Abstract
Pentoxifylline has immunomodulatory properties and has been shown to decrease organ damage and improve survival in animals with gram-negative sepsis or endotoxemia. This effect is mediated by a reduction in endotoxin-induced production of tumor necrosis factor alpha (TNF-α) by the host. In earlier studies, we observed an unexpected increase in mortality in mice infected with Candida albicans that were given pentoxifylline even though concentrations of TNF-α in serum were not affected. The current study was designed to determine whether the pharmacokinetics of pentoxifylline and its metabolites were altered in C. albicans-infected mice and, if so, whether these changes could have contributed to the increased mortality. Noninfected mice and mice infected with C. albicans were treated with pentoxifylline (60 mg/kg of body weight) intraperitoneally every 8 h. Serum was collected from animals after one (day 0), four (day 1), or seven (day 2) injections of pentoxifylline or saline (controls). The first dose was administered 6 h after C. albicans infection. Serum was pooled. Concentrations of pentoxifylline and metabolites I, IV, and V were determined by capillary gas chromatography. Renal function and hepatic profiles were assessed. Pharmacokinetic parameters (maximum concentration of pentoxifylline in serum, half-life, and area under the concentration-time curve from 0 h to infinity [AUC0–∞]) for all noninfected mice were similar and did not differ from those for day 0-infected mice. For day 1-infected mice, values of these three pharmacokinetic parameters for pentoxifylline and metabolite I were increased two- to fourfold over values for noninfected and day 0-infected mice. For metabolites IV and V, the AUC0–∞ was increased approximately eightfold over control values. In addition, day 1-infected mice demonstrated evidence of renal and hepatic dysfunction. In summary, C. albicans infection produced marked changes in the pharmacokinetics of pentoxifylline and its metabolites in the mice. The high concentrations of pentoxifylline and its metabolites in serum attained in infected mice may have contributed to the increased mortality of mice with systemic candidiasis.
Pentoxifylline is one of several methylxanthine compounds that has immunomodulatory properties (10). In vitro, pentoxifylline pretreatment can attenuate the production of interleukin 1 and tumor necrosis factor alpha by human mononuclear cells in response to bacterial endotoxin (12). In addition, it can decrease endotoxin-mediated migration, adherence, and production of superoxide radicals by phagocytic cells (13). In vivo, pentoxifylline improves the outcome for animals challenged with high-level inocula of gram-negative bacilli or endotoxin, suggesting that pentoxifylline or related compounds might be of value in treating sepsis (6, 7). These observations have led to its extensive use in animal models of sepsis, burns, and traumatic shock (4).
In a series of experiments to determine the role of cytokine activation in systemic Candida albicans infection, we observed decreased survival rates in pentoxifylline-treated mice (5). However, pentoxifylline administration did not affect the production of tumor necrosis factor alpha or interleukin 6 in these infected mice. It was possible that altered pharmacokinetics of pentoxifylline and its metabolites in systemic C. albicans infection were responsible for the shortened survival times. However, a review of the literature failed to provide information about the metabolism and clearance of pentoxifylline in infected versus healthy animal models. Therefore, the purpose of this study was to determine the pharmacokinetics, including clearance, of pentoxifylline in a murine model of systemic candidiasis in an attempt to define a possible pharmacologic cause for the unexpected results of our previous studies (5).
MATERIALS AND METHODS
C. albicans.
Strain 88-689-6 was isolated from the blood of a neutropenic patient. The microorganism was maintained on Sabouraud dextrose agar (BBL Microbiology Systems, Cockeysville, Md.) at 22°C until use. For each study, two or three colonies of C. albicans were subcultured onto potato dextrose agar (BBL) and incubated at 35°C for 48 h. A fungal suspension was prepared with sterile, pyrogen-free, phosphate-buffered saline (PBS; Gibco-BRL Inc., Grand Island, N.Y.) and quantified with a hemocytometer.
Morphologic examination demonstrated that >99% of the organisms were blastoconidia. The viability of the yeast was found to be >95% by trypan blue exclusion and quantitative cultures. The endotoxin concentration in the fungal suspension was found to be <0.05 endotoxin units (EU) per ml by a competitive endotoxin enzyme-linked immunosorbent assay (PyroChek competitive lipopolysaccharide ELISA; ALerCHEK, Portland, Maine).
Mice.
Female, 18- to 20-g NYLAR white mice were raised at the Animal Research Facility of the Wadsworth Center for Laboratories and Research (Griffin Laboratories, Guilderland, N.Y.). These outbred Swiss mice were housed in hanging metal cages and received food and water ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committees of the respective institutions.
Pentoxifylline.
Pentoxifylline powder was provided by William Novick, Jr. (Hoechst-Roussel, Somerville, N.J.). The potency of the drug was confirmed by the manufacturer just prior to the initiation of this study. Pentoxifylline was dissolved in sterile, pyrogen-free water to produce a stock solution at a concentration of 20 mg/ml. The stock solution was passed through a 0.45-μm-pore-size filter (Lida Manufacturing, Kenosha, Wis.). The drug was further diluted to the desired concentrations with sterile, pyrogen-free PBS and was used immediately. The solutions contained less than 0.25 EU of endotoxin per ml, as determined by Limulus amebocyte lysate assay (Whittaker M. A. Bioproducts, Walkersville, Md.).
Systemic candidiasis model.
Mice were injected intravenously with 106 CFU of C. albicans or PBS in a 0.2-ml volume. Mice received 60 mg of pentoxifylline per kg of body weight intraperitoneally (i.p.) at 8-h intervals beginning 6 h after the intravenous injection of C. albicans or PBS. In a previous study (5), this pentoxifylline regimen resulted in a statistically significant decrease in mean survival of NYLAR mice systemically infected with C. albicans. The volume of each i.p. injection was 0.1 ml. Animals were assessed prior to each injection for mortality. Previous studies demonstrated that mice died within 4 h after becoming moribund; therefore, moribund mice were sacrificed.
Measurement of serum levels of pentoxifylline and its metabolites by capillary gas chromatography.
The method used in the assay of plasma pentoxifylline and metabolites I, IV, and V was described by Burrows (2). The procedure included a single extraction of all species from plasma into chloroform, derivative formation (the trifluoracetyl derivative in the case of metabolite I and the methyl ester derivatives in the case of metabolites IV and V and their internal standard), and analysis by capillary gas chromatography. An analog of pentoxifylline (Hoechst EH79-0254) was employed as the internal standard for pentoxifylline and metabolite I, while 1-(5′-carboxypentyl)-3,7-dimethylxanthine (Hoechst-Roussel) was used as the internal standard for measuring metabolites IV and V.
Standard curves were generated for pentoxifylline, metabolite I, and metabolite IV in the range of 5 to 2,000 ng/ml, and for metabolite V in the range of 25 to 10,000 ng/ml. Capillary gas chromatography was performed with a Varian model 3700 gas chromatograph, modified for split-injection capillary column analysis and fitted with a thermionic radiation-specific (nitrogen/phosphorus) detector. The separation was conducted with a DB-5 phenylmethyl column (30 by 0.32 [inside diameter] mm; Varian model JW123503-20) fitted with a precolumn glass insert guard column and packed with quartz fiberglass (CapSaver; N-Phase, Inc.). The linearities, sensitivities, and percent coefficients of variation at high and low concentrations of pentoxifylline and metabolites I, IV, and V are given in Table 1.
TABLE 1.
The linearities, sensitivities, and percentages of coefficient variation at high and low concentrations of pentoxifylline and its metabolites I, IV, and V
| Compound | Concn (ng/ml) rangea | Linearity | Sensitivity limit (ng/ml) | % Coefficient variation at:
|
|
|---|---|---|---|---|---|
| High concn | Low concn | ||||
| Pentoxifylline | 5–2,000 | 0.997 | 5 | 1.1 | 3.3 |
| Metabolite I | 5–2,000 | 0.999 | 5 | 2.3 | 4.4 |
| Metabolite IV | 5–2,000 | 0.997 | 5 | 4.0 | 2.8 |
| Metabolite V | 25–10,000 | 0.995 | 25 | 3.6 | 5.6 |
Range over which standard curve was generated.
Pharmacokinetic study.
To determine the pharmacokinetics of pentoxifylline and metabolites in noninfected and infected mice, three or four animals in each group were sacrificed by CO2 asphyxiation at 0, 5, 15, 30, 60, and 90 (infected animals only) min after the first (day 0), fourth (day 1), and seventh (day 2) doses of pentoxifylline. The first dose of drug was administered 6 h after mice were injected with C. albicans or PBS. None of the infected mice survived to day 2. Blood was collected by cardiac puncture and allowed to clot at 4°C. At each time point the serum was separated from the clot by centrifugation, pooled, and stored at −70°C until analyzed.
Pharmacokinetic analysis.
Pharmacokinetic parameters were determined by standard techniques. The concentration-time data for pentoxifylline and its three metabolites were analyzed separately. The elimination rate constant (kel) was determined by linear regression of the log concentration-time data, and the half-lives (t1/2) were calculated as t1/2 = 0.693/kel. The t1/2 of metabolites IV and V could not be determined in any treatment group because concentrations were essentially unchanging over the measurement interval.
The linear-trapezoidal rule was used to calculate the area under the concentration-time curve to the last measured value (AUCτ), and the residual area, AUCres, was calculated by dividing the last measured concentration (Cτ) by kel. The total area under the curve (AUC0–∞) = AUCτ + AUCres.
RESULTS
Pharmacokinetics.
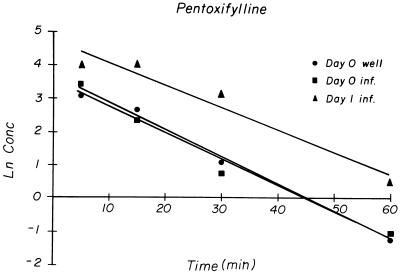
The maximum plasma concentration (Cmax) of pentoxifylline occurred at the first sampling time, 5 min after injection. For the noninfected mice on days 0, 1, and 2 and the infected mice on day 0, the Cmax, t1/2, and AUC0–∞ values for pentoxifylline were similar, but the Cmax, t1/2, and AUC0–∞ values were higher on day 1 in infected mice (Table 2). In all treatment groups, pentoxifylline concentrations declined over time in a log-linear fashion (Fig. 1). The pharmacokinetic parameters for noninfected mice on all three treatment days were similar and, thus, were combined to obtain an estimate of the within-treatment variability of pentoxifylline pharmacokinetics. The pharmacokinetic parameters for noninfected mice are reported as the means ± standard deviations.
TABLE 2.
Pharmacokinetics, including AUC, t1/2, and Cmax, of pentoxifylline and its metabolites for noninfected mice and mice infected with C. albicans on days 0, 1, and 2 after fungal infectionsa
| Mice | Day(s) | Com- poundd | AUC0–∞ (μg · min/ml) | t1/2 min | Cmax (μg/ml) |
|---|---|---|---|---|---|
| Noninfected | 0, 1, 2 | Pentox | 427 ± 78 | 6.6 ± 1.6 | 31.8 ± 8.1 |
| Infected | 0 | Pentox | 415 | 8.6 | 30.5 |
| Infected | 1 | Pentox | 1,673 | 13.1 | 54.5 |
| Noninfected | 0, 1, 2 | Met I | 136 ± 32 | 6.3 ± 2.8 | 8.9 ± 1.9 |
| Infected | 0 | Met I | 121 | 8.3 | 7.9 |
| Infected | 1 | Met I | 444 | 14.5 | 14.5 |
| Noninfected | 0, 1, 2 | Met IV | 6.3 ± 2.3b | N/Cc | 0.40 ± 0.2 |
| Infected | 0 | Met IV | 5.42b | N/C | 0.24 |
| Infected | 1 | Met IV | 51.1b | N/C | 0.65 |
| Noninfected | 0, 1, 2 | Met V | 766 ± 89 | 9.4 ± 0.4 | 31.8 ± 4.8 |
| Infected | 0 | Met V | 759 | 12.6 | 26.4 |
| Infected | 1 | Met V | 6,280 | N/C | 103.8 |
Results for days 0, 1, and 2 for infected mice were similar; therefore, they were combined (mean ± standard deviation).
Value for AUCτ, because kel value could not be determined.
N/C, not calculated.
Pentox, pentoxifylline; Met, metabolite.
FIG. 1.
Natural logarithm of concentrations of pentoxifylline in serum over time after i.p. dosing. Well, uninfected mice; inf., infected mice. All sera for each time point and group were pooled.
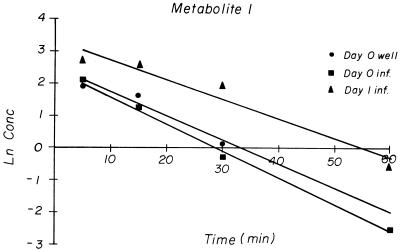
The Cmax of metabolite I in all treatment groups also occurred at the first sampling time. The Cmax, t1/2, and AUC0–∞ values for metabolite I on days 0, 1, and 2 for noninfected mice and on day 0 for infected mice were similar, but all parameters were higher for the day 1-infected mice (Table 2). Metabolite I concentrations declined in a log-linear fashion in all treatment groups (Fig. 2). The pharmacokinetic parameters of metabolite I for noninfected mice on all treatment days were also combined and reported as the means ± standard deviations.
FIG. 2.
Natural logarithm of concentrations of pentoxifylline metabolite I in serum over time after i.p. dosing. Well, uninfected mice; inf., infected mice. All sera for each time point and group were pooled.
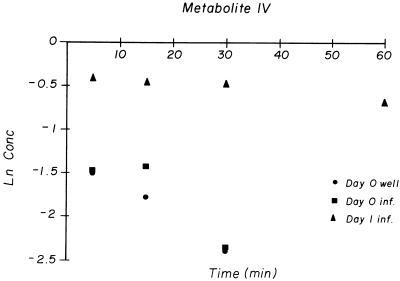
Metabolite IV (1-[4-carboxybutyl]-3,7-dimethylxanthine), which is important in humans (1, 11), was present in very low concentrations in all treatment groups (Fig. 3). The time to Cmax (Tmax) varied in the noninfected mice, occurring at 5 min in two groups and at 30 min in one group. It was not possible to obtain an accurate estimate of the t1/2 of metabolite IV in any treatment group. The values of AUC0–τ of metabolite IV for the day 1-infected group were dramatically higher than those for the noninfected and day 0-infected mice (Table 2).
FIG. 3.
Natural logarithm of concentrations of pentoxifylline metabolite IV in serum over time after i.p. dosing. Well, uninfected mice; inf., infected mice. All sera for each time point and group were pooled.
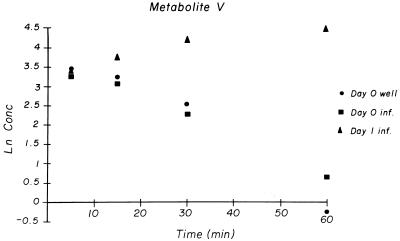
The Tmax of metabolite V, the major urinary metabolite of pentoxifylline in humans (3-carboxypropyl), occurred at 5 min in two noninfected-mouse groups and at 15 min in one noninfected-mouse group. For day 0-infected mice, metabolite V concentrations and pharmacokinetic parameters were similar to those for noninfected mice. In day 1-infected mice, metabolite V levels increased throughout the sampling protocol so that Cmax, t1/2, and AUC0–∞ could not be accurately determined (Fig. 4). However, the AUCτ for metabolite V for the day 1-infected mice was almost 10-fold greater than the AUC0–∞ for the noninfected and day 0-infected mice, and the Cmax for the day 1-infected mice was more than 3-fold higher than those for the noninfected and day 0-infected mice (Table 2).
FIG. 4.
Natural logarithm of concentrations of pentoxifylline metabolite V in serum over time after i.p. dosing. Well, uninfected mice; inf., infected mice. All sera for each time point and group were pooled.
Organ function.
Values of serum creatinine, lactate dehydrogenase, alanine transaminase, aspartate transaminase, and alkaline phosphatase, measured in aliquots of drug concentration samples, revealed that in day 0-infected mice, renal and hepatic functions were still within normal limits while in day 1-infected mice, renal and hepatic functions were significantly compromised, indicating organ injury (Table 3).
TABLE 3.
Mean serum creatinine, lactate dehydrogenase, alanine transaminase, aspartate transaminase, and alkaline phosphatase levels in noninfected mice and mice infected with C. albicans before and during pentoxifylline administration
| Mice | Day | Time (min) | Mean level ofc:
|
||||
|---|---|---|---|---|---|---|---|
| Creatinine (mg/dl) | LD (U/ml) | ALT (U/ml) | AST (U/ml) | AP (U/ml) | |||
| Untreated, noninfected mice | N/Aa | N/A | 0.2 | 812 | 54 | 330 | 222 |
| Noninfected | 0 | −20d | 0.2 | 1,530 | 64 | 517 | 249 |
| 5 | 0.4 | 1,022 | 49 | 192 | 260 | ||
| 15 | 0.3 | 891 | 49 | 190 | 276 | ||
| 30 | 0.3 | 1,205 | 61 | 297 | 266 | ||
| 60 | 0.1 | 1,740 | 71 | 370 | 275 | ||
| Noninfected | 1 | −20 | 0.1 | 1,592 | 86 | 631 | 248 |
| 5 | 0.3 | 1,105 | 40 | 358 | 244 | ||
| 15 | 0.3 | 1,360 | 36 | 447 | 255 | ||
| 30 | 0.2 | 1,262 | 42 | 393 | 252 | ||
| 60 | 0.2 | 840 | 54 | 319 | 255 | ||
| Noninfected | 2 | −20 | 0.2 | 1,762 | 68 | 405 | 251 |
| 5 | 0.3 | 1,483 | 44 | 364 | 234 | ||
| 15 | 0.2 | 903 | 46 | 232 | 254 | ||
| 30 | 0.2 | 833 | 49 | 225 | 231 | ||
| 60 | 0.2 | 904 | 68 | 441 | 219 | ||
| Infected | 0 | −20 | 0.2 | 2,359 | 52 | 324 | 223 |
| 5 | 0.1 | 1,659 | 49 | 370 | 190 | ||
| 15 | 0.2 | 1,354 | 54 | 402 | 240 | ||
| 30 | 0.2 | 1,455 | 48 | 334 | 229 | ||
| 60 | 0.1 | 1,510 | 65 | 484 | 209 | ||
| Infected | 1 | −20 | 1.2 | >600b | 296 | >1,000b | 112 |
| 5 | 1.1 | >600b | 342 | >1,000b | 128 | ||
| 15 | 1.2 | >600b | 371 | >1,000b | 100 | ||
| 30 | 1.2 | 9,564 | 331 | 5,243 | 103 | ||
| 60 | 1.1 | 4,835 | 161 | 3,089 | 97 | ||
| 90 | 1.1 | >600b | 203 | >1,000b | >1,000b | ||
N/A, not applicable.
Insufficient sample to further quantify value.
LD, lactate dehydrogenase; ALT, alanine transaminase; AST, aspartate transaminase; AP, alkaline phosphatase.
Levels were measured 20 min before drug administration.
DISCUSSION
Pentoxifylline was eliminated very rapidly after absorption in both the infected and noninfected mice. The t1/2 of both pentoxifylline and its metabolites in noninfected mice were within the ranges reported for healthy mice by other investigators (3, 9). There was also an increase in the AUCs for pentoxifylline (fourfold), metabolite I (threefold), and metabolites IV and V (greater than fourfold) in the infected mice (Table 2). These large increases in the AUCs of pentoxifylline and its metabolites in infected mice resulted from decreased hepatic and renal elimination due to the impaired liver and kidney functions, and from increased pentoxifylline absorption following i.p. administration. Raju et al. reported a 29% bioavailability for i.p. administration compared to that for subcutaneous administration for healthy mice (8). These authors speculated that this low i.p. bioavailability was due to extensive “first pass” hepatic metabolism of pentoxifylline. In our study, increased pentoxifylline bioavailability resulting from decreased hepatic elimination during the first pass through the liver would have contributed to an increase in both the AUC and Cmax of pentoxifylline in the infected mice. Pentoxifylline metabolite formation would also have increased as a result of the increased bioavailability of pentoxifylline in the infected mice.
Murine pentoxifylline pharmacokinetics differ among various mouse strains, resulting in differences in the extent of metabolite formation. This study and that of Honess et al. (3) found measurable concentrations of metabolites I, IV, and V at comparable doses. Conversely, Raju et al. (8) were not able to detect metabolite IV in plasma following i.p. or subcutaneous administration of pentoxifylline. Honess et al. (3) found that metabolite I and IV concentrations were approximately 1/10 that of pentoxifylline, while in the present study metabolite I and IV concentrations were 1/4 and 1/100, respectively, those of pentoxifylline. Raju et al. (8) found that metabolite I levels were about one-fourth those of pentoxifylline, while metabolite V concentrations were somewhat higher than those of pentoxifylline. Metabolite V concentrations were of the same order of magnitude as pentoxifylline concentrations in all three studies. These differences in the metabolite patterns of pentoxifylline, particularly pharmacologically active metabolite I, caution against any extrapolation of the physiological effects of pentoxifylline among mouse strains, unless the pharmacokinetics and metabolic profiles are known.
Did the changes in the pharmacokinetics of pentoxifylline and its metabolites caused by the C. albicans infection contribute to a reduction in mouse survival time and greater mortality? The magnitudes of the AUCs of pentoxifylline and metabolites I and V for the infected mice would only be achieved for noninfected mice with doses greater than 240 mg/kg, assuming dose-independent pharmacokinetics. Honess et al. reported that single i.p. doses of up to 200 mg/kg were well tolerated by healthy mice but that 400 mg/kg was highly toxic (3). However, in our study, the day 1-infected mice received three doses of pentoxifylline, and as the C. albicans infection progressed, liver and kidney functions became progressively impaired. Thus, with each successive dose of pentoxifylline different pharmacokinetics were exhibited, reflective of successively increasing doses. The combination of the infection-induced organ function impairment and the resulting increasing systemic exposure to pentoxifylline and its metabolites likely was responsible for the shorter survival times and greater mortality of the infected animals.
REFERENCES
- 1.Beermann B, Ings R, Mansby J, Chamberlain J, McDonald A. Kinetics of intravenous and oral pentoxifylline in healthy subjects. Clin Pharmacol Ther. 1985;37:25–28. doi: 10.1038/clpt.1985.6. [DOI] [PubMed] [Google Scholar]
- 2.Burrows J L. Determination of oxpentifylline and three metabolites in plasma by automated capillary gas chromatography using nitrogen-selective detection. J Chromatogr. 1987;423:139–146. doi: 10.1016/0378-4347(87)80336-5. [DOI] [PubMed] [Google Scholar]
- 3.Honess D J, Dennis I F, Bleehen N M. Pentoxifylline: its pharmacokinetics and ability to improve tumor perfusion and radiosensitivity in mice. Radiother Oncol. 1993;28:208–218. doi: 10.1016/0167-8140(93)90060-l. [DOI] [PubMed] [Google Scholar]
- 4.Lechner A J, Rouben L R, Potthoff L H, Tredway T L, Matuschak G M. Effects of pentoxifylline on tumor necrosis factor production and survival during lethal E. coli sepsis vs. disseminated candidiasis with fungal septic shock. Circ Shock. 1993;39:306–315. [PubMed] [Google Scholar]
- 5.Louie A, Baltch A L, Franke M A, Ritz W J, Smith R P, Singh J K, Gordon M A. Effect of pentoxifylline on the course of systemic Candida albicans infection in mice. J Antimicrob Chemother. 1996;37:943–954. doi: 10.1093/jac/37.5.943. [DOI] [PubMed] [Google Scholar]
- 6.Noel P, Nelson S, Bokulic R, Bagby G, Lippton H, Lipscomb G, et al. Pentoxifylline inhibits lipopolysaccharide-induced serum tumor necrosis factor and mortality. Life Sci. 1990;47:1023–1029. doi: 10.1016/0024-3205(90)90474-6. [DOI] [PubMed] [Google Scholar]
- 7.Opal S M, Cross A S, Kelly N M, Sadoff G C, Bodmer M W, Palardy J E. Efficacy of a monoclonal antibody directed against tumor necrosis factor in protecting neutropenic rats from lethal infection with Pseudomonas aeruginosa. J Infect Dis. 1990;161:1148–1152. doi: 10.1093/infdis/161.6.1148. [DOI] [PubMed] [Google Scholar]
- 8.Raju P I, Tolman K C, Davis P J, Ludden T M, Roy T K, Johnson F E. Distribution and metabolism of pentoxifylline in non-tumor-bearing mice. J Med (Westbury) 1993;24:353–368. [PubMed] [Google Scholar]
- 9.Rowland M, Tozer T N, editors. Clinical pharmacokinetics. Philadelphia, Pa: Lea and Febiger; 1989. pp. 12–13. [Google Scholar]
- 10.Sáez-Llorens X, Ramilo O, Mustafa M M, Mertsola J, de Alba C, Hansen E, McCracken G H., Jr Pentoxifylline modulates meningeal inflammation in experimental bacterial meningitis. Antimicrob Agents Chemother. 1990;34:837–843. doi: 10.1128/aac.34.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R V, Waller E S, Doluisio J T, Bauza M T, Surendra K P, Ho I, Lassman H B. Pharmacokinetics of orally administered pentoxifylline in humans. J Pharm Sci. 1986;75:47–52. doi: 10.1002/jps.2600750111. [DOI] [PubMed] [Google Scholar]
- 12.Strieter R M, Remick D G, Ward P A, Spengler R N, Lynch III J P, Larrick J, Kunkel S L. Cellular and molecular regulation of tumour necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155:1230–1260. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan G W, Carper H T, Novick W J, Jr, Mandell G L. Inhibition of the inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by pentoxifylline. Infect Immun. 1988;56:1722–1729. doi: 10.1128/iai.56.7.1722-1729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]