ABSTRACT
Small intestinal lipomatosis is a rare condition with a poorly understood epidemiology and pathophysiology. Cases of small intestinal lipomatosis have been documented in multiple countries over the last century, yet little has been published regarding the natural history of this disease. Therapeutic options are largely surgical and based on limited evidence. We report a unique case of diffuse jejunal lipomatosis in a 62-year-old man with complications of small bowel obstruction, small bowel volvulus, jejunal diverticulosis, pneumatosis intestinalis, malnutrition, small intestinal bacterial overgrowth, and intestinal dysmotility developing over a 12-year period.
KEYWORDS: jejunum, lipomatosis, diverticulosis, volvulus, dysmotility
INTRODUCTION
Small intestinal lipomas represent rare, largely benign lesions ranging from solitary masses to diffuse intestinal lipomatosis. In a large autopsy series of 1,319 subjects, the prevalence of intestinal lipomatosis was 1.5%.1 Patients with intestinal lipomatosis may present with nonspecific symptoms of abdominal pain, altered bowel habits, nausea, emesis, weight loss, and obstruction.2 Despite early reports of intestinal lipomatosis in 1906 by Hellström,3 the natural history of the disease remains poorly understood. Case reports from across the globe have previously associated small intestinal lipomatosis with various causes of intestinal obstruction, diverticulosis, intestinal bleeding, intestinal infarction, malabsorption, and small intestinal bacterial overgrowth (SIBO).4–12 Here, we present the case of a man with diffuse jejunal lipomatosis and chronicle his disease progression with multiple complications.
CASE REPORT
In 2010, a previously healthy 50-year-old man presented to a peripheral center with abdominal pain, emesis, and diarrhea. An abdominal computed tomography (CT) scan demonstrated jejunal dilation and marked intramural fat deposition in keeping with diffuse jejunal lipomatosis (Figure 1). Fluoroscopy demonstrated large jejunal diverticulae (Figure 2). Images were reviewed at Brigham and Women's Hospital to confirm the diagnosis. He was managed with opioid analgesia and a short period of total parenteral nutrition (TPN) until he was able to tolerate oral intake.
Figure 1.
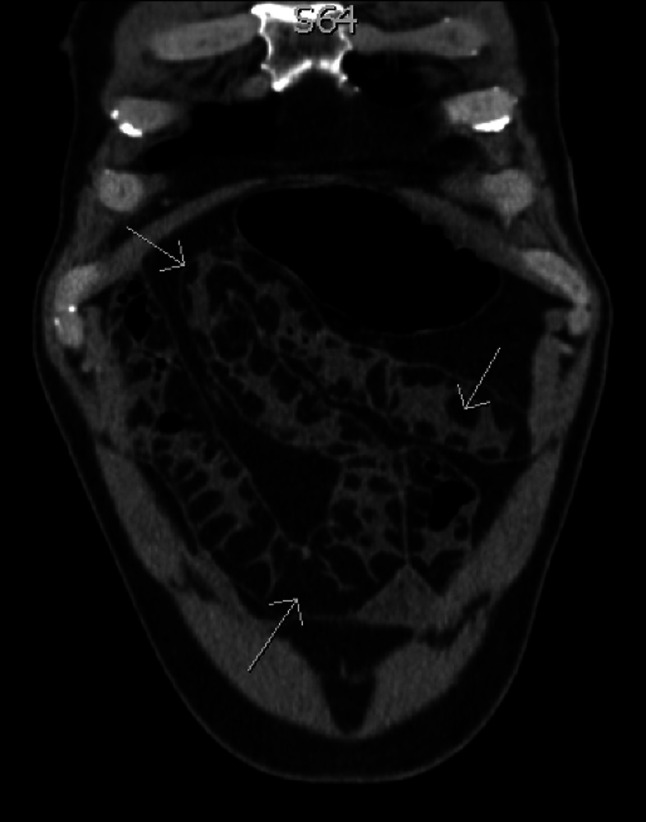
Coronal section from an abdominal computed tomography demonstrates diffuse intramural fat deposition within the jejunum (narrow arrowhead).
Figure 2.
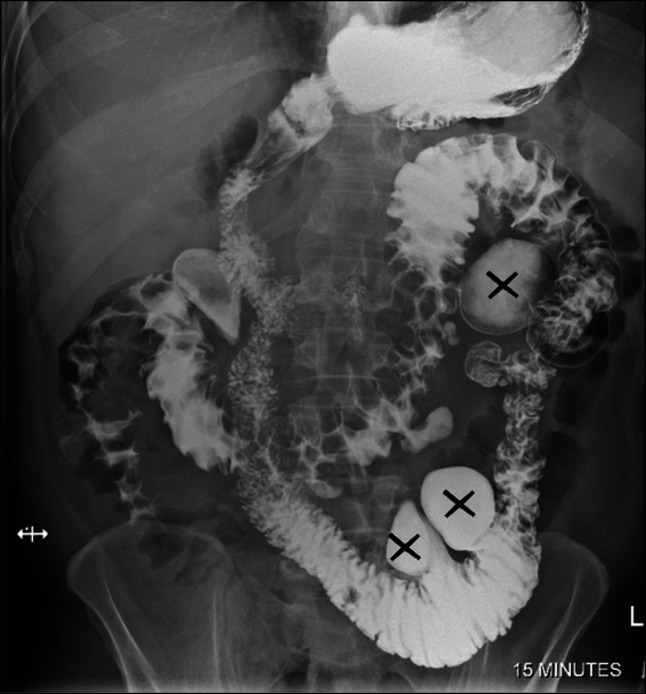
Small bowel fluoroscopy demonstrating multiple, large jejunal diverticulae (X).
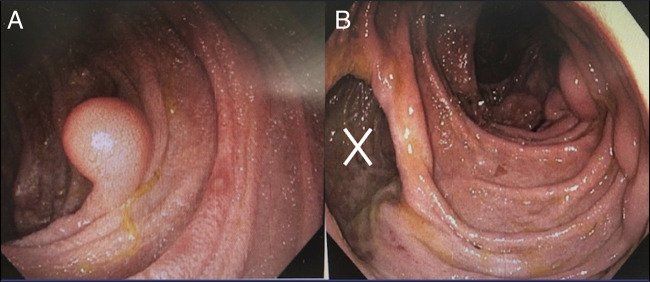
He required 5 admissions from 2010 to 2019 due to complications, including a nonlocalized perforation with pneumatosis intestinalis, and a small bowel volvulus. Three laparotomies were performed, including a Ladd procedure given intraoperative evidence of incomplete intestinal malrotation. Owing to the extent of jejunal lipomatosis, resection was not deemed appropriate. In 2019, he was admitted for disseminated nocardiosis with low serum IgG and IgM immunoglobulins, 6.00 g/L (ref: 6.80–18.00 g/L) and 0.38 g/L (ref: 0.50–3.00 g/L), respectively, along with evidence of malnutrition. A push enteroscopy revealed jejunal submucosal lesions and giant diverticulae (Figure 3). Pathology of the submucosal lesions confirmed lipomatosis. Endoscopic and histologic assessment of his colon was unremarkable.
Figure 3.
(A) Endoscopic view of a large jejunal lipoma. (B) Endoscopic view demonstrating diffuse jejunal lipomatosis with large jejunal diverticulae (X).
At the age of 60, he was referred to our center for evaluation of his symptoms and malnutrition. He reported ongoing weight loss, abdominal distension, intermittent diarrhea, nausea, decreased oral intake, and postprandial abdominal pain. Physical examination demonstrated muscle wasting with a body mass index of 23.6 kg/m2. He was moderately malnourished based on a subjective global assessment. Initial laboratory investigations including a complete blood count, liver enzymes, lipase, and renal function were within normal limits. His lipid panel was also normal with a total cholesterol of 2.47 mmol/L, triglyceride level of 1.08 mmol/L (ref: 0.00–1.70 mmol/L), low-density lipoprotein cholesterol of 0.80 mmol/L (ref: 0.00–3.40 mmol/L), and non-HDL cholesterol of 1.29 mmol/L (ref: 0.00–4.20 mmol/L). Abnormal investigations included a serum magnesium of 0.48 mmol/L (ref: 0.70–1.00 mmol/L), vitamin B12 of 127 pmol/L (ref: >159 pmol/L), vitamin A of 1.0 umol/L (ref: 1.5–3.5 umol/L), and 25-hydroxyvitamin D of 59.6 nmol/L (ref: 80–200 nmol/L). His serum folate was elevated at 45.3 nmol/L (9.0–40.0 nmol/L). Magnetic resonance enterography revealed pronounced bowel dilatation. Cine sequences showed hyperperistaltic bowel with to-and-fro motion of intraluminal contents rather than predominately antegrade flow, suggesting diffuse dysfunctional peristalsis.
He received micronutrient replacement, enteral feeds, and antibiotics for SIBO. This combination of treatments improved his symptoms of distension and diarrhea. A course of prucalopride did not improve his symptoms. Unfortunately, he was unable to maintain his nutritional requirements with oral intake alone and was admitted to our center in 2021 for a trial of nasogastric feeding (which he did not tolerate) and, eventually, transitioned to TPN. Thereafter, he was briefly admitted to his home hospital in 2022 with recurrence of pneumatosis intestinalis and pneumoperitoneum which was managed nonoperatively (Figure 4).
Figure 4.
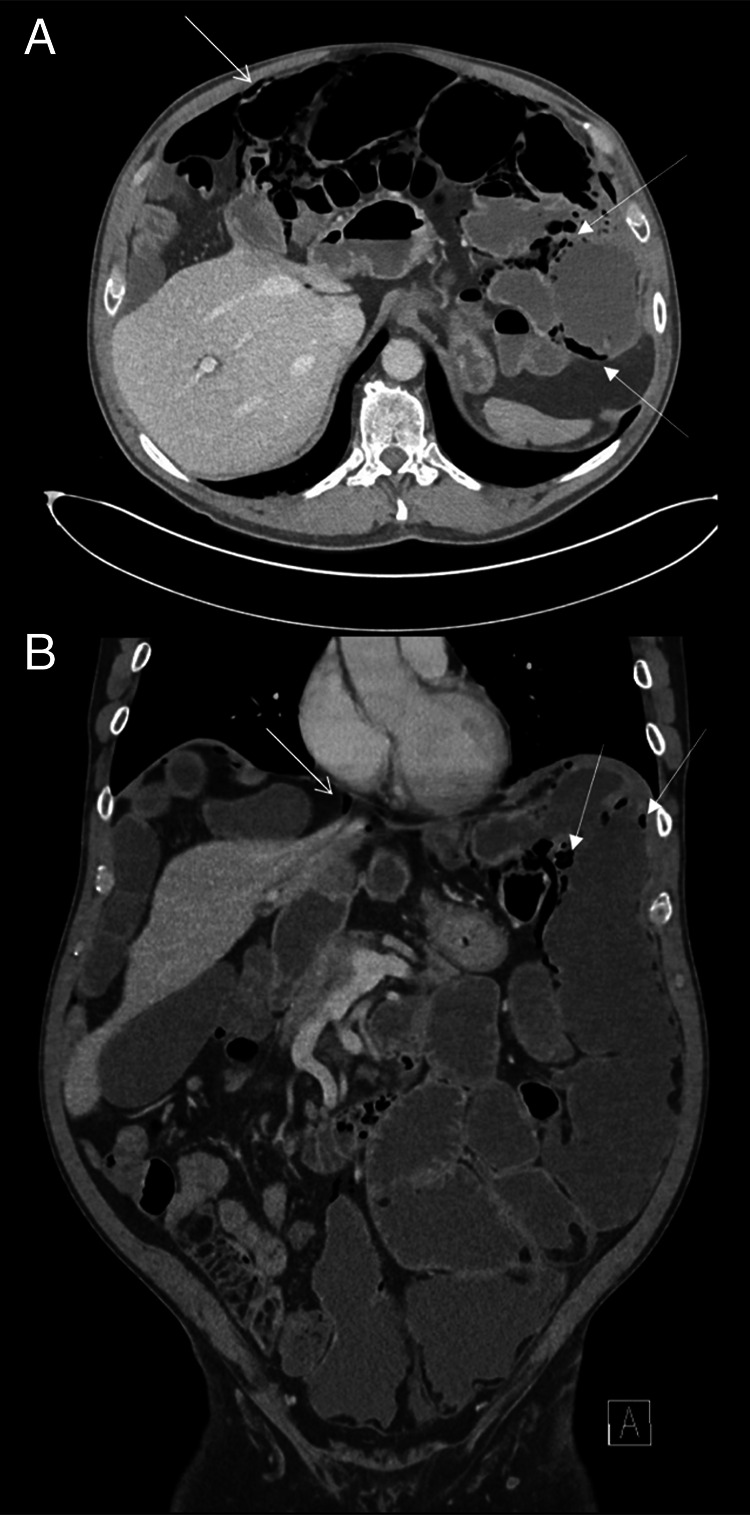
(A) Axial section from an abdominal computed tomography (CT) showing pneumoperitoneum (narrow arrowhead) and pneumatosis intestinalis (thick arrowhead). (B) Coronal section from an abdominal CT showing pneumoperitoneum (narrow arrowhead) and pneumatosis intestinalis (thick arrowhead). Jejunal lipomas and dilation remain evident.
Given the extensive nature of this patient's prior complications, now including intestinal dysmotility, consultation from our colleagues in rheumatology, neurology, immunology, pathology, and medical genetics was sought. Ultimately, further investigations were not suggestive of an alternative, unifying diagnosis. His cytogenetic testing, however, identified a de novo 10p12.31 duplication, which is currently of unknown significance. Whole exome sequencing of 20,000 genes, including testing for mitochondrial neurogastrointestinal encephalomyopathy, was uninformative.
Today, TPN has become his primary source of nutrition despite patient attempts to wean off. He is maintaining a weight of 82 kg (previously 74 kg). The patient does continue some oral intake for comfort and has required rotating antimicrobials with rifaximin, amoxicillin-clavulanate, nystatin, and metronidazole. He is awaiting surgical reevaluation at our center for a potential partial bowel resection.
DISCUSSION
This case of jejunal lipomatosis is novel and adds to our understanding of the natural history of the disease in 2 ways. We report the first case of intestinal dysmotility in a patient with intestinal lipomatosis as evidenced by the findings documented on magnetic resonance enterography cine sequences. Furthermore, the complications of small bowel obstruction, volvulus, jejunal diverticulosis, malabsorption, and SIBO have been previously reported in patients with intestinal lipomatosis,4,5,13 but not within the same individual, as in our case. The progressive development of complications we have described may reflect the natural history of intestinal lipomatosis as we are the first to report a prolonged follow-up period of 12 years in a patient with the disease. Anticipating that extensive complications can develop over time in a single patient with intestinal lipomatosis may lead to a timelier diagnosis and management of such complications.
Lipomatous involvement of the small bowel can result in intraluminal obstruction or provide a lead point for extraluminal causes of obstruction including volvulus. Jejunal diverticulosis outside cases of intestinal lipomatosis represents a rare phenomenon with an estimated prevalence of 0.3%–2.3%.14 It is postulated that intestinal lipomatosis results in intestinal wall weakening,12 which combined with increased intraluminal pressures (be it from obstruction and/or dysmotility as in our case) foster the formation of propulsion-based jejunal diverticular disease. The patient's large jejunal diverticulae likely promoted bacterial overgrowth secondary to stasis, resulting in SIBO for which his symptoms improved with treatment. Although no other investigations have been completed to assess dysmotility in our patient, we suspect that the patient's jejunal lipomatosis and diverticulosis-induced abnormal motility and evacuation resulting in the findings noted on his imaging. Unsurprisingly, our patient developed signs and symptoms of malnutrition related to his gastrointestinal complications, severe enough to require TPN. His immunodeficiency was deemed to be transient and likely secondary to gastrointestinal losses and his malnourished state as his immunoglobulin levels normalized on correction of his malnutrition.
The pathophysiology of intestinal lipomatosis remains unknown but may be related to dysregulation of adipose metabolism, ectopic adipose deposition during embryogenesis, chemotherapy, or as part of genetic syndromes.10,15 Our patient's incidental finding of incomplete intestinal malrotation may support a disruption in embryogenesis as a potential etiology. FLNA gene mutations, and PTEN hamartoma syndromes, including Cowden syndrome have been associated with intestinal lipomatosis,16 yet despite extensive testing, no known genetic predisposition was identified in this patient. However, genetic testing in such syndromes is not always diagnostic, as prevalence rates for PTEN-associated mutations in patients with confirmed Cowden syndrome is only 25%–35%.16 Our patient's de novo 10p12.31 duplication may be related to his disease process, but this has yet to be confirmed in the literature. Importantly, our patient had no family history of similar pathology.
Small intestinal lipomatosis can be diagnosed by CT and magnetic resonance imaging or endoscopically, including endoscopic ultrasound.17–19 Typically, CT demonstrates masses with smooth, regular edges and Hounsfield unit densities of adipose tissue.17 Endoscopic maneuver's eliciting features such as “pillow” or “cushion” sign have been reported to have high specificity (98%) but low sensitivity (40%) for intestinal lipoma identification.18
Limited evidence and agreement exist on the appropriate management for patients with intestinal lipomatosis. Surgery, including bowel resection, has been effective in some patients but may be detrimental depending on the extent of bowel involved and should be approached cautiously.6,9,12 It was previously felt that a bowel resection would result in unfavorable outcomes for our patient due to the degree of jejunum involved; however, he is currently awaiting a second surgical opinion. Various methods of endoscopic resection for solitary lipomas have been effective but are unlikely feasible in cases of diffuse lipomatosis such as ours.20 This case highlights that clinicians caring for patients with intestinal lipomatosis should monitor patients closely for developing complications associated with the disease. Medical treatment for associated SIBO and malnutrition can improve symptoms. Furthermore, an understanding of the surgical emergencies that patients with intestinal lipomatosis may present with may be lifesaving and prevent further morbidity. Given the paucity of evidence, the management of small intestinal lipomatosis remains largely anecdotal and would benefit from an improved understanding of the disease epidemiology, pathogenesis, and sharing of our clinical experience with this rare condition.
DISCLOSURES
Author contributions: B. Tkachuk and R. Collins assisted in data collection and writing of the manuscript. D. Ng provided reference for radiographic images included in the report. I. Stukalin, M. Gupta, and H. Jijon conceived the study design and confirmed accuracy in case reporting. All authors approved the final manuscript. H. Jijon is the article guarantor.
Financial disclosure: M. Gupta sits on advisory boards for and reports speaker fees from Sanofi, AVIR Pharmaceuticals, Bausch Health, Takeda, and Astra Zeneca. The remaining authors report no conflicts of interest pertaining to financial, consultant, or institutional interests.
Informed consent was obtained for this case report.
Footnotes
Bryce Tkachuk and Reid Collins contributed equally to this work.
Contributor Information
Reid Collins, Email: red.collins@ucalgary.ca.
Igor Stukalin, Email: igor.stukalin@albertahealthservices.ca.
Milli Gupta, Email: milli.gupta@albertahealthservices.ca.
Danny Ng, Email: danny.ng@albertahealthservices.ca.
Humberto Jijon, Email: hbjijon@gmail.com.
REFERENCES
- 1.Weinberg T, Feldman M, Sr. Lipomas of the gastrointestinal tract. Am J Clin Pathol 1955;25(3):272–81. [DOI] [PubMed] [Google Scholar]
- 2.Farkas N, Wong J, Bethel J, Monib S, Frampton A, Thomson S. A systematic review of symptomatic small bowel lipomas of the jejunum and ileum. Ann Med Surg 2020;58:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellström N. Kasuistische Beiträge zur Kenntnis des Intestinallipoms. Deutsche Z für Chirurgie. 1906;84:488–511. [Google Scholar]
- 4.Ranchod M, French T, Novis Β, Bank S, Marks I. Diffuse nodular lipomatosis and diverticulosis of the small intestine. Gastroenterology 1972;63(4):667–71. [PubMed] [Google Scholar]
- 5.Ceretto S, Alessandria C, Marzano A. Bacterial overgrowth in intestinal lipomatosis with pandiverticulosis. Clin Gastroenterol Hepatol 2007;5(7):A30. [DOI] [PubMed] [Google Scholar]
- 6.Jayasundara J, Sellahewa C, Hall A, Patel R. A case of gastroduodenal lipomatosis. Ann The R Coll Surgeons Engl 2016;98(8):e203–e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zirpe D, Wani M, Tiwari P, Ramaswamy PK, Kumar RP. Duodenal lipomatosis as a curious cause of upper gastrointestinal bleed: A report with review of literature. J Clin Diagn Res 2016;10(5):PE01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei M, Hu M, Chen W, Qin C. Multiple duodenal lipomas as a rare cause of upper gastrointestinal obstruction: Case report and literature review. Gastroenterol Res 2017;10(2):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabone RA, DeGreve T, Webb P, Yuide P. Jejuno-jejunal intussusception secondary to diffuse intestinal lipomatosis. J Surg Case Rep 2019;2019(11):rjz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cojocari N, David L. Acute intestinal infarction due to diffuse jejunoileal and mesenteric lipomatosis in a 39-year-old woman. Am J Case Rep 2020;21:e922830–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arango LA, Díaz CP, Londoño M, Ortiz P, Cano LM. Intestinal lipomatosis: Report of two cases. Rev Colombiana Gastroenterol. 2020;35(2):212–5. [Google Scholar]
- 12.Grudzińska E, Mrowiec S, Pilch-Kowalczyk J, Ciupińska M, Kusnierz K. Small intestinal intussusception due to complicated giant jejunal diverticulosis. Medicina 2021;57(2):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leigh N, Sullivan BJ, Anteby R, Talbert S. Perforated jejunal diverticulitis: A rare but important differential in the acute abdomen. Surg Case Rep 2020;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebert P, Millet I, Ernst O, et al. Acute jejunoileal diverticulitis: Multicenter descriptive study of 33 patients. Am J Roentgenol. 2018:1245–51. [DOI] [PubMed] [Google Scholar]
- 15.Hu S, Mojtahed A, Covington A, Thompson W, Volpicelli N, McCarthy D. Intestinal lipomatosis and chemotherapy: A growing concern. Dig Dis Sci 2016;61:3151–4. [DOI] [PubMed] [Google Scholar]
- 16.Caliskan A, Kohlmann WK, Affolter KE, Downs-Kelly E, Kanth P, Bronner MP. Intramucosal lipomas of the colon implicate Cowden syndrome. Mod Pathol 2018;31(4):643–51. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z-W, Liang P, Li Z-L, et al. Diagnostic value and potential clinical significance of duodenal lipoma based on computed tomography imaging data. Medicine 2021;100(33):e26944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim GH. Systematic endoscopic approach for diagnosing gastric subepithelial tumors. Gut and Liver 2022;16(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kameyama H, Niwa Y, Arisawa T, Goto H, Hayakawa T. Endoscopic ultrasonography in the diagnosis of submucosal lesions of the large intestine. Gastrointest Endosc 1997;46(5):406–11. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi R, Inoue K, Hirose R, et al. Obscure gastrointestinal bleeding from a large jejunal lipoma treated using an endoscopic unroofing technique with double balloon enteroscopy: A case study. Clin J Gastroenterol 2023;16(1):32–8. [DOI] [PubMed] [Google Scholar]