Abstract
Neurological disorders are a common feature in patients who recover from severe acute pneumonia. However, the underlying mechanisms remain poorly understood. Here, we show that the neurological syndromes after severe acute pneumonia are partly attributed to the translocation of endogenous bacteria from the lung to the brain during pneumonia. Using principal components analysis, similarities were found between the brain’s flora species and those of the lungs, indicating that the bacteria detected in the brain may originate from the lungs. We also observed impairment of both the lung-blood and brain-blood barriers, allowing endogenous lung bacteria to invade the brain during pneumonia. An elevated microglia and astrocyte activation signature via bacterial infection–related pathways was observed, indicating a bacterial-induced disruption of brain homeostasis. Collectively, we identify endogenous lung bacteria that play a role in altering brain homeostasis, which provides insight into the mechanism of neurological syndromes after severe pneumonia.
Translocation of endogenous bacteria from the lung to the brain during pneumonia infection contributes the neurological disorders
INTRODUCTION
Acute pneumonia is mostly caused by infectious pathogens (e.g., bacteria, viruses, and fungi), leading to an inflammatory condition of the lungs. Acute pneumonia has been one of the major causes of death in the past several yearshttps://covid19.who.int. In addition to acute pneumonia-related respiratory failure, neurological disorders after severe acute pneumonia are common complications that reduce patient’s quality of life. A very recent example is “brain fog” occurring in “long coronavirus disease (long-COVID)” (1, 2). Some people who have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) struggle for weeks to months with concentration issues and other cognitive symptoms that are associated with changes in the brain structure (3). Neurological syndromes after severe pneumonia exist in recovered patients with many respiratory diseases (4, 5). One study showed that the frequencies of most neurological manifestations did not differ after coronavirus disease 2019 (COVID-19), influenza, and bacterial pneumonia (6), indicating a potential general mechanism involved in all acute pneumonia–induced neurological disorders. In most patients suffering from neurological syndromes after severe acute pneumonia and also experimental models, hyperactive astrocytic and microglial activity is observed in the brain, accompanied by axonal damage and β-amyloid (Aβ) formation (7, 8). Given the major role of astrocytes and microglia in neuroinflammation, there is growing evidence suggesting that a detrimental immune response in the brain contributes to neurological symptoms after severe pneumonia (7). Yet, it still remains controversial whether the alteration of the brain is induced by direct inflammatory responses from the lung or the bystander effects of pneumonia. The relationship between acute pneumonia and brain activity has not been elucidated.
Bidirectional interactions in the lung-brain axis have been reported (9). The central nervous system (CNS) extensively communicates with other systems. Studies have shown that the brain and lung can communicate through a variety of ways, including the neuroanatomical pathway, endocrine pathway, and immune pathway (10). Typically, the lung is an organ that contains microbiota (11). The microbial community in the lung is composed of about 140 distinct families, while most of their functions in healthy individuals are still unknown (11). In contrast, the brain is considered to be a sterile environment [or much fewer microbes (12)] under normal conditions, as the blood-brain barrier (BBB) is able to prevent bacteria from entering the brain tissue (13).
In this study, we accidentally found that neurological disorders after severe acute pneumonia are associated with the translocation of endogenous bacteria from the lung to the brain. In the lipopolysaccharide (LPS)–induced experimental severe pneumonia mouse model, we detected an emergence of bacteria in the brain tissue. Similarities were found between the brain’s flora species and those of the lungs, indicating that the bacteria in the brain may come from the lungs during pneumonia. We also observed simultaneous lung-blood barrier and BBB impairment, during acute pneumonia, which may explain how lung bacteria invade the brain during acute pneumonia. The existence of bacteria in the brain tissue results in the disruption of brain homeostasis, especially for astrocytes and microglia.
Platelet-derived extracellular vesicles (PEVs) could serve as a general platform for inflammatory cells targeting drug delivery, which has been demonstrated by our group in previous studies (14–17). By loading rapamycin into PEVs (rapamycin-PEVs), we showed that intranasal delivery of rapamycin-PEVs rescued the dysfunction of astrocytes and microglia caused by severe acute pneumonia effectively alleviated the neurological disorders after infection. Our work provides insight into the mechanism of neurological syndromes after severe acute pneumonia, which is partly attributed to the translocation of endogenous bacteria from the lung to the brain during pneumonia.
RESULTS
Severe acute pneumonia leads to neurological disorders in the brain
To avoid interference with exogenous pathogens, LPS-induced acute pneumonia was established to study neurological disorders in mice (Fig. 1A). It was found that after intratracheal LPS challenge (4 mg kg−1), the mice experienced momentary weight loss but quickly regained. The body weight of the treated mice was similar to that of untreated mice at day 30 after LPS challenge (Fig. 1B). The blood oxygen saturation and cytokine levels within the lungs such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor (TNF) were all gradually recovered, with no difference from the untreated mice after 30 days (Fig. 1, C to F), indicating that the function of lungs in treated mice can be recovered from LPS-induced pneumonia under normal conditions. To test whether these mice suffer from a neurological disorder, the behavior of the mice recovered from pneumonia was tested. In the open-field experiment, the recovered mice were found to be more huddled in the corner and immobile compared with untreated mice, with reduced total activity path, central activity path, and central activity time as well as anxiety-like behavior (Fig. 1, G to K). In the new object recognition experiment, the discrimination index and preference index for new objects were significantly lower in mice that recovered from pneumonia, indicating impaired cognitive ability and short-term memory (Fig. 1, L to N). In the Morris water maze experiment, mice that recovered from pneumonia took a much longer time to reach the station (Fig. 1, O and P), indicating a decrease in spatial memory ability. In addition, we also observed similar behavior abnormal in the mice after 60 days of LPS treatment, indicating a long-term impact on behavior and motor function (fig. S1). Furthermore, immunofluorescence of Aβ protein in microglia and astrocytes in the hippocampus of the recovered mice confirmed an altered function of these cells (Fig. 1, Q to T). A decrease in the length and number of microglia branches indicated that microglia were in an activated state (Fig. 1, U and V). It is noteworthy that inflammatory markers in the brain tissue after pneumonia declined; however, they were still higher than baseline (Fig. 1, W to Y). These data showed that the defect in brain function is a common feature in mice recovered from severe pneumonia.
Fig. 1. Severe acute pneumonia leads to neurological disorders in the brain.
(A) Schematic of the experimental timeline. (B) Body weight of untreated and LPS-treated mice. (C) Blood oxygen saturation of untreated and LPS-treated mice (n = 8). (D to F) Inflammatory factors including IL-1β (D), IL-6 (E), and TNF-α (F) of lung tissue homogenate after LPS challenge (n = 4). (G) Schematic illustration of the open-field test. (H) Representative paths of mice in the open-field test. (I) Total distance for mice in the open-field test. (J) Central distance for mice in the open-field test. (K) Central time for mice in the open-field test. (L) Schematic illustration of the novel object recognition test. (M) Discrimination index in the novel object recognition. (N) Preference index in the novel object recognition. (O) Representative plot of Morris water maze images and (P) quantification of escape latency. (Q) Confocal microscopy images of Aβ protein in microglia. Red, Aβ; green, IBA1; blue, 4′,6-diamidino-2-phenylindole (DAPI). Scale bar, 20 μm. (R) Mean fluorescence quantification of Aβ protein in microglia (n = 4). (S) Confocal microscopy images of Aβ protein in astrocytes. Red, Aβ; green, glial fibrillary acidic protein (GFAP); blue, DAPI. Scale bar, 20 μm. (T) Mean fluorescence quantification of Aβ protein in astrocytes (n = 4). (U) Plot of microglia branches and length and (V) corresponding quantitative analysis. (W to Y) Inflammatory factors including IL-1β (W), IL-6 (X), and TNF-α (Y) of brain tissue homogenate after LPS challenge (n = 4). Data are shown as means ± SD. Statistical significance was calculated by Student’s t-test (two-tailed) and one-way analysis of variance (ANOVA) using Tukey’s posttest. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; UNTX, untreated; i.t., intratracheal injection; n.s., nonsignificant; a.u., arbitrary units; p.i., post-infection.
Bacteria were observed in the brain during acute pneumonia
Lung tissue has been demonstrated to harbor a microbial community that may regulate brain autoimmunity (18). We questioned whether alterations in the homeostasis of the brain were induced by the influence of the lung microbiome. Whole brain and lung tissue were collected, homogenized, and plated on Luria-Bertani (LB) plates at different time points after LPS challenge (Fig. 2A). Bacterial colonies were quantified by counting colony-forming units (CFUs) (Fig. 2, B to E). In accordance with the brain being considered a sterile organ, there were no colonies in the brains of mice before LPS treatment. Unexpectedly, we observed that CFUs in the brain tissue emerged and increased on days 1 and 7 after LPS challenge and then gradually reduced (but not eliminated) within 30 days after pneumonia (Fig. 2, B and C). There was no obvious change in the lung CFU during the whole period of pneumonia remission (Fig. 2, D and E).
Fig. 2. Bacteria were observed in the brain during acute pneumonia.
(A) Schematic of the experimental timeline. (B) Representative plot of bacterial colony growth after 24 hours of brain tissue homogenate and (C) corresponding quantification results (CFU) (n = 4). (D) Representative plot of bacterial colony growth after 24 hours of lung tissue homogenate and (E) corresponding quantification results (n = 4). (F) Genomes of the lung and whole brain were extracted, and the V3-V4 detection region was selected for polymerase chain reaction amplification. Representative agarose gel electrophoresis for DNA detection and (G) corresponding quantitative analysis (n = 4). Standard bacterial genomic DNA mix was used as a positive control. (H) Relative abundance of lung and brain bacterial inhabitants at the phylum level [n = 5 (Lung) and n = 6 (brain)]. (I) Relative abundance of lung and brain bacterial inhabitants at the family level [n = 5 (lung) and n = 6 (brain)]. (J) Principal components analysis on the lung and brain bacterial inhabitants 1 day after LPS challenge (n = 5). (K) Schematic of the experimental timeline. (L) Representative plot of bacterial colony growth after 24 hours of brain tissue homogenate (n = 4). (M) Inflammatory factors expression of lung tissue homogenate at days 1 and 30 (n = 6). (N) Inflammatory factors expression of brain tissue homogenate at days 1 and 30 (n = 6). (O) Confocal microscopy images of Aβ protein in microglia. Red, Aβ; green, IBA1; blue, DAPI. Scale bar, 20 μm. (P) Mean fluorescence quantification of Aβ protein in microglia (n = 4). (Q) Confocal microscopy images of Aβ protein in astrocytes at day 30. Red, Aβ; green, GFAP; blue, DAPI. Scale bar, 20 μm. (R) Mean fluorescence quantification of Aβ protein in astrocytes (n = 4). Data are shown as means ± SD. Statistical significance was calculated by Student’s t-test (two-tailed) and one-way ANOVA using the Tukey post-test. ****P < 0.0001. i.g., intragavage.
To confirm the existence of bacteria in the brain tissue, the genomes of the lung and whole brain were extracted and the V3-V4 detection region [fragment in 16S ribosomal DNA (rDNA) gene] was selected for polymerase chain reaction amplification. Standard bacterial genomic DNA mix was used as a positive control. The amplified products were subjected to agarose gel electrophoresis. The results showed that an obvious amount of bacterial genes were present in the brain genome of mice 1 day after LPS challenge. In contrast, negligible bacterial genes were detected in the brains of untreated mice (Fig. 2, F and G). Furthermore, we evaluated the microbial composition in the brain tissue and lung tissue of the mice by 16S rDNA amplicon sequencing. According to the database, the composition of the identified flora was analyzed at the level of phylum, class, order, family, genus, and species (Fig. 2, H and I, and fig. S2). It was found that the relative abundance between the brain and the lung flora species was similar at the level of phylum and family classification (Fig. 2, H and I). It is worth noting that there is no substantial change in the composition of lung microbiota before and after LPS challenge (fig. S3). Principal components analysis (PCA) further determined the similarity in sample composition between the brain and lung microbiota, suggesting that the emerging bacteria in the brain may originate from the lung (Fig. 2J). Furthermore, there is a significant difference in the composition of fecal microbiota and brain microbiota, suggesting that the microorganisms in the brain unlikely originated from the intestine (figs. S4 and S5). Notably, hematoxylin and eosin staining indicated that the influx of bacteria after LPS challenge had no obvious impact on the entire brain pathology (fig. S6A). In addition, we verified the existence of bacteria in the brain by Gram staining (fig. S6B). This series of experiments indicates that the brain can be invaded by bacteria that may originate from the lung after pneumonia.
The results above prompted us to explore whether alterations in the brain were caused by invading bacteria. We indiscriminately eliminated the bacteria by antibiotic cocktail treatment (fig. S7). Following this, the mice were administered the same dose of LPS to induce pneumonia (Fig. 2K). We confirmed that the brain tissue of mice receiving antibiotic therapy did not have any bacteria (Fig. 2L). After 30 days, inflammatory factors were determined in both lung and brain tissues. Notably, when the lung inflammation subsided 30 days after LPS challenge, the brain inflammation of germ-free mice also recovered to baseline (Fig. 2, M and N). Immunofluorescence images of the hippocampus of the brain revealed no obvious increase in Aβ protein content in microglia and astrocytes (Fig. 2, O to R), indicating that these cells were not dysfunctional. These results suggested that the alterations in the brain were associated with invaded bacteria.
Bacteria translocated from the lung to the brain during acute pneumonia is associated with increased lung and brain permeability
We next sought to determine the mechanism by which bacteria translocated from the lung to the brain. The above observations are indicative of the scenario in which the lung-blood barrier and the BBB are leaky during acute pneumonia, leading to an open access route for bacteria translocation. To validate this hypothesis, we analyzed the diffusion of high–molecular weight (4 kDa) fluorescein isothiocyanate (FITC)–conjugated dextran (FITC-DXT) in mice following pneumonia. FITC-DXT is a general tool to assess the integrity of the BBB (19). An increased permeability of the BBB is associated with the accumulation of FITC-DXT in the brain. In the LPS-induced pneumonia mouse model, we found that as early as 2 hours after injection, accumulation of dextran in the brain tissue was observed (Fig. 3A and fig. S8). This was further confirmed by confocal imaging and flow cytometry of brain cells (Fig. 3, B to D). These findings are consistent with a previous study that reported that the most prominent sign of severe COVID is BBB impairment (20). We further found that the extent of BBB permeability was related to the magnitude of the pneumonia response. Although LPS (0.1 mg/kg) challenge caused breathing difficulties in mice (fig. S9A), there was no obvious signal of FITC-DXT in the brain. However, at an LPS dose higher than 1 mg/kg, FITC-DXT was more obviously enriched in the brain (fig. S9, B to D), suggesting that BBB permeability correlated with the level of pneumonia, while mild pneumonia did not induce BBB impairment in our experiment. In addition, the dose of LPS also correlated with behavioral disorders (fig. S9, E to L). We analyzed the bacterial burden in the brain under different LPS doses, showing that the bacterial burden was related to the LPS dosage. Through 16S RNA sequencing, it was found that there was no substantial difference in the composition of brain microbiota among different LPS doses (fig. S10).
Fig. 3. Severe acute pneumonia leads to alteration of brain and lung permeability.
(A) We analyzed the diffusion of high–molecular weight (4 kDa) FITC-DXT in mice following pneumonia. Ex vivo imaging showed biodistribution of 4-kDa FITC-DXT. In the LPS-induced pneumonia mouse model, accumulation of dextran in the brain tissue was observed. (B) Confocal fluorescence imaging of brain tissue slices of mice after different treatments as indicated. Scale bar, 50 μm. (C) Representative flow cytometric analysis of 4-kDa FITC-DXT signal and (D) corresponding quantification results of mean fluorescence intensity (MFI) of FITC (n = 4). (E) Representative confocal images showing PV1 (red) detection in CD31+ (purple) blood vessels and ZO-1 (green) in the lung of mice after different treatments as indicated. Scale bars, 10 μm. (F and G) Corresponding quantitative analysis (n = 3). (H) Representative confocal images showing PV1 (red) detection in CD31+ (purple) blood vessels and ZO-1 (green) in the brain of mice after different treatments as indicated. Scale bars, 10 μm. (I and J) Corresponding quantitative analysis (n = 3). Data are shown as mean ± SD. Statistical significance was calculated by Student’s t test (two-tailed) and one-way ANOVA using Tukey’s post-test. *P < 0.05, **P < 0.01, and ***P < 0.001.
We next examined the vascular endothelial barrier and epithelial barrier in both the lung and brain 1 day after pneumonia. Plasmalemmal vesicle-associated protein 1 (PV1) is a plasma membrane vesicle-associated protein on endothelial cells and is an indicator of inflammation-induced permeability (21). On day 1 after LPS challenge, PV1 expression increased significantly in the lung and brain (Fig. 3, E to J), indicating that the vascular barrier was disrupted. These results were consistent with the accumulation of FITC-DXT in the brain, as endothelial cells control the passage of molecules from the blood to the matrix. We also observed that PV1 expression in the lung and brain returned to baseline levels on day 30 after LPS challenge (Fig. 3, E to J), suggesting that the vascular barrier was closed at that time. Next, we examined the tight junction protein zonula occludens-1 (ZO-1) (22). We observed a decrease in ZO-1 levels on day 1 and day 30 after LPS challenge, suggesting dysfunction of tight junctions of the epithelial barrier in both the lung and the brain for a long time (Fig. 3, E to J). The gut microbiota represents a significantly higher biomass than the lung microbiota. To further prove that the emerging bacteria in the brain were not from the intestinal microbiome, we examined intestinal permeability on day 1 after pneumonia. The results showed no obvious change in intestinal permeability (fig. S11). In addition, we used FITC-DXT to detect intestinal leakage and found that pneumonia did not result in intestinal leakage (fig. S12). These results corroborate our hypothesis that both the lung-blood barrier and the BBB are leaky during pneumonia, allowing the translocation of endogenous bacteria from the lung to the brain.
Altered brain homeostasis is induced by bacterial translocation
To further characterize the brain microenvironment of the mice suffering from neurological disorders, we performed single-cell RNA sequencing of whole brain cells from the mice recovered from pneumonia at day 30. Brain cells from individual mice were barcoded before single-cell RNA sequencing using the droplet-based system of 10x Genomics. Cells were clustered on the basis of gene expression using an unsupervised inference analysis using the Seurat v4 pipeline. The clusters were organized into seven “meta-clusters” including astrocytes, microglia, oligodendrocytes, T cells, monocytes, neurons, and granulocytes (Fig. 4, A and B, and fig. S13, A to C). In addition, there was obvious monocyte infiltration to brain tissue, suggesting neuroinflammation (fig. S14). The frequency of different types of cells was not changed significantly (Fig. 4C). However, enrichment analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of differential genes in all brain cells showed a signature related to Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, retrograde nerve signaling, and GABAergic synaptic pathway (Fig. 4D), indicating that the mice exhibited brain dysfunction and development of neurological disorders at day 30 after pneumonia. Notably, we also observed an association with Salmonella infection pathways in KEGG analysis. In addition, as shown in the volcano map, the expression of genes (Rhog, Il1a, Arf1, etc.) related to the bacterial infection pathway was significantly up-regulated (Fig. 4E). These data further confirmed our finding that bacteria existed in the brain.
Fig. 4. The altered brain homeostasis induced by bacterial translocation.
(A and B) Uniform manifold approximation and projection (UMAP) showing brain cell clustering and (C) the percentage of each cluster. The clusters were organized into seven meta-clusters including astrocytes, microglia, oligodendrocytes, T cells, monocytes, neurons, and granulocytes. (D) Enrichment analysis of KEGG pathways of differential genes in all brain cells. (E) Volcano plot of various genes differential expression of the brain. (F) Schematic diagram of bacterial infection to produce proinflammatory factors through the NF-κB pathway. (G) Representative flow cytometric analysis of CD14 and (H) corresponding quantification results of MFI of CD14. (I) Dotplot of differentially expressed genes related to the LPS signaling pathway in the brain. (J) Highlighted UMAP plot of representative genes of Jun, H2-DMa, Cd14, Il1a, Ctsd, Fcgr3, Fcer1g, and Fos. Data are shown as means ± SD (n = 4). Statistical significance was calculated by Student’s t test (two-tailed) and one-way ANOVA using Tukey’s post-test. **P < 0.01. TRAM, TRIF-related adaptor molecular; TIRAP, toll-interleukin 1 receptor domain-containing adaptor protein; PGN, peptidoglycan; LTA, lipoteichoicacid; TLR, Toll-like receptors; TCA, tricarboxylic acid; FC, fold change.
Bacteria-related pathogen-associated molecular patterns (PAMPs) can bind to pattern recognition receptors (PRRs) on various types of cells to produce proinflammatory factors through the nuclear factor–κB (NF-κB) pathway (Fig. 4F) (23). LPS is one such typical PAMP molecule derived from Gram-negative bacteria. We verified by flow cytometry that the expression of the LPS-related receptor CD14 in the brains of mice was significantly increased at day 30 (Fig. 4, G and H), indicating the existence of an inflammatory immune response to bacteria in the brain tissue. In addition, the genes (Myd88, Ticam2, Ly96, Tlr4, Nfkb1, etc.) related to the LPS signaling pathway were all up-regulated (Fig. 4, I and J) (24). To rule out the possibility of the effect of the injected LPS, 1 day after LPS challenge, the mice were given intravenous antibiotic treatment, which eliminated the bacterial flora in the brain (fig. S15). On the basis of our data, antibiotic-treated (ABx-treated) mice exhibited no brain inflammatory response and low CD14 expression at day 30, and their behavioral activities were normal, indicating that the LPS-related pathway was unlikely to be induced by injected LPS 30 days before.
The gene vitrification map showed that the increased expression of genes (Jun, H2-DMa, Cd14, Il1a, Ctsd, Fcgr3, Fcer1g, Fos, etc.) related to the bacterial infection pathway was mainly distributed in microglia and astrocytes, which are the two primary cell types that mediate neuroinflammation (Fig. 4J and fig. S15D). Microglial cells are the major group of macrophages in the parenchyma of the CNS and are very sensitive to brain bacteria. As expected, the expression of microglial genes (Rhog, Il1a, Arf1, etc.) related to bacterial infection pathways was significantly up-regulated (Fig. 5A), and the KEGG pathway showed that this cluster was involved in Salmonella and Staphylococcus infections (Fig. 5B). At the same time, microglial cells were activated as shown by a variety of markers, such as C1qa, C1qb, and C1qc (complement pathway genes); Ctss and Ctsb (lysosome pathway genes); Ccl3 and Ccl4 (chemokines); Rtp4 and Bst2 (interferon response genes); and Lamp1, Lamp2, H2-D1, P2ry12, Hexb, and Trem2 (microglia activation-related genes) (25, 26) (Fig. 5C and fig. S9A), and NLRP3 dominated the inflammasome pathway (fig. S16A). Similarly, in astrocytes, we found that the expression of genes (Rhog, Il1a, Arf1, etc.) associated with bacterial infection pathways was also remarkably up-regulated (Fig. 5D). The KEGG pathway analysis of astrocytes showed that this cluster is involved in Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, retrograde nerve signaling, GABAergic synapses, and lysosomal pathways (Fig. 5E), indicating that astrocyte reactivity is associated with neurological disorders. Moreover, the expression of reactivity marker genes of astrocytes—such as Id3, Npc2, and Prdx6 (27); Alzheimer’s disease risk-related genes Ctsb, Ctsd, Ctsl, S100a6, Itgab5, and Vsir (28); and interferon-stimulated genes Gbp3, Gbp2, Irgm1, Iigp1, Igtp, and Cxcl10—were all up-regulated in the astrocyte of the brain (Fig. 5F and fig. S16B) (29–31). All these data suggest that the bacteria are involved in alternations of brain homeostasis by switching both microglia and astrocytes from quiescence to activation through bacterial infection–related pathways. Meanwhile, the activation of microglia and astrocytes is linked to neurological diseases.
Fig. 5. Dysfunction of astrocytes and microglia.
Microglia and astrocytes are the two primary cell types that mediate neuroinflammation. (A) Volcano plot of gene differential expression of microglia. (B) KEGG enrichment scatter plot of microglia. (C) Violin plots of representative differential gene expression in the microglial cluster. (D) Volcano plot of gene differential expression of astrocytes. (E) KEGG enrichment scatter plot of astrocytes. The expression of genes associated with bacterial infection pathways was remarkably up-regulated. (F) Violin plots of representative differential gene expression in astrocytes cluster. (G) Dotplot of differentially expressed genes in microglia. (H) Dotplot of differentially expressed genes in astrocytes. (I) Western blot analysis of the expression of various types of proteins in the brain cells after various treatments as indicated and (J) the relative expression of proteins compared to the untreated group. (K) Schematic diagram of the mammalian target of rapamycin (mTOR) signaling pathway. Data are shown as means ± SD (n = 3). Statistical significance was calculated by Student’s t test (two-tailed) and one-way ANOVA using Tukey’s post-test. *P < 0.05 and **P < 0.01.
In particular, we found the up-regulation of phosphatidylinositol 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) signaling in both microglial (Fig. 5G) and astrocytic (Fig. 5H) clusters, which was further validated by Western blotting approach (Fig. 5, I and J). Numerous studies have shown that the PI3K-AKT-mTOR pathway is a key signaling pathway used by microglia and astrocytes to respond to extracellular stimuli including bacteria. This pathway is considered central for maintaining brain homeostasis while abnormal PI3K-AKT-mTOR signaling is linked to various neurological diseases, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. Given the central role of PI3K-AKT-mTOR signaling in neurological homeostasis (Fig. 5K and Fig. S17), it may be a therapeutic target in pneumonia-induced neurological disorders.
Rapamycin rescues brain homeostasis and neurological disorders
Rapamycin is used extensively for selective mTOR inhibition. Studies have shown that rapamycin reduces inflammation and inhibits the pathological processes of neurodegenerative disorders by inhibiting mTOR (32, 33). Therefore, we hypothesized that treatment with rapamycin may rescue the pneumonia-induced neurological disorders (Fig. 6, A and B). As the rapamycin we used here was relatively insoluble and unstable (Fig. 6A), its distribution in the brain was quite limited by intravenous injection in our preliminary data. In our previous studies, we demonstrated that PEVs could serve as a universal platform to selectively target various inflammatory cells and tissues (14–17) (Fig. 6C). To effectively deliver rapamycin to the brain, PEVs were used as carriers for direct nasal delivery of rapamycin, which is an effective and reliable way to bypass the BBB and deliver drugs into the CNS (34).
Fig. 6. Characterization and brain accumulation of rapamycin-PEVs.
(A) Chemical structure and high-performance liquid chromatography characteristic peak of rapamycin. mAU, milli absorbance unit. (B) Schematic diagram of the mTOR pathway. (C) Scheme of the preparation of PEVs. (D) Morphology of PEVs by TEM. (E) PEVs size distribution measured by DLS. (F) Western blot results of platelet lysate and PEVs. (G) Drug loading amount and efficacy of rapamycin to the PEVs. (H) In vitro release profile of drug-PEVs in the phosphate-buffered saline over 48 hours. (I) Schematic diagram of intranasal administration and in vivo fluorescence imaging of the mice after intranasal administration DiR-labeled PEVs. (J) Ex vivo fluorescence imaging of the brain and (K) corresponding quantitative analysis. (L) Confocal fluorescence imaging of brain tissue slices of mice after different treatments as indicated. Scale bar, 50 μm. (M) Representative flow cytometric analysis of DiR-PEVs and corresponding quantification results of MFI of DiR. Data are shown as means ± SD (n = 3). RAPA, rapamycin. Statistical significance was calculated by Student’s t test (two-tailed) and one-way ANOVA using Tukey’s post-test. ***P < 0.001 and ****P < 0.0001. Na+,K+-ATPase, Na+- and K+-dependent ATPase.
Transmission electron microscopy (TEM) and dynamic light scattering (DLS) analysis showed that PEVs had a round shape with a diameter of approximately 80 to 150 nm (Fig. 6, D and E). PEVs retained adhesion molecules from platelets, including CD41 and P-selectin (Fig. 6F and Fig. S18A), which can bind to activated microglia and astrocytes. According to our established protocol (14–17), rapamycin can be loaded onto PEVs by hydrophobic interactions (fig. S18B). The loading did not change the PEVs significantly (fig. S18, C and D). At a rapamycin concentration of 100 μg ml−1, the drug-loading rate (loading/adding rapamycin) was approximately 11.73% (Fig. 6G). Meanwhile, 80.01% of rapamycin was released from PEVs within 48 hours, showing a sustained release profile (Fig. 6H).
We next examined brain accumulation of the rapamycin-PEVs via intranasal administration (Fig. 6I). In our experiment, 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyaine iodide(DiR)-labeled rapamycin-PEVs were intransally administrated to the mice with pneumonia on day 1. Naïve mice were used as controls. The mice were imaged at the experimentally designed time points using an in vivo near-infrared fluorescence (NIRF) system. As expected, a remarkable accumulation of DiR-rapamycin-PEVs was observed in the brains of pneumonia mice, which was more significant than that in normal mice (Fig. 6I). This can be explained by a targeting effect of PEVs to activated microglia and astrocytes. In addition, the PEVs in the brain reached the maximum enrichment at 12 hours, and the retention time of PEVs was significantly longer than that of controls (Fig. 6I). Ex vivo NIRF imaging also revealed substantial accumulation of PEV in the brains of mice with pneumonia (Fig. 6, J and K). These findings are supported by confocal imaging (Fig. 6L) and flow analysis, which showed a higher number of DiR+ cells in the brain after intranasal administration (Fig. 6M). Together, these data suggested that rapamycin can be effectively delivered into brain tissue with the help of PEVs.
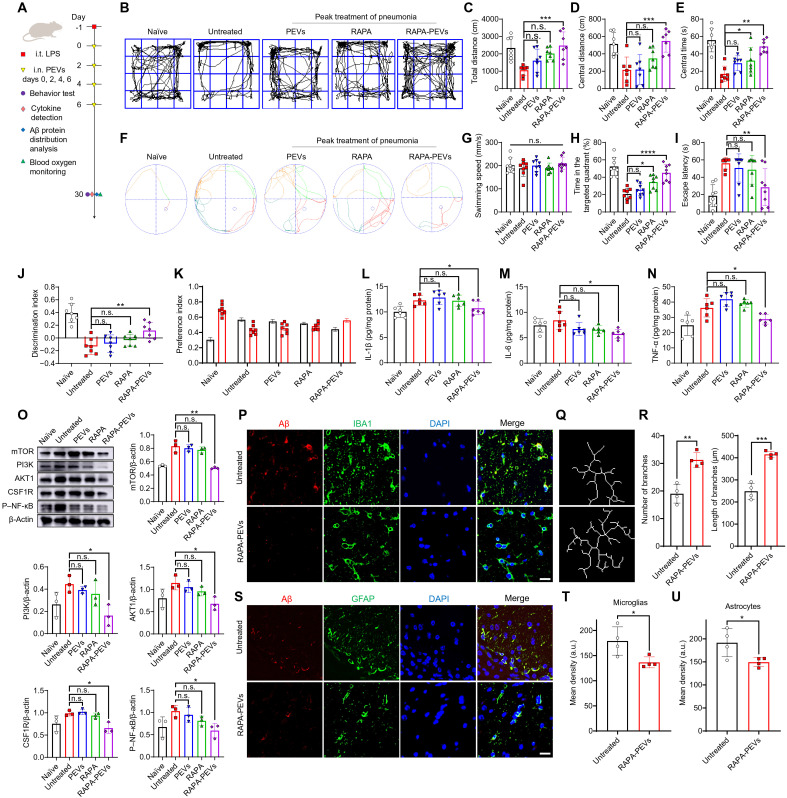
Next, we assessed the therapeutic efficacy of rapamycin-PEVs on mice suffering from pneumonia-induced neurological disorders (Fig. 7A). One day after LPS challenge, mice received intranasal administration of rapamycin-PEVs once every 2 days for a total of four times. Naïve mice, untreated mice, and mice treated with PEVs or rapamycin alone were used as controls. Behavioral tests were performed on day 30. The results of the open-field experiment showed that rapamycin-PEVs effectively alleviated the reduced motor activity induced by pneumonia-induced neurological disorder in untreated mice (Fig. 7, B to E). For the water maze, intranasal administration of rapamycin-PEVs significantly rescued the probability of mice entering the target quadrant and reduced the time required to reach the target platform compared to the untreated mice, while there was no difference in swimming speed (Fig. 7, F to I). In addition, mice receiving rapamycin-PEVs significantly mitigated the reduction in the new object preference index after pneumonia (Fig. 7, J and K). Free rapamycin or PEVs alone had inferior or limited therapeutic effects (Fig. 7, B to K). These results suggest that intranasal delivery of rapamycin-PEVs can significantly rescue pneumonia-induced behavioral disorders.
Fig. 7. Therapeutic effect of rapamycin-PEVs.
(A) Schematic of the experimental timeline. One day after LPS challenge, mice received intranasal administration of rapamycin-PEVs once every 2 days for a total of four times. Naïve mice, untreated mice, and mice treated with PEV or rapamycin alone were used as controls (n = 7). Behavioral testing and sample collection were conducted on day 30. (B) Representative paths of mice in the open-field test and quantitative analysis of (C) the total distance, (D) the central distance, and (E) the central time. (F) Representative plot of Morris water maze images and quantification of (G) swimming speed, (H) the ratio of the time in the target quadrant to the total time, and (I) escape latency. (J) Discrimination index in the novel object recognition. (K) Preference index in the novel object recognition. (L to N) Inflammatory factors including IL-1β (L), IL-6 (M), and TNF-α (N) of the brain tissue homogenate (n = 6). (O) Western blot analysis of the expression of various types of proteins in the brain cells after various treatments as indicated (n = 3). (P) Confocal microscopy images of Aβ protein in microglia. Red, Aβ; green, IBA1; blue, DAPI. Scale bar, 20 μm. (Q) Plot of microglial branches and (R) corresponding quantitative analysis (n = 4). (S) Confocal microscopy images of Aβ protein in astrocytes. Red, Aβ; green, GFAP; blue, DAPI. Scale bar, 20 μm. (T and U) Mean fluorescence quantification of Aβ protein in microglia (T) and astrocytes (U) (n = 4). Data are shown as means ± SD. Statistical significance was calculated by Student’s t test (two-tailed) and one-way ANOVA using Tukey’s post-test. *P < 0.05, **P < 0.01, and ***P < 0.001.
In addition, rapamycin-PEVs administration also recovered the levels of the proinflammatory cytokines IL-1β, IL-6, and TNF in the brain homogenate at day 30 (Fig. 7, L to N). After rapamycin-PEVs treatment, the number of bacteria in the brain also decreased (fig. S19), partly owing to the recovered BBB leakage after reduced brain neuroinflammation. Moreover, the expression of mTOR, the target protein of rapamycin, was decreased, and the expression of PI3K, AKT1, colony-stimulating factor 1receptor (CSF1R), and phosphorylated NF-κB (P–NF-κB) were down-regulated (Fig. 7O), indicating that rapamycin-PEVs treatment could effectively relieve the microglial and astrocytic dysfunction in the brain following pneumonia. In addition, the expression of Aβ protein in the hippocampus decreased after rapamycin-PEVs treatment and the increase of microglial branch length and number indicated that microglia restored homeostasis (Fig. 7, P and U). Rapamycin-PEVs treatment also showed good safety in treated mice (fig. S20). These data suggest that intranasal delivery of rapamycin-PEVs showed impressive therapeutic efficiency in rescuing the brain homeostatic processes disrupted by bacterial translocation.
DISCUSSION
Brain-related abnormalities are frequently reported in patients who have recovered from pneumonia. For example, the inability to concentrate is the major problem of people recovering from COVID-19 in the United States and Europe (35). Neurological disorders also exist in recovered patients with other respiratory diseases, such as influenza (36) and bacterial pneumonia (5). In this paper, we accidentally found that neurological disorders after severe pneumonia are associated with the translocation of endogenous bacteria from the lung to the brain. The lung is frequently exposed to air and contains a pulmonary microbial community, while its function is still controversial. Changes in the pulmonary microbial community seem to play a role in the progression of pulmonary disorders (11). In contrast, the brain should be in a sterile environment under normal conditions, as the BBB is able to prevent bacteria from entering the brain tissue. However, in acute pneumonia condition, we detected bacteria in the brain tissue. We observed bacterial growth in the LB plates containing mouse brain tissue–homogenizing fluid following days of pneumonia. The peak of the bacterial amount in the brain was approximately 7 days after pneumonia. The existence of bacteria in the brain tissue was further confirmed by 16S rDNA gene detection and analysis. To rule out the possibility of exogenous bacterial contamination during surgery or operation, single-cell RNA sequences of brain cells indicated that genes related to the bacterial infection pathway were significantly up-regulated even 30 days later. From the 16S rDNA gene analysis and PCA, we found a similarity in the diversity and abundance of microbiota in the brain and lung, which is indicative of the scenario that the emerging bacteria in the brain originate from the lung.
In previous studies, bacteria or their products served as direct mediators of the brain-lung axis. Cryptococcus is a fungal pathogen that causes disease in humans and enters the body primarily through inhalation. In some cases, it progresses to pneumonia followed by the spread of the infection to the CNS, resulting in meningoencephalitis (37). Other evidence suggests that the lung microbiome contributes to brain-related diseases. Chronic obstructive pulmonary disease alters the respiratory microbiota to increase the risk of Parkinson’s disease and Alzheimer’s disease (38). In addition, studies have confirmed that similar microbiomes were detected in both bronchoalveolar lavage fluid and blood in LPS-induced pneumonia, suggesting the migration of bacteria from the lung to the blood circulation (39, 40). In our study, we further validated that both the lung-blood barrier and the BBB are leaky during pneumonia, allowing the translocation of bacteria from the lung to the brain via the bloodstream.
Furthermore, the level of BBB leakage is highly correlated with the severity of pneumonia; the more severe the pneumonia, the more leaking the BBB. This phenomenon may explain the data of many previous studies. For example, a study of the medical records of U.S. veterans analyzed various health burdens in the 6 months after COVID-19 infection, which indicated that neurological abnormalities were significantly higher in severe (requiring hospitalization) patients than in mild (not requiring hospitalization) patients (41). Another study analyzed long COVID symptoms in 2020 and 2021, which once again showed that the proportion of sequelae was related to the severity of pneumonia at the time of infection (35).
The existence of bacteria in the brain tissue results in the disruption of brain homeostasis, especially for astrocytes and microglia, which are the two primary cell types that mediate neuroinflammation (42). According to an automated single-cell RNA sequencing study, dysregulated astrocytic and microglial signatures were displayed in COVID-19 brains (43). Activated microglia and astrocytes are sensitive indications in response to bacterial infection by producing cytokines, chemokines, and reactive oxygen species that are beneficial in killing bacteria (44, 45). However, the by-product of this local inflammation results in short-term damage to the CNS and neurological diseases (46, 47). The invasion of bacteria promotes inflammation in the brain, and there is a positive correlation between brain inflammation and an increase in Aβ protein. Although the bacteria in the brain can be eliminated completely by the activated immune system, the switching of microglia and astrocytes from activation to quiescence takes more time. A study has shown that patients with post–COVID-19 brain fog recover completely over the course of 6 to 9 months (48), indicating that these neurological disorders can be recovered after a period of time, which is consistent with our findings.
To prevent potential damage to the normal CNS and speed up the process of microglia and astrocytes from activation to quiescence, we used rapamycin as a therapeutic agent for treatment. We found the up-regulation of PI3K-AKT-mTOR signaling in both microglia and astrocyte clusters from single-cell RNA sequencing data, while PI3K-AKT-mTOR is a key signaling pathway for maintaining brain homeostasis. These data suggest that mTOR may be a therapeutic target in pneumonia-induced brain dysfunction. Rapamycin as a selective mTOR inhibitor has been demonstrated to treat pathological processes of neurodegenerative changes previously (32, 33). In addition, we used PEVs as a drug delivery platform for effectively delivering rapamycin into brain tissue, thus reducing the dosage and potential sideeffects of rapamycin to the whole body. Behavioral impairments in mice were alleviated by intranasal administration of rapamycin-PEVs, thereby providing an effective low-cost approach to reduce potential damage in the brain during pneumonia. After rapamycin-PEVs treatment, the number of bacteria in the brain also decreased (fig. S19), partly owing to the recovered BBB leakage after reduced brain neuroinflammation that was able to prevent bacteria infiltration. Other clinically approved anti-inflammatory agents can be examined in the future.
This study has a few limitations that warrant discussion. First, we only used LPS to establish a pneumonia model; however, whether virus-induced pneumonia has a similar effect needs further examination. Second, although some viruses, such as SARS-CoV-2, have rarely been detected in the cerebrospinal fluid of patients with neurological symptoms (49, 50), we cannot rule out the possibility that the other exogenous pathogens translocate into the brain (51). In addition, microbial nutrients or their derivatives, such as short-chain fatty acids or bacterial outer membrane vesicles, can also influence the activation of astrocytes and microglia, which should be investigated in the feature.
In conclusion, our study found that neurological disorders after severe pneumonia were associated with the translocation of endogenous bacteria from the lung to the brain. We observed the existence of bacteria in the brain tissue, which might result from the leakage of both the lung-blood barrier and the BBB during pneumonia. Using single-cell RNA sequencing technology, we identified PI3K-AKT-mTOR signaling pathway disruption in both microglia and astrocyte clusters. Administration of rapamycin-PEVs could speed up the recovery of brain homeostasis, and alleviate behavioral impairments in mice suffering from severe pneumonia-induced brain symptoms. Our work provides insights into the mechanism of neurological syndromes after severe pneumonia, which is partly attributed to the translocation of endogenous bacteria from the lung to the brain.
MATERIALS AND METHODS
Materials
Rapamycin and antibodies applied in this study are shown in Table S1.
Animals
Female Kunming (KM) mice aged from 4 weeks were purchased from Nanjing Peng Sheng Biological Technology Co. Ltd. There was an at least 7-day gap between the time of purchasing mice and our experiment on them to ensure that they were accustomed to the conditions of the laboratory. The mice were housed in a vivarium maintained at 20° ± 2°C, 55% humidity, with a 12-hour light/12-hour dark cycle, and free access to food and water. The housing group was five at maximum for mice in each group. All animal tests were conducted with the approval of Soochow University Laboratory Animal Center and the Institutional Review Committee, in accordance with relevant ethical and moral standards (no. SUDA20200512A01).
We used the Animal Research: Reporting in Vivo Experiments (ARRIVE) reporting guidelines. The aim of this study was to investigate the relationship between neurological disorders following severe pneumonia and the pulmonary microbial community. Animal experiments shall be approved and supervised by the Animal Welfare Review Committee after the purpose, method, and ethics of the experiments are clarified. Mice were randomly divided into groups. No animals were excluded from the study, and the researchers conducted the experiments independently and evaluated the results. The mice were euthanized at the end of the experiment or when they had health problems. All experiments were repeated at least three times.
Animal model induction and treatment
For LPS treatment, healthy mice were anesthetized with isoflurane. We placed each mouse in an air-numbed chamber and adjusted the oxygen flowmeter to between 0.6 and 1.2 liter/min. Once fully anesthetized, the mice were fixed in the supine position. The mouth of the mice was opened, the tongue was picked out with forceps and placed in the lateral position, and the exposed tracheal hole was observed under the spotlight. A total of 50 μl of LPS (4 mg/kg; Biosharp) was injected into the trachea through a syringe. LPS-challenged mice and untreated healthy mice as controls were weighed at the same time and monitored every 2 days until 30 days after modeling. In addition, blood oxygen saturation was measured by a MouseOx (STARR) oxygen detector at days −1, 0, 7, 14, and 30 of LPS challenge. The mice were included in the study if the blood oxygen saturation dropped remarkably, and these mice were randomly divided into four groups, treated with intranasal/pulmonary delivery of rapamycin (1 mg/kg; Energy Chemical) or rapamycin-PEVs (rapamycin equal to 1 mg/kg), respectively. Some healthy mice of the same age without any treatment were used as controls.
Antibiotic cocktail therapy
As previously described, 12 mice were gavaged with sterilized drinking water supplemented with the following broad-spectrum antibiotic cocktails: ampicillin (1 mg/ml; Aladdin), gentamicin (1 mg/ml; Aladdin), metronidazole (1 mg/ml; Energy Chemical), and vancomycin (0.5 mg/ml; Aladdin) continuously administered for 10 days to prepare germ-free mice. In addition, six sterile mice were selected for acute pneumonia modeling, and appropriate experiments were conducted 1 and 30 days after modeling. The remaining six sterile mice were used as controls. In addition, eight acute pneumonia mouse models were established separately. One day after the modeling, four acute pneumonia mice were randomly selected to receive tail vein antibiotic treatment, and then appropriate experiments were conducted 1 and 30 days after treatment. The remaining four acute pneumonia mice were used as controls.
Cytokine detection
After the tissue samples were rinsed with pre-cooled phosphate-buffered saline (PBS), samples with the same weight were weighed and grinded in a tissue grinder to make a 10% tissue homogenate. The prepared homogenate was centrifuged at 6000g for 10 min. The supernatant of each homogenate sample was collected, and the total protein content of each tissue homogenate sample was determined by the bicinchoninic acid (BCA) assay method. After that, the reaction was performed on the precoated enzyme-labeled plate, followed by washing, enzyme-labeled antibody coupling, substrate reaction, and termination of the reaction operation. Last, the multifunctional enzyme-labeled instrument was used for detection at the wavelengths of 450 and 570 nm.
Behavioral tests
Each group of seven mice underwent the open-field test, new object recognition test, and Morris water maze test in turn. Before the experiment, the mice were acclimated in the test room. The experiment will be conducted on consecutive days, and the same batch of mice will be used for testing. The tests were run in the same order as on day 1. In addition, behavioral tests themselves affect the brain, so we performed brain tissue analysis on mice that did not undergo behavioral tests.
Open-field test
The experimental device was a three-dimensional blank rectangular open-field box (60 cm by 60 cm by 60 cm). The mice were quickly placed in the central area of the experimental box, and the tracking software was opened to automatically record the behavior of the mice in the box for 10 min. The open-field box was wiped between each group of tests to remove the residual odor of mice. Tracking software was used to collect the following data: central movement time, central movement distance, total movement distance, and movement speed.
Novel object recognition test
The day before training, the mice were exposed to an open-field box for 10 min without an object to form a habit. During the training phase, in the presence of two identical objects, the mice were placed in an open-field box and allowed to explore the object for 5 min. Twenty-four hours later, one of the objects was replaced with a new one, and the mice were again placed in the open field and allowed to explore for 5 min. The discrimination index and preference index were used to assess novel object recognition, and this index illustrates the differences in exploration time. Tracking software was used to collect behavioral data of mice, and statistical time was used to evaluate the mice’s preferred behavior. The discrimination index was calculated as the time spent exploring the novel object minus the time spent exploring the familiar object, divided by the total exploration time. The preference index was calculated as the proportion of total time spent exploring new or old objects. [Discrimination index = Tnovel − Tfamiliar/(Tnovel + Tfamiliar), Preference index = Tnovel or Tfamiliar/Tnovel + Tfamiliar)]. All discrimination index values fall between −1 and + 1, and preference index values fall between 0 and 1.
Morris water maze test
The water maze was located in a separate laboratory to ensure that the experiment was quiet and free from influence. The pool was divided into four quadrants, and different symbols (circle, pentagram, triangle, and square) were affixed to the walls of each quadrant to provide additional spatial clues to the maze. The water temperature was maintained at 23° ± 1°C, and all water maze experiments were conducted daily at the same time points. Before the experiment, the mice were conditioned in a water maze room for 2 hours. Each mouse was trained to find a hidden target platform for 5 days with two tests per day, with a 4-hour intertrial interval. The mice were placed gently in either of the pool quadrants and protected from human influence and facing away from the pool wall. During training, if the platform was found by the mouse for more than 60 s, the mice were guided onto a platform and stayed there for 10 seconds. Twenty-four hours after the training, the platform was removed and the 60-s exploratory test began. The mice were placed in the water facing the quadrant opposite the target quadrant. The time spent in the target quadrant and the time taken to reach the platform location were recorded as indicators of spatial memory.
Immunofluorescence analysis
The tissue was fixed with 4% paraformaldehyde and then buried overnight using an optimal cutting service temperature (OCT) compound. The embedded organ was then cut into 4-μm slices using a cryo-slicer. After being sealed with 10% fetal bovine serum for 20 min, the slices were incubated with rabbit anti-Aβ, anti-ionized calcium-binding adapter molecule 1 (IBA1), anti–glial fibrillary acidic protein, anti-CD31, anti-PV1, and anti–ZO-1 (1:500; applicable to each of these antibodies, Serviceio) at 4°C overnight. After being placed at room temperature for 30 min on the second day, the slices were incubated with a fluorescent goat anti-rabbit secondary antibody (1:500; Serviceio) for 2 hours and incubated with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml; Beyotime) for 10 min. Images were obtained by confocal microscopy, and the image data were processed by ImageJ software package. In addition, the behavior test itself may affect the brain, so we performed immunofluorescence analysis of the brain tissue in mice that did not undergo the behavior test.
Tissue sample plate coating
Mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium, and sterile PBS was infused from the left ventricle to remove circulating blood cells in the tissue. Acquired organs (whole brain, lung, and colon) were weighed on a sterile super clean table and ground into a 5% tissue homogenate liquid. The sample was diluted with sterilized water. A certain amount of sample liquid was absorbed with a sterile pipette and coated evenly on a plate containing LB medium (Beyotime) prepared in advance after high-temperature sterilization. All reagents and consumables were sterilized before use, and the entire operation was carried out on an ultraclean platform to ensure no bacterial contamination. LB medium was inverted in an incubator for 24 hours, and quantitative CFUs were analyzed using a colony counter.
16S rDNA amplicon sequencing
16S rDNA amplicon sequencing was performed by Genesky Biotechnologies Inc., Shanghai, 201315 (China). In short, whole brain and lung total genomic DNA were extracted using the FastDNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA) according to the manufacturer’s instructions. The integrity and quality of genomic DNA were determined by agarose gel electrophoresis, and the concentration and purity of genomic DNA were determined by NanoDrop 2000 and Qubit3.0 Spectrophotometer. The V3-V4 hypervariable regions of the 16S rDNA gene were amplified with the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) and then sequenced using an Illumina NovaSeq 6000 sequencer.
Brain permeability assay to 4-kDa dextran molecule
According to the experimental design, three mice were in each group, including untreated mice, mice treated with LPS (0.1, 1, and 4 mg/kg) after 1 day; and mice treated with LPS (4 mg/kg) after 30 days, respectively. A total of 500 μg of 4-kDa FITC-DXT (MedChemExpress, MCE) was injected into the tail vein of mice. Twelve hours after the injection, the major organs of mice were collected for NIRF imaging. Then, the brain tissues were sectioned by OCT and observed under a confocal microscope with DAPI staining. Fluorescence histograms of brain cells were recorded using BD FACSCalibur flow cytometer, and FITC fluorescence was detected using Flowjo_V10 software based on 10,000 gated events.
Single-cell RNA sequencing of brain
Mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium, and sterile PBS was infused from the left ventricle to remove circulating blood cells in the tissue. Single-cell RNA sequence were obtained from the whole brain of untreated or 30 days post–LPS-treated mice. The tissue was digested with enzymes and processed into a single-cell suspension. The oligo(dT)-based complementary DNA database is a droplet-partioning barcode using the Chromium Single Cell Controller (10-fold genomics) system in the National Cancer Institute– Center for Cancer Research (NCI-CCR) single-cell analysis tool. We carried out the removal of dead cells and adjusted the cells to the required concentration, and approximately 8000 cells were detected on the machine. Sequencing was performed on a NovaSeq (Illumina) at the NCI-CCR sequencing facility. Bioinformatic analysis was performed using the OmicStudio tools at www.omicstudio.cn/tool.
Western blotting
After the brain tissue of each experimental group was obtained, a mixed solution of radio immunoprecipitation assay lysate and phenylmethylsulfonyl fluoride (100:1) was added. After lysis in an ice bath, the protein supernatant was obtained by centrifugation at 12,000 rpm, and the protein was quantified by a BCA protein quantification kit. The denatured protein was mixed with 5× load buffer and isolated by 12.5% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gel (Epizyme) electrophoresis at 60 V for 30 min and 120 V for 90 min. After that, the protein was transferred to polyvinylidene difluoride membrane by an ice bath for 100 min and then blocked in 5% skim milk powder solution for 1 hour. After that, the protein was incubated with β-actin (1:2000; Serviceio), anti-mTOR (1:1500; Serviceio), anti-PI3K (1:1500; Abclonal), AKT1 (1:1500; Serviceio), CSF1R (1:1500; Abclonal), and P–NF-κB antibodies (1:1500; Abclonal) at 4°C overnight. After that, goat anti-rabbit secondary antibody (1:5000; Absin) coupled with horseradish peroxidase was incubated at room temperature for 1 hour, bands were displayed by chemiluminescence development, and data quantitative analysis was performed by ImageJ software package (fig. S12).
PEVs fabrication
Extracellular vesicles of platelet origin were collected through natural platelet activation secretion and superfast centrifugation concentration. In short, fresh blood was collected from the fundus venous plexus of healthy KM mice and then centrifuged at 100g for 15 min to extract supernatant platelet-rich plasma (PRP). The pelleted PRP was resuspended in PBS containing prostaglandin E1 (2 μM; Absin) and EDTA (5 mM; Sigma-Aldrich) to prevent platelet activation, and platelets were obtained after the centrifugation of PRP at 800g for 20 min. To extract PEVs, platelet concentrate was activated by thrombin (2 U ml−1, Solarbio) in a low-speed shaker at room temperature for 30 min and was centrifuged at 800g for 20 min to obtain a supernatant-enriched PEV solution. Then, the supernatant was centrifuged at 100,000g for 70 min. The vesicle-containing particles were washed in PBS and centrifuged at 100,000g for 70 min. The particles containing purified vesicles were resuspended in 200 μl of sterilized PBS. Each centrifugation was performed at 4°C. The particle size distribution and zeta potential of PEVs in aqueous solution were then measured using DLS. The morphology of PEVs was observed by TEM. The protein expression of platelet lysates and PEVs was determined by SDS-PAGE and Western blotting.
Preparation and characterization of rapamycin-loaded PEVs
The insoluble rapamycin was first dissolved in a small amount of dimethyl sulfoxide and incubated with PEVs. The rapamycin-loaded PEVs were separated again by high-speed centrifugation. The size distribution and zeta potential of rapamycin-loaded PEVs were measured by DLS. Furthermore, the properties of drug loading and drug release were determined by high-performance liquid chromatography. Perform the following liquid phase conditions: chromatographic column C18, mobile phase V (acetonitrile):V (water) = 51:49. The flow rate was 1.0 ml/min, UV detection wavelength was 278 nm, the injection volume was 20 μl, and the column temperature was 27°C. The mass fraction of rapamycin-PEVs was 11.73% when the drug concentration was 100 μg ml−1.
Intranasal delivery of PEVs
We intranasally delivered rapamycin-PEVs to mice after shallow anesthesia using isoflurane gas containing oxygen, ensuring that the mice recovered within minutes, with seven mice in each group. We placed each mouse in an air-numbed chamber and adjusted the oxygen flowmeter to 0.6 to 1.2 liter/min. Once fully anesthetized, the animals lay on their backs at a 60° angle and a steady rate of respiration was monitored. We then slowly injected 20 μl of PEVs into the nostrils at a rate of 5 μl per drop. We stopped the drug for 3 to 4 min after each administration to ensure that the mice inhaled the drops and breathed at a steady rate, carefully watching the nostrils for signs of blockage or irritation. After the full dose was given, each mouse recovered from anesthesia before being transferred to its cage.
Biodistribution of PEVs
Mice were first anesthetized with isoflurane mixed with oxygen, followed by intranasal administration of DiR-labeled PEVs or free DiR, with four mice in each group. The IVIS Spectral Imaging System (PerkinElmer Ltd.) was used to monitor NIRF imaging for different groups at different time points over a 24-hour period. Then, major organs were collected for in vitro NIRF imaging. Fluorescence intensity was quantified as average radiance (photons s−1 cm−2 sr−1) with IVIS Living Image 4.2. In addition, brain single-cell suspensions were prepared for flow analysis of PEVs uptake. The expression level of DiR in the brain tissue was observed by confocal imaging.
Histological analysis
After treatment, major organs of mice in each group were obtained, cleaned in PBS to remove excess blood, fixed in 4% paraformaldehyde solution, and then embedded in paraffin. The paraffin sample was cut into 4-μm-thick slices for hematoxylin and eosin staining. Then, the pathological status of the samples was observed and analyzed by optical microscopy. In addition, behavioral tests themselves affect the brain, so we performed brain tissue analysis on mice that did not undergo behavioral tests.
Flow cytometric immunoassay of mouse tissue
According to the experimental groups, the brain tissue of mice was obtained, and the tissue cell suspension was obtained using a tissue grinder. Then, the single-cell suspension was removed through the filter screen, washed and centrifuged with PBS, and resuspended in fluorescence-activated cell sorting buffer solution (PBS containing 3% bovine serum albumin). Furthermore, the cells were stained with anti–CD45-phycoerythrin, anti–CD14-allophycocyanin (BioLegend). The stained cells were analyzed using BD Accuri C6 flow cytometer and analyzed using Flowjo_V10 software based on 100,000 gated events.
Statistical analysis
All data in the present study are means ± SDs. The significance of differences between two groups was calculated by a two-tailed unpaired Student’s t test. In addition, analysis of variance (ANOVA) comparisons and Tukey’s post hoc tests were performed between more than two groups (multiple comparisons). All statistical analyses were performed using GraphPrism (v5.0). Values of P = 0.05 or less were considered significant. All intensities of fluorescence expression in the experiments were further calculated by ImageJ software. The standard symbols were presented as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Acknowledgments
We thank the use of the instrumentation facility at FUNSOM and Soochow University.
Funding: This work was supported by the National Key Research and Development Program of China (2022YFB3808100), the National Natural Science Foundation of China (no. 32022043, T2321005, 32371476), and the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China (Grant No. 22KJA180003). This work is partly supported by Collaborative Innovation Center of Suzhou Nano Science & Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the 111 Project.
Author contributions: C.W. designed the project. Q.M., C.Y., Y.W., and H.W. performed the experiments and collected the data. All authors analyzed and interpreted the data, contributed to the writing of the manuscript, discussed the results and implications, and edited the manuscript at all stages.
Competing interests: The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S21
Table S1
REFERENCES AND NOTES
- 1.I. Ahmad, F. A. Rathore, Neurological manifestations and complications of COVID-19: A literature review. J. Clin. Neurosci. 77, 8–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.C. H. Sudre, B. Murray, T. Varsavsky, M. S. Graham, R. S. Penfold, R. C. Bowyer, J. C. Pujol, K. Klaser, M. Antonelli, L. S. Canas, E. Molteni, M. Modat, M. J. Cardoso, A. May, S. Ganesh, R. Davies, L. H. Nguyen, D. A. Drew, C. M. Astley, A. D. Joshi, J. Merino, N. Tsereteli, T. Fall, M. F. Gomez, E. L. Duncan, C. Menni, F. M. K. Williams, P. W. Franks, A. T. Chan, J. Wolf, S. Ourselin, T. Spector, C. J. Steves, Attributes and predictors of long COVID. Nat. Med. 27, 626–631 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.G. Douaud, S. Lee, F. Alfaro-Almagro, C. Arthofer, C. Wang, P. McCarthy, F. Lange, J. L. R. Andersson, L. Griffanti, E. Duff, S. Jbabdi, B. Taschler, P. Keating, A. M. Winkler, R. Collins, P. M. Matthews, N. Allen, K. L. Miller, T. E. Nichols, S. M. Smith, SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604, 697–707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.P. Zarifkar, C. Peinkhofer, M. Benros, D. Kondziella, The risk of neurological diseases after COVID-19, influenza A/B or communityacquired pneumonia infection. Eur. J. Neurol. 29, 71 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.K. M. Blackburn, C. Wang, Post-infectious neurological disorders. Ther. Adv. Neurol. Disord. 13, 1756286420952901 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.P. Zarifkar, C. Peinkhofer, M. E. Benros, D. Kondziella, Frequency of neurological diseases after COVID-19, influenza A/B and bacterial pneumonia. Front. Neurol. 13, 904796 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.J. A. Frontera, S. Sabadia, R. Lalchan, T. Fang, B. Flusty, P. Millar-Vernetti, T. Snyder, S. Berger, D. Yang, A. Granger, N. Morgan, P. Patel, J. Gutman, K. Melmed, S. Agarwal, M. Bokhari, A. Andino, E. Valdes, M. Omari, A. Kvernland, K. Lillemoe, S. H.-Y. Chou, M. M. Nett, R. Helbok, S. Mainali, E. L. Fink, C. Robertson, M. Schober, J. I. Suarez, W. Ziai, D. Menon, D. Friedman, D. Friedman, M. Holmes, J. Huang, S. Thawani, J. Howard, N. Abou-Fayssal, P. Krieger, A. Lewis, A. S. Lord, T. Zhou, D. E. Kahn, B. M. Czeisler, J. Torres, S. Yaghi, K. Ishida, E. Scher, A. de Havenon, D. Placantonakis, M. Liu, T. Wisniewski, A. B. Troxel, L. Balcer, S. Galetta, A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology 96, e575–e586 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J. J. Matschke, M. Lütgehetmann, C. Hagel, J. P. Sperhake, A. S. Schröder, C. Edler, H. Mushumba, A. Fitzek, L. Allweiss, P. M. Dandri, M. Dottermusch, A. Heinemann, S. Pfefferle, M. Schwabenland, D. S. Magruder, P. S. Bonn, P. M. Prinz, P. C. Gerloff, P. K. Püschel, S. Krasemann, P. M. Aepfelbacher, P. M. Glatzel, Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 19, 919–929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O. Bajinka, L. Simbilyabo, Y. Tan, J. Jabang, S. A. Saleem, Lung-brain axis. Crit. Rev. Microbiol. 48, 257–269 (2022). [DOI] [PubMed] [Google Scholar]
- 10.C. Li, W. Chen, F. Lin, W. Li, P. Wang, G. Liao, L. Zhang, Functional two-way crosstalk between brain and lung: The brain–lung axis. Cell. Mol. Neurobiol. 43, 991–1003 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J. G. Natalini, S. Singh, L. N. Segal, The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 21, 222–235 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.C. D. Link, Is there a brain microbiome? Neurosci. Insights 16, 263310552110187 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.N. J. Abbott, A. A. Patabendige, D. E. Dolman, S. R. Yusof, D. J. Begley, Structure and function of the blood–brain barrier. Neurobiol. Dis. 37, 13–25 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Q. L. Ma, Q. Fan, X. Han, Z. L. Dong, J. L. Xu, J. Y. Bai, W. W. Tao, D. D. Sun, C. Wang, Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J. Control. Release 329, 445–453 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Q. Ma, C. Yao, H. Shi, J. Xu, H. Dai, Z. Fei, Y. Wu, T. Lu, C. Wang, Targeted delivery of dexamethasone in acute pneumonia. Biomater. Sci. 9, 5569–5576 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Q. Ma, Q. Fan, J. Xu, J. Bai, X. Han, Z. Dong, X. Zhou, Z. Liu, Z. Gu, C. Wang, Calming cytokine storm in pneumonia by targeted delivery of TPCA-1 using platelet-derived extracellular vesicles. Matter 3, 287–301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Q. Ma, J. Bai, J. Xu, H. Dai, Q. Fan, Z. Fei, J. Chu, C. Yao, H. Shi, X. Zhou, L. Bo, C. Wang, Reshaping the inflammatory environment in rheumatoid arthritis joints by targeting delivery of berberine with platelet-derived extracellular vesicles. Adv. Nanobiomed. Res. 1, 2100071 (2021). [Google Scholar]
- 18.L. Hosang, R. C. Canals, F. J. van der Flier, J. Hollensteiner, R. Daniel, A. Flügel, F. Odoardi, The lung microbiome regulates brain autoimmunity. Nature 603, 138–144 (2022). [DOI] [PubMed] [Google Scholar]
- 19.R. Natarajan, N. Northrop, B. Yamamoto, Fluorescein isothiocyanate (FITC)-dextran extravasation as a measure of blood-brain barrier permeability. Curr. Protoc. Neurosci. 79, 9.58. 1–9.58. 15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.M. M. Etter, T. A. Martins, L. Kulsvehagen, E. Pössnecker, W. Duchemin, S. Hogan, G. Sanabria-Diaz, J. Müller, A. Chiappini, J. Rychen, N. Eberhard, R. Guzman, L. Mariani, L. Melie-Garcia, E. Keller, I. Jelcic, H. Pargger, M. Siegemund, J. Kuhle, J. Oechtering, C. Eich, A. Tzankov, M. S. Matter, S. Uzun, Ö. Yaldizli, J. M. Lieb, M.-N. Psychogios, K. Leuzinger, H. H. Hirsch, C. Granziera, A.-K. Pröbstel, G. Hutter, Severe Neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: A prospective cross-sectional study. Nat. Commun. 13, 6777–6721 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A. Bertocchi, S. Carloni, P. S. Ravenda, G. Bertalot, I. Spadoni, A. Lo Cascio, S. Gandini, M. Lizier, D. Braga, F. Asnicar, N. Segata, C. Klaver, P. Brescia, E. Rossi, A. Anselmo, S. Guglietta, A. Maroli, P. Spaggiari, N. Tarazona, A. Cervantes, S. Marsoni, L. Lazzari, M. G. Jodice, C. Luise, M. Erreni, S. Pece, P. P. Di Fiore, G. Viale, A. Spinelli, C. Pozzi, G. Penna, M. Rescigno, Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39, 708–724.e11 (2021). [DOI] [PubMed] [Google Scholar]
- 22.J. Mouries, P. Brescia, A. Silvestri, I. Spadoni, M. Sorribas, R. Wiest, E. Mileti, M. Galbiati, P. Invernizzi, L. Adorini, G. Penna, M. Rescigno, Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 71, 1216–1228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A. K. Bhunia, in Foodborne Microbial Pathogens: Mechanisms and Pathogenesis, A. K. Bhunia, Ed. (Springer, 2018), pp. 25–42. [Google Scholar]
- 24.H. Björkbacka, K. A. Fitzgerald, F. Huet, X. Li, J. A. Gregory, M. A. Lee, C. M. Ordija, N. E. Dowley, D. T. Golenbock, M. W. Freeman, The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol. Genomics 19, 319–330 (2004). [DOI] [PubMed] [Google Scholar]
- 25.T. Masuda, L. Amann, R. Sankowski, O. Staszewski, M. Lenz, P. D. Errico, N. Snaidero, M. J. C. Jordão, C. Böttcher, K. Kierdorf, S. Jung, J. Priller, T. Misgeld, A. Vlachos, M. Meyer-Luehmann, K.-P. Knobeloch, M. Prinz, Novel Hexb-based tools for studying microglia in the CNS. Nat. Immunol. 21, 802–815 (2020). [DOI] [PubMed] [Google Scholar]
- 26.F. Leng, P. Edison, Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 17, 157–172 (2021). [DOI] [PubMed] [Google Scholar]
- 27.C. Escartin, E. Galea, A. Lakatos, J. P. O’Callaghan, G. C. Petzold, A. Serrano-Pozo, C. Steinhäuser, A. Volterra, G. Carmignoto, A. Agarwal, N. J. Allen, A. Araque, L. Barbeito, A. Barzilai, D. E. Bergles, G. Bonvento, A. M. Butt, W.-T. Chen, M. Cohen-Salmon, C. Cunningham, B. Deneen, B. De Strooper, B. Díaz-Castro, C. Farina, M. Freeman, V. Gallo, J. E. Goldman, S. A. Goldman, M. Götz, A. Gutiérrez, P. G. Haydon, D. H. Heiland, E. M. Hol, M. G. Holt, M. Iino, K. V. Kastanenka, H. Kettenmann, B. S. Khakh, S. Koizumi, C. J. Lee, S. A. Liddelow, B. A. MacVicar, P. Magistretti, A. Messing, A. Mishra, A. V. Molofsky, K. K. Murai, C. M. Norris, S. Okada, S. H. R. Oliet, J. F. Oliveira, A. Panatier, V. Parpura, M. Pekna, M. Pekny, L. Pellerin, G. Perea, B. G. Pérez-Nievas, F. W. Pfrieger, K. E. Poskanzer, F. J. Quintana, R. M. Ransohoff, M. Riquelme-Perez, S. Robel, C. R. Rose, J. D. Rothstein, N. Rouach, D. H. Rowitch, A. Semyanov, S. Sirko, H. Sontheimer, R. A. Swanson, J. Vitorica, I.-B. Wanner, L. B. Wood, J. Wu, B. Zheng, E. R. Zimmer, R. Zorec, M. V. Sofroniew, A. Verkhratsky, Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.N. Habib, C. McCabe, S. Medina, M. Varshavsky, D. Kitsberg, R. Dvir-Szternfeld, G. Green, D. Dionne, L. Nguyen, J. L. Marshall, F. Chen, F. Zhang, T. Kaplan, A. Regev, M. Schwartz, Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A. N. Brandebura, A. Paumier, T. S. Onur, N. J. Allen, Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 24, 23–39 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Z. Jiwaji, S. S. Tiwari, R. X. Avilés-Reyes, M. Hooley, D. Hampton, M. Torvell, D. A. Johnson, J. McQueen, P. Baxter, K. Sabari-Sankar, J. Qiu, X. He, J. Fowler, J. Febery, J. Gregory, J. Rose, J. Tulloch, J. Loan, D. Story, K. McDade, A. M. Smith, P. Greer, M. Ball, P. C. Kind, P. M. Matthews, C. Smith, O. Dando, T. L. Spires-Jones, J. A. Johnson, S. Chandran, G. E. Hardingham, Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Aß pathology. Nat. Commun. 13, 135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.P. Hasel, I. V. L. Rose, J. S. Sadick, R. D. Kim, S. A. Liddelow, Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 24, 1475–1487 (2021). [DOI] [PubMed] [Google Scholar]
- 32.P. B. Crino, The mTOR signalling cascade: Paving new roads to cure neurological disease. Nat. Rev. Neurol. 12, 379–392 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Z. Z. Chong, Y. C. Shang, S. Wang, K. Maiese, Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog. Neurobiol. 99, 128–148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.S. Herman, I. Fishel, D. Offen, Intranasal delivery of mesenchymal stem cells-derived extracellular vesicles for the treatment of neurological diseases. Stem Cells 39, 1589–1600 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Global Burden of Disease Long COVID Collaborators, S. W. Hanson, C. Abbafati, J. G. Aerts, Z. Al-Aly, C. Ashbaugh, T. Ballouz, O. Blyuss, P. Bobkova, G. Bonsel, S. Borzakova, D. Buonsenso, D. Butnaru, A. Carter, H. Chu, C. De Rose, M. M. Diab, E. Ekbom, M. E. Tantawi, V. Fomin, R. Frithiof, A. Gamirova, P. V. Glybochko, J. A. Haagsma, S. H. Javanmard, E. B. Hamilton, G. Harris, M. H. Heijenbrok-Kal, R. Helbok, M. E. Hellemons, D. Hillus, S. M. Huijts, M. Hultström, W. Jassat, F. Kurth, I.-M. Larsson, M. Lipcsey, C. Liu, C. D. Loflin, A. Malinovschi, W. Mao, L. Mazankova, D. M. Culloch, D. Menges, N. Mohammadifard, D. Munblit, N. A. Nekliudov, O. Ogbuoji, I. M. Osmanov, J. L. Peñalvo, M. S. Petersen, M. A. Puhan, M. Rahman, V. Rass, N. Reinig, G. M. Ribbers, A. Ricchiuto, S. Rubertsson, E. Samitova, N. Sarrafzadegan, A. Shikhaleva, K. E. Simpson, D. Sinatti, J. B. Soriano, E. Spiridonova, F. Steinbeis, A. A. Svistunov, P. Valentini, B. J. van de Water, R. van den Berg-Emons, E. Wallin, M. Witzenrath, Y. Wu, H. Xu, T. Zoller, C. Adolph, J. Albright, J. O. Amlag, A. Y. Aravkin, B. L. Bang-Jensen, C. Bisignano, R. Castellano, E. Castro, S. Chakrabarti, J. K. Collins, X. Dai, F. Daoud, C. Dapper, A. Deen, B. B. Duncan, M. Erickson, S. B. Ewald, A. J. Ferrari, A. D. Flaxman, N. Fullman, A. Gamkrelidze, J. R. Giles, G. Guo, S. I. Hay, J. He, M. Helak, E. N. Hulland, M. Kereselidze, K. J. Krohn, A. Lazzar-Atwood, A. Lindstrom, R. Lozano, D. C. Malta, J. Månsson, A. M. M. Herrera, A. H. Mokdad, L. Monasta, S. Nomura, M. Pasovic, D. M. Pigott, R. C. Reiner Jr., G. Reinke, A. L. P. Ribeiro, D. F. Santomauro, A. Sholokhov, E. E. Spurlock, R. Walcott, A. Walker, C. S. Wiysonge, P. Zheng, J. P. Bettger, C. J. L. Murray, T. Vos, Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328, 1604–1615 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.J. J. Ekstrand, in Seminars in pediatric neurology. (Elsevier, 2012), vol. 19, pp. 96–100. [Google Scholar]
- 37.A. Miyazato, Mechanism of Cryptococcus Meningoencephalitis. Med. Mycol. J. 57, J27–J32 (2016). [DOI] [PubMed] [Google Scholar]
- 38.J. S. Bell, J. I. Spencer, R. L. Yates, S. A. Yee, B. M. Jacobs, G. C. DeLuca, Invited Review: From nose to gut - the role of the microbiome in neurological disease. Neuropathol. Appl. Neurobiol. 45, 195–215 (2019). [DOI] [PubMed] [Google Scholar]
- 39.D. N. Doll, H. Hu, J. Sun, S. E. Lewis, J. W. Simpkins, X. Ren, Mitochondrial crisis in cerebrovascular endothelial cells opens the blood-brain barrier. Stroke 46, 1681–1689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M. A. Sze, M. Tsuruta, S.-W. J. Yang, Y. Oh, S. F. Man, J. C. Hogg, D. D. Sin, Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLOS ONE 9, e111228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Z. Al-Aly, B. Bowe, Y. Xie, Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28, 1461–1467 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.H. S. Kwon, S.-H. Koh, Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 9, 42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.N. Deigendesch, L. Sironi, M. Kutza, S. Wischnewski, V. Fuchs, J. Hench, A. Frank, R. Nienhold, K. D. Mertz, G. Cathomas, M. S. Matter, M. Siegemund, M. Tolnay, L. Schirmer, A.-K. Pröbstel, A. Tzankov, S. Frank, Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 140, 583–586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.M. M. Mariani, T. Kielian, Microglia in infectious diseases of the central nervous system. J. Neuroimmune Pharmacol. 4, 448–461 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.S. Geyer, M. Jacobs, N.-J. Hsu, Immunity against bacterial infection of the central nervous system: An astrocyte perspective. Front. Mol. Neurosci. 12, 57 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D. Kaur, V. Sharma, R. Deshmukh, Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 27, 663–677 (2019). [DOI] [PubMed] [Google Scholar]
- 47.T. H. Palpagama, H. J. Waldvogel, R. L. Faull, A. Kwakowsky, The role of microglia and astrocytes in Huntington’s disease. Front. Mol. Neurosci. 12, 258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A. A. Asadi-Pooya, A. Akbari, A. Emami, M. Lotfi, M. Rostamihosseinkhani, H. Nemati, Z. Barzegar, M. Kabiri, Z. Zeraatpisheh, M. Farjoud-Kouhanjani, A. Jafari, S. Sasannia, S. Ashrafi, M. Nazeri, S. Nasiri, M. Shahisavandi, Long COVID syndrome-associated brain fog. J. Med. Virol. 94, 979–984 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.B. Neumann, M. L. Schmidbauer, K. Dimitriadis, S. Otto, B. Knier, W.-D. Niesen, J. A. Hosp, A. Günther, S. Lindemann, G. Nagy, T. Steinberg, R. A. Linker, B. Hemmer, J. Bösel; PANDEMIC and the IGNITE study groups , Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 418, 117090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.M. Bellon, C. Schweblin, N. Lambeng, P. Cherpillod, J. Vazquez, P. H. Lalive, M. Schibler, C. Deffert, Cerebrospinal fluid features in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) positive patients. Clin. Infect. Dis. 73, e3102–e3105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.G. U. Jeong, H.-J. Kwon, W. H. Ng, X. Liu, H. W. Moon, G. Y. Yoon, H. J. Shin, I.-C. Lee, Z. L. Ling, A. G. Spiteri, N. J. C. King, A. Taylor, J. S. Chae, C. Kim, D.-G. Ahn, K.-D. Kim, Y. B. Ryu, S.-J. Kim, S. Mahalingam, Y.-C. Kwon, Ocular tropism of SARS-CoV-2 in animal models with retinal inflammation via neuronal invasion following intranasal inoculation. Nat. Commun. 13, 7675 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S21
Table S1