Abstract
The pharmacokinetic properties of meropenem were investigated in nine critically ill patients treated by continuous venovenous hemofiltration (CVVH). All patients received one dose of 1 g of meropenem intravenously. High-flux polysulfone membranes were used as dialyzers. Meropenem levels were measured in plasma and ultrafiltrate by high-performance liquid chromatography. The total body clearance and elimination half-life were 143.7 ± 18.6 ml/min and 2.46 ± 0.41 h, respectively. The post- to prehemofiltration ratio of meropenem was 0.24 ± 0.06. Peak plasma drug concentrations measured 60 min postinfusion were 28.1 ± 2.7 μg/ml, and trough levels after 6 h of CVVH were 6.6 ± 1.5 μg/ml. The calculated total daily meropenem requirement in these patients with acute renal failure and undergoing CVVH was 2,482 ± 321 mg. Based on these data, we conclude that patients with severe infections who are undergoing CVVH can be treated effectively with 1 g of meropenem every 8 h.
Meropenem is a new carbapenem antibiotic with a broad spectrum of antibacterial activity against gram-positive as well as gram-negative pathogens, including beta-lactamase producers and Pseudomonas aeruginosa (9, 17). The drug is therefore frequently employed in critically ill patients with severe infections or sepsis when the causative organism is unknown and beta-lactamase-mediated resistance has to be considered.
In patients with normal glomerular filtration rates, intravenous administration of a standard dose of 1 g of meropenem (molecular mass, 437.51 Da; protein binding, 2%) results in peak plasma drug concentrations of 54.8 μg/ml. The elimination half-life (t1/2) is approximately 1 h. The area under the plasma concentration-time curve (AUC) is 77.2 mg · h/liter, increasing linearly in a dose-related manner. The volume of distribution is 21 liters. The drug is eliminated by metabolism as well as excretion. In healthy volunteers, approximately 70% of the compound is excreted unchanged in urine within 12 h (1, 4, 14). The dosing interval has to be prolonged as creatinine clearance decreases. During hemodialysis, the plasma half-life of about 7 h in patients with end-stage renal disease (ESRD) is shortened to 2.9 h (3). In patients receiving hemodialysis, dosing after each hemodialysis session is recommended.
In intensive-care patients suffering from sepsis and multiple organ failure, continuous venovenous hemofiltration (CVVH) is an important supportive extracorporeal renal replacement therapy. Pharmacokinetic studies of antibiotics in critically ill patients being treated with CVVH, however, are scarce. Most authors have studied continuous arteriovenous hemofiltration or hemodiafiltration. CVVH is characterized by a high clearance rate of up to 25 to 50 ml/min (19, 26). The elimination of any given drug by renal replacement therapy is determined by several major factors: the characteristics of the membrane used (pore size, filter surface area, adsorption, electrostatic charge, and filter material), the physicochemical properties of the drug, (molecular weight, protein binding, water solubility, and molecular charge) and the characteristics of the renal replacement technique used (5).
No dosage recommendations for meropenem are available for patients undergoing continuous renal replacement therapy. Because beta-lactam drug levels need to be above the minimal inhibitory concentration (MIC) for the organism (6, 10) during at least 40 to 50% of the dosing interval for optimal efficacy, underdosing might impair clinical outcome.
This study was designed to investigate the influence of CVVH on the pharmacokinetics of a single 1-g dose of meropenem in patients with acute renal failure.
MATERIALS AND METHODS
Patients.
Nine intensive-care patients (three females and six males) with acute renal failure and suspected or proven gram-positive or gram-negative infections were included in the study (Table 1). The mean age and body weight were 57.9 ± 5.7 years and 86.7 ± 23.1 kg, respectively. All patients were anuric. Hemodialysis was not employed during this study. Concomitant drug therapy consisted mainly of intravenous catecholamines (n = 9), anticoagulation with heparin (n = 9), morphine derivatives (n = 2), digitoxin (n = 3), and sucralfate (n = 9). All patients received parenteral nutrition and required mechanical ventilation. None of the patients had a known hypersensitivity to meropenem, imipenem-cilastatin or other beta-lactam antibiotics. Patients with bleeding disorders or a history of convulsions were excluded. All concomitant drugs were administered as clinically indicated.
TABLE 1.
Patient characteristics
| Patient no. | Sexa | Age (yr)b | Body weight (kg)c | Diagnosis | Clinical outcome |
|---|---|---|---|---|---|
| 1 | F | 55 | 100 | Myocardial infarction, aorto-coronary bypass | Died |
| 2 | M | 52 | 82 | Liver cirrhosis, sepsis | Survived |
| 3 | M | 65 | 70 | Aorto-coronary bypass | Survived |
| 4 | F | 61 | 70 | Kidney transplantation, sepsis | Died |
| 5 | F | 57 | 70 | Liver cirrhosis, sepsis | Died |
| 6 | M | 58 | 68 | Colitis, double lung transplantation, sepsis | Died |
| 7 | M | 52 | 145 | Liver cirrhosis, sepsis | Died |
| 8 | M | 69 | 85 | Aorto-coronary bypass, prosthetic aortic valve | Survived |
| 9 | M | 53 | 90 | Myocardial infarction, sepsis | Died |
F, female; M, male.
Mean age ± standard deviation, 57.9 ± 5.7 years.
Mean body weight ± standard deviation, 86.7 ± 23.1 kg.
CVVH.
CVVH was performed as described by Canaud et al. with a high-flux polysulfone capillary hemofilter with a membrane surface area of 0.43 m2 (Diafilter-30; Amicon, Limerick, Ireland) (2). Dialyzers and lines were steam sterilized. The standard blood flow rate was 150 ml/min. The ultrafiltrate pump rate was adjusted to 50 ml/min, and the average ultrafiltration rate was 45.8 ± 6.2 ml/min. Ringer’s lactate was infused as substitution fluid into the venous line (postdilution) at a rate of 43.2 ± 9.9 ml/min depending on a balanced fluid therapy during the six study hours.
Drug administration and sampling.
All patients received a single dose of 1 g of meropenem (Zeneca Pharmaceuticals, Macclesfield, United Kingdom) dissolved in 100 ml of physiological saline solution and injected over a period of 15 min into a central venous catheter different from the venous catheter used for CVVH. Blood samples were drawn from the arterial and venous lines of the extracorporeal circuit at 60, 90, 150, 210, 270, and 360 min after the start of the infusion. Patient 9 received 1 g of meropenem every 8 h over a period of 24 h. Plasma and ultrafiltration samples, collected from the outlet of the ultrafiltrate compartment of the hemofilter, were taken at corresponding times, separated immediately, and stored at −70°C until analysis.
Drug assay.
Meropenem concentrations in plasma and filtrate samples were measured by high-performance liquid chromatography according to the method described by Burman et al. (1). The metabolite ICI-213,689 was not measured.
The chromatographic system consisted of a Shimadzu SIL6B autoinjection port and a Shimadzu LC10 workstation (Shimadzu, Tokyo, Japan). Stainless steel columns (200 by 4 mm) were slurry packed with 5-μm-particle-size nucleosil C18 (Macherery-Nagel, Düren, Germany). Detection was by UV absorbance at 296 nm. The mobile phase consisted of 800 ml of 0.01 M potassium phosphate and 200 ml of methanol (pH 7.4). The mobile phase flow rate was 1 ml/min. Plasma samples were diluted 1:4 with distilled water and filtered through 0.2-μm-pore-size filters (Waters-Millipore, Molsheim, France). Standards were prepared in drug-free human serum in concentrations ranging between 1.0 and 25 mg/liter. The detection limit was 0.5 mg/liter. The interassay coefficient of variation was <6%.
Pharmacokinetic analysis.
An open one-compartment model was applied. The elimination half-life was calculated as t1/2 = ln2/kel, where kel is the elimination rate constant as published previously (25). The AUC was determined by the trapezoidal rule and by extrapolation of the terminal slope to infinity. The total clearance (CLtot) was estimated as CLtot = intravenous dose/AUC, and the volume of distribution (V) was estimated as V = CL/kel. The clearance of hemofiltration (CLHF) was determined according to the formula CLHF = (UFR · CUF)/CA, where UFR is the ultrafiltration rate and CUF and CA are ultrafiltrate and arterial plasma meropenem concentrations, respectively. The sieving coefficient (SC) was calculated as SC = CUF/CA (8, 20). Total removal (RE) of the drug during hemofiltration was calculated as RE = (CA60 − CA360)/CA0 · 100, where CA60 and CA360 are arterial plasma drug concentrations at the peak level (after the first hour) and at the end of the study, respectively. The fraction of the extracorporeal eliminated dosage (FHF) was calculated as FHF = CLHF/(CLHF + CLNR), where CLNR refers to the nonrenal clearance. Because all patients were anuric, the CLNR was calculated as the difference between CLtot and CLHF.
Based on the CLtot data from each patient, a meropenem dosing regimen was calculated with the following equation: 24-h dose (D24h) = CLtot (liters/h) · 12 mg/liter · 24 h, maintaining a middosing interval plasma drug concentration of 12 μg/ml. A midpoint concentration of 12 μg of meropenem/ml was chosen to guarantee plasma meropenem concentrations above the MIC at which 90% of the isolates are inhibited (MIC90) for intermediately resistant P. aeruginosa during 40 to 50% of the dosing interval (15, 16). Data are presented as means ± standard deviations.
RESULTS
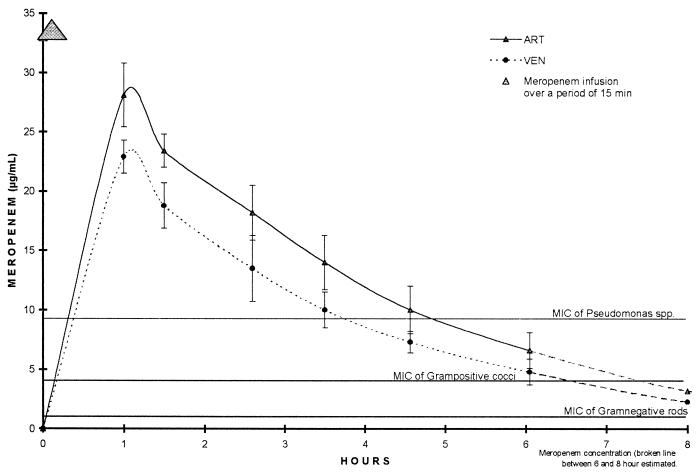
All patients tolerated the intravenous infusion of 1 g of meropenem (12.2 ± 2.4 mg/kg of body weight) over a period of 15 min without apparent side effects. One hour after infusion, plasma drug concentrations were 28.1 ± 2.7 μg/ml at the arterial port and 22.9 ± 1.4 μg/ml at the venous port. Trough plasma drug levels after 6 h of CVVH were 6.6 ± 1.5 μg/ml at the arterial port (Fig. 1). The post- to prehemodialysis ratio was 0.24 ± 0.06, total removal was 35.8 ± 10.1%, and the mean difference in meropenem concentration between the arterial and venous lines was 23.4 ± 4.9%.
FIG. 1.
Concentrations of meropenem in arterial (ART) and venous (VEN) plasma.
Patient 9 received a dosage of 1 g of meropenem every 8 h during hemofiltration. One hour after each intravenous infusion, peak plasma drug concentrations were 31.4, 33.8, and 30.6 μg/ml, and trough levels measured immediately before the next meropenem dose were 6.9 and 8.9 μg/ml.
Table 2 presents the meropenem pharmacokinetic parameters calculated from the plasma and ultrafiltrate concentration data. The mean total clearance was 143.7 ± 18.6 ml/min, and the hemofiltration clearance was 49.7 ± 8.3 ml/min. The volume of distribution (VD) of meropenem was 29.9 ± 2.7 liters, and the calculated AUC was 118.0 ± 15.8 mg/liters · h. The sieving coefficient was 1.09 ± 0.10.
TABLE 2.
Pharmacokinetic parameters for meropenem
| Patient no. | Cmax (μg/ml)a | CLtot (ml/min) | CLHF (ml/min) | kel (h−1) | V (liters) | t1/2 (h) | AUC (mg/liter · h) |
|---|---|---|---|---|---|---|---|
| 1 | 30.9 | 128.7 | 59.3 | 0.27 | 28.9 | 2.59 | 129.5 |
| 2 | 27.5 | 145.4 | 36.3 | 0.29 | 30.1 | 2.39 | 114.7 |
| 3 | 32.1 | 162.8 | 51.4 | 0.40 | 24.5 | 1.74 | 102.4 |
| 4 | 24.8 | 115.5 | 57.9 | 0.23 | 30.1 | 3.00 | 144.3 |
| 5 | 28.8 | 163.1 | 41.8 | 0.35 | 28.2 | 2.00 | 102.2 |
| 6 | 27.3 | 161.5 | 41.5 | 0.31 | 31.0 | 2.22 | 103.2 |
| 7 | 25.0 | 163.6 | 44.3 | 0.29 | 33.9 | 2.39 | 101.9 |
| 8 | 25.1 | 129.8 | 57.1 | 0.23 | 33.9 | 2.46 | 118.0 |
| 9 | 31.4 | 122.7 | 57.4 | 0.25 | 29.3 | 2.76 | 135.9 |
| Mean ± SD | 28.1 ± 2.5 | 143.7 ± 18.6 | 49.7 ± 8.3 | 0.31 ± 0.05 | 29.5 ± 2.7 | 2.33 ± 0.38 | 118.0 ± 15.8 |
Cmax, peak concentration of meropenem in plasma.
Based on meropenem clearance data, a dosing regimen was calculated postulating a desired midpoint concentration of 12 μg/ml (15, 23). In six of our patients, a total daily meropenem dose of 2,482 ± 321 mg ensured meropenem concentrations above the MIC90 for intermediately resistant P. aeruginosa strains.
DISCUSSION
Continuous renal replacement therapy is likely to alter the pharmacokinetics of many antibiotics in intensive-care patients with acute renal failure, but data on drug characteristics under such conditions are rare. Because a routine dosing regimen cannot be applied, clinicians risk missing bactericidal plasma drug levels or causing severe side effects. Our study provides the first data on the pharmacokinetics of meropenem during CVVH with modern polysulfone high-flux membranes.
Meropenem is a new carbapenem antibiotic which differs chemically from imipenem-cilastatin in having a 1-beta-methyl substitution, providing it with excellent intrinsic stability to human renal dehydropeptidase-I. The compound displays linear pharmacokinetics over a dose range of 250 mg to 2 g (3). Patients without renal impairment will receive standard doses of 0.5 to 2 g of meropenem at 8-h intervals. In patients with ESRD, a prolonged half-life of 6.8 h compared to 0.9 h in healthy volunteers has been described (4). The recommended doses for patients with ESRD are 0.25 to 0.5 g once a day. Hemodialysis shortens the elimination half-life to 1.4 to 2.9 h (3, 12). Elimination of the drug through CVVH with a polysulfone membrane results in a half-life of 2.46 ± 0.41 h (Table 2).
Sixty minutes after the start of the meropenem infusion, plasma drug concentrations were 28.1 ± 2.7 μg/ml. Six hours later, the concentrations in plasma had decreased to trough levels between 4.3 and 8.9 μg/ml (mean, 6.6 ± 1.5 μg/ml). The extracorporeal removal of meropenem by CVVH (calculated as AUCUF · UFR) was 35.8% ± 10.1%. In patients on chronic hemodialysis, approximately 51.5% of the drug is eliminated (12). However, the total decline of the plasma meropenem concentration during the study period was 76.1% ± 5.8%, which is significantly higher than the calculated extracorporeal removal of 35.8%. This difference might be explained by adsorption of meropenem to the hemofilter, an occurrence that has been described for glycopeptides (11, 13, 18, 24). In these studies, only a small amount of the drug was found in the dialysate-ultrafiltrate, although more than 90% of the drug was eliminated (18, 24). The large amount of the apparently lost drug was bound to the dialyzer membrane. Additionally, differences in the electrical charges of membrane material and meropenem or the precipitation of the drug with heparin, which is commonly used in hemodialysis to prevent clotting, might in part be responsible for the discrepancies. In contrast, a recently published abstract documented the use of an in vitro continuous renal replacement therapy model with which there was no influence of various filter membranes on the elimination of meropenem (21). However, up to 91.4% of the maximum meropenem concentration was eliminated over 2 h.
Meropenem concentrations in plasma in the range of 0.5 to 4 μg/ml are considered adequate for the successful treatment of bacterial strains for which MIC90s are below 0.5 μg/ml. As beta-lactam antibiotics and carbapenems are thought to act only if their concentrations are well above the MIC for the pathogen, these levels should ideally be maintained for a maximum amount of time. Other authors have postulated that a drug level four- to eightfold the MIC90 for the target pathogen is required. The proposed susceptibility breakpoints for meropenem are 4 μg/ml (full susceptibility), 8 μg/ml (intermediate susceptibility), and ≥16 μg/ml (resistant) (7). The MIC90s of meropenem for all pathogens tested, with the exceptions of coagulase-negative staphylococci and P. aeruginosa, are below 0.1 to 0.2 μg/ml (22). In our patients, plasma meropenem concentrations were above 10 μg/ml for 4.5 h. During these first 270 min, plasma drug concentrations decreased from 28.1 ± 2.7 μg/ml (at 60 min) to 10.0 ± 2.0 μg/ml (at 270 min). Absolute meropenem levels in plasma ranged between 4.3 and 31.4 μg/ml during the 6-h observation period during CVVH. Based on our clearance data, a necessary total dose of 2,482 ± 321 mg of meropenem in 24 h was calculated to guarantee a midpoint concentration of no less than 12 μg/ml. We therefore recommend a dose of 1 g of meropenem every 8 h in anuric patients undergoing CVVH with a polysulfone high-flux membrane. Similar results were reported by Tegeder et al. in a recent study performed with imipenem-cilastatin in critically ill patients undergoing CVVH (23).
Patient 9 received 1 g of meropenem thrice daily for a multiple-dose pharmacokinetic study. The peak drug concentrations after each infusion were 31.4, 33.8, and 30.6 μg/ml, respectively. Trough levels were 6.9 and 8.9 μg/ml just before the second and third meropenem doses. No cumulation of the drug was seen. However, longer observation periods with multiple dosing of 1 g of meropenem every 8 h will be necessary to confirm these data.
In conclusion, the elimination of meropenem by CVVH in patients with acute renal failure is comparable to the elimination characteristics in patients without renal failure. No dose reduction or additional dosing is required in patients with acute renal failure during CVVH, and dosing recommendations for patients with normal renal function can be applied. Trough levels of 6.6 ± 1.5 μg/ml after 1-g doses of meropenem are above the MIC90s for gram-negative bacteria and for the most important gram-positive bacteria. Even in severe infections, 1 g of meropenem every 8 h during CVVH will result in sufficient plasma antibiotic levels to cover the vast majority of pathogens in this critically ill patient group.
REFERENCES
- 1.Burman L A, Nilsson-Ehle I, Hutchinson M, Haworth S J. Pharmacokinetics of meropenem and its metabolite ICI 213,689 in healthy subjects with known renal metabolism of imipenem. J Antimicrob Chemother. 1991;27:219–224. doi: 10.1093/jac/27.2.219. [DOI] [PubMed] [Google Scholar]
- 2.Canaud B, Garred L J, Christol J P, Aubas S, Béraud J J, Mion C. Pump assisted continuous venovenous hemofiltration for treating acute uremia. Kidney Int. 1988;33:154–156. [PubMed] [Google Scholar]
- 3.Chimata M, Nagase M, Suzuki Y, Shimomura M, Kakuta S. Pharmacokinetics of meropenem in patients with various degrees of renal function, including patients with end-stage renal disease. Antimicrob Agents Chemother. 1993;37:229–233. doi: 10.1128/aac.37.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensson B A, Nilsson-Ehle I, Hutchison M, Haworth S J, Öqvist B, Norrby S R. Pharmacokinetics of meropenem in subjects with various degrees of renal impairment. Antimicrob Agents Chemother. 1992;36:1532–1537. doi: 10.1128/aac.36.7.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotterill S. Antimicrobial prescribing in patients on haemofiltration. J Antimicrob Chemother. 1995;36:773–780. doi: 10.1093/jac/36.5.773. [DOI] [PubMed] [Google Scholar]
- 6.Craig W A, Ebert S. Continuous infusion of β-lactam antibiotics. Antimicrob Agents Chemother. 1992;36:2577–2583. doi: 10.1128/aac.36.12.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, J. R., and P. J. Turner. 1995. Laboratory data which differentiate meropenem and imipenem. Scand. J. Infect. Dis. 96(Suppl.):S5–S10. [PubMed]
- 8.Golper T A. Drug removal during continuous hemofiltration or hemodialysis. Contrib Nephrol. 1991;93:110–116. doi: 10.1159/000420197. [DOI] [PubMed] [Google Scholar]
- 9.Jones R N, Barry A L, Thornsberry C. In vitro studies of meropenem. J Antimicrob Chemother. 1989;24:S9–S29. doi: 10.1093/jac/24.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 10.Keil S, Wiedemann B. Antimicrobial effects of continuous versus intermittent administration of carbapenem antibiotics in an in vitro dynamic model. Antimicrob Agents Chemother. 1997;41:1215–1219. doi: 10.1128/aac.41.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanese D M, Alfrey P S, Molitoris B A. Markedly increased clearance of vancomycin during hemodialysis using polysulfone dialyzers. Kidney Int. 1989;35:1409–1412. doi: 10.1038/ki.1989.141. [DOI] [PubMed] [Google Scholar]
- 12.Leroy A, Fillastre J P, Etienne I, Borsa-Lebas F, Humbert G. Pharmacokinetics of meropenem in subjects with renal insufficiency. Eur J Clin Pharmacol. 1992;42:535–538. doi: 10.1007/BF00314864. [DOI] [PubMed] [Google Scholar]
- 13.Menth M, Fiegel P. Elimination von Teicoplanin über verschiedene Dialysemembranen. Fortschr Antimikrob Antineoplastischen Chemother. 1992;11:599–606. [Google Scholar]
- 14.Mouton J W, van den Anker J N. Meropenem clinical pharmacokinetics. Clin Pharmacokinet. 1995;28:275–286. doi: 10.2165/00003088-199528040-00002. [DOI] [PubMed] [Google Scholar]
- 15.Mueller B A, Scarim S K, Macias W L. Comparison of imipenem pharmacokinetics in patients with acute or chronic renal failure treated with continuous hemofiltration. Am J Kidney Dis. 1993;21:172–179. doi: 10.1016/s0272-6386(12)81089-4. [DOI] [PubMed] [Google Scholar]
- 16.Pitkin, D. H., W. Sheikh, and H. L. Nadler. 1997. Comparative in vitro activity of meropenem versus other extended-spectrum antimicrobials against randomly chosen and selected resistant clinical isolates tested in 26 North American centers. Clin. Infect. Dis. 24(Suppl. 2):238–248. [DOI] [PubMed]
- 17.Pryka R D, Haig G M. Meropenem: a new carbapenem antimicrobial. Ann Pharmacother. 1994;28:1045–1054. doi: 10.1177/106002809402800910. [DOI] [PubMed] [Google Scholar]
- 18.Quale J M, O’Halloran J J, de Vicenzo N, Barth R H. Removal of vancomycin by high-flux hemodialysis membranes. Antimicrob Agents Chemother. 1992;36:1424–1426. doi: 10.1128/aac.36.7.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronco C, Bellomo R. Basic mechanisms and definitions for continuous renal replacement therapies. Int J Artif Organs. 1996;19:95–99. [PubMed] [Google Scholar]
- 20.Schetz M, Ferdinande P, Van den Berge G, Verwaest C, Lauwers P. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med. 1995;21:612–620. doi: 10.1007/BF01700172. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder, T. H., M. Hansen, E. Hoffmann, and W. A. Krueger. 1997. Simulation der Pharmakokinetik von Meropenem bei kontinuierlichen Nierenersatzverfahren in vitro. Anaesthesiol. Intensivmed. Notfallmed. Schmerzther. 32(Suppl. 1):S162.
- 22.Sumita Y, Inoue M, Mitsuhashi S. In vitro antibacterial activity and beta-lactamase stability of the new carbapenem SM-7338. Eur J Clin Microbiol Infect Dis. 1989;8:908–916. doi: 10.1007/BF01963782. [DOI] [PubMed] [Google Scholar]
- 23.Tegeder I, Bremer F, Oelkers R, Schobel H, Schüttler J, Brune K, Geisslinger G. Pharmacokinetics of imipenem-cilastatin in critically ill patients undergoing continuous venovenous hemofiltration. Antimicrob Agents Chemother. 1997;41:2640–2645. doi: 10.1128/aac.41.12.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thalhammer F, Rosenkranz A R, Burgmann H, Graninger W, Hollenstein U, Schenk P, Thalhammer-Scherrer R, Traindl O, Hörl W H, Breyer S. Single-dose pharmacokinetics of teicoplanin during hemodialysis therapy using high-flux polysulfone membranes. Wien Klin Wochenschr. 1997;109:362–365. [PubMed] [Google Scholar]
- 25.Thalhammer F, Schmaldienst S, El Menyawi I, Atteneder M, Burgmann H, Hollenstein U, Georgopoulos A, Graninger W, Putz D, Rosenkranz A R, Mayer G, Hörl W H, Breyer S. Multiple-dose pharmacokinetics of cefpirome in long-term hemodialysis with high-flux membranes. Clin Pharmacol Ther. 1996;60:645–650. doi: 10.1016/S0009-9236(96)90212-X. [DOI] [PubMed] [Google Scholar]
- 26.van der Werf T S, Mulder P O M, Zijstra J G, Uges D R A, Stegeman C A. Pharmacokinetics of piperacillin and tazobactam in critically ill patients with renal failure, treated with continuous venovenous hemofiltration. Intensive Care Med. 1997;23:873–877. doi: 10.1007/s001340050424. [DOI] [PubMed] [Google Scholar]