Abstract
Proteins that contain basic helix-loop-helix (bHLH) and Per-Arnt-Sim motifs (PAS) function as transcription factors. bHLH–PAS proteins exhibit essential and diverse functions throughout the body, from cell specification and differentiation in embryonic development to the proper function of organs like the brain and liver in adulthood. bHLH–PAS proteins are divided into two classes, which form heterodimers to regulate transcription. Class I bHLH–PAS proteins are typically activated in response to specific stimuli, while class II proteins are expressed more ubiquitously. Here, we discuss the general structure and functions of bHLH–PAS proteins throughout the animal kingdom, including family members that do not fit neatly into the class I-class II organization. We review heterodimerization between class I and class II bHLH–PAS proteins, binding partner selectivity and functional redundancy. Finally, we discuss the evolution of bHLH–PAS proteins, and why a class I protein essential for cardiovascular development in vertebrates like chicken and fish is absent from mammals.
Introduction
The basic helix-loop-helix Per-Arnt-Sim family of proteins (bHLH–PAS) are transcription factors that regulate diverse biological functions (Figure 1). bHLH–PAS proteins are present throughout the animal kingdom and contain several conserved functional domains: a bHLH domain at the N-terminus that facilitates binding to DNA, two tandem PAS domains (PAS-A, PAS-B), and a domain at the C-terminus that activates or blocks transcription of target genes (Figure 1B). bHLH–PAS proteins are divided into two classes based on their dimerization properties. Class I bHLH–PAS proteins are typically specialized transcription factors that are activated in response to a stimulus such as a small molecule ligand, oxygen levels or neuronal activity. Class II bHLH–PAS proteins, which are often widely expressed, dimerize with class I proteins to form a functional transcriptional complex. The expression patterns of class I and class II proteins, together with the stimulus-dependent activation of class I proteins, allows bHLH–PAS protein heterodimers to activate or repress target genes in a spatially and temporally distinct manner. In this article, we will review the general structure and functions of the bHLH and PAS domains from bHLH–PAS proteins throughout the animal kingdom, focusing on animals commonly used in biomedical research. We highlight bHLH–PAS family members that contain only one PAS domain and do not fit neatly into the class I-class II organization. We discuss heterodimerization between class I and class II bHLH–PAS proteins, binding partner selectivity and functional redundancy. Some class II bHLH–PAS proteins may form homodimers, while some class I proteins may have functions other than as transcription factors. Finally, we discuss the evolution of bHLH–PAS proteins, and why a class I protein essential for cardiovascular development in vertebrates like chicken and fish is absent from mammals.
Figure 1.
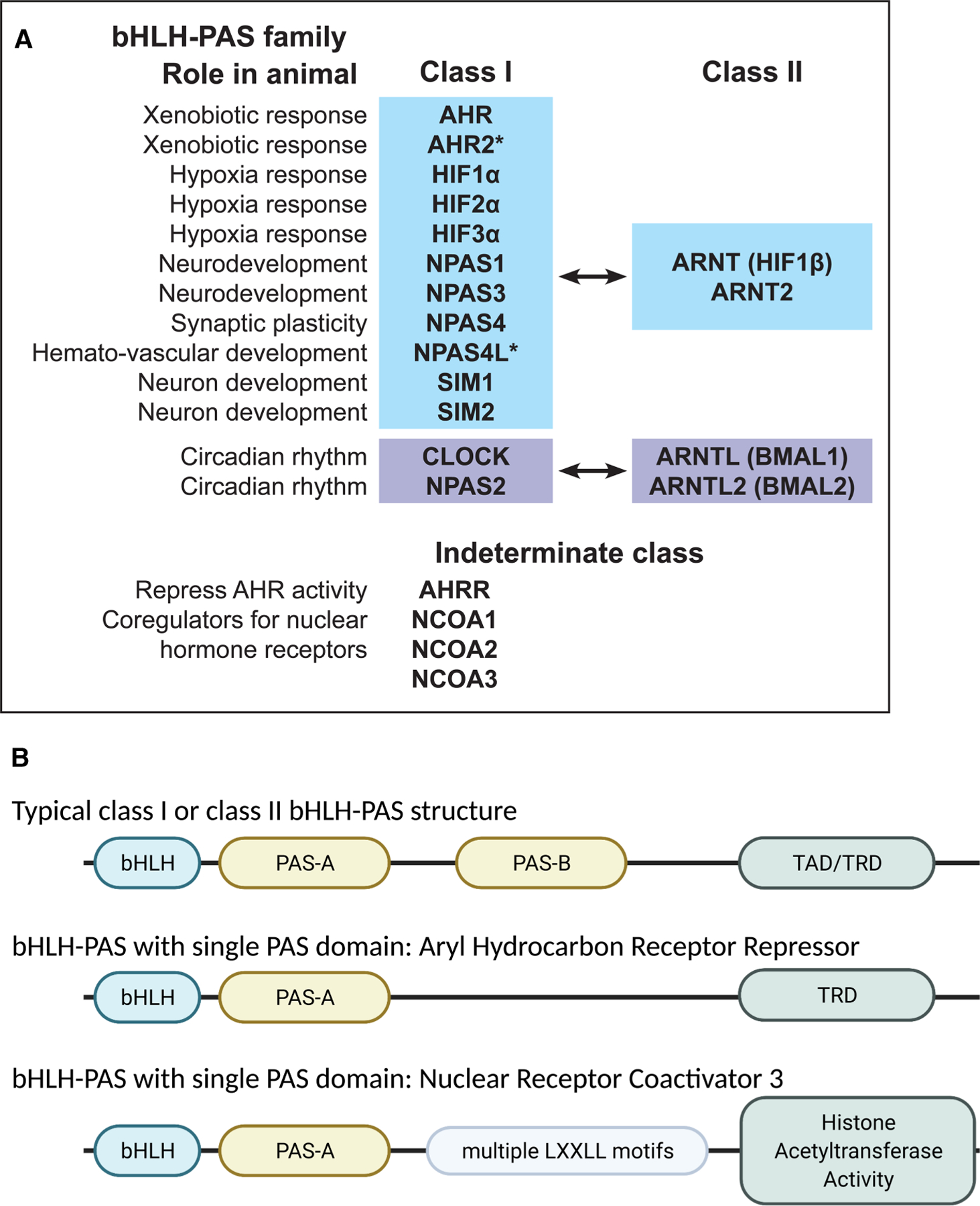
Structure and function of bHLH– PAS proteins.(A) bHLH–PAS transcription factors act as heterodimers, where a class I and indicated class II protein interact and bind DNA. Asterisks indicate proteins encoded by genes present in vertebrates but absent from mammals. Proteins in the indeterminate class can bind class I proteins or act independently of class I and class II proteins. (B) Cartoon structure of representative bHLH–PAS proteins indicating key functional domains: basic helix-loop-helix (bHLH), Per-Arnt-Sim motifs (PAS-A, PAS-B), transcriptional activation or repression domains (TAD, TRD), LxxLL motifs, which modulate protein–protein interactions that influence transcription.
Functions of bHLH–PAS proteins
bHLH–PAS proteins function as transcription factors that regulate gene expression. bHLH–PAS proteins regulate gene expression in many cell types, during multiple stages of organismal development and adulthood (Table 1). Aryl hydrocarbon receptor (AHR) is a ligand-dependent transcription factor that is activated by environmental hydrocarbons and endogenous ligands such as tryptophan metabolites [1,2]. AHR up-regulates enzymes that metabolize and detoxify environmental small molecules. AHR also regulates the development and function of the liver, intestine and the immune system [3–8]. Hypoxia inducible factors (HIF1α, HIF2α, HIF3α) are activated by hypoxia and regulate the growth of blood vessels and differentiation of blood cells [9–17]. Circadian Locomotor Output Cycles Kaput (CLOCK), neuronal PAS domain protein 2 (NPAS2), aryl hydrocarbon receptor nuclear translocator like proteins (ARNTL, ARNTL2, also known as brain and muscle ARNT-like 1 and 2 or BMAL1, BMAL2) regulate circadian rhythms [18–26]. Neuronal PAS domain proteins 1, 3 and 4 (NPAS1, NPAS3, NPAS4) and SIM bHLH transcription factors 1 and 2 (SIM1, SIM2) regulate neuronal development and synaptic plasticity [27–34]. Aryl hydrocarbon receptor nuclear translocator proteins (ARNT, ARNT2) are essential coregulators for class I bHLH–PAS transcription factors [33,35–43] (discussed below), while nuclear receptor co-activators (NCOA1, NCOA2, NCOA3, also known as SRC-1, −2, −3) are essential coregulators for nuclear hormone receptors [44–52] (Figure 1).
Table 1.
List of bHLH-PAS genes and their functions from animals commonly studied for biomedical research
| Species | bHLH–PAS gene name |
bHLH–PAS protein name | Protein class | Function of protein | Reference (PMID) |
|---|---|---|---|---|---|
| Caenorhabditis elegans | aha-1 | AHA-1 | II | Mutation is larval lethal Neuronal specification with ahr-1 Hypoxic transcriptional response with hif-1 Ortholog of human ARNT/ARNT2 |
14757639 19632181 |
| Mus musculus | Ahr | AHR | I | Angiogenesis and hematopoiesis Xenobiotic metabolism Development and function of the immune system Immune checkpoint regulation Regulation of viral replication Liver development and function |
15590894 16443691 33941684 7732381 8806883 34446714 33408169 33239445 32690969 27783946 27158906 26005855 21068375 20676095 20676092 |
| Homo sapiens | AHR | AHR | I | Angiogenesis and hematopoiesis Xenobiotic metabolism Immune response Mutation associated with retinitis pigmentosa |
21976023 21999944 23723449 29726989 |
| Caenorhabditis elegans | ahr-1 | AHR-1 | I | Neuron development Aggregation on lawns of bacterial food Lifespan |
33349622 24655420 16919260 15136141 14757639 |
| Danio rerio | ahr1a | Ahr1a | I | Unclear ( possible role in transcription in endothelial cells) | 30907958 28817646 |
| Danio rerio | ahr1b | Ahr1b | I | Unclear ( possible role in transcription in endothelial cells) | 30907958 28817646 |
| Danio rerio | ahr2 | Ahr2 | I | Xenobiotic metabolism Immune response Fin development Vascular morphology Craniofacial development |
30907958 22242167 28817646 32384158 29494622 |
| Mus musculus | Ahrr | AHRR | indeterminate | Regulate AHR activity Modulate xenobiotic metabolism |
17949687 9887096 |
| Homo sapiens | AHRR | AHRR | indeterminate | Represses AHR transcription activity Possible tumor supressor in cancers |
17890447 18172554 |
| Danio rerio | ahrra | Ahrra | indeterminate | Modulate Ahr2 activation by dioxins Possible role in hematopoiesis |
19494032 24675095 18000031 |
| Danio rerio | ahrrb | Ahrrb | indeterminate | Modulate Ahr2 activation by dioxins | 19494032 18000031 |
| Mus musculus | Arnt | ARNT (HIF1β) | II | Binding partner of multiple Class I bHLH–PAS transcription factors | 27782878 26245371 28602820 7592839 17023418 |
| Homo sapiens | ARNT | ARNT (HIF1β) | II | Binding partner of multiple Class I bHLH–PAS transcription factors | 8662957 27782878 |
| Danio rerio | arnt | Arnt (Hif1β) | II | Binding partner of multiple Class I bHLH–PAS transcription factors | 16306231 16936225 Edwards et al. bioRxiv 2022 |
| Mus musculus | Arnt2 | ARNT2 | II | Binding partner of multiple Class I bHLH–PAS transcription factors | 8657146 10873592 31585079 |
| Homo sapiens | ARNT2 | ARNT2 | II | Binding partner of multiple Class I bHLH–PAS transcription factors Mutation causes post-natal microcephaly, visual and renal anomalies |
24022475 27782878 |
| Danio rerio | arnt2 | Arnt2 | II | Binding partner of multiple Class I bHLH–PAS transcription factors | 19374551 19234064 16936225 Edwards et al. bioRxiv 2022 |
| Mus musculus | Arntl | ARNTL (BMAL1) | II | Binding partner of CLOCK and NPAS2 Circadian rhythm regulation |
17417633 9160755 9616112 |
| Homo sapiens | ARNTL | ARNTL (BMAL1) | II | Binding partner of CLOCK and NPAS2 Circadian rhythm regulation |
17417633 9616112 27782878 |
| Danio rerio | arntl1a | Arntl1a | II | Circadian rhythm regulation with clock genes Ortholog of human ARNTL |
23468616 |
| Danio rerio | arntl1b | Arntl1b | II | Circadian rhythm regulation with clock genes Ortholog of human ARNTL |
23468616 |
| Mus musculus | ARNTL2 | ARNTL2 (BMAL2) | II | Binding partner of CLOCK and NPAS2 Circadian rhythm regulation |
11207387 20153195 |
| Homo sapiens | ARNTL2 | ARNTL2 (BMAL2) | II | Binding partner of CLOCK and NPAS2 Circadian rhythm regulation | 10964693 |
| Danio rerio | arntl2 | Arntl2 (Bmal2) | II | Circadian rhythm regulation | 11517315 |
| Caenorhabditis elegans | cky-1 | CKY-1 | I | Speculated to be functionally similar to NPAS4 | 14701734 19284974 |
| Drosophila melanogaster | clk | Clock | I | Circadian regulation with cyc Regulation of circadian genes per and tim |
11106063 |
| Mus musculus | Clock | CLOCK | I | Circadian rhythm regulation Circadian regulation of genes in multiple tissues |
17417633 9160755 9616112 |
| Homo sapiens | CLOCK | CLOCK | I | Circadian rhythm regulation Circadian regulation of genes in multiple tissues |
17417633 9616112 |
| Danio rerio | clocka | Clocka | I | Circadian rhythm regulation Mesoderm patterning |
18800057 28687631 23468616 18569451 |
| Danio rerio | clockb | Clockb | I | Speculated function circadian rhythm regulation | 23468616 |
| Drosophila melanogaster | cyc | Cycle | II | Circadian regulation with clk Sensitization to cocaine |
11106063 10409723 10446052 |
| Drosophila melanogaster | dysf | Dysfusion | I | Required for tracheal migration, adhesion and fusion during development Joint formation in legs |
16914738 12897136 17652079 25329825 30080872 |
| Drosophila melanogaster | gce | Germ-cell expressed bHLH–PAS | indeterminate | Responds to juvenile hormone Can form heterodimer with met |
26161662 16516852 |
| Caenorhabditis elegans | hif-1 | HIF-1 | I | Stimulates gene expression in response to hypoxia Essential for heat acclimation |
11427734 30696318 12686697 |
| Mus musculus | Hif1a | HIF1A | I | Up-regulates genes in response to hypoxia Hematopoiesis |
24141110 9436976 |
| Homo sapiens | HIF1A | HIF1A | I | Regulates genes in response to hypoxia Vascular devleopment |
9436976 9782081 |
| Danio rerio | hif1aa | Hif1aa | I | Up-regulates genes in response to hypoxia Hematopoesis Neural protective |
29339404 31314708 |
| Danio rerio | hif1ab | Hif1ab | I | Up-regulates genes in response to hypoxia Hematopoesis Maturation of intestinal goblet cells |
29339404 31314708 |
| Danio rerio | hif1al | Hif1al | I | Up-regulates genes in response to hypoxia Regulates erythropoiesis Ortholog of human HIF3A |
33037038 23034716 26052946 |
| Danio rerio | hif1al2 | Hif1al2 | I | Maturation of intestinal goblet cells Speculated up-regulates genes in response to hypoxia |
27222589 |
| Mus musculus | Epas1 | HIF2A | I | Regulates gene expression in response to hypoxia Hematopoesis Regulates vascular endothelial growth factor (VEGF) Spermatogenesis |
15626745 20181618 15851592 |
| Homo sapiens | EPAS1 | HIF2A | I | Regulates gene expression in response to hypoxia Vascular development Mutation causes familial erythrocytosis |
15851592 18184961 |
| Danio rerio | epas1a | Epas1a | I | Up-regulates genes in response to hypoxia hematopoesis ortholog of human EPAS1 (HIF2A) |
29339404 |
| Danio rerio | epas1b | Epas1b | I | Up-regulates genes in response to hypoxia Hematopoesis ortholog of human EPAS1 (HIF2A) |
29339404 |
| Mus musculus | Hif3a | HIF3A | I | Inhibits/represses expression of hypoxia-induced genes | 25626335 9840812 11734856 |
| Homo sapiens | HIF3A | HIF3A | I | Modulates expression of hypoxia-induced genes Dominant negative regulator of HIF1A |
11573933 16126907 19694616 9840812 |
| Caenorhabditis elegans | hlh-34 | HLH-34 | I | Unknown, expressed in a subset of interneurons Ortholog of human NPAS3 |
34604715 |
| Drosophila melanogaster | met | Methoprene-tolerant | indeterminate | Responds to juvenile hormone Mutants are resistant to insecticide methoprene Can form heterodimer with Germ-cell expressed |
1592245 26161662 9501163 15720391 16516852 |
| Mus musculus | Ncoa1 | NCOA1 | indeterminate | Transciptional coactivator of steroid hormone receptors Energy metabolism and obesity Possible regulator of hematopoiesis |
8616895 7481822 12954634 12507421 27958775 |
| Homo sapiens | NCOA1 | NCOA1 | indeterminate | Transcriptional coactivator of steroid hormone receptors Transcriptional coactivator of STAT5 transcription factor |
9427757 7481822 9223431 12954634 9252329 |
| Danio rerio | ncoa1 | Ncoa1 | indeterminate | Transcriptional coactivator of steroid hormone receptors Hematopoiesis |
27958775 30448381 27156127 |
| Mus musculus | Ncoa2 | NCOA2 | indeterminate | Transcriptional coactivator of steroid hormone receptors Energy metabolism and obesity Fertility |
9238002 8670870 12507241 12138202 |
| Homo sapiens | NCOA2 | NCOA2 | indeterminate | Transcriptional coactivator of steroid hormone receptors | 9238002 8670870 |
| Danio rerio | ncoa2 | Ncoa2 | indeterminate | Possible regulator of hematopoiesis Transcriptional coactivator of steroid hormone receptors |
18295965 18248177 |
| Mus musculus | Ncoa3 | NCOA3 | indeterminate | Transcriptional coactivator of steroid hormone receptors Important for hearing Regulates adipogenesis hematopoiesis Inflammation |
33326993 9765300 |
| Homo sapiens | NCOA3 | NCOA3 | indeterminate | Transcriptional coactivator of steroid hormone receptors Mutation associated with progressive hearing loss |
33326993 9765300 |
| Danio rerio | ncoa3 | Ncoa3 | indeterminate | Development and function of otoliths Proposed trancriptional coactivator | 33326993 |
| Mus musculus | Npas1 | NPAS1 | I | Neural development Behavior |
15347806 9012850 28499489 |
| Homo sapiens | NPAS1 | NPAS1 | I | Neuronal devleopment Regulates genes involved in psychiatric diseases |
9012850 28499489 |
| Danio rerio | npas1 | Npas1 | I | Expressed in adult telencephalon Speculated to be involved in neural development |
25556858 |
| Mus musculus | Npas2 | NPAS2 | I | Circadian rhythm regulation | 11441147 26895328 11163178 17417633 |
| Homo sapiens | NPAS2 | NPAS2 | I | Circadian rhythm regulation Mutation associated with nonobstructive azoospermia |
11441147 11163178 25956372 |
| Danio rerio | npas2 | Npas2 | I | Not characterized | none |
| Mus musculus | Npas3 | NPAS3 | I | Neural development behavior Lung development and function |
15347806 28499489 19581591 |
| Homo sapiens | NPAS3 | NPAS3 | I | Neural development Mutations associated with schizophrenia |
12746393 21709683 22228753 15924306 |
| Danio rerio | npas3 | Npas3 | I | Insufficent data Speculated to be involved in neuronal development |
- |
| Mus musculus | Npas4 | NPAS4 | I | Synaptic plasticity Memory |
24201284 22194569 18815592 |
| Homo sapiens | NPAS4 | NPAS4 | I | Variants associated with intellectual disability | 33758288 24465693 |
| Danio rerio | npas4a | Npas4a | I | Forebrain development Angiogenesis of intersegmental vessicles |
25538572 28082451 |
| Danio rerio | npas4b | Npas4b | I | Angiogenesis of intersegmental vessicles | 28082451 |
| Danio rerio | npas4l | Npas4l | I | Master regulator of hemato-vascular specification Formation of lens cataract |
27411634 7588049 30861003 |
| Drosophila melanogaster | sim | Single-minded | I | Midline central nervous system development Axonal extension |
3345560 3345559 2242162 1760843 16439478 |
| Mus musculus | Sim1 | SIM1 | I | Neuronal development Hypothalamus development and function Obesity |
9784500 16291793 12947113 10587584 |
| Homo sapiens | SIM1 | SIM1 | I | Obesity Neuroendocrine development |
10587584 16924270 33434169 |
| Danio rerio | sim1a | Sim1a | I | Hypothalamus development and function Axonal guidance |
19234064 23222439 16691572 |
| Danio rerio | sim1b | Sim1b | I | Hypothalamus development and function | 19234064 16691572 |
| Mus musculus | Sim2 | SIM1 | I | Development and function of neuroendocrine cells Axon guidance Overexpression causes Down syndrome phenotypes Growth and integrity of skeleton |
14988428 12024028 16291793 10400987 10915774 |
| Homo sapiens | SIM2 | SIM2 | I | Promotes glioblastoma cell invasion Part of Down syndrome critical region on chromosome 21 |
7647800 7568099 20448453 15946822 |
| Danio rerio | sim2 | Sim2 | I | Not characterized | none |
| Drosophila melanogaster | sima | Similar | I | Hypoxic response Trachea development Ortholog of human HIF1A |
12215541 19285057 |
| Drosophila melanogaster | ss | Spineless | I | Distal limb formation Mutants have reduced sensory bristles Ortholog of human AHR |
10433921 17084833 9573046 |
| Drosophila melanogaster | tgo | Tango | II | Neuronal devleopment Glial migration Trachea development Limb formation Coregulator of class I genes Ortholog of human ARNT/ARNT2 |
8557198 10433921 9409674 |
| Drosophila melanogaster | trh | Trachealess | I | Development of salivary duct Development of trachea distal limb Development Most similar to human NPAS1, NPAS3 |
17187773 8557198 |
Some bHLH–PAS proteins have additional functions beyond transcription. AHR is not only a transcription factor, AHR also functions as an E3 ubiquitin ligase that targets proteins for degradation [53]. NCOA1 is a histone acetyl transferase and increases the accessibility of chromatin for transcription [54]. It remains to be seen whether other bHLH–PAS proteins have additional functions beyond transcription.
Functional motifs in bHLH–PAS proteins
There are hundreds of proteins that contain either a bHLH domain or PAS domains [55–57]. bHLH domains bind DNA [58,59]. PAS domains detect a plethora of stimuli and regulate diverse biological processes throughout the animal kingdom, like nitrogen fixation in bacteria [60], growth in response to light in plants [61], circadian rhythms in fungi, insects, and vertebrates [18,62–65], and ion channel gating in vertebrates [66].
We define bHLH–PAS proteins as any protein containing both a bHLH domain and a PAS domain, of which there are 19 in humans. Most bHLH–PAS proteins contain a bHLH domain, two PAS domains, and a transrepression or transactivation domain (Figure 1B). Some bHLH–PAS proteins contain one PAS domain instead of two (Figure 1). bHLH–PAS proteins are divided into two classes, where class I proteins form dimers with class II proteins. There are only four class II proteins: ARNT (also known as HIF1β), ARNT2, ARNTL (BMAL1), ARNTL2 (BMAL2) versus a dozen class I proteins. The same class II protein can dimerize with multiple different class I proteins. Dimerization is restricted: class II proteins ARNTL and ARNTL2 appear to dimerize only with class I proteins CLOCK and NPAS2, while class II proteins ARNT and ARNT2 appear to dimerize with all other class I proteins except for CLOCK and NPAS2 [37] (Figure 1).
Some bHLH–PAS domain proteins contain only a single PAS domain, such as the aryl hydrocarbon receptor repressor (AHRR) and NCOA1, NCOA2 and NCOA3 (Figure 1). These proteins do not fit neatly into the class I and II dichotomy. AHRR behaves like a class I protein, in that it can bind ARNT and DNA and repress transcription [67]. However, AHRR proteins with mutations that prevent DNA binding or prevent dimerization with ARNT still retain the ability to repress AHR activation [68], suggesting that AHRR may block AHR activity using additional mechanisms besides forming a complex with ARNT and DNA.
It is also difficult to classify NCOA proteins as class I or class II. NCOA1, NCOA2 and NCOA3 proteins were originally identified as coregulators of nuclear hormone receptors, ligand-dependent transcription factors that are not members of the bHLH–PAS family [44,48,49,51]. NCOA1–3 act as coregulators for a variety of transcription factors, including class I bHLH–PAS proteins like the aryl hydrocarbon receptor [69–71]. Whether NCOA1–3 proteins form a transcriptional complex in the absence of class II bHLH–PAS proteins is not clear. NCOA1 and NCOA2 are associated with ARNT at the promoter region of CYP1A1, an AHR target gene, while NCOA1 was required for AHR-dependent reporter gene expression [70]. Overexpression of NCOA2 reduced expression of HIF1α-dependent reporter gene by competing with HIF1 α to bind ARNT [72]. These studies suggest that NCOA proteins bind ARNT, typical of class I proteins. However, in many cases where NCOA proteins form a transcriptional complex with nuclear receptors, there is absence of evidence as to whether ARNT or other class II bHLH–PAS proteins are part of the transcriptional complex. Mice and worms with mutations in Arnt (or aha-1, the C elegans ortholog) do not survive beyond embryo or larval stages [73–75], so it is difficult to test whether nuclear steroid receptor function, which requires NCOAs, is abnormal in the absence of ARNT proteins.
Class I and Class II dimerization selectivity
For a given class I protein, what regulates its dimerization with ARNT vs ARNT2 (or ARNTL vs ARNTL2)? What are the functional differences, if any, when a class I protein dimerizes with ARNT vs ARNT2? Alternatively, do ARNT and ARNT2 act redundantly? Below we provide examples using different class I proteins. Redundant functions of ARNT and ARNT2 may depend on several factors that remain to be explored, such as cell type, developmental stage, and identity of the Class I protein.
NPAS4 forms stimulus-dependent dimers with ARNT or ARNT2 in the brain
NPAS4 regulates the structure and strength of synapses in the nervous system and is required for contextual memory formation in mice [29,76,77]. NPAS4 is expressed following two different stimuli, action potentials and excitatory post synaptic potentials. In the hippocampus, when NPAS4 was induced by action potentials, it formed a complex with ARNT2 but not with ARNT or BMAL1, two other class II proteins expressed in the hippocampus. In contrast, when NPAS4 was induced by excitatory post synaptic potentials, NPAS4 formed a complex with ARNT exclusively [43]. NPAS4–ARNT and NPAS4–ARNT2 heterodimers bind different regions of the genome, suggesting that they target expression of different genes. NPAS4–ARNT was enriched at enhancer regions, while NPAS4–ARNT2 and NPAS4–ARNT were present at gene promoters [43].
Arnt and Arnt2 act redundantly with HIF1α and NPAS4L
In cultured mouse and human cell lines (Hepa1c4, HeLa), ARNT and ARNT2 appeared redundant in their ability to drive the expression of a hypoxia response element (HIF-dependent) reporter gene [78]. In zebrafish embryos, Npas4l is required for the specification of blood cells and most blood vessels (endothelial cells). Zebrafish embryos with mutations in both arnt and arnt2 genes, but not single arnt or arnt2 mutants, exhibit the same phenotype as npas4l mutants, suggesting that ARNT and ARNT2 act redundantly with NPAS4L [79].
AHR preferentially dimerizes with ARNT
The aryl hydrocarbon receptor (AHR) is a ligand-dependent class I protein. In zebrafish embryos, exposure to the AHR ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) causes cardiac toxicity. Arnt2 mutants are sensitive to TCDD-dependent cardiac toxicity, whereas arnt1 mutants have normal cardiac morphology following TCDD exposure [79]. These results demonstrate that in zebrafish, the aryl hydrocarbon receptor preferentially interacts with Arnt and not Arnt2 in response to TCDD. Similar results were obtained in cultured cells, where activation of an AHR-dependent reporter gene in response to the AHR ligand 3MC was robust in the presence of ARNT and low in the presence of ARNT2. Additionally, 3MC-dependent expression of the endogenous AHR target gene CYP1A1 was robust in the presence of ARNT, but absent from cells expressing ARNT2 [78]. Mutating a single amino acid in the PAS-B region of ARNT prevented 3MC-dependent reporter gene expression. This mutation had no effect on HIF1α reporter gene expression [78]. Together, these results suggest that AHR preferentially dimerizes with ARNT and that differences in the ARNT and ARNT2 PAS-B regions are responsible for the preferential dimerization of AHR with ARNT vs ARNT2.
It is likely that the degree to which class II proteins act redundantly, or form selective dimers with class I proteins, depends on cell type, developmental stage, and relative expression levels. Much remains to be learned about class I-class II dimerization selectivity.
Evidence that ARNT can function without other bHLH–PAS proteins
ARNT can form homodimers in vitro. ARNT homodimers bound E-box core DNA sequence in vitro, consistent with DNA binding properties of the ARNT bHLH domain, and drove reporter gene expression from an E-box-containing promoter, the adenovirus major late promoter [38,80]. Structural studies of the human ARNT PAS-B domain demonstrated that it can form a homodimer [81]. To what extent ARNT forms homodimers in vivo is not known. It is not known whether ARNT2 can form homodimers.
ARNT and ARNT2 may also act as transcriptional coregulators with non- bHLH–PAS transcription factors. ARNT and ARNT2 enhanced the transcriptional activity of nuclear estrogen receptors alpha and beta (ERα, ERβ) [82]. Formation of an ER–ARNT complex required the C-terminal domain of ARNT and did not require the bHLH or PAS domains [82]. This contrasts with the AHR–ARNT complex, which requires ARNT bHLH and PAS domains for dimerization and formation of a transcriptional complex. Moreover, ARNTL (BMAL1) was unable to act as an ER coregulator [82]. Reducing the amount of available ARNT, either by small interfering RNAs targeting ARNT or by activating AHR or HIF1α signaling to compete for binding to ARNT, reduced the transcription activity of ERs [83]. Together, these results suggest that ARNT acts as a transcriptional coregulator for nuclear estrogen receptors, separate from its role as a bHLH–PAS dimer. A caveat is that NCOA proteins might be present in ER–ARNT transcriptional complexes.
ARNT can function as part of the NF-kappaB protein complex to regulate gene expression. In cultured lymphoma cells, ARNT binds the NF-kappaB subunit protein RelB. ARNT binding to RelB enhanced the binding of the ARNT-RelB-p50 protein complex to DNA, which inhibited expression of target genes [84]. These results suggests that ARNT acts as a transcriptional coregulator for NF-kappaB. However, it is not known whether any class I bHLH–PAS proteins are present in the NF-kappaB complex with ARNT in lymphoma cells. Class I proteins such as AHR and HIF2α interact with RelA, RelB and other NF-kappaB proteins in other contexts [85–90], though it remains to be seen whether ARNT or other class II proteins are necessary for NF-kappaB function in these contexts.
Evidence that AHR can function without class II bHLH–PAS proteins
ARNT is considered an obligate binding partner of AHR [39]. However, recent studies suggest that under certain conditions AHR can act independently of ARNT to up-regulate gene expression. In mouse liver, TCDD-AHR was bound to the promoter of, and up-regulated, the Serpine1 gene, but no ARNT was detected at the promoter and knockdown of ARNT did not reduce expression [91]. In mouse hepatoma 1c1c7 cells, exposure to N-acetylsphingosine caused apoptosis in an AHR-dependent manner but reducing ARNT levels had no effect [92]. In a rat liver epithelial cell line (WB-F344), TCDD exposure increased JunD and cyclin A expression in an AHR-dependent manner, while knockdown of ARNT had no effect [93]. In human macrophages, AHR interacts with RelB to regulate the expression of several chemokine genes. Gel shift assays identified the promoter DNA sequence to which AHR and RelB bind, however ARNT was not detected in this complex [88]. Taken together, these results suggest that TCDD-AHR can up-regulate gene expression independently of ARNT. A limitation of these studies is that none considered ARNT2. ARNT2 was shown to interact with AHR and DNA in vitro [35,94,95], but to our knowledge there is an absence of evidence of whether ARNT2 interacts with AHR in vivo. DNA binding, coregulator recruitment and target gene expression could be different between AHR–ARNT and AHR–ARNT2 transcriptional complexes.
When AHR acts as a ubiquitin ligase, is ARNT required? In MCF7 breast cancer cells, knockdown of ARNT reduced AHR-dependent transcription but did not reduce AHR E3 ubiquitin ligase activity [96]. In contrast, in cultured HeLa cells overexpressing AHR, both AHR and ARNT were associated in a complex of ubiquitin ligase core proteins [53]. This result could be an artifact of AHR overexpression and not reflect what occurs in vivo. Alternatively, ARNT may act as a coregulator of AHR ubiquitin ligase activity in some cell types and not others.
Alternative splicing alters function of bHLH–PAS transcription factors
DNA is transcribed to produce precursor messenger RNA ( pre-mRNA). Portions of pre-mRNA are then excised or spliced out, leaving a mature messenger RNA (mRNA) that serves as a template for protein translation. The splicing process may occur such that the same pre-RNA produces multiple different mRNAs. Such alternatively spliced mRNAs may be translated into proteins with different functions, despite being transcribed from the same gene [97–100]. Alternative splicing occurs throughout the genome in many different cell types at all stages of development and adulthood. Aberrant splicing can cause or exacerbate diseases in humans [101–104]. How does alternative splicing influence the function of bHLH–PAS proteins? We know the most about alternative splicing of ARNT pre-mRNA.
Alternative splicing produces different ARNT proteins
The ARNT pre-mRNA is alternatively spliced to produce two different protein isoforms: ARNT isoform 1, which includes 15 amino acids encoded by exon 5, and ARNT isoform 3, which lacks exon 5 and those 15 amino acids [105]. The additional amino acids in isoform 1 include an amino acid phosphorylated by casein kinase 2, a phosphorylation site that is absent from isoform 3 [106].
In human multiple myeloma and anaplastic large cell lymphoma cell lines (KMS-18, Karpas 299), knockdown of the ARNT isoform 1 induced cell-cycle arrest and apoptosis [107]. Moreover, six different B- and T-cell cancer cell lines exhibited increased isoform 1 expression, or increased ratio of expression of isoform 1 to isoform 3, compared with normal human primary B and T cells [107]. Thus, isoform 1 confers a proliferation advantage.
How do cells regulate production of ARNT isoform 1 vs isoform 3? The RNA-binding protein RBFOX2 promotes the inclusion of exon 5 in the conversion of ARNT pre-mRNA to mRNA, leading to production of ARNT isoform 1 [108]. RBFOX2 gene expression was up-regulated in two human malignant T cell lines ( Jurkat, Karpas 299) compared with primary T cells. Knockdown of RBFOX2 expression led to a reduction in ARNT isoform 1 protein levels and a reduction in cell proliferation [108].
Do different ARNT isoforms influence the dimerization or function of class I proteins differently? This is best studied in the context of AHR, a ligand-dependent class I protein. In Karpas299 cells exposed to the AHR ligand TCDD, AHR target gene expression was different depending on the relative levels of ARNT isoform 1 vs isoform 3 [109]. Additionally, phosphorylation of the casein kinase 2 phosphorylation site, present exclusively in isoform 1, is required for optimal AHR activity [109].
These studies demonstrate clear functional differences between ARNT isoform 1 and ARNT isoform 3 proteins, differences that affect the function of one class I protein (AHR), cause differential target gene expression, and promote or reduce cell proliferation. Many exciting questions remain. How does alternative splicing of ARNT affect the function of other class I proteins besides AHR? Does alternative splicing of other class II proteins occur, and if so, how does it affect their function? To what degree is bHLH–PAS protein alternative splicing conserved in different species? ARNT isoforms 1 and 3 appear to be present in mice, although mice may have additional ARNT isoforms whose function is not known [110]. NPAS3 alternatively spliced forms appear to be similar in humans and chicken, however the functional differences, if any, are not known [111]. In zebrafish, there are three different isoforms of ARNT2. Overexpression of zebrafish AHR together with different ARNT2 isoforms in COS-7 monkey cells demonstrated that, in response to TCDD, one ARNT2 isoform up-regulated a TCDD reporter gene while the other two isoforms did not [94]. Meanwhile, all three isoforms induced gene expression similarly with a different class I protein, HIF2α [94], suggesting that ARNT2 isoforms affect the function of some, but not all, class I proteins. While it is not clear whether ARNT2 is similarly spliced in humans, the idea that alternative splicing affects bHLH–PAS protein function is supported by studies of ARNT isoforms in humans and ARNT2 isoforms in zebrafish.
Evolution of bHLH–PAS proteins
bHLH–PAS transcription factors containing PAS-A and PAS-B domains (eg canonical class I and class II proteins) are only found in metazoans [112]. Most invertebrate genomes contain less than a dozen bHLH–PAS genes, while vertebrate genomes contain dozens [112]. One gene is particularly interesting: npas4l, present in the genome of non-mammalian vertebrates such as fish but absent from mammals (Figure 2). In chicken and zebrafish embryos, Npas4l is essential for the development of blood cells and blood vessels [113–115]. How is such an essential gene absent from mammalian genomes? The closest related gene, Npas4, does not seem to be required for blood cell or blood vessel formation in mice. The functional homolog(s) of Npas4l in mammalian embryonic development are not known. One possibility is that, in the relatively hypoxic environment of the placenta, the expression of HIF genes changed in response to the low oxygen environment, and these class I bHLH–PAS proteins took over the role of Npas4l in specifying hemato-vascular progenitor cells. Zebrafish embryos develop outside of the mother, with free access to oxygen from the environment, via diffusion through embryonic tissue. In this high-oxygen environment, Npas4l is sufficient for driving hemato-vascular specification. In both zebrafish and mice, Arnt and Arnt2 genes are required for the proper development of blood cells and endothelial cells [74,79,116]. The same class II proteins might be used in all species, but the class I partners may have changed depending on evolutionary pressure.
Figure 2. Phylogenetic tree of bHLH–PAS proteins.

Unrooted phylogenetic tree of bHLH–PAS proteins from humans and commonly studied animal models of human development. The neighbor-joining tree was built using multiple alignments of the indicated bHLH–PAS amino acid sequences. To assess the degree of confidence of the nodes, bootstrap with 1000 repetitions was performed.
Perspectives.
bHLH–PAS transcription factors regulate diverse biological processes throughout the body, from cell specification and differentiation in embryos to organ function in adults. Understanding the molecular mechanisms by which bHLH–PAS proteins regulate transcription is a prerequisite for understanding how bHLH–PAS function is abnormal in birth defects and disease.
Current thinking indicates that bHLH–PAS proteins form heterodimers, where class I proteins interact with class II proteins. The class II proteins ARNT and ARNT2 interact with a variety of class I proteins, whereas the class II proteins ARNTL and ARNTL2 interact with only two class I proteins, CLOCK and NPAS2.
Important future questions include to what degree class II proteins act redundantly and how specific bHLH–PAS proteins, such as NPAS4L, evolved to have essential roles in some animals but not in others.
Acknowledgements
We thank members of the Gorelick laboratory for helpful discussions. Figure 1 was generated with Biorender.com.
Funding
Work in this manuscript was supported by the National Institutes of Health R01ES026337 and P30ES030285.
Abbreviations
- AHR
aryl hydrocarbon receptor
- AHRR
aryl hydrocarbon receptor repressor
- bHLH–PAS
basic helix-loop-helix Per-Arnt-Sim family of proteins
- NPAS2
neuronal PAS domain protein 2
- PAS
Per-Arnt-Sim motifs
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K et al. (1997) Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 2, 645–654 10.1046/j.1365-2443.1997.1490345.x [DOI] [PubMed] [Google Scholar]
- 2.Burbach KM, Poland A and Bradfield CA (1992) Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A 89, 8185–8189 10.1073/pnas.89.17.8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt JV, Su GH, Reddy JK, Simon MC and Bradfield CA (1996) Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl Acad. Sci. U.S.A 93, 6731–6736 10.1073/pnas.93.13.6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S et al. (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 10.1038/nature10491 [DOI] [PubMed] [Google Scholar]
- 5.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C et al. (2011) Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 10.1126/science.1214914 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF et al. (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 10.1016/j.cell.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 7.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 22, 586–597 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E et al. (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 10.1038/nature06880 [DOI] [PubMed] [Google Scholar]
- 9.Gerri C, Marass M, Rossi A and Stainier DYR (2018) Hif-1α and Hif-2α regulate hemogenic endothelium and hematopoietic stem cell formation in zebrafish. Blood 131, 963–973 10.1182/blood-2017-07-797795 [DOI] [PubMed] [Google Scholar]
- 10.Scortegagna M, Morris MA, Oktay Y, Bennett M and Garcia JA (2003) The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood 102, 1634–1640 10.1182/blood-2003-02-0448 [DOI] [PubMed] [Google Scholar]
- 11.Duan L-J, Zhang-Benoit Y and Fong G-H (2005) Endothelium-intrinsic requirement for Hif-2alpha during vascular development. Circulation 111, 2227–2232 10.1161/01.CIR.0000163580.98098.A3 [DOI] [PubMed] [Google Scholar]
- 12.Kotch LE, Iyer NV, Laughner E and Semenza GL (1999) Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev. Biol 209, 254–267 10.1006/dbio.1999.9253 [DOI] [PubMed] [Google Scholar]
- 13.Ramírez-Bergeron DL, Runge A, Adelman DM, Gohil M and Simon MC (2006) HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev. Cell 11, 81–92 10.1016/j.devcel.2006.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y et al. (2010) Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7, 391–402 10.1016/j.stem.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 15.Tsang KM, Hyun JS, Cheng KT, Vargas M, Mehta D, Ushio-Fukai M et al. (2017) Embryonic stem cell differentiation to functional arterial endothelial cells through sequential activation of ETV2 and NOTCH1 signaling by HIF1α. Stem Cell Rep 9, 796–806 10.1016/j.stemcr.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH et al. (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12, 149–162 10.1101/gad.12.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang GL, Jiang BH, Rue EA and Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. U.S.A 92, 5510–5514 10.1073/pnas.92.12.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP et al. (1997) Positional cloning of the mouse circadian clock gene. Cell 89, 641–653 10.1016/S0092-8674(00)80245-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reick M, Garcia JA, Dudley C and McKnight SL (2001) NPAS2: an analog of clock operative in the mammalian forebrain. Science 293, 506–509 10.1126/science.1060699 [DOI] [PubMed] [Google Scholar]
- 20.Cermakian N, Whitmore D, Foulkes NS and Sassone-Corsi P (2000) Asynchronous oscillations of two zebrafish CLOCK partners reveal differential clock control and function. Proc. Natl Acad. Sci. U.S.A 97, 4339–4344 10.1073/pnas.97.8.4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP et al. (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 10.1126/science.280.5369.1564 [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M and Nomura M (1997) cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS protein (BMAL1) and identification of alternatively spliced variants with alternative translation initiation site usage. Biochem. Biophys. Res. Commun 233, 258–264 10.1006/bbrc.1997.6371 [DOI] [PubMed] [Google Scholar]
- 23.Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TD et al. (1998) Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 280, 1599–1603 10.1126/science.280.5369.1599 [DOI] [PubMed] [Google Scholar]
- 24.Rutila JE, Suri V, Le M, So WV, Rosbash M and Hall JC (1998) CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93, 805–814 10.1016/S0092-8674(00)81441-5 [DOI] [PubMed] [Google Scholar]
- 25.Ikeda M, Yu W, Hirai M, Ebisawa T, Honma S, Yoshimura K et al. (2000) cDNA cloning of a novel bHLH-PAS transcription factor superfamily gene, BMAL2: its mRNA expression, subcellular distribution, and chromosomal localization. Biochem. Biophys. Res. Commun 275, 493–502 10.1006/bbrc.2000.3248 [DOI] [PubMed] [Google Scholar]
- 26.Pando MP, Pinchak AB, Cermakian N and Sassone-Corsi P (2001) A cell-based system that recapitulates the dynamic light-dependent regulation of the vertebrate clock. Proc. Natl Acad. Sci. U.S.A 98, 10178–10183 10.1073/pnas.181228598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erbel-Sieler C, Dudley C, Zhou Y, Wu X, Estill SJ, Han T et al. (2004) Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc. Natl Acad. Sci. U.S.A 101, 13648–13653 10.1073/pnas.0405310101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloodgood BL, Sharma N, Browne HA, Trepman AZ and Greenberg ME (2013) The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 503, 121–125 10.1038/nature12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL et al. (2011) Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334, 1669–1675 10.1126/science.1208049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaud JL, Rosenquist T, May NR and Fan CM (1998) Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev 12, 3264–3275 10.1101/gad.12.20.3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crews ST, Thomas JB and Goodman CS (1988) The Drosophila single-minded gene encodes a nuclear protein with sequence similarity to the per gene product. Cell 52, 143–151 10.1016/0092-8674(88)90538-7 [DOI] [PubMed] [Google Scholar]
- 32.Löhr H, Ryu S and Driever W (2009) Zebrafish diencephalic A11-related dopaminergic neurons share a conserved transcriptional network with neuroendocrine cell lineages. Development 136, 1007–1017 10.1242/dev.033878 [DOI] [PubMed] [Google Scholar]
- 33.Probst MR, Fan CM, Tessier-Lavigne M and Hankinson O (1997) Two murine homologs of the Drosophila single-minded protein that interact with the mouse aryl hydrocarbon receptor nuclear translocator protein. J. Biol. Chem 272, 4451–4457 10.1074/jbc.272.7.4451 [DOI] [PubMed] [Google Scholar]
- 34.Fan CM, Kuwana E, Bulfone A, Fletcher CF, Copeland NG, Jenkins NA et al. (1996) Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Mol. Cell. Neurosci 7, 1–16 10.1006/mcne.1996.0001 [DOI] [PubMed] [Google Scholar]
- 35.Hirose K, Morita M, Ema M, Mimura J, Hamada H, Fujii H et al. (1996) cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS factor (Arnt2) with close sequence similarity to the aryl hydrocarbon receptor nuclear translocator (Arnt). Mol. Cell. Biol 16, 1706–1713 10.1128/MCB.16.4.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood SM, Gleadle JM, Pugh CW, Hankinson O and Ratcliffe PJ (1996) The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J. Biol. Chem 271, 15117–15123 10.1074/jbc.271.25.15117 [DOI] [PubMed] [Google Scholar]
- 37.Wu D, Su X, Potluri N, Kim Y and Rastinejad F (2016) NPAS1-ARNT and NPAS3-ARNT crystal structures implicate the bHLH-PAS family as multi-ligand binding transcription factors. eLife 5, e18790 10.7554/eLife.18790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson HI, Chan WK and Bradfield CA (1995) DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J. Biol. Chem 270, 26292–26302 10.1074/jbc.270.44.26292 [DOI] [PubMed] [Google Scholar]
- 39.Reyes H, Reisz-Porszasz S and Hankinson O (1992) Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 256, 1193–1195 10.1126/science.256.5060.1193 [DOI] [PubMed] [Google Scholar]
- 40.Adelman DM, Maltepe E and Simon MC (1999) Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev 13, 2478–2483 10.1101/gad.13.19.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaud JL, DeRossi C, May NR, Holdener BC and Fan CM (2000) ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mech. Dev 90, 253–261 10.1016/S0925-4773(99)00328-7 [DOI] [PubMed] [Google Scholar]
- 42.Sonnenfeld M, Ward M, Nystrom G, Mosher J, Stahl S and Crews S (1997) The Drosophila tango gene encodes a bHLH-PAS protein that is orthologous to mammalian Arnt and controls CNS midline and tracheal development. Development 124, 4571–4582 10.1242/dev.124.22.4571 [DOI] [PubMed] [Google Scholar]
- 43.Brigidi GS, Hayes MGB, Delos Santos NP, Hartzell AL, Texari L, Lin P-A et al. (2019) Genomic decoding of neuronal depolarization by stimulus-specific NPAS4 heterodimers. Cell 179, 373–391.e27 10.1016/j.cell.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Oñate SA, Tsai SY, Tsai MJ and O’Malley BW (1995) Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270, 1354–1357 10.1126/science.270.5240.1354 [DOI] [PubMed] [Google Scholar]
- 45.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY et al. (1997) AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277, 965–968 10.1126/science.277.5328.965 [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L et al. (1997) Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90, 569–580 10.1016/S0092-8674(00)80516-4 [DOI] [PubMed] [Google Scholar]
- 47.Hong H, Kohli K, Trivedi A, Johnson DL and Stallcup MR (1996) GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc. Natl Acad. Sci. U.S.A 93, 4948–4952 10.1073/pnas.93.10.4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Gomes PJ and Chen JD (1997) RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl Acad. Sci. U.S.A 94, 8479–8484 10.1073/pnas.94.16.8479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR and Frail DE (1998) A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J. Biol. Chem 273, 27645–27653 10.1074/jbc.273.42.27645 [DOI] [PubMed] [Google Scholar]
- 50.Takeshita A, Cardona GR, Koibuchi N, Suen CS and Chin WW (1997) TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J. Biol. Chem 272, 27629–27634 10.1074/jbc.272.44.27629 [DOI] [PubMed] [Google Scholar]
- 51.Voegel JJ, Heine MJ, Zechel C, Chambon P and Gronemeyer H (1996) TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15, 3667–3675 10.1002/j.1460-2075.1996.tb00736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK et al. (1997) The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387, 677–684 10.1038/42652 [DOI] [PubMed] [Google Scholar]
- 53.Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H et al. (2007) Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature 446, 562–566 10.1038/nature05683 [DOI] [PubMed] [Google Scholar]
- 54.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA et al. (1997) Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389, 194–198 10.1038/38304 [DOI] [PubMed] [Google Scholar]
- 55.Murre C (2019) Helix-loop-helix proteins and the advent of cellular diversity: 30 years of discovery. Genes Dev 33, 6–25 10.1101/gad.320663.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor BL and Zhulin IB (1999) PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev 63, 479–506 10.1128/MMBR.63.2.479-506.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Möglich A, Ayers RA and Moffat K (2009) Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17, 1282–1294 10.1016/j.str.2009.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellenberger T, Fass D, Arnaud M and Harrison SC (1994) Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev 8, 970–980 10.1101/gad.8.8.970 [DOI] [PubMed] [Google Scholar]
- 59.Ma PC, Rould MA, Weintraub H and Pabo CO (1994) Crystal structure of MyoD bHLH domain-DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell 77, 451–459 10.1016/0092-8674(94)90159-7 [DOI] [PubMed] [Google Scholar]
- 60.Tuckerman JR, Gonzalez G and Gilles-Gonzalez MA (2001) Complexation precedes phosphorylation for two-component regulatory system FixL/FixJ of sinorhizobium meliloti. J. Mol. Biol 308, 449–455 10.1006/jmbi.2001.4591 [DOI] [PubMed] [Google Scholar]
- 61.Christie JM, Swartz TE, Bogomolni RA and Briggs WR (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J 32, 205–219 10.1046/j.1365-313X.2002.01415.x [DOI] [PubMed] [Google Scholar]
- 62.Huang ZJ, Curtin KD and Rosbash M (1995) PER protein interactions and temperature compensation of a circadian clock in Drosophila. Science 267, 1169–1172 10.1126/science.7855598 [DOI] [PubMed] [Google Scholar]
- 63.Bae K, Lee C, Sidote D, Chuang KY and Edery I (1998) Circadian regulation of a Drosophila homolog of the mammalian clock gene: PER and TIM function as positive regulators. Mol. Cell. Biol 18, 6142–6151 10.1128/MCB.18.10.6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crosthwaite SK, Dunlap JC and Loros JJ (1997) Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276, 763–769 10.1126/science.276.5313.763 [DOI] [PubMed] [Google Scholar]
- 65.Vosshall LB, Price JL, Sehgal A, Saez L and Young MW (1994) Block in nuclear localization of period protein by a second clock mutation, timeless. Science 263, 1606–1609 10.1126/science.8128247 [DOI] [PubMed] [Google Scholar]
- 66.Morais Cabral JH, Lee A, Cohen SL, Chait BT, Li M and Mackinnon R (1998) Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell 95, 649–655 10.1016/S0092-8674(00)81635-9 [DOI] [PubMed] [Google Scholar]
- 67.Mimura J, Ema M, Sogawa K and Fujii-Kuriyama Y (1999) Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev 13, 20–25 10.1101/gad.13.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans BR, Karchner SI, Allan LL, Pollenz RS, Tanguay RL, Jenny MJ et al. (2008) Repression of aryl hydrocarbon receptor (AHR) signaling by AHR repressor: role of DNA binding and competition for AHR nuclear translocator. Mol. Pharmacol 73, 387–398 10.1124/mol.107.040204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar MB and Perdew GH (1999) Nuclear receptor coactivator SRC-1 interacts with the Q-rich subdomain of the AhR and modulates its transactivation potential. Gene Expr 8, 273–286 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6157383/ [PMC free article] [PubMed] [Google Scholar]
- 70.Beischlag TV, Wang S, Rose DW, Torchia J, Reisz-Porszasz S, Muhammad K et al. (2002) Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Mol. Cell. Biol 22, 4319–4333 10.1128/MCB.22.12.4319-4333.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor RT, Wang F, Hsu EL and Hankinson O (2009) Roles of coactivator proteins in dioxin induction of CYP1A1 and CYP1B1 in human breast cancer cells. Toxicol. Sci 107, 1–8 10.1093/toxsci/kfn217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai C-H, Li C-H, Liao P-L, Cheng Y-W, Lin C-H, Huang S-H et al. (2015) NcoA2-dependent inhibition of HIF-1α activation is regulated via AhR. Toxicol. Sci 148, 517–530 10.1093/toxsci/kfv199 [DOI] [PubMed] [Google Scholar]
- 73.Kozak KR, Abbott B and Hankinson O (1997) ARNT-deficient mice and placental differentiation. Dev. Biol 191, 297–305 10.1006/dbio.1997.8758 [DOI] [PubMed] [Google Scholar]
- 74.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA and Simon MC (1997) Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403–407 10.1038/386403a0 [DOI] [PubMed] [Google Scholar]
- 75.Huang X, Powell-Coffman JA and Jin Y (2004) The AHR-1 aryl hydrocarbon receptor and its co-factor the AHA-1 aryl hydrocarbon receptor nuclear translocator specify GABAergic neuron cell fate in C. elegans. Development 131, 819–828 10.1242/dev.00959 [DOI] [PubMed] [Google Scholar]
- 76.Weng F-J, Garcia RI, Lutzu S, Alviña K, Zhang Y, Dushko M et al. (2018) Npas4 is a critical regulator of learning-induced plasticity at mossy fiber-CA3 synapses during contextual memory formation. Neuron 97, 1137–1152.e5 10.1016/j.neuron.2018.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spiegel I, Mardinly AR, Gabel HW, Bazinet JE, Couch CH, Tzeng CP et al. (2014) Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157, 1216–1229 10.1016/j.cell.2014.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sekine H, Mimura J, Yamamoto M and Fujii-Kuriyama Y (2006) Unique and overlapping transcriptional roles of arylhydrocarbon receptor nuclear translocator (Arnt) and Arnt2 in xenobiotic and hypoxic responses. J. Biol. Chem 281, 37507–37516 10.1074/jbc.M606910200 [DOI] [PubMed] [Google Scholar]
- 79.Edwards HE, Souder JP and Gorelick DA (2022) Hemato-vascular specification requires arnt1 and arnt2 genes in zebrafish embryos. BioRxiv https://www.biorxiv.org/content/10.1101/2022.01.04.474920v1 https://doi.org/10.1101/2022.01.04.474920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sogawa K, Nakano R, Kobayashi A, Kikuchi Y, Ohe N, Matsushita N et al. (1995) Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation. Proc. Natl Acad. Sci. U.S.A 92, 1936–1940 10.1073/pnas.92.6.1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Card PB, Erbel PJA and Gardner KH (2005) Structural basis of ARNT PAS-B dimerization: use of a common beta-sheet interface for hetero- and homodimerization. J. Mol. Biol 353, 664–677 10.1016/j.jmb.2005.08.043 [DOI] [PubMed] [Google Scholar]
- 82.Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A and Pongratz I (2003) The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc. Natl Acad. Sci. U.S.A 100, 6517–6522 10.1073/pnas.1136688100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rüegg J, Swedenborg E, Wahlström D, Escande A, Balaguer P, Pettersson K et al. (2008) The transcription factor aryl hydrocarbon receptor nuclear translocator functions as an estrogen receptor beta-selective coactivator, and its recruitment to alternative pathways mediates antiestrogenic effects of dioxin. Mol. Endocrinol 22, 304–316 10.1210/me.2007-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wright CW and Duckett CS (2009) The aryl hydrocarbon nuclear translocator alters CD30-mediated NF-kappaB-dependent transcription. Science 323, 251–255 10.1126/science.1162818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vogel CFA, Sciullo E, Li W, Wong P, Lazennec G and Matsumura F (2007) Relb, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol 21, 2941–2955 10.1210/me.2007-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishihara Y, Kado SY, Hoeper C, Harel S and Vogel CFA (2019) Role of NF-kB RelB in aryl hydrocarbon receptor-mediated ligand specific effects. Int. J. Mol. Sci 20, 2652 10.3390/ijms20112652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH and Sonenshein GE (2000) The relA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 19, 5498–5506 10.1038/sj.onc.1203945 [DOI] [PubMed] [Google Scholar]
- 88.Vogel CFA, Sciullo E and Matsumura F (2007) Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun 363, 722–726 10.1016/j.bbrc.2007.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bracken CP, Whitelaw ML and Peet DJ (2005) Activity of hypoxia-inducible factor 2alpha is regulated by association with the NF-kappaB essential modulator. J. Biol. Chem 280, 14240–14251 10.1074/jbc.M409987200 [DOI] [PubMed] [Google Scholar]
- 90.Tian Y, Ke S, Denison MS, Rabson AB and Gallo MA (1999) Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J. Biol. Chem 274, 510–515 10.1074/jbc.274.1.510 [DOI] [PubMed] [Google Scholar]
- 91.Huang G and Elferink CJ (2012) A novel nonconsensus xenobiotic response element capable of mediating aryl hydrocarbon receptor-dependent gene expression. Mol. Pharmacol 81, 338–347 10.1124/mol.111.075952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reiners JJ and Clift RE (1999) Aryl hydrocarbon receptor regulation of ceramide-induced apoptosis in murine hepatoma 1c1c7 cells. A function independent of aryl hydrocarbon receptor nuclear translocator. J. Biol. Chem 274, 2502–2510 10.1074/jbc.274.4.2502 [DOI] [PubMed] [Google Scholar]
- 93.Weiss C, Faust D, Schreck I, Ruff A, Farwerck T, Melenberg A et al. (2008) TCDD deregulates contact inhibition in rat liver oval cells via Ah receptor, JunD and cyclin A. Oncogene 27, 2198–2207 10.1038/sj.onc.1210859 [DOI] [PubMed] [Google Scholar]
- 94.Tanguay RL, Andreasen E, Heideman W and Peterson RE (2000) Identification and expression of alternatively spliced aryl hydrocarbon nuclear translocator 2 (ARNT2) cDNAs from zebrafish with distinct functions. Biochim. Biophys. Acta 1494, 117–128 10.1016/S0167-4781(00)00225-6 [DOI] [PubMed] [Google Scholar]
- 95.Rowatt AJ, DePowell JJ and Powell WH (2003) ARNT gene multiplicity in amphibians: characterization of ARNT2 from the frog Xenopus laevis. J. Exp. Zool. B Mol. Dev. Evol 300, 48–57 10.1002/jez.b.45 [DOI] [PubMed] [Google Scholar]
- 96.Luecke-Johansson S, Gralla M, Rundqvist H, Ho JC, Johnson RS, Gradin K et al. (2017) A molecular mechanism to switch the aryl hydrocarbon receptor from a transcription factor to an E3 ubiquitin ligase. Mol. Cell. Biol 37, e00630–16 10.1128/MCB.00630-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reixachs-Solé M and Eyras E (2022) Uncovering the impacts of alternative splicing on the proteome with current omics techniques. Wiley Interdiscip. Rev. RNA, e1707 https://wires.onlinelibrary.wiley.com/doi/10.1002/wrna.1707 https://doi.org/10.1002/wrna.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kastner B, Will CL, Stark H and Lührmann R (2019) Structural insights into nuclear pre-mRNA splicing in higher eukaryotes. Cold Spring Harb. Perspect. Biol 11, a032417 10.1101/cshperspect.a032417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee Y and Rio DC (2015) Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem 84, 291–323 10.1146/annurev-biochem-060614-034316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pai AA and Luca F (2019) Environmental influences on RNA processing: biochemical, molecular and genetic regulators of cellular response. Wiley Interdiscip. Rev. RNA 10, e1503 10.1002/wrna.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Öther-Gee Pohl S and Myant KB (2022) Alternative RNA splicing in tumour heterogeneity, plasticity and therapy. Dis. Model. Mech 15, dmm049233 10.1242/dmm.049233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Titus MB, Chang AW and Olesnicky EC (2021) Exploring the diverse functional and regulatory consequences of alternative splicing in development and disease. Front. Genet 12, 775395 10.3389/fgene.2021.775395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corbett AH (2018) Post-transcriptional regulation of gene expression and human disease. Curr. Opin. Cell Biol 52, 96–104 10.1016/j.ceb.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chabot B and Shkreta L (2016) Defective control of pre-messenger RNA splicing in human disease. J. Cell Biol 212, 13–27 10.1083/jcb.201510032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA et al. (1991) Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252, 954–958 10.1126/science.1852076 [DOI] [PubMed] [Google Scholar]
- 106.Kewley RJ and Whitelaw ML (2005) Phosphorylation inhibits DNA-binding of alternatively spliced aryl hydrocarbon receptor nuclear translocator. Biochem. Biophys. Res. Commun 338, 660–667 10.1016/j.bbrc.2005.08.073 [DOI] [PubMed] [Google Scholar]
- 107.Gardella KA, Muro I, Fang G, Sarkar K, Mendez O and Wright CW (2016) Aryl hydrocarbon receptor nuclear translocator (ARNT) isoforms control lymphoid cancer cell proliferation through differentially regulating tumor suppressor p53 activity. Oncotarget 7, 10710–10722 10.18632/oncotarget.7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooper AM, Nutter CA, Kuyumcu-Martinez MN and Wright CW (2022) Alternative splicing of the aryl hydrocarbon receptor nuclear translocator (ARNT) is regulated by RBFOX2 in lymphoid malignancies. Mol. Cell. Biol 42, e0050321 10.1128/mcb.00503-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bourner LA, Muro I, Cooper AM, Choudhury BK, Bailey AO, Russell WK et al. (2022) Ahr promotes phosphorylation of ARNT isoform 1 in human T cell malignancies as a switch for optimal AhR activity. Proc. Natl Acad. Sci. U.S.A 119, e2114336119 10.1073/pnas.2114336119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ishqi HM, Ur Rehman S, Sarwar T, Husain MA and Tabish M (2016) Identification of differentially expressed three novel transcript variants of mouse ARNT gene. IUBMB Life 68, 122–135 10.1002/iub.1464 [DOI] [PubMed] [Google Scholar]
- 111.Shin J and Kim J (2013) Novel alternative splice variants of chicken NPAS3 are expressed in the developing central nervous system. Gene 530, 222–228 10.1016/j.gene.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 112.Yan J, Ma Z, Xu X and Guo A-Y (2014) Evolution, functional divergence and conserved exon-intron structure of bHLH/PAS gene family. Mol. Genet. Genomics 289, 25–36 10.1007/s00438-013-0786-0 [DOI] [PubMed] [Google Scholar]
- 113.Weng W, Nagai H, Hamidi S and Sheng G (2020) NPAS4L is involved in avian hemangioblast specification. Haematologica 105, 2647–2650 10.3324/haematol.2019.239434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reischauer S, Stone OA, Villasenor A, Chi N, Jin S-W, Martin M et al. (2016) Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature 535, 294–298 10.1038/nature18614 [DOI] [PubMed] [Google Scholar]
- 115.Stainier DY, Weinstein BM, Detrich HW, Zon LI and Fishman MC (1995) Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121, 3141–3150 10.1242/dev.121.10.3141 [DOI] [PubMed] [Google Scholar]
- 116.Keith B, Adelman DM and Simon MC (2001) Targeted mutation of the murine arylhydrocarbon receptor nuclear translocator 2 (Arnt2) gene reveals partial redundancy with Arnt. Proc. Natl Acad. Sci. U.S.A 98, 6692–6697 10.1073/pnas.121494298 [DOI] [PMC free article] [PubMed] [Google Scholar]


