Abstract
People with type 2 diabetes mellitus have a greater risk of developing cardiovascular problems. Since cardiovascular diseases are a major cause of mortality all over the world, we need to find more efficient measures to control this risk in the diabetes population in addition to conventional glycemic control. In this systematic review, we aim to explore the latest findings on the cardiovascular effects of glucagon-like peptide-1 (GLP-1) agonists and dual GLP-1/glucose-dependent insulinotropic peptide (GIP) agonists in patients with type 2 diabetes mellitus. We conducted a comprehensive literature search using PubMed and Google Scholar as the main sources for data collection. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 recommendations for conducting this review. The outcomes of interest included mortality due to cardiovascular causes, non-fatal myocardial infarction, stroke, effects on cardiovascular risk factors, heart failure, and development of arrhythmias. After thorough literature screening and quality analysis, 14 articles were finally included for qualitative synthesis. GLP-1 receptor agonists appeared to be effective in reducing the risk of cardiovascular mortality, myocardial infarction, and stroke. They were found to reduce the risk of composite major adverse cardiovascular event (MACE) outcomes by 12-14% when compared to placebo. Their role in preventing heart failure and arrhythmias is uncertain, and further trials are needed to confirm the same. The cardiovascular outcomes of GLP-1/GIP dual agonists are currently under investigation. Studies completed to date show that they do not increase the risk of cardiovascular disease when compared to placebo.
Keywords: cardiovascular adverse events, glp-1-gip co-agonist, cardiovascular outcomes, diabetes mellitus type 2, glp-1 receptor agonists
Introduction and background
Cardiovascular diseases (CVDs) are a leading cause of mortality worldwide [1]. Ischemic heart disease (IHD) and stroke were the top two causes of disability in people over 50 in 2019 and the two leading causes of disease burden in adults between the ages of 25 and 49 [1]. In the United States alone, about 697,000 people died from heart disease in the year 2020, which accounts for one in every five deaths [2,3]. Diabetes mellitus (DM) is an important risk factor for CVD. A large study published in the European Journal of Preventive Cardiology found that the prevalence of diabetes in patients with coronary heart disease was about 30% compared to 10.5% in the general population [4,5]. Unlike the microvascular complications of diabetes, glycemic control alone does not reduce the risk of mortality due to cardiovascular problems [6]. Hence, it is imperative that we find more ways to reduce this risk in the diabetes population.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) were initially developed as antihyperglycemic agents and have shown positive results in large-scale cardiovascular outcome trials (CVOTs). They primarily act by inducing glucose-dependent insulin secretion from pancreatic β cells, inhibiting glucagon release from pancreatic α cells, and delaying gastric emptying. These actions lead to a reduction in blood glucose and improved postprandial glucose metabolism. GLP-1 agonists are also known to induce satiety and weight loss through their action on hypothalamic neurons. As a result, liraglutide and semaglutide have been approved for weight management in people with obesity or overweight [7]. Another drug class recently grabbing attention is the dual GLP-1/glucose-dependent insulinotropic polypeptide (GIP) agonists. Tirzepatide (a new dual GLP-1/GIP agonist) displayed better and clinically relevant hemoglobin A1c (HbA1c) reduction when compared to insulin glargine in persons with type 2 diabetes (T2D) and increased cardiovascular risk, with a reduced incidence of hypoglycemia [8].
In December 2008, the US FDA issued a notice mandating manufacturers to conduct additional studies on the effect of drugs used for type 2 DM (T2DM) on atherosclerotic cardiovascular risk [9]. The FDA implemented this requirement in response to a suspected cardiovascular safety concern regarding rosiglitazone, a concern that was later dismissed [10]. Since that time, several studies have been completed across all medication classes, with most of them showing no concern for elevated atherosclerotic cardiovascular risk. Surprisingly, some of these studies have provided evidence for a reduction in major adverse cardiovascular events (MACEs) in addition to the other benefits [11].
In this systematic review, we aim to explore and aggregate the latest literature findings on the cardiovascular effects of GLP-1 agonists and dual GLP-1/GIP agonists in T2DM with or without pre-existing cardiovascular risk factors. Our outcomes of interest included mortality due to cardiovascular causes, non-fatal myocardial infarction (MI), stroke, effects on cardiovascular risk factors, heart failure (HF), and development of arrhythmias. We included clinical trials, meta-analyses, and traditional and systematic reviews that were published in the English language from 2018 to 2023.
Review
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 recommendations [12].
Database and Search Strategy
We conducted our data search from April 16, 2023 to April 23, 2023. We used PubMed and Google Scholar libraries as sources for data collection. We looked for studies related to the cardiovascular effects of GLP-1 agonists and dual GIP/GLP-1 RAs in patients with T2DM. Our detailed search strategy, keywords, and Medical Subject Headings (MeSH) terms used are elaborated in Table 1. Additionally, we also looked for relevant articles in the reference section of selected papers.
Table 1. Search strategy.
GLP: glucagon-like peptide-1; GIP: glucose-dependent insulinotropic polypeptide; RCT: randomized controlled trial.
| Database | Search strategy | No. of records before applying filters | Filters applied | No. of records after applying filters |
| PubMed | ((GLP 1 agonists OR semaglutide OR dulaglutide OR exenatide OR liraglutide OR lixisenatide OR (("Glucagon-Like Peptide 1/adverse effects"[Majr] OR "Glucagon-Like Peptide 1/agonists"[Majr] OR "Glucagon-Like Peptide 1/analogs and derivatives"[Majr] OR "Glucagon-Like Peptide 1/drug effects"[Majr] OR "Glucagon-Like Peptide 1/pharmacology"[Majr] OR "Glucagon-Like Peptide 1/therapeutic use"[Majr])) OR ("Glucagon-Like Peptide 1/adverse effects"[Mesh:NoExp] OR "Glucagon-Like Peptide 1/agonists"[Mesh:NoExp] OR "Glucagon-Like Peptide 1/analogs and derivatives"[Mesh:NoExp] OR "Glucagon-Like Peptide 1/drug effects"[Mesh:NoExp] OR "Glucagon-Like Peptide 1/pharmacology"[Mesh:NoExp] OR "Glucagon-Like Peptide 1/therapeutic use"[Mesh:NoExp])) AND (Tirzepatide OR glucose-dependent insulinotropic polypeptide/glucagon-like peptide 1 (GLP-1) receptor agonist OR Twincretin OR dual glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) receptor agonist OR (("Gastric Inhibitory Polypeptide/adverse effects"[Majr] OR "Gastric Inhibitory Polypeptide/agonists"[Majr] OR "Gastric Inhibitory Polypeptide/analogs and derivatives"[Majr] OR "Gastric Inhibitory Polypeptide/drug effects"[Majr] OR "Gastric Inhibitory Polypeptide/pharmacology"[Majr] OR "Gastric Inhibitory Polypeptide/therapeutic use"[Majr])) OR ("Gastric Inhibitory Polypeptide/adverse effects"[Mesh:NoExp] OR "Gastric Inhibitory Polypeptide/agonists"[Mesh:NoExp] OR "Gastric Inhibitory Polypeptide/analogs and derivatives"[Mesh:NoExp] OR "Gastric Inhibitory Polypeptide/drug effects"[Mesh:NoExp] OR "Gastric Inhibitory Polypeptide/pharmacology"[Mesh:NoExp] OR "Gastric Inhibitory Polypeptide/therapeutic use"[Mesh:NoExp])) AND (Diabetes Mellitus OR Diabetes OR (("Diabetes Mellitus, Type 2/drug therapy"[Majr] OR "Diabetes Mellitus, Type 2/therapy"[Majr])) OR ("Diabetes Mellitus, Type 2/drug therapy"[Mesh:NoExp] OR "Diabetes Mellitus, Type 2/therapy"[Mesh:NoExp])) AND (Cardiovascular outcomes OR Cardiovascular events OR Major Cardiovascular adverse events OR Cardiac OR Cardiovascular OR MACE OR (("Cardiovascular Diseases/drug therapy"[Majr] OR "Cardiovascular Diseases/prevention and control"[Majr] OR "Cardiovascular Diseases/therapy"[Majr])) OR ("Cardiovascular Diseases/drug therapy"[Mesh:NoExp] OR "Cardiovascular Diseases/prevention and control"[Mesh:NoExp] OR "Cardiovascular Diseases/therapy"[Mesh:NoExp]))) OR ((GLP 1 agonists OR semaglutide OR dulaglutide OR exenatide OR liraglutide OR lixisenatide) AND (Diabetes Mellitus OR Diabetes) AND (Cardiovascular outcomes OR Cardiovascular events OR Major Cardiovascular adverse events OR Cardiac OR Cardiovascular OR MACE)) OR ((Tirzepatide OR glucose-dependent insulinotropic polypeptide/glucagon-like peptide 1 (GLP-1) receptor agonist OR Twincretin OR dual glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) receptor agonist) AND (Diabetes Mellitus OR Diabetes) AND (Cardiovascular outcomes OR Cardiovascular events OR Major Cardiovascular adverse events OR Cardiac OR Cardiovascular OR MACE)) | 3251 | Free full text, clinical trial, meta-analysis, RCT, review, systematic review, English, 2018-2023 | 583 |
| Google Scholar | (GLP 1 agonists OR dual GLP 1/GIP agonist OR (GLP 1 agonists AND dual GLP 1/GIP agonist)) AND diabetes mellitus type 2 AND (cardiovascular effects OR cardiovascular outcomes) | 1310 | 2018-2023, English | 760 |
Inclusion Criteria
We included peer-reviewed articles and studies published in the English language from 2018 to 2023. The types of studies chosen were clinical trials, meta-analyses, and traditional and systematic reviews. Only the articles available as free full texts were considered.
Exclusion Criteria
We excluded gray literature, studies conducted before 2018, and those published in languages other than English. We also excluded any studies on children, adolescents, animals, and patients with type 1 DM.
Data Extraction and Quality Assessment
After removing duplicate articles from the search, two authors independently conducted screening and data extraction based on inclusion and exclusion criteria. Any disagreements were resolved by consensus, and the opinion of a third reviewer was sought when required. The tools used for critical appraisal were the Cochrane Risk of Bias tool for randomized controlled trials (RCTs) [13], the New Castle Ottawa scale for observational studies [14], the AMSTAR (A MeaSurement Tool to Assess systematic Reviews) checklist for systematic reviews and meta-analysis [15], and the SANRA (Scale for the Assessment of Narrative Review Articles) checklist for narrative reviews [16]. We excluded studies that were of low quality.
Results
Search Results
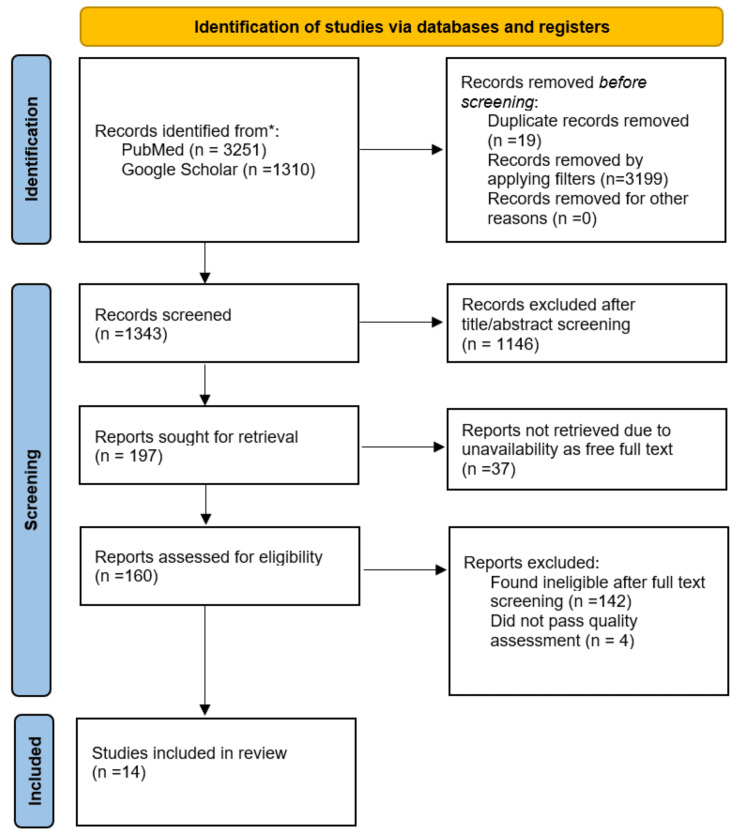
Our literature search yielded a total of 4561 articles through PubMed and Google Scholar. Upon applying filters and removing duplicate studies, 1343 articles were retrieved for title and abstract screening. Two individual reviewers conducted the screening independently and excluded 1146 articles for being irrelevant. A similar method of independent screening by two reviewers was used to read the full text, which led to the exclusion of 142 articles. Finally, 18 articles were selected for critical appraisal, of which 14 passed our assessment and were included in the study. Figure 1 shows the detailed PRISMA flow diagram [12].
Figure 1. PRISMA flow diagram.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Critical Appraisal
We assessed the quality of our selected papers using quality appraisal tools. We used the AMSTAR (a measurement tool to assess the methodological quality of systematic reviews) for systematic reviews and meta-analyses and the SANRA (Scale for the Assessment of Narrative Review Articles) for traditional reviews. The detailed quality assessment of the included studies is shown in Table 2. Only those studies that scored above 75% were finally included.
Table 2. Critical appraisal of included studies.
AMSTAR: A MeaSurement Tool to Assess systematic Reviews.
| Title | Author | Type of study | Quality assessment tool used | Quality score |
| The longer-term benefits and harms of glucagon-like peptide-1 receptor agonists: a systematic review and meta-analysis | Alexander et al. [17] | Systematic review and meta-analysis | AMSTAR | 91% |
| Heterogeneity of antidiabetic treatment effect on the risk of major adverse cardiovascular events in type 2 diabetes: a systematic review and meta‑analysis | D’Andrea et al. [18] | Systematic review and meta-analysis | AMSTAR | 100% |
| GLP‑1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta‑analysis of eight CVOTs | Giugliano et al. [19] | Meta-analysis | AMSTAR | 91% |
| The effect of DPP‑4 inhibitors, GLP‑1 receptor agonists and SGLT‑2 inhibitors on cardiorenal outcomes: a network meta‑analysis of 23 CVOTs | Giugliano et al. [20] | Meta-analysis | AMSTAR | 91% |
| Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis | Kanie et al. [21] | Meta-analysis | AMSTAR | 100% |
| Effects of GLP-1 receptor agonists on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease: a systematic review and meta-analysis | Kelly et al. [22] | Systematic review and meta-analysis | AMSTAR | 82% |
| A meta-analysis evaluating indirectly GLP-1 receptor agonists and arrhythmias in patients with type 2 diabetes and myocardial infarction | Liu et al. [23] | Meta-analysis | AMSTAR | 82% |
| Glucagon‑like peptide‑1 (GLP‑1) receptor agonists and cardiovascular events in patients with type 2 diabetes mellitus: a meta‑analysis of double‑blind, randomized, placebo‑controlled clinical trials | Qin et al. [24] | Meta-analysis | AMSTAR | 100% |
| Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis | Sattar et al. [25] | Meta-analysis | AMSTAR | 82% |
| Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: a systematic review and meta-analysis | Wei et al. [26] | Systematic review and meta-analysis | AMSTAR | 91% |
| Risk of stroke and retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: an eight RCTs meta-analysis | Wei et al. [27] | Meta-analysis | AMSTAR | 82% |
| Cardiovascular and renal outcomes with SGLT‑2 inhibitors versus GLP‑1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta‑analysis | Yamada et al. [28] | Systematic review and meta-analysis | AMSTAR | 91% |
| The potential of glucagon-like peptide-1 receptor agonists in heart failure | Kreiner et al. [29] | Narrative review | SANRA | 92% |
| GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes | Marx et al. [30] | Narrative review | SANRA | 92% |
Study Characteristics
At the end of the screening and quality check, 14 articles were finally included for review. Out of the total, 12 were meta-analyses and two were traditional reviews. Table 3 shows the study characteristics in detail.
Table 3. Characteristics of included studies.
MACEs: major adverse cardiovascular events; GLP-1 RA: glucagon-like peptide-1 receptor agonists; CVD: cardiovascular disease; CVOTs: cardiovascular outcome trials; SGLT-2: sodium-glucose cotransporter-2; RCTs: randomized controlled trials; T2DM: type 2 diabetes mellitus; CKD: chronic kidney disease; RR: relative risk; MI: myocardial infarction; eGFR: estimated glomerular filtration rate; HFpEF: heart failure with preserved ejection fraction; ASCVD: atherosclerotic cardiovascular disease; HbA1c: hemoglobin A1c.
| Author | Name of journal and publication year | Type of study | Patient population | Outcome |
| Alexander et al. [17] | Journal of General Internal Medicine, 2022 | Systematic review and meta-analysis | 45 trials comprising 71,517 patients were included | The three-component MACE outcome favored GLP-1 RA as compared to placebo (RR: 0.87, 95% CI: 0.82–0.93, I2 = 23%). GLP-1 RAs led to fewer strokes (RR: 0.86, 95% CI: 0.78–0.95, I2 = 0%). GLP-1 RAs compared to placebo were also associated with significant reductions in cardiovascular risk factors |
| D’Andrea et al. [18] | Cardiovascular Diabetology, 2020 | Systematic review and meta-analysis | 10 trials enrolling 89,790 patients were included | GLP-1 RA drugs showed a 12% overall reduction in MACEs (HR: 0.88; 95% CI: 0.82–0.94) |
| Giugliano et al. [19] | Cardiovascular Diabetology, 2021 | Meta-analysis | Eight CVOTs enrolling 60,080 patients were included of which 72.4% had established CVD | GLP-1 RAs reduce the risk of MACE by 14% compared to placebo in patients with T2DM over a period of 1.3-5.4 years (HR: 0.86; 95% CI: 0.79–0.94; P = 0.006) |
| Giugliano et al. [20] | Cardiovascular Diabetology, 2022 | Meta-analysis | 23 trials enrolling a total number of 181,143 participants were included | GLP-1 RA can reduce MACE by 13% and the risk of non-fatal stroke compared to placebo; SGLT-2 inhibitors are superior in reducing cardiovascular death, hospitalization for HF |
| Kanie et al. [21] | Cochrane Database of Systematic Reviews, 2021 | Meta-analysis | 20 RCTs enrolling 129,465 participants were included in meta-analysis (31 studies were used for qualitative analysis) | GLP-1 RA may lower the risk of CVD mortality and all-cause mortality in patients with established CVD, according to meta-analyses of moderate- to high-certainty evidence; moderate-certainty evidence is probable in favor of using GLP-1 RA to lower fatal and non-fatal stroke |
| Kelly et al. [22] | Pharmacotherapy, 2022 | Systematic review and meta-analysis | Four RCTs comprising 7130 patients with T2DM and CKD were included | In a subset population with T2DM and CKD, GLP-1 RAs were not linked with a lower risk of the composite cardiovascular endpoint (three-point composite MACE), compared to placebo (odds ratio: 0.80; 95% CI: 0.59–1.07; P = 0.13) |
| Liu et al. [23] | Frontiers in Cardiovascular Medicine, 2022 | Meta-analysis | Five RCTs with 31,314 patients, which had at least 30% patients with T2DM and MI, were included | GLP-1 RAs may be linked with reduced risk for atrial arrhythmias (RR: 0.81; 95% CI: 0.70–0.95).; GLP-1 RAs appear to have a stronger anti-atrial arrhythmia impact in patients with T2DM and MI |
| Qin et al. [24] | BMC Endocrine Disorders, 2022 | Meta-analysis | Six RCTs with a total of 52,821 patients were included | GLP-1 RA therapy reduced mortality from cardiovascular causes (RR: 0.90; 95% CI: 0.83–0.97; P = 0.004) and fatal or non-fatal stroke (RR: 0.85; 95% CI: 0.77–0.94; P = 0.001) |
| Sattar et al. [25] | Nature Medicine, 2022 | Meta-analysis | Seven RCTs with a total of 7215 patients | Participants with T2DM who took tirzepatide experienced no increased risk of serious cardiovascular problems across a spectrum of T2DM duration and cardiovascular risk levels (HR: 0.80; 95% CI: 0.57–1.11) |
| Wei et al. [26] | Frontiers in Endocrinology, 2022 | Systematic review and meta-analysis | Eight CVOTs were included with a total of 60,081 participants | GLP-1 RA therapy has no discernible impact on the risk of severe arrhythmias in T2DM patients (RR: 0.96; 95% CI: 0.96–1.05; P = 0.36) |
| Wei et al [27] | Frontiers in Endocrinology, 2022 | Meta-analysis | Eight RCTs with a total patient population of 60081 were included | GLP-1 RA significantly reduces the risk of total stroke (RR: 0.83; 95%CI: 0.73-0.95; P = 0.005), as well as ischemic stroke (RR: 0.83; 95%CI: 0.73-0.95; P = 0.008) in type 2 diabetes with cardiovascular risk factors |
| Yamada et al. [28] | Cardiovascular Diabetology, 2021 | Systematic review and meta-analysis | 13 studies were selected with a total of 32,949 patients | GLP-1 RAs did not lead to significantly lower cardiovascular endpoints in patients with T2DM and CKD (eGFR < 60 mL/min/1.73 m2) (RR: 0.91; 95% CI: 0.80–1.04) |
| Kreiner et al. [29] | Frontiers in Physiology, 2022 | Narrative review | Eight CVOTs with a total of 60,081 participants and one meta-analysis were included | People with HF who have or are at risk of having obesity-related HFpEF are most likely to benefit from GLP-1 RA therapy |
| Marx et al. [30] | Circulation, 2022 | Narrative review | Eight CVOTs with a total of 60,081 participants were included | Numerous studies have demonstrated that GLP-1 RAs lower cardiovascular risk in individuals with diabetes and ASCVD, or diabetes and high cardiovascular risk without regard to HbA1c |
Discussion
In this systematic review, we found that GLP-1 RAs have a positive effect on cardiovascular outcomes in people with T2DM. High certainty evidence indicates a reduction of both cardiovascular mortality and all-cause mortality by GLP-1 RAs when compared with placebo [21].
Effects on MACE
Several RCTs have been conducted to date to study the cardiovascular outcomes of GLP-1 RA. A meta-analysis of these trials conducted by Giugliano et al. found that when compared to a placebo, GLP-1 RA lowers MACE risk in T2DM patients by 14% [19]. The three MACE components, CV mortality (reduced by 13%), non-fatal stroke (reduced by 16%), and non-fatal MI (reduced by 9%, although the level of reduction was not statistically significant), are likewise decreased by GLP-1 RA. This reduction in risk was independent of the chemical makeup of these medications (exendin-4-based or human equivalents). Additionally, Giugliano et al. discovered that these medications decreased the risk of MACE to a greater extent in patients with known cardiovascular illness compared to those without established CVD (16% vs. 6% reduction, respectively). A similar finding was observed by D’Andrea et al., wherein patients with pre-existing CVD showed a 14% reduction of MACE (HR: 0.86; CI: 0.80-0.93) on GLP-1 RA, while those at high risk of CVD, but without a history of cardiovascular events, seemed to have minimal or no effect (HR: 0.94; CI: 0.82-1.07) [18]. In the same analysis, baseline HbA1c level was discovered to be an effect modifier in addition to cardiovascular history. Individuals with baseline HbA1c levels that were equal to or higher than 8% showed trends toward significant decreases in the risk of MACE compared to individuals with baseline HbA1c values below 8%. The trials included in the meta-analysis, however, enrolled a majority of, and occasionally only, patients with established CVD and used inconsistent definitions of established CVD. Therefore, additional research focusing specifically on this population would be required to determine whether or not GLP-1 RAs are effective for the primary prevention of cardiovascular events.
Another study conducted by Alexander et al. focused mainly on the longer-term benefits and harms of GLP-1 RAs on the cardiovascular system. Their results provide reassurance that the reduction in MACE and cardiovascular risk factors associated with GLP-1 RA use as compared to placebo was maintained at the end of one year [17]. GLP-1 RAs have been found to significantly reduce the risk of non-fatal stroke when compared with placebo [17,19,21]. According to a study conducted on the GLP-1 RA CVOTs, these medications reduced the risk of total stroke as well as ischemic stroke by approximately 17% in T2D patients [27]. However, they did not lower the risk for hemorrhagic stroke with statistical significance, although the relative risk (RR) was similar to that for ischemic stroke. Among Asian patients with T2DM who had dyslipidemia or hypertension but no known atherosclerotic cardiovascular illnesses, retrospective cohort research published in 2022 found a connection between longer use and larger dosages of GLP-1 RAs and a reduced risk of hospitalization for ischemic stroke [31]. In a further investigation in 2021 examining the efficacy and safety of sodium/glucose cotransporter-2 inhibitor (SGLT2i), GLP-1 RA, and dipeptidyl peptidase 4 inhibitor (DPP4i), it was discovered that just GLP-1 RAs were connected to a lower chance of stroke in comparison with placebo (RR: 0.85, 95% CI: 0.76-0.94) [32].
Effects on HF
Currently, there is sparse evidence on the effects of GLP-1 RAs on HF. Sattar et al. conducted a meta-analysis of the data available from the CVOTs. According to their research, the GLP-1 RA could considerably lower the incidence of HF hospital admission by 11% (HR vs. placebo of 0.89; 95% CI: 0.82-0.98) [33]. All eight CVOTs for GLP-1 RAs, with the exception of semaglutide (HR: 1.11, 95% CI: 0.77-1.61), show HRs of <1. For this secondary outcome, only efpeglenatide and albiglutide demonstrated statistical significance. This may imply that Sattar et al.'s meta-analysis, which identified a benefit of GLP-1 RAs on HF hospitalization, was influenced by the data from the efpeglenatide and albiglutide CVOTs. Post hoc analyses of the CVOT for efpeglenatide revealed a significant 39% RR reduction in HF requiring hospitalization (HR: 0.61; 95% CI: 0.38-0.98). Even though the CVOTs frequently featured a significant count of people with T2D and HF at enrollment, it must be highlighted that the majority of the CVOTs did not analyze outcomes based on the presence of HF at enrollment. Additionally, the studies' definitions of HF varied and were generally vague [29].
Two studies, LIVE (24-week study in adults with stable chronic HF with or without diabetes) and FIGHT (effects of liraglutide on clinical stability among patients with advanced HF and reduced ejection fraction) [34,35], were conducted to examine the effect of liraglutide on HF. In either experiment, liraglutide was unable to show any statistically significant advantages or disadvantages. As a result, the findings regarding GLP-1 RA's effects on HF are mostly equivocal, and additional studies are required to validate such effects.
Cardiovascular Effects in Patients With CKD
Two of the included studies focused on the cardiovascular outcomes of GLP-1 RAs in patients with T2DM and pre-existing CKD. CKD was defined as a reduced baseline eGFR below 60 mL/min/1.73 m2. Neither of the studies observed a statistically significant benefit of GLP-1 RAs in reducing composite cardiovascular outcomes in this subset of patients [22,28]. However, a subsequent analysis among GLP-1 RA subclasses revealed that GLP-1 analogs significantly reduced the risk of MACE, while exendin-4 analogs did not. Hence, the final results of the above two studies appear to be influenced by the neutral cardiovascular effects of exenatide (HR: 0.91; 95% CI: 0.83-1.00) and the fact that exenatide CVOT had the largest sample size among the GLP-1 RA CVOT included in the analysis. However, Kelly et al.'s meta-analysis revealed that other GLP-1 RAs were linked to lower cardiovascular event rates in CKD patients, with liraglutide and once-weekly semaglutide showing the greatest absolute reductions [22]. Natriuresis-induced BP reduction, a decrease in reactive oxygen species and inflammation, and an improvement in endothelial function are some potential pathways for these positive benefits. To confirm these effects of GLP-1 RAs in patients with CKD, additional research is required.
Arrhythmias
Wei et al. conducted a systematic review and meta-analysis of data extracted from eight CVOTs for GLP-1 RA to explore their effects on different arrhythmias. They included 60,081 participants of which 76.7% had a history of cardiovascular disease (CVD). Their research revealed that neither the risk of specific types of arrhythmias nor the risk of all arrhythmias was affected by GLP-1 RA therapy (RR: 0.96, 95% CI: 0.96-1.05, P = 0.36) [26]. The use of GLP-1 RAs, however, may be linked to a reduced risk of atrial arrhythmias, according to Liu et al.'s meta-analysis of five trials including 31,314 patients [23]. Only semaglutide was found to lower the risk of atrial arrhythmias and AF, according to the subgroup analysis; the other GLP-1 RAs showed no anti-arrhythmic effect. To be included in this analysis, however, trials had to have at least 30% of their patients with T2DM and MI. The study's findings thus imply that individuals with T2DM and MI appear to be more responsive to the anti-atrial arrhythmia action of GLP-1 RAs.
Effects on Cardiovascular Risk Factors
A meta-analysis of 45 trials comprising 71,517 patients conducted by Alexander et al. in 2022 observed favorable effects of GLP-1 RAs on cardiovascular risk factors at the end of at least one year. GLP-1 RAs led to lower HbA1c levels compared to placebo (MD = −0.67%; 95% CI: −0.77 to −0.58%; I2 = 93%) [17]. Trials where participants received background medications at baseline as well as those where participants did not receive other anti-hyperglycemic medications in addition to the study drug showed a similar reduction in HbA1c. GLP-1 RAs also reduced systolic blood pressure (MD = −1.75 mmHg; 95% CI: −2.14 to −1.35 mmHg; I2 = 48%), weight (MD −1.84 kg; 95% CI: −2.37 to −1.30 kg; I2 = 95%), BMI (MD = −1.12 kg/m2; 95% CI: −1.67 to −0.57 kg/m2; I2 = 96%), and low-density lipoprotein (LDL) (MD = −0.04 mmol/L; 95% CI: −0.06 to −0.02; I2 = 0%) when compared to placebo. On the other hand, heart rate increased by about 2 bpm (MD = 2.22 bpm; 95% CI: 1.69-2.75 bpm; I2 = 88%). It must be noted that there was significant heterogeneity among several of the above outcomes. However, subgroup analysis generated consistent results.
Dual GLP-1/GIP Agonists
Tirzepatide is a single modified peptide with GIP and GLP‐1 RA approved for the treatment of people with T2D in the United States. Its cardiovascular outcomes are currently under investigation. The drug is also being investigated for its effects on chronic weight management, heart failure with preserved ejection fraction (HFpEF), obesity, and non‐alcoholic steatohepatitis. In a pre-specified meta-analysis of phase two and phase three trials, Sattar et al. observed that tirzepatide was not associated with increased risk of the MACE-4 (composite of cardiovascular death, MI, stroke, and hospitalization because of unstable angina) outcome (HR: 0.80; 95% CI: 0.57-1.11), cardiovascular death (HR: 0.90; 95% CI: 0.50-1.61), MI (HR: 0.76; 95% CI: 0.45-1.28), stroke (HR: 0.81; 95% CI: 0.39-1.68), and hospitalization for unstable angina (HR: 0.46; 95% CI: 0.15-1.41) [25]. The overall cardiovascular results show that tirzepatide treatment, when compared to placebo or comparators not known to be cardioprotective, is not associated with an increased risk of CVD. This treatment was given for a median of just over a year at a mean randomization dose of 9.9 mg per week to a population in which just over one-third had already developed CVD. In subgroup analyses by sex, age, baseline HbA1c, race, US or non-US clinical sites, or baseline SGLT2i use for the primary MACE-4 outcome, no significant effect modification was discovered. According to studies, tirzepatide was superior to placebo, semaglutide 1 mg/week, dulaglutide 1.5 mg/week, insulin degludec 100 U/mL, and semaglutide or insulin glargine 100 U/mL in lowering HbA1c and weight in T2D patients throughout a 26- to 52-week treatment period [36,37]. Once the tirzepatide CVOT results are released, it will be known if or not this advantage over GLP-1 RA will extend to cardiovascular outcomes.
Strengths and Limitations
The strengths of this systematic review are the inclusion of the most recent studies on cardiovascular outcomes of GLP-1 RAs, the large sample sizes in almost all the studies, and the high quality of trials on which the studies were based. We included only those studies that had a low risk of bias. Limitations include excluding studies conducted before 2018, those published in languages other than English, and articles unavailable as free full text. Our review was mainly based on meta-analyses and narrative reviews of previously conducted RCTs. While this provides a good picture of the overall effects of GLP-1 RA as a class, we may have missed the specific effects of individual drugs belonging to the class.
Conclusions
In patients with T2DM, GLP-1 RAs are associated with a significant 12-14% reduction in three-point composite MACE outcome consisting of cardiovascular mortality, non-fatal MI, and non-fatal stroke compared to placebo. They also significantly reduce the risk of ischemic stroke in T2D patients with cardiovascular risk factors. These effects appeared to be more pronounced in people with pre-existing CVD. Further studies are required to elucidate the role of GLP-1 RA in HF and cardiac arrhythmias. Tirzepatide, the dual GLP-1/GIP agonist approved for treating T2DM, is being investigated for cardiovascular outcomes. This novel class of drugs needs further exploration to unravel its benefits or harms in the treatment of DM. Additionally, it is unknown whether these novel antihyperglycemic medications have cardioprotective properties irrespective of diabetic status, and further investigation may hold the potential to pave the way to a new era of cardiovascular risk management.
The authors have declared that no competing interests exist.
References
- 1.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. GBD 2019 Diseases and Injuries Collaborators. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Health Statistics. About Multiple Cause of Death, 1999-2020. CDC WONDER Online Database website. Atlanta, GA: Centers for Disease Control and Prevention; 2022. [ Apr; 2023 ];https://wonder.cdc.gov/ 2022 21:2022. [Google Scholar]
- 3.Heart Disease and Stroke Statistics-2022 Update: a report from the American Heart Association. Tsao CW, Aday AW, Almarzooq ZI, et al. Circulation. 2022;145:0–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 4.Prevalence of diabetes and impact on cardiovascular events and mortality in patients with chronic coronary syndromes, across multiple geographical regions and ethnicities. Mak KH, Vidal-Petiot E, Young R, et al. Eur J Prev Cardiol. 2022;28:1795–1806. doi: 10.1093/eurjpc/zwab011. [DOI] [PubMed] [Google Scholar]
- 5.International Diabetes Federation. Vol. 10. Brussels, Belgium: International Diabetes Federation; [ Apr; 2023 ]. 2021. IDF Diabetes Atlas, 10th Edition ; p. 2021. [Google Scholar]
- 6.Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 7.Efficacy of GLP-1 RA approved for weight management in patients with or without diabetes: a narrative review. Jensterle M, Rizzo M, Haluzík M, Janež A. Adv Ther. 2022;39:2452–2467. doi: 10.1007/s12325-022-02153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Del Prato S, Kahn SE, Pavo I, et al. Lancet. 2021;398:1811–1824. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 9.US Food Drug Admin. 2008. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Center for Drug Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services, Silver Spring, MD. Res., US Food Drug Admin., US Dep. Health Hum. Serv., Silver. 2008. https://www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-for-industry-on-diabetes-mellitus-evaluating-cardiovascular-risk-in-new-antidiabetic https://www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-for-industry-on-diabetes-mellitus-evaluating-cardiovascular-risk-in-new-antidiabetic
- 10.Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. Nissen SE, Wolski K. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 11.More than 7 years of hindsight: revisiting the FDA’s 2008 guidance on cardiovascular outcomes trials for type 2 diabetes medications. Regier EE, Venkat MV, Close KL. Clin Diabetes. 2016;34:173–180. doi: 10.2337/cd16-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RoB 2: a revised tool for assessing risk of bias in randomised trials. Sterne JA, Savović J, Page MJ, et al. BMJ. 2019;366:0. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [ Apr; 2023 ]. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 15.Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. Shea BJ, Grimshaw JM, Wells GA, et al. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SANRA-a scale for the quality assessment of narrative review articles. Baethge C, Goldbeck-Wood S, Mertens S. Res Integr Peer Rev. 2019;4:5. doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The longer-term benefits and harms of Glucagon-Like Peptide-1 receptor agonists: a systematic review and meta-analysis. Alexander JT, Staab EM, Wan W, et al. J Gen Intern Med. 2022;37:415–438. doi: 10.1007/s11606-021-07105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heterogeneity of antidiabetic treatment effect on the risk of major adverse cardiovascular events in type 2 diabetes: a systematic review and meta-analysis. D'Andrea E, Kesselheim AS, Franklin JM, Jung EH, Hey SP, Patorno E. Cardiovasc Diabetol. 2020;19:154. doi: 10.1186/s12933-020-01133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Giugliano D, Scappaticcio L, Longo M, et al. Cardiovasc Diabetol. 2021;20:189. doi: 10.1186/s12933-021-01366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: a network meta-analysis of 23 CVOTs. Giugliano D, Longo M, Signoriello S, Maiorino MI, Solerte B, Chiodini P, Esposito K. Cardiovasc Diabetol. 2022;21:42. doi: 10.1186/s12933-022-01474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis. Kanie T, Mizuno A, Takaoka Y, et al. Cochrane Database Syst Rev. 2021;10:0. doi: 10.1002/14651858.CD013650.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effects of GLP-1 receptor agonists on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease: a systematic review and meta-analysis. Kelly M, Lewis J, Rao H, Carter J, Portillo I, Beuttler R. Pharmacotherapy. 2022;42:921–928. doi: 10.1002/phar.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A meta-analysis evaluating indirectly GLP-1 receptor agonists and arrhythmias in patients with type 2 diabetes and myocardial infarction. Liu Z, Bian N, Wu S, et al. Front Cardiovasc Med. 2022;9:1019120. doi: 10.3389/fcvm.2022.1019120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glucagon-like peptide-1 (GLP-1) receptor agonists and cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of double-blind, randomized, placebo-controlled clinical trials. Qin J, Song L. BMC Endocr Disord. 2022;22:125. doi: 10.1186/s12902-022-01036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, Zoungas S. Nat Med. 2022;28:591–598. doi: 10.1038/s41591-022-01707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: a systematic review and meta-analysis. Wei J, Wang R, Ye H, Wang Y, Wang L, Zhang X. Front Endocrinol (Lausanne) 2022;13:910256. doi: 10.3389/fendo.2022.910256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risk of stroke and retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: an eight RCTs meta-analysis. Wei J, Yang B, Wang R, Ye H, Wang Y, Wang L, Zhang X. Front Endocrinol (Lausanne) 2022;13:1007980. doi: 10.3389/fendo.2022.1007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Yamada T, Wakabayashi M, Bhalla A, et al. Cardiovasc Diabetol. 2021;20:14. doi: 10.1186/s12933-020-01197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The potential of glucagon-like peptide-1 receptor agonists in heart failure. Kreiner FF, Hovingh GK, von Scholten BJ. Front Physiol. 2022;13:983961. doi: 10.3389/fphys.2022.983961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Marx N, Husain M, Lehrke M, Verma S, Sattar N. Circulation. 2022;146:1882–1894. doi: 10.1161/CIRCULATIONAHA.122.059595. [DOI] [PubMed] [Google Scholar]
- 31.GLP-1RAs for ischemic stroke prevention in patients with type 2 diabetes without established atherosclerotic cardiovascular disease. Yang YS, Chen HH, Huang CN, Hsu CY, Hu KC, Kao CH. Diabetes Care. 2022;45:1184–1192. doi: 10.2337/dc21-1993. [DOI] [PubMed] [Google Scholar]
- 32.The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: a network meta-analysis of randomised clinical trials. Lin DS, Lee JK, Hung CS, Chen WJ. Diabetologia. 2021;64:2676–2686. doi: 10.1007/s00125-021-05529-w. [DOI] [PubMed] [Google Scholar]
- 33.Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Sattar N, Lee MMY, Kristensen SL, et al. Lancet Diabetes Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 34.Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Jorsal A, Kistorp C, Holmager P, et al. Eur J Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 35.Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. Margulies KB, Hernandez AF, Redfield MM, et al. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS- 1): a double-blind, randomised, phase 3 trial. Rosenstock J, Wysham C, Frias JP, et al. Lancet. 2021;398:143–155. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 37.Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. Frías JP, Davies MJ, Rosenstock J, et al. N Engl J Med. 2021;385:503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]