Abstract
BACKGROUND
Erdheim-Chester disease (ECD) is a rare non–Langerhans cell histiocytosis characterized histologically by foamy histiocytes and Touton giant cells in a background of fibrosis. Bone pain with long bone osteosclerosis is highly specific for ECD. Central nervous system involvement is rare, although dural, hypothalamic, cerebellar, brainstem, and sellar region involvement has been described.
OBSERVATIONS
A 59-year-old man with a history of ureteral obstruction, medically managed petit mal seizures, and a left temporal lesion followed with serial magnetic resonance imaging (MRI) presented with worsening seizure control. Repeat MRI identified bilateral amygdala region lesions. Gradual growth of the left temporal lesion over 1 year with increasing seizure frequency prompted resection. A non–Langerhans cell histiocytosis with a BRAF V600E mutation was identified on pathology. Imaging findings demonstrated retroperitoneal fibrosis and long bone osteosclerosis with increased fluorodeoxyglucose uptake that, together with the neuropathologic findings, were diagnostic of ECD.
LESSONS
This case of biopsy-proven ECD is unique in that the singular symptom was seizures well controlled with medical management in the presence of similarly located bilateral anterior mesial temporal lobe lesions. Although ECD is rare intracranially, its variable imaging presentation, including the potential to mimic seizure-associated medial temporal lobe tumors, emphasizes the need for a wide differential diagnosis.
Keywords: Erdheim-Chester disease, non-Langerhans cell histiocytosis, seizures
ABBREVIATIONS: CNS = central nervous system, CT = computed tomography, ECD = Erdheim-Chester disease, FDG = fluorodeoxyglucose, LCH = Langerhans cell histiocytosis, MRI = magnetic resonance imaging, PET = positron emission tomography, RDD = Rosai-Dorfman disease, SUV = standardized uptake value
Erdheim-Chester disease (ECD) is a rare, idiopathic non–Langerhans cell histiocytosis characterized histologically by xanthogranulomatous infiltrate and on imaging by osteosclerosis of long bones.1,2 Erdheim-Chester disease most commonly affects middle-aged adults and is considered nonfamilial.3 Symmetric, bilateral osteosclerosis of long bones is a highly specific finding for ECD, but cardiovascular, pulmonary, central nervous system (CNS), retroperitoneal, and adrenal involvement is seen in half of patients with ECD.1 Clinical symptomatology can range from systemic disease to focal, asymptomatic processes.
Central nervous system involvement is rare in ECD, most commonly manifesting as diabetes insipidus, orbital lesions, cerebellar syndromes, or extra-axial masses involving the dura.4 Only a third of patients with ECD report neurological symptoms.5 In most cases, patients already have systemic disease by the time an intracranial lesion is identified.3 We present the unique case of a middle-aged patient with bilateral anterior mesial temporal lobe ECD presenting with chronic adult-onset seizures. The nearly 5 years of medical management and imaging follow-up in this patient prior to surgery highlights the indolent nature of ECD and the need for a wide differential diagnosis for intracranial lesions.
Illustrative Case
A 59-year-old man with a past medical history of hypertension, rheumatoid arthritis, retroperitoneal fibrosis, bilateral ureteral obstruction requiring bilateral ureteral stents, and type 2 diabetes mellitus presented for treatment consultation of a known “brain tumor.” Four years earlier, the patient had experienced a grand mal seizure, and magnetic resonance imaging (MRI) showed a left temporal lesion. The patient was placed on mycophenolate for autoimmune encephalitis and levetiracetam and was advised to follow the lesion’s growth with serial imaging. Two years passed, and the patient began experiencing petit mal seizures. Lamotrigine was added to his medication regimen, and watchful management given the lack of lesion growth was continued.
One year later, in June 2020, 4 years since the first seizure event, the patient presented to our institutional clinic. He was still taking mycophenolate, levetiracetam, and lamotrigine; however, the medications in combination were sedating, impairing his quality of life and only resulted in partial seizure control. The frequency of seizures ranged from 1 or 2 per week to several months, and the majority were characterized as petit mal seizures.
The patient had recently consulted elsewhere, and brain MRI showed a nodular-enhancing lesion without significant surrounding edema or mass effect in the left anterior mesial temporal lobe. The differential diagnosis included a low-grade glioma or glioneuronal tumor such as a ganglioglioma, pleomorphic xanthoastrocytoma, or dysembryoplastic neuroepithelial tumor. However, further review of imaging revealed a second similar, but smaller, nodular-enhancing lesion in the contralateral right anterior mesial temporal lobe. The possibility of brain metastases was proposed but considered unusual given the nearly symmetric locations of the lesions and chronic seizure history (Fig. 1).
FIG. 1.
Magnetic resonance imaging (MRI) findings at clinical presentation. Axial postcontrast T1-weighted imaging (T1WI; A) shows a mildly lobulated nodular enhancing lesion in the region of the left amygdala measuring 13 mm × 9 mm × 8 mm. Additionally, there is a smaller 5-mm enhancing lesion in the region of the right amygdala (B). Coronal postcontrast T1WI (C) shows the left-sided enhancing lesion and partially the right-sided enhancing lesion. Noncontrast T1WI (D) and T2-weighted imaging (T2WI; E) show subtle mild T1 hypointensity and mild T2 hyperintensity, respectively, of the dominant left-sided lesion.
Further imaging was performed for lesion characterization and staging. Brain and whole-body positron emission tomography (PET)/computed tomography (CT) showed avid fluorodeoxyglucose (FDG) uptake (standardized uptake value [SUV] 9.7) of the dominant nodular-enhancing lesion in the left anterior mesial temporal lobe concerning for malignancy but no other significantly increased FDG uptake in the brain. Mildly increased FDG uptake (SUV 3.0–4.8) was noted in the long bones of the bilateral upper and lower extremities in addition to sclerotic bone lesions interpreted as indeterminate for benign versus malignant etiologies, although no definitive primary cancer was identified. Brain proton magnetic resonance spectroscopy of the left anterior mesial temporal lesion showed mildly increased choline, mildly decreased N-acetylaspartate, and no identifiable lactate or lipid peak, which was nonspecific but suggested high-grade glioma as unlikely. Magnetic resonance perfusion showed minimally increased cerebral blood flow in the left anterior mesial temporal lobe.
Results of neurocognitive testing indicated difficulties on several left hemisphere tasks, including verbal intelligence, naming to confrontation, and 3 of 4 episodic verbal memory tasks, although all right hemisphere functions were intact. Options including continuing conservative management with serial scans, stereotactic radiosurgery, laser interstitial thermal therapy, or open surgical excision of the left-sided lesion were discussed. The patient believed his current medication regimen was making him worse; however, concern for surgery worsening his deficit and the potential that the seizures were not exclusively left-sided in origin prompted continued conservative management. The patient returned for follow-up serial scans in January and July 2021, which revealed gradual growth of the dominant left-sided lesion (Fig. 2). Interestingly, the lesion on the right appeared slightly decreased in size. As there were 2 consecutive scans indicating growth of the dominant left-sided lesion, craniotomy and left-sided biopsy versus resection, depending on frozen pathology, was planned.
FIG. 2.

Follow-up MRI findings. Axial (A) and coronal (B) postcontrast T1WI show the nodular enhancing lesion in the region of the left amygdala has slightly increased in size, measuring 13 mm × 11 mm × 10 mm. Axial postcontrast T1WI (C) shows the nodular enhancing lesion in the region of the right amygdala lesion as stable to slightly decreasing in size, measuring 3 mm.
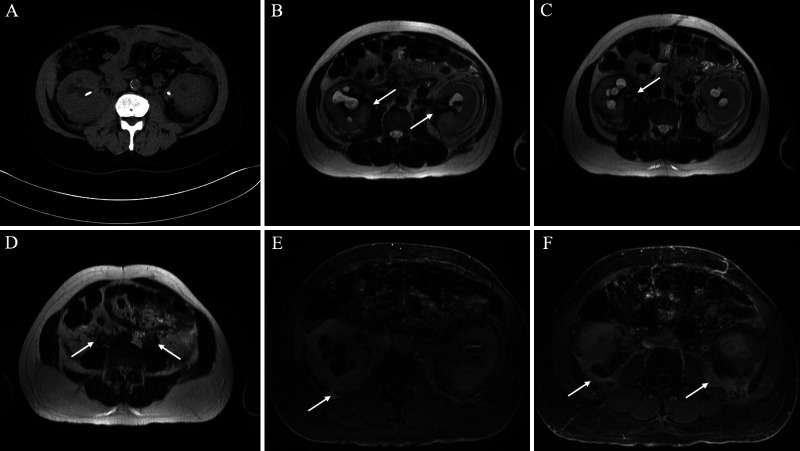
During this time, the patient continued follow-up with his nephrology and primary care teams for his history of ureteral obstruction requiring ureteral stent placement. Computed tomography and MRI of the abdomen and pelvis were notable for complications of retroperitoneal fibrosis (Fig. 3). A review of PET/CT scans obtained earlier indicated FDG uptake in the region of retroperitoneal fibrosis.
FIG. 3.
Computed tomography (CT) and MRI findings of retroperitoneal fibrosis. A: Axial noncontrast CT shows mildly enlarged kidneys with nonspecific perinephric fat stranding, bilateral hydronephrosis, and partially visualized bilateral ureteral stents. B–D: Axial T2WI shows T2-hypointense signal encasing the kidneys and proximal ureters (arrows). E–F: Postcontrast T1WI shows delayed enhancement of the posterior perinephric space with extension into the posterior pararenal space (arrows). Additionally, there is thickening of the perirenal septa suggesting a “hairy kidney sign” (pathognomonic for Erdheim-Chester disease [ECD]). The constellation of findings is compatible with retroperitoneal fibrosis.
In December 2021, 4 months since imaging confirmation of growth of the dominant left-sided lesion, the patient underwent left temporal craniotomy. Several abnormal regions were encountered in the tract to the main lesion, with frozen sections demonstrating inflammatory infiltrates. A distinct, firm, yellowish, and rubbery mass was attached to the very anterior aspect of the temporal horn located within the medial temporal lobe. Multiple frozen sections (7) were performed, and the frozen-section diagnosis in the most pathologic focus was “inflammatory lesion with histiocytes, lymphocytes, and multinucleated giant cells.” Additional pieces from the same anatomical focus were requested for permanent section evaluation and molecular studies. Frozen sections excluded the presence of a neoplasm, which was in the radiological differential. A gross-total excision was achieved.
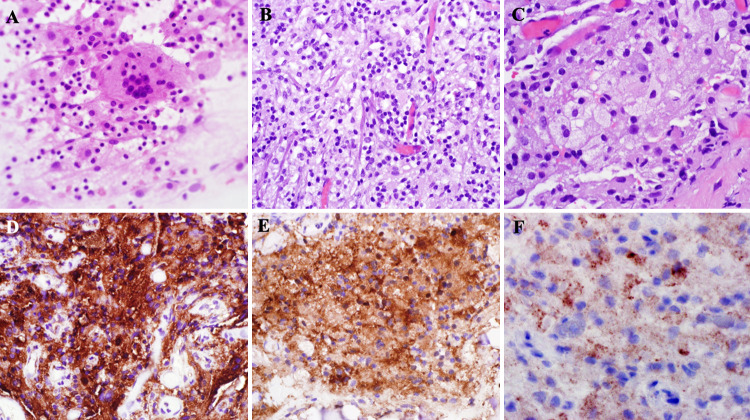
Further neuropathologic evaluation showed foamy histiocytes and multinucleated Touton-type giant cells in a background of lymphocytic inflammation (Fig. 4). These foamy histiocytes were positive for CD68, CD163, and factor XIIIa, as are characteristic of ECD. No Langerhans cells or eosinophils were identified, and CD1a was negative, excluding a Langerhans cell histiocytosis. S100 staining was negative in histiocytes, and engulfment of lymphocytes by histiocytes (emperipolesis) was not identified, excluding Rosai-Dorfman disease (RDD). Special stains for organisms were negative, and there was no granulomatous inflammation. Electron microscopy studies of the biopsy showed histiocytes with cytoplasmic vacuoles, including lipid, lysosomes, and dense granules, as well as invaginated nuclei. No Birbeck granules were noted, further excluding the presence of Langerhans cells. A BRAF V600E mutation was subsequently confirmed using next-generation sequencing, as well as immunohistochemistry for mutant BRAF V600E protein. Based on these findings, a pathologic diagnosis of “non–Langerhans cell histiocytosis, BRAF V600E-mutated” was made.
FIG. 4.
Non–Langerhans cell histiocytosis specimen. Cytological preparations (A, original magnification ×400) at a frozen section showed many foamy histiocytes and scattered multinucleated giant cells. Permanent hematoxylin-and-eosin–stained sections (B, original magnification ×400) showed a histocyte-rich lesion with many foamy histocytes (C, original magnification ×600) and multinucleated giant cells, including those of a Touton type as well as background lymphocytes. Immunohistochemistry workup showed strong, diffuse expression of CD68 (D, original magnification ×400), CD163 (not shown), and factor XIII (E) within the histiocytes and multinucleated giant cells. CD1a (not shown) and S100 (not shown) were negative. BRAF p.V600E staining (F, original magnification ×400, enlarged for clarity) showed strong, patchy cytoplasmic staining, and a BRAF p.V600E mutation was later confirmed by next-generation sequencing.
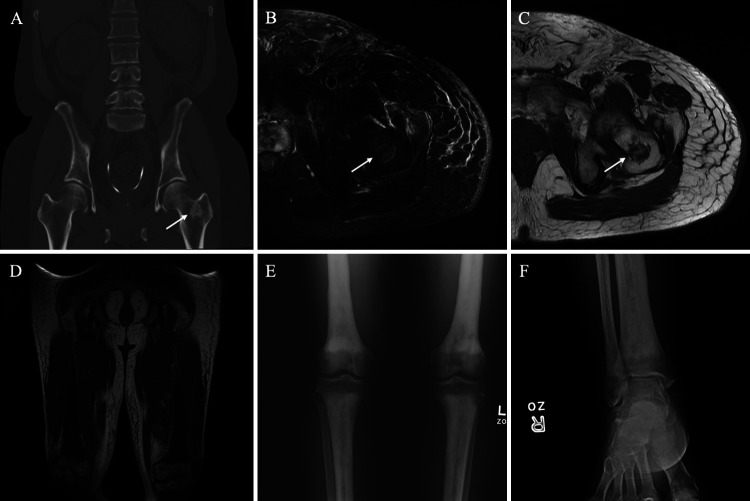
The patient was referred to hematology-oncology for treatment. At the 1-month follow-up, the patient was seizure free on lamotrigine and levetiracetam with further improvement in cognitive function. A follow-up MRI bone survey was significant for low T1-weighted marrow signal in the mid- and distal femoral shaft and midportion of the humerus, suggestive of a marrow proliferative or dysplastic process, although the patient still had no complaints of bone pain. Magnetic resonance imaging of the lower extremity identified a benign fibro-osseous lesion in the proximal femoral intertrochanteric region on the left. Additionally, symmetric hypointense cortical hypertrophy within the mid- to distal femoral shaft with epiphysis sparing supported the MRI bone survey’s suggestion of an atypical red marrow conversion or other marrow proliferative process (Fig. 5). These findings supported a prior PET/CT, which had revealed mild FDG uptake and sclerosis within the long bones of the bilateral upper and lower extremities. However, an initial bone survey completed in 2018, 2 years into the patient’s treatment for seizures, had been negative for aggressive osseous lesions. Thus, there was progression over this nearly 4-year span.
FIG. 5.
Erdheim-Chester disease skeletal changes. Coronal CT (A) shows a 2.0-cm sclerotic lesion in the left femoral neck (arrow). Axial T2WI with fat saturation (B) and axial T1WI (C) show a T2-hyperintense and T1-hypointense irregular lesion (arrows) centered at the left femoral neck corresponding to the CT finding. Coronal T1WI (D) shows diffuse symmetric T1 hypointensity involving bilateral mid-distal femurs and proximal tibiae, sparing the epiphysis. A frontal radiograph (E) of the bilateral knees shows bilateral symmetric metaphyseal and diaphyseal sclerotic changes of the tibiae, fibulae, and femurs with epiphyseal sparing. A frontal radiograph (F) of the right ankle shows similar sclerotic changes of the distal tibia and fibula, sparing the epiphysis. Symmetric findings were seen on the contralateral ankle. The constellation of findings is consistent with sclerotic changes secondary to ECD.
From the combination of imaging and pathological findings, the patient’s final diagnosis was ECD, a rare form of non–Langerhans cell histiocytosis identified on pathology by xanthogranulomatous infiltrate and on imaging by osteosclerosis with FDG uptake of long bones, retroperitoneal involvement with hairy kidney sign, and ureteral obstruction. The patient is currently being treated with trametinib and dabrafenib.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Non–Langerhans cell histiocytosis is a diagnostic category that includes RDD, xanthogranuloma, and ECD. In addition to RDD, Langerhans cell histiocytosis (LCH) and sarcoidosis are 2 other histiocytic disorders rarely characterized by a single mass lesion.3 Immunohistological and microscopic characteristics of histiocytes differentiate ECD from LCH, with histiocytes in ECD positive for CD68 and negative for CD1a, S100 protein, and OKT6.6 In contrast, LCH is CD1a and S100 positive, and Birbeck granules can be found on electron microscopy.7,8 Histopathology with immunohistochemistry paired with the clinical findings of long bone sclerosis confirmed ECD in this patient, and notably showed BRAF V600E protein, which was further confirmed by next-generation sequencing studies.
Neurological symptoms such as seizures are rare in ECD and can lead to severe disability and death.6 Lachenal et al.5 reported 6 cases of ECD with neurological symptoms and identified 60 additional cases on literature review. In 22% of patients, as in our patient, neurological symptoms were the first manifestation of ECD. Unlike our case, the majority of the 66 cases had multiple neurological symptoms, whereas our patient presented with just 1 symptom, seizures.
Isolated craniocerebral involvement is reported in fewer than 50% of patients with ECD.9 Even when CNS lesions are present, the majority of patients are asymptomatic, with diabetes insipidus and ataxia being the most common clinical manifestations in symptomatic patients.1 Jain et al.10 reported a case of biopsy-confirmed ECD with recurrent, secondarily generalized seizures. Unlike this case, where osteosclerosis in the long bones was identified, Jain et al. reported no extracranial involvement even after 12 years of follow-up, and the osteolytic lesions isolated to the skull vault resolved spontaneously.10 Rushing et al.3 reported a case of isolated intracranial ECD mimicking a primary brain tumor.3 As in our case, the patient had seizures as his primary symptom, a temporoparietal mass was identified on imaging, and skeletal involvement was not identified until a postoperative bone scan.
In this case, PET/CT imaging identified both CNS and long bone lesions, even in the absence of bone pain, and retrospectively correlated the histopathologic diagnosis of ECD with prior imaging findings. In a review of 32 patients with 18F-FDG PET/CT imaging, Young et al.11 found that PET/CT imaging impacted patient management in half of the patients, especially in those with CNS involvement. Sioka et al.12 used PET/CT imaging in a patient with ECD previously treated with interferon alpha-2b to retrospectively identify lesions in the heart, mediastinum, abdomen, and brain, including, as in this patient, a left medial temporal lobe lesion, thus illustrating the idle, silent progression of ECD.
Observations
It is important to note the progression of differential diagnoses in this case alongside the serial imaging findings, with many conclusions considered in retrospect. Seizure activity prompted the initial clinical and imaging workup of the patient. The identification of 2 enhancing brain lesions on serial imaging 4 years after the initial presentation raised concern for metastatic disease. However, the location of these lesions in the symmetric opposite anterior mesial temporal lobes, the lack of association with any significant T2 fluid-attenuated inversion recovery (FLAIR) signal abnormality or mass effect, and a 3-year seizure history made this less likely. As a primary brain tumor was on the differential, surgery for resection and immunohistopathologic analysis was a topic of significant discussion. However, the location of the lesion in the left temporal lobe raised concerns for worsening language and verbal memory difficulties with surgery. Thus, an initial watch-and-wait approach was chosen given the presence of bilateral lesions, the risks of surgery, and the well-controlled seizures. Later growth of the dominant left-sided lesion on serial imaging, combined with the risk of continued growth and transformation into a more aggressive pathology, hastened the need for diagnosis and was the indication for surgery. Concerns for worsening function in this patient following surgery fortunately did not materialize. After 1 month of postoperative follow-up, the patient did not have significant deficits and continued the treatment regimen of trametinib and dabrafenib.
Lessons
This case of biopsy-proven ECD is unique in that the patient presented with bilateral symmetric temporal lobe lesions and a singular symptom of seizures controlled for years with medical management. While most cases of ECD that present with neurological findings have cerebellar, brainstem, or pituitary involvement on imaging, this patient’s intracranial lesion was restricted to bilateral anterior mesial temporal lobes.13 Although ECD is rarely found intracranially, its potential to mimic brain tumors emphasizes the need for a wide differential in patients presenting with a suspicious lesion or lesions on MRI. A biopsy with established mutational and immunohistopathologic confirmation is required to confirm ECD diagnosis and guide targeted therapy.
Acknowledgments
This work was supported by the Donna and Kenneth R. Peak Foundation, The Kenneth R. Peak Brain and Pituitary Center at Houston Methodist Hospital, The Taub Foundation, The Blanche Green Estate Fund of the Pauline Sterne Wolff Memorial Foundation, The John S. Dunn Foundation, Kelly Kicking Cancer, The Marilee A. and Gary M. Schwarz Foundation, The Houston Methodist Hospital Foundation, and the Verelan Foundation.
Author Contributions
Conception and design: Baskin, Stuebe, Jenson, Cykowski, McClain. Acquisition of data: Baskin, Stuebe, Jenson, Lines, Cykowski, McClain. Analysis and interpretation of data: Stuebe, Lines, Cykowski, Fung, Fisher, McClain. Drafting of the article: Stuebe, Jenson, Holloman, Cykowski, Fung, McClain. Critically revising the article: Baskin, Stuebe, Holloman, Cykowski, Fung. Reviewed submitted version of the manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Baskin. Administrative/technical/material support: Baskin, Jenson. Study supervision: Baskin.
References
- 1. Drier A, Haroche J, Savatovsky J, et al. Cerebral, facial, and orbital involvement in Erdheim-Chester disease: CT and MR imaging findings. Radiology. 2010;255(2):586–594. doi: 10.1148/radiol.10090320. [DOI] [PubMed] [Google Scholar]
- 2. Adle-Biassette H, Chetritt J, Bergemer-Fouquet AM, Wechsler J, Mussini JM, Gray F. Pathology of the central nervous system in Chester-Erdheim disease: report of three cases. J Neuropathol Exp Neurol. 1997;56(11):1207–1216. doi: 10.1097/00005072-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 3. Rushing EJ, Bouffard JP, Neal CJ, et al. Erdheim-Chester disease mimicking a primary brain tumor. Case report. J Neurosurg. 2004;100(6):1115–1118. doi: 10.3171/jns.2004.100.6.1115. [DOI] [PubMed] [Google Scholar]
- 4. Wright RA, Hermann RC, Parisi JE. Neurological manifestations of Erdheim-Chester disease. J Neurol Neurosurg Psychiatry. 1999;66(1):72–75. doi: 10.1136/jnnp.66.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lachenal F, Cotton F, Desmurs-Clavel H, et al. Neurological manifestations and neuroradiological presentation of Erdheim-Chester disease: report of 6 cases and systematic review of the literature. J Neurol. 2006;253(10):1267–1277. doi: 10.1007/s00415-006-0160-9. [DOI] [PubMed] [Google Scholar]
- 6. Arnaud L, Pierre I, Beigelman-Aubry C, et al. Pulmonary involvement in Erdheim-Chester disease: a single-center study of thirty-four patients and a review of the literature. Arthritis Rheum. 2010;62(11):3504–3512. doi: 10.1002/art.27672. [DOI] [PubMed] [Google Scholar]
- 7. Adem C, Hélie O, Lévêque C, Taillia H, Cordoliani YS. Case 78: Erdheim-Chester disease with central nervous system involvement. Radiology. 2005;234(1):111–115. doi: 10.1148/radiol.2341021806. [DOI] [PubMed] [Google Scholar]
- 8. Mahnel R, Tan KH, Fahlbusch R, et al. Problems in differential diagnosis of non Langerhans cell histiocytosis with pituitary involvement: case report and review of literature. Endocr Pathol. 2002;13(4):361–368. doi: 10.1385/ep:13:4:361. [DOI] [PubMed] [Google Scholar]
- 9. Adam Z, Balsíková K, Pour L, et al. Diabetes insipidus followed, after 4 years, with dysarthria and mild right-sided hemiparesis—the first clinical signs of Erdheim-Chester disease. Description and depiction of a case with a review of information on the disease. Vnitr Lek. 2009;55(12):1173–1188. Article in Czech. [PubMed] [Google Scholar]
- 10. Jain RS, Sannegowda RB, Jain R, Mathur T. Erdheim-Chester disease with isolated craniocerebral involvement. BMJ Case Rep. 2013;2013:bcr2012006823. doi: 10.1136/bcr-2012-006823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young JR, Johnson GB, Murphy RC, Go RS, Broski SM. 18F-FDG PET/CT in Erdheim-Chester disease: imaging findings and potential BRAF mutation biomarker. J Nucl Med. 2018;59(5):774–779. doi: 10.2967/jnumed.117.200741. [DOI] [PubMed] [Google Scholar]
- 12. Sioka C, Estrada-Veras J, Maric I, Gahl WA, Chen CC. FDG PET images in a patient with Erdheim-Chester disease. Clin Nucl Med. 2014;39(2):170–177. doi: 10.1097/RLU.0b013e31828da5e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parks NE, Goyal G, Go RS, Mandrekar J, Tobin WO. Neuroradiologic manifestations of Erdheim-Chester disease. Neurol Clin Pract. 2018;8(1):15–20. doi: 10.1212/CPJ.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]