Abstract
BACKGROUND
Herein, the authors describe the successful utilization of 5-aminolevulinic acid (5-ALA) and the first case of GammaTile cesium-131 therapy in a pediatric patient with recurrent high-grade glioma. 5-ALA was utilized to optimize gross-total resection prior to GammaTile implantation. After conversion to an equivalent dose in 2-Gy fractions (EQD2), a composite was made of the GammaTile dose with the initial external beam radiotherapy. Two hypothetical plans consisting of a standard hypofractionated strategy for glioma reirradiation and a CyberKnife plan using GammaTile’s planning target volume were developed and likewise underwent EQD2 conversion and composite plan generation with the initial radiotherapy.
OBSERVATIONS
5-ALA was useful in achieving gross-total resection with no acute toxicity from the surgery or GammaTile irradiation. When compared with the hypothetical composite doses, GammaTile’s composite, axium point dose (D0.03cc) to the brainstem was 32.9 Gy less than the hypofractionated and the CyberKnife composite plans at 38.7 Gy and 40.2 Gy, respectively. The right hippocampus demonstrated a substantially reduced composite plan dose with GammaTile with a D0.03cc of 62.4 Gy versus 71.7 and 80.7 Gy for the hypofractionated and CyberKnife composite plans, respectively.
LESSONS
Utilization of 5-ALA and GammaTile therapy yielded clinically superior tumor debulking and effective radiotherapy dose localization with sparing of organs at risk, respectively.
Keywords: GammaTile, 5-aminolevulinic acid, 5-ALA, high-grade glioma, HGG, brachytherapy, pediatric radiotherapy, recurrent glioma
ABBREVIATIONS: DVH = dose-volume histogram, EBRT = external beam radiotherapy, EQD = equivalent dose in 2-Gy fractions, FDA = Food and Drug Administration, HGG = high-grade glioma, MRI = magnetic resonance imaging, OAR = organ at risk, PpIX = protoporphyrinogen IX, PTV = planning target volume, RTOG = Radiation Therapy Oncology Group, TMZ = temozolomide, WHO = World Health Organization, 5-ALA = 5-aminolevulinic acid
High-grade gliomas (HGGs) are intracranial neoplasms that have poor outcomes in both the adult and pediatric populations despite multimodal treatments.1,2 Modern research efforts have focused on making advances in surgery, radiotherapy, and chemotherapy to help improve outcomes. Many advancements have been made to improve radiotherapy, which is largely delivered via external beam radiotherapy (EBRT). However, radiation necrosis remains a significant concern for reirradiation in areas of recurrent disease. This is particularly true in the pediatric population, which faces not only conventional reirradiation risks, such as secondary neoplasms and cognitive decline, but also an elevated risk of radiation necrosis.3–5
In pursuit of safer alternatives, there has been recently renewed interest in intracranial brachytherapy, specifically with the development of GammaTile (GT Medical Technologies). GammaTile is a brachytherapy platform that originally received Food and Drug Administration (FDA) clearance with no age restriction in 2018 for the treatment of recurrent brain neoplasms and subsequent clearance for any newly diagnosed malignant brain tumors (primary or metastatic). Each tile is 2-cm × 2-cm × 4-mm thick and consists of a biocompatible collagen sponge containing 4 cesium-131 radiation-emitting sources.3 GammaTile therapy differs from alternative intracranial brachytherapy strategies in 2 ways. First, the incorporation of cesium sources within the thickness of the collagen matrix provides a barrier between the radioisotope sources and brain parenchyma. In effect, the barrier diminishes the radio-necrosis risk by reducing the potential “hot spots” that were characteristic of former brachytherapy.6 Second, suspending cesium-131 sources within the collagen scaffolding permits homogeneous spacing of the sources both within and between adjoining tiles. This construct facilitates straightforward application of the sources evenly within the operative cavity, allowing for the administration of 60 Gy to 5-mm depth with homogeneity superior to that in former strategies.7 Although initial interest in GammaTile was limited to application in resected brain metastasis, emerging single-institution data have demonstrated long-term efficacy and safety in the reirradiation of World Health Organization (WHO) grade 3 and 4 gliomas. In 1 instance, the application of GammaTile in such patients with more than 2 years’ mean follow-up resulted in a 1-year local control rate of 81%, a median overall survival of 20 months, and no radiotherapy-related toxicity.8
In addition to GammaTile, neurosurgeons have increasingly utilized 5-aminolevulinic acid (5-ALA) to improve maximal safe resection when anatomically feasible. 5-ALA, approved by the FDA in 2017, is an endogenous, naturally occurring product of succinyl-CoA metabolism and is normally converted intracellularly into protoporphyrinogen IX (PpIX), a fluorescent intermediate in the heme synthesis pathway.9,10 For reasons not entirely understood, PpIX has been observed to accumulate in HGGs but not in low-grade gliomas or normal brain tissue. This offers neurosurgeons real-time assistance in the intraoperative identification of malignant tissue. Although 5-ALA has been increasingly utilized in the management of adult HGG, its use in pediatric populations is not widely established.11
We present the first documented application of GammaTile in a pediatric patient for a recurrent HGG while also highlighting the beneficial utilization of 5-ALA. By comparing the patient’s composite GammaTile dose to hypothetical EBRT plans, we concluded that GammaTile was able to deliver a more intense and evenly distributed dose to the surgical bed while reducing the dose to critical organs at risk (OARs).
Illustrative Case
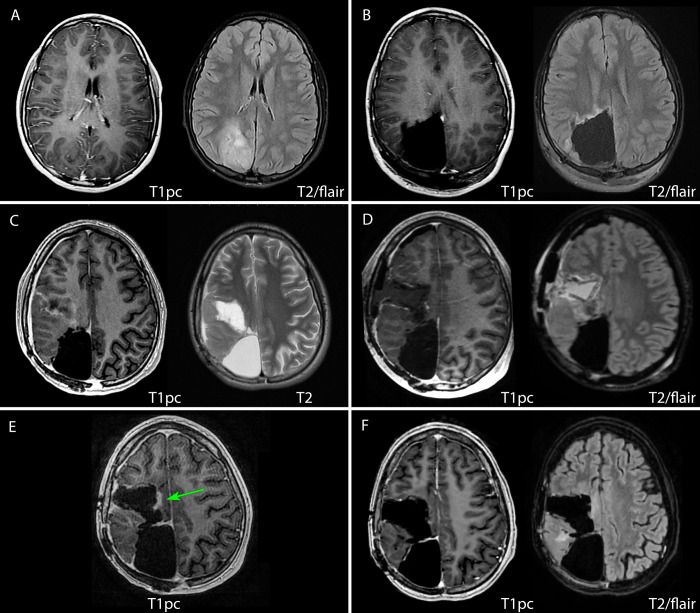
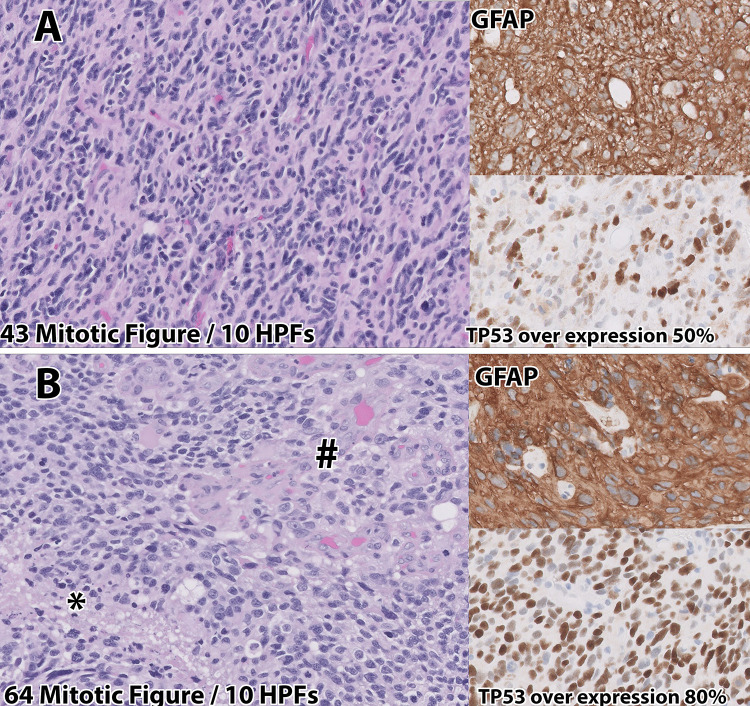
A 13-year-old male presented to our institution with new-onset seizures, altered mental status, and headaches. He underwent magnetic resonance imaging (MRI) that demonstrated an intra-axial mass measuring approximately 5.8 cm × 3.6 cm × 4.6 cm in the right occipitoparietal lobe (Fig. 1A). The patient underwent a right craniotomy with maximal safe resection, no evidence of residual disease, and minimal postoperative changes on MRI (Fig. 1B). Surgery resulted in permanent left hemiparesis. Histological evaluation of the resected lesion demonstrated a WHO grade 3 anaplastic astrocytoma according to the 2016 criteria12 (Fig. 2A). Molecular evaluation revealed a histone H3–3A G34R mutation, mutations in ATRX and TP53, PDGFRA amplification, and MGMT promoter hypermethylation. The patient then underwent chemoradiation with 59.4 Gy in 33 fractions with concurrent and adjuvant temozolomide (TMZ) as per the Children’s Oncology Group (study no. ACNS0822).13 Radiation delivered to the resection cavity expanded by 15 mm to anatomy for the clinical target volume and then by 5 mm to the planning target volume (PTV). Contouring was performed in Velocity v4.1 (Varian Medical Systems), and planning was performed in Eclipse v15.5 (Varian Medical Systems). Figure 3 demonstrates the resulting dose cloud from this PTV (Fig. 3A and B) and a dose-volume histogram (DVH) of nearby pertinent OARs (Fig. 3C), which showed a moderate dose to the right hippocampus.
FIG. 1.
Magnetic resonance imaging (MRI) demonstrating the course of the patient’s disease. Preoperatively, the initial G3 glioma (A) demonstrated increased signal on T2 fluid-attenuated inversion recovery (FLAIR) sequences in the right occipital lobe with no postcontrast T1 enhancement. Immediate postoperative imaging (B) did not demonstrate any clear residual disease with mild postsurgical T2 FLAIR signal along the resection cavity. Twenty-seven months following initial glioma resection, and several months following initial recurrence with a second resection (not pictured), imaging demonstrated yet another, second recurrence. Preoperatively (C), the lesion demonstrated peripheral contrast enhancement of central necrosis not seen on previous imaging. Immediate postoperative imaging (D) demonstrates a thin area of residual enhancement, possibly due to hemorrhage within the resection cavity, and the GammaTiles are most easily seen lining the cavity on the axial T2 FLAIR sequence. Approximately 5 months following this latest resection, imaging demonstrated nodular enhancement (green arrow) of the medial margin of the resection cavity (E) to which Gamma Knife radiosurgery was applied. At 3 months following Gamma Knife radiosurgery, the most recent imaging (F) exhibited no evidence of disease, edema, or radio-necrosis. pc = postcontrast images.
FIG. 2.
Histology from original right occipital (A) lesion and the second tumor recurrence 27 months later (B). Histologically, the initial lesion was consistent with a grade 3 anaplastic astrocytoma using World Health Organization (WHO) 2016 criteria, with GFAP expression and TP53 overexpression by neoplastic cells. The patient’s second recurrent lesion (B) histologically and by molecular evaluation met criteria for the newly recognized entity diffuse hemispheric glioma with GFAP and increased TP53 overexpression, increased mitotic figures, areas of necrosis (*) and vascular proliferation (#). HPF = high-power field.
FIG. 3.
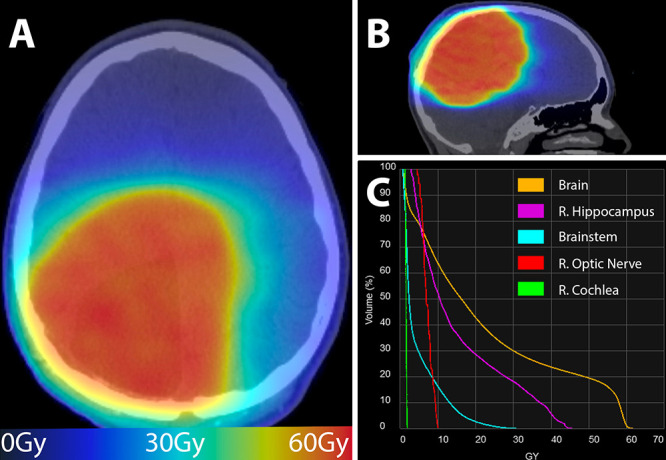
Adjuvant dose cloud (A and B) after WHO grade 3 astrocytoma resection and corresponding dose-volume histogram (DVH) (C). The axial (A) and sagittal (B) dose cloud displayed over the simulation computed tomography (CT) shows that a large volume of brain parenchyma received approximately 60 Gy. This is reflected in the DVH with the right hippocampus, brainstem, and overall brain tissue receiving a significant amount of dose approximately 27 months prior to the most recent disease recurrence. The resulting DVH shows very little dose to the optic structures and brainstem but a moderate dose to the underlying right hippocampus. The color gradient corresponds to the dose according to bottom line ribbon. R. = right.
After approximately 1 year of maintenance TMZ, a focal, gadolinium-enhancing nodularity was noted along the prior resection bed on MRI (not pictured). The patient underwent subsequent repeat resection, which resulted in increased left-sided weakness, and pathology was consistent with the prior glioma. The patient then received salvage oral everolimus and dasatinib.14 He remained without evidence of recurrence clinically and radiographically until 27 months following the initial craniotomy, at which time a new 5.7-cm × 3.6-cm × 4.5-cm mass was appreciated anterior to the initial tumor bed on MRI (Fig. 1C). This new, centrally necrotic lesion demonstrated peripheral enhancement with surrounding edema consistent radiographically with an HGG.
In light of the short intervals between each recurrence, the team believed that any surgical intervention would necessitate adjuvant radiotherapy to have durable benefit. Considering both the substantial overall dose volume and the specific doses delivered to OARs during the patient’s initial EBRT, we concluded that any meaningful EBRT reirradiation strategy would present a high risk of radiotherapy-related morbidity. Given our institution’s experience treating operable, recurrent HGG with GammaTile, we foresaw this treatment strategy as the best means of delivering a significant dose to the patient while safely limiting the dose to OARs. Furthermore, analysis of the preoperative MRI suggested an area of central necrosis within the lesion, indicating that the creation of a suitable cavity in which to place the GammaTiles was feasible. We opted to utilize 5-ALA to potentially allow greater gross-total resection of the tumor without damaging normal brain parenchyma and causing further motor morbidity.
The tumor surface of enhancing disease was estimated to be 74 cm2 via preoperative imaging. Taking into consideration immediate contraction of the site and no need for coverage along the roof of the surgical bed, an estimated 8–9 tiles were deemed necessary (1 tile per 4-cc surface area to achieve 60 Gy at a 5-mm depth). The patient was given an oral dose of 5-ALA, per the manufacturer’s instructions. Abnormal tissue appreciated at the brain surface was sent for frozen-section pathology and was consistent with HGG. After frozen-section pathology confirmation that the pinkish-red tissue under fluorescence was disease, further resection was aided by examination of the fluorescence of 5-ALA. After the removal of all fluorescent tissue, a clean resection cavity remained, which permitted uninterrupted abutment of tiles along the resection cavity surface. GammaTile placement took approximately 3.5 minutes, and all 9 tiles were utilized, 2 of which were cut in half to aid in complete coverage. Final pathology was consistent with a WHO grade 4 glioma (Fig. 2B), specifically the newly recognized entity diffuse hemispheric glioma, H3 G34-mutant using the 2021 WHO criteria.15
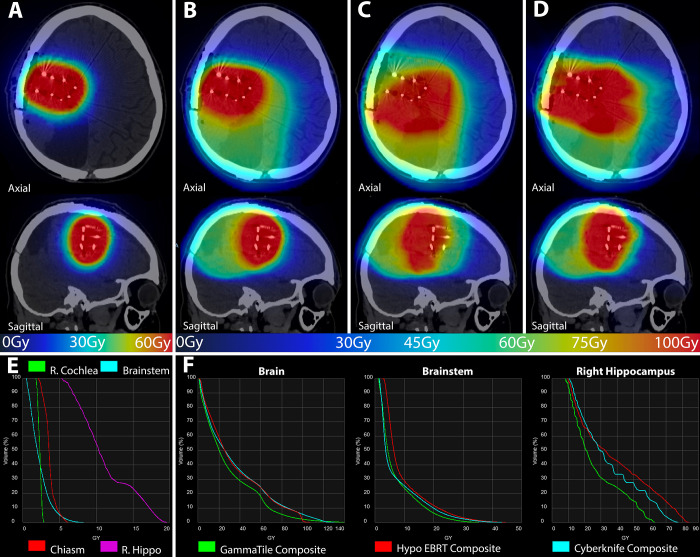
On postoperative day 1, the patient underwent MRI, which demonstrated no residual enhancement and tiles adhered to the cavity surface (Fig. 1D). The patient’s postoperative course was unremarkable, and he did not experience any adverse reaction to the 5-ALA. To manage post–5-ALA photosensitivity,16 the patient remained in a darkened room for 48 hours after surgery without issue. Three weeks after surgery, he remained asymptomatic and had regained some of the facial symmetry lost prior to surgery. In particular, he did not have any significant fatigue or skin irritation from his GammaTile implantation. Postimplant dosimetry was completed in Eclipse and Brachyvision 16.15.49 (Varian Medical Systems) with the PTV consisting of a 5-mm expansion on the resection cavity surface that had disease contact. The labeled sources were evenly distributed throughout the resection cavity, resulting in a mean dose to 90% of normal brain volume (D90) of 118% and a 100% isodose volume of 60 Gy (V10060Gy) of 98%. To better determine the dose experienced by the nearby OARs, this dose cloud was transferred to Velocity and converted to an equivalent dose in 2-Gy fractions (EQD2). As shown in Fig. 4A, the resulting EQD2 dose cloud and corresponding DVH (Fig. 4E) demonstrate conformality to the resection cavity with a sharp dose falloff and minimal dose to most concerning structures, especially with sparing of the right hippocampus.
FIG. 4.
GammaTile dose cloud converted to equivalent dose in 2-Gy fractions (EQD2) (A) to resected recurrent high-grade glioma (HGG) resection cavity and corresponding DVH (E). The axial and sagittal (A) dose cloud displayed over the simulation CT shows the 60-Gy prescription dose conforms well to the resection cavity. There is minimal dose to the brainstem, optic structures, and right cochlea, with sparing of the right hippocampus. The composite dose cloud of the initial external beam radiotherapy (EBRT) with the delivered GammaTile dose (B), the composite dose cloud of the initial EBRT with a hypothetical hypofractionated EBRT volume (C), and the composite dose cloud of the initial EBRT with a hypothetical CyberKnife plan attempting the same volume as GammaTile (D) are also shown, with all doses converted to EQD2. Panels B–D demonstrate that the GammaTile plan was able to deliver a greater amount of conformal dose to the recurrent disease resection bed, while at the same time decreasing the dose to both untreated and previously treated healthy tissue. The color gradient corresponds to the dose according to bottom line ribbon for panels A–D. Panel F demonstrates DVHs for normal brain parenchyma, the brainstem, and the right hippocampus from the composite of the patient’s original EBRT and the delivered GammaTile dose (green), the hypothetical standard hypofractionated EBRT dose (red), and the hypothetical standard fractionation CyberKnife dose (blue). All composite doses were converted to EQD2, which demonstrates that the GammaTile composite delivers less volume at most doses to the brain. The GammaTile composite plan also results in a decreased maximal dose to the brainstem and a significant decrease in both maximum and average dose to the right hippocampus. Hippo = hippocampus; Hypo = hypofractionated.
To see how alternative EBRT strategies would have fared compared with GammaTile, 2 hypothetical plans were constructed. The first plan was a standard hypofractionated strategy for HGG reirradiation, such as that used in Radiation Therapy Oncology Group (RTOG) 120517 to 35 Gy in 10 fractions (10-mm expansion to anatomy for computed tomography, subsequent 5-mm expansion to PTV). We also developed a plan on CyberKnife using the same PTV as GammaTile to make a more volumetrically equivalent comparison, taking the volume to 59.4 Gy in 33 fractions. Contouring for the hypofractionated volume and the CyberKnife volume was completed in Velocity, while the plans were developed in Eclipse and Precision 3.1.0.0 (Accuray, Inc.), respectively. These generated dose volumes, as well as the already delivered initial 59.4-Gy volume, were then transferred to Velocity and converted to EQD2 dose clouds. To compare the overall dose coverage, volume/dose of treatment overlap, and total dose delivered to OARs, 3 separate EQD2 composites were created in Velocity as follows: the initial delivered EBRT and delivered GammaTile dose (Fig. 4B), the initial delivered EBRT and hypothetical hypofractionated EBRT plan (Fig. 4C), and the initial delivered EBRT and hypothetical CyberKnife plan (Fig. 4D).
The composite GammaTile dose cloud (Fig. 4B) demonstrated greater conformality to the resection bed with less moderate/low-dose overlap with the prior EBRT dose compared with both hypothetical plans (Fig. 4C and D). Figure 4F demonstrates the composite DVH of the 3 plans for the most relevant OARs. In the case of optic structures, none of the 3 composite plans contributed a significant dose. Aside from tissue immediately adjacent to the resection cavity, the GammaTile composite plan delivered less dose per volume to normal brain parenchyma in the range of 5–20 Gy (Table 1). The maximum point dose (D0.03cc) to the brainstem was 32.9 Gy from GammaTile compared with both the hypofractionated composite plan and the CyberKnife composite plan at 38.7 Gy and 40.2 Gy, respectively. The most significant difference in dose was in the right hippocampus where the maximum dose (D0.03cc) was 62.4 Gy in the GammaTile composite, 71.7 Gy in the hypofractionated EBRT composite, and 80.7 Gy in the CyberKnife composite, with further large differences in the mean dose. Additional dosimetric factors are summarized in Table 1.
TABLE 1.
Composite dose to organs at risk
| Organ at Risk | Parameter | Composite Plan (Gy) |
||
|---|---|---|---|---|
| GammaTile | Hypo EBRT | CyberKnife | ||
| Brain |
D5% |
81.9 |
97.7 |
102.0 |
| Brain |
D30% |
38.1 |
49.0 |
49.8 |
| Brain |
D50% |
20.2 |
25.3 |
25.4 |
| Brain |
Mean |
29.6 |
35.6 |
35.9 |
| Brainstem |
D0.03cc |
32.9 |
41.6 |
41.9 |
| Brainstem |
Mean |
7.1 |
9.6 |
7.5 |
| Rt hippocampus |
D0.03cc |
62.4 |
71.7 |
80.7 |
| Rt hippocampus |
Mean |
26.7 |
38.2 |
34.8 |
| Rt hippocampus |
D100% |
8.2 |
9.5 |
10.5 |
| Total hippocampus | Mean | 18.4 | 25.8 | 23.3 |
Hypo = hypofractionated; Rt = right.
Following the latest resection, the patient was started on procarbazine, lomustine, and vincristine until approximately 5 months later, at which time he was admitted for neutropenic fever. Magnetic resonance imaging demonstrated nodular enhancement to the medial margin of the resection cavity, as highlighted in Fig. 1E, representing disease progression versus treatment effect. Given the small volume of this area, it was determined that Gamma Knife radiosurgery could be applied with minimal risk. The nodularity was treated to 16 Gy, and the patient has since continued on trimetazidine, Avastin, and irinotecan. At 8 months after surgery, his most recent MRI exhibited no evidence of disease, edema, or radionecrosis (Fig. 1F). At the time of publication, the patient had no functional deficits from his recent craniotomy and had improved facial symmetry. Given the lack of disease in his most recent MRI and lack of symptomatic progression, we believe that the enhancing nodularity was a treatment effect rather than actual disease progression.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
The precision of GammaTile’s radiation was made possible by the inherently sharp dose falloff that is a general characteristic of brachytherapy sources.18 In conjunction with GammaTile’s superior conformability and dose intensity, this attribute permitted considerable radiation localization to targeted parenchyma while minimizing damage to sensitive surrounding structures (Fig. 4). These findings were further supported when we compared GammaTile’s OAR radiation with that of EBRT, with the former displaying a considerable reduction in dose at all volumes compared with the latter (Table 1). Most notable was the dose sparing of the right hippocampus, especially since excess radiation to this region, particularly in the developing brains of children, is known to impact memory and cognition.19 At 8.2 Gy, only the GammaTile composite was able to keep the right hippocampal dose covering 100% of the volume (D100) under 9 Gy; at 18.4 Gy, GammaTile was the closest of all modalities to the objective Dmax of 16 Gy outlined by RTOG 0993.20 In all likelihood, the EBRT plans would have brought a delayed, but significant, impact on quality of life because of the dose that the right hippocampus would have received. This is suggested by recent analysis of a randomized trial of pediatric medulloblastoma where, for every increase of 1 Gy in mean hippocampal dose, the odds of a decline in composite processing speed and associative memory increased by 25%.21 Another dosimetric objective that demonstrated a significant difference was the maximum and moderate dose to the brainstem (Table 1). Although all 3 composite plans achieved maximum doses to the brainstem within tissue tolerance, GammaTile’s approximate 9-Gy decrease in maximum dose compared with the other composites allowed greater potential for future intracranial radiotherapy, if needed.
In addition to dosimetric advantages, GammaTile presented the added benefit of not requiring multiple fractions of radiotherapy. This is significant in the pediatric population, as many of these patients require sedation for the administration of EBRT and may have a restricted ability to travel the distances often necessary to obtain radiotherapy from a radiation oncologist with pediatric experience. Another benefit of GammaTile was the immediate delivery of therapy that this technique offered, contrasting with the 4- to 6-week waiting period required for healing prior to EBRT initiation postoperatively. Not only are these waiting periods inconvenient for the patient but they also pose potential risks of decreased tumor control to those with rapidly growing malignancies such as HGG.1
Despite its superior radiotherapy localization and patient satisfaction, concerns for GammaTile placement include prolongation of the time for the surgical procedure.3,22 However, our 3.5-minute implantation was in line with the published placement time of under 5 minutes.3,22 Other potential complications of GammaTile placement include cerebral edema, infection, cerebrospinal fluid leakage, and seizures.3,22 To address these concerns, we prophylactically administered mannitol intraoperatively, prolonged the conventional postoperative steroid taper, and maintained the patient on his home seizure medication. As a result, the patient did not experience any of these complications.
Another inherent concern with GammaTile placement is bystander radiation. In the case of our patient, Geiger counter readings demonstrated 9 µSv at a distance of approximately 1 m while the skull flap was still removed. Once the skull flap was attached, this dosage dropped to 2.8 µSv. While this finding was acceptable, it still necessitated several precautionary measures. At our institution, pregnant caregivers are restricted from providing postoperative care to patients who have received GammaTile brachytherapy. This may complicate postoperative care, particularly since most of our patient’s potential caregivers were female. In conjunction with the patient’s promising follow-up course and formerly described dosimetric advantages over conventional therapies, these results demonstrate GammaTile to be a treatment modality that is not only safe but also effective. Recent literature further supports this finding, with 1 study in particular concluding favorable exploratory safety and outcome data in 22 patients who were prospectively followed after undergoing maximal safe tumor resection and GammaTile placement.8
The utilization of 5-ALA was indispensable in optimally debulking the tumor without damaging normal parenchyma, allowing a cavity for optimal GammaTile placement and dose delivery. Although deemed safe for all ages, some data have suggested diminished efficacy of 5-ALA in younger individuals. For example, Milos et al.23 demonstrated only 21% of primary central nervous system tumors in pediatric patients showing fluorescence compared with 95% of matched adults. Another study evaluating the utility of 5-ALA in pediatric brainstem gliomas found a total fluorescence rate of 62.5% but a subjectively assessed “usefulness” rate of only 37.5%.24 Although there are various hypotheses to explain the difference in the degree of pediatric tumor fluorescence, these remain controversial as pharmacodynamic and pharmacokinetic studies on 5-ALA have been limited to adults and animal studies.25 One such hypothesis suggests age-related differences in gastrointestinal uptake of 5-ALA, while yet another proposition has attributed this phenomenon to the fact that pediatric tumors have differing properties that affect uptake and fluorescence compared with tumors in adults.23 In our case, the tumor was initially identified under the microscope with frozen pathologic confirmation that the pinkish-red tissue under fluorescence was disease before depending on 5-ALA for resection of less conspicuous tumor tissue. It should be noted that 5-ALA has also been associated with several side effects, notably photosensitivity, hypotension, and impaired liver function,26 none of which were experienced by our patient. In this case, we ultimately found 5-ALA guided surgery to be both safe and useful and therefore argue in support of its future applications in the pediatric population.
Lessons
The simultaneous utilization of 2 emerging strategies in a pediatric patient allowed for superior tumor debulking and radiotherapy dose localization with OAR sparing that could not have been achieved using conventional techniques. We determined that GammaTile’s ability to deliver 60 Gy to the immediate rind of the resection bed leads to clear dosimetric advantages over traditional radiation modalities, such as EBRT. Additionally, 5-ALA was indispensable for tumor debulking, creating a resection bed for GammaTile placement, and limiting complications. Although this study was limited by its inclusion of only a single patient and by its retrospective nature, we believe both fluorescence-guided surgery using 5-ALA and GammaTile brachytherapy to be effective technologies in pediatric populations. We advocate for further longitudinal studies in the treatment of pediatric recurrent gliomas using these techniques.
Author Contributions
Conception and design: Knudson, Gordon, Pasli, Lee, Peach. Acquisition of data: Gordon, Connor, Boyer, Lee, Peach. Analysis and interpretation of data: Gordon, Cook, Connor, Boyer, Peach. Drafting of the article: Knudson, Gordon, Pasli, Peach. Critically revising the article: Knudson, Gordon, Cook, Boyer, Ju, Lee, Peach. Reviewed submitted version of the manuscript: Knudson, Gordon, Pasli, Cook, Boyer, Ju, Lee, Peach. Approved the final version of the manuscript on behalf of all authors: Knudson. Statistical analysis: Gordon. Administrative/technical/material support: Gordon.
Supplemental Information
Previous Presentations
Portions of the content in this article were presented during Research & Creative Achievement Week at East Carolina University in Greenville, NC, on April 4, 2023, in the form of a brief PowerPoint presentation.
References
- 1.Das KK, Kumar R. Pediatric glioblastoma. In: De Vleeschouwer S, editor. Glioblastoma. Codon Publications; 2017. Accessed August 24, 2023. http://www.ncbi.nlm.nih.gov/books/NBK469983/ [PubMed] [Google Scholar]
- 2. Merchant TE, Pollack IF, Loeffler JS. Brain tumors across the age spectrum: biology, therapy, and late effects. Semin Radiat Oncol. 2010;20(1):58–66. doi: 10.1016/j.semradonc.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ekhator C, Nwankwo I, Rak E, Homayoonfar A, Fonkem E, Rak R. GammaTile: comprehensive review of a novel radioactive intraoperative seed-loading device for the treatment of brain tumors. Cureus. 2022;14(10):e29970. doi: 10.7759/cureus.29970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guckenberger M, Andratschke N, Alheit H, et al. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol. 2014;190(1):26–33. doi: 10.1007/s00066-013-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigues AJ, Jin MC, Wu A, Bhambhvani HP, Li G, Grant GA. Risk of secondary neoplasms after external-beam radiation therapy treatment of pediatric low-grade gliomas: a SEER analysis, 1973-2015. J Neurosurg Pediatr. 2021;28(3):306–314. doi: 10.3171/2021.1.PEDS20859. [DOI] [PubMed] [Google Scholar]
- 6. Penoncello GP, Gagneur JD, Vora SA, et al. Comprehensive commissioning and clinical implementation of GammaTiles STaRT for intracranial brain cancer. Adv Radiat Oncol. 2022;7(4):100910. doi: 10.1016/j.adro.2022.100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Odia Y, Gutierrez AN, Kotecha R. Surgically targeted radiation therapy (STaRT) trials for brain neoplasms: a comprehensive review. Neuro Oncol. 2022;24(suppl 6):S16–S24. doi: 10.1093/neuonc/noac130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gessler DJ, Neil EC, Shah R, et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neurooncol Adv. 2022;4(1):vdab185. doi: 10.1093/noajnl/vdab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eatz TA, Eichberg DG, Lu VM, Di L, Komotar RJ, Ivan ME. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol. 2022;156(2):233–256. doi: 10.1007/s11060-021-03901-9. [DOI] [PubMed] [Google Scholar]
- 10. Kim JE, Cho HR, Xu WJ, et al. Mechanism for enhanced 5-aminolevulinic acid fluorescence in isocitrate dehydrogenase 1 mutant malignant gliomas. Oncotarget. 2015;6(24):20266–20277. doi: 10.18632/oncotarget.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwake M, Schipmann S, Müther M, Köchling M, Brentrup A, Stummer W. 5-ALA fluorescence-guided surgery in pediatric brain tumors—a systematic review. Acta Neurochir (Wien) 2019;161(6):1099–1108. doi: 10.1007/s00701-019-03898-1. [DOI] [PubMed] [Google Scholar]
- 12. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman LM, Geller J, Leach J, et al. TR-14 * A feasibility and randomized phase ii study of vorinostat, bevacizumab, or temozolomide during radiation followed by maintenance chemotherapy in newly-diagnosed pediatric high-grade glioma: Children’s Oncology Group Study ACNS0822. Neuro Oncol. 2015;17(suppl 3):iii39–iii40. [Google Scholar]
- 14. Miklja Z, Yadav VN, Cartaxo RT, et al. Everolimus improves the efficacy of dasatinib in PDGFRα-driven glioma. J Clin Invest. 2020;130(10):5313–5325. doi: 10.1172/JCI133310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yahanda AT, Dunn GP, Chicoine MR. Photosensitivity reaction from operating room lights after oral administration of 5-aminolevulinic acid for fluorescence-guided resection of a malignant glioma. Cureus. 2021;13(2):e13442. doi: 10.7759/cureus.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsien CI, Pugh SL, Dicker AP, et al. NRG Oncology/RTOG1205: a randomized phase ii trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J Clin Oncol. 2023;41(6):1285–1295. doi: 10.1200/JCO.22.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karagiannis E, Strouthos I, Leczynski A, Zamboglou N, Ferentinos K. Narrative review of high-dose-rate interstitial brachytherapy in primary or secondary liver tumors. Front Oncol. 2022;12:800920. doi: 10.3389/fonc.2022.800920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Allmen DY, Wurmitzer K, Martin E, Klaver P. Neural activity in the hippocampus predicts individual visual short-term memory capacity. Hippocampus. 2013;23(7):606–615. doi: 10.1002/hipo.22121. [DOI] [PubMed] [Google Scholar]
- 20. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Acharya S, Guo Y, Patni T, et al. Association between brain substructure dose and cognitive outcomes in children with medulloblastoma treated on SJMB03: a step toward substructure-informed planning. J Clin Oncol. 2022;40(1):83–95. doi: 10.1200/JCO.21.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi M, Zabramski JM. Re-irradiation using brachytherapy for recurrent intracranial tumors: a systematic review and meta-analysis of the literature. Cureus. 2020;12(8):e9666. doi: 10.7759/cureus.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Milos P, Haj-Hosseini N, Hillman J, Wårdell K. 5-ALA fluorescence in randomly selected pediatric brain tumors assessed by spectroscopy and surgical microscope. Acta Neurochir (Wien) 2023;165(1):71–81. doi: 10.1007/s00701-022-05360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Labuschagne J. 5-Aminolevulinic acid-guided surgery for focal pediatric brainstem gliomas: a preliminary study. Surg Neurol Int. 2020;11:334. doi: 10.25259/SNI_246_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.“Gliolan: European Public Assessment Report (EPAR) scientific discussion” Photonamic GmbH & Co, KG, Pinneberg, Germany. Accessed August 9, 2023. https://www.ema.europa.eu/en/documents/scientific-discussion/gliolan-epar-scientific-discussion_en.pdf. [Google Scholar]
- 26. Chung IWH, Eljamel S. Risk factors for developing oral 5-aminolevulinic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagn Photodyn Ther. 2013;10(4):362–367. doi: 10.1016/j.pdpdt.2013.03.007. [DOI] [PubMed] [Google Scholar]