Abstract
The use of the antimalarial agent amodiaquine has been curtailed due to drug-induced idiosyncratic reactions. These have been attributed to the formation of a protein-reactive quinoneimine species via oxidation of the 4-aminophenol group. Therefore, the effects of chemical modifications on the disposition of amodiaquine in relation to its metabolism, distribution, and pharmacological activity have been investigated. The inclusion of a group at the C-5′ position of amodiaquine reduced or eliminated bioactivation, as determined by glutathione conjugate formation in vivo. This can be seen in two series of C-5′-substituted compounds: the bis-Mannich antimalarial agents, including cycloquine and pyronaridine, and mono-Mannich antimalarial agents containing a 5′-chlorophenyl group (tebuquine and 5′-ClPAQ). Chemical substitution at the C-5′ position also resulted in compounds which underwent slower elimination (<5% of the dose excreted into bile and urine, compared with 50% for amodiaquine) and increased levels of accumulation in tissue (10% of the dose in the liver at 48 h compared with 1% with amodiaquine). This may be due to an increase in either the lipophilicity or the basicity of the analogs and may reflect the lack of metabolic clearance for these compounds. The alteration in the disposition following the introduction of the C-5′ substituent resulted in an increased duration of antimalarial activity in the mouse compared with that for amodiaquine. While this is desirable in the treatment of malaria, repeated administration for prophylaxis may induce toxicity through accumulation. Therefore, by simple chemical modification it is possible to block the bioactivation of amodiaquine while maintaining and in some cases extending the duration of antimalarial activity.
The antimalarial agent amodiaquine is effective against both chloroquine-resistant and -sensitive strains of Plasmodium falciparum (24). However, its use has been curtailed due to the development of adverse reactions to the drug during prophylactic administration (7, 11). These drug-induced reactions have been attributed to the ability of amodiaquine to undergo oxidative metabolism to a chemically reactive quinoneimine species, detected in vivo as the excretion of glutathione conjugates in experimental animals (6, 10, 19).
Formation of this metabolite requires the presence of a 4-aminophenol group allowing formation of an electrophilic quinoneimine. To date, amodiaquine is unique among antimalarial agents in its ability to form this type of chemically reactive metabolite and to produce idiosyncratic drug reactions in humans. Most toxicities associated with the use of antimalarial agents are related to the dose (9) and have been ascribed to the ability of these compounds to accumulate in acidic cellular lysosomes. However, this mechanism of accumulation has also been proposed as a requirement for the pharmacological activities of some antimalarial agents (4, 8, 30). It has been shown that both the basicity and the lipophilicity have a great influence on the ability of compounds to accumulate (8) and on the site of accumulation (3). The extent of tissue storage is, in turn, known to influence the pharmacokinetics, and tissue distribution plays a major part in the pharmacological profile of a compound with respect to its duration of action, its potency in vivo, and its toxicology. A review of novel and therapeutically used compounds revealed that many antimalarial agents contain a 4-aminophenol group, which may influence their toxicological profiles. These compounds, analogs of amodiaquine, can be classed as Mannich antimalarial agents due to the incorporation of an amine group in their side chains.
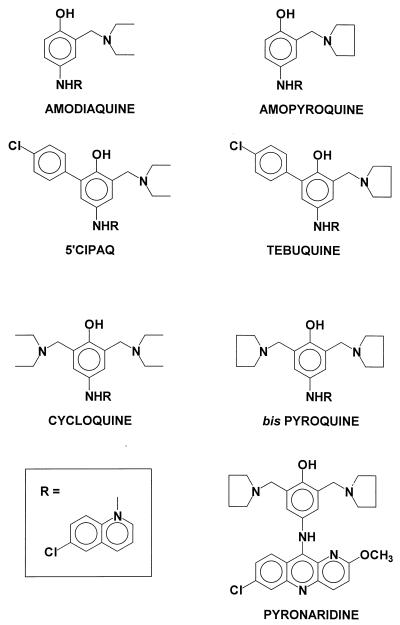
The introduction of a C-5′-chlorophenyl group (5′-ClPAQ and tebuquine; Fig. 1) increased the in vivo activity of amodiaquine 20-fold against Plasmodium berghei in the mouse (26). However, neither of these compounds has been used in the clinic. In the case of tebuquine, this was due to chronic toxicity seen during tests with animals, including the appearance of foamy macrophages (25a), a histological finding also seen following the chronic administration of chloroquine to humans (29). Tissue accumulation has been implicated in chloroquine toxicity (9), and the mechanism of tebuquine toxicity may also be a consequence of accumulation.
FIG. 1.
Chemical structures of amodiaquine and its analogs.
Previous studies have shown that bis-Mannich analogs of amodiaquine (cycloquine and bis-pyroquine; Fig. 1) have higher levels of activity than their mono-Mannich derivatives against both chloroquine-resistant and -sensitive strains of the malarial parasite in vitro (17). In vivo, the activities of bis-Mannich and mono-Mannich compounds are comparable against Plasmodium vinckei vinckei after intraperitoneal (i.p.) administration (1). However, the inclusion of the extra group at the C-5′ position of amodiaquine increases the duration of action for bis-Mannich antimalarial agents. Pyronaridine (Fig. 1), a bis-Mannich antimalarial substituted at the C-5′ with a pyrrolidine group and an acridine rather than a quinoline nucleus is currently undergoing clinical trials in China and has already been used effectively in the treatment of resistant strains of P. falciparum both in vitro (17) and in the clinic (20, 27).
The aim of this study was to investigate the influence of pharmacokinetics on pharmacodynamics with reference to three types of Mannich antimalarial compounds: the mono-Mannich compound amodiaquine, which has two sites of protonation; 5′-ClPAQ, which contains a chlorophenyl group at C-5′ of amodiaquine and which has a higher level of lipophilicity than amodiaquine; and the bis-Mannich cycloquine, which has three sites of protonation and which has a greater basicity than amodiaquine. These results have been extrapolated to related compounds to see whether physiochemical properties can explain the observed duration of antimalarial activity through changes in the disposition of the compound in vivo.
MATERIALS AND METHODS
Materials.
Amodiaquine, polyethylene glycol 200 (PEG 200), and 1-octanol were purchased from Sigma Chemical Co. (Poole, United Kingdom). Monopoly resolving medium (density, 1.114 g · ml−1; Ficoll Hypaque) was obtained from ICN (High Wycombe, United Kingdom), and Lymphoprep (density, 1.077 g · ml−1) was obtained from Nycomed (Birmingham, United Kingdom). Tissue solubilizer was obtained from Wallac UK (Milton Keynes, United Kingdom), Giemsa solution was obtained from BDH (Poole, United Kingdom), and sodium pentobarbitone (Sagatal) was obtained from Rhone Merieux (Harlow, United Kingdom). Tebuquine and pyronaridine were gifts from D. C. Warhurst (London School of Hygiene and Tropical Medicine, London, United Kingdom). [3H]amodiaquine was synthesized by following methods based on those of Burckhalter et al. (5). 5′-ClPAQ was synthesized by the methods of O’Neill et al. (16), and its radiolabelled form ([3H]5′-ClPAQ) was synthesized by the same method by substituting 4,7-dichloro-[G-3H]quinoline (213 mCi/mmol; radiolabelled purity, >98% by thin-layer chromatography [TLC]; Amersham International PLC, Bucks, United Kingdom) for 4,7-dichloroquinoline. Cycloquine and bis-pyroquine were synthesized by the methods of O’Neill et al. (16a). All other reagents were of analytical grade.
Synthesis of [3H]cycloquine.
4-Hydroxyacetanilide was allowed to react with excess diethylamine and aqueous formaldehyde in refluxing ethanol for 48 h. The solvent was removed under reduced pressure, and the intermediate was purified by silica gel column chromatography with methanol-dichloromethane (20:80 [vol/vol]). Hydrolysis of the amide function of the bis-(diethylaminomethyl) derivative with HCl (5 M) produced the corresponding 4-aminophenol derivative, which was subsequently allowed to react with tritium-labelled 4,7-dichloroquinoline in refluxing ethanol. The reaction was followed by TLC, and the product was purified by preparative TLC with methanol-dichloromethane (35:65 [vol/vol]).
Determination of drug lipophilicity.
The relative lipophilicity of each of the compounds used in the study was assessed at pH 7.4 and 5.0 by a method adapted from that of Zamora et al. (31). The lipophilicity (log D) was determined by measuring the relative partitioning of each drug between a lipophilic layer (1-octanol) and a hydrophilic layer (0.1 M phosphate-buffered saline [PBS; pH 7.4] or 0.1 M acetate buffer [pH 5.0]). Each compound was dissolved in dimethyl sulfoxide (DMSO), and the solution was added to a mixture of 1-octanol presaturated with buffer (1 ml) and buffer presaturated with 1-octanol (1 ml) to give a final drug concentration of 100 μM. The tubes were tumbled for 15 min and were then left to equilibrate overnight. The partition coefficient, D, is the ratio of the absorbance at λmax (340 nm) of the 1-octanol phase and the aqueous phase. The log D was then used as a measure of lipophilicity.
Isolation of peripheral blood cells.
Human polymorphonuclear leukocytes (PMNs), mononuclear leukocytes (MNLs), and erythrocytes (RBCs) were isolated from the venous blood of healthy volunteers (age, 21 to 40 years) by using a dual density gradient of Lymphoprep (4 ml) layered on monopoly resolving medium (8 ml). After centrifugation (750 × g, 45 min), the PMNs were relayered onto monopoly resolving medium (5 ml) to remove RBC contamination, and MNLs were relayered onto Lymphoprep (5 ml) to remove platelets. Both were centrifuged (750 × g) for a further 5 min. PMNs, MNLs, and RBCs were washed twice in PBS and were resuspended in platelet-poor plasma. The purity of the cell preparation (PMNs and MNLs) was assessed by staining with Wright’s stain (>99%), and the viability (PMNs and MNLs) was assessed by trypan blue dye exclusion (>95%).
Accumulation of [3H]amodiaquine and [3H]cycloquine in peripheral blood cells.
Freshly isolated PMNs, MNLs, or RBCs (4 × 106 cells · ml−1) were incubated separately with either [3H]amodiaquine or [3H]cycloquine (0.1 μCi, 10 μM, 2 h) at 37°C in platelet-poor plasma (1 ml). The accumulation was terminated by centrifugation (750 × g, 5 min), and the supernatant was assayed for radioactivity in scintillant (4 ml). The pelleted cells were washed in PBS, and the cellular accumulation of drug was assessed by scintillation counting (4 ml) following cell lysis. The results are expressed as the percent accumulation of radiolabel into each cell type.
Bioactivation of antimalarial agents in vivo.
Male CD1 mice (weight, 35 to 45 g) were anaesthetized with pentobarbitone (60 mg · kg−1 of body weight in 0.9% [wt/vol] saline), and tracheal and bile duct cannulae were inserted. Compounds were administered via the jugular vein. [3H]5′-ClPAQ, tebuquine, and bis-pyroquine (54 μmol · kg−1) were administered in DMSO (2.3 ml · kg−1); amopyroquine and pyronaridine (54 μmol · kg−1) were administered as their tetraphosphate salts in PEG 200–saline–ethanol (3:3:1 [vol/vol/vol]; 2.3 ml · kg−1). [3H]cycloquine was administered intravenously at a dose of 25 μmol · kg−1 in DMSO (2.3 ml · kg−1) because a dose of 54 μmol · kg−1 resulted in acute toxicity. A dose of 25 μmol · kg−1 and multiples thereof were used in all subsequent experiments.
Further studies were conducted with male Wistar rats (weight 200 to 250 g; n = 4/group) anaesthetized with urethane (7% [wt/vol] in 0.9% [wt/vol] saline; 20 ml · kg−1). All compounds were administered via the hepatic portal vein at the doses stated above in the appropriate vehicle (1 ml · kg−1).
Bile was collected over 3 h and was kept at −20°C until it was analyzed, after which time the animals were killed by cervical dislocation. Aliquots (10 μl) of bile from animals dosed with [3H]5′-ClPAQ and [3H]cycloquine were counted by liquid scintillation (4 ml), and the percentage of the dose excreted was calculated. This was compared with the data obtained for [3H]amodiaquine (19).
Investigation of the tissue distribution and urinary excretion of [3H]amodiaquine, [3H]5′-ClPAQ, and [3H]cycloquine over 48 h.
[3H]amodiaquine (54 μmol · kg−1; 20 μCi · kg−1), [3H]5′-ClPAQ (54 μmol · kg−1; 20 μCi · kg−1), or [3H]cycloquine (25 μmol · kg−1; 20 μCi · kg−1) was administered in PEG 200–ethanol (6:1 [vol/vol]; 2.3 ml · kg−1, i.p.) to male CD1 mice (weight, 30 to 40 g), which were housed in metabolism cages with access to water. Urine and feces were collected at 3, 6, 18, 24, and 48 h. After each time point the animals (n = 4 per time point) were anaesthetized with pentobarbitone (200 μl) and exsanguinated, and the liver, spleen, kidneys, gastrointestinal tract, heart, lungs, and brain were removed and stored at −20°C until they were required. Blood was kept at 4°C overnight, and serum and packed cells were separated and stored at −20°C until they were assayed for radioactivity.
Portions of each solid tissue (100 mg) and aliquots (two aliquots of 200 μl each) of blood were taken, tissue solubilizer was added (1 ml), and the mixtures were left overnight at 50°C. The solutions were cooled to room temperature, decolorized with hydrogen peroxide (ca. 200 μl), and neutralized with glacial acetic acid (50 μl). Samples were left overnight in darkness before being assayed for radioactivity in scintillant (16 ml). Results were expressed as a percentage of the dose for the whole organ. Blood volume was taken to be 3 ml (1, 23). The gastrointestinal tract was dissolved as a whole in tissue solubilizer (12 ml) and was left overnight as described above, and aliquots (two aliquots of 1 ml each) were taken and treated as described above. Feces were treated as described above but with the addition of distilled water (1 ml) to aid the solubilization. Aliquots (two aliquots of 0.5 ml each) of cage washings were taken and assayed for radioactivity; the values obtained were added to those of the radioactivity excreted into urine.
Identification of biliary and urinary metabolites.
Aliquots of bile from animals administered [3H]5′-ClPAQ or tebuquine (10 μl) were analyzed by LCMS with a gradient of 20 to 45% acetonitrile over 20 min with ammonium formate (20 mM; pH 3.5), a μBondapak C18 column (3.9 by 300 mm), and a flow rate of 1 ml · min−1. Aliquots (10 μl) of bile collected from rats following the administration of amopyroquine were analyzed with a gradient of 10 to 30% acetonitrile over 30 min with ammonium formate (20 mM; pH 3.5). Bile from animals dosed with pyronaridine was analyzed on a Spherisorb column (4.6 mm by 29 cm) with a gradient of 5 to 20% acetonitrile over 20 min with ammonium formate (20 mM; pH 3.5) at a flow rate of 1 ml · min−1. Bile from animals dosed with [3H]cycloquine was not analyzed further because no radioactivity was excreted into the bile. The limit of detection for all parent compounds was found to be 10 nmol. The coefficients of variation for all parent compounds were less than 6.2 (10 nmol) and 9.3 (30 nmol).
In vivo antimalarial activities of Mannich antimalarial agents after administration of a single dose.
The antimalarial activities of amodiaquine and its related compounds were assessed by the methods of Barlin and Tan (1) allowing concurrent evaluation of both parasite clearance time and duration of action.
Male CD1 mice (weight, 25 to 30 g) were inoculated i.p. with 106 parasitized RBCs (P. berghei N). Once the infection had reached a baseline of at least 20%, the mice were divided into groups (n = 6 per group) and were administered either amodiaquine cycloquine, or pyronaridine at 0 to 250 μmol · kg−1 i.p. or 5′-ClPAQ, tebuquine, amopyroquine, or bis-pyroquine at 25 μmol · kg−1 i.p. in PEG 200–ethanol (6:1 [vol/vol]; 100 μl/animal). Thin blood films from tail snips were taken 24, 48, 72, and 96 h and 7, 10, 14, 18, 21, and 28 days after drug administration. The films were fixed in methanol and stained for 20 min with 10% (wt/vol) Giemsa solution in PBS (pH 7.4). The level of parasitemia was measured as a percentage of RBCs infected with parasites in either a minimum of five fields of view or 1,000 RBCs.
Statistics.
All results are expressed as means ± standard deviations (SDs). Statistical analysis was performed by one-way analysis of variance with Bonferroni’s correction, with a P value of <0.05 deemed significant. Survival data were analyzed by the Mantel-Haenszel test, and survival rates were compared with those for mice receiving either the control treatment (no treatment) or amodiaquine (25 μmol · kg−1) treatment.
RESULTS
Drug lipophilicity.
All the compounds investigated were found to be highly lipophilic at pH 7.4, with the majority of the compound residing in the octanol phase of the octanol-buffer mixture. However, the introduction of a lipophilic chlorophenyl group into the C-5′ position of amodiaquine, seen in 5′-ClPAQ and tebuquine, was found to produce the greatest increase in the lipophilicity of the parent compound, amodiaquine (Table 1).
TABLE 1.
Effect of chemical modification on the lipophilicities of antimalarial agents as measured by octanol:water partitioning at pH 5.0 and pH 7.4
| Drug | Lipophilicity (log D)
|
|
|---|---|---|
| pH 5.0 | pH 7.4 | |
| Amodiaquine | −1.16 | 0.93 |
| Amopyroquine | −1.15 | 0.68 |
| 5′-C1PAQ | 1.20 | 1.18 |
| Tebuquine | 0.99 | 1.08 |
| Cycloquine | −1.35 | 0.98 |
| Bis-pyroquine | −1.27 | 0.71 |
| Pyronaridine | −0.88 | 0.34 |
Introduction of extra nitrogen groups into amodiaquine in the form of the bis-Mannich antimalarial agents (cycloquine and bis-pyroquine) caused the greatest level of reduction in lipophilicity compared with that caused by amodiaquine at pH 5, suggesting an increase in the basicity of the compound. Direct basicity, measured as pKa, could not be determined due to the inherent insolubility of these compounds in aqueous environments. The further inclusion of an acridine group (pyronaridine) was seen to reverse the decrease in both the lipophilicities and basicities of the bis-Mannich compounds.
Accumulation of amodiaquine and cycloquine in peripheral blood cells.
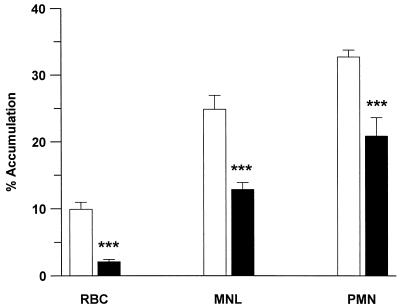
The relative patterns of accumulation of [3H]amodiaquine and [3H]cycloquine in cells were similar, with the greatest accumulation seen in PMNs (Fig. 2). The accumulation of radioactivity in MNLs was significantly less for both compounds (P < 0.01), with little accumulation in RBCs after 2 h (P < 0.001; Fig. 2). The level of [3H]cycloquine accumulation was significantly (P < 0.001) higher than the level of [3H]amodiaquine accumulation in all cell types.
FIG. 2.
Accumulation of [3H]amodiaquine (10 μM; ■) and [3H]cycloquine (10 μM; □) in PMNs, MNLs, and RBCs from platelet-poor plasma. ∗∗∗, P < 0.001 compared with amodiaquine.
Tissue distribution of radiolabelled compound.
The tissue distribution of radioactivity revealed that the gastrointestinal tract is the primary organ of accumulation of amodiaquine, 5′-ClPAQ, and cycloquine (Table 2). Other sites of accumulation included the liver, kidney, lung, and spleen. In all organs, 5′-ClPAQ was seen to accumulate to significantly (P < 0.001) higher levels than cycloquine, and both accumulated to significantly (P < 0.001) higher levels than amodiaquine. The levels of radioactivity decreased with time, although the rates of clearance for 5′-ClPAQ and cycloquine appeared to be slower than those for amodiaquine. The majority of the radioactivity measured within blood (<1% of the dose administered for amodiaquine, 5′-ClPAQ, and cycloquine) resided within the cellular fraction (>80%).
TABLE 2.
Distribution of radioactivity in tissue or blood over 48 h following administration of single i.p. doses of [3H]amodiaquine, [3H]5′-ClPAQ, and [3H]cycloquine to CD1 mice
| Compartment | Druga | % Dose/compartment at the following timesb:
|
||||
|---|---|---|---|---|---|---|
| 3 h | 6 h | 18 h | 24 h | 48 h | ||
| Liver | AQ | 4.3 ± 1.2 | 3.5 ± 1.0 | 9.2 ± 8.5 | 2.9 ± 1.1 | 0.9 ± 0.2 |
| 5′-ClPAQ | 13.8 ± 2.7** | 13.6 ± 2.5*** | 17.8 ± 1.6 | 14.8 ± 3.7*** | 10.7 ± 1.6*** | |
| CYC | 12.9 ± 5.9** | 12.4 ± 2.0*** | 10.8 ± 1.8 | 10.4 ± 2.2*** | 13.3 ± 1.3***,†† | |
| Kidney | AQ | 1.5 ± 0.1 | 1.3 ± 0.4 | 0.6 ± 0.1 | 0.5 ± 0.2 | 0.7 ± 1.3 |
| 5′-ClPAQ | 3.8 ± 1.1** | 4.5 ± 1.2*** | 9.0 ± 0.6*** | 4.4 ± 1.2*** | 6.0 ± 1.1*** | |
| CYC | 2.2 ± 0.9† | 1.8 ± 0.2††† | 1.7 ± 0.1***,††† | 1.7 ± 0.5*,††† | 2.3 ± 0.3*,††† | |
| Lung | AQ | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.01 |
| 5′-ClPAQ | 1.4 ± 0.4 | 2.1 ± 0.7*** | 4.5 ± 0.6*** | 2.7 ± 0.9*** | 2.1 ± 0.4*** | |
| CYC | 1.3 ± 0.7 | 1.0 ± 0.2*,† | 0.4 ± 0.1††† | 0.4 ± 0.3**,††† | 0.8 ± 0.2***,††† | |
| Spleen | AQ | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.05 ± 0.1 | 0.01 ± 0.05 |
| 5′-ClPAQ | 0.9 ± 0.2* | 1.9 ± 0.5*** | 2.3 ± 0.2*** | 2.7 ± 0.7*** | 1.3 ± 0.2*** | |
| CYC | 0.6 ± 0.3† | 0.6 ± 0.1††† | 0.4 ± 0.1*,††† | 0.6 ± 0.1††† | 0.7 ± 0.1***,††† | |
| Gastrointestinal tract | AQ | 1.4 ± 1.9 | 18.9 ± 4.5 | 24.3 ± 13.6 | 21.7 ± 9.6 | 3.4 ± 2.7 |
| 5′-ClPAQ | 19.6 ± 4.5** | 18.3 ± 3.4 | 34.1 ± 3.3 | 19.4 ± 4.7 | 10.5 ± 1.2 | |
| CYC | 22.4 ± 7.6*** | 17.3 ± 0.9 | 18.7 ± 4.8 | 21.2 ± 3.3 | 5.9 ± 3.8 | |
| Blood | AQ | 0.4 ± 0.5 | 1.3 ± 0.2 | 0.2 ± 0.2 | 0.7 ± 0.1 | 0.3 ± 0.01 |
| 5′-ClPAQ | 0.3 ± 0.1 | 5.2 ± 3.8 | 1.0 ± 0.1 | 1.0 ± 0.05 | 0.4 ± 0.05 | |
| CYC | 0.3 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.3 ± 0.1 | |
The drug doses are given in the text. AQ, [3H]amodiaquine; 5′-ClPAQ, [3H]5′-ClPAQ; CYC, [3H]cycloquine.
Data are means ± SDs (n = four mice per time point). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with amodiaquine; †, P < 0.05; ††, P < 0.01; †††, P < 0.001 for cycloquine compared with 5′-ClPAQ.
After 24 h, 80% of the dose could be accounted for following [3H]amodiaquine administration, and 50 to 60% was accounted for following the administration of [3H]5′-ClPAQ or [3H]cycloquine.
Excretion of radiolabelled compound.
The level of excretion of radioactivity into urine and feces following the i.p. administration of the compounds is shown in Table 3. Fifty percent of the amodiaquine dose was accounted for, whereas introduction of either a 5′-chlorophenyl group (5′-ClPAQ) or another Mannich group (cycloquine) reduced the level of excretion of radiolabelled material significantly (P < 0.001); 3 to 8% of the dose was excreted into the urine and feces following the administration of 5′-ClPAQ, and no radiolabelled compound (<1% of the dose) was detected in the urine of mice following the administration of [3H]cycloquine, with fecal excretion accounting for only 5% of the administered dose.
TABLE 3.
Excretion of radioactivity in urine and feces over 48 h following administration of a single i.p. dose of [3H]amodiaquine, [3H]5′-ClPAQ, and [3H]cycloquine to male CD1 mice and excretion into bile following intravenous administration.
| Compartment | Druga | % Dose/sample at the following timesb:
|
||||
|---|---|---|---|---|---|---|
| 3 h | 6 h | 18 h | 24 h | 48 h | ||
| Feces | AQ | 6.3 ± 0.6 | 6.5 ± 3.8 | 14.9 ± 7.0 | 22.7 ± 10.5 | NMc |
| 5′-ClPAQ | 0.1 ± 0.2 | 0.1 ± 0.1** | 2.4 ± 1.0*** | 2.1 ± 0.1*** | 3.4 ± 0.7*** | |
| CYC | NM | 0.2 ± 0.2** | 4.5 ± 0.5** | 3.3 ± 1.4** | NM | |
| Urine | AQ | 7.8 ± 0.1 | 8.4 ± 4.2 | 14.4 ± 3.7 | 22.7 ± 6.0 | NM |
| 5′-ClPAQ | 3.6 ± 1.9 | 2.3 ± 1.3** | 4.3 ± 4.1*** | 4.6 ± 1.4*** | 2.7 ± 1.3*** | |
| CYC | NM | 0.1 ± 0.0*** | 0.2 ± 0.1*** | 0.2 ± 0.1*** | NM | |
| Bile | AQ | 28.5 ± 6.7d | ||||
| 5′-ClPAQ | 3.0 ± 0.9 | |||||
| CYC | 0.6 ± 0.2 | |||||
The drug doses are given in the text. AQ, [3H]amodiaquine; 5′-ClPAQ, [3H]5′-ClPAQ; CYC, [3H]cycloquine.
Values are means ± SDs (n = four mice per time point). **, P < 0.01; ***, P < 0.001 compared with amodiaquine.
NM, not measured.
Data taken from reference 19.
Over 3 h biliary excretion followed the excretion of radiolabelled compound into feces, with <4% of the dose being detected in bile following the administration of either 5′-ClPAQ or cycloquine but with 28% of the dose being detected in bile following the administration of amodiaquine (19).
Identification of glutathione conjugates formed in vivo.
Insufficient bile and urine were obtained from mice for chemical analysis and characterization of metabolites. However, following the administration of 5′-ClPAQ to rats, two glutathione conjugates of the parent compound were identified in bile by LCMS (Table 4). Although both metabolites produced a mass ion current at m/z 771 and gave [M+1] molecular ions at m/z 773/771, the alteration in retention times (12 and 14 min) and the different fragmentation patterns suggest the presence of two glutathione metabolites rather than one. This suggests that glutathione has conjugated at both the C-2′ and C-6′ positions of 5′-ClPAQ.
TABLE 4.
Molecular ions and principal fragments of the biliary metabolites of 5′-ClPAQ in the rata
| Metaboliteb | Retention time (min) | m/z (relative intensity) |
|---|---|---|
| 5′-ClPAAQI | 24 | 466/464 (M+1, 80/100), 393 (52) |
| GS-5′-ClPAQ | 12 | 773/771 (M+1, 90/100), 698 (100), 425 (100), 386 (95) |
| GS-5′-ClPAQ | 14 | 773/771 (M+1, 73/100), 698 (68) |
Four rats were used.
5′-ClPAAQI, 5′-chlorophenylamodiaquine quinoneimine; GS-5′-ClPAQ, 5′-chlorophenylamodiaquine quinoneimine-glutathione adduct.
Interestingly, it appears that the quinoneimine metabolite of 5′-ClPAQ is stable enough to be excreted into the bile of rats, giving [M+1] ions at m/z 466/464 (Table 4), 2 amu below that of the parent compound, although UV analysis suggests that this is only a minor metabolite (data not shown).
However, all these metabolites plus the parent compound (5′-ClPAQ), which was also detected in the bile of rats, accounted for only 7% of the administered dose.
No glutathione conjugates or quinoneimine metabolites could be detected in bile samples from animals dosed with tebuquine, amopyroquine, pyronaridine, bis-pyroquine, or cycloquine.
Antimalarial activities of Mannich antimalarial agents.
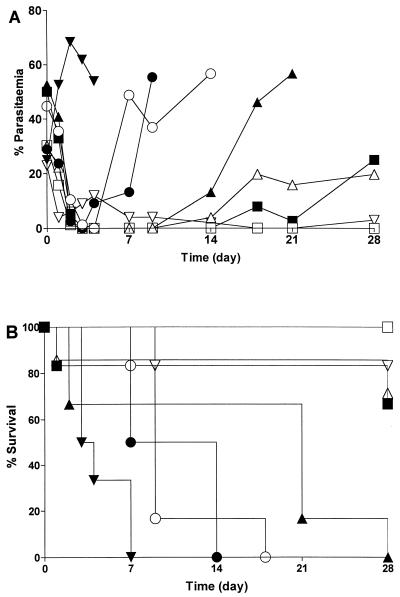
At doses of 2.5 μmol/kg and lower, amodiaquine, pyronaridine, and cycloquine had no effect on the level of parasitemia in the mouse or on the survival rate compared with those for the controls (Fig. 3). At concentrations of 25 μmol · kg−1 and above all compounds were equally active, clearing parasites from the blood by day 2. All three compounds significantly (P < 0.01) increased the survival rates for treated animals compared with those for solvent-treated control animals.
FIG. 3.
Effect of C-5′-substituted compounds (25 μmol · kg−1 i.p.) on the percentage of parasitemia (A) and the survival rate of mice infected with P. berghei (B). ▾, vehicle; ○, amodiaquine; •, amopyroquine; ▵, 5′-ClPAQ; ▴, tebuquine; □, cycloquine; ■, bis-pyroquine; ▿, pyronaridine.
The introduction of a C-5′ substituent into the amodiaquine molecule had profound effects on the duration of antimalarial action. The introduction of a lipophilic chlorophenyl group at the C-5′ position of amodiaquine extended the duration of action. Recrudescence occurred at day 4 after amodiaquine (25 μmol · kg−1) administration, whereas it occurred at day 14 after 5′-ClPAQ and tebuquine (25 μmol · kg−1) administration (Fig. 3A); at day 28, four of six mice survived after 5′-ClPAQ administration (P < 0.01 compared with amodiaquine) and two of six mice survived after tebuquine administration (Fig. 3B). In contrast, all the mice administered amodiaquine (25 μmol · kg−1) were dead by day 14.
Following the administration of the bis-Mannich antimalarial agents no recrudescence occurred after the administration of cycloquine (25 to 250 μmol · kg−1) during the 28 days of the study. The levels of parasitemia recrudesced to <20% at day 4 following administration of pyronaridine, with levels decreasing to zero by day 28; bis-pyroquine was found to be active until day 28 (Fig. 3A). The prolonged activity of the bis-Mannich antimalarial agents resulted in a significant (P < 0.01) increase in the rate of survival of mice treated with all three compounds until day 28 compared with that for mice treated with amodiaquine (Fig. 3B).
DISCUSSION
In vivo studies revealed that the introduction of a 5′ substituent into amodiaquine had a profound effect on drug excretion. While substantial amounts of radioactivity were excreted into both the bile and the urine of mice following the administration of amodiaquine (28 and 22%, respectively [19]), there was a significant decrease in the level of excretion upon the introduction of either a lipophilic chlorophenyl group (5′-ClPAQ) or an amphiphilic diethylamino group (cycloquine). Metabolism studies with the mouse suggest that the decreased clearance of these C-5′-substituted compounds may be due to a decreased metabolic clearance through the prevention of glutathione conjugation. Only following the administration of 5′-ClPAQ were glutathione conjugates found in the bile of mice. However, the levels of radioactivity associated with these metabolites were significantly lower compared with those found following amodiaquine administration (19).
The excretion of glutathione conjugates following the administration of amodiaquine has been associated with the prior formation of a reactive quinoneimine metabolite (6). Both direct and indirect causal mechanisms (15, 18) have been proposed to explain the idiosyncratic toxicity associated with the prophylactic administration of amodiaquine. However, the formation of a reactive quinoneimine metabolite from amodiaquine in both the liver and leukocytes is thought to be central to amodiaquine toxicity due to the ability of the quinoneimine metabolite to bind to protein (6, 22).
The lack of glutathione conjugate excretion following the introduction of a C-5′ group suggests that either quinoneimine formation has been precluded or conjugation to glutathione has been blocked through either steric constraints or electronic effects. Parallel in vitro studies (14) suggest the latter, because the depletion of glutathione from stimulated PMNs seen with amodiaquine was inhibited by the introduction of a group at the C-5′ position of amodiaquine. Interestingly, no glutathione conjugates were detected in rat or mouse bile following the administration of amopyroquine, a close structural analog of amodiaquine which does not contain a group at the C-5′ position. However, in vitro studies revealed the comparable abilities of amopyroquine and amodiaquine to deplete intracellular levels of glutathione from PMNs (14), suggesting that minor chemical alterations to the diethyl side chain of amodiaquine alter hepatic metabolism.
Both the ability of a compound to pass freely through cellular lipid layers and its ability to become trapped within the acidic environment of a cellular lysosome are known to affect the movement of a compound into and out of a cell or tissue into the blood and thus its safe excretion. Therefore, the reduced clearance of these compounds may be influenced by an alteration in the tissue residency times of cycloquine and 5′-ClPAQ compared with that of amodiaquine.
While the sites of accumulation appeared to be the same for all compounds, with the major organ being the gastrointestinal tract, the extent of accumulation and the elimination time from tissues was markedly different. Following the accumulation of amodiaquine there was a substantial decline in the maximum levels of radioactivity over 48 h. However, there was no significant change in tissue distribution following the administration of either cycloquine or 5′-ClPAQ, with the levels in the liver still being above 10% of the administered dose after 48 h, whereas the levels in liver were 1% of the administered dose after amodiaquine administration. The increased level of accumulation of cycloquine was also observed in vitro in human peripheral blood cells, with cycloquine accumulating to a greater extent than amodiaquine, although both compounds underwent greater accumulation in the leukocyte fraction, i.e., the PMNs and MNLs, with little accumulation in RBCs. Following the in vivo administration of all three compounds to mice, the ability of cells to accumulate drug resulted in detectable levels of radioactivity being found only within the packed blood cell fraction; little radioactivity was seen within the plasma. This confirms the results from other studies, in which both amodiaquine and its major plasma metabolite, desethylamodiaquine, are concentrated within the leukocyte fraction under physiological conditions (12, 28).
Studies with desipramine revealed that introduction of an N-acetyl group prevented desipramine from becoming charged without altering its lipophilic properties, resulting in a considerable shift in the tissue residency time, with the tissue half-lives being greatly reduced following administration of the nonbasic compound (13). This resulted in a greatly increased elimination rate from the animal. In reverse, this could be applied to cycloquine. The inclusion of the third protonation site in cycloquine increases the basicity compared with that of amodiaquine, resulting in a decrease in the lipophilicity of the compound at pH 5. This would trap cycloquine within the acidic vacuoles of the tissue by reducing its ability to pass through lipid membranes. These changes in lipophilicity resulted in a significantly longer residence time within the tissues, and this occurred to such an extent that a reduction in levels in tissue could not be measured over a 48-h period.
A different mechanism may be used to explain the increased tissue residency time seen following 5′-ClPAQ administration. In this instance, the increase in lipophilicity without any alteration in basicity resulted in a compound which undergoes extensive accumulation in tissue by residing within the lipid membranes of the cell rather than the acidic vacuoles.
However, the total radioactivity accounted for after 5′-ClPAQ and cycloquine administration was only 50 to 60% of the administered dose, suggesting that there are other storage tissues within the animal. A high proportion of the radioactivity following the administration of all three compounds was found within the gastrointestinal tract. In the cases of cycloquine and 5′-ClPAQ, this could not be accounted for as radioactivity within the fecal material of the lower gastrointestinal tract. It has been shown that i.p. administration differs from other routes of administration in that a proportion of the drug remains at the site of injection (3). Therefore, the unaccounted for radioactivity may, in part, reside in the remaining adipose tissue of the peritoneal cavity, and that radioactivity was not measured during this experiment. An increased ability to reside in tissue compartments has been implicated in the cardiotoxicity seen after chronic administration of chloroquine (9).
Successive chemical alteration of the amodiaquine molecule did not have a major effect on the initial pharmacological activity of amodiaquine. All of the compounds studied increased the survival time above that for the controls (which received vehicle only) and reduced the level of parasitemia to <1% by 48 h after the administration of a single dose (≥25 μmol · kg−1). These results are due to the maintenance of the groups known to be essential to the antimalarial activity of the aminoquinoline antimalarial agents: the 7-chloro group and the four-carbon bridge between the 4-amino group and the side chain nitrogen (21).
However, the chemical alterations did have a significant effect on the duration of pharmacological action. As discussed above, chemical alteration can affect the accumulation of a drug in tissues. Thus, tissues can act as storage compartments and as such may influence the pharmacokinetics and subsequently the therapeutic actions of drugs. The introduction of a lipophilic chlorophenyl group into the C-5′ position of amodiaquine was seen to increase the lipophilicity of the compound (5′-ClPAQ and tebuquine) and hence to increase the tissue residency time of 5′-ClPAQ. This may explain the prolonged durations of action of 5′-ClPAQ and tebuquine compared with that of amodiaquine. Similarly, the increased tissue residency time of cycloquine due to the extra protonation site may explain the increased durations of activity for cycloquine and bis-pyroquine. This would suggest that the long duration of pyronaridine activity compared with that of amodiaquine activity is also due, in part, to an increase in the level of accumulation in tissue. In vitro antimalarial tests suggest that pyronaridine has a higher intrinsic level of activity (17) than amodiaquine and cycloquine. The increased intrinsic level of activity of pyronaridine may result in a drug that is effective at lower concentrations in plasma and that therefore does not require excess levels of accumulation in tissues.
Thought to be central to both amodiaquine toxicity and its elimination is its ability to form a protein-reactive quinoneimine metabolite that is excreted into bile as a glutathione conjugate (6). Studies in vitro with human PMN cells (14) suggest that introduction of a C-5′ group into amodiaquine inhibits the formation of such a metabolite, and this is confirmed in this study by the lack of glutathione conjugate formation in vivo. Other antimalarial agents such as chloroquine are cardiotoxic upon repeated administration (9) due to their ability to accumulate. However, while cycloquine is no longer used as an antimalarial agent, its ability to accumulate accompanied by the obvious reduced level of elimination suggests that accumulation rather than protein reactivity may play a significant role in any toxicity seen with novel bis-Mannich antimalarial agents once they are present in humans. Pyronaridine, a bis-Mannich antimalarial agent currently undergoing clinical trials, has been used successfully as a treatment for malaria and is being heralded as a promising new antimalarial agent (2). While studies with humans suggest that pyronaridine has no side effects following the administration of a single dose, care must be taken, and further investigation is required to ascertain the toxic potential of pyronaridine, especially after repeated administration, considering its apparent similarity to cycloquine in these tests.
It appears from the results of the present investigation that increases in both lipophilicity and basicity by substitution at the C-5′ position of amodiaquine have a profound effect on the ability of antimalarial agents to accumulate and reside within tissues. The increase in the tissue residency time of C-5′-substituted compounds in turn increases the duration of their pharmacological activity through maintenance of therapeutically active concentrations in plasma. However, while these properties may appear to be valuable for treatment, they may also increase the toxicity of the compound if it is given for prophylaxis, for which multiple doses are used. The increase in half-life may also have repercussions on the development of parasitic resistance to such compounds through the prolonged exposure of parasites to subtherapeutic drug concentrations (25).
ACKNOWLEDGMENTS
We thank S. Newby for technical assistance and J. L. Maggs for analysis of spectra by LCMS.
This work was funded by The Wellcome Trust (J.E.R., M.D.T., S.A.W., and B.K.P.) and Roche (P.M.O.).
REFERENCES
- 1.Barlin G B, Tan W L. Potential antimalarials. V. 4-(7′-Trifluoromethylquinolin-4′-ylamino)phenols, 4-[2′7′- and 2′8′-bis(trifluoromethyl)quinolin-4′-ylamino] phenols and N4-substituted 2,7-(and 2,8-)bis(triefluoromethyl)quinolin-4-amines. Austr J Chem. 1985;38:1827–1835. [Google Scholar]
- 2.Basco L K, Le Bras J. In vitro activity of pyronaridine against African strains of Plasmodium falciparum. Ann Trop Med Parasitol. 1992;86:447–454. doi: 10.1080/00034983.1992.11812693. [DOI] [PubMed] [Google Scholar]
- 3.Betschart H R, Jondorf W R, Bickel M H. Differences in adipose tissue distribution of basic lipophilic drugs between intraperitoneal and other routes of administration. Xenobiotica. 1988;18:113–121. doi: 10.3109/00498258809055142. [DOI] [PubMed] [Google Scholar]
- 4.Bray G, Ward S A. Malaria chemotherapy: resistance to quinoline containing drugs in Plasmodium falciparum. FEMS Microbiol Lett. 1993;113:1–8. doi: 10.1111/j.1574-6968.1993.tb06479.x. [DOI] [PubMed] [Google Scholar]
- 5.Burckhalter J H, Tendwick J H, Jones F H, Jones P A, Holcome W F, Rawlins A L. Aminoalkylphenols as antimalarials. II. (Heterocyclic-amino)-α-amino-o-cresols: the synthesis of camoquine. J Am Chem Soc. 1948;70:1363–1373. doi: 10.1021/ja01184a023. [DOI] [PubMed] [Google Scholar]
- 6.Harrison A C, Kitteringham N R, Clarke J B, Park B K. The mechanism of bioactivation and antigen formation of amodiaquine in the rat. Biochem Pharmacol. 1992;43:1412–1430. doi: 10.1016/0006-2952(92)90198-r. [DOI] [PubMed] [Google Scholar]
- 7.Hatton C S R, Peto T E A, Bunch C, Pasvol G. Frequency of severe neutropenia associated with amodiaquine prophylaxis against malaria. Lancet. 1986;i:411–413. doi: 10.1016/s0140-6736(86)92371-8. [DOI] [PubMed] [Google Scholar]
- 8.Hawley S R, Bray P G, Ward S A. Amodiaquine accumulation in Plasmodium falciparum as a possible explanation for its superior antimalarial activity over chloroquine. Mol Biochem Parasitol. 1996;80:15–25. doi: 10.1016/0166-6851(96)02655-2. [DOI] [PubMed] [Google Scholar]
- 9.Jaeger A, Sauder P, Kopferschmitt J, Flesch F. Clinical features and management of poisoning due to antimalarial drugs. Med Toxicol. 1987;2:242–273. doi: 10.1007/BF03259868. [DOI] [PubMed] [Google Scholar]
- 10.Jewell H, Maggs J L, Harrison A C, O’Neill P M, Ruscoe J E, Park B K. The role of hepatic metabolism in the bioactivation and detoxication of amodiaquine. Xenobiotica. 1995;25:199–217. doi: 10.3109/00498259509061845. [DOI] [PubMed] [Google Scholar]
- 11.Larrey D, Castot A, Pessayre D, Merigot P, Machayekhy J P, Feldamn G, Lenoir A, Ruelt B, Benhamov J P. Amodiaquine-induced hepatitis. Ann Int Med. 1986;104:801–803. doi: 10.7326/0003-4819-104-6-801. [DOI] [PubMed] [Google Scholar]
- 12.Laurent F, Saivin S, Chretien P, Magnaval J F, Peyron F, Sqalli A, Tufenkji A E, Coulais Y, Baba H, Campistron G, Regis H, Ambroise-Thomas P, Bryskier A, Houin G. Pharmacokinetic and pharmacodynamic study of amodiaquine and its two metabolites after single oral dose in human volunteers. Arzneim-Forsch/Drug Res. 1993;43:612–616. [PubMed] [Google Scholar]
- 13.Moor M J, Steiner S H, Jachertz G, Bickel M H. Adipose tissue distribution and chemical structure of basic lipophilic drugs: desipramine, N-acetyl desipramine, and haloperidol. Pharmacol Toxicol. 1992;70:121–124. doi: 10.1111/j.1600-0773.1992.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 14.Naisbitt D J, Ruscoe J E, Williams D, O’Neill P M, Pirmohamed M, Park B K. Disposition of amodiaquine and related antimalarials in human neutrophils. Implications for drug design. J Pharmacol Exp Ther. 1997;280:884–893. [PubMed] [Google Scholar]
- 15.Neftel K A, Woodtly W, Schmid M, Frink P G, Fehr J. Amodiaquine induced agranulocytosis and liver damage. Br Med J. 1986;292:721–723. doi: 10.1136/bmj.292.6522.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill P M, Willcock D J, Hawley S R, Bray P G, Storr R C, Ward S A, Park B K. 4-Aminoquinolines—past, present and future: a chemical perspective. 1997. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 16a.O’Neill, P. M., et al. Unpublished data.
- 17.Peters W, Robinson B L. The chemotherapy of rodent malaria. XLVII. Studies on pyronaridine and other Mannich base antimalarials. Ann Trop Med Parasitol. 1992;86:455–465. doi: 10.1080/00034983.1992.11812694. [DOI] [PubMed] [Google Scholar]
- 18.Rouveix B, Coulombel L, Aymard J P, Chau F, Abel L. Amodiaquine-induced immune agranulocytosis. Br J Haematol. 1989;71:7–11. doi: 10.1111/j.1365-2141.1989.tb06266.x. [DOI] [PubMed] [Google Scholar]
- 19.Ruscoe J E, Jewell H, Maggs J L, O’Neill P M, Storr C R, Ward S A, Park B K. The effect of chemical substitution on the metabolic activation, metabolic detoxication, and pharmacological activity of amodiaquine in the mouse. J Pharmacol Exp Ther. 1995;273:393–404. [PubMed] [Google Scholar]
- 20.Shao B R. A review of antimalarial drug pyronaridine. Chin Med J. 1990;103:428–434. [PubMed] [Google Scholar]
- 21.Thomson P E, Werbel L M. Antimalarial agents: chemistry and pharmacology. New York, N.Y: Academic Press, Inc.; 1972. [Google Scholar]
- 22.Tingle M D, Jewell H, Maggs J L, O’Neill P M, Park B K. The bioactivation of amodiaquine by human polymorphonuclear leucocytes in vitro: chemical mechanisms and the effects of fluorine substitution. Biochem Pharmacol. 1995;50:1113–1119. doi: 10.1016/0006-2952(95)00236-s. [DOI] [PubMed] [Google Scholar]
- 23.Tuffery A A. Laboratory animals: an introduction for new experimenters. Chichester, United Kingdom: Wiley; 1987. [Google Scholar]
- 24.Watkins W, Sixsmith D, Spencer H, Boriga D, Kariuka D, Kipingor T. Effectiveness of amodiaquine as treatment for chloroquine-resistant Plasmodium falciparum infections in Kenya. Lancet. 1984;i:357–359. doi: 10.1016/s0140-6736(84)90410-0. [DOI] [PubMed] [Google Scholar]
- 25.Watkins W M, Mosobo M. Treatment of Plasmodium falciparum malaria with pyrimethamine and sulphadoxine: a selective pressure for resistance is a function of long elimination half-life. Trans R Soc Trop Med Hyg. 1993;87:75–79. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- 25a.Werbel, L. M., and H. Chung (Walter Reed Army Institute of Research). Personal communication.
- 26.Werbel L M, Cook P D, Elslager E F, Hung J H, Johnson J L, Keston S J, McNamara D J, Ortwine D F, Wroth D F. Synthesis, antimalarial activity and quantitative structure-activity relationships of tebuquine and a series of related 5-[(7-chloro-4quinolinyl)amino]-3-[(alkylamino)methyl][1,1′-biphenyl]-2-ols and N-oxides. J Med Chem. 1986;29:924–939. doi: 10.1021/jm00156a009. [DOI] [PubMed] [Google Scholar]
- 27.Winstanley P A. Pyronaridine: a promising drug for Africa. Lancet. 1996;347:2–3. doi: 10.1016/s0140-6736(96)91548-2. [DOI] [PubMed] [Google Scholar]
- 28.Winstanley P A, Edwards I G, Orme M L, Breckenridge A M. The disposition of amodiaquine in man after oral administration. Br J Clin Pharmacol. 1987;23:1–7. doi: 10.1111/j.1365-2125.1987.tb03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto A, Adachi S, Matsuzawa Y, Kitani T, Hiraoka A, Seki K. Studies on drug induced lipidosis. VII. Effects of bis-(-diethyl-aminoethylther of hexestrol, chloroquine, homochlorocyclizine, prenylamine and diazacholestrol on the lipid composition of rat liver and kidney. Lipid. 1976;11:616–622. doi: 10.1007/BF02532875. [DOI] [PubMed] [Google Scholar]
- 30.Yayon A, Cabantchik Z I, Ginsburg H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984;11:2695–2700. doi: 10.1002/j.1460-2075.1984.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamora J M, Pearce H L, Beck W T. Physical-chemical properties shared by compounds that modulate multidrug resistance in human leukaemic cells. Mol Pharmacol. 1988;33:454–462. [PubMed] [Google Scholar]