progression, and emerging therapeutic strategies to restrict fission-induced cardiovascular pathophysiology.
Mechanisms of mitochondrial fission
Mitochondrial machinery for fission and fusion
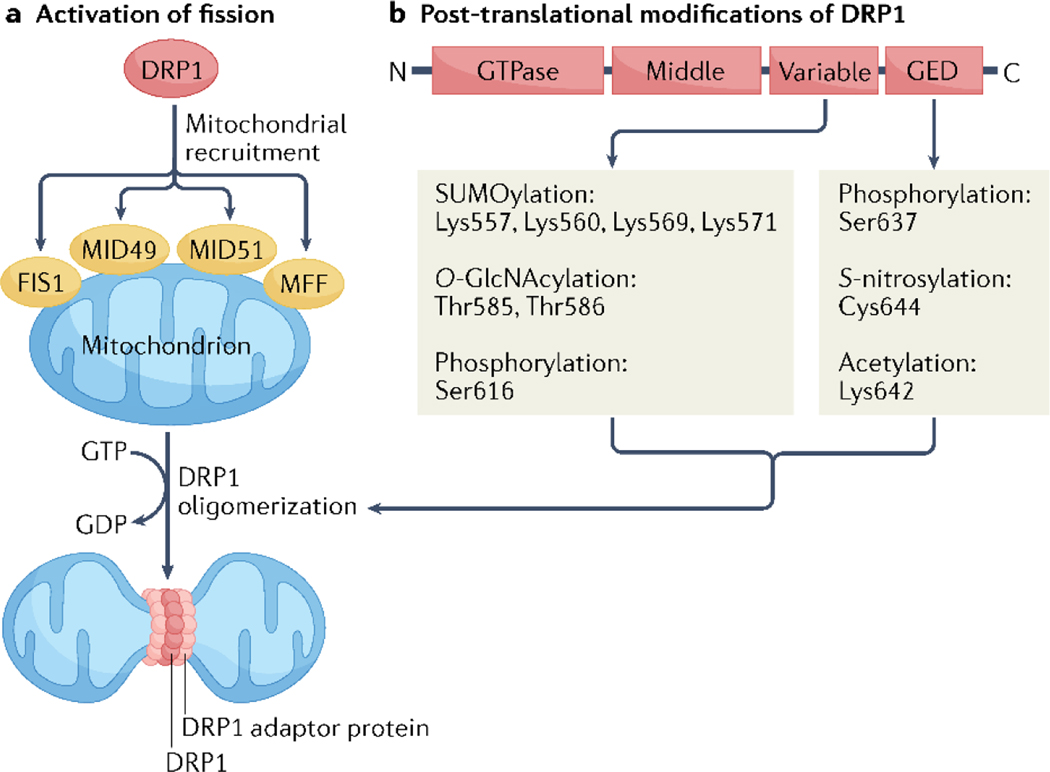
DNML1, a member of the dynamin family of GTP-binding proteins, is the primary mediator of mitochondrial fission. DNML1 translocates from the cytosol to mitochondria where it assembles into helical oligomers that wrap around the outer mitochondrial membrane (OMM) to facilitate constriction and scission in a GTP-dependent manner13,14 (FIG. 1a). Classical dynamins directly bind phospholipids in membranes through their pleckstrin homology domain, which is lacking in DNML1 and so adaptor proteins such as mitochondrial fission 1 (FIS1), mitochondrial fission factor (MFF), mitochondrial dynamics protein 49 (MID49) and MID51 are used to anchor DNML1 to the mitochondrial surface. FIS1 was the first protein reported to function as a mitochondrial adaptor protein for DNML1 in cells15–17. However, whether FIS1 is an essential regulator of DNML1-mediated fission is unclear, because FIS1-deficient mammalian cells have mild or no fission defects16–20. Other studies have identified that FIS1 specifically functions in mitophagy21–23. Subsequent identification of MFF, MID49, and MID51 have led to additional questions about the specific functions of these adaptor proteins in regulating DNML1-mediated fission. For example, knockdown of MFF, but not FIS1, in HeLa cells leads to mitochondrial elongation, and reduces recruitment of DNML1 to mitochondria20. Similarly, MFF-deficient mouse embryonic fibroblasts (MEFs) have more elongated mitochondria compared with Fis1−/− MEFs, and simultaneous deletion of Fis1 and Mff is required to recapitulate the mitochondrial phenotype seen in Dnml1−/− MEFs18. However, in contrast to these findings, Osellame et al. reported that single deletion of MiD49, MiD51, Mff or Fis1 has no effect on mitochondrial morphology or recruitment of DNML1 in MEFs24. Only simultaneous deletion of multiple adaptor proteins disrupts recruitment of DNML1 and results in a fused mitochondrial network24, suggesting that redundancies exist among the various adaptor proteins. Thus, the specific roles of individual DNML1 adaptors and their regulation is still not well understood. Other proteins, including the BCL-2 family members BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) and induced myeloid leukaemia cell differentiation protein Mcl-1 (MCL-1), as well as FUN14 domain-containing protein 1 (FUNDC1), have also been reported to recruit DNML1 to the OMM to induce fission25–27.
Fig. 1 |. Structure and function of DNM1L in mitochondrial fission.
a | Cytosolic dynamin-1-like protein (DNML1; also known as dynamin-related protein 1 (DRP1) monomers are recruited to the outer mitochondrial membrane by the adaptor proteins mitochondrial fission 1 (FIS1), mitochondrial dynamics protein 49 (MID49) or MID51 and mitochondrial fission factor (MFF), which facilitates the formation of helical ring DNML1 oligomers. GTP hydrolysis by DNML1 stimulates OMM constriction and subsequent scission. b | Several post-translational modifications within the variable region and GTPase effector domain (GED) of DNML1 regulate fission. O-GlcNAcylation, O-linked-N-acetylglucosaminylation.
Studies in DNML1-deficient mice have confirmed the importance of functional mitochondrial fission for cardiac health (TABLE 1). Global DNML1-deficiency is embryonically lethal28,29, whereas myocyte-specific DNML1-deficient mice develop severe cardiac dysfunction after birth and die within 10 days30,31. Deletion of Dnml1 in the hearts of adult mice also results in rapid heart failure and premature death8,32. Interestingly, however, doxycycline-inducible expression of the dominant-negative DNML1K38A for up to 6 months in transgenic mice is reported to have no systemic effect33. On the basis of phenotypes in mice with genetic deletion of Dnml1, fission is not likely to be completely abrogated in DNML1K38A transgenic mice. Little is known about the various adaptor proteins in the heart, but Chen and colleagues found that MFF is important for heart function because Mff-deficient mice develop dilated cardiomyopathy and die from heart failure by 13 weeks of age34. The milder cardiac phenotype in MFF deficiency compared with the lethality in Dnml1-knockout mice suggests that DNML1-mediated mitochondrial fission can still function in the absence of MFF probably due to partial compensation by other adaptor proteins. However, the specific development of heart failure in mice with global MFF deficiency suggests that MFF has a more important role in regulating DNML1-mediated fission in the mature heart relative to other tissues.
Table 1 |.
Systemic and cardiac-specific genetic mouse models of mitochondrial dynamics
| Target(s) | Genetic model | Outcome | Refs | |
|---|---|---|---|---|
| Basal characterization | Effects of pathological stress | |||
| Mff | Systemic homozygous knockout | Lethal cardiomyopathy at 13 weeks. Neuromuscular and fertility defects. Impaired mitochondrial respiration and ATP production. Loss of pro-fusion MFN1 rescues phenotype. | NR | 34 |
| Dnm1l | Systemic homozygous knockout | Lethality by embryonic day 10.5. Developmental defects due to brain hypoplasia and apoptosis. | NR | 28,29 |
| Systemic heterozygous knockout | Viable with no obvious cardiac phenotype. | Reduced immune infiltration and protection against angiotensin II-mediated AAA. No effect in AAV9–PCSK9 atherosclerosis model. Protection against TNF-driven endothelial leukocyte adhesion. | 12,29,147,155 | |
| Systemic C452F mutation | Progressive cardiac mitochondrial dysfunction and sterile inflammation resulting in heart failure. | NR | 92 | |
| Cardiac-specific homozygous knockout | Lethal dilated cardiomyopathy by postnatal day 7. Cardiac mitochondrial respiratory defects, mitophagy impairment and mtDNA nucleoid aggregation. | NR | 30–32 | |
| Cardiac-specific heterozygous knockout | Normal cardiac structure and function despite reduced mitochondrial ATP production. | Increased myocardial IRI. Impaired mitophagy and exacerbated cardiac pathophysiology following pressure overload. | 8,31,90 | |
| Tamoxifen-inducible, cardiac-specific knockout | Lethal dilated cardiomyopathy at 4–8 weeks post-tamoxifen. Mitochondrial dysfunction evident prior to cardiac pathophysiology. Dysregulation of mitophagy and activation of apoptosis in the heart. Diminished maximal exercise performance. | NR | 3,8,32,79 | |
| Systemic, doxycycline-inducible, K38A dominant negative transgenic | No phenotype evident after modest induction of transgene for 6 months. | Protection against diabetes-induced oxidative stress in the kidney and liver. Protection against vascular hyperplasia following arterial injury. | 33,154 | |
| Cardiac-specific inducible bitransgenic (tetracycline-off) | Normal mitochondrial and cardiac function. | NR | 41 | |
| Mfn1 | Systemic homozygous knockout | Lethality by embryonic day 11.5. | NR | 36 |
| Mfn2 | Systemic homozygous knockout | Lethality by embryonic day 10.5. | NR | 35 |
| Mfn1, Mfn2 | Cardiac-specific double homozygous knockout | Lethality by embryonic day 9.5 when Cre recombinase expression is driven by the NKX-2.5 promoter. Lethal cardiomyopathy when cre expression is driven by the MYH6 promoter. Abnormal mitochondrial structure and reduced mtDNA content. | NR | 37,38 |
| Mfn1, Mfn2 | Tamoxifen-inducible, cardiac-specific double knockout | Eccentric ventricular remodelling and wall thickening at 6 weeks after gene deletion. Reduced mitochondrial size and activation of the mitochondrial unfolded protein response. 50% premature mortality by 9 weeks post-tamoxifen. | Protection against myocardial IRI when induced ~4 weeks after gene deletion (prior to cardiomyopathy) due to impaired mitochondria-sarcoplasmic reticulum tethering. | 32,41,170 |
| Dnm1l, Mfn1, Mfn2 | Tamoxifen-inducible, cardiac-specific triple knockout | Concentric cardiac hypertrophy eventually progressing to heart failure, despite minimal changes in myocyte viability. Extended survival with mfn1-mfn2-dnml1 triple knockout compared with mfn1-mfn2 double knockout or dnm1l single knockout (7 vs 17 weeks post-tamoxifen). | NR | 41 |
AAA, abdominal aortic aneurysm; AAV9, adeno-associated virus serotype 9; DNM1L, dynamin-1-like protein (also known as dynamin-related protein 1 (DRP1)); IRI, ischaemia–reperfusion injury; mtDNA, mitochondrial DNA; MFN, mitofusin; MYH6, myosin-6; NKX-2.5, homeobox protein Nkx-2.5; NR, not reported; PCSK9, proprotein convertase subtilisin/kexin type 9; TNF, tumour necrosis factor.
DNML1-mediated mitochondrial fission is opposed by fusion between mitochondria, which is driven by GTPases mitofusin-1 (MFN1) and MFN2 in the outer membrane and dynamin-like 120 kDa protein, mitochondrial (also known as optic atrophy protein 1; OPA1) in the inner membrane. The loss of these fusion factors is sufficient to cause a similar fragmented mitochondrial phenotype seen with DNML1 activation35. Studies in mice have demonstrated the importance of mitochondrial fusion in cardiac homeostasis (TABLE 1). Despite the overlapping function between MFN1 and MFN2 in promoting mitochondrial fusion, deletion of either Mfn1 or Mfn2 causes embryonic lethality36 suggesting that these proteins have tissue-specific expression or function during development. By contrast, mice with cardiac-specific deletion of either Mfn1 or Mfn2 is well tolerated with mice developing normally, whereas simultaneous ablation of Mfn1 and Mfn2 in hearts results lethality during development37 or shortly after birth38. Similarly, a truncating nonsense mutation in Opa1 is embryonically lethal in mice39 and imbalanced OPA1 processing leads to pronounced mitochondrial fission in the myocardium of mice eventually leading to heart failure40. Interestingly, an imbalance in mitochondrial fission or fusion contributes to the development of heart failure. The lethal cardiomyopathy that develops in mice lacking the fission protein MFF is rescued with the concomitant deletion of Mfn134. Similarly, Mfn1/Mfn2/Dnml1 cardiac triple-knockout mice with disruptions in both fission and fusion are viable with longer survival and delayed development of cardiomyopathy compared with cardiac-specific Drp1- or Mfn1/2-deficient mice41. The fact that cardiomyocyte-specific genetic disruption of either mitochondrial fission or fusion leads to faster development of heart failure than with deletion of DRP1 or MFN1/MFN2 alone suggest that disproportionate fission or fusion is more deleterious that a simultaneous disruption in both processes.
Post-translational modifications in DNML1
DNML1 is subjected to various post-translational modifications that regulate its activity in response to changes in metabolic or redox status (FIG. 1b). Phosphorylation of DNML1 is well known to stimulate or inhibit fission depending on the specific residue targeted. Phosphorylation of DNML1 at Ser616 and Ser637 — corresponding to mouse Ser579 and Ser600, respectively — are among the most studied sites in fission regulation. Phosphorylation at Ser616 within the GTPase effector domain is associated with translocation of DNML1 to the mitochondria and activation of fission42–44, whereas phosphorylation at Ser637 remains controversial. Ser637 phosphorylation was initially reported to restrict fission through cytosolic retention of DNML145,46. However, DNML1 has still been observed to translocate to the mitochondria with Ser637 phosphorylation47, and Ser637 phosphorylation by protein kinase D and Rho-associated protein kinase 1 is associated with increased fission in myocytes and endothelial cells, respectively48,49. Interestingly, in a study published in 2021, Ser637 phosphorylation was shown to promote subsequent Ser616 phosphorylation and phosphorylation at both sites was necessary for maximal fission in MEFs50. However, blocking Ser616 phosphorylation downstream of Ser637 resulted in mitochondrial elongation rather than fission50. Thus, although the precise mechanism for their cross-talk remains elusive, Ser616 phosphorylation status seems to dictate whether phosphorylation at Ser637 will promote fission or fusion. Additional post-translational modifications could potentially coordinate with Ser637 phosphorylation to determine its effect on mitochondrial morphology. A comprehensive understanding of kinases and phosphatases responsible for Ser616 and Ser637 modulation, and the potential coordination with additional post-translational modifications, is needed and might explain the discrepancy in the findings for Ser637.
In addition to phosphorylation, DNML1 activity is also regulated by SUMOylation51–53, acetylation54, O-linked-N-acetylglucosaminylation (O-GlcNAcylation)55, and S-nitrosylation56–59 (FIG. 1b). Protein SUMOylation involves the covalent attachment of small ubiquitin-related modifier (SUMO) proteins to lysine residues. By contrast to ubiquitination, SUMOylation does not label a protein for degradation but serves to modify function or location. DNML1 can be SUMOylated by both SUMO1 and SUMO2 or SUMO3 at one or more lysine clusters within the variable region52,53. Interestingly, SUMO1 and SUMO2/3 have opposite effects on DNML1 activity, where conjugation of SUMO1 promotes DNML1 association with the mitochondrial membrane60, whereas SUMO2/3 reduce its binding53. The SUMOylation of DNML1 prevents it from interacting with MFF at the OMM51, and a SUMOylation-resistant mutant of DNML1 exhibits increased interaction with MFF and association with mitochondria51. This finding suggests that DNML1 must be deSUMOylated for induction of mitochondrial fission. Moreover, whereas addition of SUMO2/3 prevents mitochondrial fission and cell death during ischaemia51, SUMO1 conjugation to DNML1 promotes fission and cell death following treatment with the apoptosis inducer staurosporine61. Complex regulation of DNML1 SUMOylation clearly exists depending on the cellular context.
Acetylation is another major post-translational modification in cells, involving the transfer of an acetyl group onto a lysine residue in a target protein. Protein acetylation rates vary according to nutrient status, and hyperacetylation of mitochondrial proteins is well known to accompany metabolic remodelling during heart failure62. Indeed, nutrient overload is associated with DNML1 acetylation at Lys632, which promotes mitochondrial fission in mice and primates fed a high-fat diet54. The modification of serine and threonine residues by O-GlcNAcylation is also regulated by metabolic status, and O-GlcNAcylation of DNML1 is evident in cultured neonatal cardiomyocytes as well as in the hearts of mice with type 2 diabetes mellitus55. Importantly, both DNML1 acetylation and O-GlcNAcylation seem to facilitate increased Ser616 phosphorylation and subsequent translocation of DNML1 to the mitochondria54,55,. Cysteine residues are highly sensitive to oxidative modification, such as nitrosylation, which involves the coupling of a nitric oxide moiety to a reactive cysteine thiol63. DNML1 activity can be regulated by S-nitrosylation at Cys644, a residue that is conserved from flies to humans57. Although researchers agree that S-nitrosylation of DNML1 promotes mitochondrial fission, the mechanism by which this post-translational modification regulates DNML1 activity is not well understood. For example, although S-nitrosylation of DNML1 generally coincides with Ser616 phosphorylation, whether nitrosylation directly stimulates DNML1 GTPase activity remains controversial56–59. Considering the pivotal role of nitric oxide in vascular tone and cardiac function64, investigation into the role of DNML1 nitrosylation in cardiovascular disease is warranted.
Overall, these studies demonstrate clearly that DNML1 is subject to highly sophisticated regulation. Considering the pleiotropic effects of mitochondrial fission, any single post-translational modification is unlikely to underlie the physiological versus pathological effects of this pathway. Rather, multiple post-translational modifications on DNML1 are likely to have a synergistic or additive effect. Therefore, simultaneous assessment of multiple post-translational modifications in a given cellular context could provide insight into the relative tone of fission activation, which is believed to be a major factor determining functional outcomes.
Interorganelle contacts
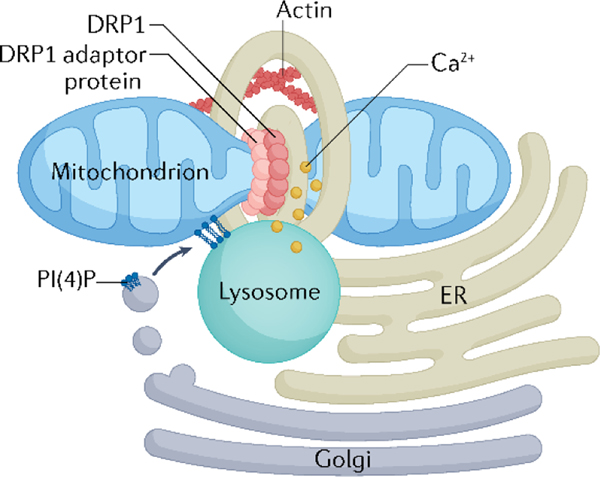
Mitochondrial fission is not an autonomous process, but involves the actin cytoskeleton and other organelles, including the endoplasmic reticulum (ER), Golgi and lysosomes. Mitochondria form physical contacts with the ER, which are known as mitochondria–ER association membranes (MAMs, or mitochondria–ER contact sites, MERCSs). These contact sites are important for phospholipid synthesis, calcium signalling and autophagosome formation65. MAMs are also sites where fission takes place66. Using powerful, high-resolution microscopy techniques, Friedman et al. observed that mitochondrial division events predominantly occur where DNML1 and its adaptor MFF associate with MAMs66. In a subsequent study, DNML1 oligomers were reported to assemble at the ER prior to transfer to the mitochondria, suggesting that the ER might function as a platform for DNML1 oligomerization67. The ER is also important in driving constriction of the OMM, as ER tubules wrap around the mitochondria during fission (FIG. 2)66. In addition, the cytoskeleton positively regulates fission, as actin filaments at MAMs promote DNML1 recruitment and stimulate its GTPase activity68–70. Independent of changes in GTPase activity, a study published in 2022 showed that DNML1 undergoes retrograde transport along actin filaments through the PDZ domain-containing protein GIPC1 (also known as GAIP/RGS19-interacting protein), a scaffolding protein involved in trafficking71. This active transport allows for effective delivery of peripheral cytosolic DNML1 to distal mitochondria in the perinuclear region.
Fig. 2 |. Interorganelle contacts promote mitochondrial fission.
Mitochondria–endoplasmic reticulum (ER) association membranes, in conjunction with polymerized actin, promote DNML1 recruitment and outer mitochondrial membrane OMM constriction. Uptake of calcium (Ca2+) into the mitochondria stimulates inner membrane constriction. phosphatidylinositol 4-phosphate (PI4P) is also critical for the execution of mitochondrial fission and can be transferred to the OMM through lysosomes or trans-Golgi-derived vesicles.
In addition to the ER and cytoskeleton, phosphatidylinositol 4-phosphate (PI(4)P) has emerged as a critical factor in the regulation of fission. Vesicles from the trans-Golgi network enriched in PI(4)P are routed to MAM constriction sites downstream of DNML1 translocation immediately prior to mitochondrial division72. Studies have demonstrated that PI(4)P can also be delivered by lysosomes to the sites of fission73,74. However, because depletion of PI(4)P on lysosomes still leads to a decline in mitochondrial fission, PI(4)P-enriched vesicles from the Golgi seem to be unable to compensate for the loss of lysosomal PI(4)P74. Although lysosomal contacts with the mitochondria might initially be important for PI(4)P transfer, untethering of lysosome-mitochondrial contacts is clearly necessary for the execution of fission. Here, the DNML1 adaptor FIS1 recruits the GTPase activating protein TBC1 domain family member 15 (TBC1D15), which facilitates the inactivation of Ras-related protein Rab-7 and subsequent organelle untethering for the completion of mitochondrial fission73. Interestingly, myocardial infarction is associated with prolonged mitochondria-lysosomal contacts, and TBC1D15 overexpression in mice reverses these outcomes to restore cardiac function after injury75. Although the enrichment of PI(4)P at the site of fission could be involved in recruiting proteins required for actin polymerization76, the exact function for this lipid in fission is unclear and requires further investigation.
Mitochondrial calcium levels
A transient increase in mitochondrial calcium uptake is a general feature of fission, where the influx of calcium into the mitochondrial matrix stimulates inner mitochondrial membrane (IMM) constriction prior to fission77. In addition, ER contact sites with the mitochondria and polymerization of actin are both needed for fission-induced mitochondrial calcium uptake78. Transport of calcium across the IMM into the mitochondrial matrix occurs through the mitochondrial calcium uniporter (MCU). The MCU is essential for calcium-mediated IMM constriction, and MCU depletion reduces both basal and stimulated mitochondrial fission78. Interestingly, loss of DNML1 results in upregulation of MCU in mouse heart79 and skeletal muscle80, and disrupts calcium homeostasis in myofibers due to abnormally elevated MCU-mediated mitochondrial uptake80. Altogether, these data suggest that DNML1 and MCU coordinate to maintain mitochondrial fission and calcium homeostasis in cells.
Physiological roles of mitochondrial fission
Mitochondrial division and mitophagy
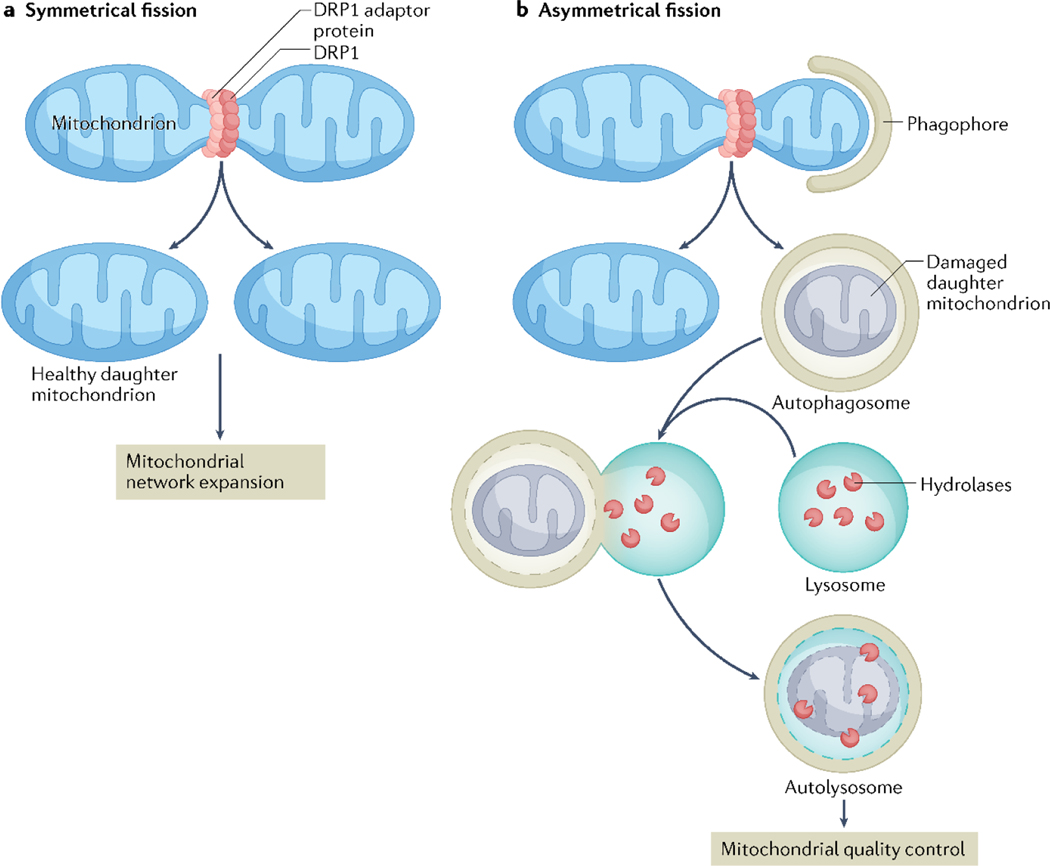
Mitochondrial fission has a role in many basic cellular functions, including cell division. Symmetrical mitochondrial fission (also known as replicative fission) generates two functional daughter mitochondria (FIG. 3a) and is closely coordinated with the cell cycle to facilitate the equal segregation of mitochondria during cell division81,82. Indeed, loss of DNML1 reduces proliferation of myofibroblasts83, vascular smooth muscle84 and pulmonary artery smooth muscle cells85, confirming the importance of DNML1 in cell division. Mitochondria contain their own genome that encodes respiratory chain proteins, mitochondrial ribosomal and tRNA. The genetic material is packaged into structures known as nucleoids that contain several copies of mitochondrial DNA (mtDNA) and proteins involved in transcription. Mitochondrial dynamics ensure the correct distribution of mtDNA in cells; defects in fission leads to formation of abnormally large mtDNA nucleoids that cluster in fused mitochondria, which correlates with abnormal heart development in mice30,86. Importantly, these abnormal mtDNA nucleoids disrupt mitochondrial respiration and sarcomere organization in developing myocytes30.
Fig. 3 |. Distinct mitochondrial fission subtypes produce divergent fates.
a | Symmetric fission generates two healthy daughter mitochondria during cellular division. b | Asymmetric fission facilitates engulfment of damaged mitochondria by autophagosomes, which fuse with lysosomes for organelle degradation via mitophagy.
Another key function of fission is the segregation of damaged mitochondrial components through asymmetrical division. This form of fission leads to the formation of a healthy mitochondrion and a dysfunctional fragment, which is subsequently labelled for mitophagy87 (FIG. 3b). The process of mitophagy involves the formation of a double-membraned autophagosome, which engulfs damaged mitochondria and delivers them to the lysosome for degradation by hydrolytic enzymes5. Interestingly, in 2021, Kleele et al. reported that FIS1 recruits DNML1 to the periphery of the mitochondrion for asymmetrical fragmentation, whereas MFF seems to be uniquely involved in symmetrical fission at the midpoint of a mitochondrion22. Mitophagy of dysfunctional mitochondria is primarily regulated by loss of membrane potential, whereas daughter mitochondria arising from symmetrical fission retain membrane polarization and re-fuse with the existing mitochondrial network22,87,88. DNML1 interacts with zinc transporter ZIP1 at the site of mitochondrial fission to regulate membrane potential89. Specifically, DNML1–ZIP1 interaction promotes the influx of Zn2+ into the mitochondrial matrix leading to a reduction in mitochondrial membrane potential, which enables the clearance of depolarized mitochondrial fragments through mitophagy89. Despite normal mitochondria and cardiac function at baseline, hemizygous Dnml1-knockout mice exhibit impaired mitophagy during pathological stress90, and complete loss of DNML1 in cardiac myocytes results in accumulation of dysfunctional mitochondria in cultured cells91 and mouse hearts8. Similarly, mutations in Dnml1 that prevent its disassembly during execution of fission also result in lethal heart failure due to defects in mitophagy92. Therefore, although the myocardium can tolerate reduced rates of mitochondrial fission under baseline conditions, a minimal threshold of DNML1-dependent fission is essential for mitochondrial quality via mitophagy. Although DNML1-mediated fission is clearly sufficient to promote mitophagy, some studies have demonstrated that DNML1 is not necessary for mitophagy32,93,94. Moreover, Song and colleagues reported that preventing mitophagy delays cardiomyopathy in cardiac-specific Dnml1-knockout mice95. Despite the controversy about whether fission is an essential prerequisite for mitophagy, the studies discussed here confirm the importance of DNML1-mediated asymmetrical fission in segregating damaged mitochondria from the healthy network for mitophagy.
Bioenergetics
Mitochondrial morphology is closely linked to respiration and ATP generation. Studies have shown that inhibition or depletion of DNML1 in cultured cardiac myocytes is associated with reduced mitochondrial respiration8,91,96. This finding is consistent with impaired mitochondrial function and respiratory chain complex activities in cardiac-specific Dnml1-knockout mice30,31. Cardiac respiratory chain deficiencies are also evident with the loss of MFF in vivo, confirming the importance of mitochondrial fission for respiratory function34. A portion of the mitochondrial respiratory chain complexes form supercomplex structures to ensure efficient energy production97. Interestingly, mice with muscle-specific loss of DNML1 have impaired respiratory chain supercomplex assembly80, suggesting that functional mitochondrial fission is important for their formation, stability or both. In addition to supporting basal respiration in myocytes, DNML1-dependent fission in the heart is critical for increased mitochondrial respiratory capacity during exercise3. DNML1 is rapidly recruited to cardiac mitochondria following intense exercise; however, elevated basal fission prior to exercise reduces endurance, suggesting that excessive fission could exhaust respiratory function25. Indeed, sustained fission through DNML1 in various myocyte models of ischaemia–reperfusion10,98,99, proteotoxicity100,101, lipotoxicity102,103 or inflammation104,105 is consistently associated with mitochondrial bioenergetic defects. These relationships are also conserved in endothelial cells106,107 and vascular smooth muscle cells (VSMCs)108 exposed to pathological conditions. Therefore, although physiological fission supports mitochondrial function by facilitating supercomplex assembly and respiration, its hyperactivation during pathological stress often perturbs respiratory capacity.
Cell death
Mitochondrial fragmentation is often present during activation of cell death, and many studies have demonstrated that DNML1-mediated fission is directly involved in apoptosis. BCL2 Associated X, Apoptosis Regulator (BAX) is a pro-apoptotic protein that translocates to the mitochondria upon activation of apoptosis, where it induces mitochondrial outer membrane permeabilization (MOMP) resulting in cytochrome c release and downstream caspase activation. Activated BAX oligomers cluster at mitochondrial fission sites109, and DNML1-dependent mitochondrial membrane remodelling directly promotes BAX oligomerization during apoptosis110. As such, genetic and pharmacological inhibition of DNML1 is sufficient to prevent BAX-mediated MOMP2,111, consistent with the apoptosis resistance observed in Dnml1-null embryonic mouse stem cells28 as well as MEFs lacking DNML1 adaptor proteins24. In a study published in 2022, DNML1 was shown to directly interact with BAX, and forced dimerization of these two proteins was sufficient to cause mitochondrial remodelling, membrane permeabilization and cell death even in the absence of apoptotic stimuli112. Clearly, DNML1-dependent mitochondrial fission promotes BAX-dependent MOMP, cytochrome c release and caspase activation in cells. However, mitochondrial fission can still occur in MEFs lacking DNML1 or BAX, indicating that multiple pathways exist for mitochondrial fission during apoptosis110. Although the specific functions of individual DNML1 adaptor proteins remain unclear, MID49 and MID51 seem to be uniquely essential for DNML1-dependent cristae remodelling during apoptosis in vitro113. The mitochondrial permeability transition pore (mPTP) is a non-selective channel that allows the passage of any molecule with a molecular mass <1.5 kDa. Excessive opening of the mPTP leads to the influx of solutes into the matrix, swelling and rupture of the OMM114. Studies have shown that DNML1 induces opening of the mPTP in response to various stressors42,115,116. Chronic fission is also linked to mPTP opening in cardiovascular pathophysiology116,117.
Notably, DNML1-mediated fission alone is not sufficient to induce cell death, as overexpression of DNML1 in cells does not cause death41,118,119. Furthermore, transgenic mice overexpressing DNML1 in the heart have normal cardiac function despite extensive mitochondrial fission41. This finding suggests that additional stimuli are needed for mitochondrial fission to switch into a pro-death pathway via mPTP opening or BAX-mediated MOMP. Additional studies are needed to determine the molecular mechanism underlying DNML1-mediated cell death and the specific conditions that activate this mechanism.
Immune function
Immune cells defend organisms against infections and other foreign material. Research has demonstrated a role for mitochondrial fission in immune cell function. During chemotaxis, mitochondrial network organization via mitochondrial fission facilitates immune cell migration120. Simula et al. reported that T cell infiltration into tumours is dependent on DNML1, and ablation of DNML1 in the T-cell lineage disrupts infiltration of these cells in solid tumours121 (TABLE 2). Thus, immune surveillance against tumours is compromised in mice with a specific DNML1-deficiency in the T-cell lineage. Moreover, when the immune response is activated, immune cells undergo a metabolic transition from a quiescent to an active state. Naïve T cells in the thymus rely on mitochondrial oxidative phosphorylation, whereas activated T cells depend on glycolysis for cytokine production122. As such, mitochondrial dynamics regulate T-cell fate through metabolic reprogramming where mitochondrial fission reduces oxidative phosphorylation by promoting cristae remodelling and disassembly of the electron transport chain complexes to shift substrate utilization to glycolysis121,123. The importance of mitochondrial fission for glycolysis during immune cell activation is also evident in myeloid dendritic cells124 and macrophages125. Efferocytosis is a process by which macrophages engulf and eliminate apoptotic cells. Studies have shown that phagocytic uptake of apoptotic cells requires DNML1-mediated mitochondrial fission, and silencing Dnml1 in macrophages leads to impaired efferocytosis126. Thus, mitochondrial fission directly contributes to immune cell activation and function.
Table 2 |.
Genetic mouse models of Dnm1l knockout in non-cardiac tissues
| Genetic model | Outcome | Refs | |
|---|---|---|---|
| Basal characterization | Effects of pathological stress | ||
| Skeletal muscle-specific knockout | Reduced body growth, muscle fibre number and size. Defects in respiratory complex assembly and function. Lethality by postnatal day 30. | NR | 80 |
| Tamoxifen-inducible, skeletal muscle-specific knockout | Reduced body weight at 50 days post-tamoxifen. Reduced muscle mass and fibre size 70–180 days post-tamoxifen. Mitochondrial dysfunction and impaired mitophagy. | NR | 80 |
| T cell-specific knockout | Impaired migration and expansion of developing thymocytes. | Accelerated tumour growth. | 121 |
| Myeloid/macrophage-specific knockout | Impaired macrophage-mediated uptake of apoptotic cells (i.e. efferocytosis). | Accelerated plaque necrosis in the Ldlr−/− model of atherosclerosis. Protection against vascular remodelling and fibrosis following arterial injury. Reduced macrophage activation and cell proliferation in injured arteries. | 126,153 |
| Liver-specific knockout | Reduced liver and white adipose tissue weights. Reduced serum triacylglycerol and total cholesterol levels. | Protection from high-fat diet-induced obesity. | 12,152 |
| Endothelial cell-specific knockout | NR | Protection against TNF-driven endothelial leukocyte adhesion. | 155 |
DNM1L, dynamin-1-like protein (also known as dynamin-related protein 1 (DRP1)); LDLR, low-density lipoprotein receptor; NR, not reported; TNF, tumour necrosis factor.
Dysregulation of mitochondrial fission
Mitochondrial fusion in heart disease
The balance between mitochondrial fission and fusion dictates the outcome on organelle morphology. An overall decrease in fusion can shift the balance towards fission, leading to excessive mitochondrial fission. Although our knowledge of how mitochondrial fusion machinery is changed in cardiovascular disease remains limited, studies have shown that levels of mitochondrial fusion proteins are decreased during cardiac hypertrophy and in failing hearts. For instance, MFN2 is downregulated in mouse hearts at 1 and 3 weeks after transverse aortic constriction (TAC) and in hypertrophied hearts from 10-month-old spontaneously hypertensive rats127. Another study demonstrated that levels of MFN2 and OPA1 are reduced, whereas DNML1 and FIS1 levels are increased in failing hearts from dogs and humans128. In 2021, Hsiao et al. reported that levels of MFN1 are decreased in patients with idiopathic dilated cardiomyopathy who did not respond to established heart failure treatments, and that the reduction in MFN1 correlates with increased mitochondrial fragmentation in human hearts129. Thus, the excessive mitochondrial fission that contributes to cardiovascular disease is likely to be caused by a combination of increased DNML1 activation and reduced mitochondrial fusion. However, further studies are clearly needed to determine how altered mitochondrial fusion contributes to the development of cardiovascular disease, as the vast majority of existing mechanistic data to date have focused on DNML1 activation. In the remainder of this Review, we discuss the role of DNML1-driven mitochondrial fission in cardiovascular pathogenesis.
Cardiac hypertrophy
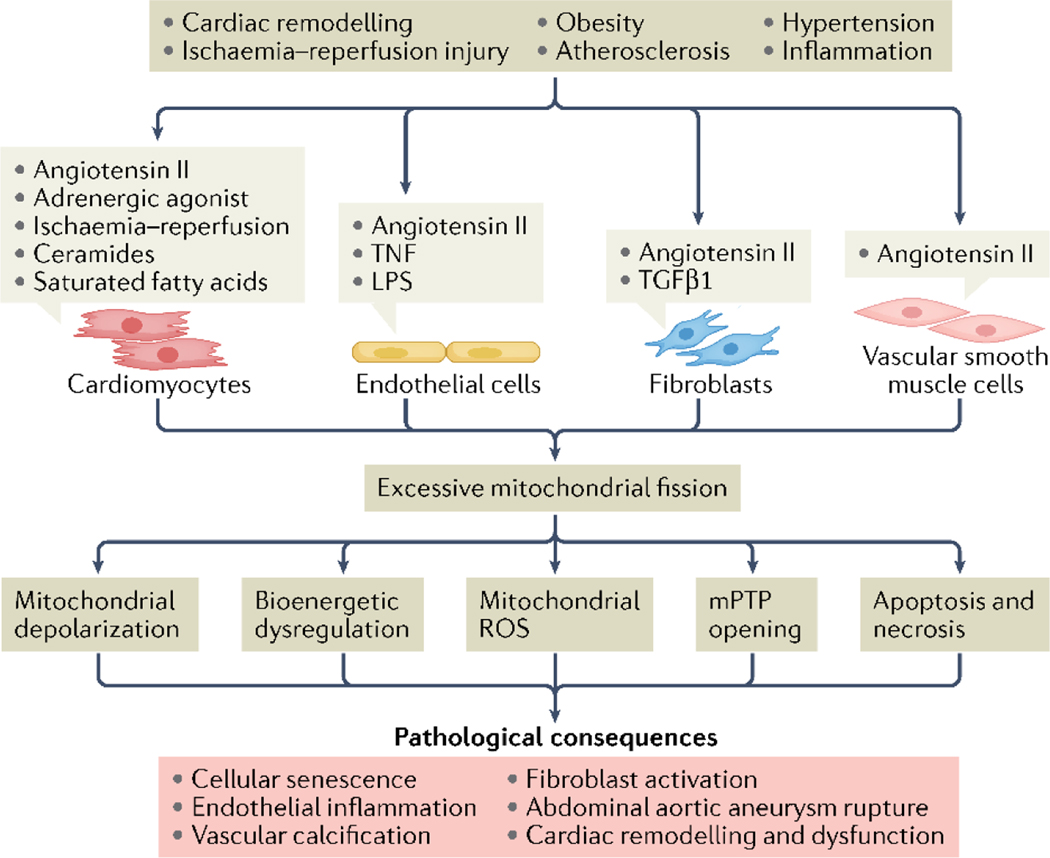
Although mitochondrial fission has an important regulatory role in cellular homeostasis, it is becoming increasingly clear that aberrant activation of fission during cardiovascular disease directly contributes to pathophysiology (FIG. 4). Cardiac myocytes undergo hypertrophy as an adaptive response to increased workload (exercise) or haemodynamic overload (hypertension) to maintain contractility and reduce ventricular wall stress. A growing body of evidence suggests that mitochondrial fission is activated during myocyte hypertrophy. For instance, treatment of neonatal rat myocytes with the hypertrophic agonist norepinephrine activates mitochondrial fission, whereas overexpression of a dominant-negative DNML1 inhibits both mitochondrial fission and hypertrophic growth of myocytes11. Exercise is associated with physiological hypertrophy, and DNML1-mediated mitochondrial fission is rapidly activated in hearts as an adaptive response3,25. Although enhanced fission alters bioenergetics, it is also possible that DNML1 is activated during exercise to facilitate mitochondrial clearance as exercise is linked to mitophagy in trained skeletal muscle130 and in mouse hearts exposed to irradiation therapy131. Physiological hypertrophy through exercise is reversible, however, persistent pathological stress such as chronic hypertension can lead to a transition to pathological hypertrophy with loss of myocytes, cardiac remodelling and development of heart failure132. Evidence exists that excessive fission is an underlying factor in this transition. For example, sustained fission contributes to loss of myocytes and cardiac dysfunction in hypertensive rats133. Treatment of myocytes with angiotensin II, a major contributor to hypertension, induces pronounced mitochondrial fission and downstream activation of apoptosis133. In a TAC mouse model of pressure overload, DNML1 is rapidly phosphorylated and recruited to the mitochondria, and pharmacological inhibition of DNML1 reduces cardiac hypertrophy134. Although this finding suggests that fission contributes to maladaptive cardiac remodelling, not all studies have shown a pathological role for fission in cardiac hypertrophy. For instance, rats subjected to ascending aortic constriction develop diastolic dysfunction concurrent with increased DNML1-mediated fission, activation of mitochondrial biogenesis and enhanced mitochondrial respiration135. Interestingly, relief of the pressure overload by aortic de-banding leads to reversal of cardiac hypertrophy and restoration of cardiac function, despite sustained induction of mitochondrial fission135. Similarly, Shirakabe et al. found that DNML1 is essential for mitophagy in mouse hearts during TAC90. In this study, haploinsufficent Dnml1 mice had exacerbated development of both mitochondrial dysfunction and heart failure in response to TAC due to impaired mitophagy. Taken together, these studies suggest that mitochondrial fission is an adaptive component of the hypertrophic response, enhancing bioenergetics and facilitating mitophagy of damaged mitochondria, but that persistent fission during pressure overload becomes detrimental to the heart potentially through excessive mitophagy and activation of apoptosis or necrosis.
Fig. 4 |. Sustained mitochondrial fission promotes cardiovascular pathophysiology.
Several upstream signals shared and distinct among cardiac myocytes, endothelial cells, fibroblasts and vascular smooth muscle cells (VSMC) cause excessive mitochondrial fission, which culminates in organelle dysfunction and cell death. These effects directly promote cardiac and vascular pathophysiology across multiple disease models. AAA, abdominal aortic aneurysm; FA, fatty acids; LPS, lipopolysaccharide; mPTP, mitochondrial permeability transition pore; ROS, reactive oxygen species; TGF- β1, transforming growth factor β1; TNF, tumour necrosis factor.
Myocardial ischaemic injury
Both coronary occlusion and subsequent reperfusion to restore blood and oxygen delivery to the heart can lead to extensive death of myocytes and irreversible tissue damage. A growing body of evidence suggests that excessive mitochondrial fission is an underlying factor of myocyte death in IRI. Mitochondrial fission is initially activated during ischaemia and is sustained during reperfusion, which contributes to increased mitochondrial generation of reactive oxygen species, calcium overload and mPTP opening in myocytes9,10,136. In addition, DNML1 is activated in the peri-infarct region of mouse heart tissue, resulting in pronounced mitochondrial fission and cardiac dysfunction137. Excessive mitochondrial fission during ischaemia–reperfusion also disrupts endothelial barrier function and integrity in the heart, and restricting fission in cardiac microvascular endothelial cells reverses mitochondrial damage and cell death in studies of simulated ischaemia–reperfusion138. Pharmacologic or genetic inhibition of DNML1-mediated fission reduces mPTP opening and cell death in cardiac myocytes subjected to ischaemia–reperfusion9,136 and inhibiting fission at the onset of reperfusion prevents long-term cardiac dysfunction9,136. By contrast, another study indicates that defective mitophagy in cardiac-specific Dnml1 heterozygous knockout mice exacerbates myocardial IRI8. Conflicting results in these studies could be related to pharmacological targeting of fission in non-myocytes compared with cardiac specificity in genetic models.
Fibrosis and calcification
Mitochondrial fission has also been implicated in cardiac fibrosis, an important component of the wound repair process following myocardial injury. However, excessive extracellular matrix deposition induces maladaptive fibrotic remodelling and disrupts electrical signalling, ultimately leading to heart failure139. Transforming growth factor β (TGFβ) drives cardiac fibrosis by activating fibroblasts and the extracellular matrix gene programme. Interestingly, stimulation of cardiac fibroblasts with TGFβ also activates mitochondrial fission, and inhibition of DNML1 reduces TGFβ-stimulated fibroblast proliferation, migration and extracellular matrix synthesis83. This finding suggests that mitochondrial fission is directly linked to fibroblast activation in vitro, and that fission is likely to be an early catalyst driving fibrotic remodelling in the heart. Sustained mitochondrial fission also drives proliferation of right ventricular fibroblasts isolated from an animal model of pulmonary arterial hypertension140 as well as human cardiac fibroblasts stimulated with lysophosphatidylcholine, a lipid known to directly promote collagen production141. Moreover, pharmacological inhibition of DNML1 reduces fibrotic remodelling of hearts in hypertensive rats142. These studies suggest that the concurrent activation of mitochondrial fission in fibroblasts during cardiac stress also contributes to pathological remodelling and fibrosis in the heart.
Ectopic calcification is an important factor in the progression of atherosclerosis, and evidence exists that DNML1-mediated mitochondrial fission has a direct role in cardiovascular calcification. DNML1 is enriched in human carotid atherosclerotic plaques and calcified aortic valves143. VSMCs initiate calcification by differentiating into osteogenic or chondrocytic cells144 and knockdown or inhibition of DNML1 attenuates VSMC-mediated calcification in vitro143. Similarly, preventing mitochondrial fission reduces aortic calcification in rats fed an adenine-rich diet145. Although mitochondrial fission is clearly linked to osteogenic differentiation, the precise mechanism by which mitochondrial fission promotes calcification remains unclear.
Abdominal aortic aneurysm (AAA) is another increasingly common cardiovascular disease that is characterized by dilation of the abdominal aorta, and its rupture can be fatal. Calcification is a risk factor for AAA severity and rupture146. In 2021, Cooper et al. reported that expression of DNML1 is increased in AAA patient samples compared to healthy controls, and that inhibition of DNML1 protects against aortic dilation and rupture in a mouse model of AAA147. During AAA, adventitial fibroblasts undergo a phenotypic switch towards the myofibroblast phenotype, which is a crucial component of vascular remodelling. DNML1-dependent fission is required for adventitial fibroblast activation in vitro, and strategies to mitigate mitochondrial fission also reduce adventitial thickness and fibrosis in mice148.
Metabolic cardiomyopathy
Obesity and Type 2 diabetes mellitus are associated with cardiac lipid overload due to increased myocyte fatty acid uptake and oxidation, and accumulation of lipids in diabetic cardiomyopathy is an independent predictor of contractile dysfunction in patients149. Excessive lipid uptake is also linked to changes in mitochondrial morphology, where lipids can activate mitochondrial fission. For instance, ceramide is a reactive lipid that triggers rapid mitochondrial fission in cardiac myocytes and in the hearts of rats with diabetes150,151. Blocking ceramide biosynthesis in induced pluripotent stem cell-derived myocytes reverses mitochondrial dysfunction and apoptosis by restricting mitochondrial fragmentation102. The saturated fatty acid palmitate is also associated with diabetic cardiomyopathy. Increased palmitate levels in the myocardium of mice and primates fed high-fat diets promote mitochondrial fission through Lys624 acetylation and activation of DNML154. Abrogating Lys642 acetylation in adult myocytes restricts fission, thereby preventing apoptosis and contractile deficits imposed by palmitate54. In the liver, mitochondrial fission also contributes to systemic metabolism, as hepatocyte-specific Dnml1-knockout mice exhibit reduced cholesterol levels and are protected from high-fat diet-induced obesity152. Overall, these studies demonstrate that metabolic dysregulation during lipid overload promotes aberrant fission and mitochondrial dysfunction, which contributes to cardiac pathophysiology.
Vascular inflammation
Mitochondrial fission has also been implicated as an important driver of vascular inflammation. Chronic inflammation of the arterial wall and migration of immune cells and VSCMs within the vasculature contribute to the formation of atherosclerotic lesions. An atherogenic role for mitochondrial fission in activated immune cells has been identified, as macrophage-specific Dnml1-knockout mice are protected from intimal thickening and fibrosis following arterial injury153. Loss of DNML1 disrupts macrophage activation through reduced pro-inflammatory cytokine production and chemotaxis, which ultimately abolishes VSMC migration in co-culture studies153. These findings are consistent with another study in which reduced VSMC migration and intimal hyperplasia was reported in DNML1K38A transgenic mice subjected to arterial injury154. In addition, pharmacological inhibition of DNML1 reduces macrophage accumulation and advanced atherosclerotic plaque formation in the aortic roots of Apoe−/− mice with diabetes, highlighting the function of mitochondrial fission in vascular inflammation12.
Mitochondrial fission in cell types other than macrophages also contributes to vascular inflammation. Forrester et al. found that loss of DNML1 in endothelial cells reduces leukocyte adhesion in mouse mesenteric post-capillary venules following immune stimulation with tumour necrosis factor (TNF)155. Aberrant mitochondrial fission in endothelial cells also leads to senescence106, which is a critical component of atherosclerosis156. Whereas senescent cells secrete pro-inflammatory factors for immune recruitment, inhibition of DNML1 reverses these outcomes in endothelial cells treated with angiotensin II157. This finding suggests that abnormal mitochondrial fission is sufficient to cause endothelial dysfunction, and point to fission as a potent inducer of vascular inflammation during cardiovascular disease.
Therapeutic approaches
Specific DNML1 inhibitors
Collectively, the plethora of studies on mitochondrial dynamics in cells, animal models and human tissue all indicate that dysregulation of mitochondrial fission contributes to the development of several cardiovascular pathologies. Thus, targeting proteins in this pathway has strong therapeutic potential for treating cardiovascular disease.
Mitochondrial division inhibitor 1 (mdivi-1) is a small molecule inhibitor of DNML1 GTPase activity that has been widely used to abrogate mitochondrial fission in vitro and in vivo. Consistent with a role for fission in immune cell activation and migration, mdivi-1 infusion prevents leukocyte–endothelial cell adhesion in mice treated with TNF, providing similar vascular protection to that seen with genetic deletion of DNML1155. Similarly, mdivi-1 administration reduces calcification and vascular remodelling in mouse models of atherosclerosis12 and AAA147, respectively. Mdivi-1 also attenuates myocardial infarct size and cardiac dysfunction in mouse models of ischaemia–reperfusion9,10. These studies suggest that inhibition of DNML1-mediated fission by mdivi-1 provides protection against cardiovascular disease development. However, a report published in 2017 questioned the specificity of mdivi-1, because this compound also inhibits respiratory complex I (also known as mitochondrial complex I) in a DNML1-independent manner158. The study also suggested that mdivi-1 does not inhibit the GTPase activity of recombinant human DNML1 protein158. These findings remain controversial, as other investigators have noted the unreliability of assays with recombinant DNML1 and demonstrate that mdivi-1 inhibits GTPase activity in DNML1 immunoprecipitated from human alveolar cells159. Considering that DNML1 is necessary for the assembly of respiratory complex I in mouse skeletal muscle80, and respiratory complex I protein levels are similarly decreased in neonatal rat cardiac myocytes exposed to mdivi-1 or Dnml1 knockdown91, additional studies are needed to clarify potential off-target effects of mdivi-1. Application of mdivi-1 in larger mammals is also needed, as a pilot study published in 2019 showed a lack of cardioprotection in a more clinically relevant swine model of myocardial ischaemia–reperfusion160.
Additional inhibitors of DNML1 have also been developed. The peptide inhibitor P110 specifically disrupts the interaction between DNML1 and FIS1, and performs similarly to mdivi-1 in protecting against myocardial IRI136. P110 also alleviates cardiac dysfunction and mitochondrial damage in sepsis, suggesting that the interaction between DRP1 and FIS1 specifically has a critical role in promoting pathological mitochondrial fission. By contrast, another peptide inhibitor P259 abrogates DNML1–MFF binding161, although animal studies are needed to test the therapeutic potential of P259 in cardiovascular disease. The newly developed DNML1 inhibitor Drpitor1a has greater potency than mdivi-1 and protects rat hearts during ischaemia–reperfusion ex vivo159. On the basis of in vitro and in vivo studies in rodents, pharmacological inhibition of mitochondrial fission clearly represents a promising therapeutic strategy to protect against cardiovascular disease. However, this approach needs to be further explored and confirmed in large-animal models.
Indirect regulation of fission
In addition to specific inhibitors of DNML1, many FDA-approved compounds have been reported to repress aberrant mitochondrial fission. For example, the non-steroidal anti-inflammatory drug sodium salicylate limits mitochondrial fission in endothelial cells, thereby reducing immune cell adhesion in mice treated with TNF, and afforded a similar level of protection against endothelial inflammation as seen with mdivi-1155. Metformin is used to treat type 2 diabetes and its administration in mice reduces DNML1 protein levels and mitochondrial fragmentation in the aortic endothelium of ApoE−/− mice with diabetes162. Importantly, restricting mitochondrial fission through metformin attenuates diabetes-induced endothelial dysfunction and inflammation independent of changes in blood glucose162. Empagliflozin, a sodium–glucose co-transporter 2 inhibitor, is also approved for the treatment of type 2 diabetes and its administration in mice limits hyperglycaemia-induced mitochondrial fission, thereby attenuating cardiac microvascular remodelling and dysfunction in vivo163.
Two different FDA-approved drugs used to treat hypertension have also been shown to inhibit mitochondrial fission. Hydralazine causes vasodilation by interfering with calcium transport to relax arteriolar smooth muscle cells. Interestingly, acute infusion of hydralazine immediately prior to reperfusion protects against myocardial IRI in mice164. Similarly, treatment of cultured adult myocytes with hydralazine significantly reduces mitochondrial fission and cell death in response to simulated ischaemia–reperfusion164. Molecular docking analysis indicates that hydralazine directly binds to the Asp218, Asn246 and Ser248 residues of DNML1, which leads to inhibition of GTPase activity. Cilnidipine is a dihydropyridine calcium channel blocker that is used clinically to treat hypertension. Although this drug acts predominately on VSMCs, Nishimura et al. reported that cilnidipine also has off-target effects that are independent of this mechanism137. Specifically, cilnidipine prevents hypoxia-induced mitochondrial fission in cardiac myocytes, thereby improving cardiac function in mice subjected to myocardial infarction137.
In addition to these exogenous compounds, research has identified that some endogenous hormones can regulate mitochondrial fission. For example, melatonin supplementation protects cardiac myocytes165, endothelial cells117 and VSMCs166 from pathological DNML1-mediated fission in multiple disease models. In addition, treatment with the steroid hormone vitamin D3 prevents mitochondrial fragmentation, oxidative stress and myocyte death induced by ischaemia–reperfusion in vitro and attenuates cardiac injury in mice167. Together, these data lend further support for targeting mitochondrial fission in cardiovascular disease and indicate that several FDA-approved drugs, as well as endogenous peptides, could potentially be repositioned for this purpose.
Conclusions
Aberrant mitochondrial fission through DNML1 directly contributes to cardiovascular pathophysiology. Genetic ablation of DNML1 is well-characterized to restrict pathological cardiac and vascular remodelling in mice, and proof of concept in genetic models is validated by pharmacological inhibitors. Despite strong therapeutic promise, the physiological functions of mitochondrial fission warrant careful consideration when developing treatment strategies. Along these lines, fission is needed for mitochondrial division, which supports mtDNA segregation7 and cellular proliferation81. As such, restricting fission might not be advisable during particular developmental stages, or in patients with heritable mtDNA mutations. Mitochondrial fission also has an important role in facilitating mitophagy8,90. Therefore, pharmacological strategies to limit fission must consider dose and duration of treatment with respect to mitochondrial quality control through mitophagy. Nevertheless, the fact that pharmacological inhibition of unchecked mitophagy during anthracycline cardiotoxicity also restricts excessive mitochondrial fission168 indicates that the coupling of these two processes could synergistically promote cardiovascular pathophysiology in particular situations. Cell-type specificity is a major obstacle when evaluating drug efficacy and safety, and could be a potential barrier for targeting mitochondrial fission, as macrophages153 and endothelial cells155 are purported to drive vascular inflammation through hyperactive fission. Because DNML1 is essential for immune cell maturation121 and phagocytosis126, ensuring that inhibitor regimens do not disrupt physiological immune function will be vital before animal studies can be translated to the clinic. Targeting specific DNML1 adaptor proteins could enable regulation of distinct subtypes of mitochondrial fission contributing to pathogenesis, while leaving physiological mitochondrial division intact22. However, more studies are needed to determine whether this strategy is viable. Currently, there are no ongoing clinical trials evaluating the effects of specific inhibitors for mitochondrial fission169. However, a wealth of data indicating a distinct pathological threshold for this process, coupled with the repurposing of several FDA-approved compounds, argues for increased translational application. In summary, mitochondrial fission has emerged as a pivotal hallmark of cardiovascular disease and future research is likely to pave the way for novel therapies in patients.
Key points.
Mitochondria are involved in regulating many important cellular processes, including metabolism, ATP generation, immune response and activation of cell death pathways.
Mitochondria are dynamic and undergo changes in morphology in response to various environmental cues, which impacts organelle function.
Mitochondrial fission is subject to sophisticated regulation, and activation involves various post-translational modifications of dynamin-1-like protein (DNM1L), actin polymerization and the involvement of other cell organelles.
Although mitochondrial fission is critical for cardiac homeostasis, strong evidence exists that dysregulation of DNM1L-mediated fission contributes to the development of several cardiovascular pathologies.
Targeting proteins that regulate mitochondrial dynamics has strong therapeutic potential for cardiovascular disease.
Acknowledgements
J.M.Q. is supported by a postdoctoral fellowship from the AHA (#830983). Å.B.G. is supported by NIH grants R01HL138560, R01HL132300, R01HL155281 and R01HL157265.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Bock FJ & Tait SWG Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21, 85–100, doi: 10.1038/s41580-019-0173-8 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Frank S. et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Developmental cell 1, 515–525 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Coronado M. et al. Physiological mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand. Circulation research 122, 282–295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoque A. et al. Mitochondrial fission protein Drp1 inhibition promotes cardiac mesodermal differentiation of human pluripotent stem cells. Cell death discovery 4, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafsson AB & Dorn GW, 2nd. Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol Rev 99, 853–892, doi: 10.1152/physrev.00005.2018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi N, Ishihara N, Jofuku A, Oka T. & Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. Journal of Biological Chemistry 282, 11521–11529 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Lewis SC, Uchiyama LF & Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 353, 261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda Y. et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116, 264–278, doi: 10.1161/CIRCRESAHA.116.303356 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Ong SB et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121, 2012–2022, doi:CIRCULATIONAHA.109.906610 [pii] 10.1161/CIRCULATIONAHA.109.906610 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Sharp WW et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28, 316–326, doi: 10.1096/fj.12-226225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennanen C. et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. Journal of cell science 127, 2659–2671 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers MA et al. Dynamin-related protein 1 inhibition reduces hepatic PCSK9 secretion. Cardiovascular Research 117, 2340–2353 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalia R. et al. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature 558, 401–405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fröhlich C. et al. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1‐like protein. The EMBO journal 32, 1280–1292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozdy AD, McCaffery JM & Shaw JM Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol 151, 367–380 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James DI, Parone PA, Mattenberger Y. & Martinou J-C hFis1, a novel component of the mammalian mitochondrial fission machinery. Journal of Biological Chemistry 278, 36373–36379 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Yoon Y, Krueger EW, Oswald BJ & McNiven MA The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Molecular and cellular biology 23, 5409–5420 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loson OC, Song Z, Chen H. & Chan DC Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24, 659–667, doi: 10.1091/mbc.E12-10-0721 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y. j., Jeong S-Y, Karbowski M, Smith CL & Youle RJ Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Molecular biology of the cell 15, 5001–5011 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otera H. et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. Journal of Cell Biology 191, 1141–1158 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Q. et al. Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Mol Biol Cell 25, 145–159, doi: 10.1091/mbc.E13-09-0525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleele T. et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593, 435–439 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Varuzhanyan G. et al. Fis1 ablation in the male germline disrupts mitochondrial morphology and mitophagy, and arrests spermatid maturation. Development 148, 199686 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osellame LD et al. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J Cell Sci 129, 2170–2181, doi: 10.1242/jcs.185165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyzis AG et al. Mcl-1-mediated mitochondrial fission protects against stress but impairs cardiac adaptation to exercise. Journal of Molecular and Cellular Cardiology 146, 109–120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Lee H-Y, Hanna RA & Gustafsson ÅB Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. American journal of physiology-heart and circulatory physiology 301, H1924–H1931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M. et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 12, 689–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishihara N. et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nature cell biology 11, 958–966 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Wakabayashi J. et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. Journal of Cell Biology 186, 805–816 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishihara T. et al. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol 35, 211–223, doi: 10.1128/MCB.01054-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kageyama Y. et al. Parkin‐independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. The EMBO journal 33, 2798–2813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song M, Mihara K, Chen Y, Scorrano L. & Dorn II GW Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell metabolism 21, 273–286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galloway CA et al. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes 61, 2093–2104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H. et al. Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. Journal Of Cell Biology 211, 795–805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao S. & Hu J. Mitochondrial fusion: the machineries in and out. Trends in Cell Biology 31, 62–74 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Chen H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. The Journal of cell biology 160, 189–200 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Liu Y. & Dorn GW Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circulation research 109, 1327–1331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papanicolaou KN et al. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circulation research 111, 1012–1026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies VJ et al. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Human molecular genetics 16, 1307–1318 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Wai T. et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350, aad0116 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Song M, Franco A, Fleischer JA, Zhang L. & Dorn II GW Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell metabolism 26, 872–883 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S. et al. CaMKII induces permeability transition through Drp1 phosphorylation during chronic β-AR stimulation. Nature communications 7, 1–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito T. et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. The Journal of clinical investigation 129, 802–819 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han H. et al. PINK 1 phosphorylates Drp1S616 to regulate mitophagy‐independent mitochondrial dynamics. EMBO reports 21, e48686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cribbs JT & Strack S. Reversible phosphorylation of Drp1 by cyclic AMP‐dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO reports 8, 939–944 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cereghetti G. et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proceedings of the National Academy of Sciences 105, 15803–15808 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu R. et al. The phosphorylation status of Ser-637 in dynamin-related protein 1 (Drp1) does not determine Drp1 recruitment to mitochondria. Journal of Biological Chemistry 294, 17262–17277 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jhun BS et al. Protein kinase D activation induces mitochondrial fragmentation and dysfunction in cardiomyocytes. The Journal of physiology 596, 827–855 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W. et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell metabolism 15, 186–200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valera-Alberni M. et al. Crosstalk between Drp1 phosphorylation sites during mitochondrial remodeling and their impact on metabolic adaptation. Cell Reports 36, 109565 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo C, Wilkinson KA, Evans AJ, Rubin PP & Henley JM SENP3-mediated deSUMOylation of Drp1 facilitates interaction with Mff to promote cell death. Sci Rep 7, 43811, doi: 10.1038/srep43811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueroa-Romero C. et al. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. FASEB J 23, 3917–3927, doi: 10.1096/fj.09-136630 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo C. et al. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J 32, 1514–1528, doi: 10.1038/emboj.2013.65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu Q. et al. Increased Drp1 acetylation by lipid overload induces cardiomyocyte death and heart dysfunction. Circulation research 126, 456–470 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gawlowski T. et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem 287, 30024–30034, doi: 10.1074/jbc.M112.390682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee DS & Kim JE PDI-mediated S-nitrosylation of DRP1 facilitates DRP1-S616 phosphorylation and mitochondrial fission in CA1 neurons. Cell Death Dis 9, 869, doi: 10.1038/s41419-018-0910-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho DH et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324, 102–105, doi: 10.1126/science.1171091 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bossy B. et al. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J Alzheimers Dis 20 Suppl 2, S513–526, doi: 10.3233/JAD-2010-100552 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rizza S. et al. S-nitrosylation drives cell senescence and aging in mammals by controlling mitochondrial dynamics and mitophagy. Proceedings of the National Academy of Sciences 115, E3388–E3397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wasiak S, Zunino R. & McBride HM Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177, 439–450, doi: 10.1083/jcb.200610042 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prudent J. et al. MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Molecular cell 59, 941–955 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Bertero E. & Maack C. Metabolic remodelling in heart failure. Nature Reviews Cardiology 15, 457–470 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Hess DT & Stamler JS Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem 287, 4411–4418, doi: 10.1074/jbc.R111.285742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farah C, Michel LY & Balligand J-L Nitric oxide signalling in cardiovascular health and disease. Nature Reviews Cardiology 15, 292–316 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Prinz WA, Toulmay A. & Balla T. The functional universe of membrane contact sites. Nature Reviews Molecular Cell Biology 21, 7–24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman JR et al. ER tubules mark sites of mitochondrial division. Science 334, 358–362, doi: 10.1126/science.1207385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji WK et al. Receptor-mediated Drp1 oligomerization on endoplasmic reticulum. J Cell Biol 216, 4123–4139, doi: 10.1083/jcb.201610057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korobova F, Ramabhadran V. & Higgs HN An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 339, 464–467 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji W. k., Hatch AL, Merrill RA, Strack S. & Higgs HN Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife 4, e11553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore AS, Wong YC, Simpson CL & Holzbaur EL Dynamic actin cycling through mitochondrial subpopulations locally regulates the fission–fusion balance within mitochondrial networks. Nature communications 7, 1–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramonett A. et al. Regulation of mitochondrial fission by GIPC-mediated Drp1 retrograde transport. Molecular biology of the cell 33, ar4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagashima S. et al. Golgi-derived PI(4)P-containing vesicles drive late steps of mitochondrial division. Science 367, 1366–1371, doi: 10.1126/science.aax6089 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Wong YC, Ysselstein D. & Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386, doi: 10.1038/nature25486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boutry M. & Kim PK ORP1L mediated PI(4)P signaling at ER-lysosome-mitochondrion three-way contact contributes to mitochondrial division. Nat Commun 12, 5354, doi: 10.1038/s41467-021-25621-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu W. et al. TBC1D15/RAB7-regulated mitochondria-lysosome interaction confers cardioprotection against acute myocardial infarction-induced cardiac injury. Theranostics 10, 11244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong R. et al. Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell 166, 408–423, doi: 10.1016/j.cell.2016.06.037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho B. et al. Constriction of the mitochondrial inner compartment is a priming event for mitochondrial division. Nature communications 8, 1–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chakrabarti R. et al. INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. Journal of Cell Biology 217, 251–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qin Y. et al. Mitochondrial fusion mediated by fusion promotion and fission inhibition directs adult mouse heart function toward a different direction. The FASEB Journal 34, 663–675 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Favaro G. et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nature communications 10, 1–17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pangou E. et al. A PKD-MFF signaling axis couples mitochondrial fission to mitotic progression. Cell Reports 35, 109129 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Kashatus DF et al. RALA and RALBP1 regulate mitochondrial fission at mitosis. Nature cell biology 13, 1108–1115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao Q-Y et al. Mitochondrial fission and mitophagy reciprocally orchestrate cardiac fibroblasts activation. Frontiers in cell and developmental biology 8, 1741 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Zhang X. et al. Involvement of mitochondrial fission in calcium sensing receptor-mediated vascular smooth muscle cells proliferation during hypertension. Biochemical and biophysical research communications 495, 454–460 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Chen K-H et al. Epigenetic dysregulation of the dynamin-related protein 1 binding partners MiD49 and MiD51 increases mitotic mitochondrial fission and promotes pulmonary arterial hypertension: mechanistic and therapeutic implications. Circulation 138, 287–304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ban-Ishihara R, Ishihara T, Sasaki N, Mihara K. & Ishihara N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proceedings of the National Academy of Sciences 110, 11863–11868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Twig G. et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal 27, 433–446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narendra D, Tanaka A, Suen DF & Youle RJ Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183, 795–803, doi:jcb.200809125 [pii] 10.1083/jcb.200809125 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho HM et al. Drp1-Zip1 Interaction Regulates Mitochondrial Quality Surveillance System. Mol Cell 73, 364–376 e368, doi: 10.1016/j.molcel.2018.11.009 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Shirakabe A. et al. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation 133, 1249–1263, doi: 10.1161/CIRCULATIONAHA.115.020502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aishwarya R. et al. Pleiotropic effects of mdivi-1 in altering mitochondrial dynamics, respiration, and autophagy in cardiomyocytes. Redox biology 36, 101660 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cahill TJ et al. Resistance of dynamin-related protein 1 oligomers to disassembly impairs mitophagy, resulting in myocardial inflammation and heart failure. Journal of Biological Chemistry 290, 25907–25919 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hammerling BC et al. A Rab5 endosomal pathway mediates Parkin-dependent mitochondrial clearance. Nat Commun 8, 14050, doi: 10.1038/ncomms14050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamashita SI et al. Mitochondrial division occurs concurrently with autophagosome formation but independently of Drp1 during mitophagy. J Cell Biol 215, 649–665, doi: 10.1083/jcb.201605093 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song M. et al. Interdependence of Parkin-Mediated Mitophagy and Mitochondrial Fission in Adult Mouse Hearts. Circ Res 117, 346–351, doi: 10.1161/CIRCRESAHA.117.306859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang H. et al. A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration. Cardiovascular research 113, 160–170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vercellino I. & Sazanov LA The assembly, regulation and function of the mitochondrial respiratory chain. Nature Reviews Molecular Cell Biology 23, 141–161 (2021). [DOI] [PubMed] [Google Scholar]
- 98.Zepeda R. et al. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. Journal of cardiovascular pharmacology 63, 477–487 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Deng Y. et al. AMPKα2 Overexpression Reduces Cardiomyocyte Ischemia-Reperfusion Injury Through Normalization of Mitochondrial Dynamics. Frontiers in Cell and Developmental Biology 8, 833 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alam S. et al. Aberrant mitochondrial fission is maladaptive in desmin mutation–induced cardiac proteotoxicity. Journal of the American Heart Association 7, e009289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Joshi A, Ebert A, Haileselassie B. & Mochly-Rosen D. Drp1/Fis1-mediated mitochondrial fragmentation leads to lysosomal dysfunction in cardiac models of Huntington’s disease. Journal of molecular and cellular cardiology 127, 125–133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bekhite M. et al. The role of ceramide accumulation in human induced pluripotent stem cell-derived cardiomyocytes on mitochondrial oxidative stress and mitophagy. Free Radical Biology and Medicine 167, 66–80 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Tsushima K. et al. Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of AKAP121, DRP1, and OPA1 that promote mitochondrial fission. Circulation research 122, 58–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haileselassie B. et al. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. Journal of molecular and cellular cardiology 130, 160–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y, Yu W, Shi C. & Hu P. Crocetin Attenuates Sepsis-Induced Cardiac Dysfunction via Regulation of Inflammatory Response and Mitochondrial Function. Frontiers in Physiology 11, 514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim Y-M et al. Redox regulation of mitochondrial fission protein Drp1 by protein disulfide isomerase limits endothelial senescence. Cell reports 23, 3565–3578 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim D, Sankaramoorthy A. & Roy S. Downregulation of Drp1 and Fis1 inhibits mitochondrial fission and prevents high glucose-induced apoptosis in retinal endothelial cells. Cells 9, 1662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Y. et al. Lactate accelerates vascular calcification through NR4A1-regulated mitochondrial fission and BNIP3-related mitophagy. Apoptosis 25, 321–340 (2020). [DOI] [PubMed] [Google Scholar]
- 109.Karbowski M. et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. The Journal of cell biology 159, 931–938 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oettinghaus B. et al. DRP1-dependent apoptotic mitochondrial fission occurs independently of BAX, BAK and APAF1 to amplify cell death by BID and oxidative stress. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1857, 1267–1276 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Cassidy-Stone A. et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Developmental cell 14, 193–204 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jenner A. et al. DRP1 interacts directly with BAX to induce its activation and apoptosis. The EMBO Journal Online ahead of print, e108587 (2022). [DOI] [PMC free article] [PubMed]
- 113.Otera H, Miyata N, Kuge O. & Mihara K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. Journal of Cell Biology 212, 531–544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bauer TM & Murphy E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circulation research 126, 280–293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duan C. et al. Mitochondrial Drp1 recognizes and induces excessive mPTP opening after hypoxia through BAX-PiC and LRRK2-HK2. Cell Death Dis 12, 1050, doi: 10.1038/s41419-021-04343-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou H. et al. Mff-Dependent Mitochondrial Fission Contributes to the Pathogenesis of Cardiac Microvasculature Ischemia/Reperfusion Injury via Induction of mROS-Mediated Cardiolipin Oxidation and HK2/VDAC1 Disassociation-Involved mPTP Opening. J Am Heart Assoc 6, doi: 10.1161/JAHA.116.005328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou H. et al. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission‐VDAC 1‐HK 2‐mPTP‐mitophagy axis. Journal of pineal research 63, e12413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Touvier T. et al. Muscle-specific Drp1 overexpression impairs skeletal muscle growth via translational attenuation. Cell death & disease 6, e1663-e1663 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smirnova E, Griparic L, Shurland D-L & Van Der Bliek AM Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Molecular biology of the cell 12, 2245–2256 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Campello S. et al. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. The Journal of experimental medicine 203, 2879–2886 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simula L. et al. Drp1 Controls Effective T Cell Immune-Surveillance by Regulating T Cell Migration, Proliferation, and cMyc-Dependent Metabolic Reprogramming. Cell Rep 25, 3059–3073 e3010, doi: 10.1016/j.celrep.2018.11.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang C-H et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buck MD et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 166, 63–76, doi: 10.1016/j.cell.2016.05.035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Basit F, Mathan T, Sancho D. & de Vries IJM Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Frontiers in immunology 9, 2489 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao Z. et al. Mitochondrial dynamics controls anti-tumour innate immunity by regulating CHIP-IRF1 axis stability. Nature communications 8, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y. et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell 171, 331–345. e322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fang L. et al. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life sciences 80, 2154–2160 (2007). [DOI] [PubMed] [Google Scholar]
- 128.Sabbah HN, Gupta RC, Singh-Gupta V, Zhang K. & Lanfear DE Abnormalities of mitochondrial dynamics in the failing heart: normalization following long-term therapy with elamipretide. Cardiovascular drugs and therapy 32, 319–328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsiao YT et al. Cardiac mitofusin-1 is reduced in non-responding patients with idiopathic dilated cardiomyopathy. Scientific reports 11, 1–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moore TM et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol Metab 21, 51–67, doi: 10.1016/j.molmet.2018.11.012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He W. et al. Exercise Enhanced Cardiac Function in Mice With Radiation-Induced Heart Disease via the FNDC5/Irisin-Dependent Mitochondrial Turnover Pathway. Frontiers in physiology 12, 739485. e Collection 732021. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakamura M. & Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nature Reviews Cardiology 15, 387–407 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Qi J. et al. Mitochondrial fission is required for angiotensin II-induced cardiomyocyte apoptosis mediated by a Sirt1-p53 signaling pathway. Frontiers in pharmacology 9, 176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang Y-W et al. Quantitative phosphoproteomic study of pressure-overloaded mouse heart reveals dynamin-related protein 1 as a modulator of cardiac hypertrophy. Molecular & Cellular Proteomics 12, 3094–3107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miranda-Silva D. et al. Mitochondrial Reversible Changes Determine Diastolic Function Adaptations During Myocardial (Reverse) Remodeling. Circulation: Heart Failure 13, e006170 (2020). [DOI] [PubMed] [Google Scholar]
- 136.Disatnik MH et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long‐term cardiac dysfunction. Journal of the American Heart Association 2, e000461 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nishimura A. et al. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Sci Signal 11, doi: 10.1126/scisignal.aat5185 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Zhou H. et al. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2α. Basic research in cardiology 113, 1–20 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Hinderer S. & Schenke-Layland K. Cardiac fibrosis–A short review of causes and therapeutic strategies. Advanced drug delivery reviews 146, 77–82 (2019). [DOI] [PubMed] [Google Scholar]
- 140.Tian L. et al. Increased Drp1-mediated mitochondrial fission promotes proliferation and collagen production by right ventricular fibroblasts in experimental pulmonary arterial hypertension. Frontiers in physiology 9, 828 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tseng H-C, Lin C-C, Hsiao L-D & Yang C-M Lysophosphatidylcholine-induced mitochondrial fission contributes to collagen production in human cardiac fibroblasts [S]. Journal of lipid research 60, 1573–1589 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hasan P. et al. Mitochondrial fission protein, dynamin-related protein 1, contributes to the promotion of hypertensive cardiac hypertrophy and fibrosis in Dahl-salt sensitive rats. Journal of molecular and cellular cardiology 121, 103–106 (2018). [DOI] [PubMed] [Google Scholar]
- 143.Rogers MA et al. Dynamin-related protein 1 inhibition attenuates cardiovascular calcification in the presence of oxidative stress. Circulation research 121, 220–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Durham AL, Speer MY, Scatena M, Giachelli CM & Shanahan CM Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovascular research 114, 590–600 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cui L, Li Z, Chang X, Cong G. & Hao L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vascular pharmacology 88, 21–29 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Buijs RV et al. Calcification as a risk factor for rupture of abdominal aortic aneurysm. European Journal of Vascular and Endovascular Surgery 46, 542–548 (2013). [DOI] [PubMed] [Google Scholar]
- 147.Cooper HA et al. Targeting mitochondrial fission as a potential therapeutic for abdominal aortic aneurysm. Cardiovascular research 117, 971–982 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang G. et al. Targeting HSP90 attenuates angiotensin II-induced adventitial remodelling via suppression of mitochondrial fission. Cardiovascular research 116, 1071–1084 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Rijzewijk LJ et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. Journal of the American College of Cardiology 52, 1793–1799 (2008). [DOI] [PubMed] [Google Scholar]
- 150.Parra V. et al. Calcium and mitochondrial metabolism in ceramide-induced cardiomyocyte death. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1832, 1334–1344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu Q-R et al. High glucose induces Drp1-mediated mitochondrial fission via the Orai1 calcium channel to participate in diabetic cardiomyocyte hypertrophy. Cell death & disease 12, 1–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang L. et al. Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia 58, 2371–2380 (2015). [DOI] [PubMed] [Google Scholar]
- 153.Umezu R. et al. Macrophage (Drp1) dynamin-related protein 1 accelerates intimal thickening after vascular injury. Arteriosclerosis, thrombosis, and vascular biology 40, e214–e226 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Wang L. et al. Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovascular research 106, 272–283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Forrester SJ et al. Mitochondrial fission mediates endothelial inflammation. Hypertension 76, 267–276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Childs BG et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miyao M. et al. Involvement of senescence and mitochondrial fission in endothelial cell pro-inflammatory phenotype induced by angiotensin ii. International journal of molecular sciences 21, 3112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bordt EA et al. The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Developmental cell 40, 583–594. e586 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu D. et al. Identification of novel dynamin‐related protein 1 (Drp1) GTPase inhibitors: Therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia‐reperfusion injury. The FASEB Journal 34, 1447–1464 (2020). [DOI] [PubMed] [Google Scholar]
- 160.Ong S-B et al. Targeting mitochondrial fission using Mdivi-1 in a clinically relevant large animal model of acute myocardial infarction: a pilot study. International journal of molecular sciences 20, 3972 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kornfeld OS et al. Interaction of mitochondrial fission factor with dynamin related protein 1 governs physiological mitochondrial function in vivo. Scientific reports 8, 1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang Q. et al. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes 66, 193–205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou H. et al. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox biology 15, 335–346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kalkhoran SB et al. Hydralazine protects the heart against acute ischemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission. Cardiovascular Research 118, 282–294 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu LM et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia‐reperfusion injury by improving mitochondrial quality control: Role of SIRT6. Journal of pineal research 70, e12698 (2021). [DOI] [PubMed] [Google Scholar]
- 166.Chen WR et al. Melatonin attenuates vascular calcification by inhibiting mitochondria fission via an AMPK/Drp1 signalling pathway. Journal of cellular and molecular medicine 24, 6043–6054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee T-L et al. Vitamin D attenuates ischemia/reperfusion-induced cardiac injury by reducing mitochondrial fission and mitophagy. Frontiers in Pharmacology 11, 604700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liang X. et al. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of Drp1-mediated maladaptive mitochondrial fission. Pharmacological Research 157, 104846 (2020). [DOI] [PubMed] [Google Scholar]
- 169.Singh A, Faccenda D. & Campanella M. Pharmacological advances in mitochondrial therapy. EBioMedicine 65, 103244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hall A. et al. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell death & disease 7, e2238-e2238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]