Abstract
The limited availability of freshwater in renewable energy-rich areas has led to the exploration of seawater electrolysis for green hydrogen production. However, the complex composition of seawater presents substantial challenges such as electrode corrosion and electrolyzer failure, calling into question the technological and economic feasibility of direct seawater splitting. Despite many efforts, a comprehensive overview and analysis of seawater electrolysis, including electrochemical fundamentals, materials, and technologies of recent breakthroughs, is still lacking. In this review, we systematically examine recent advances in electrocatalytic seawater splitting and critically evaluate the obstacles to optimizing water supply, materials, and devices for stable hydrogen production from seawater. We demonstrate that robust materials and innovative technologies, especially selective catalysts and high-performance devices, are critical for efficient seawater electrolysis. We then outline and discuss future directions that could advance the techno-economic feasibility of this emerging field, providing a roadmap toward the design and commercialization of materials that can enable efficient, cost-effective, and sustainable seawater electrolysis.
Recent advances in materials and technologies for electrocatalytic green hydrogen production from seawater are analyzed.
INTRODUCTION
As the global movement toward achieving Net Zero Emissions by 2050 gains momentum, green hydrogen is increasingly recognized as a key player in both environmental and economic sustainability (1, 2). Produced by water electrolysis using renewable energy sources, green hydrogen can be used electrochemically in fuel cells or thermochemically in the synthesis of various commodity chemicals such as methane, methanol, hydrocarbons, or oxygenates, which have a high potential to facilitate industrial greening (3, 4). However, the high cost and energy consumption of electrocatalytic water splitting hinder its large-scale application in both industry and daily life (5). Currently, many offshore and coastal renewable energy plants have been established to reduce renewable electricity costs and promote green hydrogen production (6). However, conventional water electrolyzers, such as proton exchange membrane water electrolyzers (PEMWEs) that use highly active platinum and iridium as electrode catalysts, require ultrahigh-purity water as feedstock to ensure the longevity of the hydrogen plant infrastructure, which relies on sufficient high-purity freshwater supplies (7). Therefore, building large-scale hydrogen plants in such arid areas is challenging due to economic and technological problems like freshwater scarcity (8). For example, recent cancelations of large-scale electrolysis projects due to water supply concerns highlight the need for more sustainable and flexible solutions (9). As a result, green hydrogen production by electrocatalytic seawater splitting is a more attractive alternative to freshwater electrolysis because seawater is considered an infinite water supply on our planet (7, 10–12).
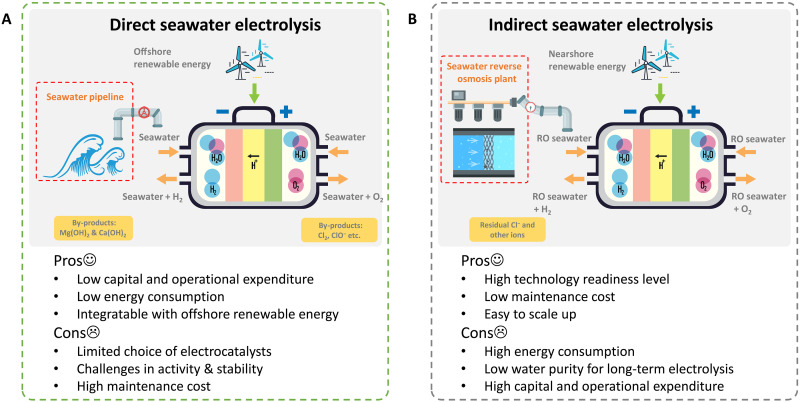
Despite the potential benefits, the complex composition of seawater poses substantial challenges to efficient and sustainable electrolysis (13). The anodic chlorine chemistry, cathodic magnesium/calcium hydroxide precipitation, and microorganism fouling cause severe electrode corrosion and electrolyzer failure (14). For example, in natural seawater, chloride ions are adsorbed onto the surface of the steel, destabilizing and degrading the corrosion protection layer. Meanwhile, oxidative Cl2 (acidic seawater) or ClO− (alkaline seawater) are generated on the anode, leading to the corrosion of electrocatalysts (15). Therefore, pretreatments are necessary to deionize seawater and enable successful electrolysis, driving the development of direct and indirect seawater splitting (7, 16, 17). Direct electrolysis, without external seawater desalination and subsequent purification, is a promising technology with lower infrastructure and capital requirements (Fig. 1A) (15, 17–20). However, direct seawater splitting is still at the early development stage due to the challenges in the advancement of catalysts and technology. Most electrocatalysts are not stable in seawater-based electrolytes due to undesirable ions and microorganisms (21). For example, commercial Pt-based electrocatalysts can be only operated in seawater for less than 1 hour due to the poison matter in natural seawater (11). Therefore, a collaboration between scientists and the energy industry is necessary to push direct seawater splitting technology from technology readiness level 3 (TRL3) to TRL 5 and beyond (15, 22, 23). A feasible prototype with a TRL over 6 is urgently needed to meet the 2030 target.
Fig. 1. Direct and indirect seawater electrolysis.
Comparison of (A) direct and (B) indirect seawater electrolysis from the perspective of technological and economic feasibility. RO, reverse osmosis.
Indirect seawater electrolysis, which integrates mature desalination technologies such as seawater reverse osmosis (SWRO) with commercial water electrolyzers with a TRL of 8 to 9, is considered more practical for real-world applications due to its lower initial seawater purification cost (Fig. 1B) (13, 24, 25). The economic value of direct versus indirect methods for seawater splitting is a topic of debate in the research community (13, 24, 25). The cost of electrocatalytic hydrogen production is primarily driven by electricity consumption, with capital cost being an essential factor for PEMWEs. Seawater desalination using reverse osmosis (RO) is a relatively minor factor in comparison, with only 3 to 4 kWh of electricity required per ton H2O (~4.75 × 104 kWh ton−1 H2 in commercial electrolyzers) (25). Therefore, some studies have conducted feasibility analysis and suggested that indirect seawater electrolysis, which integrates desalination technologies with pure water electrolyzers, may be more promising than direct seawater electrolysis (25). However, simply integrating seawater RO with conventional electrolysis, such as PEMWE is not technologically feasible due to the impurities of RO seawater (13, 26). Further deionization will substantially increase the cost. As a result, an alternative pathway should be explored, and the water supply chain should be considered. Researchers have investigated various aspects such as mechanism, catalyst synthesis, electrolyte engineering, cathodic-coupled reactions, and in situ desalination to design efficient electrolyzers for both direct and indirect seawater splitting. Although some reviews of seawater electrolysis have been reported, they have typically focused either on materials or economic values of the process (13, 24, 25), which fail to cover the whole blueprint of this field.
In this review, we examine recent studies to identify the latest advancements and prospects of seawater electrolysis. By analyzing the essential role of water supply in green hydrogen production, we found that direct seawater splitting is more suitable for long-term sustainable development. Our analysis indicates that optimizing materials, electrolytes, anodic chlorine chemistry, and electrolyzer configuration could potentially overcome critical catalytic obstacles in direct seawater splitting. Furthermore, we discuss the design and construction of innovative electrolyzers, which play a crucial role in enhancing both direct and indirect seawater electrolysis performance. To accelerate the widespread implementation of direct seawater splitting for sustainable and cost-effective applications, several important challenges must be addressed, such as the establishment of standardized testing criteria, the development of stable electrocatalysts, and efficient in situ desalination components. By creating dependable and effective seawater electrolysis systems, we can harness the vast potential of the world’s oceans to generate clean and sustainable energy.
IMPORTANCE OF USING SEAWATER
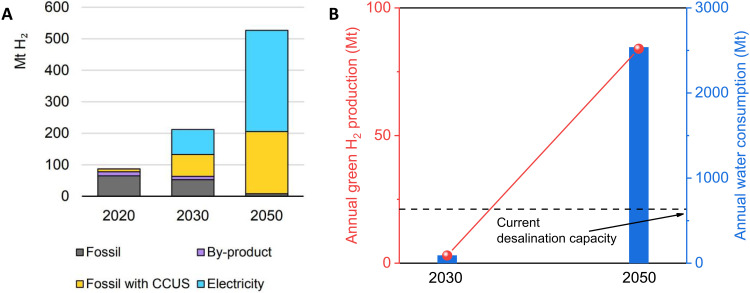
According to the International Energy Agency report (1, 27), the global demand for hydrogen is expected to reach 115 million tonnes (Mt) by 2030, with 24 Mt coming from water electrolysis (Fig. 2A). Europe and Australia are among the front runners in hydrogen production projects using water electrolysis. For instance, Australia has a substantial pipeline of projects that could produce 3 Mt of hydrogen from renewable electricity by 2030, equivalent to an electrolyzer capacity of almost 50 GW (8). If Australia keeps contributing 28% electrolyzer capacity, the value will potentially increase to 84 Mt by 2050. However, hydrogen production using conventional water electrolysis requires a substantial amount of fresh water. Theoretically, producing 1 kg of H2 consumes 9 kg of water (28). When considering water demineralization, the actual water usage could increase to 30.2 kg per kg H2 produced (29). This means that the targeted water usage in Australia for water electrolysis by 2050 could reach 2530 Mt, much higher than Australia’s current seawater desalination ability of ~630 Mt/year (Fig. 2B) (30). Unfortunately, the water scarcity issue in Australia is inevitable due to the relatively dry and variable climate. The capacity of seawater desalination cannot meet the increasing demand for hydrogen production by water electrolysis as the RO seawater is mainly aimed to be used for living needs (drinking and agricultural operations) (31, 32). This makes it impractical to rely on either conventional freshwater electrolysis or indirect seawater electrolysis for large-scale green hydrogen production in renewable energy-rich arid areas, such as Australia, Africa, the Middle East, and the West Coast of the United States. To address this issue, there is a need to develop a direct seawater electrolysis technology without SWRO treatment or a route with energy-saving desalination to balance the supply of SWRO between living requirements and hydrogen production. This development would enable the production of green hydrogen in water-scarce regions without further straining the water supply.
Fig. 2. Water demand for green hydrogen production.
(A) Global hydrogen demand for 2020, 2030, and 2050. CCUS, carbon capture, utilization, and storage. Copied with permission from the International Energy Agency (27). (B) Australia’s planned green hydrogen production by water electrolysis and the required amount of water and seawater desalination capacity.
CHALLENGES AND FEASIBILITY OF SEAWATER ELECTROLYSIS
Seawater is highly corrosive due to its high salinity and chlorine ions. In addition, the pH of natural seawater is susceptible (7.5 to 8.4) depending on the influence of water intake depth, latitude, and other conditions (15). On the basis of the currently renewable energy plant distribution, surface seawater electrolysis is more feasible, compared to deep ocean water electrolysis. Therefore, developing advanced desalination technologies and robust electrocatalysts is very important in improving the stability of seawater electrolyzers. In this regard, specific scientific and technological challenges should be addressed. In this section, we summarize the challenges and possible solutions for this emerging field.
Challenges in seawater splitting
The primary challenge for direct seawater electrolysis is the complex composition of seawater (7). Seawater contains dissolved inorganic salts (Na+, Mg2+, Ca2+, Cl−, SO42−, etc.), small organic molecules, microplastics, living organisms, and dissolved gasses, all of which can deactivate catalysts, electrodes, or membranes (33). Moreover, the low ionic concentration of seawater (averaging 3.5 wt %) increases energy consumption and makes the process economically inefficient (15, 19, 20). The pH fluctuations near electrode surfaces during electrolysis lead to catalyst degradation, and carbonates in seawater cannot prevent pH fluctuations locally at the cathode and anode surface, leading to the precipitation of Mg(OH)2 and Ca(OH)2 that can block the cathode and decay active sites for the hydrogen evolution reaction (HER) (11, 20). To overcome these challenges, the addition of supporting electrolytes such as alkali and buffer is recommended (11, 34, 35). However, the alkali ($800 tonnes−1 KOH) additive causes additional costs. Therefore, alternative pathways to lower the overall cost of electrolyte modification are needed. Note that Mg(OH)2 holds a higher value compared to KOH and NaOH due to the widespread applications of magnesium alloys in various fields (36). Consequently, establishing a continuous magnesium resources recovery system using water electrolysis presents a sustainable pathway with a high potential for recovering valuable resources from seawater.
Another major challenge in the field is the industrialization of seawater electrolysis. Conventional electrolyzers face substantial obstacles in using seawater as a feedstock, mainly due to the corrosion of electrocatalysts and bipolar plates. To address this issue, the industry must prioritize the research and development of innovative materials and devices capable of overcoming these limitations. In addition, it is essential to explore both direct and indirect seawater electrolysis approaches simultaneously to accelerate industrial advancement in this promising field.
For indirect seawater splitting, integrated SWRO with PEMWEs will become a feasible pathway if more SWRO plants are built because seawater desalination only contributes 0.1% energy and 0.5% operating cost of electrolysis (25). While some argue that the quality of water from one-time SWRO is not sufficient, residual trace Na+, Mg2+, and Cl− can poison the active Pt- and Ir-based electrocatalysts in PEMWEs. Impurities like iron, chromium, copper, and others can also adversely affect several components such as the diaphragm, catalysts, membrane, and porous transport layer for PEMWEs (16). In addition, it is important to reduce the microorganism fouling to improve the performance SWRO plant, which needs additional pretreatments. To meet the required standards, the water must have a conductivity of less than 1 μS cm−1 and a total organic carbon of less than 50 μg liter−1 (37). Therefore, considering and evaluating these costs are necessary before pursuing seawater desalination to solve water scarcity. In addition, the disposal of brine generated by seawater desalination is a substantial challenge that must be addressed to prevent potential harm to the local marine ecosystem (38). One viable solution is to treat the brine to reduce its environmental impact. However, this option would come at an additional cost of $0.6 to $2.4 m−3 for the water (39). The feasibility and economic value of indirect seawater splitting raise substantial concerns due to the inadequate purification of RO water obtained from one-time RO for the sustained operation of PEMWE (14, 25). To ensure the sustainable development of green hydrogen from seawater, a more viable approach is proposed, involving direct seawater splitting using robust electrocatalysts and stable electrolyzers. A crucial challenge in this regard is to reduce the cost and energy consumption of the next-generation seawater electrolyzers by innovatively designing affordable and efficient infrastructures.
Solutions for seawater splitting
1) To achieve efficient seawater electrolysis, the development of high-performance electrocatalysts and electrodes is crucial. The design of stable materials must focus on activity, stability, and selectivity. In addition, the criteria and principles for designing cathodes and anodes differ due to different catalytic challenges. It is essential to specify design criteria for various seawater electrolysis systems such as acidic, alkaline, and neutral seawater. By focusing on the design of stable and efficient electrocatalysts and electrodes, seawater electrolysis can become a sustainable and viable method for producing hydrogen and other valuable products.
2) The development of seawater electrolysis requires the modification of key components in electrolyzers, such as membranes and bipolar plates. However, current studies on seawater splitting use different testing protocols and systems, making it challenging to compare the performance of catalysts and electrolyzers across studies (17, 40). To enable direct comparisons, benchmarks and standards for seawater electrolysis are necessary. Benchmarking and standardized testing protocols, as well as the developed low-cost and efficient seawater pretreatment systems, will guide the research community in accurately assessing the performance of electrocatalysts and electrolyzers.
3) Pretreating seawater for electrolysis is a substantial challenge as the presence of impurities such as salts, microorganisms, or heavy metal ions can affect electrode activity. Using alkaline/buffered seawater can remove many impurities and enhance electrolyte conductivity. However, there is a pressing need for low-cost and highly efficient seawater pretreatment systems that can be readily integrated with electrolyzers (41, 42). In addition, developing a standard electrolyte, such as natural seawater, would facilitate accurate testing and promote the development of efficient electrocatalysts.
4) The inadequate salt concentration in seawater contributes to limited electrical conductivity, resulting in substantial energy losses during the electrolysis process. In addition, constructing anticorrosive hydrogen generation plants requires additional engineering solutions, and conventional water splitting systems such as alkaline water electrolyzers (AWEs), PEMWEs, and anion exchange membrane water electrolyzers (AEMWEs), which are not fully compatible with direct seawater splitting (12). Therefore, careful consideration of the technological and economic feasibility of direct seawater electrolysis is necessary.
DESIGN OF MATERIALS AND ELECTROLYTES
The design of materials and electrolytes that are active in saline environments, with tunable functions and chemical stability under chlorine-contained media, is essential for enhancing the comprehensive performance of direct seawater electrolysis. In this section, we only present a selected list of representative examples encompassing advanced materials, electrolytes and coupling reactions on the basis of the knowledge of the aforementioned challenges and solutions in seawater splitting. More detailed reviews on the design of materials for seawater electrolysis are available (7, 21, 43).
Engineering electrocatalysts for cathode
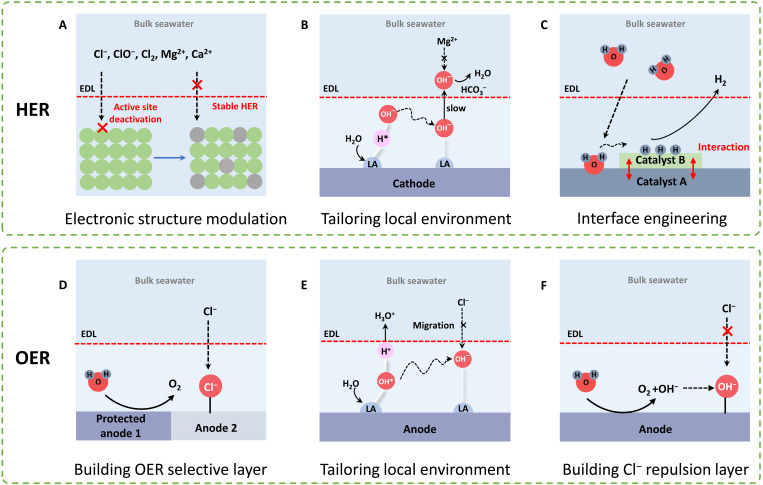
When seawater splitting is operated in a neutral solution, the pH value on the catalyst’s surface will increase due to the consumption of proton, causing the formation of precipitations, such as Mg(OH)2 and Ca(OH)2 (14, 44). Specifically, the Mg(OH)2 forms when the pH value exceeds 10.7 (45). Kirk and Ledas (42) found that, in the case of testing in neutral seawater, the HER current was reduced by half after the precipitation occurred. Consequently, the main goal for HER in seawater is the development of catalysts with anti-Cl− toxicity and anti-precipitation properties. The materials for HER catalysts are mainly based on transition metals (TMs) due to their low cost and excellent corrosion resistance (11, 34). Physical and chemical modifications of TM-based catalysts are expected to modulate their HER activity and stability (46, 47). In this section, we analyze several strategies to promote HER in seawater, including electronic structure modulation, local environment customization, and interface engineering.
Electronic structure modulation
A straightforward strategy to enhance HER performance is the modulation of electronic structure to optimize the adsorption of the intermediates and enhance the ability to anti-position associated with Mg2+, Ca2+, or Cl− (Fig. 3A). For example, the TM-based nitrides have been regarded as alternatives to Pt for HER in purified water due to the noble metal–like electronic structures and anticorrosive properties (47). In addition, increasing the valence state of the metal site enhances the antifouling ability against deleterious seawater ions (18). Jin et al. (18) developed two-dimensional Mo5N6 nanosheets with nitrogen-rich properties for HER in natural seawater, with the HER activity outperforming other benchmark catalysts such as TMs and Pt/C. The incorporation of oversaturated nitrogen atoms resulted in tunning the inherent properties, improving the Mo valence. Other compounds, such as modified phosphides, sulfides, and selenides, have also demonstrated superior HER activity and stability in seawater (34, 47, 48).
Fig. 3. Engineering electrocatalysts for direct seawater electrolysis.
Protocols for cathode modification of (A) electronic structure modulation, (B) tailoring local environment, and (C) interface engineering. Protocols for anode modification of (D) building protective layer, (E) tailoring local environment, and (F) building Cl− repulsion layer. (B) and (E) are reproduced with permission from the Nature Publishing Group (54). OER, oxygen evolution reaction. EDL, electrical double layer; HER, hydrogen evolution reaction; LA, Lewis acid.
Local environment customization
The local environment generally includes the nanoscale space at the interface of the catalyst and electrolyte (49–52). The Local environment is believed to greatly affect the kinetics and selectivity of reactions (Fig. 3B) (53). Compared with the modification of catalysts, the regulation of the local environment is more difficult because of the interference from bulk electrolytes. Recently, a breakthrough in direct seawater electrolysis was realized by adjusting the local pH value of a catalyst. Guo et al. (54) achieved both excellent HER activity and anti-precipitation by introducing a Lewis acid (LA) layer of CrOx on TM oxides (Fig. 3B). LA layer dynamically splits water molecules and captures hydroxyl anions (OH−). Such an OH−-captured behavior led to the rapid increase of local pH on the cathode surface, which facilitated the HER kinetics, accompanied by improved HER activity. Meantime, precipitation did not occur during electrolysis. This is because the Cr2O3 LA layer attracts OH− and restricts it within the electrical double layer. Thus, the pH value of bulk seawater was kept under 8.5, lower than that required for the precipitation.
Interface engineering
It is suggested that interface engineering also affects the HER performance (Fig. 3C) (55, 56). For example, Xiu et al. (57) designed a Pt-MXene interface for stable alkaline water and natural seawater electrolysis. The Pt-MXene multifunctional electrocatalytic interface has high atomic utilization efficiency, good conductivity, efficient H+/water adsorption-activation, and fast ionic/mass accessibility, facilitating stable and efficient seawater electrolysis. Jiang et al. (58) synthesized a heterostructure nanosheet composed of NiFe layered double hydroxide (LDH)/FeOOH. The as-prepared NiFe LDH/FeOOH allowed strong electron interactions at the interface and prevented the corrosion of Cl− on LDH. Current studies on the interface engineering of HER electrocatalysts are mainly focused on alkaline seawater electrolysis, especially on the development of more functional catalysts in the future for HER in natural seawater.
Engineering electrocatalysts for anode
The major challenge for engineering anode for seawater electrocatalysis is related to Cl−-induced corrosion, the dominant anion in seawater (0.55 M) (19). The chlorine evolution reaction (ClER) competes with the oxygen evolution reaction (OER) and the oxidized products seriously corrode the electrolyzer device. Considering the thermodynamics and kinetics of OER and ClER, it becomes evident that alkaline conditions offer a substantially broader overpotential range (~0.48 V) for anode materials in comparison to acidic conditions (19). To prevent ClER and attain a high level of OER selectivity, the development of high-performance catalysts for OER and HER in alkaline environments is considered a promising approach. Nonetheless, achieving the desired high current density at an overpotential lower than 0.48 V proves to be a challenging task. In this regard, intensive studies have been undertaken to improve the selectivity toward OER and protect the anode catalysts against corrosion. In this section, we aim to discuss three typical strategies to overcome this challenge.
Building OER selective layer
The use of seawater in PEMWEs, where the OER proceeds in an acidic environment, is faced with a severe problem of ClER as the thermodynamic onset potentials for ClER and OER are close in an acidic medium (59–61). Unfortunately, Ru- or Ir-based catalysts, the typical anode materials for PEMWEs, also show superior catalytic activity for ClER (62, 63). Inspired by the fact that MnOx tends to promote the OER in acidic saline water selectively, Vos et al. (64) electrodeposited a thin film consisting of MnOx on the surface of IrOx to suppress the ClER (Fig. 3D). It was demonstrated that the multilayer catalyst decreased the ClER selectivity from 86% to lower than 7%. MnOx as a permeable layer stops the transport of Cl−, making it a suitable catalyst for acidic seawater splitting. The metal oxide coating may present two drawbacks: (i) a decrease in the current density due to active site blocking and (ii) metal dissolution under the highly oxidative potential of OER. Therefore, it is necessary to design efficient strategies to enhance the selectivity and stability of coating materials. Heteroatom doping has proven to be an effective strategy as it not only enhances the selectivity of the coating layer for OER but also reinforces the metal-oxygen bond, thereby improving stability (65). For instance, Jiang and Meng (66) investigated the durability of different elements of doped MnO2-coated IrO2 for OER in seawater electrolysis.
Local environment customization
The OER that occurs in neutral seawater is hindered by the slow OER kinetics and competing ClER, which make the use of neutral seawater more difficult than alkalized or acidified seawater (67). According to previous works (19, 68), the onset potential for ClER would be 0.48 V higher than OER in an alkaline solution, which means ClER is thermodynamically unfavorable. As mentioned above, introducing the LA layer of CrOx on the catalyst enables a localized alkaline environment, acting as an electrical barrier to repel Cl− ions (Fig. 3E) (54). Simultaneously, these LA sites also overcome the slow kinetics of OER in neutral water as the OER occurs in a strong alkali environment. Notably, natural seawater without the purification or addition of buffer was used as feedstock, and 1 A cm−2 was reached at 1.87 V when operated at 60°C. The direct electrolysis of natural seawater has been achieved at an industrial-level current density. In addition, palladium-doped cobalt oxide (Co3-xPdxO4), as a strong-proton-adsorption material, was developed by Wang et al. (69) to facilitate water dissociation. Thus, only an overpotential of 370 mV was required to achieve 10 mA cm−2. Moreover, the catalyst was evaluated in an anion exchange membrane AEM–based water electrolysis system, showing stable OER performance for 450 hours at 200 mA cm−2.
Building Cl− repulsion layer
It is a general solution to construct a Cl− repulsion layer to isolate Cl− ions and anode catalyst (Fig. 3F) (70). This method aims to achieve analogous pure water splitting on the catalyst surface with seawater as a feedstock. As shown elsewhere (71), the CeOx-based coating on the surface of NiFeOx was effective to block the penetration of Cl− selectively. The preparation of an isolation layer on the catalyst assured a stable performance in the presence of Cl−. Such a protective layer was able to show superior OER selectivity but may decrease catalytic activity. Therefore, it is desirable to form a layer with proper permselectivity to minimize its influence on the OER activity. A sulfate decoration strategy was proposed to not only repel Cl− ions for high OER selectivity but also to assure excellent catalytic activity for OER (19). Specifically, NiSx was deposited on the surface of NiFe LDH, serving as a sulfur source to produce sulfate (72). The in situ generated sulfate acted as an electrostatic repulsive layer, blocking the contact between Cl− and anode catalyst. In addition, the evolved sulfate layer was shown to be helpful for the OER performance as widely reported (72, 73).
Electrolyte engineering
Acid, base, or buffers are usually chosen to improve the conductivity of the electrolyte and enhance the kinetics of half-reactions (35, 64, 68). However, the marked Cl− oxidation in acid seawater and/or slow dynamics of HER in alkaline seawater are still insufficient to satisfy stable electrolysis; an additional modulation of electrolyte is required to control the structure of water and related microenvironment (74, 75). For example, the effect of electrolytes on the connectivity of the hydrogen bonding network and HER in seawater should be considered (74). As regards the channels for proton transport, the poor proton transport capability of the hydrogen bonding network affects the continuous transfer of protons, causing kinetic problems at the catalytic interface (76). In seawater, the complex composition with crowded alkali metal cations (Na+ and K+) substantially improves the discontinuity of the hydrogen bonding network (Fig. 4). Thus, the hydrogen bonding network and related double-layer regulation in seawater have been considered. Zhao et al. (77) improved the hydrogen bonding environment by introducing theophylline derivatives, and the 7-n-butyl theophylline–decorated Pt demonstrated three times higher intrinsic HER activity. At the anode, electrolyte modulation can also provide an effective strategy to inhibit chlorine oxidation (Fig. 4). For example, Ma et al. (78) reported three to five times higher stability of NiFe-LDH in seawater by adding SO42−. The observed phenomenon was explained by the preferential adsorption of the additive SO42− on the surface of the anode, repulsing Cl− in the bulk phase by the electrostatic repulsive forces. Further theoretical calculations and in situ experiments confirmed the preferential adsorption of SO42− as compared to Cl− preventing effectively the diffusion of Cl− from the electrolyte to the catalyst surface.
Fig. 4. Electrolyte engineering for direct seawater splitting.
Illustration of electrolyte engineering that can improve the discontinuity of the hydrogen bonding network for HER and prevent the diffusion of chloride ions for OER.
Coupling seawater electrolysis with other reactions
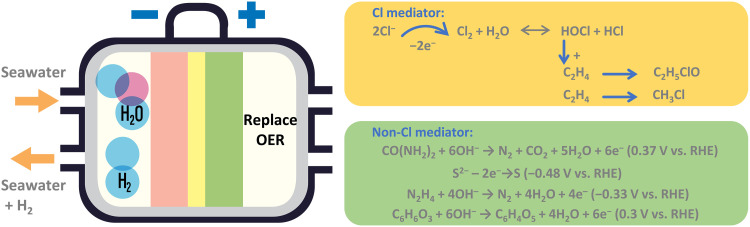
The slow dynamics of OER and the corrosion problems of ClER on the anode substantially increase electricity and maintenance costs. Therefore, coupling the anode of seawater electrolysis with small-molecule oxidation reactions to produce hydrogen and organic products is a promising strategy (79). Compared with OER, many small-molecule oxidation reactions have facile thermodynamic equilibrium potential with favorable dynamic performance, leading to lower full-cell voltage and energy consumption, while generating high-value-added products (10, 11, 80, 81). In addition, since natural seawater contains considerable amounts of chloride, bromide, and iodide ions, it provides a favorable environment for oxidation of some small organic molecules because halogen ions have the potential to facilitate the activation of O─H and C─H bonds or form halogen-mediated intermediates for many small organic molecules (82, 83). In this section, our primary focus is on the design of coupled oxidation reactions by substituting OER. We have categorized the reactions into two groups based on the involvement of the Cl-mediated pathway (Fig. 5).
Fig. 5. Coupling advanced oxidation reactions on the anode.
Schematic illustration of the replacement of OER with advanced oxidation reactions including Cl-mediated and non-Cl-mediated pathways.
Cl-mediated pathway
The Cl-mediated oxidation of alkanes (alkenes) provides an efficient and environmentally friendly route for green synthesis of commodity chemicals (83). Leow et al. (83) reported ampere-level oxidation of ethylene and propylene by using chloride as the redox mediator without CO2 emission. In KCl electrolyte, about 70% of faradaic efficiency is achieved together with 97% of product selectivity. The use of high concentrations of chloride ions to mediate ethylene and propylene oxidation is the key to this reaction, which has great potential in seawater electrolysis. In addition, Wang et al. (81) reported a CH4 oxidation reaction to produce chloromethane (CH3Cl) by chlorine intermediates without using plasma. Besides, bromine in seawater also demonstrates great advantages in the oxidation of alkanes (alkenes). For example, Wang et al. (82) reported bromine-assisted ethylene oxidation to generate 2-bromoethanol, due to the fast rate constant of HOBr reacting with C═C bond, high partial current density together with 87.2% of faradaic efficiency achieved.
Non-Cl-mediated pathway
The primary advantage of non-Cl-mediated oxidation is the reduction of the cell voltage of electrolyzers (11). Therefore, the reactions with lower overpotentials than OER and ClER are preferred (Fig. 5). For example, the urea oxidation reaction has a low equilibrium potential (0.37 V versus reversible hydrogen electrode) (84). Sulfion oxidation reaction is another option owing to its relatively low oxidation potential and rapid kinetics involving fewer electrons (10). Hydrazine oxidation reaction has excellent reaction thermodynamics and kinetics, and the oxidation products N2 and H2O are also safe and harmless (80, 85). Several favorable alcohol oxidation reactions (methanol, ethanol, glycerol, and benzyl alcohol) can also be integrated with seawater electrolysis to obtain value-added organic acids and hydrogen simultaneously (79, 86). In addition, electrocatalytic dual hydrogenation systems that can produce hydrogen on both the cathode and anode are promising in seawater electrolysis (87, 88).
DESIGN OF TECHNOLOGIES AND DEVICES
Designing practical and reliable devices is essential for the successful scaling up the seawater electrolysis technology. This section aims to clarify the advantages and gaps in various device structures and create a possible roadmap for future development in this field.
Commercial electrolyzers for seawater electrolysis
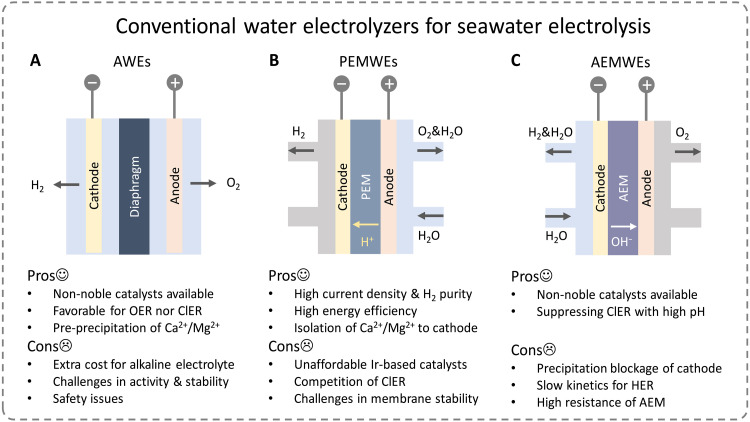
Direct seawater electrolysis has high requirements for the design of electrolyzers, where the influence of impurities in seawater needs to be evaluated in relation to efficiency and lifetime. Conventional water electrolyzers, such as AWEs, PEMWEs, and AEMWEs, exhibit their pros and cons in dealing with the above problems (Fig. 6).
Fig. 6. Seawater electrolysis based on conventional water electrolyzers.
Illustration and feasibility analysis of (A) AWEs, (B) PEMWEs, and (C) AEMWEs.
AWE
AWE is a mature technology that usually works with a porous diaphragm (e.g., Zirfon) separating cathode and anode in 30–40 wt % KOH (89). The high concentration of OH− and ppm level impurities are required for high performance and stability. With the concentrated OH−, the OER would be more favorable than the ClER. Also, Ca2+/Mg2+ can be pre-precipitated and the cathode blockage can be avoided. Therefore, alkaline seawater electrolysis has received an extensive attention. However, the concentrated alkaline increases the expense and may lead to safety issues. In addition, the porous diaphragm and other components in electrolyzers are for high ion permeability and gas diffusion. The ions and impurities in seawater system can cause unavoidable membrane blockage and gaseous crossover (90), leading to safety issues.
PEMWE
PEMWE based on Nafion membranes can deliver a high current density (1 to 3 A cm−2) and obtain high-purity hydrogen (>99.999%) with deionized water (91). Membrane electrode assemblies (MEA) and innovative strategies substantially increase the energy efficiency of conventional water electrolysis, leading to lower energy consumption (92). However, MEAs for PEMWE are all based on noble metal catalysts, leading to a high capital cost. Typically, seawater is fed to the anode of PEMWEs only, therefore greatly decreasing precipitation at cathode. However, the formation of chlorine and other oxychlorides in acidic environment is easy to corrode membranes, catalysts, bipolar plates, and other related accessories, reducing the efficiency and lifetime of the electrolyzers. Anticorrosive titanium-based bipolar plates would largely increase the cost. Also, the sodium ions will compete with protons across the membrane, which can lead to instability because protons cannot be efficiently transferred to the cathode, which results in lowering the local pH of anode and increasing Nernst overpotential. In order to solve these challenges, a vapor-fed anode and saline catholyte to manage ion transport in the PEMWEs have been proposed by Rossi et al. (93) to achieve a similar performance to conventional PEMWEs up to 1 A cm−2 when both anode and cathode are fed with deionized water.
AEMWE
AEMWE is also proposed to be directly applied to seawater electrolysis (94). The chloride oxidation can be considerably hampered due to the alkaline environment. On the other hand, the resistance for hydroxide transport in AEM is much slower than the proton transfer in the Nafion membrane (95), thus its electrolysis efficiency is much lower than that of PEMWE. In addition, the membrane resistivity is easily affected by chloride ions (96). In the AEMWE configuration, water directly feeds the cathode; therefore, Ca2+/Mg2+ ions precipitate rapidly under high currents, which greatly reduces the effective working area of the cathode catalyst and easily blocks the cathode flow field.
Innovations in membrane electrolyzers
Our analysis reveals that conventional pure water electrolyzers have limitations for direct seawater electrolysis. Therefore, innovations in membranes, especially for Na+ and Cl− ion conduction, are crucial for the development of efficient seawater electrolyzers. For instance, Shi et al. (97) designed a Na+ exchange membrane that effectively prevents Cl− from passing through to the anode, thereby avoiding undesired ClER. The difference in chemical potentials between the cathode and anode electrolytes can be harnessed to reduce the energy cost of hydrogen production. In this section, we discuss the recent advances in the innovations of membrane electrolyzers.
Bipolar membranes water electrolyzer
As discussed above, optimizing the local pH environment of a membrane electrode is of great significance for concurrently improving the kinetics of the anodic reactions and avoiding chloride oxidation and precipitation of Ca2+/Mg2+. Therefore, bipolar membranes (BPMs) have been proposed, which consist of a polymeric cation-exchange layer (CEL) and anion-exchange layer (AEL) (Fig. 7A). BPMs make it possible to couple different pH environments into a single electrolyzer so the optimal pH conditions can be selected independently for each half-reaction. It is promising to flow seawater at the anode, which is a local alkaline environment, so the chloride oxidation can be greatly hampered and precipitation can be avoided (98). For the cathode, the BPMs can block the transport of Ca2+/Mg2+, preventing their precipitation and reducing the maintenance cost. However, two points are important for the application of BPMs. One is sufficient water supply to the bipolar interface in the case of membrane drying and a standstill of the reaction; another one is the ratio of AEM and PEM thickness, which greatly affects the performance of the electrolyzer. As a solution, Oener et al. (99) proved that a thin CEL could enable high-current-density BPMs water electrolyzer (BPMsWE) via improved water transport. Similarly, Mayerhöfer et al. (100) proposed that a thinner AEL would enhance the efficiency of BPMsWE as the proton migrates within PEM two to eight times faster as the OH− transport through AEM. In addition, instability and high overpotential requirements of BPMs are major challenges for practical applications. To circumvent these membrane issues, the development of efficient strategies is desired to boost the polarization process of water molecules.
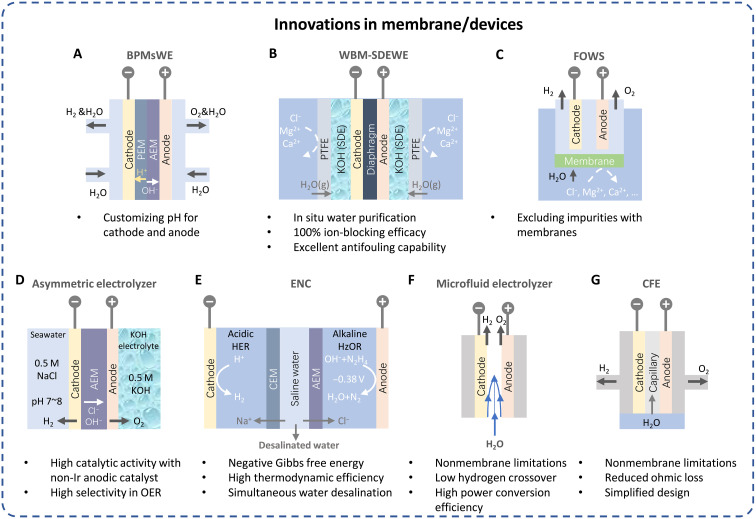
Fig. 7. Innovative membrane devices for seawater electrolysis.
Illustration of (A) bipolar membranes water electrolyzer (BPMsWE), (B) waterproof breathable membrane combined with self-dampening electrolyte water electrolyzer (WBM-SDEWE), (C) forward-osmosis water splitting (FOWS), (D) asymmetric electrolyzer, (E) electrochemical neutralization cell (ENC), (F) microfluid electrolyzer, and (G) capillary-fed electrolyzer (CFE). CEM, cation exchange membrane. PTFE, polytetrafluoroethylene.
Waterproof breathable membrane combined with self-dampening electrolyte water electrolyzer
Recently, Xie et al. (12) reported a self-driven phase transition mechanism on a membrane-based seawater electrolyzer that was demonstrated to operate stably at a current density of 250 mA cm−2 for over 3200 hours under practical application conditions. A hydrophobic porous polytetrafluoroethylene-based waterproof breathable membrane (WBM) as a gas-path interface and concentrated KOH solution as a self-dampening electrolyte (SDE) were adopted (Fig. 7B). Because of the difference in the water vapor pressure between seawater and SDE, water can migrate from seawater across the membrane to the SDE, which is self-driven by a liquid-gas-liquid phase transition mechanism. This contributes to in situ water purification with 100% ion-blocking efficiency. In addition, a pilot-scale SDE water electrolyzer at an H2 generation rate (386 liters hour−1) was fabricated. The system is compact with dimensions of 82 cm by 62 cm by 70.5 cm and consists of 11 cells with a total effective geometric electrode surface area of 3696 cm2. This serves as an excellent example of retrofitting commercial AWE for sustainable seawater electrolysis.
Forward-osmosis water splitting
This approach couples the forward osmosis with water splitting, where the anions and cations in seawater, such as Ca2+, Mg2+, CO2, and Cl−, are excluded from the water splitting compartment by cellulose acetate membranes (Fig. 7C) (101). The rate of H2O influx via forward osmosis is set equal to H2O outflux via water oxidation, thus H2O can be continually extracted from seawater and in situ purified. The obstacles of forward-osmosis water splitting approach largely rely on the selectivity of the semipermeable membrane. The anti-biofouling coatings will benefit the approach for long-term operation.
Asymmetric electrolyzer
Asymmetric electrolyte feeds have been applied in direct seawater electrolysis (Fig. 7D) (17, 97, 102). By adopting this method, it becomes feasible to directly introduce neutral seawater to the cathode in a single flow, while simultaneously circulating pure KOH electrolyte at the anode. The high alkalinization was avoided in this design and only trace amounts of Cl− crossed the membrane to the anode. The substitution of Ir-based anodic catalysts with NiFe-LDH showcased remarkable performance and selectivity in the OER.
Electrochemical neutralization cell
An electrochemical neutralization cell (ENC) was created by combining the acidic HER and alkaline hydrazine oxidation with ionic exchange, enabling sustainable hydrogen production alongside electricity generation (Fig. 7E) (103). This innovative cell efficiently harnesses energy from both chemical reactions and low-grade heat from the surroundings to drive hydrogen production and/or electricity generation. Simultaneously, it achieves the rapid removal of salt from saline water by a unique cation exchange membrane/AEM structure, ensuring efficient water desalination.
Innovations in nonmembrane electrolyzers
As discussed above, even though membrane-separated electrolyzers could facilitate the formation of pure gas products and are much safer because of reducing the mix of oxygen and hydrogen, the stability of the membrane used could challenge the performance of electrolyzers. Therefore, several nonmembrane-separated electrolyzers have been reported.
Microfluid electrolyzer
A microfluidic electrolyzer with a microscale interelectrode distance offers several advantages, including reduced undesirable ohmic losses, improved material utilization, and higher energy density. In this system, two parallel electrodes are set at a distance of around a hundred micrometers and the generated gas can move near the electrode with a distance of 0.6 R from the tube’s center according to the Segré-Silberberg effect (Fig. 7F) (104). Last, the product gasses can be efficiently removed by the delicate balance between fluid mechanic forces, where the water splitting at current densities over 300 mA cm−2 with more than 42% power conversion efficiency, and the crossover of hydrogen gas can be controlled as low as 0.4% (105). By scaling out this design with multistack panels or with large area electrodes, once suitable electrocatalysts were applied, continuous hydrogen fuel could be produced without the limitation of membrane, where the cost can be also greatly reduced.
Capillary-fed electrolyzer
It was reported recently by Hodges et al. (106) (Fig. 7G), where a thin layer of electrolyte is constantly supplied to the electrodes by the capillary action. The formed hydrogen and oxygen gasses can be readily transported through the thin liquid layer covering the respective electrodes, thus the bubbles masking the electrodes can be avoided and access to the active sites can be maintained. A cell voltage of 0.5 mA cm−2 and 85°C of only 1.15 V is achieved, which equates to 98% energy efficiency with an energy consumption of 40.4 kWh kg−1 hydrogen (~47.5 kWh kg−1 in commercial electrolysis cells). This design allows for a notably simplified balance of plant and reduced energy consumption. It is also promising for seawater splitting with suitable electrocatalysts assembled. The precipitation should be prevented by adjusting the pH or removing the Ca2+/Mg2+ in advance of clogging the capillary.
SUMMARY AND OUTLOOK
Although tremendous efforts have been made to develop efficient seawater-splitting technology, several challenges must be addressed to make this technology viable, namely:
1) Developing robust and selective electrocatalysts for both anode and cathode is the core of sustainable direct seawater electrolysis. Materials science and chemistry offer opportunities to design highly efficient electrocatalysts, but careful tuning of the materials at the atomic level is required. A successful demonstration of stable seawater electrolysis in natural seawater without treatment (54) should be the focus of future studies on natural seawater electrolysis by tuning the local environment. Furthermore, there is a need for standardization of testing criteria for catalysts to ensure consistent evaluations. Standardization of components for seawater electrolysis is also essential, including electrolyte composition, electrolyzers, benchmark catalyst, and testing parameters. Environmental sustainability is also a key consideration in the design of practical devices.
2) The feasibility of other components in the electrolysis system should be carefully considered, particularly the corrosion of the bipolar plates and the water recycling system. Therefore, the designs for seawater electrolysis must encompass all the key components in the electrolyzers, comparing them with commercial pure water electrolyzers. Furthermore, note that most commercial pure water electrolyzers operate with hot water (50° to 90°C) to achieve high cell efficiency. As a result, the stability of components under such harsh conditions (50° to 90°C seawater) should be considered. Bridging this gap necessitates collaboration between scientists and the energy industry to advance this technology from TRL 3 to TRL 5.
3) The cost of catalysts and capital is another challenge that must be addressed. Although the capital and operational costs of the current desalination and purification units are competitive compared to seawater electrolysis, future efforts are needed to develop efficient materials and technologies for hydrogen production via direct seawater electrolysis. The offshore renewable energy plant offers an ideal platform for developing direct seawater splitting, easily integrated with low-cost marine transportation and existing offshore oil-gas infrastructure. Therefore, policies must carefully evaluate the impact on the marine ecosystem to ensure environmental sustainability.
4) In addition to fuel production, direct seawater splitting is a multifunctional technology that has the potential to address water desalination and management issues. This is because direct seawater electrolysis extracts hydrogen and oxygen from saline water. Further application of hydrogen in fuel cells could generate deionized water. As a result, fresh water is produced from seawater by a carbon-free electrochemical loop, which could be collected and used directly for daily life or to recharge brackish and coastal land. Therefore, further study and quantification of the feasibility and economic benefits of seawater splitting for surface salty water desalination and saline land remediation are encouraged.
5) Direct electrolysis and indirect electrolysis of seawater show different requirements for the design of electrolysis systems and also generate different by-products for various applications. For indirect electrolysis of seawater, it is necessary to find an energy-saving and efficient seawater desalination technology to match the large-scale demand for hydrogen production from industrial water electrolysis. For the direct electrolysis of seawater, it is urgent to finely design the electrolyzer with anticorrosion and antifouling capacity. It is worth looking if the anode product selectivity can be adjusted by rationally designing the catalyst: For the OER-dominated catalysts, high-value products hydrochloric acid and sodium hydroxide can be produced from the anode at the same time through electrolytic cell design (107). Meanwhile, for the ClER-dominated catalysts, chlorine is produced from the anode and can be applied for in situ conversion of other stable, harmless, and high-value chlorine-containing industrial products. This is seldom explored due to the complexity of the system design, but it is attractive for the optimal utilization of seawater resources.
Acknowledgments
Funding: H.J. gratefully acknowledges financial support from Institute for Sustainability, Energy and Resources, The University of Adelaide, Future Making Fellowship. J.X. acknowledges the financial support from the Chinese CSC scholarship Program. This work was supported by Australian Research Council grant FL170100154, Australian Research Council grant FT200100062, Australian Research Council grant DP230102027, Australian Research Council grant DP220102596, Australian Research Council grant DP190103472, Australian Research Council grant DP200100159, Australian Research Council grant LP210301397, and Australian Research Council grant CE230100032.
Author contributions: Supervision: S.-Z.Q. and Y.Z. Conceptualization: H.J., Y.Z., and S.-Z.Q. Methodology: H.J. and Y.Z. Investigation: H.J., J.X., H.L., H.S., and H.Y. Writing—original draft: H.J., J.X., H.L., H.S., and H.Y. Writing—review and editing: S.-Z.Q., Y.Z., M.J., and H.J.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper.
REFERENCES AND NOTES
- 1.International Energy Agency, Net Zero by 2050: A Roadmap for the Global Energy Sector (International Energy Agency, 2021); https://iea.blob.core.windows.net/assets/deebef5d-0c34-4539-9d0c-10b13d840027/NetZeroby2050-ARoadmapfortheGlobalEnergySector_CORR.pdf.
- 2.B. Lee, L. Wang, Z. Wang, N. J. Cooper, M. Elimelech, Directing the research agenda on water and energy technologies with process and economic analysis. Energ. Environ. Sci. 16, 714–722 (2023). [Google Scholar]
- 3.J. A. Turner, Sustainable hydrogen production. Science 305, 972–974 (2004). [DOI] [PubMed] [Google Scholar]
- 4.R. Subbaraman, D. Tripkovic, K.-C. Chang, D. Strmcnik, A. P. Paulikas, P. Hirunsit, M. Chan, J. Greeley, V. Stamenkovic, N. M. Markovic, Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012). [DOI] [PubMed] [Google Scholar]
- 5.M. F. Lagadec, A. Grimaud, Water electrolysers with closed and open electrochemical systems. Nat. Mater. 19, 1140–1150 (2020). [DOI] [PubMed] [Google Scholar]
- 6.A. Crivellari, V. Cozzani, Offshore renewable energy exploitation strategies in remote areas by power-to-gas and power-to-liquid conversion. Int. J. Hydrogen Energy 45, 2936–2953 (2020). [Google Scholar]
- 7.W. Tong, M. Forster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan, P. Farràs, Electrolysis of low-grade and saline surface water. Nat. Energy 5, 367–377 (2020). [Google Scholar]
- 8.International Energy Agency, Global Hydrogen Review 2022 (International Energy Agency, 2022); www.iea.org/reports/global-hydrogen-review-2022.
- 9.B. Peacock, Green Hydrogen Megaproject in SA Discontinued (PV magazine, 2022); www.pv-magazine-australia.com/2022/05/31/green-hydrogen-megaproject-in-sa-discontinued/.
- 10.L. Zhang, Z. Wang, J. Qiu, Energy-saving hydrogen production by seawater electrolysis coupling sulfion degradation. Adv. Mater. 34, e2109321 (2022). [DOI] [PubMed] [Google Scholar]
- 11.H. Jin, X. Wang, C. Tang, A. Vasileff, L. Li, A. Slattery, S.-Z. Qiao, Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv. Mater. 33, e2007508 (2021). [DOI] [PubMed] [Google Scholar]
- 12.H. Xie, Z. Zhao, T. Liu, Y. Wu, C. Lan, W. Jiang, L. Zhu, Y. Wang, D. Yang, Z. Shao, A membrane-based seawater electrolyser for hydrogen generation. Nature 612, 673–678 (2022). [DOI] [PubMed] [Google Scholar]
- 13.P. Farràs, P. Strasser, A. J. Cowan, Water electrolysis: Direct from the sea or not to be? Joule 5, 1921–1923 (2021). [Google Scholar]
- 14.S. Dresp, F. Dionigi, M. Klingenhof, P. Strasser, Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 4, 933–942 (2019). [Google Scholar]
- 15.H. Yu, J. Wan, M. Goodsite, H. Jin, Advancing direct seawater electrocatalysis for green and affordable hydrogen. One Earth 6, 267–277 (2023). [Google Scholar]
- 16.G. A. Lindquist, Q. Xu, S. Z. Oener, S. W. Boettcher, Membrane electrolyzers for impure-water splitting. Joule 4, 2549–2561 (2020). [Google Scholar]
- 17.S. Dresp, T. Ngo Thanh, M. Klingenhof, S. Brückner, P. Hauke, P. Strasser, Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energ. Environ. Sci. 13, 1725–1729 (2020). [Google Scholar]
- 18.H. Jin, X. Liu, A. Vasileff, Y. Jiao, Y. Zhao, Y. Zheng, S.-Z. Qiao, Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 12, 12761–12769 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Y. Kuang, M. J. Kenney, Y. Meng, W.-H. Hung, Y. Liu, J. E. Huang, R. Prasanna, P. Li, Y. Li, L. Wang, M.-C. Lin, M. D. McGehee, X. Sun, H. Dai, Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. U.S.A. 116, 6624–6629 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.X. Lu, J. Pan, E. Lovell, T. H. Tan, Y. H. Ng, R. Amal, A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energ. Environ. Sci. 11, 1898–1910 (2018). [Google Scholar]
- 21.J. Liu, S. Duan, H. Shi, T. Wang, X. Yang, Y. Huang, G. Wu, Q. Li, Rationally designing efficient electrocatalysts for direct seawater splitting: Challenges, achievements, and promises. Angew. Chem. Int. Ed. 61, e202210753 (2022). [DOI] [PubMed] [Google Scholar]
- 22.R. d’Amore-Domenech, T. J. Leo, Sustainable hydrogen production from offshore marine renewable farms: Techno-energetic insight on seawater electrolysis technologies. ACS Sustain. Chem. Eng. 7, 8006–8022 (2019). [Google Scholar]
- 23.M. A. Ahsan, T. He, J. C. Noveron, K. Reuter, A. R. Puente-Santiago, R. Luque, Low-dimensional heterostructures for advanced electrocatalysis: An experimental and computational perspective. Chem. Soc. Rev. 51, 812–828 (2022). [DOI] [PubMed] [Google Scholar]
- 24.M. A. Khan, T. Al-Attas, S. Roy, M. M. Rahman, N. Ghaffour, V. Thangadurai, S. Larter, J. Hu, P. M. Ajayan, M. G. Kibria, Seawater electrolysis for hydrogen production: A solution looking for a problem? Energ. Environ. Sci. 14, 4831–4839 (2021). [Google Scholar]
- 25.J. N. Hausmann, R. Schlögl, P. W. Menezes, M. Driess, Is direct seawater splitting economically meaningful? Energ. Environ. Sci. 14, 3679–3685 (2021). [Google Scholar]
- 26.Y. Zheng, S.-Z. Qiao, Direct seawater splitting to hydrogen by a membrane electrolyzer. Joule 7, 20–22 (2023). [Google Scholar]
- 27.International Energy Agency, Global Hydrogen Review 2021 (International Energy Agency, 2021); https://iea.blob.core.windows.net/assets/5bd46d7b-906a-4429-abda-e9c507a62341/GlobalHydrogenReview2021.pdf.
- 28.R. R. Beswick, A. M. Oliveira, Y. Yan, Does the green hydrogen economy have a water problem? ACS Energy Lett. 6, 3167–3169 (2021). [Google Scholar]
- 29.D. J. Lampert, H. Cai, A. Elgowainy, Wells to wheels: Water consumption for transportation fuels in the United States. Energ. Environ. Sci. 9, 787–802 (2016). [Google Scholar]
- 30.Wikipedia, List of Desalination Plants in Australia (Wikipedia, 2023); https://en.wikipedia.org/wiki/List_of_desalination_plants_in_Australia.
- 31.K. Zuo, W. Wang, A. Deshmukh, S. Jia, H. Guo, R. Xin, M. Elimelech, P. M. Ajayan, J. Lou, Q. Li, Multifunctional nanocoated membranes for high-rate electrothermal desalination of hypersaline waters. Nat. Nanotech. 15, 1025–1032 (2020). [DOI] [PubMed] [Google Scholar]
- 32.C. He, Z. Liu, J. Wu, X. Pan, Z. Fang, J. Li, B. A. Bryan, Future global urban water scarcity and potential solutions. Nat. Commun. 12, 4667 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.M. Maril, J.-L. Delplancke, N. Cisternas, P. Tobosque, Y. Maril, C. Carrasco, Critical aspects in the development of anodes for use in seawater electrolysis. Int. J. Hydrogen Energy 47, 3532–3549 (2022). [Google Scholar]
- 34.L. Wu, F. Zhang, S. Song, M. Ning, Q. Zhu, J. Zhou, G. Gao, Z. Chen, Q. Zhou, X. Xing, T. Tong, Y. Yao, J. Bao, L. Yu, S. Chen, Z. Ren, Efficient alkaline water/seawater hydrogen evolution by a nanorod-nanoparticle-structured Ni-MoN catalyst with fast water-dissociation kinetics. Adv. Mater. 34, e2201774 (2022). [DOI] [PubMed] [Google Scholar]
- 35.S.-H. Hsu, J. Miao, L. Zhang, J. Gao, H. Wang, H. Tao, S. F. Hung, A. Vasileff, Z. Qiao Shi, B. Liu, An earth-abundant catalyst-based seawater photoelectrolysis system with 17.9% solar-to-hydrogen efficiency. Adv. Mater. 30, e1707261 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Y. Sano, Y. Hao, F. Kuwahara, Development of an electrolysis based system to continuously recover magnesium from seawater. Heliyon 4, e00923 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.H. Blanco, Hydrogen Production in 2050: How Much Water Will 74EJ Need? (energypost.eu, 2021); https://energypost.eu/hydrogen-production-in-2050-how-much-water-will-74ej-need/.
- 38.Y. Jin, P. Behrens, A. Tukker, L. Scherer, Water use of electricity technologies: A global meta-analysis. Renew. Sustain. Energy Rev. 115, 109391 (2019). [Google Scholar]
- 39.A. Panagopoulos, K.-J. Haralambous, M. Loizidou, Desalination brine disposal methods and treatment technologies - A review. Sci. Total Environ. 693, 133545 (2019). [DOI] [PubMed] [Google Scholar]
- 40.L. Yu, L. Wu, S. Song, B. McElhenny, F. Zhang, S. Chen, Z. Ren, Hydrogen generation from seawater electrolysis over a sandwich-like NiCoN|NixP|NiCoN microsheet array catalyst. ACS Energy Lett. 5, 2681–2689 (2020). [Google Scholar]
- 41.R. d’Amore-Domenech, Ó. Santiago, T. J. Leo, Multicriteria analysis of seawater electrolysis technologies for green hydrogen production at sea. Renew. Sustain. Energy Rev. 133, 110166 (2020). [Google Scholar]
- 42.D. W. Kirk, A. E. Ledas, Precipitate formation during sea water electrolysis. Int. J. Hydrogen Energy 7, 925–932 (1982). [Google Scholar]
- 43.R. J. Ouimet, J. R. Glenn, D. De Porcellinis, A. R. Motz, M. Carmo, K. E. Ayers, The role of electrocatalysts in the development of gigawatt-scale PEM electrolyzers. ACS Catal. 12, 6159–6171 (2022). [Google Scholar]
- 44.E. M. Kapp, The precipitation of calcium and magnesium from sea water by sodium hydroxide. Biol. Bull. 55, 453–458 (1928). [Google Scholar]
- 45.J. E. Bennett, Electrodes for generation of hydrogen and oxygen from seawater. Int. J. Hydrogen Energy 5, 401–408 (1980). [Google Scholar]
- 46.Y. Zhao, B. Jin, Y. Zheng, H. Jin, Y. Jiao, S.-Z. Qiao, Charge state manipulation of cobalt selenide catalyst for overall seawater electrolysis. Adv. Energy Mater. 8, 1801926 (2018). [Google Scholar]
- 47.L. Yu, Q. Zhu, S. Song, B. McElhenny, D. Wang, C. Wu, Z. Qin, J. Bao, Y. Yu, S. Chen, Z. Ren, Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 10, 5106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Q. Lv, J. Han, X. Tan, W. Wang, L. Cao, B. Dong, Featherlike NiCoP holey nanoarrys for efficient and stable seawater splitting. ACS Appl. Energy Mater. 2, 3910–3917 (2019). [Google Scholar]
- 49.X. Wang, C. Xu, M. Jaroniec, Y. Zheng, S.-Z. Qiao, Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10, 4876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.J.-J. Lv, R. Yin, L. Zhou, J. Li, R. Kikas, T. Xu, Z.-J. Wang, H. Jin, X. Wang, S. Wang, Microenvironment engineering for the electrocatalytic CO2 reduction reaction. Angew. Chem. Int. Ed. Engl. 61, e202207252 (2022). [DOI] [PubMed] [Google Scholar]
- 51.Y. Zhao, L. Hao, A. Ozden, S. Liu, R. K. Miao, P. Ou, T. Alkayyali, S. Zhang, J. Ning, Y. Liang, Y. Xu, M. Fan, Y. Chen, J. E. Huang, K. Xie, J. Zhang, C. P. O’Brien, F. Li, E. H. Sargent, D. Sinton, Conversion of CO2 to multicarbon products in strong acid by controlling the catalyst microenvironment. Nat. Synth. 2, 403–412 (2023). [Google Scholar]
- 52.H. S. Shafaat, J. Y. Yang, Uniting biological and chemical strategies for selective CO2 reduction. Nat. Catal. 4, 928–933 (2021). [Google Scholar]
- 53.X. Wang, Y. Jiao, L. Li, Y. Zheng, S.-Z. Qiao, Local environment determined reactant adsorption configuration for enhanced electrocatalytic acetone hydrogenation to propane. Angew. Chem. Int. Ed. 61, e202114253 (2022). [DOI] [PubMed] [Google Scholar]
- 54.J. Guo, Y. Zheng, Z. Hu, C. Zheng, J. Mao, K. Du, M. Jaroniec, S.-Z. Qiao, T. Ling, Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 8, 264–272 (2023). [Google Scholar]
- 55.F. Song, W. Li, J. Yang, G. Han, P. Liao, Y. Sun, Interfacing nickel nitride and nickel boosts both electrocatalytic hydrogen evolution and oxidation reactions. Nat. Commun. 9, 4531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.M. Zhou, Q. Weng, Z. I. Popov, Y. Yang, L. Y. Antipina, P. B. Sorokin, X. Wang, Y. Bando, D. Golberg, Construction of polarized carbon–nickel catalytic surfaces for potent, durable, and economic hydrogen evolution reactions. ACS Nano 12, 4148–4155 (2018). [DOI] [PubMed] [Google Scholar]
- 57.L. Xiu, W. Pei, S. Zhou, Z. Wang, P. Yang, J. Zhao, J. Qiu, Multilevel hollow MXene tailored low-Pt catalyst for efficient hydrogen Evolution in full-pH range and seawater. Adv. Funct. Mater. 30, 1910028 (2020). [Google Scholar]
- 58.K. Jiang, W. Liu, W. Lai, M. Wang, Q. Li, Z. Wang, J. Yuan, Y. Deng, J. Bao, H. Ji, NiFe layered double hydroxide/FeOOH heterostructure nanosheets as an efficient and durable bifunctional electrocatalyst for overall seawater splitting. Inorg. Chem. 60, 17371–17378 (2021). [DOI] [PubMed] [Google Scholar]
- 59.T. Lim, G. Y. Jung, J. H. Kim, S. O. Park, J. Park, Y.-T. Kim, S. J. Kang, H. Y. Jeong, S. K. Kwak, S. H. Joo, Atomically dispersed Pt–N4 sites as efficient and selective electrocatalysts for the chlorine evolution reaction. Nat. Commun. 11, 412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R. R. Rao, M. J. Kolb, L. Giordano, A. F. Pedersen, Y. Katayama, J. Hwang, A. Mehta, H. You, J. R. Lunger, H. Zhou, N. B. Halck, T. Vegge, I. Chorkendorff, I. E. L. Stephens, Y. Shao-Horn, Operando identification of site-dependent water oxidation activity on ruthenium dioxide single-crystal surfaces. Nat. Catal. 3, 516–525 (2020). [Google Scholar]
- 61.R. K. B. Karlsson, A. Cornell, Selectivity between oxygen and chlorine evolution in the chlor-alkali and chlorate processes. Chem. Rev. 116, 2982–3028 (2016). [DOI] [PubMed] [Google Scholar]
- 62.J. Wang, H. Yang, F. Li, L. Li, J. Wu, S. Liu, T. Cheng, Y. Xu, Q. Shao, X. Huang, Single-site Pt-doped RuO2 hollow nanospheres with interstitial C for high-performance acidic overall water splitting. Sci. Adv. 8, eabl9271 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.M. Retuerto, L. Pascual, F. Calle-Vallejo, P. Ferrer, D. Gianolio, A. G. Pereira, Á. García, J. Torrero, M. T. Fernández-Díaz, P. Bencok, M. A. Peña, J. L. G. Fierro, S. Rojas, Na-doped ruthenium perovskite electrocatalysts with improved oxygen evolution activity and durability in acidic media. Nat. Commun. 10, 2041 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.J. G. Vos, T. A. Wezendonk, A. W. Jeremiasse, M. T. M. Koper, MnOx/IrOx as selective oxygen evolution electrocatalyst in acidic chloride solution. J. Am. Chem. Soc. 140, 10270–10281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.H. Jin, X. Liu, P. An, C. Tang, H. Yu, Q. Zhang, H.-J. Peng, L. Gu, Y. Zheng, T. Song, K. Davey, U. Paik, J. Dong, S.-Z. Qiao, Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 14, 354 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.N. Jiang, H.-M. Meng, The durability of different elements doped manganese dioxide-coated anodes for oxygen evolution in seawater electrolysis. Surf. Coat. Technol. 206, 4362–4367 (2012). [Google Scholar]
- 67.F. Cheng, X. Feng, X. Chen, W. Lin, J. Rong, W. Yang, Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH. Electrochim. Acta 251, 336–343 (2017). [Google Scholar]
- 68.F. Dionigi, T. Reier, Z. Pawolek, M. Gliech, P. Strasser, Design criteria, operating conditions, and nickel–iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 9, 962–972 (2016). [DOI] [PubMed] [Google Scholar]
- 69.N. Wang, P. Ou, S.-F. Hung, J. E. Huang, A. Ozden, J. Abed, I. Grigioni, C. Chen, R. K. Miao, Y. Yan, J. Zhang, Z. Wang, R. Dorakhan, A. Badreldin, A. Abdel-Wahab, D. Sinton, Y. Liu, H. Liang, E. H. Sargent, Strong-proton-adsorption Co-based electrocatalysts achieve active and stable neutral seawater splitting. Adv. Mater. 35, 2210057 (2023). [DOI] [PubMed] [Google Scholar]
- 70.J. Li, Y. Liu, H. Chen, Z. Zhang, X. Zou, Design of a multilayered oxygen-evolution electrode with high catalytic activity and corrosion resistance for saline water splitting. Adv. Funct. Mater. 31, 2101820 (2021). [Google Scholar]
- 71.K. Obata, K. Takanabe, A permselective CeOx coating to improve the stability of oxygen evolution electrocatalysts. Angew. Chem. Int. Ed. Engl. 57, 1616–1620 (2018). [DOI] [PubMed] [Google Scholar]
- 72.C. Qiao, Z. Usman, T. Cao, S. Rafai, Z. Wang, Y. Zhu, C. Cao, J. Zhang, High-valence Ni and Fe sites on sulfated NiFe-LDH nanosheets to enhance O-O coupling for water oxidation. Chem. Eng. J. 426, 130873 (2021). [Google Scholar]
- 73.L. Tan, J. Yu, C. Wang, H. Wang, X. Liu, H. Gao, L. Xin, D. Liu, W. Hou, T. Zhan, Partial sulfidation strategy to NiFe-LDH@FeNi2S4 heterostructure enable high-performance water/seawater oxidation. Adv. Funct. Mater. 32, 2200951 (2022). [Google Scholar]
- 74.Y.-H. Wang, S. Zheng, W.-M. Yang, R.-Y. Zhou, Q.-F. He, P. Radjenovic, J.-C. Dong, S. Li, J. Zheng, Z.-L. Yang, G. Attard, F. Pan, Z.-Q. Tian, J.-F. Li, In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021). [DOI] [PubMed] [Google Scholar]
- 75.A. T. Chu, Y. Surendranath, Aprotic solvent exposes an altered mechanism for copper-catalyzed ethylene electrosynthesis. J. Am. Chem. Soc. 144, 5359–5365 (2022). [DOI] [PubMed] [Google Scholar]
- 76.P. Li, Y. Jiang, Y. Hu, Y. Men, Y. Liu, W. Cai, S. Chen, Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal. 5, 900–911 (2022). [Google Scholar]
- 77.K. Zhao, X. Chang, H.-S. Su, Y. Nie, Q. Lu, B. Xu, Enhancing hydrogen oxidation and evolution kinetics by tuning the interfacial hydrogen-bonding environment on functionalized platinum surfaces. Angew. Chem. Int. Ed. 61, e202207197 (2022). [DOI] [PubMed] [Google Scholar]
- 78.T. Ma, W. Xu, B. Li, X. Chen, J. Zhao, S. Wan, K. Jiang, S. Zhang, Z. Wang, Z. Tian, Z. Lu, L. Chen, The critical role of additive sulfate for stable alkaline seawater oxidation on nickel-based electrodes. Angew. Chem. Int. Ed. 60, 22740–22744 (2021). [DOI] [PubMed] [Google Scholar]
- 79.T. Wang, X. Cao, L. Jiao, Progress in hydrogen production coupled with electrochemical oxidation of small molecules. Angew. Chem. Int. Ed. 61, e202213328 (2022). [DOI] [PubMed] [Google Scholar]
- 80.F. Sun, J. Qin, Z. Wang, M. Yu, X. Wu, X. Sun, J. Qiu, Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat. Commun. 12, 4182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Q. Wang, T. Li, C. Yang, M. Chen, A. Guan, L. Yang, S. Li, X. Lv, Y. Wang, G. Zheng, Electrocatalytic methane oxidation greatly promoted by chlorine intermediates. Angew. Chem. Int. Ed. 60, 17398–17403 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Q. Wang, C. Yang, Y. Yan, H. Yu, A. Guan, M. Kan, Q. Zhang, L. Zhang, G. Zheng, Electrocatalytic CO2 upgrading to triethanolamine by bromine-assisted C2H4 oxidation. Angew. Chem. Int. Ed. 62, e202212733 (2023). [DOI] [PubMed] [Google Scholar]
- 83.W. R. Leow, Y. Lum, A. Ozden, Y. Wang, D.-H. Nam, B. Chen, J. Wicks, T.-T. Zhuang, F. Li, D. Sinton, E. H. Sargent, Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368, 1228–1233 (2020). [DOI] [PubMed] [Google Scholar]
- 84.S.-K. Geng, Y. Zheng, S.-Q. Li, H. Su, X. Zhao, J. Hu, H.-B. Shu, M. Jaroniec, P. Chen, Q.-H. Liu, S.-Z. Qiao, Nickel ferrocyanide as a high-performance urea oxidation electrocatalyst. Nat. Energy 6, 904–912 (2021). [Google Scholar]
- 85.X. Liu, J. He, S. Zhao, Y. Liu, Z. Zhao, J. Luo, G. Hu, X. Sun, Y. Ding, Self-powered H2 production with bifunctional hydrazine as sole consumable. Nat. Commun. 9, 4365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.B. You, X. Liu, N. Jiang, Y. Sun, A general strategy for decoupled hydrogen production from water splitting by integrating oxidative biomass valorization. J. Am. Chem. Soc. 138, 13639–13646 (2016). [DOI] [PubMed] [Google Scholar]
- 87.T. Wang, L. Tao, X. Zhu, C. Chen, W. Chen, S. Du, Y. Zhou, B. Zhou, D. Wang, C. Xie, P. Long, W. Li, Y. Wang, R. Chen, Y. Zou, X.-Z. Fu, Y. Li, X. Duan, S. Wang, Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat. Catal. 5, 66–73 (2022). [Google Scholar]
- 88.G. Han, G. Li, Y. Sun, Electrocatalytic dual hydrogenation of organic substrates with a Faradaic efficiency approaching 200%. Nat. Catal. 6, 224–233 (2023). [Google Scholar]
- 89.K. Zeng, D. Zhang, Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 36, 307–326 (2010). [Google Scholar]
- 90.M. Schalenbach, W. Lueke, D. Stolten, Hydrogen diffusivity and electrolyte permeability of the Zirfon PERL separator for alkaline water electrolysis. J. Electrochem. Soc. 163, F1480–F1488 (2016). [Google Scholar]
- 91.M. Carmo, D. L. Fritz, J. Mergel, D. Stolten, A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 38, 4901–4934 (2013). [Google Scholar]
- 92.J. Xu, H. Jin, T. Lu, J. Li, Y. Liu, K. Davey, Y. Zheng, S.-Z. Qiao, IrOx·nH2O with lattice water–assisted oxygen exchange for high-performance proton exchange membrane water electrolyzers. Sci. Adv. 9, eadh1718 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.R. Rossi, D. M. Hall, L. Shi, N. R. Cross, C. A. Gorski, M. A. Hickner, B. E. Logan, Using a vapor-fed anode and saline catholyte to manage ion transport in a proton exchange membrane electrolyzer. Energ. Environ. Sci. 14, 6041–6049 (2021). [Google Scholar]
- 94.Y. S. Park, J. Lee, M. J. Jang, J. Yang, J. Jeong, J. Park, Y. Kim, M. H. Seo, Z. Chen, S. M. Choi, High-performance anion exchange membrane alkaline seawater electrolysis. J. Mater. Chem. A 9, 9586–9592 (2021). [Google Scholar]
- 95.S. Gottesfeld, D. R. Dekel, M. Page, C. Bae, Y. Yan, P. Zelenay, Y. S. Kim, Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 375, 170–184 (2018). [Google Scholar]
- 96.S. Dresp, F. Dionigi, S. Loos, J. F. de Araujo, C. Spori, M. Gliech, H. Dau, P. Strasser, Direct electrolytic splitting of seawater: Activity, selectivity, degradation, and recovery studied from the molecular catalyst structure to the electrolyzer cell level. Adv. Energy Mater. 8, 1800338 (2018). [Google Scholar]
- 97.H. Shi, T. Wang, J. Liu, W. Chen, S. Li, J. Liang, S. Liu, X. Liu, Z. Cai, C. Wang, D. Su, Y. Huang, L. Elbaz, Q. Li, A sodium-ion-conducted asymmetric electrolyzer to lower the operation voltage for direct seawater electrolysis. Nat. Commun. 14, 3934 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.S. Z. Oener, M. J. Foster, S. W. Boettcher, Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 369, 1099–1103 (2020). [DOI] [PubMed] [Google Scholar]
- 99.S. Z. Oener, L. P. Twight, G. A. Lindquist, S. W. Boettcher, Thin cation-exchange layers enable high-current-density bipolar membrane electrolyzers via improved water transport. ACS Energy Lett. 6, 1–8 (2021). [Google Scholar]
- 100.B. Mayerhöfer, D. McLaughlin, T. Böhm, M. Hegelheimer, D. Seeberger, S. Thiele, Bipolar membrane electrode assemblies for water electrolysis. ACS Appl. Energy Mater. 3, 9635–9644 (2020). [Google Scholar]
- 101.S. S. Veroneau, A. C. Hartnett, A. E. Thorarinsdottir, D. G. Nocera, Direct seawater splitting by forward osmosis coupled to water electrolysis. ACS Appl. Energy Mater. 5, 1403–1408 (2022). [Google Scholar]
- 102.M. L. Frisch, T. N. Thanh, A. Arinchtein, L. Hager, J. Schmidt, S. Brückner, J. Kerres, P. Strasser, Seawater electrolysis using all-PGM-free catalysts and cell components in an asymmetric feed. ACS Energy Lett. 8, 2387–2394 (2023). [Google Scholar]
- 103.F. Sun, D. He, K. Yang, J. Qiu, Z. Wang, Hydrogen production and water desalination with on-demand electricity output enabled by electrochemical neutralization chemistry. Angew. Chem. Int. Ed. 61, e202203929 (2022). [DOI] [PubMed] [Google Scholar]
- 104.G. Segre, A. Silberberg, Radial particle displacements in poiseuille flow of suspensions. Nature 189, 209–210 (1961). [Google Scholar]
- 105.S. M. H. Hashemi, M. A. Modestino, D. Psaltis, A membrane-less electrolyzer for hydrogen production across the pH scale. Energ. Environ. Sci. 8, 2003–2009 (2015). [Google Scholar]
- 106.A. Hodges, A. L. Hoang, G. Tsekouras, K. Wagner, C.-Y. Lee, G. F. Swiegers, G. G. Wallace, A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 13, 1304 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.A. Kumar, K. R. Phillips, G. P. Thiel, U. Schröder, J. H. Lienhard, Direct electrosynthesis of sodium hydroxide and hydrochloric acid from brine streams. Nat. Catal. 2, 106–113 (2019). [Google Scholar]